Abstract
Cannulas and voice prostheses are mechanical aids for patients who had to undergo tracheotomy or laryngectomy for different reasons. For better understanding of the function of those artificial devices, first the indications and particularities of the previous surgical intervention are described in the context of this review. Despite the established procedure of percutaneous dilatation tracheotomy e.g. in intensive care units, the application of epithelised tracheostomas has its own position, especially when airway obstruction is persistent (e.g. caused by traumata, inflammations, or tumors) and a longer artificial ventilation or special care of the patient are required. In order to keep the airways open after tracheotomy, tracheostomy cannulas of different materials with different functions are available. For each patient the most appropriate type of cannula must be found. Voice prostheses are meanwhile the device of choice for rapid and efficient voice rehabilitation after laryngectomy. Individual sizes and materials allow adaptation of the voice prostheses to the individual anatomical situation of the patients. The combined application of voice prostheses with HME (Head and Moisture Exchanger) allows a good vocal as well as pulmonary rehabilitation. Precondition for efficient voice prosthesis is the observation of certain surgical principles during laryngectomy. The duration of the prosthesis mainly depends on material properties and biofilms, mostly consisting of funguses and bacteries. The quality of voice with valve prosthesis is clearly superior to esophagus prosthesis or electro-laryngeal voice. Whenever possible, tracheostoma valves for free-hand speech should be applied. Physicians taking care of patients with speech prostheses after laryngectomy should know exactly what to do in case the device fails or gets lost.
Keywords: tracheostomy cannula, voice prosthesis, tracheotomy, laryngectomy, history, design and material, function and efficiency, fixation, care, complication, duration of stay, microbial settlement, biofilms
Introduction
The main aspects of the present lecture are first the cannulas inserted in the trachea after tracheotomy and second the valve prostheses for voice rehabilitation after laryngectomy. For both applications, first the indications are described as well as those that justify the previously performed surgical intervention before emphasizing on the configurations and the specifications of the tubes.
1 Tracheostomy cannulas
Tracheostomy cannulas are inserted into the trachea through a tracheostoma, they keep it open and facilitate the access for care and ventilation. Before describing the single cannulas, the indications and methods of tracheotomy will be explained.
1.1 History of tracheotomy and tracheostomy cannulas
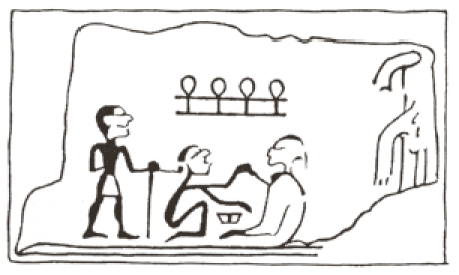
The opening of the trachea belongs to one of the oldest surgical interventions in medicine. Already in times before Christ people thought about the possibility of life-saving methods in cases of upper airway obstruction because those obstructing processes caused by inflammations, tumors, or traumas were already known since the beginning of human being. In 2006, Sosath presented an extended historical description of classic tracheotomy as well as percutaneous dilation procedures [1]. In the medical literature dealing with the historic origin of tracheotomy, two Egyptian tables are mentioned from Abydos and Saqqara. They date from the first dynasty of 2950–2800 before Christ (Figure 1 (Fig. 1)). On the table from Abydos, the sign of “ankh” representing life is shown above the heads between the person that is assumed to be the surgeon and the patient. It is interpreted in that way that life is given from the one to the other person [2]. Another hint to surgeries in the neck area is given on the papyrus Ebers. Its origin is dated to the year 1550 B.C. and it is said to be the best preserved papyrus. In this document, neck surgery is described in general, not particularly the opening of the trachea [3]. The opus of Hippokrates does not mention tracheotomy, however, intubation by means of a small tube is described which has to be inserted into the throat of a choking patient. The earliest hint to tracheotomy is found in the work of Asklepiades (128 to about 60 B.C.) while the original source has been lost. According to Claudius Galenus (about 129–199 B.C.) he recommended opening of the upper tracheal part as ultimate possibility to avoid suffocation [4].
Figure 1. Tablet from Abydos (taken from [2]).
Antyllus (3rd/4th century A.D.) got back to this surgery which is described in detail by Paulus of Aegina [5]. Antyllus recommended tracheotomy in order to reconstitute breathing in cases of diphtheria. It is noticeable that no details are given on how to keep the tracheal wound open. The eldest elaborate description of tracheotomy which was called “laryngotomia” at that time can be found in the manual of Paulus of Aegina [6].
