Abstract
The cochlear implant (CI) represents, for almost 25 years now, the gold standard in the treatment of children born deaf and for postlingually deafened adults. These devices thus constitute the greatest success story in the field of ‘neurobionic’ prostheses. Their (now routine) fitting in adults, and especially in young children and even babies, places exacting demands on these implants, particularly with regard to the biocompatibility of a CI’s surface components. Furthermore, certain parts of the implant face considerable mechanical challenges, such as the need for the electrode array to be flexible and resistant to breakage, and for the implant casing to be able to withstand external forces.
As these implants are in the immediate vicinity of the middle-ear mucosa and of the junction to the perilymph of the cochlea, the risk exists – at least in principle – that bacteria may spread along the electrode array into the cochlea. The wide-ranging requirements made of the CI in terms of biocompatibility and the electrode mechanism mean that there is still further scope – despite the fact that CIs are already technically highly sophisticated – for ongoing improvements to the properties of these implants and their constituent materials, thus enhancing the effectiveness of these devices.
This paper will therefore discuss fundamental material aspects of CIs as well as the potential for their future development.
Keywords: cochlear implant, biomaterials, biocompatibility, electrode, inner ear, cochleostomy, surface functionalization, drug delivery, nanoparticles, coating
1 The cochlear implant
1.1 Clinical requirements
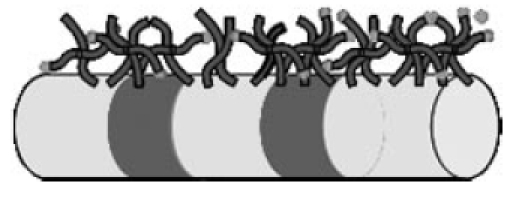
The chief clinical requirements that have to be fulfilled by a cochlear implant derive from the main functional principle of the implant: the differential, site-specific transfer of charge to the auditory nerve and the tonotopic hearing sensation that this generates. The CI systems currently available have developed largely out of cardiac pacemaker technology. In this regard it has proved possible to draw on broad empirical experience, not only in relation to the use of suitable materials (silicone, ceramics, titanium and platinum), but also in terms of benefiting from the already existing production technology (Figure 1 (Fig. 1)). In the early phase when CIs were first being introduced, this meant that even at that initial stage these implants had good biocompatibility [1]. Ensuring the long-term stability and long-term function of CIs is one of the main clinical needs within the requirement profile for these implants. The reasons for this are twofold: the early age at implantation, and the high average life expectancy of the recipients, who continue to receive technical intervention into their senior years. The intention, therefore, is to give implants a functional life of several decades. The choice of implant materials is thus of crucial significance – the aim being, as far as possible, to prevent the need for revision surgery or for operations to replace the implant, as this delays auditory rehabilitation and places a strain on the recipient.
Figure 1. The implantable portion of the cochlear implant: (1) Transmitter coil in a silicone sheath; (2): Electronics enclosed in a titanium casing; (3): Electrode with platinum contacts in a silicone array; (4): Cochleostomy site; (5): Silicone cable in the middle-ear/mastoid region.

As well as meeting the material requirements geared to long-term stability, the materials used must (as far as possible) be hypoallergenic. In addition to their biological compatibility, the long-term technical integrity of the implants is therefore a further important necessity. The clinical demands made of a CI were described as follows in 1992 by E. Lehnhardt (modified after [2]):
The materials used must be biocompatible (i.e. physically tolerated);
The insertion of the electrode array should not cause any additional damage;
The surgical technique used should be as non-invasive as possible;
There needs to be efficient, non-damaging electrical stimulation of the auditory nerve on a sustained basis;
There must be no increased risk of infection caused by the implant and by the access route to the fluid-filled spaces of the cochlea.
1.2 Clinical provision: status
At present, some 2,000 cochlear implants are fitted in Germany every year. By way of comparison, around 66,000 cardiac pacemakers are implanted there annually [3]. The technical execution of the surgical procedure is now standard medical practice. Alongside routine unilateral implantation, bilateral CI fitting has become an established approach in recent years, which makes possible a considerable improvement in both spatial hearing and speech understanding for CI users [4]. Bilateral implantation, especially among recipients fitted in early childhood, has now become virtually the standard therapy for deaf-born children.
The large incision (“Hannover C-incision”, Figure 2 (Fig. 2)) formerly used has now been abandoned by most surgeons in favour of considerably smaller, primarily retroauricular access routes (Figure 3 (Fig. 3)) [5]. This has made for a considerable reduction in the rate of postoperative infection [6], [7]. A possible cause of this could be the fact that, where the smaller incision is used, this allows the superior blood supply to the skin flap to be preserved [8]. The surgical procedure generally involves mastoidectomy, posterior tympanotomy, and the drilling of a cochleostomy in order to insert the intracochlear electrode array. Many surgeons create a bony recess in the bone of the skull, which serves to accommodate the implant (i.e. as an implant bed) and thus provide protection against undesired mobilization of the device. A connecting tunnel between the implant bed and the mastoid is often also used, which allows protection of the electrodes at the point where they exit the implant, as well as serving to additionally fix the device in place within the bone. The use of auxiliary materials [9], [10], [11] to hold the implant in position (such as sutures, osteosynthetic material, bone cement, Dacron sutures and titanium clips) has therefore been discontinued by many surgeons as the implant can be adequately secured by modifying the surgical technique accordingly (i.e. by creating a bony implant bed and bony projections in the area of the mastoid in order to protect the electrode). The main reason for this change in practices lies in the observation that, viewed in the long term, some of the auxiliary materials used show insufficient biocompatibility or have led to local irritation. These include the formation of cochleastomas following the use of Dacron sutures for securing the electrode array in the rear wall of the ear canal. Overall, both the surgical procedure and the long-term stability of the materials used have resulted in a high degree of technical reliability and surgical safety.
Figure 2. Preoperative marking of the incision line for the “Hannover C” incision formerly used, showing the large wound surface that results.
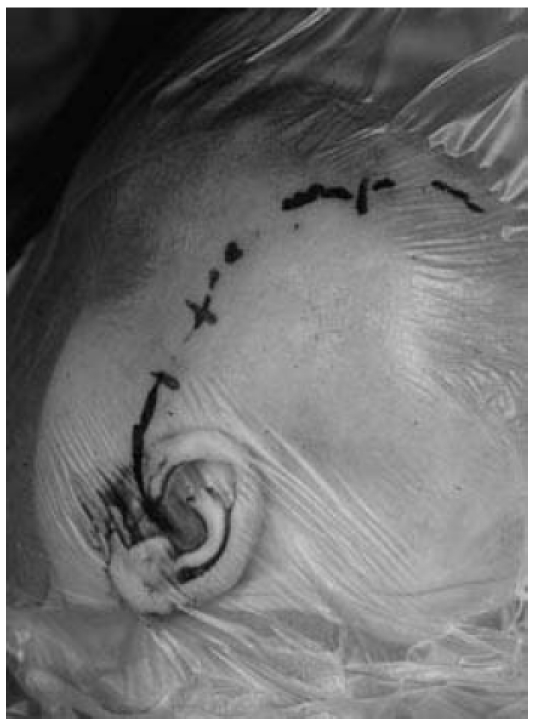
Figure 3. Smaller, retroauricular incision in use today (left ear, dummy implant).
2 General material requirements for a cochlear implant
A cochlear implant, unlike many other implants used in human applications, has a number of very different requirements to fulfil. These include mechanical stability and charge transfer to the auditory nerve, as well as biocompatibility and long-term stability.
2.1 Mechanical requirements
The mechanical requirements for a cochlear implant vary greatly with regard to the individual components. The implant’s outer casing needs to be a stable and fluid-tight enclosure that securely houses the electronics. This is a not insignificant aspect in terms of ensuring the device’s long-term functioning. Important materials that have found application over the years include titanium and ceramics, each of which combines a number of beneficial properties. Although ceramic materials have high resistance to breakage, almost all CI manufacturers now favour titanium instead for housing the implant’s electronic components. This is because ceramics have proved prone to a lack of leak-tightness, leading to implant failure, and because their mechanical resistance to external forces (such as impact trauma to the head) is poorer than that of titanium. Moreover, the most important parts of the implant casing are coated with a layer of silicone (Figure 1 (Fig. 1)). In a 2002 analysis (carried out at Hannover Medical School) of the reasons for removal of CIs manufactured by Advanced Bionics and Cochlear, a technical defect was identified in 97 cases (i.e. 66.4% of these explantations), of which 13 were attributable to a lack of leak-tightness and a further 54 to impact damage [12]. The investigations carried out during this study related to the augmentation of the standard that governs “active implantable medical devices” specifically for cochlear CIs. In this connection, proposals for a uniform reporting standard to be followed by manufacturers and implanting centres in the event of implant failure have been drawn up by Battmer et al. [13].
The second part of the implant, the electrode array, needs to exhibit not only great flexibility but also long-term mechanical stability if it is not to give rise to cable breaks in the bend of the array, or to a lack of leak-tightness (the latter being caused by short-circuiting). Modern CI electrodes usually consist of a silicone carrier material in which the platinum contacts and the input wires (platinum-iridium 90/10 with insulating Teflon coating) are embedded.
Certain CI electrodes place complex demands on mechanical geometry, as the electrode bodies are preformed; example include the Nucleus Contour system (Cochlear Ltd.) or the Helix electrode from Advanced Bionics. The bent shape of the electrode (Figure 4 (Fig. 4)) is designed to reduce the distance between the electrode and the neuronal cells in the modiolus, thus leading to focused stimulation. Elastic materials are able to reduce insertion trauma, although the electrode must have the necessary stiffness to ensure it can be inserted atraumatically, and be positioned safely and reproducibly within the cochlea. Cochlear’s Contour electrode has an elasticity of 4.8x108 Pa at the tip and 4.0x108 Pa at the base. The side adjacent to the modiolus is somewhat more elastic than the outer side. The Nucleus straight electrode, however, has lower flexural rigidity at the tip. The elasticity module at the tip is 1.8x108 Pa, whereas the flexural rigidity of the base (4.9x108 Pa) [14], [15] is similar to that of the Contour electrode. Electrode stiffness is thus determined less by the carrier material (i.e. silicone, with an elasticity module of 4.5x105 Pa) and more (indeed, chiefly) by the internal Pt/Ir wires, whose geometric configuration is the main influence on the electrode’s mechanical properties [15]. It is frictional forces that play the most significant role in terms of electrode insertion [16], [17]. Comparative studies have revealed Contour electrodes and the Nucleus straight electrode to have similar frictional coefficients on the modiolar side, with the Contour electrode exhibiting considerably lower frictional coefficients on the lateral side [18].
Figure 4. View of preformed electrode with modified tip (Softip®, Cochlear Ltd).
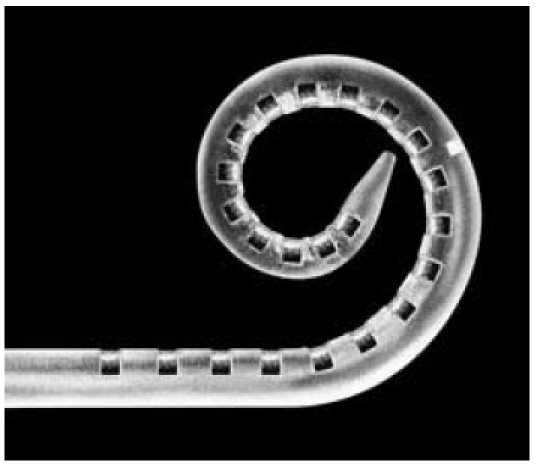
The choice of material plays a major part, especially with regard to the development of new electrode designs, as here the electrode is required to meet other requirements in terms of its mechanical properties. Examples include electrodes with a soft tip and low contact pressure on the basilar membrane and the outer wall of the cochlea (Softip®, Cochlear Ltd., Figure 4 (Fig. 4)) [19], as well as the development of new access routes to the cochlea, as for example with the endosteal electrode [20], [21]. Here, the use of novel carrier materials (such as new polymers) could lead to the electrodes’ mechanical properties being optimized [22]. Furthermore, the literature contains individual reports on applications in animal experiments involving electrode arrays, surface-coated with polymers, which bring about alterations in the electrode mechanism [23], [24].
2.2 Biological requirements
With regard to the middle-ear region, the biological requirements for cochlear implant electrode arrays relate to the possibility of contact with bacteria and the potential spread of pathogens along the electrode array into the recipient’s inner ear [25]. It is important to ensure that the cochleostomy is well-sealed and to prevent a sheath of connective tissue forming around the electrode array (Figure 5 (Fig. 5)) [26]. The fact that pathogens come into contact with the electrode surface relatively frequently in the middle-ear region means a risk exists that a biofilm will form or, in extreme cases, that bacteria will spread from the middle ear into the inner ear [27], [28], [29] or the cerebrospinal fluid, and that meningitis may occur. It is thus especially important to securely seal the cochleostomy (Figure 6 (Fig. 6)) [30], [31].
Figure 5. Histological section through the cochlea of a guinea pig showing formation of new connective tissue and bone in the area of the scala tympani (EK: electrode channel; scala vestibuli (Sc. V.); scala media (Sc. M.); RK: Rosenthal channel, BDG: connective tissue, K: bone).
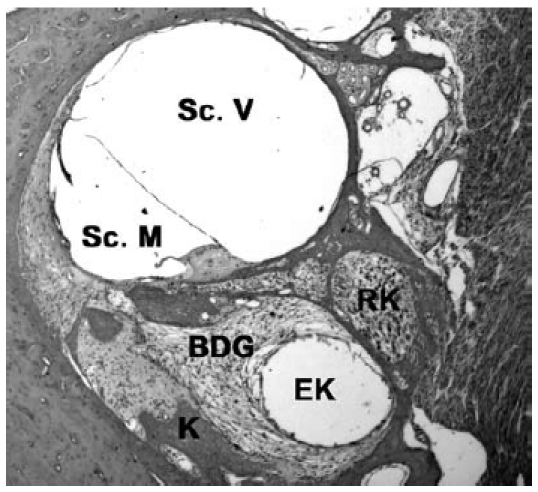
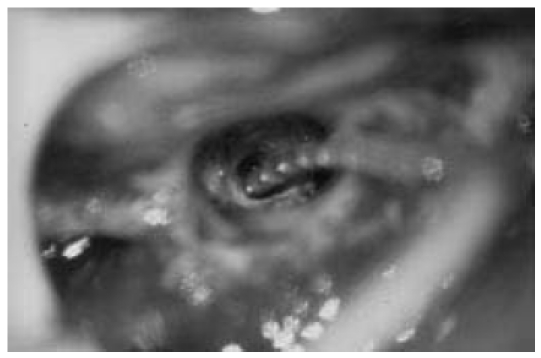
Figure 6. View of the cochleostomy during the insertion of a cochlear implant electrode.
2.3 Electrical requirements
The chief function of the cochlear implant involves facilitating charge transfer from the electrode array to the auditory nerve and the hearing sensation that this generates. At present, all CI manufacturers use platinum contacts in electrode production [2]. Moreover, iridium oxide coatings have been investigated in certain studies, which showed beneficial effects in terms of what happens to impedance following implantation [32], [33].
The demands placed on electrode contacts are highly exacting with regard to the potential functional life of implants (>20 years). It is essential to avoid corrosion [34] and to prevent the electrode contacts being damaged or destroyed. Long-term stimulation of neural tissue involving a charge density of <30 µC/cm-2 geometric/phase is generally regarded as safe [35], provided the stimulation is pulsatile in nature and the electrode surface is relatively large [2]. This value is well below those at which electrochemical responses can be expected. With platinum electrodes, gas is observed to form at values of 300 µC/cm-2 geometric/phase and above, owing to electrochemical changes [36]. If neural damage occurs even at low values, this should be interpreted as the result of electrical stimulation of the neural structures and not as being attributable to noxious chemical substances generated by undesired electrochemical responses [2]. Corrosion of the platinum can be observed at 500 µs/pulse and above, or at or above a current density of 2 mA/mm2 [37]. These values, too, are well below the parameters used in clinical practice (namely 0.9 mA and 25 µs/phase). Platinum is thus an extremely safe material for human applications involving cochlear implantation.
2.4 Long-term stability
Alongside mechanical considerations (such as the need to prevent electrode breakage and thus short-circuiting), density-related aspects play a particularly important role in determining implants’ long-term stability. Implants sometimes fail a number of years after implantation, although it is most likely to happen within the first two years. For example, a study by Maurer et al. [38], involving three different implant types, revealed the cumulative survival rate to be 93.2% two years after implantation and 92.7% five years post-implantation. These findings show that the technical integrity of implants in present-day use has reached a high level but still has scope for further improvement.
