Abstract
Biomaterials for reconstruction of bony defects of the skull comprise of osteosynthetic materials applied after osteotomies or traumatic fractures and materials to fill bony defects which result from malformation, trauma or tumor resections. Other applications concern functional augmentations for dental implants or aesthetic augmentations in the facial region.
For ostheosynthesis, mini- and microplates made from titanium alloys provide major advantages concerning biocompatibility, stability and individual fitting to the implant bed. The necessity of removing asymptomatic plates and screws after fracture healing is still a controversial issue. Risks and costs of secondary surgery for removal face a low rate of complications (due to corrosion products) when the material remains in situ. Resorbable osteosynthesis systems have similar mechanical stability and are especially useful in the growing skull.
The huge variety of biomaterials for the reconstruction of bony defects makes it difficult to decide which material is adequate for which indication and for which site. The optimal biomaterial that meets every requirement (e.g. biocompatibility, stability, intraoperative fitting, product safety, low costs etc.) does not exist. The different material types are (autogenic) bone and many alloplastics such as metals (mainly titanium), ceramics, plastics and composites. Future developments aim to improve physical and biological properties, especially regarding surface interactions. To date, tissue engineered bone is far from routine clinical application.
Keywords: osteosynthesis, bone replacement, biomaterials, titanium, craniofacial reconstruction
1 Introduction
In 2004 Warnke et al. [157] described a near total reconstruction of the mandibular arch, applying a computer aid designed (CAD) individual-fit titanium mesh-“cage” filled with xenograft bone-minerals, autograft bone marrow and recombinant human Bone-Morphogenetic-Protein(BMP)-7, which was implanted into a latissimus dorsi muscle pouch in order to allow ossification. In a second stage it was transplanted to the mandibular region with microvascular anastomoses. This case report exemplarily demonstrates the broad spectrum of the present options in reconstructive surgery and surgical use of biomaterials for bone replacement in the skull: Autograft tissue, alloplastic materials, recombinant engineering, hormone-induced bone formation, microvascular surgical techniques, CAD und CAM (Computer Aided Manufacturing).
From a plentitude of data available in the literature, the following survey aims to outline the substantial issues of biomaterials for craniofacial reconstruction important in otolaryngology, head and neck surgery.
Two major groups of biomaterials have to be differentiated in this context: Biomaterials for osteosynthesis in traumatology and biomaterials as substitute or augmentation of bone. Biomaterials for ossiculoplasty and for rhino- and otoplasty have already been subject of circumstantial reviews in this rubric [13], [44]. Their particularities shall not be repeated here although some overlapping contents cannot be avoided.
2 Osteosynthesis
2.1 History
Dr. Carl Hansmann (1852–1917) who worked in the hospital “St. Georg” in Hamburg, Germany pioneered in plate fixation of fractures with a self-manufactured plate osteosynthesis system in 1886 [68]. William Halsted from Baltimore improved the system around 1893 by implanting the screws subcutaneously rather than percutaneously as Hansmann did [129]. Nevertheless, severe corrosion of the historical materials, poor hygiene and the lack of antibiotics led to frequent cases of osteomyelitis and implant-fracture. Only with the availability of antibiotics, modern materials and the principal of axial compression, proclaimed by Robert Danis in 1949, plate fixation became a routine procedure in traumatology.
Luhr [101] realized the principal of axial compression for mandibular osteosynthesis in the 1960s by using self-locking compression plates with tapered screw heads and excentric plate bore-holes. This mandibularcompression-screw (MCS) plate is still used today.
Materials in frequent use for craniofacial applications were stainless steel [140] and vitallium [101], an alloy from cobalt, chrome und molybdenum. Although titanium had been in clinical use since 1966 [74], it was Brånemark in 1983 [21] who outlined the superior biocompatibility and favorable mechanical properties of this metal. In addition, biodegradable plates and screws were available since the mid 1990s.
2.2 Metals
2.2.1 Stainless steel
High-alloyed stainless steel contains variable amounts of nickel, chrome, manganese, vanadium and/or molybdenum. In vivo, the corrosion resistance may be impaired under certain ambient conditions which may lead to crevice corrosion. Clinically, the corrosion products may lead to the formation of granulation tissue on the surface thus causing sensitization. As steel shows a higher elasticity modulus than bone tissue (ratio of the uniaxial stress over the uniaxial strain in the range of stress) stress shielding effects may occur and lead to bone resorption in such cases of long bone osteotomy where the metal material carries the greatest part of the load. Even though great mechanical stress is rather rare in craniofacial surgery (an exception may be the mandible) stainless steel is rarely used for craniofacial osetosynthesis today.
2.2.2 Cobalt based alloys
In the 20th century, new developments in the metalworking industry led to the production of an iron-free molybdenous cast alloy on a chrome and cobalt basis. These were introduced to the market under the name Vitallium® by Dres Reiner Erdle and Charles Prange (Stryker Howmedica, former Austenal Laboratories), at first only for dental indications. Thereafter Vitallium® served as a very corrosion-resistant material for several in dications in the field of endoprosthesis and osteosynthesis. Luhr was the pioneer of craniofacial mini-osteosynthesis-systems based on Vitallium (Luhr® Modular Craniomaxillofacial/Mandibular Fixation System, Leibinger Co.) [99], [100], [101]. Similar to stainless steel, cobalt alloys may also cause unwanted secondary effects due to corrosion products. This includes elevation [164] and even accumulation [17] of metal ions in the blood. Regarding the great number of implants throughout the world these sporadic cases of secondary effects are more or less negligible [131], [138], this being the reason for the systems successful use today.
2.2.3 Titanium
Titanium is the most biocompatible and corrosion-resistant metal [4], [127], its elasticity modulus corresponds to the elasticity modulus of the bone more than any other metal does [70], [83], therefore titanium is increasingly driving other metals out of the “craniofacial osteosynthesis market”.
2.2.3.1 Chemical and mechanical properties
Titanium is a white metallic transition element with an atomic number of 22. Even though titanium is commonly found in the lithosphere the industrial production of “pure” titanium is complex and therefore expensive, as it must be extracted from iron ore via highly energy intensive methods. Commercially obtainable “pure” titanium is classified into four quality grades. “Pure” titanium refers to an amount of less than 1% of additives like nitrate, carbon, hydrogen, oxygen or iron. These are specified in ISO 5832-2:2000-08. Titanium osteosynthesis material regularly consists of alloys, e.g. Ti-6Al-4V (6% aluminium, 4% vanadium) or Ti-6Al-7Nb (6% aluminium, 7% niobium), as standardized in ISO 5832-3.
Titanium is characterized by high stability at a light mass. This makes titanium a popular material for mechanically stressed and equally lightweight components (e.g. aerospace industry). Its elasticity modulus (105 kN/mm2) is well above the modulus of the human bone (approx. 20 kN/mm2) but only half as large as the modulus of stainless steel or cobalt alloys, therefore making stress shielding – effects less likely.
An approx. 10 µm thick superficial layer of titanium-oxide develops spontaneously, this is not only responsible for corrosion-resistance but also for the adhesion of glycoproteins in vivo, therefore being of great importance for the biocompatibility. In despite of this protectional layer a slight amount of corrosion occurs as a result of flexure under mechanical stress and also friction between osteosynthesis screws and plates [139]. This phenomenon may be intensified in the presence of macrophages through the production of H2O2 [109].
2.2.3.2 Toxicity
Titanium shows very low toxicity both in its ionic and also in its particle form. Titanium ions are subject to renal excretion [78]. Rulite, a titanium corrosion product, accumulates in lymph nodes, liver, spleen, bone marrow and the brain [29].
Higher toxicity potential arises from alloy additives:
Aluminium ions, which are also subject to renal excretion [108], may accumulate in cases of impaired renal function [55] and act neurotoxic. However all reported cases of aluminium accumulation detectable in the serum and hair relate to hip implants and not to craniofacial application [42], [150]. Also there have not yet been stated any cases of clinically relevant toxic accumulations, therefore the systemic toxicity may be regarded as negligible.
Vanadium is considered to be a micronutrient with a yet unidentified function. Vanadium only causes systemic toxic effects in high concentrations [38], [43].
In a synopsis of the literature there currently is no indication for clinically relevant toxicity caused by titanium-alloyed craniofacial osteosynthesis systems. Possible toxicity in the tissue surrounding the implants is also negligible. Animal tests also have shown no effects on skeletal muscles [121]. While localized reaction of mucous membranes are extensively described in dental implantology [112], only very few reports exist about localized reactions caused by screws protruding into nasal sinuses and therefore gaining contact to the mucosa. According to Brunner [23] this mucosal contact may give rise to inflammatory complications. The direct contact of mucosa and titanium does not cause clinical problems, as has been shown in titaniummesh reconstruction of the frontal and maxillary sinuses (see below) [84], [93].
2.2.3.3 Sensitization
Other than with nickel, cobalt und chromium, sensitization through titanium is rare [147]. Though individual cases have been described for orthopedic applications and pacemakerimplants, no type IV-reactions have been described for craniofacial applications to date. This also applies to aluminium und vanadium. Overall, allergic reactions do not seem to play any role concerning the craniofacial application of titanium alloys.
2.2.3.4 Cancerogenity
Animal tests do not show any indication of cancerogenity of titanium alloys [97], [144]. Individual cases of malignant tumors in the tissue surrounding titanium implants (pacemakers [56], mandibular plates [58]) remain speculation, pathogenically. Regarding the great amount of titanium implants used throughout the world cancerogenity is practically ruled out.
2.2.4 Systems
Today, a wide variety of systems with numerous forms of plates (miniplates, microplates, Figure 1 (Fig. 1) and Figure 2 (Fig. 2)), meshes and screws are available for clinical use [31], [138]. The individual colour of the plates and screws does not depend on pigmentation but is due to surface anodisation with which the manufacturers try to give their products a certain recognition value. Meanwhile rectangular plates, which were commonly used in earlier years, have been replaced by modular elementary plates and narrow, round-edged arched plates mainly because of their improved intraoperative adaptability [118]. Unlike with the former AO-system (Arbeitsgemeinschaft für Osteosynthesefragen), it now is not necessary to tap a thread but simply to pre-bore for the self-tapping screws. Thanks to their ductility (plasticity under overstressing) titanium plates can de adjusted easily intra-operatively. These systems may be sterilised and are re-usable. The mechanical integrity – as measured by maximal flexure before breaking – is not significantly reduced even after 50 cycles of autoclaving [1].
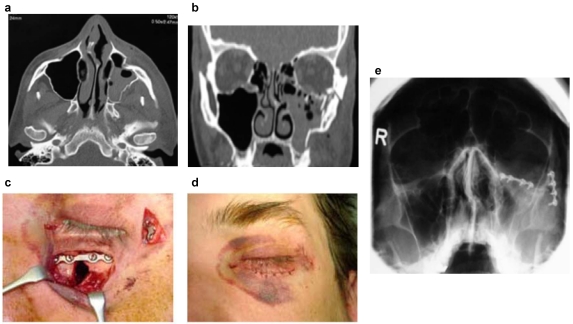
Figure 1. Osteosynthesis of a fracture of the mid-face (“Tripod”-fracture) with microplates and screws from titanium alloys:
a and b: Axial und coronal computer tomography with demonstration of the fractures; c and d: Intra- and postoperative demonstration of the miniplate osteosynthesis; e: Evaluation of postoperative position of the materials by conventional x-ray.
Figure 2. Osteosynthetic care of a fracture of the anterior frontal sinus wall:
a: Axial CT with demonstration of the fracture; b: Operative management by means of miniplate osteosynthesis from titanium after coronal incision.
2.2.5 Removal of osteosynthesis material
Titanium plates and screws that become symptomatic during the healing phase of a fracture, must be removed. Symptoms include: infection, foreign body response, wound dehiscence, extrusion, plate fracture, migration, pain, thermal sensitivity, growth disturbance in children, renal failure (possible accumulation of corrosion products). Clinical trials detect infection as the most common reason for the removal of osteosynthesis material: Over a 4-year period Bhatt et al. [16] had to remove 32 of 308 oro-maxillo-facial mini-plates implanted in 153 patients due to infectious complications. Murthy et al. [111] removed only 6 of 163 craniofacial titanium plates (76 patients) over a 10-year period, all cases due to infections. Other authors declare infection rates at approx. 7–10% [9], [77], [128].
The removal of asymptomatic plates and screws after complete fracture healing is subject to many controversies:
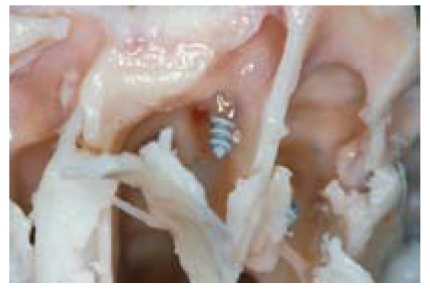
Brunner generally recommends plate removal referring to possible infectious complications [23], [24] this being less based on the material properties but on the fact that screws which protrude into the nasal sinuses (Figure 3 (Fig. 3)) and therefore gain contact to the highly reactive respiratory and potentially contaminated mucosa provide an ideal infection-duct which enables bacterial dissemination and therefore osteitic complications [23], [24]. The local immune reaction is mainly based on macrophageal interaction with the implant surface [7]. Titanium ions may enhance implant-conducted bone-resorption in vitro and in vivo through activation and secretion of cytokines [156] which in turn facilitates infection. Other reasons for the removal of implanted material may comprise: patients requests, radiological artefacts, palpability, visibility, thermal sensitivity.