In his “Canon Medicinae”, the most important representative of Arabic medicine called “Avicenna” (Ibn Sina, 980–1037) recommended the application of tracheotomy in cases of acute risk of suffocation [7], Pietro de Abano (1250–1315) favoured tracheotomy in sitting position. If this procedure has been performed by him cannot be seen from the literature [8].
Andreas Vesalius (1514–1564) performed tracheotomy in a pig [9]. The first proven tracheotomy performed in a human patient was penned by the Italian doctor Antonio Benivienius around 1502 [10]. The Italian anatomist and surgeon Hieronymus Fabricius ab Aquapendente (1533–1619) described extensively the indication for tracheotomy with insertion of tracheal cannula [11]. However, he never performed tracheotomy himself. Julius Casserius (1545–1616) who was a student of Fabricius and his successor on the chair of anatomy in Padua, Italy, further developed the technique of tracheotomy and showed the most ancient description of tracheotomy and the instruments that were used [12] (Figure 2 (Fig. 2)). Casserius introduced a modified crooked silver cannula that could be fastened with threads around the throat [13]. This improved type of cannula fell into oblivion and mostly straight cannulas were inserted [14]. Nicolaus Habicot (1550–1624), surgeon and anatomist from Paris, France, achieved a wide-spread notification of tracheotomy due to successfully performed interventions. He described four patients who had undergone tracheotomy; one young man with a foreign body choke and three others who suffered from trauma [15]. Friedrich Dekkers (1648–1720) introduced the instrument of tracheotoma in order to perform puncture tracheotomy [16] (Figure 3 (Fig. 3)).
Figure 2. Oldest description of tracheostomy (“laryngotomia”) in a manual of the Italian surgeon and anatomist Giulio Casseri (Julius Casserius, 1552–1616) (taken from [12]).
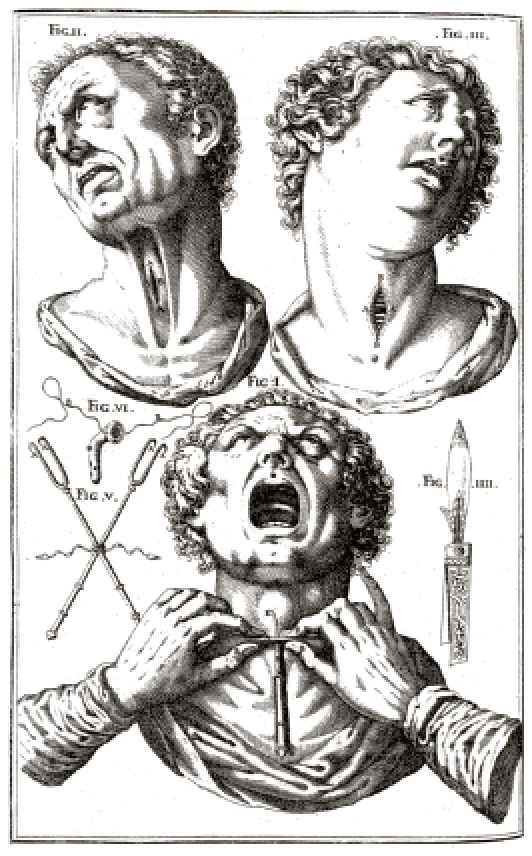
Figure 3. Tracheotome of Dekkers (1648–1720) (taken from [16]).