3 Currently used materials and their properties
3.1 Overview
The use of cochlear implants involves the following materials coming into contact with the human body: silicone, platinum, titanium and ceramics. Platinum is used as the electrode contact. For the Teflon-coated wires between the receiver/stimulator and the electrode contacts, platinum/iridium 90/10 is used. The wires are embedded in silicone, and thus do not come into contact with human tissue. The electronics within the implant body are housed in a tightly sealed casing which – depending on manufacturer and implant series – is made of either ceramics or titanium. The materials that come into contact with the human body are dealt with more fully below.
3.2 Silicone
Silicones are polymers that are built around a frame of silicon and oxygen atoms, on which hydrocarbon radicals (which, in the case of silicones used in medicine, are usually methyl groups [39]), are often present. The individual polymer chains may differ in length and in the degree of crosslinking. Chain length and cross-linkage are critical in determining the physical properties of silicones [40]. These materials have been used in medicine for more than 60 years now [39].
Silicones for use in medicine are regarded as having good biostability [39]. Owing to their flexibility and stability, these materials are used for various prostheses (including blood vessels, finger joints, heart valves and prosthetic outer ears) [41]. There are, however, indications that silicones' properties change following implantation; in other words, a kind of ‘ageing’ process sets in [42], [43]. Furthermore, Leslie et al. [44] have been able to show that the internal environment within the body also affects this process of deterioration in silicones. This means that silicones, despite their excellent biocompatibility, still have potential for improvement.
3.3 Platinum
Precious metals such as platinum are preferred as contact materials where electrical stimulation is involved, as they have low chemical reactivity and are highly resistant to corrosion. However, even precious metals can, upon electrical stimulation, partially dissolve or form unstable surface films. The extent of these undesired effects depends upon the density of the charge that is transmitted during electrical stimulation and on the polarity of the stimulus [45], [46]. Platinum is very well tolerated by the body and, as it is relatively soft in comparison with iridium, is easier to work with. Even after around 2,000 hours of stimulation testing in animal experiments, and as much as 10,000 hours of such testing in humans, no stimulus-induced corrosion has been detected [34]. Platinum therefore constitutes the best electrode material currently available.
3.4 Titanium
Titanium is particularly suitable for applications in which rigidity, low weight and high resistance to corrosion are essential. Titanium is an inert and solid metal. Its use for the casing of the receiver/stimulator has its origins in experience with cardiac pacemaker technology. In order to securely seal the pacemaker housing, a ceramic material has been developed that bonds permanently to both the metal of the wires and to the titanium of the casing [35]. This became the standard technique for sealing the point where the wires exit the pacemaker and was adopted in the development of certain cochlear implants [47]. Where titanium is used as a casing material, the receiver coil must be placed outside the casing. Titanium is therefore the material most commonly used by manufacturers for enclosing the electronic components of CIs.
3.5 Ceramics
Ceramic materials are non-metallic composites that frequently consist of a matrix made of a base material permeated with various other substances. The materials that a given ceramic contains determine its subsequent properties, as does the nature of the production process. Ceramics used for technical applications are commonly based on aluminium oxide (Al2O3).
Ceramics are used (see section 4.4) for sealing the points where the wires emerge from the implant casing. They also find (or found) application as a casing material for enclosing the electronic components. An advantage of using ceramic materials for this purpose is that the receiver coil can be placed within the casing, as ceramics do not greatly affect signal transmission. However, the material is more brittle than titanium and thus more prone to breakage under significant mechanical stress (Figure 7 (Fig. 7)).
Figure 7. Cochlear implant with cracked casing (here, ceramic casing) following application of mechanical force caused by the wearer falling on the implant.
4 Clinical application of biomaterials used
4.1 Allergic reaction/material incompatibility
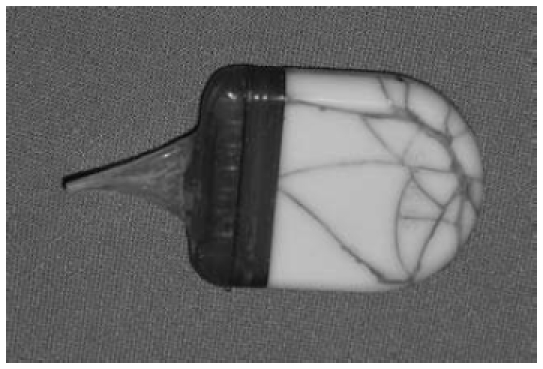
The literature contains only isolated instances in which a demonstrable allergic reaction or intolerance to silicone (where it is the principal material in cochlear implants) is described [48], [49], [50]. These cases, however, have prompted the provision of test kits in which material samples are made available by the manufacturers in laminar or rod form for implantation at a site (such as the forearm) that is well away from the ear (Figure 8 (Fig. 8)). This procedure allows individualized compatibility testing – involving an observation period of around six weeks – intended to rule out allergic responses to the CI material which is to be implanted.
Figure 8. Individual compatibility test designed to rule out allergic reactions to the cochlear implant material. Left: preparation of the forearm; centre: incision for the creation of a subcutaneous pocket; right: positioning of the test material beneath the skin.

Taking into account the total number of cochlear implantations, the probability of silicone intolerance occurring is low [51]. In the majority of cases where an allergy is suspected, the actual problem is more likely to involve a chronic infection or the formation of bacterial biofilm.
4.2 Formation of connective tissue around the implant (electrode and casing)
Although silicone possesses a number of beneficial properties that make it possible to use it for producing both flexible electrodes and biocompatible implant sheaths, it does also have certain disadvantageous characteristics. These include a relatively strong ability to induce connective-tissue growth, which can be observed around both the electrode array [52] and the implant casing. Encapsulation of the implant casing can yield definite advantages, as this provides additional fixation for the implant and makes unwanted displacement less likely. Formation of connective tissue around the cochleostomy site is also desirable, as this seals off the inner ear from the middle ear, which both prevents perilymph leaking out of the inner ear and makes it harder for bacteria to ascend from the middle ear into the inner ear. Within the cochlea, however, the formation of connective tissue is undesirable, as this sheath of connective tissue around the electrodes acts as an insulating layer that impedes the transfer of charge from the electrode array to the auditory nerve and thus leads to a postoperative increase in electrical impedance [53]. As stable, low impedance is essential if the electrical stimulation of the cochlea’s neuronal structures is to be effective and safe, reduction of connective-tissue growth (or even its avoidance altogether) is to be sought [54]. Intraoperative administration of steroids could reduce postoperative tissue response [55]. Various studies have investigated the reductive effect of a range of steroid preparations on postoperative fibroblast growth in cochlear implantation, although a solution of triamcinolone acetonide (Kenacort® A; 40 mg/ml) has proven highly effective [55]. Measurements reveal that patients in whom Triamcinoline (Triamhexal 40®) was used have significantly lower impedance than a control group [33]. A long-term study by a research team headed by De Ceulaer, in which the effect of one-time intracochlear administration of steroids immediately prior to electrode insertion was investigated, revealed a significant reduction in impedance even two months after implantation [56].
4.3 Revision surgery
Clinical observations made in relation to revision surgery not only show marked formation of connective and scar tissue around the implant and/or electrode array, but also reveal the sheathing of the array by connective tissue in the cochlea [2], [35]. In the event that the implant needs replacing, the electrode can be successfully reinserted in the vast majority of cases [57], so that – despite the electrode array being enveloped in connective tissue within the cochlea – the creation and dilatation of a passage with the electrode of the new implant prove successful. Rühl et al. [58] were able to demonstrate that the outcome of speech understanding tests one year on from reimplantation was as good as, if not better than, that before reimplantation. The literature contains descriptions of how, in exceptional cases, the existing electrode array is held in place within the cochlea by encircling it with a “wall” of scar tissue or bone [59]. The difficulty in such instances is that further attempts to extract the array can only partially remove it, with a residual portion remaining within the cochlea [60]. This calls for an intraoperative decision as to whether only one electrode – albeit incomplete and basally positioned – can be inserted or whether a double array can be introduced into the cochlea by means of two cochleostomies.
4.4 Formation of biofilm
The formation of a biofilm constitutes as as-yet-unsolved problem in cochlear implantation. Biofilms result when micro-organisms colonize boundary surfaces. They consist of a thin layer of mucous (i.e. a film) and form primarily in aqueous or moist systems, either at the aqueous surface or at its interface with a solid.
Cochlear implantation provides a good example of how these infections can occur as introduced infections that gain access during surgery; in principle, middle-ear infections [61] [62], [63] can also spread and thus result in bacterial colonies that form on components of the implant. Infections usually affect the magnetic unit, with the electrode less often affected [64]. The silicone surface of the magnet unit provides bacteria or biofilms with a good point of adhesion, making them virtually inaccessible to conventional, local or systemically applied antibiotics. In cochlear implant revision surgery, S. aureus has often been found [65], [66]. Clinically, the cases in question are marked by a postoperative healing process that is initially normal [66]. Over the subsequent weeks and months, however, recurrent swelling – which often spontaneously opens – occurs at the implant site or the mastoid (Figure 9 (Fig. 9)). It tends to discharge only a relatively small amount of putrid secretion and there is often strong formation of granulation tissue (Figure 10 (Fig. 10)) around the implant [67]. The implant surface is usually already infected in these cases, i.e. a biofilm has already formed on the implant surface by this time. Despite antibiotic therapy and rinsing with disinfectant solutions, these biofilms are generally prone to recurrence, so that there is often no choice but to remove the implant. The implant usually shows no macroscopic changes in these cases.
Figure 9. Swelling around the implant site in a case where the implant is chronically infected. Right of picture: spontaneous perforation of the skin.
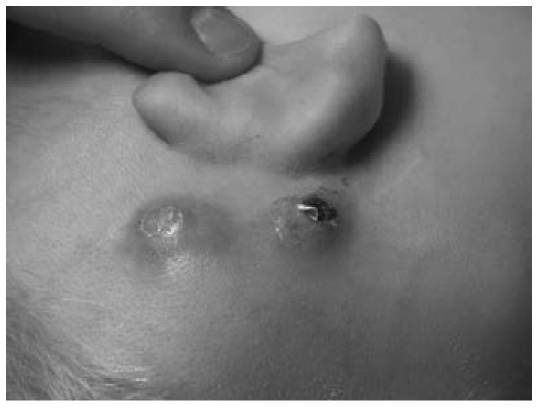
Figure 10. Infected cochlear implant with marked formation of granulation tissue around the implant.

Formation of biofilm is a clinically relevant problem as, in general, despite several swab samples being taken, it is not possible to clearly identify the pathogens involved, and treatment with antibiotics tends to lead to only a short-term improvement in the situation [68]. Often the only clinical option in these cases is to remove the implant, leaving the intracochlear electrode in place as an intracochlear placeholder. The intracochlear electrode is usually unaffected by the infection. After an infection-free phase of around three months, the reimplantation of the CI can then be carried out.
Changes in design – in order to avoid dead volume, for example – can be instrumental in reducing the risk of infection [69], whereas infections of the middle ear increase the probability that the implant will become infected [70].
It is not always easy to make a clinical distinction between an infection of the implant or formation of biofilm on the one hand, and intolerance of the implant materials (especially silicone) on the other; this task occasionally poses the physician a difficult challenge. In individual cases, an allergy test using test electrode materials, as described above, may be helpful in resolving this.
One complication of cochlear implantation is a heightened risk of meningitis [71]. As the mechanisms of infection have not yet, however, been fully clarified, various mechanisms are discussed in the literature [72]. The CI reduces the pathogen threshold required to induce meningitis. The cochleostomy in and of itself, however, does not increase the risk of infection [73]. Animal experiments show that the infection occurs only via the direct route (i.e. the middle ear or inner ear) and not systemically or intraperitoneally [74].
4.5 Clinical recommendations for action
In view of the large numbers of implants and the relatively low rates of infection and intolerance, preoperative testing with implant materials would not appear justified. However, it is essential that implantation be performed under completely sterile conditions so that the implant is not placed at risk through bacterial contamination, and in order that reimplantation – which is expensive, as well as time-consuming for the recipient – can be avoided.
5 Prospects for biomaterials development in cochlear implants
5.1 The implant of the future – objectives for new developments in implants
5.1.1 Clinical requirements
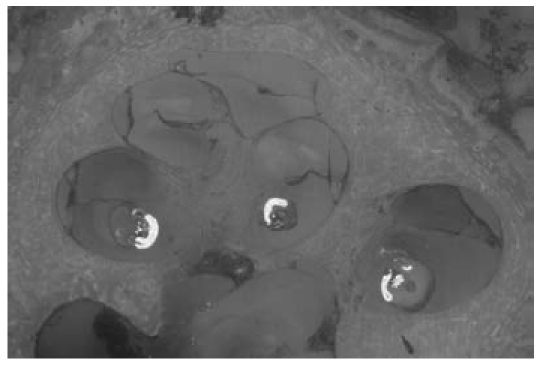
Implant development is set to pursue a number of clinical objectives into the future. The focus will be on enhancing biomaterial properties with regard to biocompatibility and optimizing the nerve-electrode interface (Figure 11 (Fig. 11)), as well as on reducing size (especially that of the intracochlear electrode array) and increasing the number of channels that can be stimulated independently of each other.
Figure 11. Section through a temporal bone and the cochlea with inserted perimodiolar cochlear implant electrodes in the scala tympani, showing the electrode contact surfaces facing towards the modiolus.
The effectiveness of electrical auditory prostheses must be optimized to the extent that hearing ability can be achieved which is as natural as possible. This entails, among other things, a considerable improvement in electrical channel separation and thus stimulus selectivity, a reduction in unspecific tissue growth, protection of neuronal cells and the regeneration of neuronal dendrites that form a cell-specific contact with the electrically conducting electrode material. The current objectives in this field revolve around the development of new multifunctional electrodes that couple conventional electrode functions with new bioactive functions. Current approaches to optimization take in the entire process chain – involving design, material, electrode production and surface functionalization, and including the individual adjustments made to each recipient’s own particular anatomy.
With regard to optimizing the biocompatibility properties of the cochlear implant’s surface materials which come into contact with body tissue, the dominant notion is that of preventing the formation of connective tissue around the intracochlear electrode array, in order to facilitate improved charge transfer to the auditory nerve at this point (Figure 5 (Fig. 5)). In addition to the use of completely new implant materials – in particular, new silicone substances – the functionalization of conventional silicones also merits consideration here. This can be achieved by surface structuring (i.e. physical functionalization), by the binding or release of drugs at the electrode array (i.e. drug delivery functionalization), by coating with polymers (i.e. chemical functionalization), by applying a layer of cells in order to release biological substances (i.e. biological functionalization), or by coating with polymers and signal proteins (i.e. biochemical functionalization) (Figure 12 (Fig. 12)).
Figure 12. The development of biomimetic implants: different methods of surface functionalization.
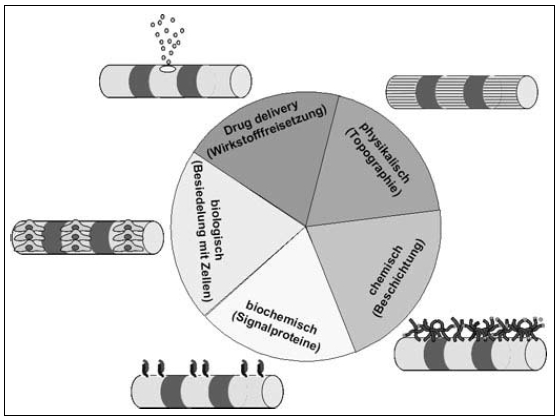
Two goals closely related to these ideas are optimizing the CI’s nerve-electrode interface by means of releasing substances into the inner ear (known as local drug delivery) and developing these approaches to the stage where they are technically feasible, as specifically applied to CI systems currently in use (involving, for example, structural modification of electrode arrays for fluid release). The development of biomimetic implants requires them to be specifically designed with the target tissue in mind.
5.1.2 Biomaterial requirements
If cochlear implant technology is to continue to advance, especially in terms of surgery aimed at preserving hearing (advances in which include Cochlear Ltd.'s Hybrid L system and MedEl GmbH’s Flex EAS), this necessitates the further miniaturization of the electrode systems in order to minimize both the insertion trauma caused and the resulting formation of connective tissue around the intracochlear part of the electrode. Moreover, the materials employed should make it possible to use a larger number of electrode contacts than has previously been the case, thus allowing better intracochlear frequency resolution. The technology that might be used here could borrow from chip technology, with switching circuits sited directly on the electrode array and active electronic elements on the intracochlear electrode array.