Figure 3. Osteosynthetic screw protruding into the lumen of the frontal sinus (screw from silicon nitride, animal experiment, minipig).
Arguments against the removal of asymptomatic plates are mainly bases on the need for subsequent removal with accordingly (low) morbidity and on the high biocompatibility of the implant material. Other titanium implants remain inside the patient (pacemakers, articular implants), the difference here being that titanium plates and screws are practically “functionless” after complete fracture healing.
Steinhart and Schröder [141] recommend a removal depending on the localisation (maxillary sinus wall, alveolar ridge) and also generally for children.
In summary there is no consent in the question of removal of asymptomatic titanium plates and screws in craniofacial applications because of the lack of scientifically proven indications or contraindications for either approaches. Other than in most European countries, the majority of asymptomatic plates and screws are not removed in the U.S. [151]. It is subject to discussion if economical reasons are of greater relevance in those cases.
2.3 Resorbable osteosynthesis systems
The demand for resorbable osteosynthesis systems for facial fractures and osteotomy stabilization arises from the above mentioned controversially discussed disadvantages of metal implants:
Necessity of a second operation for the removal of implants due to loosening of screws, palpability or visibility of implants
Thermal sensitivity
Radiological artefacts
Implant translocation in the growing skull of children
The avoidance of immobilization-caused osteoporosis may be seen as a biological argument for the use of resorbable material. Studies have shown that the resorption-dependant weakening of the implants leads to an earlier functional exposure and therefore faster restructuring of the fracture gap [60].
Even though the resorbable features were already well known from suturing material (Dexon®, Maxon®, Vicryl®, PDS®), the era of synthetical resorbable implants made from lactic acid and glycolic acid (Polylactic acid – PLA, Polyglycolic acid – PGA) began in the 1960s with studies of Kulkarni et al. [90], [91]. It was not until the 1990s that resorbable miniplates and screws were widely introduced to clinical routine. There are other resorbable polyesters known – apart from the above mentioned polyesters and their copolymers: Polycaprolaktone, polyhydroxybutyrate, polytrimethylcarbonate, polyurethane. Today there is a vast number of publications on the subject of resorbable polymers.
2.3.1 Structure of resorbable polymers
The basic elements of polylactic acid (PLA) and polyglycolic acid (PGA) are units of lactic acid or glycolic acid. Lactic acid is a chiral molecule (two optical active forms) and may be present in its L- or D-configuration. Hence polylactides are only composed of molecules of the same configuration, e.g. poly-L-lactide (PLLA) or of the sterically differing basic molecules, e.g. poly-D, L-lactide (PDLLA). By catalytically mediated ring-opening polymerization high-molecular polymers are synthesized from the basic molecules. The physical properties of the high-molecular polymers depend on molecular weight, linear or branched architecture and amorphous or crystalline structure of the polymer chains. By alteration of the components polymers and copolymers (e.g. PLLA/PDLLA) with different properties (tensile strength, flexural strength etc.) may be synthesized [12], [39], [60], [67], [123], [148], [152], [154].
2.3.2 Degradation and degradation time
Resorption of polymers generally occurs either by photo-, thermo-, mechanical oder chemical degradation. In vivo, chemical degradation plays the most important role, therefore an aqueous environment is necessary to enable hydrolysis to degrade the polymers into short-chained fragments. These lowmolecular fragments are phagocytosed and metabolized by macrophages and polymorphonuclear leukocytes. The resulting monomers are natural byproducts of anaerobic metabolism. Lactide is converted to pyruvate by lactate dehydrogenase and is then either used for gluconeogenesis or is degraded to carbondioxide and water via the citric acid cycle. These final products are either exhaled or excreted [20].
The time period of hydrolytic fragmentation depends on temperature, pH-value, availability of water, mechanical strain and also on the configuration of the polymers (composition, production, molecular weight, crystal linity etc.): Low pH-values (as in inflammated tissue) increases degradation in terms of an autocatalytic process through the released acidic monomeres. Polymers with highly crystalline regions are degraded more slowly due to the fact that hydrolytic degradation commences in the amorphous regions.
As the degradation speed is mainly determined by hydrolytic degradation – and not so much by phagocytosis and metabolisation – it may be directed by the configuration of the polymer.
The absolute degradation times quoted in the literature differ remarkably, depending on the configuration of the individual polymers and also because of the different types of studies (animal test – clinical trial). High crystalline polymers made from PGA may be entirely resorbed after 6 months time [155], whereas high crystalline residues of PLLA-implants can be traced after more than 5 years time [14]. Overlooking the abundance of publications on this topic a standard reference degradation time of approx. 12 months may be regarded for the broadly available PLLA and PGA copolymers.
2.3.3 Mechanical properties of resorbable polymers
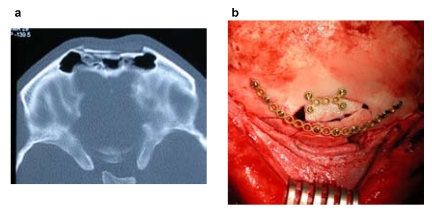
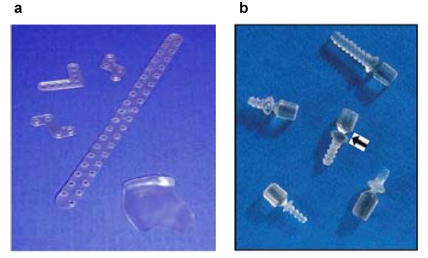
Compared to metallic systems resorbable material shows lesser tensile strength. This however has less influence on the immobilisation of fractures yet large influence on the handling of resorbable plates and screws during implantation, for instance threads still have to be tapped as self tapping screws do not yet exist. In case the screws do not exactly pass through the thread, friction force may cause breaking of the screw. This circumstance was considered when adding a predetermined breaking point above the screw head (Figure 4 (Fig. 4)). Because of the same problem there are no plate systems for interfragmental compression, having mentioned that this is not regularly necessary apart from the mandible. Another essential difference to the metallic systems is the fitting of the plate to the individual flexion of the implant bearing. Flexure of the plates is only possible at a temperature above the particular glass transition temperature (Tg) of approx. 60°C. Today the manufacturing companies offer several methods for short term heating of the plates (heating pads and probes), even short heating in heated baths is possible. One must keep in mind that unregulated heating may alter the molecular structure thus effecting stability and degradation properties. This equally applies to sterilization as there are no resterilisable reserve containers.
Figure 4. Resorbable osteofixation system from a copolymer (PLLA/PGA ratio 82/18, Lactosorb®): a: Different shapes of the plates, right below: orbital floor plate; b: Predetermined breaking point (arrow) of the screws.
Just like the degradation properties the mechanical properties also depend on the configuration of the polymer, crystallinity, molecular weight and hence on the degradation time. The manufacturing process (injection moulding) and sterilization process may also lead to an alteration of stability [61]. At the time of implantation the plates and screws are equally firm as titanium plates. A loss of solidness due to hydrolysis varies enormously determined by its composition. A copolymer miniplate and miniscrew system for facial fractures (PLLA/PGA ratio of 82/18, Lactosorb®) described in 1996 by Eppley et al. [51] showed 70% of the initial stability after 2 months time [123]. Animal tests [162] and clinical trials [8], [52], [67], [76], [92], [151] also show sufficient stability. Implant fractures are rare occasions [66], [86].
2.3.4 Clinical use
The shaping effects of the available systems are similar to those of metallic systems (Figure 4 (Fig. 4)).
In 1999 Wiltfang [162] investigated the problem of passive intraosseous translocation compared to titanium in the growing minpig skull. Passive translocation is defined as an intracranial displacement of the plates due to the growth of the skull. The study also showed translocation of resorbable plates however this never exceeded the tabula interna. In clinical routine the resorbable systems have proved themselves for for cases of osteosynthesis in adolescence [8], [49], [62], [76], [142].
Larger plates also qualify for reconstruction of the floor of the orbit in cases of blowout fractures [5]. One should also regard that resorbable systems have successfully been used for refixation of fractures and after chondrotomy/osteotomy of the thyroid cartilage [15].
2.3.5 Disadvantages of resorbable osteosynthesis systems
Polymers are considered to be primarily biocompatible, as their degradation products are entirely metabolized, yet their resorption resembles a foreign body response with accumulation of macrophages and granulocytes. Cases of fistulas, osteolysis and also soft tissue swelling have been described [19], [146], [159].
The necessity to tap a thread and to exactly fit the screw into it requires time and may give rise to screw breakage and an increased waste of material. Newer technologies are aimed at producing self-tapping (e.g. TACKER®-System, Inion Ltd. Tampere, Finland). Another time-saving alternative may be the “Ultrasonic Bone Welding”-technique in which resorbable pins are applied in place of screws. Subsequently the polymers are – to make it simple – liquified through ultrasound energy, then compressed into the Haversian Canals where they can resolidify. This results in an intense and reinforced implant-bonecompound. Clinical trial results are available meanwhile [47].
2.4 Alternative materials
The above mentioned disadvantages of metallic and resorbable osteosynthesis material justify the need to search for alternative materials, as the currently known systems are not entirely ideal.
Silicon Nitride
The mechanical strength of silicon nitride (Si3N4) is approx. twice as large as the strength of aluminium-oxide ceramics (Al2O3) used for hip implants, therefore Si3N4 qualifies as implant-biomaterial for indications with high mechanical strain. The biocompatibility of Si3N4 has been proven in many studies [6], [41], [75], [88], [114], [115]. So far, experiences with ossicular prostheses made from Al2O3 have shown that ceramic materials have good biocompatibility and mechanical strength even when gaining contact to respiratory mucosa [79], [125]. A ceramic osteosynthesis prototype [117] in the minipig model showed satisfactory intraoperative handling, reliable stabilisation of the fracture gap and good healing attributes for osteosynthesis of the anterior wall of the frontal sinus. Both the finite element analysis and also histological preparation and practical experience showed that especially the ceramic screws proved to be mechanically reliable and bioinert (Figure 3 (Fig. 3)).
The essential disadvantage of ceramic plates is their lack of ductility which in turn does not allow any modelling of the plates to the shape of the bone. Combinations of titanium plates or resorbable plates and Si3N4-screws might be worth taking into consideration [23]. The infection problem of screws protruding into the sinuses does not apply to the inert Si3N4-screws. This could make a removal of implant material superfluous. The technically complex production of ceramic screws remains as the main problem making a clinical application in the near future rather unlikely. Apart from osteosynthesis purposes the material may qualify for other indications in craniofacial bone substitution.
3 Bone substitutes
3.1 General considerations
The bone metabolism is a complex mechanism which is not fully understood to date. The bones ability for regeneration is dependant on the size of the substantial defect. Above a “critical size defect” regeneration by means of autologous bone material does not occur. These cases (i.e. congenital, traumatic and tumorous defects) require an application of bone substitutes. Other indications may concern either functional, e.g. reinforced bearing for dental implants (Sinus lift, Alveolar ridge augmentation) or aesthetical matters, e.g. facial augmentation (chin, zygomatic arch, nose).
The biological interaction of bone and bone substitution material is characterised as follows:
Substitution: Complete resorption and replacement of the implant by autologous bone material. In cases of to fast resorption an (unwanted) fibrous interlayer may occur.
Osteoconduction: This describes the materials property to direct the growth of bone tissue due to its geometric configuration. For bony regeneration it is necessary to have a scaffolding along which the osteoblasts can migrate into the defect. Evidence suggests that interconnecting pores of 150 to 450 µm are ideal for that. For example calcium phosphate and bioglass have favorable osteoconductive properties.
Osteoinduction: Describes the differentiation of mesenchymal precursor cells into osteoprogenitor cells and subsequently endochondral ossification.
Osteostimulation: Activation of differentiated bone cells and stimulation of bone metabolism.
An ideal bone substitute material should be biocompatible, osteoinductive and osteoconductive, resorbable, malleable, mechanically stabile, synthetically fabricable, long-time storable, resterilisable and inexpensive. Unfortunately none of the materials available to date meet these requirements. This explains the large amount of materials commercially available (apart from autologous bone material). Throughout the world bone substitute material follows blood and blood-based products as the second most frequently used tissue substitute. The demand for alloplastic material is generally justified by the disadvantages of autologous bone material: Necessity to source bone graft with donorsite morbidity and scarring, prolongation of operation time, highly complex operative procedures, limited individual shape modulation, limited availability in cases of large defects. This is in opposition to the problems involved with alloplastic material: expenses, storage, biocompatibility/rejection. The field of ORL poses especially high requirements to alloplastic materials due to the proximity of bone and mucous membranes. These general problems of bone substitution are not new, as Boenninghaus mentioned in 1960 (Authors translation):
“It is indubitable that the usage of bone material is to be seen as the physiological technique and therefore once again has been recommended lately. On the other hand alloplastic material offers a series of advantages which should not be underestimated” [18].