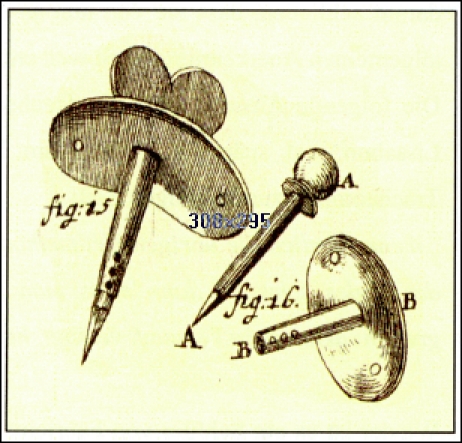
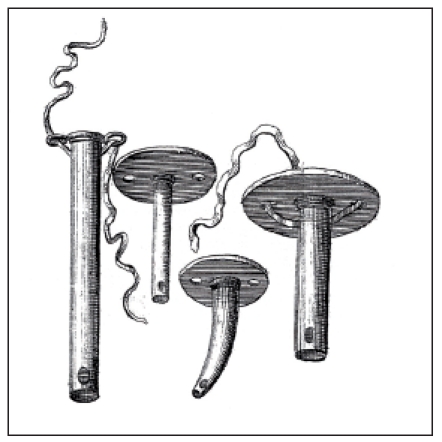
A new indication for tracheotomy was described by the professor Georg Detharding from Rostock (1671–1747). He suggested tracheotomy in drowned people and recommended to insert a small tube into the trachea in order to inflate air [17]. Lorenz Heister (1683–1758) described tracheotomy in a special chapter of his book entitled “Chirurgie” and suggested tracheal cannulas beside the tracheotoma mentioned by Dekkers [18] (Figure 4 (Fig. 4)). Foreign bodies in the trachea were an indication for tracheotomy for him. The Scottish ship’s doctor George Martin (1702–1743) performed tracheotomy in an inflammatory laryngeal disease and reported about the recommendation of one of his assistants to insert a double tube [19].
Figure 4. Tracheostomy cannulas recommended by Lorenz Heister (1683–1758) (taken from [18]).
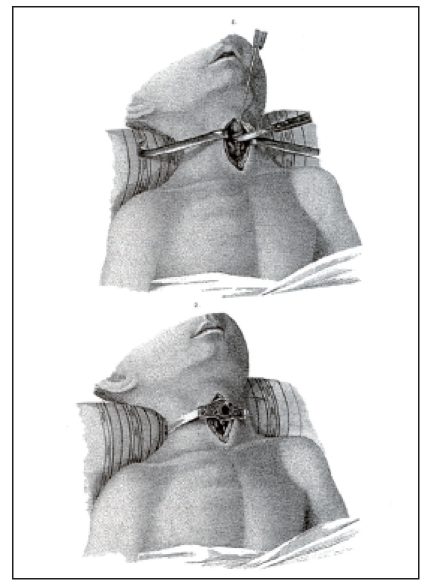
This development of the cannulas was only slowly accepted. In 1782, August Gottlieb Richter (1742–1812), Chair and surgeon in Göttingen, Germany, mentioned in his manual entitled “Anfangsgründe der Wundarzneykunst” (Beginnings of the art of wound healing) that diphtheria was the main indication for tracheotomy [20]. This disease with involvement of larynx and trachea was wide-spread in the 19th century. 30% of all infected people suffered from severe stridor accompanied by croup [21]. In 1912, more than 8000 children died from diphtheria and croup in the kingdom of Prussia [22]. Especially French physicians supported performing tracheotomy for treatment of diphtheric croup. Pierre Fidèle Bretonneau (1778–1862) performed successful tracheotomy in a child [23] which lead other Parisian physicians, in particular Armand Trousseau (1801–1867), to perform tracheotomy as standard therapy for complex diphtheria [24] (Figure 5 (Fig. 5)).
Figure 5. Tracheostomy in a child suffering from diphtheria (taken from [24]).
Around 1900, beside acute-therapeutic indications (injury, foreign body, swellings, compression of larynx and trachea) there were already preventive indications such as tracheotomy before planned laryngeal or pharyngeal interventions in order to avoid bleeding into the airways. Further, tracheotomy was recommended in cases of as
phyxia for artificial ventilation. At that time, tracheotomy was performed via a vertical incision from the lower edge of the thyroid to the jugulum and the thyroid isthmus was displaced in upward direction. The trachea was opened in longitudinal direction from bottom-up and then the tracheostomy cannula was inserted. This procedure inevitably lead to the situation that postoperatively the distance between the outer skin and the trachea was relatively wide and nearly always secondary wound infections occurred in the subcutaneous tissue. This may also be the reason for the objections of intensive care physicians against surgical tracheotomy so that they further focused on puncture or dilation tracheotomy [25]. In particular with the aim of avoiding infections, Björk [26] introduced a procedure of inserting a caudally pedunculated cartilage covering that had to be sewed in the cervical skin. With this method an easy change of the cannula is possible; however, the risk of secondary infections of the subcutaneous tissue is not eliminated. We recommend a so-called epithelised tracheostomy where not only the Björk flap but also the lateral parts and the upper parts of the trachea are joined with the skin so that easy cannula change is possible and the risk of infections is minimized.