5.2 New implant materials
5.2.1 Teflon
PTFE (poly(tetrafluoroethylene)), also known as Teflon, is a thermally and chemically highly stable polymer that is very hydrophobic. Teflon is already used to a limited extent in commercially available cochlear implants (specifically, for insulating the wires between the implant body and the electrode contact). In laboratory investigations, a movable Teflon strip has been used in order to enable the electrode array to be placed within the cochlea in a position close to the modiolus [75]. Experiments involving Teflon have also been conducted with a view to using it for sealing cochleostomy sites, although this technique did not become established [76], [29].
Among the current applications for Teflon are its use in vascular prostheses [77], [78], [79] and in investigations with a view to repairing defects in the abdominal wall [80], which have demonstrated its fundamental biocompatibility. Extending these applications to CIs would thus appear conceivable.
5.2.2 Electrically conducting polymers
Promising developments in the material sciences have demonstrated the technical feasibility of electrically conducting polymers. Depending on their chemical structure, synthetic materials may possess electrically conducting, semiconducting or isolating properties. It was through the use of these materials for electronic applications that the term “polymer electronics” was coined. Whereas electronic components made of plastic would have been inconceivable only recently, they are now well on the way towards becoming reality.
Conductive polymers are usually produced by adding small quantities of oxidizing agents such as chlorine, bromine, iodine or arsenic pentafluoride to create the polymer matrix. In the process, they acquire electrical conducting abilities comparable to those of metallic conductors [81], [82]. The first applications considered for conductive polymers included batteries and accumulators. Other semiconducting polymers are used primarily in the field of optoelectronics and in photonic components [83]. The use of such materials in medical engineering is, however, problematic in terms of how biocompatible these substances are at present. Conjugated polymers in a conductive state are, almost without exception, insoluble, infusible and not very elastic. The metal-like ability to conduct is inextricably bound up with the solid state and a crystalline arrangement. One means of producing modifiable electrically conducting polymers involves the copolymerization of electrically conducting substances with conventional polymers, such as polyacetylene in polyethylene, polystyrol or polypyrrol [84], [85].
The potential benefits of using modified polymers as electrode materials consist in the improved mechanical properties of the electrode array and in the scope for placing a considerably higher number of electrode contacts on the electrode array. This approach could lead to the electrode having considerably enhanced overall flexibility and thus to further optimization of electrodes that have the potential to help preserve hearing. However, polymer-based implants must, in terms of their long-term stability and electrical transmission properties, be held up as the benchmark for conventional implant materials, so that immediate clinical application is not yet expected at the present time.
5.2.3 Polyimides
Even in the early phase of cochlear implant development, the fundamental biocompatibility of polyimides was demonstrated. For example, the basic usability of these materials in the cochlea has been successfully shown [86]. Polyimides are polymers that are chemically stable, electrically insulating and very easy to work with [87], [88], [89], [90]. Fibroblast growth on polyimide surfaces is comparable with that on polystyrene surfaces [91]. Polyimides are already in use today for flexible electrodes [92] and as a carrier material for gold wires in the power supply system for subretinal implants [93]. Furthermore, the flexibility of these substances enables a smooth transaction to be facilitated between the electrode (with its particular mechanical properties) and the tissue, in order to prevent damage caused by minute movements of the electrodes (as during neural stimulation, for example) [94], [95], [96]. Polyimides thus constitute a promising new prospect for electrode manufacture, which may in the future be incorporated into the production process for CIs.
5.2.4 Silicon electrodes
Thanks to the very rapid development of chip technology, which has in recent years led to a radical reduction in the size of the components used, it is conceivable that these methods could also be used for multichannel electrode systems [97]. The use of this technology, i.e. the placing of active electrode systems on the intracochlear component of the electrode, could lead to automation and make the production process a good deal faster, in turn leading to a reduction in the price of implants. This technology also offers scope for optimizing the coupling between the nerve and electrode. It is conceivable that implant functions could be transferred from the implant casing to the electrode array (i.e. into the cochlea), so that these advances could ultimately lead to the creation of a purely intracochlear electrode array that replaces the implant casing used thus far. This process could draw on diverse aspects of retinal implant development, in which similar approaches are being pursued with a view to using active electrode systems for the eye. This involves silicon-based photodiode arrays being implanted under the retina; in these arrays, the stimulating electrodes stimulate the retinal neurones [98]. A key prerequisite for the application of these systems is that they are demonstrably biocompatible for the inner ear. At the present time, sufficient data relating to the cochlea – such that would indicate that this technology can be rapidly transferred into clinical use – are not available. However, the possibility of cost-effective mass production of electrodes, in particular, does make for an interesting prospect with regard to the introduction of this technology into the CI manufacturing process.
5.3 Modification of surface properties
5.3.1 Physical functionalization of surfaces
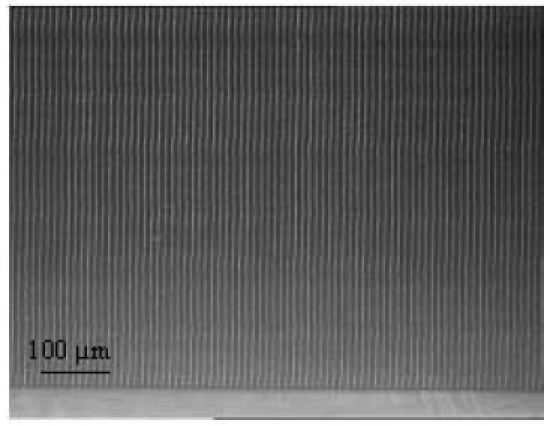
The only way to change biomaterial properties in terms of biocompatibility is to alter the surface geometry (Figure 13 (Fig. 13)). The long-term objective of optimized nerve-electrode interaction can therefore be achieved both by choosing a new carrier material [99] and through the structural modification of already known materials and their surfaces. For silicone, which is chiefly used for the surface of cochlear implants, the micro- and nanostructuring of the implant surface play a key role here. Investigations using in vitro models have already demonstrated the crucial effect of microstructuring the silicone surface in terms of reducing the growth of connective tissue. Various laser techniques are used for generating the surface structures (Figure 14 (Fig. 14)), such as laser ablation, two-photon polymerization, laser-induced melting dynamics, lithography and moulding. Nanostructures can thus specifically influence the hydrophobicity of the surface and, in turn, material-cell interaction as well.
Figure 13. Schematic representation of physical surface functionalization: alteration of surface topography.

Figure 14. Example of linear microstructure on silicone, generated through ablation by ultrashort laser pulses.
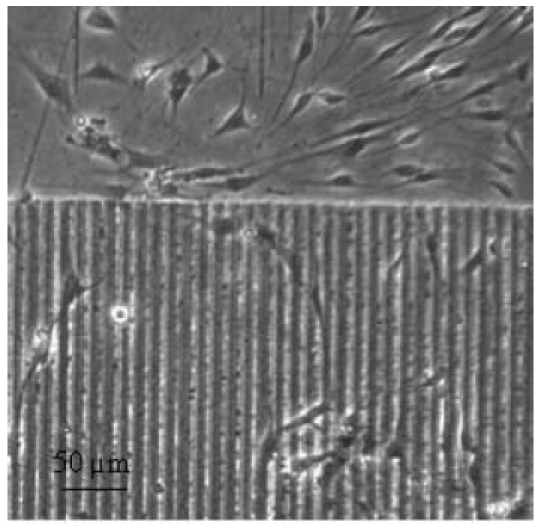
Altering surface topography by using ultra-short laser pulses provides an example of the physical functionalization of CI materials. In vitro, microstructures of linear configuration generated by means of laser ablation can both reduce the growth of connective-tissue cells (Figure 15 (Fig. 15)) [100] and influence the direction of growth of neuronal cells [101]. Individual research projects (including ventures in close collaboration with industry) are already underway, the aim of which is to translate into clinical applications the technological advances achieved with CIs (Collaborative Research Centre (SFB) 599: project T1).
Figure 15. Example of physical surface functionalization: cultivation of cells growing adherently (fibroblasts, duration of culture: three days) on laser-structured silicone surface (structural width 10 µm).
A number of research teams have also already been able to show that, by structuring the surface of materials on the nanometric scale, we can considerably influence the rate at which connective-tissue cells adhere to these surfaces. The studies by Spatz et al. [102], [103], [104] can be regarded as groundbreaking in this field; they demonstrated that structural differences of only a few nanometres in scale dramatically alter the interactive behaviour between material and cell.
The modification of surface structure is thus highly likely to lead to a change in tissue-implant interaction, despite the base material used being essentially the same. This phenomenon has also been demonstrated for various fields of application other than the cochlea, such as dental implants [105], [106], [107]. Highly promising scope for further advances in CI technology is emerging here.
5.3.2 Chemical and biological functionalization of surfaces
The use of chemically modified implant surfaces (Figure 16 (Fig. 16)) and the biochemical bonding of active agents to the implant (Figure 17 (Fig. 17)) is another promising approach by which the development of cochlear implant systems can be driven forward [108]. Not only does this idea have potential in terms of the basic applicability of implant coatings for reducing biofilm formation on implants and electrodes, it is also of particular importance in terms of optimizing nerve-electrode interaction [109]. In this regard, this approach is based on the use of biodegradable polymers as an integral part of the CI’s electrode array, which (following their degradation) lead to biologically active substances being released from the electrode array (SFB/Transregio 37: subproject C4).
Figure 16. Schematic representation of biochemical surface functionalization: binding of signal molecules onto the electrode.
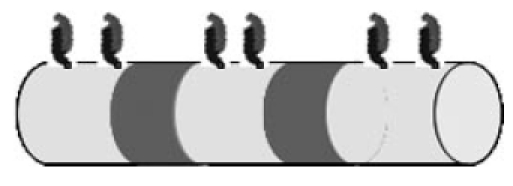
Figure 17. Schematic representation of chemical surface functionalization: binding of polymer chains onto the electrode.
Dental and orthopaedic implants are coated with growth factors (such as BMP-2) specifically in order to stimulate the formation of new bone. Various research teams have investigated a combination of BMPs and prosthetic joints in order to successfully optimize the process whereby the implant is incorporated into the surrounding tissue during the healing period [110], [111]. These generally exploit the principle of adsorption of growth factor proteins at the surface, so that the release of the substance – and, ultimately, the specific intended effect of the factor in question – is achieved over a concentration gradient. Although inductive effects have been described using this method, disadvantages are also evident. Firstly, relatively high quantities of the active substance are required in order to bring about the desired effects [112]. Secondly, the implants need to be specially pre-treated, which makes their clinical use more difficult and casts doubt on the method’s practicability.
Implants with a drug delivery function have already become routine clinical practice in other branches of medicine. A good example are coronary stents, the use of which has successfully reduced the restenosis rate of stents [113], [114], [115].
The chemical and biochemical surface functionalization of CIs is, both for the electrode array and for the implant casing, a (biomimetic) intervention option for which different biological objectives can be pursued. There is good reason to believe that findings from studies in which surfaces are coated with drugs can be transferred from the field of stent technology to that of cochlear implantation; joint interdisciplinary research projects have thus been initiated in response to this aspect (SFB 599: subproject D2).
5.3.3 Biological functionalization of surfaces
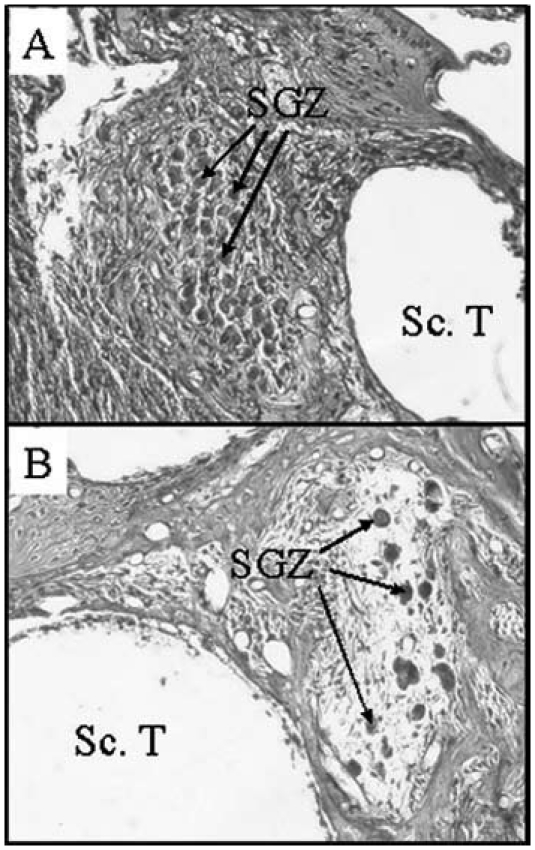
The use of genetically modified cells or stem cells as a connecting element between the residual nerve cells of the cochlea and the electrode array is a fascinating prospect – which has already been successfully demonstrated in animal experiments – in terms of facilitating initial steps in the regeneration of the inner ear (Figure 18 (Fig. 18)). Certain animal experiments have been able to show that genetically altered cells placed on an intracochlear array led to an increase in the survival rate of spiral ganglion cells (first auditory neuron of the auditory nerve; Figure 19 (Fig. 19)) following deafness. The outcome of these investigations is encouraging and suggests that the findings can, in principle, be exploited in order to optimize the nerve-electrode interface through biological functionalization of cochlear implants.
Figure 18. Schematic representation of biological surface functionalization: binding of adherent cells onto the electrode.
Figure 19. A representative example showing the consequences of deafness in the region of the spiral ganglion. Histological view of the spiral ganglion cells (SGC) in the Rosenthal channel (A) when hearing was normal, (B) six weeks after deafening, with most of the SGCs having degenerated. Sc.T. scala tympani.
These developments could ultimately lead to the CI system being used as an intracochlear therapy option (including stem cell therapy). The application of neural stem cells is conceivable here, which could create a connecting bridge between the residual nerve cells of the cochlea and the electrode array in order to facilitate enhanced charge transfer to the auditory nerve. That stem cells are generally detectable in the inner ear, and that modified cells can be used as cochlear stem cells which can be applied in the inner ear, has already been demonstrated both in vitro and in animal experiments. A combination of biological intervention strategies with conventional approaches to CI therapy offers scope both for improving the effectiveness of these implants and for drawing up strategies for regenerative therapy.
Regenerating lost hair cells by transplanting stem cells into the cochlea or through stimulation of local stem cells is a highly ambitious research approach [116], [117], [118]. However, it will probably not be possible in the near future to reproduce the complex anatomy of the organ of Corti and the perfect integration of a wide range of specialized cell types within a very compact space [119].
5.4 Fundamentals of drug delivery in the inner ear (neurotrophic effects)
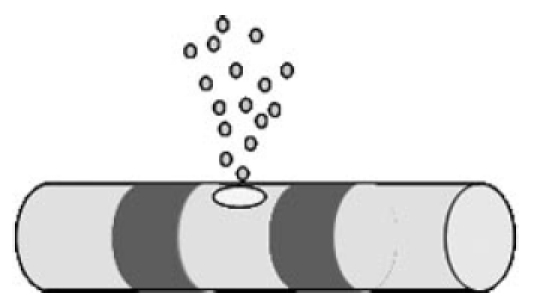
Sensorineural hearing loss leads, as a secondary effect, to the degeneration of the spiral ganglion cells, which are the target cells of electrical stimulation by the cochlear implant. In order to enhance the effectiveness of the CI, these neurones should be protected from progressive degeneration. Because of the blood-cochlear barrier [120], [121], it is not possible for many systematically applied molecules that potentially have a therapeutic action in the inner ear to reach their target location [122]. The inner ear thus constitutes a compartment or chamber that is unique within the human body, which could be treated by means of local drug delivery that uses the CI as a means of access (Figure 20 (Fig. 20)). The potential for this combined function – i.e. for using the CI electrode array to deliver drugs – is obvious and thus offers considerable possibilities for further advances in the implant system. Animal experiments have been able to show that the local application of drugs, combined with electrical stimulation by an implant, can lead to improved outcomes [123], [124]. By additionally using the CI as a drug depot, substances such as nerve growth factors could prevent progressive degeneration of the auditory neurones and thus enhance the effectiveness of these implants for long-term use [125], [122]. Steroids and other substances could also be applied in conjunction with the CI in order, for example, to minimize or even prevent tissue growth around the electrode and thus postoperatively maintain impedance at a low level.
Figure 20. Schematic representation of drug delivery from the electrode body. Use of the cochlear implant as a means of access for local drug delivery.