Using the example of bone substitution in the frontal sinuses it becomes evident, that the materials success is not only dependant on its properties but also even more on the question if sufficient air circulation is ensured and if the mucosa is fully resected in cases of obliteration. Otherwise relapse or rejection may occur independent from the material used. Boenninghaus used Supramid plates (synthetic, non-resorbable polyamide) for the “primary frontal sinus plasty” (Figure 5 (Fig. 5)) and concludes (Authors translation):
Figure 5. Historical plate of “Supramid” for reconstruction of anterior frontal sinus wall. Origin: Boenninghaus 1960 [18].
“As the plates could not be embedded into the tissue and also were not entirely surrounded by soft or bony tissue, foreign body infections occurred in the operated frontal sinuses in which plate surfaces were exposed. The secretion from the operated sinuses required removal of the plates after a few months” ([18], p. 39).
In the past, mastoid obliteration has shown rather disappointing results which are mainly attributed to the difficult implant location rather than the implant material.
Another principal question when selecting the appropriate material is always the consideration of mechanical stress to which the implant is exposed to at the specific implant location. For most materials the following rule applies: Mechanical resilience decreases with an increase of osteoconductivity (see above) and resorbability, e.g. tri-calcium phosphate ceramics: high osteoconductivity, low mechanical resilience and vice versa; aluminium oxide ceramics: no osteoconductivity, high mechanical resilience.
The porosity and pore size are also important parameters fort the possibility of tissue ingrowth into the substitute material and to what extent the material is osseointegrated or transformed into vital bone tissue. Normal cortical bone has a pore size of 1 to 100 µm, cancellous bone has a pore size of 200 to 400 µm. Size, interspace and connection of pores (interconnecting or blind pores) determine nutrient diffusion as well as cell migration and adhesion. A pore size of 100 to 500 µm is regarded as the ideal precondition for the ingrowth of surrounding bone tissue into the implanted material [89]. Pores smaller than 100 µm lead to fibrovascular encapsulation of the implant.
An extensive overview of the commercially available bone substitute materials, trade names, introduction to the market, administration form, durability etc. can be found in the internet at http://www.dentalkompakt-online.de/62_produktliste.html (see also Table 1, presentation Maier http://www.egms.de/en/journals/cto/2011-8/cto000059.shtml).
3.2 Bone
3.2.1 Autologous bone
Autologous bone has been used as bone substitute since the 19th century [102] and is considered to be the biomaterial per se. Different donor sites (calvaria, rib, iliac ala, tibia, scapula, sternum) were already described in the early 20th century (“spongiosaplasty”). Vascularised bone transplants usually derive from the tibia or iliac ala. The rate of donorsite morbidity, which is always mentioned as an argument for the usage of alloplastic material along with the time exposure, varies considerably in the literature: In an overview of more than 12,000 cranioplasties with autogenic calvarian bone the complication rate was 0.08% [85]. Other authors quote complication rates of donor sites as high as 8.6% [166]. Monocortical bone retrieval (calvarian split) is expedient in order to avoid donor-site morbidity, especially when using a coronal incision [72], [113], for example in the reconstruction of the anterior or posterior frontal sinus wall. Many studies have proven the efficacy of suchlike autologous bone transplants for the reconstruction of frontal sinus walls [95], [136], [145], [158]. Autologous bone grafts also qualify for the reconstruction of the nasal skeleton, orbit floor and anterior maxillary sinus wall, in these cases the graft can be obtained via a small retrioauricular parietotemporal incision.
The great advantage of autologous bone grafts is the complete biological integration due to their natural biocompatibility. This makes them especially suitable for cases in which alloplastic bone-plasties have been unsuccessful, in cases of infectious complication or in the situation of close contact of bone to respiratory mucosa (nasal sinuses).
The essential disadvantage of autogenic bone grafts is the limited possibility of transplant forming and modelling, especially when used not only for functional but for apparent aesthetic bone defects (e.g. large frontal defects of the margin of the orbit). Also, the data concerning resorption of autologous grafts varies considerably. Due to possible resorption autogenic grafts are not recommended for facial augmentation [32], [64], [110].
3.2.2 Allograft bone
Allograft bone is harvested from human donors. The entire organic components are industrially removed resp. denaturated, inactivated and sterilized: This happens mechanically in ultra-sound baths, through denaturating ethanol lavage, antimicrobial lavage and/or gamma radiation with the result of obtaining exclusively anorganic bone matrix for implantation. These implants have a normal bone structure. Some authors add some autologous bone marrow in order to support the transformation into vital bone substitute [143]. Still, concerns remain regarding the safety, healing rates and long-term stability of such implants [50], [69]. To date this implant form is mainly used in orthopedic surgery.
3.2.3 Xenogenic bone
The application of xenogenic bone grafts has already been taken into consideration by Adolf Bardeleben from Greifswald in 1859 (Lehrbuch der Chirurgie und Operationslehre, Zweiter Band, Georg Reimer Verlag 1859, Seite 316–317). Today immunogenity and product safety regarding the transmission of infectious diseases are the main concerns about the application of xenograft bone. Xenograft bone ist usually of bovine origin. The deproteinization process is similar to that of allogenic bone. Mineral xenograft has been used in dental surgery for many years (Bio-Oss®, Geistlich Biomaterials, Baden-Baden, Germany).
3.2.4 Demineralised bone
Dematerialized bone is also harvested from human donors. The demineralization is usually performed through acidification resulting in a matrix containing type-I-collagen and osteoinductive growth factors, particularly BMP (Bone Morphogenetic Proteine) [132]. Lyophilization makes the material storable. The porous material can be easily formed and remodelled intraoperatively. Due to its composition the material is both osteoinductive and osteoconductive. It serves as a matrix for the ingrowth of bone material but is not load resistant until fully ossified (within 4–10 months). Both manufacturers and many publications attest high safety regarding immunogenity and the transmission of infectious diseases (esp. HIV) [107], [69].
3.3 Alloplastic material
3.3.1 Metals (Titanium)
Titanium implants for the repair of calvarian, orbitofrontal and orbitozygomatic defects are available as meshes or prefabricated. The question about removal of the material does not arise, as larger defects must be bridged permanently and a removal would also demand a larger operative approach [93].
Meshes are available in two thicknesses: 0.1 mm thin meshes (e.g. M-TAM: Laser-perforated micro titanium augmentation mesh, Stryker Leibinger) can be formed and shaped individually and cut with scissors. They are fixated with titanium screws and are convenient for primary fractures with bone substance loss. Micro-Meshes have also proven appropriate for the reparation of the anterior frontal sinus wall showing successful repneumatization [93].
Thicker meshes (e.g. Micro Dynamic Mesh 0.3 and 0.6 mm Stryker Leibinger) are suitable for the treatment of contour irregularities. The area of bone loss in complex 3-dimensional reconstruction of acute traumatic defects of non load-bearing areas should not exceed 25 cm2. Meshes are also not recommended after radiation therapy [93]. For further details see chapter 3.3.1.1, presentation Maier http://www.egms.de/en/journals/cto/2011-8/cto000059.shtml.
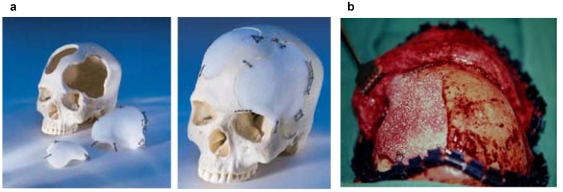
Individual titanium implants [33], [54], [133] are suitable for secondary reconstruction. Such prefabricated titanium implants are milled from a solid titanium block per Computer Aided Manufacturing (CAM) (see chapter 3.3.1.2, presentation Maier http://www.egms.de/en/journals/cto/2011-8/cto000059.shtml) after acquisition of CT-data and Computer Aided Design (CAD). The accuracy is 0.25 mm to the defect edge with a titanium plate thickness of 1.5 mm. The required equipment is expensive and not widely available.
Thermosensitivity and limited possibility of intraoperative shaping and remodelling (in contrast to glass ceramics, see below) are mentioned as the major disadvantages of metals as bone substitutes.
3.3.2 Ceramics
3.3.2.1 Bioactive glass-ceramics
The first synthetically manufactured bioactive glass-ceramic (1971) Nova Bone®, Bioglass® is composed of 45% silicon dioxide, 45% sodium oxide, 5% calcium oxide (CaO) and 5% phosphorous oxide (P2O5). The physical and biological properties of glass-ceramics can be varied by alteration of the fraction of oxides and the CaO/P2O5 ratio. The composition makes it highly reactive in liquid media: Through scission of silicon-oxagen bonds silicic acid is formed which then shows gelatine condensation at the surface and holds the glass particles together. In this gelatine layer calcium phosphate crystallises and forms an apatite layer. The latter reacts with collagen, mucopolysaccharides and glycoproteins which results in fixation of the material to the surrounding bony tissue and at the same time only showing minimal fibrous encapsulation [71]. Because of the proven osteostimulation at the surface of glass-ceramics these may be regarded as bioactive. In comparison to hydroxyapatite (see below) the ceramics show a larger amount of newly synthesized bone substance which also is more similar to natural bone substance [28], [119]. Despite the intensive bond between bone and implant, substitution of the material does not occur.
Even though the elastic modulus of glassceramics (approx. 30 kN/mm2) is near to the modulus of human bone (see above) the amorphous glass meshwork is fragile and therefore unsuitable for load-bearing indications. The main field of application remains in Dental Surgery (augmentation of alveolar ridge and periodontal defects, sinus lift) and also in the reconstruction of the calvarium [65] and the floor of the orbit [2]. Bioactive glass-ceramics (as well as hydroxyapatite) have also been used for obliteration of the frontal sinus [2], [120] (see chapter 3.3.2.1, presentation Maier http://www.egms.de/en/journals/cto/2011-8/cto000059.shtml).
Duskova et al. [46] reported an extrusion rate of 20% over a time period of 2 years for glass ceramic implants used in facial recontouring. Despite the authors’ positive resumee this complication rate seems too high. Reviewers criticize that clinical long-term results are especially poor in cases of especially voluminous bone substitution, consecutively showing poor vascularization [106], [163].
Just as titanium implants, the glass-ceramic Bioverit® established in ear-surgery by Beleites et al. [10] is available as an individual CAD/CAM implant for craniofacial reconstruction showing good clinical results [11], [137]. The implants allow intraoperative remodelling and adjustment with a bur and, in opposite to titanium implants, do not show thermosensitivity.
3.3.2.2 Calcium phosphate
This comprises: Hydroxyapatite, tricalcium phosphate, biphasic calcium phosphate (a compound of the two pre-mentioned) und unsintered calcium phosphate. Calcium phosphates do not cause inflammatory reaction or foreign body response and also are nontoxic. Basically, all Calcium phosphates are osteoconductive. Solubility and cell-mediated degradation underlie multifactorial influences, e.g. they are dependant on the different components and the manufacturing details (amongst others sintering) [96].
Hydroxyapatit (HA)
The name “apatite” is based on Greek ápatan, “to deceive”, as it is easily confused with minerals of similar appearance. Hydroxyapatite (Ca10(PO4)6(OH)2) can be found as a natural, inorganic component of teeth and bones and therefore is biocompatible. The hexagonal crystal system consists of calcium phosphates and can be manufactured synthetically through sintering. Sintering is a method for making objects from powder, by heating the material in a sintering furnace below its melting point. The source material can also be allogenic or xenogenic cancellous bone or even be phykogenic (corals).
HA is already in clinical use for approx. 30 years. Since 1993 it is available as porous granules [26], since 1992 as cement (non-ceramic form) [35]. The hydroxyapatite cement (HAC; BoneSource®, Stryker Leibinger) consists of a solid (tetra calcium phosphate and dicalcium phosphate) and a liquid component (water), which show isothermal setting within 20–30 mins after mixing. The curing may be accelerated by a sodium phosphate buffer. During the curing process the cement must not gain contact to any fluids (blood) [124]. Critics claim that a dry operating field is practically non-existent in craniofacial surgery [93]. Other cement types (Norian SRS®, Synthes) consist of modified components (mono calcium phosphate) and therefore show different curing times, physical and resorption characteristics etc. [59], [168], [134] (also see chapter 3.3.2.2, presentation Maier http://www.egms.de/en/journals/cto/2011-8/cto000059.shtml).
HA is osteoactive, meaning it can be transformed into vital bone mass by means of osteoinduction or osteoconduction. The non-linear resorption and the ingrowth of bone mass could last approx. 18 months. The question if the material is replaced by bone substance depends on form (ceramic vs. non-ceramic), porosity and volume.