1.2 Classification of tracheotomies
1.2.1 Emergency tracheotomy
The term of emergency tracheotomy means coniotomy, i.e. the opening of the larynx between the cricoid and the thyroid cartilages. This may be performed by means of scalpel incision or puncture with the according trocars. Coniotomy should be performed only in absolute emergency situations, immediately afterwards regular tracheotomy must be performed.
1.2.2 Surgical tracheotomy
As ENT specialists, we prefer an approach via a horizontal incision over the 2nd and 3rd tracheal cartilage or between the cricoid cartilage and the jugulum. Preparation is performed to the thyroid isthmus which is transected and the stumps are exactly sutured. After preparation of the anterior wall of the trachea, a horizontal incision is performed between the 2nd and 3rd tracheal cartilage with insertion of the Björk flap described above. In this context, it must be mentioned that surgeons often only limit the procedure
to the Björk flap and do not suture the lateral and upper skin edges to the trachea. However, this should always be performed in order to avoid postoperative infection.
1.2.3 Puncture dilation tracheotomy
Puncture dilation tracheotomies are performed according to the most different techniques (e.g. Ciagla [27], Griggs [28], Fantoni [29], Blue Rhino technique [30], or the Percu Twist method [31]). They are all based on puncturing the trachea under view and the canal for insertion of the tracheostomy cannula is dilated. Details are not described here, apart from the fact that nowadays the classic and the puncture tracheotomy have their indications and limits.
1.2.4 Classification according to the location
The general classification, especially supported by ancient surgeons, is based on the thyroid isthmus. They defined upper tracheotomy as opening of the trachea at the upper edge of the isthmus while lower tracheotomy was an opening at its lower edge. In cases of middle tracheotomy the isthmus was transected and the trachea was opened there. In this context it must be mentioned that the isthmus generally covers the cricoid and the first three tracheal cartilages. It should always be transected and prepared in order to get an optimal overview and to avoid bleedings from the thyroid. A clear classification is based on the tracheal segments. Upper tracheotomy is performed between the 1st and 2nd tracheal cartilage, middle tracheotomy is located between the 2nd and 3rd tracheal cartilage, and the lower one is situated between the 3rd and 4th cartilage. We favour generally middle tracheotomy.
1.3 Indications for tracheotomy from an ENT-specific point of view
Indications for application of a conventionally epithelised tracheostomy from an ENT-specific point of view are described in Table 1 (Tab. 1). Puncture dilation tracheotomy has to be recommended when the upper airways are obstructed by inflammations, traumata, or tumors and the surgeons does not find clear anatomic structures. Surgical interventions in cases of head and neck tumors become more and more extensive so that tracheotomy has to be performed in order to secure breathing. When long-term ventilation is required, epithelised tracheostoma should be applied instead of puncture tracheostoma. Puncture or dilation tracheostoma may lead to granulation and thus to obstruction of the trachea so that the situation has to change to classic tracheostoma [32]. Bilateral recurrence paresis with stridor is an emergency situation requiring rapid action and intervention by an external approach. In cases of injuries of the facial skeleton or the mandible, the upper airways are often obstructed in an unclear way so that classic tracheotomy has to be performed. In summary, a classic epithelised tracheostomy has always to be created when a long-term opening of the airways is necessay.
Table 1. Indications for creation of an epithelised tracheostoma.
1.4 Tracheotomy in children
Based on the anatomic circumstances, tracheotomy in children is a particular challenge. The larynx in children is located higher than in adults. Caudal and latero-caudal to the planned tracheostoma vessels such as the brachiocephalic trunk or the common carotid artery may be injured more easily than in adults during preparation [33], [34]. Nonetheless, tracheotomy is a secure procedure with an acceptable complication rate even in very small and immature children [35], [36], [37]. Among others, the following possible complications were described: wound healing disturbances [38], vascular arrosions [39], skin and mediastinal emphysema, pneumothorax, deshiscence of the mucocutaneous anastomosis, stoma infection, tracheitis, stoma shrinking, granulations, tracheal stenosis, and tracheomalacia [40], [41].