Various research teams have begun exploring these aspects and have attempted to modify CIs so that they can also serve as intracochlear drug depots [126], [127], [128]. Questions regarding dosage and the potential for controlling substance release are receiving a great deal of attention. Active agents must be applied at concentrations that achieve sufficiently high biological effectiveness. Furthermore, this intervention must not present the patient with an additional risk. Studies on the effectiveness and the technical feasibility of long-term application of drugs are still at the animal experimental stage. However, the findings to date are promising and suggest that this approach could soon see clinical transfer.
5.4.1 Substances
A wide range of substances are currently under discussion with a view to mediating therapeutic effects on a local basis in the inner ear. In addition to glucocorticoids (such as dexamethasone) there are a number of other factors that play a part, such as antioxidants, neurotrophic factors, neurotrophines and cytokines.
Glucocorticoids (specifically, triamcinolone) in particular have been tested in clinical trials as to their effect in reducing electrical impedance following cochlear implantation [33]. The rationale here consists in reducing growth of connective tissue around the electrode array following implantation – an effect which has also been demonstrated for the period up to initial fitting – in order to facilitate an improvement in charge transfer to the auditory nerve. Dexamethasone, by contrast, did not lead to a reduction in electrical impedance following one-time administration [129], although it does protect against further hearing loss caused by electrode insertion trauma during implantation [130].
Another class of substances that shows protective effects on spiral ganglion cells in the inner ear are the antioxidants. These can significantly increase the survival of spiral ganglion cells following deafness [131], [132], [133]. This effect has its basis in the fact that the formation of free radicals which arise during ototoxic trauma can be counteracted by the use of antioxidants such as trolox (a water-soluble analogue to vitamin E) and ascorbic acid. This leads both to improvements in the animal subjects’ electrical hearing threshold and to a significant increase (compared with a control group) in the number of surviving spiral ganglion cells [134].
Neurotrophic factors are proteins that act as regulators of neuronal differentiation. They influence both the neuronal development of the central and peripheral nervous system and the development of the auditory system. In animal experiments, the number of spiral ganglion cells that survive following deafening increased significantly (compared with untreated ears) through cochlear application of nerve growth factors [135], [136], [137], [138], [139], [140]. It has also been demonstrated that neurotrophic factors cause nerve structures to re-elongate, with the electrophysiologically relevant stimulus parameters also showing improvement upon receiving combined electrical stimulation (i.e. electrical stimulation plus drug application) [141]. In particular, brain-derived neurotrophic factor (BDNF) appears capable of locally generating protective effects within the inner ear [142], [143], [144], [145], [146], [147]. However, other research teams have shown that discontinuing local treatment of the cochlea with BDNF leads to accelerated degeneration of the spiral ganglions cells that previously were preserved by the factor [148]. It would thus appear expedient to either apply the factor over a long-term period or to maintain the protective effect by using other forms of intervention, such as electrical stimulation. The most important neurotrophic factors (fibroblast growth factor, neurotrophine, glial cell line-derived neurotrophic factor, insulin-like growth factor, and transforming growth factor β) are discussed below, as is their proven effect on spiral ganglion cells. In vitro and in vivo findings obtained using these factors will then be discussed in detail.
The fibroblast growth factor (FGF) family, which has 25 members, is involved in numerous intracellular processes such as cell differentiation, proliferation and survival [149]. In otological neurogenesis, various FGFs play a part and mediate their function by specifically binding to FGF receptors created by alternative splicing.
The family of neurotrophines (NT) is formed from small secretory proteins including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin-4/5 (NT-4/5). These proteins are important for neuronal differentiation and neuronal survival [150], [151]. They mediate their effect by means of tyrosine kinase receptors and via the low-affinity p75 neurotrophin receptor [152]. Both NT-3 and BDNF are expressed by the inner-ear epithelium. It has been shown that NT-3 promotes the survival of cochlear neurones, whereas BDNF also appears to be responsible for the survival of vestibular neurones [153]. Both the development and survival of spiral ganglion cells are influenced primarily by NT-3 and by glial cell line-derived neurotrophic factor (GDNF) [154].
The GDNF family consists of four members: GDNF, artemin (ARTN), persephin (PSP) and neurturin (NTN) [155]. All of these substances activate a common signal component, the transmembrane tyrosinkinase RET membrane proteins; these are anchored to the membrane via glycosylphosphatidyl-inositol, are known as GDNF family receptor alpha (GFRα) [156], and act as co-receptors for tyrosine kinase RET [157].
The insulin-like growth factor system (IGF) consists of the growth factors IGF I, II and relaxin, as well as various insulin-like peptides [158]. In mammals, these proteins bind to specific receptors that are located in the plasma membrane of the target cells. Studies have shown that IG I is responsible for the proliferation, differentiation and survival of neurones in the inner ear [159].
Among the neurotrophic factors, the superfamily of transforming growth factor-β-(TGF-β) is, with more than 50 members, the largest and most important group [160], [161]. The chief effects of this group of substances relate to their protective influence both on motor neurones [162] and (in particular) on the cells of the inner ear. Both protective effects on spiral ganglion cells [163], [164] and regenerative effects have been successfully demonstrated here [165], [166]. This class of substances appears particularly effective when applied shortly before ototoxic trauma [167], [168], [137].
For cytokines such as erythropoetin (EPO), too, a protective effect on the spiral ganglion cells of the inner ear has been demonstrated [169]. Erythropoetin is regarded as a regulator of the progenitor cells of erythrocytes (see overview in [170], [171]). However, EPO also helps promote the survival of neuronal cells, as for example following injury to the spinal cord [172]. Moreover, in the presence of EPO the expression and production of BDNF is increased [173]. In cultivated spiral ganglion cells, although administration of EPO had no observed effect on cell survival, it was found to increase neurite growth [174]. The expression of EPO and its receptors in the inner ear has been achieved only recently [175], paving the way for the first successful intervention in animal experiments that is aimed at protecting inner-ear structures [176], [177].
5.4.2 In vitro findings
Cell culture experiments are the first step in investigating drugs as to their neuroprotective effects on living cells. The cultivation of isolated spiral ganglion cells in neonatal rats represents a well established in vitro model for studying the impact of neurotrophic factors [178], [179], [180]. The effects of neurotrophic factors such as BDNF, GDNF, artemin, meteorin, NGF, NT-3, NT-4/5 and CNTF on the survival of spiral ganglion cells and neurite resprouting have been explored in detail. BDNF concentrations of 50 and 100 ng/ml are described as those most effective for optimal survival of spiral ganglion cells in vitro [179], [181], [182], [183]. Studies have demonstrated that GDNF has a survival-promoting effect on neonatal spiral ganglion cells in vitro at a concentration between 100 pg/ml [139], [184] and 100 ng/ml [183]. Significantly enhanced neuritogenesis, i.e. a marked increase in the length of outgrown spiral ganglion cell neurites (as compared with an untreated control group), has been achieved at a concentration between 10 and 50 ng/ml BDNF [180], [185], [186]. Trophic effects of GDNF application on cultivated spiral ganglion cells have not yet been described in the literature. These results suggest that BDNF is a potentially suitable candidate for mediating neuroprotective effects in the inner ear.
5.4.3 In vivo findings
The majority of animal experimental studies on the neuroprotective effect of BDNF, GDNF and electrical stimulation have been performed on guinea pigs [138], [187], [188], [189]. As considerations on the application of neurotrophic factors in humans with a view to treating patients with sensorineural hearing loss and subsequent degeneration of the spiral ganglion cells, many research teams initially deafen the animal subjects using ototoxins [140], [142], [146] or by exposing them to noise [139]. Kuang [140] has shown – as has Ylikoski et al. [139] – that 50 ng/ml GDNF, administered directly after deafening, can significantly protect the spiral ganglion cells from secondary degeneration. Yagi et al. [142] and Kanzaki et al. [146] applied GDNF by means of adenoviral vectors (AdGDNF) and, here too, the results of both research teams reveal significant protection of the nerve cells after deafening. Even if the onset of GDNF therapy (100 ng/ml) is delayed until three weeks post-deafening, it still has potential to protect the spiral ganglion cells [190].
Intracochlearly administered BDNF (50 ng/ml), applied for 14 days on the seventh day after ototoxic treatment, brings about significantly improved survival of the spiral ganglion cells [138]. Gillespie et al. [188] demonstrated that BDNF, NT-3, NT-4/5 and NGF – each administered at a concentration of 62.5 µg/ml – can prevent the degeneration of these cells progressing further after 14 days of deafness. Even where therapy with BDNF + FGF, NT-3 or CNTF was delayed until one to six weeks after deafening, this therapy still protected the cells from degeneration to a significant extent [191], [192], [193], [194], [145].
Through protecting the spiral ganglion cells from degenerating after deafness, and the related provision of a larger population of these cells for electrical stimulation via the CI, supportive local treatment with neurotrophic factors has great potential for enhancing the outcome of cochlear implantation.
5.5 Technical implementation of inner-ear drug delivery
5.5.1 Surface drug delivery (via coating)
The release of biologically active substances from the surface of a cochlear implant electrode array is a promising approach that is currently being addressed by a diverse range of research projects (including Transregio 37: subproject C4). The basic approach has already been outlined in section 5.3.2.
5.5.2 Fluid-based drug delivery (via pump)
Fluid-based delivery of biologically active substances by means of a cochlear implant can be made possible, for example, by using an implantable pump that is connected to the CI system. A number of individual electrode prototypes have already been produced for this purpose (Figure 21 (Fig. 21)) and have demonstrated the basic feasibility of such an approach [127], [128]. In addition to the challenge of creating a usable system that is clinically safe, an implantable, preferably refillable pump that allows fluid delivery within the cochlea will be necessary if this approach is to become reality. The production of individual prototypes (Figure 22 (Fig. 22)) has already paved the way for this.
Figure 21. Model investigation of electrode prototypes for delivery of fluids within the cochlea. Shown here: release of a dye at both the tip and side of the electrode array as a means of intracochlear fluid application.
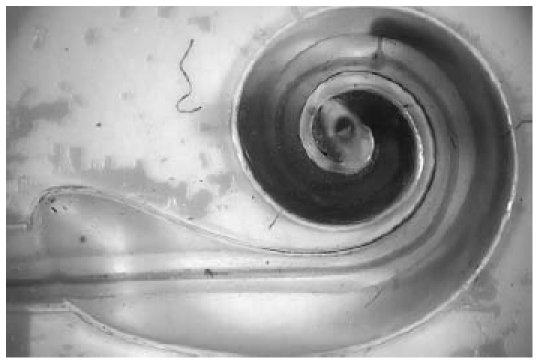
Figure 22. Schematic structure of a cochlear implant with integral micropump (MedEl GmbH) showing a septum port between the implantable pump (i.e. the circular structure on the right) and the implant (left).
Underlying investigations into fluid movements in the natural cochlear and the distribution pattern of substances applied into the cochlea have been carried out by the team headed by A. Salt [195], [196]. This group has, for example, developed a technique for sequential sampling of perilymph from the scala tympani [197]. Computer models have also been developed that allowed simulation of the distribution of the substances applied into the cochlea [198], [199].
Animal experimental studies suggest that pump rates of around 0.5 µl per hour could be used [143], [200]. These low volumes pose a considerable challenge in terms both of fluid flow within the cochlea and the positioning of the possible openings for drug delivery, as well as the accuracy of the implantable pump [201]. Here, too, initial investigations confirm the basic technical feasibility of this approach and thus lay the foundation for further upcoming clinical application studies.
5.5.3 Anti-inflammatory nanoparticles
A major focus in nanobiotechnology is antimicrobial surface coating of implants. In some studies, it was primarily nano-silver that proved more effective than other materials as an anti-inflammatory agent and infection suppressant [202], since it – in addition to the antibiotic effect – also has lower toxicity. Work on endoprosthetic hip joints has shown that reduced infection rates, enhanced biocompatibility and a reasonable useful life for the implants are possible [203]. Furthermore, the perioperative use of antibiotics can be reduced in this way, so that silver-coated implants are characterized overall by a highly favourable cost-benefit ratio. The application of these techniques for cochlear implants thus appears, in principle, to be promising.
5.5.4 Nanoparticles and cochlear implants
The miniaturization of drug carriers down to nanoscale level has led to strategies being devised whose aim is – by using nanoparticles – to allow cochlear implant-based release of drugs for local therapy of the inner ear. Nanoparticles, functioning as non-viral vectors of biogenic agents (e.g. genes, neuroptrophic factors and steroid sequences), protected from the effects of the body’s metabolism, are to be transported specifically to the desired target location and time-released. Integrating a minute (nanoscale) drug depot into a CI could for example, under this approach, lead to targeted release of neurotrophic factors and eventually to an improvement in nerve-electrode interaction.
The uptake of nanoparticles has already been demonstrated in the inner ear [204], as has the fundamental biocompatibility of the particles used in terms of preserving inner-ear structures and their function [205]. That nanoparticles can be used in the inner ear has thus already been evidenced, so that here – by combining the fluid-based application of substances and nanoparticle technology – the use of modified biomaterials for CIs is opening up a new field for inner-ear intervention.
6 Outlook
By taking materials and technologies that already exist in the field of cochlear implantation and combining them with new approaches aimed at optimizing the biomaterials used, a wide range of possibilities is opened up for further advances in these implants. In particular, the fact that scope is being incorporated for surface functionalization of conventional materials, for introducing new implant materials and also for developing strategies on local drug delivery, indicates the prospects that are emerging for additional improvements in biocompatibility and for optimizing the nerve-electrode interface. Both approaches could thus, in the long term, lead to a marked functional improvement in the implant systems currently in use.
Acknowledgements
My special thanks go to Dr. V. Scheper, Dr. U. Reich, M. Wolf, Dr. A. Warnecke and Dr. G. Paasche for their assistance to prepare the manuscript. Additionally I thank the Laserzentrum Hannover (Prof. Dr. B. Chichkov, Dr. E. Fadeeva and Dr. J. Koch) as well as Cochlear Corp. (T. Topp) and MedEl GmbH (C. Jolly) for providing related illustrations.