In animal tests Gosain et al. [64] described no significant ingrowth of bone substance into HAC-filled calvarian defects after a period of one year. The possibility of intraoperative forming and remodelling is still considered as the essential advantage of the cement [63], however apparently it only shows little to no transformation into vital bone substance. Porous HA-granulae, which were mixed with blood and the implanted into 200 patients also showed no resorption after a period of 8 years [26] so that it is now regarded to be reliable for augmentations. Overall the resorbability of HA is considerably poor.
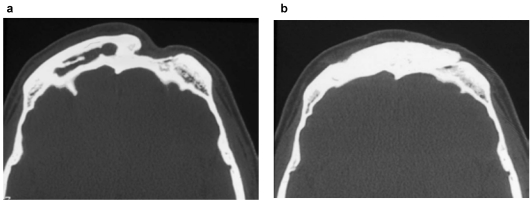
Even though HA shows high compressive strength (60MPa) it has low flexural and torsional strength. Therefore it is especially suitable for bone replacement with low to no mechanical stress (cranioplasty, skull base defects, aesthetic augmentations). Successful implantations have also been described for “unsterile” areas (sinuses, mastoid, Figure 6 (Fig. 6)) [57], [116], [130] including sealing of frontobasal CSF-filstula [37]. Contact between the hardened cement and the cerebrospinal fluid appears to be unproblematic [94].
Figure 6. Frontal sinus obliteration with hydroxyapatite cement following multiple mucocele operations:
a: Pre- and b: Postoperative (6 months) condition in CT scan
Long term results of frontal sinus obliteration using HAC are unsatisfactory, especially because of late infections [106], [110], [153]. Our own initial enthusiasm using hydroxyapatite for frontal sinus obliteration [116] was also dampened due to late infections in 1 of 4 cases making us have to remove the material. Zins et al. also advise against replacing “full-thickness cranial defects” by HAC due to high complication rates [169].
Beta-Tricalcium phosphate (β-TCP)
β-Tricalcium phosphate (Ca3(PO4)2) also is a natural component of bone. It has interconnecting pores of different sizes. Unlike ceramic hydroxyapatite, β-TCP shows resorption within 0.5–2 years [81]. As a consequence of the resorption occurring faster than the production of new bone, fibrous interlayers may result which reduce the mechanical stability. The combination of β-TCP and osteoinductive agents aims to reduce this disproportion of resorption and replacement [22]. Furthermore, modern synthetic manufacturing procedures allow a combination of interconnecting micro- (0.5–10 µm) und macropores (50–700 µm) which aims to improve the dynamics of substitution. The variety of β-TCP morphology concerning porosity, particle size, phase purity and microstructure leads to many different biological interactions [122]. To date β-TCP are mainly applied in dental surgery.
3.3.2.3 Aluminum oxide
Aluminum oxide (Al2O3) is the prototype of bioinert materials (survey see [44]). It is practically poreless and has neither osteoconductive nor osteoproductive properties. As such it is excellently appropriate to serve as ossicular replacement in reconstructive middle ear surgery [79], [125]. Otherwise its applications in craniofacial reconstruction are limited. Jahnke reported applications in the reconstruction oft the anterior skull base in cere bro-spinal fluid leakage repair [80].
3.3.2.4 Calcium sulfate
Calcium sulfate (plaster of Paris) is the oldest ceramic bone substitute (Dreesmann 1892 [45]). It sets via an exothermic reaction, has a low compressive strength (24 MPa) and is resorbed within only 2 to 6 months). There are only few reports on clinical applications as bone substitute in craniofacial reconstruction [34], [40]. A porous bone substitute composed of 51.5% nano-crystalline hydroxyapatite and 48.5% calcium sulfate (PerossalTM) has been approved especially for drug release functions (e.g. antibiotics in osteomyelitis) in other medical subspecialties [48].
3.3.3 Plastics
3.3.3.1 Acrylate
HTR
HTR (hard tissue replacement) sintered polymers consist of poly-methyl methacrylate (pMMA), poly-hydroxyethyl methacrylate (pHEMA) and calcium hydroxide, whereas only the latter two gain contact to the outer surface of the biomaterial. The operational biomaterial is characterised by its porosity (pore size 250–500 µm, material porosity: 30%), hydrophilic surface, negative surface tension (up to –15 mV) and high compressive strength (approx. 70 MPa). The porosity allows the ingrowth of blood vessels and connective tissue and therefore facilitates a firm fixation to the surrounding tissue. Prior to implantation the porous material may be imbued with fluids (e.g. antibiotics) which are then released in situ. The biological effect of the negative surface tension is currently unknown, possibly it prevents bacterial adhesion and supports tissue ingrowth [32], [53]. Different from autogenic bone material (for which resorption is described) methacrylates remain at a constant volume and are not absorbed [103].
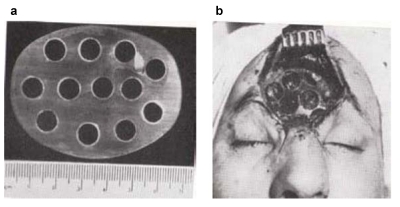
HTR are available as blocks, granula or preformed implants. They may be used for primary replacement of bone resection defects if the dimension of the resection is known in advance, e.g. cases of large bone tumours [53]. For this purpose synthetic resin models are made preoperatively based on CT-scan data (takes up to 3 days) on which the surgeon demarcates the planned resection outlines. The manufacturer – once again after a few days – then designs a CAD and CAM aided “hard tissue replacement – patient matched implant” (HTR-PMI, Figure 7 (Fig. 7)). Nowadays the entire planning procedure can be carried out online. In case the intraoperative bone resection does differ from the preoperative template, the HTR-PMI can be either burred or else modified or amended with small autologous transplants. The implant is fixated with titanium plates or resorbable plates and screws. Clinical use is mainly for large bone defects where extensive amounts of autologous bone would have to be harvested. Disadvantages surely are the costs (from 3,000 € per implant) and the delivery time of the implant (approx. 18 days). Worldwide many thousands of such implants have been used so far. Early reported complication rates of acrylates of up to 12% (1970s) [27], [161] have not been confirmed for HTR-PMI in long term studies [53]. The infection rate is increased in patients with pre-existing infections of the implant site [32] so that a history of infection is regarded as a contraindication.
Figure 7. Hard Tissue Replacement HTR: a: Selection of implants according to individual recquirements; b: Operational site following HTR implantation for calvarian repair.
3.3.3.2 Porous polyethylene
Porous polyethylene (PPE) or high density polyethylene (HDPE, Medpor®, Porex Surgical) is a linear highly compressed (sintered) aliphatic hydrocarbon. Pore-size and pore-volume are similar to those of HTR so that PPE also allows tissue ingrowth. PPE is inert and biocompatible (see page chapter 3.3.2.4, presentation Maier http://www.egms.de/en/journals/cto/2011-8/cto000059.shtml), it is preferably used for facial augmentation [13]. After heating up it is deformable so that individual remodelling and reshaping by bending and cutting are possible.
HDPE has been successfully used for various indications in bone replacement surgery: Post-traumatic or tumour resection defects of the calvarium, orbit, mandible and also aesthetic augmentation (e.g. chin). The rate of infections making a removal necessary was 3% of a total of 162 patients [165]. Other authors reported infection rates of up to 6% [30]. For a most successful implantation subperiostal positioning of the material and subsequent fixation with osteosynthesis screws is generally recommended to prevent relative movement. The ingrowth of blood vessels allows direct onlay of skin transplants onto the implant [160].
3.4 Composites
The aim of composite materials is a synthesis of the often opposing osteoconductive and mechanical properties (see above). Autogenic bone is the classic of all composites as it consists of an organic scaffolding (mainly collagen), anorganic matter (hydroxyapatite) and also growth factors and cytokines.
There are manifold possibilities of combining materials, some examples being:
Combinations of materials:
Titanium mesh with hydroxyapatite cement as calvarian substitute. Dura pulsations that could hinder the curing process of the hydroxyapatite cement are retained by the underlying titanium mesh [36], [59], [105].
Resorbable mesh with hydroxyapatite cement as calvarian substitute [25].
Titanium mesh with autogenic bone for mandibular reconstruction [98].
Composite materials:
A mixture of hydroxyapatite and beta-tricalcium phosphate (60:40) interconnected by a silicon dioxide matrix (Bonit®, DOT GmbH Rostock) combines the positive properties of calcium phosphate and bioglass. Due to the high proportion of nano-crystalline calcium phosphate particles the material is more easily resorbable than sintered β-TCP. It forms an osteoconductive scaffolding because of the interconnecting pores (porosity 60–70%). It is available for mandibular defects or sinus lift either preformed or as granulae.
Low temperature sintered (200°C) highly porous material consisting of 76% calcium phosphate and 24% silicon oxide (NanoBone®, Artoss GmbH Rostock) showed good osteoconductivity and resorption properties in animal experiments [73]. First clinical applications provide a promising outlook [149].
Further examples for composite materials can be found in chapter 3.3.2.3, presentation Maier http://www.egms.de/en/journals/cto/2011-8/cto000059.shtml.
3.5 Recent developments
Hormonal influence on bone regeneration
The very complex bone metabolism is influenced by a variety of factors [104]: Systemic influence is through vitamin D, parathyroid hormone, calcitonin, local influence through TGF-β especially BMP, IGF, TNF-α, interleukins. BMP (bone morpogenetic proteins) promote the differentiation of undifferentiated mesenchymal cells into pre-osteoblasts and osteoblasts. BMP can be extracted from bones or can be produced my recombinance. To date, recombinant BMP-7 (OP-1®, Stryker) is the only approved compound and has been evaluated in many non-craniofacial applications. Theoretically it can be combined with various biomaterials in order to enhance their osseous integration or substitution [126], [167]. According to the product information OP-1® should only be applied when other therapy methods have already failed, e.g. poor fracture healing/absent callus formation. The number of craniofacial indications is somewhat limited, as the pre-mentioned problems are mostly caused by impaired circulation which rarely occurs in the head-and-neck regions. Another aspect is the improved osseointegration of dental implants and prostheses. Possible carriers for BMP are collagen, demineralised bone, synthetic bioabsorbable polymers, calcium phosphate and surfacecoated metals.
Surface modification
Surface modification of various materials (e.g. photolitography) and selective plasma coating may lead to directed adhesion and cellular ingrowth. Especially the BMP-coating establishes many possibilities for improved and accelerated osseointegration of implants [82]. The surface coating restricts the effects to remain local effects hence preventing ectopic bone formation.
Tissue engineering
Just like in other medical subspecialties tissue engineering will increasingly gain in importance in the field of calvarian surgery. Autologous stromal bone marrow cells have already been successfully applied in a compound with calcium alginate gel for the repair of parietal bone defects in animal experiments (sheep) [135]. “Adipose derived stemcells” also show great osteogenic potential [3]. Commercially available “tissue engineered bone” has repeatedly been announced yet still is not ready for the market so far.
Bifocal distraction osteogenesis
Bifocal distraction osteogenesis describes a technique of shifting a small transport segment (autologous bone), which is much smaller than the actual defect, approx. 1 mm per day, thereby practically “stretching” the callus. Animal experiments showed successful repair of “critical size defects”, however the substitute-bone was thinner than the surrounding calvarium [87].