1.5 Types of tracheostomy cannulas
Tracheostomy cannulas allow securing the breathing by keeping open and stabilising the trachostomy by-passing the upper airways and the larynx. Special tracheostomy cannulas are used for preserving and long-term ventilation and the protection of the lower airways. The type of the inserted cannula has to meet the requirements regarding material, design, and function [42], [43].
1.5.1 Classification of tracheostomy cannulas according to the material
Generally, a difference can be made between metal and synthetic cannulas while the choice of the material depends on the requirements, comparable to the design of the cannula.
1.5.1.1 Metal tracheostomy cannulas
Metal tracheostomy cannulas consist of highquality 925 sterling silver, more rarely of steel. Because of the high material strength, silver cannulas have only very thin walls and at the same time a high air passage. Due to the increased inner volume the metal cannula is superior to the synthetic cannulas with regard to airway passage. Silver tracheostomy cannulas are very durable; they may be worked up and repaired. Another advantage is the bactericidal effect of silver as well as the possibility to sterilize it. Their very plane surface leads to rather low adhesion of secretion. The disadvantages are the missing adaptation to the anatomic situation which is due to the material, the discoloration after some time, and the high costs. In cases of probable radiotherapy and during tomographic imaging, silver cannulas must not be used because of possible metal-associated reflexion (Figure 6 (Fig. 6)).
Figure 6. Non-perforated and perforated tracheostomy cannulas made of sterling silver, with voice valve (MPV Company, Berlin, Germany).

1.5.1.2 Synthetic tracheostomy cannulas
In the group of synthetic cannulas several materials may be found such as cannulas made of silicone, mediplast, polyvinylchloride (PVC), and teflon. According to the chemical composition and additional softening agents and stabilisers, synthetic cannulas are offered as unflexible, pre-shaped tracheostomy cannulas (duroplastic) or as flexible cannulas. In comparison to metal tracheostomy cannulas, all plastic cannulas are characterised by a low weight. Cannulas made of duroplastic are inherently stable and have a larger inside diameter but their thermoplastic properties and therefore also their adaptability to anatomic situations is mostly limited.
Tracheostomy cannulas made of flexible plastic are characterised by a lower irritation potential and allow highest comfort. Thermosensitive tracheostomy cannulas are clearly more flexible, adapt to the individual anatomy of the trachea and reduce the risk of pressure marks at the tracheal mucosa. Sometimes even a contrast radiography strip is integrated [43], [44] (Figure 7 (Fig. 7)).
Figure 7. Non-perforated and perforated tracheostomy cannula made of synthetic material (Heimomed Company, Kerpen, Germany).

1.5.2 Classification of tracheostomy cannulas according to their function
With regard to the closing mechanism of the trachea the difference is made between blockable and non-blockable tracheostomy cannulas, so-called voice cannulas.
1.5.2.1 Blockable cannulas
Blockable tracheostomy cannulas are applied in cases of necessary complete closure of the trachea. In most of the cases external ventilation is needed, more rarely in cases of swallowing disorders with aspiration. The basic body of blockable tracheostomy cannulas is a plastic tube that is placed in the trachea and secures breathing. At the distal edge a cuff is found that closes the cannula against the tracheal wall (Figure 8 (Fig. 8)). Through a thin tube the cuff may the connected with an externally located valve and/or pressure compensating balloon. Generally, air is used to fill the cuff. Those cannulas are made of flexible or rigid synthetics while the advantage of flexible cannulas is that they better adapt to the anatomic circumstances.
Figure 8. Blockable spiral tracheostomy cannula (Mallinckrodt Medical Company, Athlone, Ireland).
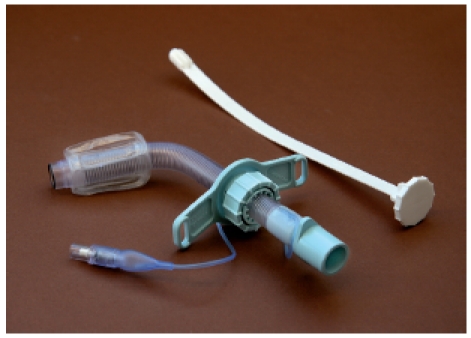
The cuff of a tracheostomy cannula must be easily compressible because every swallowing leads to a movement of the trachea that must be adjusted in order to avoid injury of the mucosa. Modern “low pressure” tracheostomy cannulas allow pressure compensation between cuff and externally located compensation balloon that balances pressure changes. Cuffs are found in globular and cylindric shapes. The globular cuffs touch the trachea only at a small surface while cylindric ones touch it with their whole external surface. Globular cuffs are less often applied because the small contact surface leads to high pressure to the tracheal mucosa and may cause edema or scarring of the mucosa. Using cylindric cuffs the pressure is distributed to a larger surface which reduces the risk of pressure-related undersupply.