References
- 1.Burgio P. Safety considerations of cochlear implantation. Otolaryngol Clin North Am. 1986;19(2):237–247. [PubMed] [Google Scholar]
- 2.Lehnhardt E. Biokompatibilität der Cochlear-implants. Eur Arch Otorhinolaryngol Suppl. 1992;1:223–233. [PubMed] [Google Scholar]
- 3.BQS Qualitätsreport. Düsseldorf: BQS Institut für Qualität und Patientensicherheit; 2007. p. 36. Available from: http://www.bqs-qualitaetsreport.de/ [Google Scholar]
- 4.Dunn CC, Tyler RS, Oakley S, Gantz BJ, Noble W. Comparison of speech recognition and localization performance in bilateral and unilateral cochlear implant users matched on duration of deafness and age at implantation. Ear Hear. 2008;29(3):352–359. doi: 10.1097/AUD.0b013e318167b870. Available from: http://dx.doi.org/10.1097/AUD.0b013e318167b870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Donoghue GM, Nikolopoulos TP. Minimal access surgery for pediatric cochlear implantation. Otol Neurotol. 2002;23(6):891–894. doi: 10.1097/00129492-200211000-00014. Available from: http://dx.doi.org/10.1097/00129492-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Ray J, Gibson W, Sanli H. Surgical complications of 844 consecutive cochlear implantations and observations on large versus small incisions. Cochlear Implants Int. 2004;5(3):87–95. doi: 10.1002/cii.132. Available from: http://dx.doi.org/10.1002/cii.132. [DOI] [PubMed] [Google Scholar]
- 7.Bajaj Y, Wyatt M, Hartley B. Small postaural incision for paediatric cochlear implantation. Cochlear Implants Int. 2005;6(2):77–84. doi: 10.1002/cii.249. Available from: http://dx.doi.org/10.1002/cii.249. [DOI] [PubMed] [Google Scholar]
- 8.Pau HW, Sievert U, Graumüller S, Wild E. Incisions for cochlear implant flaps and superficial skin temperature. Skin temperature/blood circulation in CI flaps. Otolaryngol Pol. 2004;58(4):713–719. [PubMed] [Google Scholar]
- 9.Müller J, Geyer G, Helms J. Ionomerzement in der Cochlear-Implant-Chirurgie. Laryngorhinootologie. 1993;72(1):36–38. doi: 10.1055/s-2007-997850. Available from: http://dx.doi.org/10.1055/s-2007-997850. [DOI] [PubMed] [Google Scholar]
- 10.Müller J, Schön F, Helms J. Sichere Fixierung von Cochlear-Implant-Elektrodenträgern bei Kindern und Erwachsenen - erste Erfahrungen mit einem neuen Titan-Clip. Laryngorhinootologie. 1998;77(4):238–240. doi: 10.1055/s-2007-996968. Available from: http://dx.doi.org/10.1055/s-2007-996968. [DOI] [PubMed] [Google Scholar]
- 11.Lee DJ, Driver M. Cochlear implant fixation using titanium screws. Laryngoscope. 2005;115(5):910–911. doi: 10.1097/01.MLG0000154537.05252.A0. Available from: http://dx.doi.org/10.1097/01.MLG0000154537.05252.A0. [DOI] [PubMed] [Google Scholar]
- 12.Holtkamp V. Cochlea Implantate unter Stoßbelastung - Auswertung von Unfallszenarien, Ermittlung von Beanspruchungsgrenzen und Entwicklung eines standardisierbaren Prüfverfahrens. Hannover: Medizinische Hochschule Hannover; 2004. [Google Scholar]
- 13.Battmer RD, O'Donoghue GM, Lenarz T. A multicenter study of device failure in European cochlear implant centers. Ear Hear. 2007;28(2 Suppl):95S–99S. doi: 10.1097/AUD.0b013e3180315502. Available from: http://dx.doi.org/10.1097/AUD.0b013e3180315502. [DOI] [PubMed] [Google Scholar]
- 14.Kha HN, Chen BK, Clark GM, Jones R. Stiffness properties for Nucleus standard straight and contour electrode arrays. Med Eng Phys. 2004;26(8):677–685. doi: 10.1016/j.medengphy.2004.05.001. Available from: http://dx.doi.org/10.1016/j.medengphy.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Lim YS, Park SI, Kim YH, Oh SH, Kim SJ. Three-dimensional analysis of electrode behavior in a human cochlear model. Med Eng Phys. 2005;27(8):695–703. doi: 10.1016/j.medengphy.2004.12.009. Available from: http://dx.doi.org/10.1016/j.medengphy.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Rebscher SJ, Heilmann M, Bruszewski W, Talbot NH, Snyder RL, Merzenich MM. Strategies to improve electrode positioning and safety in cochlear implants. IEEE Trans Biomed Eng. 1999;46(3):340–352. doi: 10.1109/10.748987. Available from: http://dx.doi.org/10.1109/10.748987. [DOI] [PubMed] [Google Scholar]
- 17.Rebscher SJ, Hetherington A, Bonham B, Wardrop P, Whinney D, Leake PA. Considerations for design of future cochlear implant electrode arrays: electrode array stiffness, size, and depth of insertion. J Rehabil Res Dev. 2008;45(5):731–747. doi: 10.1682/JRRD.2007.08.0119. Available from: http://dx.doi.org/10.1682/JRRD.2007.08.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kha HN, Chen BK. Determination of frictional conditions between electrode array and endosteum lining for use in cochlear implant models. J Biomech. 2006;39(9):1752–1756. doi: 10.1016/j.jbiomech.2005.04.031. Available from: http://dx.doi.org/10.1016/j.jbiomech.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 19.Kha HN, Chen BK, Clark GM. 3D finite element analyses of insertion of the Nucleus standard straight and the Contour electrode arrays into the human cochlea. J Biomech. 2007;40(12):2796–2805. doi: 10.1016/j.jbiomech.2007.01.013. Available from: http://dx.doi.org/10.1016/j.jbiomech.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Pau HW, Just T, Dommerich S, Behrend D. Temporal bone investigations on landmarks for conventional or endosteal insertion of cochlear electrodes. Acta Otolaryngol. 2007;127(9):920–926. doi: 10.1080/00016480601075423. Available from: http://dx.doi.org/10.1080/00016480601075423. [DOI] [PubMed] [Google Scholar]
- 21.Pau HW, Just T, Lehnhardt E, Hessel H, Behrend D. An "endosteal electrode" for cochlear implantation in cases with residual hearing? Feasibility study: preliminary temporal bone experiments. Otol Neurotol. 2005;26(3):448–454. doi: 10.1097/01.mao.0000169779.54162.34. Available from: http://dx.doi.org/10.1097/01.mao.0000169779.54162.34. [DOI] [PubMed] [Google Scholar]
- 22.Abbasi F, Mirzadeh H, Simjoo M. Hydrophilic interpenetrating polymer networks of poly(dimethyl siloxane) (PDMS) as biomaterial for cochlear implants. J Biomater Sci Polym Ed. 2006;17(3):341–355. doi: 10.1163/156856206775997287. Available from: http://dx.doi.org/10.1163/156856206775997287. [DOI] [PubMed] [Google Scholar]
- 23.Seldon HL, Dahm MC, Clark GM, Crowe S. Silastic with polyacrylic acid filler: swelling properties, biocompatibility and potential use in cochlear implants. Biomaterials. 1994;15(14):1161–1169. doi: 10.1016/0142-9612(94)90237-2. Available from: http://dx.doi.org/10.1016/0142-9612(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 24.Tykocinski M, Cowan RS. Poly-vinyl-alcohol (PVA) coating of cochlear implant electrode arrays: an in-vivo biosafety study. Cochlear Implants Int. 2005;6(1):16–30. doi: 10.1002/cii.17. Available from: http://dx.doi.org/10.1002/cii.17. [DOI] [PubMed] [Google Scholar]
- 25.Kim CS, Chang SO, Lee HJ, Shim WS, Oh SH, Kim YH. Cochlear implantation in patients with a history of chronic otitis media. Acta Otolaryngol. 2004;124(9):1033–1038. doi: 10.1080/00016480410017521. Available from: http://dx.doi.org/10.1080/00016480410017521. [DOI] [PubMed] [Google Scholar]
- 26.Issa TK, Bahgat MA, Linthicum FH., Jr Tissue reaction to prosthetic materials in human temporal bones. Am J Otol. 1983;5(1):40–43. [PubMed] [Google Scholar]
- 27.Clark GM, Pyman BC, Webb RL, Bailey QE, Shepherd RK. Surgery for an improved multiple-channel cochlear implant. Ann Otol Rhinol Laryngol. 1984;93(3 Pt 1):204–207. doi: 10.1177/000348948409300302. [DOI] [PubMed] [Google Scholar]
- 28.Franz B, Clark GM, Bloom DM. Permeability of the implanted round window membrane in the cat. Acta Otolaryngol (Stockh) 1984;410:17–23. [PubMed] [Google Scholar]
- 29.Clark GM, Shepherd RK, Franz B, Bloom D. Intraocular electrode implantation. Round window membrane sealing procedures and permeability studies. Acta Otolaryngol Suppl. 1984;410:1–23. [PubMed] [Google Scholar]
- 30.Dahm MC, Clark GM, Franz BK, Shepherd RK, Burton MJ, Robins-Browne R. Cochlear implantation in children: labyrinthitis following pneumococcal otitis media in unimplanted and implanted cat cochleas. Acta Otolaryngol. 1994;114(6):620–625. doi: 10.3109/00016489409126115. [DOI] [PubMed] [Google Scholar]
- 31.Nadol JB, Jr, Eddington DK. Histologic evaluation of the tissue seal and biologic response around cochlear implant electrodes in the human. Otol Neurotol. 2004;25:257–262. doi: 10.1097/00129492-200405000-00010. Available from: http://dx.doi.org/10.1097/00129492-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Hyland M, Mailley S, Savanidis C, McLaughlin J, McAdams E. Thin film platinum and iridium oxide coatings applied to gold/ flexible polymers for functional electrical stimulation electrodes. Proceedings of the 5th Annual Conference of the International Functional Electrical Stimulation Society; 2000. [Google Scholar]
- 33.Paasche G, Bockel F, Tasche C, Lesinski-Schiedat A, Lenarz T. Changes of postoperative impedances in cochlear implant patients: the short-term effects of modified electrode surfaces and intracochlear corticosteroids. Otol Neurotol. 2006;27(5):639–647. doi: 10.1097/01.mao.0000227662.88840.61. Available from: http://dx.doi.org/10.1097/01.mao.0000227662.88840.61. [DOI] [PubMed] [Google Scholar]
- 34.Clark GM, Shepherd RK, Franz BK, Dowell RC, Tong YC, Blamey PJ, Webb RL, Pyman BC, McNaughtan J, Bloom DM, et al. The histopathology of the human temporal bone and auditory central nervous system following cochlear implantation in a patient. Correlation with psychophysics and speech perception results. Acta Otolaryngol Suppl. 1988;448:1–65. doi: 10.3109/00016488809098972. [DOI] [PubMed] [Google Scholar]
- 35.Clark G. Cochlear Implants: Fundamentals and applications. New York: Springer Verlag; 2003. p. 171. [Google Scholar]
- 36.Brummer SB, McHardy J, Turner MJ. Electrical stimulation with Pt electrodes: Trace analysis for dissolved platinum and other dissolved electrochemical products. Brain Behav Evol. 1977;14(1-2):10–22. doi: 10.1159/000124611. Available from: http://dx.doi.org/10.1159/000124611. [DOI] [PubMed] [Google Scholar]
- 37.Black RC, Hannaker P. Dissolution of smooth platinum electrodes in biological fluids. Appl Neurophysiol. 1980;42(6):366–374. doi: 10.1159/000102382. [DOI] [PubMed] [Google Scholar]
- 38.Maurer J, Marangos N, Ziegler E. Reliability of cochlear implants. Otolaryngol Head Neck Surg. 2005;132(5):746–750. doi: 10.1016/j.otohns.2005.01.026. Available from: http://dx.doi.org/10.1016/j.otohns.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 39.Luria LW. The role of medical grade silicones in surgery and its topical applications. Oper Tech Plastic Reconstr Surg. 2002;9:67–74. doi: 10.1016/S1071-0949(03)90012-6. Available from: http://dx.doi.org/10.1016/S1071-0949(03)90012-6. [DOI] [Google Scholar]
- 40.Malick MH, Carr JA. Flexible elastomer molds in burn scar control. Am J Occup Ther. 1980;34(9):603–608. doi: 10.5014/ajot.34.9.603. [DOI] [PubMed] [Google Scholar]
- 41.Rosato DV. Polymers, processes and properties of medical plastics: including markets and applications. In: Szycher M, editor. Biocompatible Polymers, Metals, and Composites. Lancaster PA: Technomic Publ.; 1983. pp. 1019–1067. [Google Scholar]
- 42.Swanson JW, Lebeau JE. The effect of implantation on the physical properties of silicone rubber. J Biomed Mater Res. 1974;8(6):357–367. doi: 10.1002/jbm.820080603. Available from: http://dx.doi.org/10.1002/jbm.820080603. [DOI] [PubMed] [Google Scholar]
- 43.Dolezel B, Adamírová L, Vondrácek P, Náprstek Z. In vivo degradation of polymers. II. Change of mechanical properties and cross-link density in silicone rubber pacemaker lead insulations during long-term implantation in the human body. Biomaterials. 1989;10(6):387–392. doi: 10.1016/0142-9612(89)90130-0. Available from: http://dx.doi.org/10.1016/0142-9612(89)90130-0. [DOI] [PubMed] [Google Scholar]
- 44.Leslie LJ, Jenkins MJ, Shepherd DE, Kukureka SN. The effect of the environment on the mechanical properties of medical grade silicones. J Biomed Mater Res B Appl Biomater. 2008;86B(2):460–465. doi: 10.1002/jbm.b.31042. Available from: http://dx.doi.org/10.1002/jbm.b.31042. [DOI] [PubMed] [Google Scholar]
- 45.Robblee LS, Sweeney JD. Bioelectrodes. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials Science. London: Academic Press Ltd.; 1996. pp. 371–375. [Google Scholar]
- 46.Brummer SB, Turner MJ. Electrical stimulation of the nervous system: The principle of safe charge injection with noble metal electrodes. Bioelectrochemistry and Bioenergetics. 1975;2(1):13–25. doi: 10.1016/0302-4598(75)80002-X. Available from: http://dx.doi.org/10.1016/0302-4598(75)80002-X. [DOI] [Google Scholar]
- 47.Clark GM, Tong YC, Patrick JF, Seligman PM, Crosby PA, Kuzma JA, Money DK. A multi-channel hearing prosthesis for profound-to-total hearing loss. J Med Eng Technol. 1984;8(1):3–8. doi: 10.3109/03091908409032065. Available from: http://dx.doi.org/10.3109/03091908409032065. [DOI] [PubMed] [Google Scholar]
- 48.Puri S, Dornhoffer JL, North PE. Contact dermatitis to silicone after cochlear implantation. Laryngoscope. 2005;115(10):1760–1762. doi: 10.1097/01.mlg.0000172202.58968.41. Available from: http://dx.doi.org/10.1097/01.mlg.0000172202.58968.41. [DOI] [PubMed] [Google Scholar]
- 49.Kunda LD, Stidham KR, Inserra MM, Roland PS, Franklin D, Roberson JB., Jr Silicone allergy: A new cause for cochlear implant extrusion and its management. Otol Neurotol. 2006;27(8):1078–1082. doi: 10.1097/01.mao.0000235378.64654.4d. Available from: http://dx.doi.org/10.1097/01.mao.0000235378.64654.4d. [DOI] [PubMed] [Google Scholar]
- 50.Migirov L, Dagan E, Kronenberg J. Surgical and medical complications in different cochlear implant devices. Acta Otolaryngol. 2009;129(7):741–744. doi: 10.1080/00016480802398954. Available from: http://dx.doi.org/10.1080/00016480802398954. [DOI] [PubMed] [Google Scholar]
- 51.Stratigouleas ED, Perry BP, King SM, Syms CA., 3rd Complication rate of minimally invasive cochlear implantation. Otolaryngol Head Neck Surg. 2006;135(3):383–386. doi: 10.1016/j.otohns.2006.03.023. Available from: http://dx.doi.org/10.1016/j.otohns.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 52.Li PM, Somdas MA, Eddington DK, Nadol JB., Jr Analysis of intracochlear new bone and fibrous tissue formation in human subjects with cochlear implants. Ann Otol Rhinol Laryngol. 2007;116(10):731–738. doi: 10.1177/000348940711601004. [DOI] [PubMed] [Google Scholar]
- 53.Xu J, Shepherd RK, Millard RE, Clark GM. Chronic electrical stimulation of the auditory nerve at high stimulus rates: A physiological and histopathological study. Hear Res. 1997;105:1–29. doi: 10.1016/S0378-5955(96)00193-1. Available from: http://dx.doi.org/10.1016/S0378-5955(96)00193-1. [DOI] [PubMed] [Google Scholar]
- 54.Duan YY, Clark GM, Cowan RS. A study of intra-cochlear electrodes and tissue interface by electrochemical impedance methods in vivo. Biomaterials. 2004;25(17):3813–3828. doi: 10.1016/j.biomaterials.2003.09.107. Available from: http://dx.doi.org/10.1016/j.biomaterials.2003.09.107. [DOI] [PubMed] [Google Scholar]
- 55.Peeters S, van Immerseel L, Zarowski A, Houben V, Govaerts P, Offeciers E. New developments in cochlear implants. Acta oto-rhino-laryngologica belg. 1998;52:115–127. [PubMed] [Google Scholar]