References
- 1.Adelson RT, DeFatta RJ, Dudic Y. Integrity of craniofacial plating systems after multiple sterilization procedures. J Oral Maxillofac Surg. 2007;65:940–944. doi: 10.1016/j.joms.2005.12.059. Available from: http://dx.doi.org/10.1016/j.joms.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 2.Aitasalo KMJ, Peltola MJ. Bioactive glass hydroxyapatite in fronto-orbital defect reconstruction. Plast Reconstr Surg. 2007;120:1963–1972. doi: 10.1097/01.prs.0000287319.34425.27. Available from: http://dx.doi.org/10.1097/01.prs.0000287319.34425.27. [DOI] [PubMed] [Google Scholar]
- 3.Aksu AE, Rubin JP, Dudas JR, et al. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann Plast Surg. 2008;60:306–322. doi: 10.1097/SAP.0b013e3180621ff0. Available from: http://dx.doi.org/10.1097/SAP.0b013e3180621ff0. [DOI] [PubMed] [Google Scholar]
- 4.Alpert B, Seligson D. Removal of asymptomatic bone plates used for orthognathic surgery and facial fractures. J Oral Maxillofac Surg. 1996;54:618–621. doi: 10.1016/S0278-2391(96)90645-X. Available from: http://dx.doi.org/10.1016/S0278-2391(96)90645-X. [DOI] [PubMed] [Google Scholar]
- 5.Al-Sukhun J, Törnwall J, Lindqvist C, et al. Bioresorbable Poly-L/DL-Lactide (P[L/DL]LA 70/30) plates are reliable for repairing large inferior orbital wall bony defets: A pilot study. J Oral Maxillofac Surg. 2006;64:47–55. doi: 10.1016/j.joms.2005.09.013. Available from: http://dx.doi.org/10.1016/j.joms.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Amaral M, Lopes MA, Silva RF, et al. Densification route and mechanical properties of Si3N4-bioglass biocomposites. Biomaterials. 2002;23:857–862. doi: 10.1016/S0142-9612(01)00194-6. Available from: http://dx.doi.org/10.1016/S0142-9612(01)00194-6. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. Available from: http://dx.doi.org/10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashammakhi N, Renier D, Arnaud E, et al. Successful use of Biosorb osteofixation devices in 165 cranial and maxillofacial cases: A multicenter report. J Craniofac Surg. 2004;15:692–701. doi: 10.1097/00001665-200407000-00031. Available from: http://dx.doi.org/10.1097/00001665-200407000-00031. [DOI] [PubMed] [Google Scholar]
- 9.Bakathir AA, Margasahayam MV, Al-Ismaily MI. Removal of bone plates in patients with maxillofacial trauma: a retrospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:32–37. doi: 10.1016/j.tripleo.2008.01.006. Available from: http://dx.doi.org/10.1016/j.tripleo.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Beleites E, Neupert G, Augsten G, et al. Rasterelektronenmikroskopische Untersuchung des Zellwachstums auf maschinell bearbeitbarer Biovitrokeramik und Glaskohlenstoff in vitro und in vivo. Laryngol Rhinol Otol. 1985;64:217–220. doi: 10.1055/s-2007-1008123. Available from: http://dx.doi.org/10.1055/s-2007-1008123. [DOI] [PubMed] [Google Scholar]
- 11.Beleites E, Schneider G, Fried W, et al. 3-D-Referenzimplantate für den Gesichts- und Hirnschädel. Dtsch Ärztebl. 2001;5:209–213. [Google Scholar]
- 12.Bendix D, Liedtke H. Resorbierbare Polymere: Zusammensetzung, Eigenschaften und Anwendungen. Unfallchir. 1998;265:3–10. [Google Scholar]
- 13.Berghaus A. Implantate für die rekonstruktive Chirurgie der Nase und des Ohres. Laryngo Rhino Otol. 2007;86(Suppl 1):S67–S76. doi: 10.1055/s-2007-966301. Available from: http://dx.doi.org/10.1055/s-2007-966301. [DOI] [PubMed] [Google Scholar]
- 14.Bergsma JE, Braijn WC, Rozema FR, et al. Late degradation tissue response to poly(L-lactide) bone plates and screws. Biomaterials. 1995;16:25–31. doi: 10.1016/0142-9612(95)91092-D. Available from: http://dx.doi.org/10.1016/0142-9612(95)91092-D. [DOI] [PubMed] [Google Scholar]
- 15.Bhanot S, Alex JC, Lowlicht RA, et al. The efficacy of resorbable plates in head and neck reconstruction. Laryngoscope. 2002;112:890–898. doi: 10.1097/00005537-200205000-00021. Available from: http://dx.doi.org/10.1097/00005537-200205000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt V, Chabra P, Dover MS. Removal of miniplates in maxillofacial surgery: a follow-up study. J Oral Maxillofac Surg. 2005;63:756–760. doi: 10.1016/j.joms.2005.02.005. Available from: http://dx.doi.org/10.1016/j.joms.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Black J. In vivo corrosion of a cobalt-base alloy and its biological consequences. In: Hildebrandt HF, Champy M, editors. Biocompatibility of CO-CR-Ni alloys. New York: Plenum Press; 1988. pp. 83–100. [Google Scholar]
- 18.Boenninghaus HG. Die Behandlung der Schädelbasisbrüche. Stuttgart: Georg Thieme Verlag; 1960. [Google Scholar]
- 19.Böstman O, Portio E, Hirvensalo E, et al. Foreign-body reactions to polyglycolide screws. Acta Orthop Scand. 1992;63:173–176. doi: 10.3109/17453679209154817. Available from: http://dx.doi.org/10.3109/17453679209154817. [DOI] [PubMed] [Google Scholar]
- 20.Brady JM, Cutright DE, Miller RA, et al. Resorption rate, route of elimination and ultrustructure of the implant site of polylactid acid in the abdominal wall of the rat. J Biomed Mater Res. 1973;7:155–166. doi: 10.1002/jbm.820070204. Available from: http://dx.doi.org/10.1002/jbm.820070204. [DOI] [PubMed] [Google Scholar]
- 21.Brånemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;50:399–410. doi: 10.1016/S0022-3913(83)80101-2. Available from: http://dx.doi.org/10.1016/S0022-3913(83)80101-2. [DOI] [PubMed] [Google Scholar]
- 22.Breitbart AS, Staffenberg DA, Thome CHM, et al. Tricalcium phosphate and osteogenin: a bioactive onlay bonegraft substitute. Plast Reconstr Surg. 1995;86:699–708. doi: 10.1097/00006534-199509000-00024. Available from: http://dx.doi.org/10.1097/00006534-199509000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Brunner FX. Aktuelle Gesichtspunkte zur Osteosynthese des Mittelgesichts. HNO. 2006;54:918–921. doi: 10.1007/s00106-006-1499-z. [DOI] [PubMed] [Google Scholar]
- 24.Brunner FX. Osteosynthesematerial im Gesichtsschädelbereich - Ja oder nein? HNO. 1995;43:205–208. [PubMed] [Google Scholar]
- 25.Burstein FD, Williams JK, Hudgins R, et al. Hydroxyapatite cement in craniofacial reconstruction: Experiences in 150 patients. Plast Reconstr Surg. 2006;118:484–489. doi: 10.1097/01.prs.0000234811.48147.64. Available from: http://dx.doi.org/10.1097/01.prs.0000234811.48147.64. [DOI] [PubMed] [Google Scholar]
- 26.Byrd HS, Hobar PC, Shewmake K. Augmentation of the craniofacial skeleton with porous hydroxyapatite granules. Plast Reconstr Surg. 1993;91:15–22. doi: 10.1097/00006534-199301000-00003. Available from: http://dx.doi.org/10.1097/00006534-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Cabanela ME, Coventry MB, McCarthy CS, et al. The fate of patients with methylmethacrylate cranioplasty. J Bone Joint Surg Am. 1972;54A:278–281. [PubMed] [Google Scholar]
- 28.Cancian DC, Hochuli-Vieira E, Marcantonio RA, et al. Utilization of autogenous bone, bioactive glasses and calzium phosphate cement in surgical mandibular bone defects in Celbus paella monkeys. Int J Oral Maxillofac Impl. 2004;1:73–79. [PubMed] [Google Scholar]
- 29.Case CP, Langkamer VG, James C, et al. Widespread dissemination of metal debris from implants. J Bone joint Surg Br. 1994;76:701–712. [PubMed] [Google Scholar]
- 30.Cenzi R, Farina A, Zuccarino L, et al. Clinical Outcome of 285 Medpor grafts used for craniofacial reconstruction. J Craniofac Surg. 2005;16:526–530. doi: 10.1097/01.scs.0000168761.46700.dc. Available from: http://dx.doi.org/10.1097/01.scs.0000168761.46700.dc. [DOI] [PubMed] [Google Scholar]
- 31.Champy M, Lodde JP, Muster D, et al. Osteosynthesis using miniaturized screws on plates in facial and cranial surgery. Indications and results in 400 cases. Ann Chir Plast. 1977;22:261–264. [PubMed] [Google Scholar]
- 32.Cho YR, Gosain AK. Biomaterials in craniofacial reconstruction. Clin Plast Surg. 2004;31:377–385. doi: 10.1016/j.cps.2004.03.001. Available from: http://dx.doi.org/10.1016/j.cps.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Clijmans T, Momaerts M, Gelaude F, et al. Skull reconstruction planning transfer to the operation room by thin metallic templates: Clinical results. J Craniomaxillofac Surg. 2008;36:66–74. doi: 10.1016/j.jcms.2007.08.003. Available from: http://dx.doi.org/10.1016/j.jcms.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Coetzee AS. Regeneration of bone in the presence of calcium sulfate. Arch Otolaryngol. 1980;106:405–409. doi: 10.1001/archotol.1980.00790310029007. [DOI] [PubMed] [Google Scholar]
- 35.Costantino PD, Friedmann CD, Jones K, et al. Experimental Hydroxyapatite cement cranioplasty. Plast Reconstr Surg. 1992;90:174–191. [PubMed] [Google Scholar]
- 36.Costantino PD, Hiltzik D, Govindaraj S, et al. Bone healing and bone substitutes. Facial Plast Surg. 2002;18:13–26. doi: 10.1055/s-2002-19823. Available from: http://dx.doi.org/10.1055/s-2002-19823. [DOI] [PubMed] [Google Scholar]
- 37.Costantino PD, Hiltzik DH, Sen C, et al. Sphenoethmoid cerebrospinal fluid leak repair with hydroxyapatite cement. Arch Otolaryngol Head Neck Surg. 2001;127:588–593. doi: 10.1001/archotol.127.5.588. [DOI] [PubMed] [Google Scholar]
- 38.Dafnis E, Sabatini S. Biochemistry and pathophysiology of vanadium. Nephron. 1994;67:133–143. doi: 10.1159/000187913. Available from: http://dx.doi.org/10.1159/000187913. [DOI] [PubMed] [Google Scholar]
- 39.Daniels AU, Chang MKO, Andriano KP. Mechanical properties of biodegradable polymers and composites proposed for internal fixation of bone. J Appl Biomater. 1990;1:57–78. doi: 10.1002/jab.770010109. Available from: http://dx.doi.org/10.1002/jab.770010109. [DOI] [PubMed] [Google Scholar]
- 40.De Leonardis D, Pecora GE. Prospective study on the augmentation of the maxillary sinus with calcium sulfate: histological results. J Periodontol. 2000;71:940–947. doi: 10.1902/jop.2000.71.6.940. Available from: http://dx.doi.org/10.1902/jop.2000.71.6.940. [DOI] [PubMed] [Google Scholar]
- 41.Dion I, Bordenave L, Lefebre F, et al. Physico-chemistry and cytotoxicity of ceramics. Part II Cytotoxicity of ceramics. J Mat Sci: Mat Med. 1994;5:18–24. doi: 10.1007/BF00121148. Available from: http://dx.doi.org/10.1007/BF00121148. [DOI] [PubMed] [Google Scholar]
- 42.Dittert DD, Warnecke G, Willert HG. Aluminum levels and stores in patients with total hip endoprostheses from TiAIV or TiAINb alloys. Arch Orthop Trauma Surg. 1995;114:133–136. doi: 10.1007/BF00443386. Available from: http://dx.doi.org/10.1007/BF00443386. [DOI] [PubMed] [Google Scholar]
- 43.Domingo JL. Vanadium: a review of the reproductive and developmental toxicity. Reprod Toxicol. 1996;10:175–182. doi: 10.1016/0890-6238(96)00019-6. Available from: http://dx.doi.org/10.1016/0890-6238(96)00019-6. [DOI] [PubMed] [Google Scholar]
- 44.Dost P. Biomaterialien in der rekonstruktiven Mittelohrchirugie. Laryngo Rhino Otol. 2000;79(Suppl 2):S53–S72. doi: 10.1055/s-2000-15918. Available from: http://dx.doi.org/10.1055/s-2000-15918. [DOI] [Google Scholar]
- 45.Dreesmann H. Über Knochenplombierung. Beitr Klin Chir. 1892;9:804–810. [Google Scholar]
- 46.Dusková M, Smahel Z, Vohradník M, et al. Bioactive glass-ceramics in facial skeleton contouring. Aesthetic Plast Surg. 2002;26:274–283. doi: 10.1007/s00266-002-1032-z. Available from: http://dx.doi.org/10.1007/s00266-002-1032-z. [DOI] [PubMed] [Google Scholar]