For patients with swallowing disorders the cylindric cuff provides a better protection against aspiration. Filling the cylindric cuff, special attention must be paid that the cuff membrane is completely unfolded so that it is close to the tracheal wall. For this purpose, the cuff must be filled with moderate overpressure to be released afterwards. The pressure in the capillaries of the tracheal wall amounts about 25 mm Hg. If the cuff puts more on it, circulation of the tracheal mucosa is no longer secured; the risk of necrosis is increased. The cuff pressure must not be high-er than 35 mm Hg and should be controlled with a manometer.
In many cases, however, a cuff pressure of 25 mm Hg does not allow complete closure of the trachea against outflowing saliva. In such cases an increased cuff pressure is required. Then the tracheal mucosa must be controlled regularly and the position of the cuff must be changed in intervals. Industrially provided cannulas with two cuffs that may be alternatingly blocked and de-blocked try to implement this principle. The cuff of self-blocking cannulas contains foamed material that unfolds on its own. The air must be removed from the cuff of those cannulas before the cannula may be inserted. After positioning the cannula, the closure of the cuff tube may be opened while the cuff is filled with air on its own. To allow pressure compensation, the cuff tube must not be closed. Especially blockable cannulas are provided with an additional canal that allows suction of secretion above the cuff without changing the cannula. In particular for patients with aspiration problems, this suction possibility may be useful but it does not replace respiratory toilet. Sometimes a continuous suction via this canal is propagated; however, such a continuous suction sound is not acceptable for those critically ill patients [42], [44], [45].
1.5.2.2 Non-blockable cannulas
Non-blockable tracheostomy cannulas are provided of metal as well as rigid and soft plastic. Generally, those cannulas consist of an outer and inner tube, a core, which may be removed for cleaning without changing the cannula. In most cases cannulas made of thermoplastic are used. Rigid cannulas should only be applied in exceptional cases because of their missing adjustment to the anatomic situation. For patients requiring artificial ventilation and patients suffering from aspiration non-blockable cannulas are not always appropriate.
1.5.2.3 Tracheostomy cannulas with perforation (voice cannulas)
Tracheostomy cannulas with fenestration of a perforated area at their upper bending allow phonation because the air is lead through the opening into the larynx during exhaling. Those cannulas are called voice cannulas and are appropriate for patients with preserved and sufficient laryngeal volume. In cases of tracheostomy cannulas with closed cannula bodies, the exhaled air must be pressed with a significantly increased effort into the larynx passing the cannular wall in order to achieve phonation.
Tracheostomy cannulas with valve mechanism (voice cannulas)
In order to change the direction of the exhaled air via the fenestration of a voice cannula, the cannula entrance must be closed mechanically during exhalation. Valves with flaps, so-called speech valves that are put on the cannula, open during inspiration and close during exhalation so that the air can only pass via the fenestration in direction of the larynx and thus allow phonation. Indications for this measure were already mentioned (Figure 6 (Fig. 6)).
1.6 Special cannulas
1.6.1 Cannulas with subglottic suction and preserved voice function
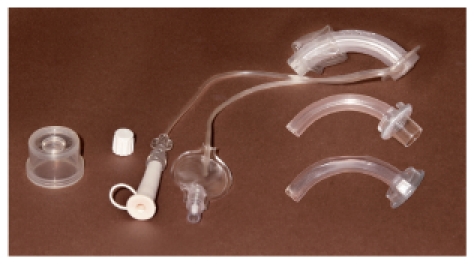
Tracheostomy cannulas are more and more distributed that combine the function of blockable cannula with a voice cannula, i.e. that those cannulas dispose of a blocking cuff as well as a perforation at the convex side of the cannula tube. However, because of the perforation the actual sense of a blockable cannula, the closure of the trachea, is not possible because aspiration through the fenestration is possible. Only insertion of a special device into the perforation area allows the complete closure of the trachea, which however makes phonation difficult. If this device is removed for phonation, the larynx and the trachea should be cleaned of saliva before. Combination cannulas are recommended only in particular cases, mostly in the care units [42], [44] (Figure 9 (Fig. 9)).