- 56.De Ceulaer G, Johnson S, Yperman M, Daemers K, Offeciers FE, O'Donoghue GM, Govaerts PJ. Long-term evaluation of the effect of intra-cochlear steroid deposition on electrode impedance in cochlear implant patients. Otol Neurotol. 2003;24:769–774. doi: 10.1097/00129492-200309000-00014. Available from: http://dx.doi.org/10.1097/00129492-200309000-00014. [DOI] [PubMed] [Google Scholar]
- 57.Rivas A, Marlowe AL, Chinnici JE, Niparko JK, Francis HW. Revision cochlear implantation surgery in adults: indications and results. Otol Neurotol. 2008;29(5):639–648. doi: 10.1097/MAO.0b013e31817e5d31. Available from: http://dx.doi.org/10.1097/MAO.0b013e31817e5d31. [DOI] [PubMed] [Google Scholar]
- 58.Rühl S, Poyda K, Lesinski-Schiedat A, Gärtner L, Lenarz T. Reimplantation bei CI-Patienten seit 1985. GMS Curr Posters Otorhinolaryngol Head Neck Surg. 2008;4:Doc16. Available from: http://www.egms.de/de/journals/cpo/2008-4/cpo000374.shtml. [Google Scholar]
- 59.Burton MJ, Shepherd RK, Clark GM. Cochlear histopathologic characteristics following long-term implantation. Safety studies in the young monkey. Arch Otolaryngol Head Neck Surg. 1996;122(10):1097–1104. doi: 10.1001/archotol.1996.01890220063011. [DOI] [PubMed] [Google Scholar]
- 60.Gray RF, Baguley DM, Harries ML, Court I, Lynch C. Profound deafness treated by the Ineraid multichannel intracochlear implant. J Laryngol Otol. 1993;107(8):673–680. doi: 10.1017/S0022215100124120. Available from: http://dx.doi.org/10.1017/S0022215100124120. [DOI] [PubMed] [Google Scholar]
- 61.Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, Forbes M, Greenberg DP, Dice B, Burrows A, Wackym PA, Stoodley P, Post JC, Ehrlich GD, Kerschner JE. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296(2):202–211. doi: 10.1001/jama.296.2.202. Available from: http://dx.doi.org/10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Franz BK, Clark GM, Bloom DM. Effect of experimentally induced otitis media on cochlear implants. Ann Otol Rhinol Laryngol. 1987;96(2 Pt 1):174–177. doi: 10.1177/000348948709600207. [DOI] [PubMed] [Google Scholar]
- 63.El-Kashlan HK, Telian SA. Cochlear implantation in the chronically diseased ear. Curr Opin Otolaryngol Head Neck Surg. 2004;12(5):384–386. doi: 10.1097/01.moo.0000134439.99357.c9. Available from: http://dx.doi.org/10.1097/01.moo.0000134439.99357.c9. [DOI] [PubMed] [Google Scholar]
- 64.Antonelli PJ, Lee JC, Burne RA. Bacterial biofilms may contribute to persistent cochlear implant infection. Otol Neurotol. 2004;25(6):953–957. doi: 10.1097/00129492-200411000-00015. Available from: http://dx.doi.org/10.1097/00129492-200411000-00015. [DOI] [PubMed] [Google Scholar]
- 65.Cunningham CD, III, Slattery WH, III, Luxford WM. Postoperative infection in cochlear implant patients. Otolaryngol Head Neck Surg. 2004;131:109–114. doi: 10.1016/j.otohns.2004.02.011. Available from: http://dx.doi.org/10.1016/j.otohns.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 66.Pawlowski KS, Wawro D, Roland PS. Bacterial biofilm formation on a human cochlear implant. Otol Neurotol. 2005;26(5):972–975. doi: 10.1097/01.mao.0000169047.38759.8b. Available from: http://dx.doi.org/10.1097/01.mao.0000169047.38759.8b. [DOI] [PubMed] [Google Scholar]
- 67.Hopfenspirger MT, Levine SC, Rimell FL. Infectious complications in pediatric cochlear implants. Laryngoscope. 2007;117(10):1825–1829. doi: 10.1097/MLG.0b013e3180de4d35. Available from: http://dx.doi.org/10.1097/MLG.0b013e3180de4d35. [DOI] [PubMed] [Google Scholar]
- 68.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/S0140-6736(01)05321-1. Available from: http://dx.doi.org/10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 69.Loeffler KA, Johnson TA, Burne RA, Antonelli PJ. Biofilm formation in an in vitro model of cochlear implants with removable magnets. Otolaryngol Head Neck Surg. 2007;136(4):583–588. doi: 10.1016/j.otohns.2006.11.005. Available from: http://dx.doi.org/10.1016/j.otohns.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 70.Cristobal R, Edmiston CE, Jr, Runge-Samuelson CL, Owen HA, Firszt JB, Wackym PA. Fungal biofilm formation on cochlear implant hardware after antibiotic-induced fungal overgrowth within the middle ear. Pediatr Infect Dis J. 2004;23(8):774–778. doi: 10.1097/01.inf.0000134315.24413.92. Available from: http://dx.doi.org/10.1097/01.inf.0000134315.24413.92. [DOI] [PubMed] [Google Scholar]
- 71.Reefhuis J, Honein MA, Whitney CG, Chamany S, Mann EA, Biernath KR, Broder K, Manning S, Avashia S, Victor M, Costa P, Devine O, Graham A, Boyle C. Risk of Bacterial Meningitis in Children with Cochlear Implants. N Engl J Med. 2003;349(5):435–445. doi: 10.1056/NEJMoa031101. Available from: http://dx.doi.org/10.1056/NEJMoa031101. [DOI] [PubMed] [Google Scholar]
- 72.Arnold W, Bredberg G, Gstöttner W, Helms J, Hildmann H, Kiratzidis T, Müller J, Ramsden RT, Roland P, Walterspiel JN. Meningitis following cochlear implantation: pathomechanisms, clinical symptoms, conservative and surgical treatments. ORL J Otorhinolaryngol Relat Spec. 2002;64(6):382–389. doi: 10.1159/000067579. Available from: http://dx.doi.org/10.1159/000067579. [DOI] [PubMed] [Google Scholar]
- 73.Wei BP, Shepherd RK, Robins-Browne RM, Clark GM, O'Leary SJ. Threshold shift: effects of cochlear implantation on the risk of pneumococcal meningitis. Otolaryngol Head Neck Surg. 2007;136(4):589–596. doi: 10.1016/j.otohns.2006.11.039. Available from: http://dx.doi.org/10.1016/j.otohns.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wei BP, Shepherd RK, Robins-Browne RM, Clark GM, O'Leary SJ. Effects of Inner Ear Trauma on the Risk of Pneumococcal Meningitis. Arch Otolaryngol Head Neck Surg. 2007;133(3):250–259. doi: 10.1001/archotol.133.3.250. Available from: http://dx.doi.org/10.1001/archotol.133.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tykocinski M, Cohen LT, Pyman BC, Roland T, Jr, Treaba C, Palamara J, Dahm MC, Shepherd RK, Xu J, Cowan RS, Cohen NL, Clark GM. Comparison of electrode position in the human cochlea using various perimodiolar electrode arrays. Am J Otol. 2000;21(2):205–211. doi: 10.1016/s0196-0709(00)80010-1. [DOI] [PubMed] [Google Scholar]
- 76.Clark GM, Pyman BC, Pavillard RE. A protocol for the prevention of infection in cochlear implant surgery. J Laryngol Otol. 1980;94(12):1377–1386. doi: 10.1017/S0022215100090204. Available from: http://dx.doi.org/10.1017/S0022215100090204. [DOI] [PubMed] [Google Scholar]
- 77.Puls R, Hoene A, Kuehn JP, Langner S, Robinson DM, Westerholt A, Hosten N. Percutaneous treatment of infrarenal aortic aneurysm with a polytetrafluoroethylene-covered nitinol stent-graft via a 10-F introducer sheath. J Vasc Interv Radiol. 2008;19(9):1378–1381. doi: 10.1016/j.jvir.2008.05.028. Available from: http://dx.doi.org/10.1016/j.jvir.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 78.Kerdjoudj H, Moby V, Berthelemy N, Gentils M, Boura C, Bordenave L, Stoltz JF, Menu P. The ideal small arterial substitute: Role of cell seeding and tissue engineering. Clin Hemorheol Microcirc. 2007;37(1-2):89–98. [PubMed] [Google Scholar]
- 79.Gordon BM, Fishbein MC, Levi DS. Polytetrafluoroethylene-covered stents in the venous and arterial system: angiographic and pathologic findings in a swine model. Cardiovasc Pathol. 2008;17(4):206–211. doi: 10.1016/j.carpath.2007.09.001. Available from: http://dx.doi.org/10.1016/j.carpath.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 80.Falcão SC, Coelho AR, Evêncio Neto J. Biomechanical evaluation of microbial cellulose (Zoogloea sp.) and expanded polytetrafluoroethylene membranes as implants in repair of produced abdominal wall defects in rats. Acta Cir Bras. 2008;23(2):184–191. doi: 10.1590/S0102-86502008000200012. Available from: http://dx.doi.org/10.1590/S0102-86502008000200012. [DOI] [PubMed] [Google Scholar]
- 81.Chiang CK, Druy MA, Gau SC, Heeger AJ, Louis EJ, MacDiarmid AG, Park YW, Shirakawa H. Synthesis of highly conducting films of derivatives of polyacetylene, (CH)x. J Am Chem Soc. 1978;100:1013–1015. doi: 10.1021/ja00471a081. Available from: http://dx.doi.org/10.1021/ja00471a081. [DOI] [Google Scholar]
- 82.Skotheim TA. Handbook of Conducting Polymers. Vol 1&2. New York: Marcel Dekker; 1986. [Google Scholar]
- 83.Rehahn M. Elektrisch leitfähige Kunststoffe. Chem unserer Zeit. 2003;37:18–30. doi: 10.1002/ciuz.200390000. Available from: http://dx.doi.org/10.1002/ciuz.200390000. [DOI] [Google Scholar]
- 84.Ferrer-Anglada N, Gomis V, El-Hachemi Z, Dettlaff Weglikovska U, Kaempgen M, Roth S. Carbon nanotube based composites for electronic applications: CNT-conducting polymers, CNT-Cu. Phys Stat Sol. 2006;203:1082–1087. doi: 10.1002/pssa.200566188. Available from: http://dx.doi.org/10.1002/pssa.200566188. [DOI] [Google Scholar]
- 85.Lee JY, Lee JW, Schmidt CE. Neuroactive conducting scaffolds: nerve growth factor conjugation on active ester-functionalized polypyrrole. J R Soc Interface. 2009;6(38):801–810. doi: 10.1098/rsif.2008.0403. Available from: http://dx.doi.org/10.1098/rsif.2008.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haggerty HS, Lusted HS. Histological reaction to polyimide films in the cochlea. Acta Otolaryngol. 1989;107(1-2):13–22. doi: 10.3109/00016488909127474. [DOI] [PubMed] [Google Scholar]
- 87.Blach-Watson JA, Watson GS, Brown CL, Myhra S. UV-patterning of polyimide: Differentiation and characterization of surface chemistry and structure. Appl Surf Sci. 2004;235:164–169. doi: 10.1016/j.apsusc.2004.05.179. Available from: http://dx.doi.org/10.1016/j.apsusc.2004.05.179. [DOI] [Google Scholar]
- 88.Nguyen LTT, Nguyen HN, La THT. Synthesis and characterization of a photosensitive polyimide precursor and its photocuring behavior for lithography applications. Optical Mater. 2007;29:610–618. doi: 10.1016/j.optmat.2005.10.010. Available from: http://dx.doi.org/10.1016/j.optmat.2005.10.010. [DOI] [Google Scholar]
- 89.Jiang X, Li H, Wang H, Shi Z, Yin J. A novel negative photoinitiator-free photosensitive polyimide. Polymer. 2006;47:2942–2945. [Google Scholar]
- 90.Hasegawa M, Horie K. Photophysics, photochemistry, and optical properties of polyimides. Prog Polym Sci. 2001;26:259–335. doi: 10.1016/S0079-6700(00)00042-3. Available from: http://dx.doi.org/10.1016/S0079-6700(00)00042-3. [DOI] [Google Scholar]
- 91.Lee KK, He J, Singh A, Massia S, Ehteshami G, Kim B, Raupp G. Polyimide-based intracortical neural implant with improved structural stiffness. J Micromech Microeng. 2004;14:32–37. doi: 10.1088/0960-1317/14/1/305. Available from: http://dx.doi.org/10.1088/0960-1317/14/1/305. [DOI] [Google Scholar]
- 92.Yeager JD, Phillips DJ, Rector DM, Bahr DF. Characterization of flexible ECoG electrode arrays for chronic recording in awake rats. J Neurosci Methods. 2008;173(2):279–285. doi: 10.1016/j.jneumeth.2008.06.024. Available from: http://dx.doi.org/10.1016/j.jneumeth.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Besch D, Sachs H, Szurman P, Gülicher D, Wilke R, Reinert S, Zrenner E, Bartz-Schmidt KU, Gekeler F. Extraocular surgery for implantation of an active subretinal visual prosthesis with external connections: feasibility and outcome in seven patients. Br J Ophthalmol. 2008;92(10):1361–1368. doi: 10.1136/bjo.2007.131961. Available from: http://dx.doi.org/10.1136/bjo.2007.131961. [DOI] [PubMed] [Google Scholar]
- 94.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. Available from: http://dx.doi.org/10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 95.Rousche PJ, Pellinen DS, Pivin DP, Jr, Williams JC, Vetter RJ, Kirke DR. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans Biomed Eng. 2001;48:361–371. doi: 10.1109/10.914800. Available from: http://dx.doi.org/10.1109/10.914800. [DOI] [PubMed] [Google Scholar]
- 96.Kim Y-T, Hitchcock RW, Bridge MJ, Tresco PA. Chronic response of adult rat brain tissue to implants anchored to the skull. Biomaterials. 2004;25:2229–2237. doi: 10.1016/j.biomaterials.2003.09.010. Available from: http://dx.doi.org/10.1016/j.biomaterials.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 97.Bulcke VM, Baert K, Beyne E, Gonzalez M, Winters C, Webers T. Active electrode arrays by chip embedding in a flexible silicone carrier. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2811–2815. doi: 10.1109/IEMBS.2006.260786. Available from: http://dx.doi.org/10.1109/IEMBS.2006.260786. [DOI] [PubMed] [Google Scholar]
- 98.Schwahn HN, Gekeler F, Kohler K, Kobuch K, Sachs HG, Schulmeyer F, Jakob W, Gabel VP, Zrenner E. Studies on the feasibility of a subretinal visual prosthesis: data from Yucatan micropig and rabbit. Graefes Arch Clin Exp Ophthalmol. 2001;239(12):961–967. doi: 10.1007/s004170100368. Available from: http://dx.doi.org/10.1007/s004170100368. [DOI] [PubMed] [Google Scholar]
- 99.Brors D, Aletsee C, Schwager K, Mlynski R, Hansen S, Schäfers M, Ryan AF, Dazert S. Interaction of spiral ganglion neuron processes with alloplastic materials in vitro(1) Hear Res. 2002;167(1-2):110–121. doi: 10.1016/S0378-5955(02)00355-6. Available from: http://dx.doi.org/10.1016/S0378-5955(02)00355-6. [DOI] [PubMed] [Google Scholar]
- 100.Reich U, Mueller PP, Fadeeva E, Chichkov BN, Stoever T, Fabian T, Lenarz T, Reuter G. Differential fine-tuning of cochlear implant material-cell interactions by femtosecond laser microstructuring. J Biomed Mater Res B Appl Biomater. 2008;87(1):146–153. doi: 10.1002/jbm.b.31084. Available from: http://dx.doi.org/10.1002/jbm.b.31084. [DOI] [PubMed] [Google Scholar]
- 101.Reich U, Reuter G, Fadeeva E, Chichkov B, Warnecke A, Stöver T. Lenarz T. Gerichtetes Wachstum neuronaler Zellen auf mikrostrukturiertem Implantatmaterial. Biomaterialien. 2007;8(3):162. [Google Scholar]
- 102.Wolfram T, Spatz JP, Burgess RW. Cell adhesion to agrin presented as a nanopatterned substrate is consistent with an interaction with the extracellular matrix and not transmembrane adhesion molecules. BMC Cell Biol. 2008;9(1):64. doi: 10.1186/1471-2121-9-64. Available from: http://dx.doi.org/10.1186/1471-2121-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Selhuber-Unkel C, López-García M, Kessler H, Spatz JP. Cooperativity in adhesion cluster formation during initial cell adhesion. Biophys J. 2008;95(11):5424–5431. doi: 10.1529/biophysj.108.139584. Available from: http://dx.doi.org/10.1529/biophysj.108.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cavalcanti-Adam EA, Volberg T, Micoulet A, Kessler H, Geiger B, Spatz JP. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys J. 2007;92(8):2964–2974. doi: 10.1529/biophysj.106.089730. Available from: http://dx.doi.org/10.1529/biophysj.106.089730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Larsson C, Emanuelsson L, Thomsen P, Ericson LE, Aronsson BO, Kasemo B, Lausmaa J. Bone response to surface modified titanium implants - studies on the tissue response after 1 year to machined and electropolished implants with different oxide thicknesses. J Mater Sci Mater Med. 1997;8(12):721–729. doi: 10.1023/A:1018548225899. Available from: http://dx.doi.org/10.1023/A:1018548225899. [DOI] [PubMed] [Google Scholar]