- 47.Eckelt U, Nitsche M, Müller A, et al. Ultrasound aided pin fixation of biodegradable osteosynthetic materials in cranioplasty for infants with craniosynostosis. J Craniomaxillofac Surg. 2007;35:218–221. doi: 10.1016/j.jcms.2007.04.005. Available from: http://dx.doi.org/10.1016/j.jcms.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Englert C, Angele P, Fierlbeck J, et al. Konduktives Knochenersatzmaterial mit variabler Antbiotikafreisetzung. Unfallchirurg. 2007;110:408–413. doi: 10.1007/s00113-007-1229-3. Available from: http://dx.doi.org/10.1007/s00113-007-1229-3. [DOI] [PubMed] [Google Scholar]
- 49.Eppley BL, Morales L, Wood R, et al. Resorbable PLLA-PGA plate and screw fixation in pediatric craniofacial surgery: Clinical experience in 1883 patients. Plast Reconstr Surg. 2004;114:850–856. doi: 10.1097/01.PRS.0000132856.69391.43. Available from: http://dx.doi.org/10.1097/01.PRS.0000132856.69391.43. [DOI] [PubMed] [Google Scholar]
- 50.Eppley BL, Pietrzak WS, Blanton MW. Allograft and alloplastic bone substitutes: A review of science and technology for the craniomaxillofacial surgeon. J Craniofac Surg. 2005;16:981–989. doi: 10.1097/01.scs.0000179662.38172.dd. Available from: http://dx.doi.org/10.1097/01.scs.0000179662.38172.dd. [DOI] [PubMed] [Google Scholar]
- 51.Eppley BL, Prevel CD, Sadove AM, et al. Resorbable bone fixation: its potential role in craniomaxillofacial trauma. J Craniomaxfac Trauma. 1996;2:56–60. [PubMed] [Google Scholar]
- 52.Eppley BL, Reilly M. Degradation characteristics of PLLA-PGA bone fixation devices. J Craniofac Surg. 1997;8:116–120. doi: 10.1097/00001665-199703000-00010. Available from: http://dx.doi.org/10.1097/00001665-199703000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Eppley BL. Craniofacial reconstruction with computer-generated HTR patient-matched implants: Use in primary bony tumor excision. J Craniofac surg. 2002;13:650–657. doi: 10.1097/00001665-200209000-00011. Available from: http://dx.doi.org/10.1097/00001665-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Eufinger H, Wehmöller M. Individual prefabricated titanium implants in reconstructive craniofacial surgery: Clinical and technical aspects of the first 22 cases. Plast Reconstr Surg. 1998;102:300–308. doi: 10.1097/00006534-199808000-00002. Available from: http://dx.doi.org/10.1097/00006534-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Exley C, Burgess E, Day JP, et al. Aluminum toxicokinetics. J Toxicol Environ Health. 1996;48:569–584. doi: 10.1080/009841096161078. Available from: http://dx.doi.org/10.1080/009841096161078. [DOI] [PubMed] [Google Scholar]
- 56.Fraedrich G, Kracht J, Scheld HH, et al. Sarcoma of the lung in a pacemaker pocket - simple coincidence or oncotaxis? Thorac Cardiovasc Surg. 1984;32:67–69. doi: 10.1055/s-2007-1023349. Available from: http://dx.doi.org/10.1055/s-2007-1023349. [DOI] [PubMed] [Google Scholar]
- 57.Friedman CD, Costantino PD, Takagi S, et al. BoneSource hydroxyapatite cement: a novel biomaterial for craniofacial skeletal tissue engineering and reconstruction. J Biomed Mat Res. 1998;43:428–432. doi: 10.1002/(SICI)1097-4636(199824)43:4<428::AID-JBM10>3.0.CO;2-0. Available from: http://dx.doi.org/10.1002/(SICI)1097-4636(199824)43:4<428::AID-JBM10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 58.Friedman KE, Vernon SE. Squamous cell carcinoma developing in conjunction with a mandibular staple bone plate. J Oral Maxillofac Surg. 1983;41:265–266. doi: 10.1016/0278-2391(83)90272-0. Available from: http://dx.doi.org/10.1016/0278-2391(83)90272-0. [DOI] [PubMed] [Google Scholar]
- 59.Genecov DG, Kremer M, Agarwal R, et al. Norian craniofacial repair system: compatibility with resorbable and nonresrbable plating materials. Plast Reconstr Surg. 2007;120:1487–1495. doi: 10.1097/01.prs.0000282034.07517.cc. Available from: http://dx.doi.org/10.1097/01.prs.0000282034.07517.cc. [DOI] [PubMed] [Google Scholar]
- 60.Gerlach KL. Resorbierbare Polymere als Osteosynthesematerialien. Mund Kiefer GesichtsChir. 2000;4(Suppl 1):S91–102. doi: 10.1007/PL00022965. [DOI] [PubMed] [Google Scholar]
- 61.Gogolewski S. Bioresorbable polymers in trauma and bone surgery. Injury. 2000;31(Suppl 4):28–32. doi: 10.1016/s0020-1383(00)80020-0. [DOI] [PubMed] [Google Scholar]
- 62.Goldstein JA, Quereshy FA, Coen AR. Early experience with biodegradable fixation for congenital pediatric craniofacial Surgery. J Craniofac Surg. 1997;8:110–115. doi: 10.1097/00001665-199703000-00009. Available from: http://dx.doi.org/10.1097/00001665-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 63.Gosain AK, Song L, Riordan P, et al. A 1-year study of osteoinduction in hydroxyapatite-derived biomaterials in an adult sheep model: part I. Plast Reconstr Surg. 2002;109:619–630. doi: 10.1097/00006534-200202000-00032. Available from: http://dx.doi.org/10.1097/00006534-200202000-00032. [DOI] [PubMed] [Google Scholar]
- 64.Gosain AK, Song L, Riordan P, et al. A 1-year study of osteoinduction in hydroxyapatite-derived biomaterials in an adult sheep model: part II. Bioengineering implants to optimize bone replacement in reconstruction of cranial defects. Plast Reconstr Surg. 2004;114:1155–1163. doi: 10.1097/01.PRS.0000135852.45465.A9. Available from: http://dx.doi.org/10.1097/01.PRS.0000135852.45465.A9. [DOI] [PubMed] [Google Scholar]
- 65.Gosain AK. Plastic Surgery Educational Foundation DATA Committee. Bioactive glass for bone replacement in craniomaxillofacial reconstruction. Plast Reconstr Surg. 2004;114:590–593. doi: 10.1097/01.PRS.0000128355.95900.DD. Available from: http://dx.doi.org/10.1097/01.PRS.0000128355.95900.DD. [DOI] [PubMed] [Google Scholar]
- 66.Haers PE, Sailer HF. Biodegradable self-reinforced poly-L/DL-lactide plates and screws in bimaxillary orthognathic surgery: short term skeletal stability and material related failures. J Craniomaxillofac Surg. 1998;26:363–372. doi: 10.1016/S1010-5182(98)80069-3. Available from: http://dx.doi.org/10.1016/S1010-5182(98)80069-3. [DOI] [PubMed] [Google Scholar]
- 67.Haers PE, Suuronen R, Lindqvist C, et al. Biodegradable poylactide plates and screws in orthognathic surgery: technical note. J Craniomaxillofac Surg. 1998;26:87–91. doi: 10.1016/S1010-5182(98)80045-0. Available from: http://dx.doi.org/10.1016/S1010-5182(98)80045-0. [DOI] [PubMed] [Google Scholar]
- 68.Hansmann W. Eine neue Methode der Fixierung der Fragmente bei complicierten Fracturen. Verh Dtsch Ges Chir. 1886;15:134. [Google Scholar]
- 69.Hardin CK. Banked bone. Otolaryngol Clin North Am. 1994;27:911–925. [PubMed] [Google Scholar]
- 70.Haug RH. Retention of asymptomatic bone plates used for orthognathic surgery and facial fractures. J Oral Maxillofac Surg. 1996;54:611–617. doi: 10.1016/S0278-2391(96)90644-8. Available from: http://dx.doi.org/10.1016/S0278-2391(96)90644-8. [DOI] [PubMed] [Google Scholar]
- 71.Hench LL, Splinter RJ, Alen WC, et al. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res. 1972;2:117–141. [Google Scholar]
- 72.Hendus J, Draf W, Bockmühl U. Tabula externa zur Reonstruktion des frontoorbitalen Knochengerüstes. Laryngo Rhino Otol. 2005;84:899–904. doi: 10.1055/s-2005-870564. Available from: http://dx.doi.org/10.1055/s-2005-870564. [DOI] [PubMed] [Google Scholar]
- 73.Henkel KO, Gerber T, Dietrich W, et al. Neuartiges Knochenaufbaumaterial auf Kalziumposphatbasis. Mund Kiefer Gesichtschir. 2004;8:277–281. doi: 10.1007/s10006-004-0561-9. Available from: http://dx.doi.org/10.1007/s10006-004-0561-9. [DOI] [PubMed] [Google Scholar]
- 74.Hille GH. Titanium for surgical implants. J Mat. 1966;1,2:373–383. [Google Scholar]
- 75.Howlett CR, McCartney E, Ching W. The effect of silicon nitride ceramic on rabbit skeletal cells and tissues. Clin Orthop. 1989;244:293–304. [PubMed] [Google Scholar]
- 76.Imola MJ, Hamlar DD, Shao W, et al. Resorbable plate fixation in pediatric craniofacial surgery. Arch Facial Plast Surg. 2001;3:79–90. doi: 10.1001/archfaci.3.2.79. Available from: http://dx.doi.org/10.1001/archfaci.3.2.79. [DOI] [PubMed] [Google Scholar]
- 77.Islamoglu K, Coskunfirat OK, Tetik G, Ozgentas HE. Complications and removal rates of miniplates and screws used for maxillofacial fractures. Ann Plast Surg. 2002;48:265–268. doi: 10.1097/00000637-200203000-00006. Available from: http://dx.doi.org/10.1097/00000637-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 78.Jacobs JJ, Skipor AJ, Black J, et al. Release and excretion of metal in patients who have a total hip replacement component made of titanium alloy. J Bone Joint Surg Am. 1991;73:1475–1486. [PubMed] [Google Scholar]
- 79.Jahnke K, Plester D, Heimke G. Al2O3-ceramic, a bioinert material in middle ear surgery. Arch Otorhinolaryngol. 1979;223:373–376. doi: 10.1007/BF01109587. Available from: http://dx.doi.org/10.1007/BF01109587. [DOI] [Google Scholar]
- 80.Jahnke K. Ceramics in reconstructive surgery of the anterior skull base and the facial bones. In: Myers EN, editor. New dimensions in otorhinolaryngology head and neck surgery. Amsterdam: Elsevier Science Publishers B.V.; 1985. pp. 185–186. [Google Scholar]
- 81.Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthoped. 1981;157:259–278. [PubMed] [Google Scholar]
- 82.Jennissen HP. Accelerated and improved osteointegration of implants biocoated with bone morphogenetic protein 2 (BMP-2) Ann N Y Acad Sci. 2002;961:139–142. doi: 10.1111/j.1749-6632.2002.tb03067.x. Available from: http://dx.doi.org/10.1111/j.1749-6632.2002.tb03067.x. [DOI] [PubMed] [Google Scholar]
- 83.Katou F, Andoh N, Motegi K, et al. Immuno-inflammatory responses in the tissue adjacent to titanium miniplates used in the treatment of mandibular fractures. J Craniomaxillofac Surg. 1996;24:155–162. doi: 10.1016/S1010-5182(96)80049-7. Available from: http://dx.doi.org/10.1016/S1010-5182(96)80049-7. [DOI] [PubMed] [Google Scholar]
- 84.Kessler P, Hardt N. Erfahrungen mit dem Micro-Titanmesh bei der Rekonstruktion von Defekten im Kieferhöhlenbereich. Dtsch Z Mund Kiefer GesichtsChir. 1996;20:55–59. [Google Scholar]
- 85.Kline RM, Wolfe SA. Complications associated with the harvesting of cranial bone grafts. Plast Reconstr Surg. 1995;95:5–13. doi: 10.1097/00006534-199501000-00002. Available from: http://dx.doi.org/10.1097/00006534-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 86.Kosaka M, Uemura F, Tomemori S, et al. Scanning electron microscopic observations of "fractured" biodegradable plates and screws. J Craniomaxillofac Surg. 2003;31:10–14. doi: 10.1016/S1010-5182(02)00166-X. Available from: http://dx.doi.org/10.1016/S1010-5182(02)00166-X. [DOI] [PubMed] [Google Scholar]
- 87.Kramer FJ, Sinikovic B, Mueller M, et al. Experimental application of a biomaterial in bifocal transport osteogenesis for craniofacial reconstruction. J Craniomaxillofac Surg. 2008;36:218–226. doi: 10.1016/j.jcms.2007.12.001. Available from: http://dx.doi.org/10.1016/j.jcms.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 88.Kue R, Sohrabi A, Nagle D, et al. Enhanced proliferation and osteocalcin production by human osteoblast-like MG63 cells on silicon nitride ceramic discs. Biomaterials. 1999;20:1195–1201. doi: 10.1016/S0142-9612(99)00007-1. Available from: http://dx.doi.org/10.1016/S0142-9612(99)00007-1. [DOI] [PubMed] [Google Scholar]