Figure 9. Combination cannula with low pressure cuff and suction possibility (Heimomed Company, Kerpen, Germany).
1.6.2 Short-term tracheostomy cannulas
Different tracheostomy placeholder may be applied in patients where a removal of the tracheostomy cannula is generally possible but who have to undergo suction regularly as for example in laryngectomised patients. In many cases the fixation of the often selfretaining placeholder is difficult; frequently they are emitted during a severe cough. More appropriate solutions are fixations via an adhesive base plate or lanyards. Those shortterm cannulas generally do not have an inner cannula system, so that the inside diameter is larger. Often those cannulas also dispose of a possibility to insert HME cassettes.
1.6.3 Tracheostomy cannulas for by-passing tracheal stenosis
Deeply located or long-distance tracheal stenosis may be by-passed by extra-long metal cannulas with flexible profile tongue at the distal edge, so-called lobster-tail cannulas. According to the indication, those custom-made devices need an individual specification regarding the bow, the length of the basic cannula, and the length of the profile tongue.
In cases of subglottic stenosis, the application of Montgomery-Safe-T-tubes is possible [46], [47], [48], [49], [50] (Figure 10 (Fig. 10)). After reconstructive measures, they keep the airways upright and support the trachea in the stenosis regions. The gouges at the extra-corporal part of the tube allow the application of the security ring to avoid accidental posterior dislocation. Further, the tube is fixed by means of this security ring; a cannula lanyard is thus not needed. According to the recommendation of the caring physician, the branches of the tube may be shortened to the desired length. To avoid irritations of the tracheal mucosa, however, no sharp edges must occur. The tube is provided in a transparent or radio-opaque design. At the inner side of the laryngeal branch of the HebelerSafe-T tube made of silicone, a balloon is found regulating the air passage through the upper tube end. During anesthesia or artificial ventilation, the balloon is filled with air for temporary closure of the upper branch. Thus a closed system is created between tracheostoma and lung.
Figure 10. Safe-T-tube made of silicone (Bess Company, Berlin, Germany).
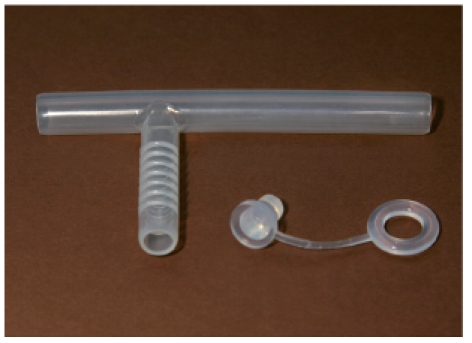
The Moore tracheostomy cannula made of silicone serves as support of a stenotic cervical or thoracic trachea and sustains the opening of the airway. The cannula is stable but flexible and adjusts to the anatomic circumstances. The standard length can be shortened individually.
Regarding Montgomery tubes see Sittel (http://www.egms.de/en/journals/cto/2011-8/cto000056.shtml).
1.6.4 Long-term cannula
Long-term cannulas made of silicone dispose of a 27° angled inner flange that is meant to keep the tube in the horizontal position after positioning in the trachea. Because of the very short inner flange there is only a contact of the cannula to the anterior tracheal wall. Thus, irritation of the mucosa is significantly reduced in comparison to conventional tracheostomy cannulas. The correct length of the cannula is found by measuring the stoma from the anterior tracheal wall to the outer stoma edge. To minimize the risk of dislocation and aspiration, a ring is attached to the extra-corporal branch that must be shortened individually.
1.7 Accessories and equipment
There is a large series of accessories and equipement that support a competent and hygienic care of tracheostomised patients. The list mentioned here describes only the basic equipment.
Lanyards are useful for safe fixation of the tracheostomy cannula. Generally they are elastic and adjustable. Patients with skin irritations may use very smooth and latex-free or even absorbing and breathable lanyards with pads.
Self-adhesive base plates are mostly used for fixation of short-term cannulas, HME cassettes, or shower devices.