- 106.Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dental Materials. 2006;23(7):844–854. doi: 10.1016/j.dental.2006.06.025. Available from: http://dx.doi.org/10.1016/j.dental.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 107.Anselme K, Bigerelle M, Noel B, Iost A, Hardouin P. Effect of grooved titanium substratum on human osteoblastic cell growth. J Biomed Mater Res. 2002;60:529–540. doi: 10.1002/jbm.10101. Available from: http://dx.doi.org/10.1002/jbm.10101. [DOI] [PubMed] [Google Scholar]
- 108.Zou J, Asukas J, Inha T, Toppila E, Kellomäki M, Pyykkö I. Biocompatibility of different biopolymers after being implanted into the rat cochlea. Otol Neurotol. 2008;29(5):714–719. doi: 10.1097/MAO.0b013e31817d874b. Available from: http://dx.doi.org/10.1097/MAO.0b013e31817d874b. [DOI] [PubMed] [Google Scholar]
- 109.Ryan AF, Wittig J, Evans A, Dazert S, Mullen L. Environmental micro-patterning for the study of spiral ganglion neurite guidance. Audiol Neurootol. 2006;11(2):134–143. doi: 10.1159/000090686. Available from: http://dx.doi.org/10.1159/000090686. [DOI] [PubMed] [Google Scholar]
- 110.Vasita R, Katti DS. Growth factor-delivery systems for tissue engineering: a materials perspective. Expert Rev Med Devices. 2006;3(1):29–47. doi: 10.1586/17434440.3.1.29. Available from: http://dx.doi.org/10.1586/17434440.3.1.29. [DOI] [PubMed] [Google Scholar]
- 111.Sachse A, Wagner A, Keller M, Wagner O, Wetzel WD, Layher F, Venbrocks RA, Hortschansky P, Pietraszczyk M, Wiederanders B, Hempel HJ, Bossert J, Horn J, Schmuck K, Mollenhauer J. Osteointegration of hydroxyapatite-titanium implants coated with nonglycosylated Recombinant human bone morphogenetic protein-2 (BMP-2) in aged sheep. Bone. 2005;37(5):699–710. doi: 10.1016/j.bone.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 112.Seeherman H, Wozney JM. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev. 2005;16(3):329–345. doi: 10.1016/j.cytogfr.2005.05.001. Available from: http://dx.doi.org/10.1016/j.cytogfr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 113.Gunn J, Cumberland D. Stent coatings and local drug delivery: State of the art. European Heart Journal. 1999;20:1693–1700. doi: 10.1053/euhj.1999.1549. Available from: http://dx.doi.org/10.1053/euhj.1999.1549. [DOI] [PubMed] [Google Scholar]
- 114.Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, Colombo A, Schampaert E, Grube E, Kirtane AJ, Cutlip DE, Fahy M, Pocock SJ, Mehran R, Leon MB. Safety and efficacy of sirolimus- and paclitaxeleluting coronary stents. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. Available from: http://dx.doi.org/10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 115.Silber S, Borggrefe M, Böhm M, Hoffmeister HM, Dietz R, Ertl G, Heuschet G. Positionspapier der Deutschen Gesellschaft für Kardiologie (DGK) zur Wirksamkeit und Sicherheit von Medikamente freisetzenden Koronarstents (DES) Der Kardiologe. 2007;1:84–111. doi: 10.1007/s12181-007-0012-6. Available from: http://dx.doi.org/10.1007/s12181-007-0012-6. [DOI] [Google Scholar]
- 116.Li H, Corrales CE, Edge A, Heller S. Stem cells as therapy for hearing loss. Trends Mol Med. 2004;10(7):309–315. doi: 10.1016/j.molmed.2004.05.008. Available from: http://dx.doi.org/10.1016/j.molmed.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 117.Senn P, Oshima K, Teo D, Grimm C, Heller S. Robust postmortem survival of murine vestibular and cochlear stem cells. J Assoc Res Otolaryngol. 2007;8(2):194–204. doi: 10.1007/s10162-007-0079-6. Available from: http://dx.doi.org/10.1007/s10162-007-0079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martinez-Monedero R, Oshima K, Heller S, Edge AS. The potential role of endogenous stem cells in regeneration of the inner ear. Hear Res. 2007;227(1-2):48–52. doi: 10.1016/j.heares.2006.12.015. Available from: http://dx.doi.org/10.1016/j.heares.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Senn P, Heller S. Stem-cell-based approaches for treating inner ear diseases. HNO. 2008;56(1):21–26. doi: 10.1007/s00106-007-1652-3. Available from: http://dx.doi.org/10.1007/s00106-007-1652-3. [DOI] [PubMed] [Google Scholar]
- 120.Juhn A, Rybak L. Labyrinthine barriers and cochlear homeostasis. Acta Otolaryngol. 1981;91:529–534. doi: 10.3109/00016488109138538. [DOI] [PubMed] [Google Scholar]
- 121.Juhn S. Barrier systems in the inner ear. Acta Otolaryngol Suppl. 1988;458:79–83. doi: 10.3109/00016488809125107. [DOI] [PubMed] [Google Scholar]
- 122.Swan EE, Mescher MJ, Sewell WF, Tao SL, Borenstein JT. Inner ear drug delivery for auditory applications. Adv Drug Deliv Rev. 2008;60(15):1583–1599. doi: 10.1016/j.addr.2008.08.001. Available from: http://dx.doi.org/10.1016/j.addr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garnham C, Reetz G, Jolly C, Miller J, Salt A, Beal F. Drug delivery to the cochlea after implantation: consideration of the risk factors. Cochlear Implants Int. 2006;6:12–14. doi: 10.1002/cii.273. Available from: http://dx.doi.org/10.1002/cii.273. [DOI] [PubMed] [Google Scholar]
- 124.Pettingill LN, Richardson RT, Wise AK, O'Leary S, Shepherd RK. Neurotrophic factors and neural prostheses: potential clinical applications based upon findings in the auditory system. IEEE Trans Biomed Eng. 2007;54:1–5. doi: 10.1109/TBME.2007.895375. Available from: http://dx.doi.org/10.1109/TBME.2007.895375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Duan ML, Ulfendahl M, Laurell G, Counter AS, Pyykko I, Borg E, Rosenhall U. Protection and treatment of sensorineural hearing disorders caused by exogeous factors: experimental findings and potential clinical application. Hear Res. 2002;169:169–178. doi: 10.1016/S0378-5955(02)00484-7. Available from: http://dx.doi.org/10.1016/S0378-5955(02)00484-7. [DOI] [PubMed] [Google Scholar]
- 126.Hochmair I, Nopp P, Jolly CN, Schmidt M, Schober H, Garnham C, Anderson I. Med-el cochlear implants: state of the art and a glimpse into the future. Trends Amplif. 2006;10:201–220. doi: 10.1177/1084713806296720. Available from: http://dx.doi.org/10.1177/1084713806296720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Paasche G, Gibson P, Averbeck T, Becker H, Lenarz T, Stover T. Technical report: modification of a cochlear implant electrode for drug delivery to the inner ear. Otol Neurotol. 2003;24:222–227. doi: 10.1097/00129492-200303000-00016. Available from: http://dx.doi.org/10.1097/00129492-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 128.Stöver T, Paasche G, Lenarz T, Ripken T, Breitenfeld P, Lubatschowski H, Fabian T. Development of a drug delivery device: using the femtosecond laser to modify cochlear implant electrodes. Cochlear Implants Int. 2007;8:38–52. doi: 10.1002/cii.329. Available from: http://dx.doi.org/10.1002/cii.329. [DOI] [PubMed] [Google Scholar]
- 129.Huang CQ, Tykocinski M, Stathopoulos D, Cowan R. Effects of steroids and lubricants on electrical impedance and tissue response following cochlear implantation. Cochlear Implants Int. 2007;8(3):123–147. doi: 10.1002/cii.336. Available from: http://dx.doi.org/10.1002/cii.336. [DOI] [PubMed] [Google Scholar]
- 130.Vivero RJ, Joseph DE, Angeli S, He J, Chen S, Eshraghi AA, Balkany TJ, Van de Water TR. Dexamethasone base conserves hearing from electrode trauma-induced hearing loss. Laryngoscope. 2008;118(11):2028–2035. doi: 10.1097/MLG.0b013e31818173ec. Available from: http://dx.doi.org/10.1097/MLG.0b013e31818173ec. [DOI] [PubMed] [Google Scholar]
- 131.Fetoni AR, Ferraresi A, Greca CL, Rizzo D, Sergi B, Tringali G, Piacentini R, Troiani D. Antioxidant protection against acoustic trauma by coadministration of idebenone and vitamin E. Neuroreport. 2008;19(3):277–281. doi: 10.1097/WNR.0b013e3282f50c66. Available from: http://dx.doi.org/10.1097/WNR.0b013e3282f50c66. [DOI] [PubMed] [Google Scholar]
- 132.Kim SJ, Jeong HJ, Myung NY, Kim MC, Lee JH, So HS, Park RK, Kim HM, Um JY, Hong SH. The protective mechanism of antioxidants in cadmium-induced ototoxicity in vitro and in vivo. Environ Health Perspect. 2008;116(7):854–862. doi: 10.1289/ehp.10467. Available from: http://dx.doi.org/10.1289/ehp.10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maruyama J, Miller JM, Ulfendahl M. Glial cell line-derived neurotrophic factor and antioxidants preserve the electrical responsiveness of the spiral ganglion neurons after experimentally induced deafness. Neurobiol Dis. 2008;29(1):14–21. doi: 10.1016/j.nbd.2007.07.026. Available from: http://dx.doi.org/10.1016/j.nbd.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Maruyama J, Yamagata T, Ulfendahl M, Bredberg G, Altschuler RA, Miller JM. Effects of antioxidants on auditory nerve function and survival in deafened guinea pigs. Neurobiol Dis. 2007;25(2):309–318. doi: 10.1016/j.nbd.2006.09.012. Available from: http://dx.doi.org/10.1016/j.nbd.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schindler RA, Gladstone HB, Scott N, Hradek GT, Williams H, Shah SB. Enhanced preservation of the auditory nerve following cochlear perfusion with nerve growth factors. Am J Otol. 1995;16(3):304–309. [PubMed] [Google Scholar]
- 136.Ernfors P, Duan ML, ElShamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat Med. 1996;2(4):463–467. doi: 10.1038/nm0496-463. Available from: http://dx.doi.org/10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- 137.Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7(4):889–894. doi: 10.1097/00001756-199603220-00011. Available from: http://dx.doi.org/10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- 138.Miller JM, Chi DH, O'Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15(4-5):631–643. doi: 10.1016/S0736-5748(96)00117-7. Available from: http://dx.doi.org/10.1016/S0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- 139.Ylikoski J, Pirvola U, Virkkala J, Suvanto P, Liang XQ, Magal E, Altschuler R, Miller JM, Saarma M. Guinea pig auditory neurons are protected by glial cell line-derived growth factor from degeneration after noise trauma. Hear Res. 1998;124(1-2):17–26. doi: 10.1016/S0378-5955(98)00095-1. Available from: http://dx.doi.org/10.1016/S0378-5955(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 140.Kuang R, Hever G, Zajic G, Yan Q, Collins F, Louis JC, Keithley E, Magal E. Glial cell line-derived neurotrophic factor. Potential for otoprotection. Ann N Y Acad Sci. 1999;884:270–291. doi: 10.1111/j.1749-6632.1999.tb08648.x. Available from: http://dx.doi.org/10.1111/j.1749-6632.1999.tb08648.x. [DOI] [PubMed] [Google Scholar]
- 141.Scheper V, Paasche G, Miller JM, Warnecke A, Berkingali N, Lenarz T, Stöver T. Effects of delayed treatment with combined GDNF and continuous electrical stimulation on spiral ganglion cell survival in deafened guinea pigs. J Neurosci Res. 2009;87(6):1389–1399. doi: 10.1002/jnr.21964. Available from: http://dx.doi.org/10.1002/jnr.21964. [DOI] [PubMed] [Google Scholar]
- 142.Yagi M, Kanzaki S, Kawamoto K, Shin B, Shah PP, Magal E, Sheng J, Raphael Y. Spiral ganglion neurons are protected from degeneration by GDNF gene therapy. J Assoc Res Otolaryngol. 2000;1(4):315–325. doi: 10.1007/s101620010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shoji F, Miller AL, Mitchell A, Yamasoba T, Altschuler RA, Miller JM. Differential protective effects of neurotrophins in the attenuation of noise-induced hair cell loss. Hear Res. 2000;146(1-2):134–142. doi: 10.1016/S0378-5955(00)00106-4. Available from: http://dx.doi.org/10.1016/S0378-5955(00)00106-4. [DOI] [PubMed] [Google Scholar]
- 144.Shoji F, Yamasoba T, Magal E, Dolan DF, Altschuler RA, Miller JM. Glial cell line-derived neurotrophic factor has a dose dependent influence on noise-induced hearing loss in the guinea pig cochlea. Hear Res. 2000;142(1-2):41–55. doi: 10.1016/S0378-5955(00)00007-1. Available from: http://dx.doi.org/10.1016/S0378-5955(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 145.Shinohara T, Bredberg G, Ulfendahl M, Pyykko I, Olivius NP, Kaksonen R, Lindstrom B, Altschuler R, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci U S A. 2002;99(3):1657–1660. doi: 10.1073/pnas.032677999. Available from: http://dx.doi.org/10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kanzaki S, Stover T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, Raphael Y. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002;454(3):350–360. doi: 10.1002/cne.10480. Available from: http://dx.doi.org/10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- 147.Nakaizumi T, Kawamoto K, Minoda R, Raphael Y. Adenovirus-mediated expression of brain-derived neurotrophic factor protects spiral ganglion neurons from ototoxic damage. Audiol Neurootol. 2004;9(3):135–143. doi: 10.1159/000077264. Available from: http://dx.doi.org/10.1159/000077264. [DOI] [PubMed] [Google Scholar]
- 148.Gillespie LN, Clark GM, Bartlett PF, Marzella PL. BDNF-induced survival of auditory neurons in vivo: Cessation of treatment leads to accelerated loss of survival effects. J Neurosci Res. 2003;71(6):785–790. doi: 10.1002/jnr.10542. Available from: http://dx.doi.org/10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- 149.Wright TJ, Mansour SL. FGF signaling in ear development and innervation. Curr Top Dev Biol. 2003;57:225–259. doi: 10.1016/S0070-2153(03)57008-9. Available from: http://dx.doi.org/10.1016/S0070-2153(03)57008-9. [DOI] [PubMed] [Google Scholar]
- 150.Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14(23):2919–2937. doi: 10.1101/gad.841400. Available from: http://dx.doi.org/10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- 151.Pirvola U, Ylikoski J. Neurotrophic factors during inner ear development. Curr Top Dev Biol. 2003;57:207–223. doi: 10.1016/S0070-2153(03)57007-7. Available from: http://dx.doi.org/10.1016/S0070-2153(03)57007-7. [DOI] [PubMed] [Google Scholar]
- 152.Chao MV, Bothwell M. Neurotrophins: to cleave or not to cleave. Neuron. 2002;33(1):9–12. doi: 10.1016/S0896-6273(01)00573-6. Available from: http://dx.doi.org/10.1016/S0896-6273(01)00573-6. [DOI] [PubMed] [Google Scholar]
- 153.Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. Available from: http://dx.doi.org/10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- 154.Roehm PC, Hansen MR. Strategies to preserve or regenerate spiral ganglion neurons. Curr Opin Otolaryngol Head Neck Surg. 2005;13(5):294–300. doi: 10.1097/01.moo.0000180919.68812.b9. Available from: http://dx.doi.org/10.1097/01.moo.0000180919.68812.b9. [DOI] [PubMed] [Google Scholar]
- 155.Baloh RH, Gorodinsky A, Golden JP, Tansey MG, Keck CL, Popescu NC, Johnson EM, Jr, Milbrandt J. GFRalpha3 is an orphan member of the GDNF/neurturin/persephin receptor family. Proc Natl Acad Sci U S A. 1998;95(10):5801–5806. doi: 10.1073/pnas.95.10.5801. Available from: http://dx.doi.org/10.1073/pnas.95.10.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nishino J, Mochida K, Ohfuji Y, Shimazaki T, Meno C, Ohishi S, Matsuda Y, Fujii H, Saijoh Y, Hamada H. GFR alpha3, a component of the artemin receptor, is required for migration and survival of the superior cervical ganglion. Neuron. 1999;23(4):725–736. doi: 10.1016/S0896-6273(01)80031-3. Available from: http://dx.doi.org/10.1016/S0896-6273(01)80031-3. [DOI] [PubMed] [Google Scholar]