- 89.Kuhne JH, Bartl R, Frisch B, et al. Bone formation in coralline hydroxyapatite. Effects of pore size studied in rabbits. Acta Ortop Scand. 1994;65:246–252. doi: 10.3109/17453679408995448. Available from: http://dx.doi.org/10.3109/17453679408995448. [DOI] [PubMed] [Google Scholar]
- 90.Kulkarni RK, Moore EG, Hegyeli AF, et al. Biodegradable poly(lactic acid)polymers. J Biomed Mat Res. 1971;5:169–181. doi: 10.1002/jbm.820050305. Available from: http://dx.doi.org/10.1002/jbm.820050305. [DOI] [PubMed] [Google Scholar]
- 91.Kulkarni RK, Pani KC, Neumann C, et al. Polylactid acid for surgical implants. Arch Surg. 1966;93:839–843. doi: 10.1001/archsurg.1966.01330050143023. [DOI] [PubMed] [Google Scholar]
- 92.Kumar AV, Staffenberg DA, Petronio JA, et al. Bioabsorbable plates and screws in pediatric craniofacial surgery: A review of 22 cases. J Craniofac Surg. 1997;8:97–99. doi: 10.1097/00001665-199703000-00006. Available from: http://dx.doi.org/10.1097/00001665-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 93.Kuttenberger JJ, Hardt N. Long-term results following reconstruction of craniofacial defect with titanium micro-mesh system. J Cranio Maxillofac Surg. 2001;29:75–81. doi: 10.1054/jcms.2001.0197. Available from: http://dx.doi.org/10.1054/jcms.2001.0197. [DOI] [PubMed] [Google Scholar]
- 94.Kveton JF, Friedman CD, Costantino PD. Indications for hydroxyapatite cement reconstruction in lateral skull base surgery. Am J Otol. 1995;16:465–469. [PubMed] [Google Scholar]
- 95.Lee C, Antonshyn OM, Forrst CR. Cranioplasty: indications, technique, and early results of autogenous split skull cranial vault reconstruction. J Cranio Maxillofac Surg. 1995;23:133–142. doi: 10.1016/S1010-5182(05)80001-0. Available from: http://dx.doi.org/10.1016/S1010-5182(05)80001-0. [DOI] [PubMed] [Google Scholar]
- 96.LeGeros RZ. Properties of osteoconductive biomaterials. Calcium phosphates. Clin Ortop. 2002;395:81–98. doi: 10.1097/00003086-200202000-00009. Available from: http://dx.doi.org/10.1097/00003086-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 97.Lewis CG, Belniak RM, Plowman MC, et al. Intraarticular carcinogenesis bioassays of CoCrMo and TiAlV alloys in rats. J Arthroplasty. 1995;10:75–82. doi: 10.1016/S0883-5403(05)80103-2. Available from: http://dx.doi.org/10.1016/S0883-5403(05)80103-2. [DOI] [PubMed] [Google Scholar]
- 98.Louis PJ, Gutta R, Said-Al-Naief N, et al. Reconstruction of the maxilla and mandible with particulate bone graft and titanium mesh for implant placement. J Oral Maxillofac Surg. 2008;66:235–245. doi: 10.1016/j.joms.2007.08.022. Available from: http://dx.doi.org/10.1016/j.joms.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 99.Luhr HG. A micro-system for cranio-maxillofacial skeletal fixation. J Craniomaxillofac Surg. 1988;16:312–314. doi: 10.1016/S1010-5182(88)80069-6. Available from: http://dx.doi.org/10.1016/S1010-5182(88)80069-6. [DOI] [PubMed] [Google Scholar]
- 100.Luhr HG. Indications for use of a microsystem for internal fixation in craniofacial surgery. J Craniofac surg. 1990;1:35–52. doi: 10.1097/00001665-199001000-00009. Available from: http://dx.doi.org/10.1097/00001665-199001000-00009. [DOI] [PubMed] [Google Scholar]
- 101.Luhr HG. Zur stabilen Osteosynthese bei Unterkieferfrakturen. Dtsch Zahnärztl Z. 1968;23:754. [PubMed] [Google Scholar]
- 102.Macewen W. Illustrative cases of cerebral surgery. Lancet. 1885;1:881–883. doi: 10.1016/S0140-6736(02)17697-5. Available from: http://dx.doi.org/10.1016/S0140-6736(02)17697-5. [DOI] [Google Scholar]
- 103.Manson PN, Crawley WA, Hoopes JE. Frontal cranioplasty: risk factors and choice of cranial vault reconstructive material. Plast Reconstr Surg. 1986;77:888–900. doi: 10.1097/00006534-198606000-00003. Available from: http://dx.doi.org/10.1097/00006534-198606000-00003. [DOI] [PubMed] [Google Scholar]
- 104.Marks SC, Popoff SN. Bone cell biology: The regulation of development, structure, and function in the skeleton. Am J Anat. 1988;183:1–44. doi: 10.1002/aja.1001830102. Available from: http://dx.doi.org/10.1002/aja.1001830102. [DOI] [PubMed] [Google Scholar]
- 105.Mathur KK, Tatum SA, Kellman RM. Carbonated apatite and hydroxyapatite in craniofacial Reconstruction. Arch Facial Plast Surg. 2003;5:379–383. doi: 10.1001/archfaci.5.5.379. Available from: http://dx.doi.org/10.1001/archfaci.5.5.379. [DOI] [PubMed] [Google Scholar]
- 106.Matic D, Manson P. Biomechanical analysis of hydroxyapatite cement cranioplasty. J Craniofac Surg. 2003;15:415–422. doi: 10.1097/00001665-200405000-00012. Available from: http://dx.doi.org/10.1097/00001665-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 107.Mellonig JT, Prewett AB, Moyer MP. HIV inactivation in a bone allograft. J Periodontol. 1992;63:979–983. doi: 10.1902/jop.1992.63.12.979. [DOI] [PubMed] [Google Scholar]
- 108.Merritt K, Margevicius RW, Brown SA. Storage and elimination of titanium, aluminum, and vanadium salts, in vivo. J Biomed Mater Res. 1992;26:1503–1515. doi: 10.1002/jbm.820261109. Available from: http://dx.doi.org/10.1002/jbm.820261109. [DOI] [PubMed] [Google Scholar]
- 109.Montague A, Merritt K, Brown S, et al. Effects of Ca and H2O2 added to RPMI on the fretting corrosion of Ti6Al4V. J Biomed Mater Res. 1996;32:519–526. doi: 10.1002/(SICI)1097-4636(199612)32:4<519::AID-JBM4>3.0.CO;2-U. Available from: http://dx.doi.org/10.1002/(SICI)1097-4636(199612)32:4<519::AID-JBM4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 110.Moreira-Gonzales A, Jackson IT, Miiyawaki T, et al. Clinical outcome in cranioplasty: Critical review in long-term follow-up. J Craniofac surg. 2003;14:144–153. doi: 10.1097/00001665-200303000-00003. Available from: http://dx.doi.org/10.1097/00001665-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 111.Murthy AS, Lehman JA., Jr Symptomatic plate removal in maxillofacial trauma: a review of 76 cases. Ann Plast Surg. 2005;55:603–607. doi: 10.1097/01.sap.0000183802.38116.37. Available from: http://dx.doi.org/10.1097/01.sap.0000183802.38116.37. [DOI] [PubMed] [Google Scholar]
- 112.Myshin HL, Wiens JP. Factors affecting soft tissue around dental implants: a review of the literature. J Prosthet Dent. 2005;94:440–444. doi: 10.1016/j.prosdent.2005.08.021. Available from: http://dx.doi.org/10.1016/j.prosdent.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 113.Nestle B, Knebel C, Cornelius CP. Kraniofaziale Techniken in der Traumatologie. Mund Kiefer GesichtsChir. 1998;2(Suppl 2):S63–S65. doi: 10.1007/PL00014483. Available from: http://dx.doi.org/10.1007/PL00014483. [DOI] [PubMed] [Google Scholar]
- 114.Neumann A, Kramps M, Ragoß C, et al. Histological and microradiographic appearances of Silicon Nitride and Aluminum Oxide in a rabbit femur implantation model. Mat Wiss Werkstofftech. 2004;35:569–573. doi: 10.1002/mawe.200400778. Available from: http://dx.doi.org/10.1002/mawe.200400778. [DOI] [Google Scholar]
- 115.Neumann A, Reske T, Held M, et al. Comparative investigation of the biocompatibility of various silicon nitride ceramic qualities in vitro. J Mat Sci Mat Med. 2004;15:1135–1140. doi: 10.1023/B:JMSM.0000046396.14073.92. Available from: http://dx.doi.org/10.1023/B:JMSM.0000046396.14073.92. [DOI] [PubMed] [Google Scholar]
- 116.Neumann A, Schultz-Coulon HJ. Obliteration of the frontal sinus with reconstruction of the facial contour using hydroxyapatite cement. In: Jahnke K, Fischer M, editors. 4th European Congress of Oto-Rhino-Laryngology Head and Neck Surgery. Monduzzi Editore S.p.A.; 2000. [Google Scholar]
- 117.Neumann A, Unkel C, Werry C, et al. Prototype of a silicon nitride ceramic-based miniplate osteofixation system for the midface. Otolaryngol Head Neck Surg. 2006;134:923–930. doi: 10.1016/j.otohns.2006.01.022. Available from: http://dx.doi.org/10.1016/j.otohns.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 118.Neumayer B. Einige grundlegende Gedanken zur Optimierung von Osteosynthese-Miniplatten aus Titan. Dtsch Z Mund Kiefer GesichtsChir. 1991;15:265–270. [PubMed] [Google Scholar]
- 119.Peltola MJ, Aitasalo KMJ, Suonpää JTK, et al. Frontal sinus and skull bone defect obliteration with three synthetic bioactive materials: A comparative study. J Biomed Mater Res B Appl Biomater. 2003;66:364–372. doi: 10.1002/jbm.b.10023. Available from: http://dx.doi.org/10.1002/jbm.b.10023. [DOI] [PubMed] [Google Scholar]
- 120.Peltola MJ, Suonpää J, Aitasalo KMJ, et al. Obliteration of frontal sinus with bioactive glass. Head Neck. 1998;20:315–319. doi: 10.1002/(SICI)1097-0347(199807)20:4<315::AID-HED6>3.0.CO;2-1. Available from: http://dx.doi.org/10.1002/(SICI)1097-0347(199807)20:4<315::AID-HED6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 121.Pennekamp PH, Gessmann J, Diedrich O, et al. Short-term microvascular response of striated muscle to cp-Ti, Ti-6Al-4V, and Ti-6Al-7Nb. J Orthop Res. 2006;24:531–540. doi: 10.1002/jor.20066. Available from: http://dx.doi.org/10.1002/jor.20066. [DOI] [PubMed] [Google Scholar]
- 122.Peters F, Reif D. Functional materials for bone regeneration from beta-tricalcium phosphate. Matwiss Werkstoftech. 2004;35:203–207. doi: 10.1002/mawe.200400735. Available from: http://dx.doi.org/10.1002/mawe.200400735. [DOI] [Google Scholar]
- 123.Pietrzak WS, Sarver DR, Verstynen ML. Bioresorbable polymer science for the practicing surgeon. J Craniofac Surg. 1997;8:87–91. doi: 10.1097/00001665-199703000-00004. Available from: http://dx.doi.org/10.1097/00001665-199703000-00004. [DOI] [PubMed] [Google Scholar]
- 124.Pistner H, Reuther E, Reinhart N, et al. Neuer Hydroxylapatitzement für die kraniofaziale Chirurgie. Mund Kiefer GesichtsChir. 1998;2(Suppl 2):S37–S40. doi: 10.1007/PL00014476. Available from: http://dx.doi.org/10.1007/PL00014476. [DOI] [PubMed] [Google Scholar]
- 125.Plester D, Jahnke K. Ceramic implants in otologic surgery. Am J Otol. 1981;3:104–108. [PubMed] [Google Scholar]
- 126.Por YC, Barceló CR, Salyer KE, et al. Bone generation in the reconstruction of a critical size calvarial defect in an experimental model. J Craniofac Surg. 2008;19:383–392. doi: 10.1097/SCS.0b013e318163e415. Available from: http://dx.doi.org/10.1097/SCS.0b013e318163e415. [DOI] [PubMed] [Google Scholar]
- 127.Rae T. The biological response to titanium and titanium-aluminim-vanadium alloy particles. I - tissue culture studies. II - long term animal studies. Biomaterials. 1986;7:30–40. doi: 10.1016/0142-9612(86)90085-2. Available from: http://dx.doi.org/10.1016/0142-9612(86)90085-2. [DOI] [PubMed] [Google Scholar]
- 128.Rallis G, Mourouzis C, Papakosta V, et al. Reasons for miniplate removal following maxillofacial trauma: a 4-year study. J Craniomaxillofac Surg. 2006;34:435–439. doi: 10.1016/j.jcms.2006.07.001. Available from: http://dx.doi.org/10.1016/j.jcms.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 129.Robinson PA. The historical background of internal fixation of fractures in North America. Bull Hist Med. 1978;52:355–382. [PubMed] [Google Scholar]