- 157.Enomoto H, Crawford PA, Gorodinsky A, Heuckeroth RO, Johnson EM, Jr, Milbrandt J. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development. 2001;128(20):3963–3974. doi: 10.1242/dev.128.20.3963. [DOI] [PubMed] [Google Scholar]
- 158.Russo VC, Schütt BS, Andaloro E, Ymer SI, Hoeflich A, Ranke MB, Bach LA, Werther GA. Insulin-like growth factor binding protein-2 binding to extracellular matrix plays a critical role in neuroblastoma cell proliferation, migration, and invasion. Endocrinology. 2005;146(10):4445–4455. doi: 10.1210/en.2005-0467. Available from: http://dx.doi.org/10.1210/en.2005-0467. [DOI] [PubMed] [Google Scholar]
- 159.Camarero G, Leon Y, Gorospe I, De Pablo F, Alsina B, Giraldez F, Varela-Nieto I. Insulin-like growth factor 1 is required for survival of transit-amplifying neuroblasts and differentiation of otic neurons. Dev Biol. 2003;262(2):242–253. doi: 10.1016/S0012-1606(03)00387-7. Available from: http://dx.doi.org/10.1016/S0012-1606(03)00387-7. [DOI] [PubMed] [Google Scholar]
- 160.Böttner M, Krieglstein K, Unsicker K. The transforming growth factor-betas: structure, signaling, and roles in nervous system development and functions. J Neurochem. 2000;75(6):2227–2240. doi: 10.1046/j.1471-4159.2000.0752227.x. Available from: http://dx.doi.org/10.1046/j.1471-4159.2000.0752227.x. [DOI] [PubMed] [Google Scholar]
- 161.Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258(11):7155–7160. [PubMed] [Google Scholar]
- 162.McLennan IS, Koishi K. The transforming growth factor-betas: multifaceted regulators of the development and maintenance of skeletal muscles, motoneurons and Schwann cells. Int J Dev Biol. 2002;46(4):559–567. [PubMed] [Google Scholar]
- 163.Weerda HG, Gamberger TI, Siegner A, Gjuric M, Tamm ER. Effects of transforming growth factor-beta1 and basic fibroblast growth factor on proliferation of cell cultures derived from human vestibular nerve schwannoma. Acta Otolaryngol. 1998;118(3):337–343. doi: 10.1080/00016489850183412. [DOI] [PubMed] [Google Scholar]
- 164.Diensthuber M, Brandis A, Lenarz T, Stöver T. Co-expression of transforming growth factor-beta1 and glial cell line-derived neurotrophic factor in vestibular schwannoma. Otol Neurotol. 2004;25(3):359–365. doi: 10.1097/00129492-200405000-00026. Available from: http://dx.doi.org/10.1097/00129492-200405000-00026. [DOI] [PubMed] [Google Scholar]
- 165.Atar O, Avraham KB. Therapeutics of hearing loss: expectations vs reality. Drug Discov Today. 2005;10(19):1323–1330. doi: 10.1016/S1359-6446(05)03618-4. Available from: http://dx.doi.org/10.1016/S1359-6446(05)03618-4. [DOI] [PubMed] [Google Scholar]
- 166.Holley MC. Keynote review: The auditory system, hearing loss and potential targets for drug development. Drug Discov Today. 2005;10(19):1269–1282. doi: 10.1016/S1359-6446(05)03595-6. Available from: http://dx.doi.org/10.1016/S1359-6446(05)03595-6. [DOI] [PubMed] [Google Scholar]
- 167.Bowers WJ, Chen X, Guo H, Frisina DR, Federoff HJ, Frisina RD. Neurotrophin-3 transduction attenuates cisplatin spiral ganglion neuron ototoxicity in the cochlea. Mol Ther. 2002;6(1):12–18. doi: 10.1006/mthe.2002.0627. Available from: http://dx.doi.org/10.1006/mthe.2002.0627. [DOI] [PubMed] [Google Scholar]
- 168.Chen X, Frisina RD, Bowers WJ, Frisina DR, Federoff HJ. HSV amplicon-mediated neurotrophin-3 expression protects murine spiral ganglion neurons from cisplatin-induced damage. Mol Ther. 2001;3(6):958–963. doi: 10.1006/mthe.2001.0334. Available from: http://dx.doi.org/10.1006/mthe.2001.0334. [DOI] [PubMed] [Google Scholar]
- 169.Ghezzi P, Brines M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004;11(Suppl 1):S37–S44. doi: 10.1038/sj.cdd.4401450. Available from: http://dx.doi.org/10.1038/sj.cdd.4401450. [DOI] [PubMed] [Google Scholar]
- 170.Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–494. doi: 10.1038/nrn1687. Available from: http://dx.doi.org/10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- 171.Krantz SB. Erythropoietin. Blood. 1991;77(3):419–434. [PubMed] [Google Scholar]
- 172.Arishima Y, Setogushi T, Yamaura I, Yone K, Komiya S. Preventive effect of erythropoietin on spinal cord cells following acute traumatic injury in rats. SPINE. 2006;21:2432–2438. doi: 10.1097/01.brs.0000239124.41410.7a. Available from: http://dx.doi.org/10.1097/01.brs.0000239124.41410.7a. [DOI] [PubMed] [Google Scholar]
- 173.Viviani B, Bartesaghi S, Corsini E, Villa P, Ghezzi P, Garau A, Galli CL, Marinivich M. Erythropoietin protects primary hippocampal neorons increasing the expression of brain-derived neurotrophic factor. J Neurochem. 2005;93(2):412–421. doi: 10.1111/j.1471-4159.2005.03033.x. Available from: http://dx.doi.org/10.1111/j.1471-4159.2005.03033.x. [DOI] [PubMed] [Google Scholar]
- 174.Berkingali N, Warnecke A, Gomes P, Paasche G, Tack J, Lenarz T, Stöver T. Neurite outgrowth on cultured spiral ganglion neurons induced by erythropoietin. Hear Res. 2008;243(1-2):121–126. doi: 10.1016/j.heares.2008.07.003. Available from: http://dx.doi.org/10.1016/j.heares.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 175.Caye-Thomasen P, Wagner N, Lidegaard Frederiksen B, Asal K, Thomsen J. Erythropoietin and erythropoietin receptor expression in the guinea pig inner ear. Hear Res. 2005;203(1-2):21–27. doi: 10.1016/j.heares.2004.11.017. Available from: http://dx.doi.org/10.1016/j.heares.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 176.Monge A, Nagy I, Bonabi S, Schmid S, Gassmann M, Bodmer D. The effect of erythropoietin on gentamicin-induced auditory hair cell loss. Laryngoscope. 2006;116(2):312–316. doi: 10.1097/01.mlg.0000199400.08550.3f. Available from: http://dx.doi.org/10.1097/01.mlg.0000199400.08550.3f. [DOI] [PubMed] [Google Scholar]
- 177.Andreeva N, Nyamaa A, Haupt H, Gross J, Mazurek B. Recombinant human erythropoietin prevents ischemia-induced apoptosis and necrosis in explant cultures of the rat organ of Corti. Neurosci Lett. 2006;396(2):86–90. doi: 10.1016/j.neulet.2005.11.013. Available from: http://dx.doi.org/10.1016/j.neulet.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 178.Malgrange B, Lefebvre P, Van de Water TR, Staecker H, Moonen G. Effects of neurotrophins on early auditory neurones in cell culture. Neuroreport. 1996;7(4):913–917. doi: 10.1097/00001756-199603220-00016. Available from: http://dx.doi.org/10.1097/00001756-199603220-00016. [DOI] [PubMed] [Google Scholar]
- 179.Hegarty JL, Kay AR, Green SH. Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cAMP and requires elevation of [Ca2+]i within a set range. J Neurosci. 1997;17:1959–1970. doi: 10.1523/JNEUROSCI.17-06-01959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gillespie LN, Clark GM, Bartlett PF, Marzella PL. LIF is more potent than BDNF in promoting neurite outgrowth of mammalian auditory neurons in vitro. Neuroreport. 2001;12:275–279. doi: 10.1097/00001756-200102120-00019. Available from: http://dx.doi.org/10.1097/00001756-200102120-00019. [DOI] [PubMed] [Google Scholar]
- 181.Mou K, Hunsberger CL, Cleary JM, Davis RL. Synergistic effects of BDNF and NT-3 on postnatal spiral ganglion neurons. J Comp Neurol. 1997;386:529–539. doi: 10.1002/(SICI)1096-9861(19971006)386:4<529::AID-CNE1>3.0.CO;2-4. Available from: http://dx.doi.org/10.1002/(SICI)1096-9861(19971006)386:4<529::AID-CNE1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 182.Marzella PL, Gillespie LN, Clark GM, Bartlett PF, Kilpatrick TJ. The neurotrophins act synergistically with LIF and members of the TGF-beta superfamily to promote the survival of spiral ganglia neurons in vitro. Hear Res. 1999;138:73–80. doi: 10.1016/S0378-5955(99)00152-5. Available from: http://dx.doi.org/10.1016/S0378-5955(99)00152-5. [DOI] [PubMed] [Google Scholar]
- 183.Wefstaedt P, Scheper V, Lenarz T, Stöver T. Brain-derived neurotrophic factor/glial cell line-derived neurotrophic factor survival effects on auditory neurons are not limited by dexamethasone. Neuroreport. 2005;16(18):2011–2014. doi: 10.1097/00001756-200512190-00008. Available from: http://dx.doi.org/10.1097/00001756-200512190-00008. [DOI] [PubMed] [Google Scholar]
- 184.Qun LX, Pirvola U, Saarma M, Ylikoski J. Neurotrophic factors in the auditory periphery. Ann N Y Acad Sci. 1999;884:292–304. doi: 10.1111/j.1749-6632.1999.tb08649.x. Available from: http://dx.doi.org/10.1111/j.1749-6632.1999.tb08649.x. [DOI] [PubMed] [Google Scholar]
- 185.Wefstaedt P. Untersuchungen zu trophischen und protektiven Effekten neurotropher Faktoren (Brain-derived neurotrophic factor, Glial cell line-derived neurotrophic factor), des Glukocorticoids Dexamethason sowie elektrischer Stimulation auf kultivierte Spiralganglienzellen der Ratte. Hannover: Aus dem Institut für Zoologie der Tierärztlichen Hochschule Hannover und der Klinik für Hals-Nasen-Ohren-Heilkunde der Medizinischen Hochschule Hannover; 2006. [Google Scholar]
- 186.Hartnick CJ, Staecker H, Malgrange B, Lefebvre PP, Liu W, Moonen G, Van De Water TR. Neurotrophic effects of BDNF and CNTF, alone and in combination, on postnatal day 5 rat acoustic ganglion neurons. J Neurobiol. 1996;30:246–254. doi: 10.1002/(SICI)1097-4695(199606)30:2<246::AID-NEU6>3.0.CO;2-5. Available from: http://dx.doi.org/10.1002/(SICI)1097-4695(199606)30:2<246::AID-NEU6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 187.Shah SB, Gladstone HB, Williams H, Hradek GT, Schindler RA. An extended study: protective effects of nerve growth factor in neomycin-induced auditory neural degeneration. Am J Otol. 1995;16(3):310–314. [PubMed] [Google Scholar]
- 188.Gillespie LN, Clark GM, Marzella PL. Delayed neurotrophin treatment supports auditory neuron survival in deaf guinea pigs. Neuroreport. 2004;15(7):1121–1125. doi: 10.1097/00001756-200405190-00008. Available from: http://dx.doi.org/10.1097/00001756-200405190-00008. [DOI] [PubMed] [Google Scholar]
- 189.Gillespie LN, Shepherd RK. Clinical application of neurotrophic factors: the potential for primary auditory neuron protection. Eur J Neurosci. 2005;22(9):2123–2133. doi: 10.1111/j.1460-9568.2005.04430.x. Available from: http://dx.doi.org/10.1111/j.1460-9568.2005.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Scheper V. Elektrophysiologische und histologische Untersuchungen zum protektiven Effekt von Glial cell line-derived neurotrophic factor, Brain-derived neurotrophic factor, Dexamethason und Elektrostimulation auf Spiralganglienzellen ertaubter Meerschweinchen. Hannover: Aus dem Institut für Zoologie der Tierärztlichen Hochschule Hannover und der Klinik für Hals-Nasen-Ohren-Heilkunde der Medizinischen Hochschule Hannover; 2007. [Google Scholar]
- 191.Miller JM, Le Prell CG, Prieskorn DM, Wys NL, Altschuler RA. Delayed neurotrophin treatment following deafness rescues spiral ganglion cells from death and promotes regrowth of auditory nerve peripheral processes: effects of brain-derived neurotrophic factor and fibroblast growth factor. J Neurosci Res. 2007;85(9):1959–1969. doi: 10.1002/jnr.21320. Available from: http://dx.doi.org/10.1002/jnr.21320. [DOI] [PubMed] [Google Scholar]
- 192.Wise AK, Richardson R, Hardman J, Clark G, O'Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487(2):147–165. doi: 10.1002/cne.20563. Available from: http://dx.doi.org/10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- 193.Yamagata T, Miller JM, Ulfendahl M, Olivius NP, Altschuler RA, Pyykko I, Bredberg G. Delayed neurotrophic treatment preserves nerve survival and electrophysiological responsiveness in neomycin-deafened guinea pigs. J Neurosci Res. 2004;78(1):75–86. doi: 10.1002/jnr.20239. Available from: http://dx.doi.org/10.1002/jnr.20239. [DOI] [PubMed] [Google Scholar]
- 194.Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486(2):145–158. doi: 10.1002/cne.20564. Available from: http://dx.doi.org/10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Thorne M, Salt AN, DeMott JE, Henson MM, Henson OW, Gewalt SL. Cochlear fluid space dimensions for six species derived from reconstructions of three-dimensional magnetic resonance images. Laryngoscope. 1999;109:1661–1668. doi: 10.1097/00005537-199910000-00021. Available from: http://dx.doi.org/10.1097/00005537-199910000-00021. [DOI] [PubMed] [Google Scholar]
- 196.Salt AN, Rask-Andersen H. Responses of the endolymphatic sac to perilymphatic injections and withdrawals: evidence for the presence of a one-way valve. Hear Res. 2004;191(1-2):90–100. doi: 10.1016/j.heares.2003.12.018. Available from: http://dx.doi.org/10.1016/j.heares.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 197.Mynatt R, Hale SA, Gill RM, Plontke SK, Salt AN. Demonstration of a longitudinal concentration gradient along scala tympani by sequential sampling of perilymph from the cochlear apex. J Assoc Res Otolaryngol. 2006;7(2):182–193. doi: 10.1007/s10162-006-0034-y. Available from: http://dx.doi.org/10.1007/s10162-006-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Plontke SK, Salt AN. Simulation of application strategies for local drug delivery to the inner ear. ORL J Otorhinolaryngol Relat Spec. 2006;68(6):386–392. doi: 10.1159/000095284. Available from: http://dx.doi.org/10.1159/000095284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Plontke SK, Siedow N, Wegener R, Zenner HP, Salt AN. Cochlear pharmacokinetics with local inner ear drug delivery using a three-dimensional finite-element computer model. Audiol Neurootol. 2007;12(1):37–48. doi: 10.1159/000097246. Available from: http://dx.doi.org/10.1159/000097246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Prieskorn DM, Miller JM. Technical report: chronic and acute intracochlear infusion in rodents. Hear Res. 2000;140:212–215. doi: 10.1016/S0378-5955(99)00193-8. Available from: http://dx.doi.org/10.1016/S0378-5955(99)00193-8. [DOI] [PubMed] [Google Scholar]
- 201.Paasche G, Bögel L, Leinung M, Lenarz T, Stöver T. Substance distribution in a cochlea model using different pump rates for cochlear implant drug delivery electrode prototypes. Hear Res. 2006;212(1-2):74–82. doi: 10.1016/j.heares.2005.10.013. Available from: http://dx.doi.org/10.1016/j.heares.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 202.Schierholz JM, Lucas LJ, Rump A, Pulverer G. Efficacy of silver-coated medical devices. J Hosp Infect. 1998;40(4):257–262. doi: 10.1016/S0195-6701(98)90301-2. Available from: http://dx.doi.org/10.1016/S0195-6701(98)90301-2. [DOI] [PubMed] [Google Scholar]
- 203.Nair LS, Laurencin CT. Nanofibers and nanoparticles for orthopaedic surgery applications. J Bone Joint Surg Am. 2008;90(Suppl 1):128–131. doi: 10.2106/JBJS.G.01520. Available from: http://dx.doi.org/10.2106/JBJS.G.01520. [DOI] [PubMed] [Google Scholar]
- 204.Zou J, Saulnier P, Perrier T, Zhang Y, Manninen T, Toppila E, Pyykkö I. Distribution of lipid nanocapsules in different cochlear cell populations after round window membrane permeation. J Biomed Mater Res B Appl Biomater. 2008;87(1):10–18. doi: 10.1002/jbm.b.31058. Available from: http://dx.doi.org/10.1002/jbm.b.31058. [DOI] [PubMed] [Google Scholar]
- 205.Tamura T, Kita T, Nakagawa T, Endo T, Kim TS, Ishihara T, Mizushima Y, Higaki M, Ito J. Drug delivery to the cochlea using PLGA nanoparticles. Laryngoscope. 2005;115(11):2000–2005. doi: 10.1097/01.mlg.0000180174.81036.5a. Available from: http://dx.doi.org/10.1097/01.mlg.0000180174.81036.5a. [DOI] [PubMed] [Google Scholar]