- 130.Rosenlicht JL, Tarnow DP. Human histologic evidence of integration of functionally loaded hydroxyapatite-coated implants placed simultaneously with sinus augmentation: a case report 2 1/2 years postplacement. J Oral Implantol. 1999;25:7–10. doi: 10.1563/1548-1336(1999)025<0007:HHEOIO>2.3.CO;2. Available from: http://dx.doi.org/10.1563/1548-1336(1999)025<0007:HHEOIO>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 131.Rubin JP, Yaremchuk MJ. Complications and toxicities of implantable biomaterials used in facial reconstructive and aesthetic surgery: A comprehensive review oft the literature. Plast Reconstr Surg. 1997;100:1336–1353. doi: 10.1097/00006534-199710000-00043. Available from: http://dx.doi.org/10.1097/00006534-199710000-00043. [DOI] [PubMed] [Google Scholar]
- 132.Salyer KE, Gendler E, Menendez JL, et al. Demineralized perforated bone implants in craniofacial surgery. J Craniofac Surg. 1992;3:55–62. doi: 10.1097/00001665-199209000-00002. Available from: http://dx.doi.org/10.1097/00001665-199209000-00002. [DOI] [PubMed] [Google Scholar]
- 133.Schipper J, Ridder GJ, Spetzger U, et al. Individual prefabricated titanium implants and titanium mesh in skull base reconstructive surgery. A report of cases. Eur Arch Otorhinolaryngol. 2004;261:282–290. doi: 10.1007/s00405-003-0686-8. Available from: http://dx.doi.org/10.1007/s00405-003-0686-8. [DOI] [PubMed] [Google Scholar]
- 134.Schmitz JP, Hollinger JO, Milam SB. Reconstruction of bone using calcium phosphate one cements: a critical review. J Oral Maxillofac Surg. 1999;57:1122–1126. doi: 10.1016/S0278-2391(99)90338-5. Available from: http://dx.doi.org/10.1016/S0278-2391(99)90338-5. [DOI] [PubMed] [Google Scholar]
- 135.Shang Q, Wang Z, Liu W, et al. Tissue-engineered bone repair of sheep cranial defects with autologous bone marrow stromal cells. J Craniofac Surg. 2001;12:586–593. doi: 10.1097/00001665-200111000-00017. Available from: http://dx.doi.org/10.1097/00001665-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 136.Shumrick KA, Smith CP. The use of cancellous bone for frontal sinus obliteration and reconstruction of frontal bony defects. Arch Otolaryngol Head Neck Surg. 1994;120:1003–1009. doi: 10.1001/archotol.1994.01880330081015. [DOI] [PubMed] [Google Scholar]
- 137.Siebert H, Schleier P, Beinemann J, et al. Evaluierung individueller, in der CAD/CAM-Technik gerfertigter Bioverit®-Keramik-Implantate zur Wiederherstellung mehrdimensionaler kraniofazialer Defekte am menschlichen Schädel. Mund Kiefer GesichtsChir. 2006;10:185–191. doi: 10.1007/s10006-006-0687-z. Available from: http://dx.doi.org/10.1007/s10006-006-0687-z. [DOI] [PubMed] [Google Scholar]
- 138.Siegert R. Metallimplantate in der Kopf- und Halschirurgie. Eur Arch Oto Rhino Laryngol. 1992;Suppl 1:97–107. [PubMed] [Google Scholar]
- 139.Solar RJ, Pollak SR, Korostoff E. In vitro corrosion testing of titanium surgical implant alloys: an approach to understanding titanium release from implants. J Biomed Mat Res. 1979;13:217–221. doi: 10.1002/jbm.820130206. Available from: http://dx.doi.org/10.1002/jbm.820130206. [DOI] [PubMed] [Google Scholar]
- 140.Spiessl B. Erfahrungen mit dem AO-Besteck bei Kieferbruchbehandlungen. Schweiz Mschr Zahnmed. 1969;79:112–113. [Google Scholar]
- 141.Steinhart H, Schroeder HG. Müssen Osteosyntheseplatten im Gesichtsschädelbereich wieder entfernt werden? Ergebnisse experimenteller und klinischer Untersuchungen. HNO. 1995;43:211–215. [PubMed] [Google Scholar]
- 142.Surpure SJ, Smith KS, Sullivan SM, et al. The use of a resorbable plating system for treatment of craniosynostosis. J Oral Maxillofac Surg. 2001;59:1271–1275. doi: 10.1053/joms.2001.27497. Available from: http://dx.doi.org/10.1053/joms.2001.27497. [DOI] [PubMed] [Google Scholar]
- 143.Taguchi Y, Pereira BP, Kour BP, et al. Autoclaved autograft bone combined with vascularized bone and bone marrow. Clin Orthop. 1995;320:220–230. [PubMed] [Google Scholar]
- 144.Takamura K, Hayashi K, Ishinishi N, et al. Evaluation of carcinogenicity and chronic toxicity associated with orthopedic implants in mice. J Biomed Mater Res. 1994;28:583–589. doi: 10.1002/jbm.820280508. Available from: http://dx.doi.org/10.1002/jbm.820280508. [DOI] [PubMed] [Google Scholar]
- 145.Tessier P, Kawamoto H, Matthews D, et al. Autogenous bone grafts and bone substitutes - tools and techniques: I. A. 20.000-case experience in maxillofacial and craniofacial surgery. Plast Reconstr Surg. 2005;116(Suppl.):6S–24S. doi: 10.1097/01.prs.0000173862.20563.12. Available from: http://dx.doi.org/10.1097/01.prs.0000173862.20563.12. [DOI] [PubMed] [Google Scholar]
- 146.Thiele A, Bilkenroth U, Blocing M, et al. Fremdkörperreaktion nach Implantation eines biokompatiblen Osteosynthesesystems. HNO. 2008;56:545–548. doi: 10.1007/s00106-006-1530-4. [DOI] [PubMed] [Google Scholar]
- 147.Thomas P, Schuh A, Ring J, et al. Gemeinsame Stellungnahme des Arbeitskreises Implantatallergie (AK 20) der Deutschen Gesellschaft für Orthopädie und Orthopädische Chirurgie, der Deutschen Kontaktallergie Gruppe und der Deutschen Gesellschaft für Allergologie und Klinische Immunologie. Hautarzt. 2008;59:220–229. doi: 10.1007/s00105-007-1453-3. Available from: http://dx.doi.org/10.1007/s00105-007-1453-3. [DOI] [PubMed] [Google Scholar]
- 148.Törmälä P. Biodegradable self-reinforced composite materials; manufacturing structure and mechanical properties. Clin Mater. 1992;10:29–24. doi: 10.1016/0267-6605(92)90081-4. Available from: http://dx.doi.org/10.1016/0267-6605(92)90081-4. [DOI] [PubMed] [Google Scholar]
- 149.Traykova T, Böttcher R, Neumann HG, et al. Silica/Calzium phosphate sol-gel derived bone grafting material from animal tests to first clinical experience. Key Engin Mat. 2004;411:679–682. doi: 10.4028/www.scientific.net/KEM.254-256.679. Available from: http://dx.doi.org/10.4028/www.scientific.net/KEM.254-256.679. [DOI] [Google Scholar]
- 150.Trinchi V, Nobis M, Cecchele D. Emission spectrophotometric analysis of titanium, aluminum, and vanadium levels in the blood, urine, and hair of patients with total hip arthroplasties. Ital J Orthop Traumatol. 1992;18:331–339. [PubMed] [Google Scholar]
- 151.Turvey TA, Bell RB, Phillips C, et al. Self-reinforced biodegradable screw fixation compared with titanium screw fixation advancement. J Oral Maxillofac Surg. 2006;64:40–46. doi: 10.1016/j.joms.2005.09.011. Available from: http://dx.doi.org/10.1016/j.joms.2005.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vainionpää S, Kilpikari J, Laiho J, et al. Strength and strength retention in vitro, of absorbable, self-reinforced polyglycolide (PGA) rods for fracture fixation. Biomaterials. 1987;8:46–48. doi: 10.1016/0142-9612(87)90028-7. Available from: http://dx.doi.org/10.1016/0142-9612(87)90028-7. [DOI] [PubMed] [Google Scholar]
- 153.Verret DJ, Dudic Y, Oxford L, et al. Hydroxyapatite cement in craniofacial reconstruction. Otolaryngol head Neck Surg. 2005;133:897–899. doi: 10.1016/j.otohns.2005.09.001. Available from: http://dx.doi.org/10.1016/j.otohns.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 154.Vert M, Chabot F, Leray J, et al. Stereoregular bioresorbable polyesters for orthopaedic surgery. Macromol Chem Suppl. 1981;5:30–41. doi: 10.1002/macp.1981.020051981103. Available from: http://dx.doi.org/10.1002/macp.1981.020051981103. [DOI] [Google Scholar]
- 155.Vert M, Christel P, Chabot F, et al. Bioresorbable plastic material for bone surgery. In: Hastings GW, Ducheyne P, editors. Macromolecular biomaterials. Boca Raton, Florida: CRC Press, Inc.; 1984. pp. 119–142. [Google Scholar]
- 156.Wang JY, Wicklund BH, Gustilo RB, et al. Prosthetic metals impair murine immune response and cytokine release in vivo and in vitro. J Orthop Res. 1997;15:688–699. doi: 10.1002/jor.1100150510. Available from: http://dx.doi.org/10.1002/jor.1100150510. [DOI] [PubMed] [Google Scholar]
- 157.Warnke PH, Springer ING, Wiltfang J, et al. Growth and transplantation of a custom vascularized bone graft in a man. Lancet. 2004;364:766–770. doi: 10.1016/S0140-6736(04)16935-3. Available from: http://dx.doi.org/10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 158.Weber R, Draf W, Kahle G, et al. Obliteration of the frontal sinus: state of the art an reflections on new materials. Rhinology. 1999;37:1–15. [PubMed] [Google Scholar]
- 159.Weiler A, Helling HJ, Kirch U, et al. Tierexperimentelle Langzeituntersuchung über Fremdkörperreaktionen und Osteolysen nach Verwendung von Polyglykolidimplantaten. Unfallchirurg. 1998;265:156–159. [Google Scholar]
- 160.Wellisz T, Kanel G, Anooshian RV. Characteristics of the tissue response to Medpor porous polyethylene implants in the human facial skeleton. J Long Term Eff Med Implants. 1993;3:223–235. [Google Scholar]
- 161.White RJ, Yashon D, Albin MS, et al. Delayed acrylic reconstruction of the skull in craniocerebral trauma. J Trauma. 1970;10:780–786. doi: 10.1097/00005373-197009000-00009. Available from: http://dx.doi.org/10.1097/00005373-197009000-00009. [DOI] [PubMed] [Google Scholar]
- 162.Wiltfang J, Merten HA, Becker HJ, et al. The resorbable miniplate system Lactosorb in a growing cranio-osteoplasty animal model. J Cranio Maxillofac Surg. 1999;27:207–210. doi: 10.1016/S1010-5182(99)80030-4. Available from: http://dx.doi.org/10.1016/S1010-5182(99)80030-4. [DOI] [PubMed] [Google Scholar]
- 163.Wolfe SA. Diskussion zu Aitasalo KMJ, Peltola MJ. Bioactive glass hydroxyapatite in fronto-orbital defect reconstruction. Plast Reconstr Surg. 2007;120:1973–1974. doi: 10.1097/01.prs.0000287325.17515.4d. Available from: http://dx.doi.org/10.1097/01.prs.0000287325.17515.4d. [DOI] [PubMed] [Google Scholar]
- 164.Woodman JL, Black J, Nunamaker DM. Release of cobalt and nickel from a new total finger joint prosthesis made of vitallium. J Biomed Mat Res. 1983;17:655–668. doi: 10.1002/jbm.820170410. Available from: http://dx.doi.org/10.1002/jbm.820170410. [DOI] [PubMed] [Google Scholar]
- 165.Yaremchuk MJ. Facial skeletal reconstruction using porous polyethylene implants. Plast Reconstr Surg. 2003;111:1818–1827. doi: 10.1097/01.PRS.0000056866.80665.7A. Available from: http://dx.doi.org/10.1097/01.PRS.0000056866.80665.7A. [DOI] [PubMed] [Google Scholar]
- 166.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. Available from: http://dx.doi.org/10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 167.Zhou AJ, Peel SA, Clokie CM. An evaluation of hydroxyapatite and biphasic calcium phosphate in combination with Pluronic F127 and BMP on bone repair. J Craniofac Surg. 2007;18:1264–1275. doi: 10.1097/scs.0b013e318158cb1a.. Available from: http://dx.doi.org/10.1097/scs.0b013e318158cb1a. [DOI] [PubMed] [Google Scholar]
- 168.Zins JE, Moreira-Gonzales A, Parikh A, et al. Biomechanical and histologic evaluation of the Norian craniofacial repair system and Norian craniofacial repair system fast set putty in the long-term reconstruction of full-thickness skull defects in a sheep model. Plas Reconstr Surg. 2008;121:271e–282e. doi: 10.1097/PRS.0b013e31816a9fd1. Available from: http://dx.doi.org/10.1097/PRS.0b013e31816a9fd1. [DOI] [PubMed] [Google Scholar]
- 169.Zins JE, Moreira-Gonzalez A, Papay FA. Use of calcium-based bone cements in the repair of large, full-thickness cranial defects: a caution. Plast Reconstr Surg. 2007;120:1332–1342. doi: 10.1097/01.prs.0000279557.29134.cd.. Available from: http://dx.doi.org/10.1097/01.prs.0000279557.29134.cd. [DOI] [PubMed] [Google Scholar]