Tracheo-compresses are used for cushioning the cannula and to absorb tracheal secretion. They are made of soft and absorbent material and protect the skin against irritations. Partly applied aluminium evaporation at the back side allows an atraumatic compress change.
Cannula cleaning powder is used for preparation of cleaning solutions for rapid and intensive removal of blood and secretion residues.
Cannula cleaning containers are used of the hygienic cleaning of the cannula. A removable filter serves to eliminate secretions. It allows the careful bathing of the tracheostomy cannula in the cleaning solution. After the cleaning bath, the cannula may then be rinsed in the filter with running water.
Cannula disinfection powder/fluid concentrates are used for cleaning and disinfection of the tracheostomy cannulas. They are bactericide, fungicide, sporicidal, and sometimes even virucidal. After disinfection, the cannula should be carefully rinsed with sterile or boiled water.
Cleaning brushes allow a cannula-preserving mechanical cleaning of secretions. For an optimal cleaning and protection of the cannula of damage a fleece head is applied. Cleaning brushes are provided in different sizes.
Stoma oil is neutral oil made of coconut oil glycerides that facilitates the insertion of the tracheostomy cannula during the change of the cannula. In cases of two-piece cannulas it makes joining of the inner and outer cannula easier and avoids adhesion and thus damage of the cannula material.
Cuff manometers are useful for filling, regulating, and supervision of the cuff pressure and reduce the risk of pressure necrosis in the trachea.
A speculum may be used for spreading and dilation of the tracheostoma. In cases of very narrow stoma, the speculum facilitates the insertion of the tracheostomy cannula. In emergency situations it is an essential means to secure the airway.
Larynx protection bibs/larynx protection cloth or also pullovers are used for optic covering and protect against dust, cooling, and desiccation.
HME cassettes are filters replacing the missing protection and filter functions of the nose and protect the trachea from desiccation, cooling, and mechanical soiling. Further, the filter increases the airway resistance and contributes to the preservation of the pulmonary function (see below).
Suction devices are essential for tracheostomised patients in order to avoid possible secretion stasis and dyspnea.
Air humidifiers increase the humidity in the housing space that must not be below 50%.
Vaporizers, especially those that secure warm and damp inhalation, are important for tracheostomised patients who use adapted masks that are placed direction on the tracheostoma.
Regarding HME, suction, and inhalation/vaporization, more detailed information can be read in the following.
1.8 Care of tracheostomized patients
To avoid complications, the cannula should be changed daily as well as tracheostoma cleaning and disinfection. Afterwards oil or a nurturing ointment may be applied. In cases of high-risk patients, a cannula change should be performed by two persons in order to be able to react rapidly and adequately. For a smooth change different measures must be prepared: applicable second cannula, guide bar, suction device, disinfectant and skin care product, slip agent for the cannula (ointment, oil), tracheal compress, lanyard, speculum, and cuff manometer for blocked cannulas.
The slightly oiled tracheostomy cannula is inserted with a turning of 90° into the tracheostoma. Any immoderate pressure must be avoided to reduce pain and injury. In an optimal situation, the insertion of the cannula can be easily performed. In difficult circumstances, an insertion probe is useful that is inserted over the still lying tracheostomy cannula. Frequently the patient develops a slight coughing during insertion of the cannula. In particular patients suffering form perceptual disorder, the insertion of the cannula may cause severe reactions and even vomiting because of the vagal irritation. For prophylaxis, the trachea of those patients should be anesthesized with a spray and/or the cannula should be rubbed with anesthetizing gel.
Blockable cannulas have reached their correct position when the ascending branch that protrudes from the skin level is located in a 90° angle to the posterior tracheal wall. It is
important to care for a sufficient mobility of the tracheostomy cannula. Cannulas that are fixed too rigidly draw the cannula in cranial direction and impair swallowing. To fill the cuff, generally cuff manometers must be used to reduce the risk of tracheal lesions. Blockable cannulas cannot avoid aspiration, but they may reduce the velocity of already aspired material in direction of the lungs [42], [43], [51]. Even this fact confirms the necessity to provide a suction device to maintain the airways.
The separation of the upper and lower airways has permanent significant effects on the respiration of the patients. The “by-passed” functions of the nose (filtering, warming, and moistening of the inhaled air) often lead to a chronic-inflammatory irritation of trachea and bronchia. Tracheostomised patients often complain about cough, excessive secretion production, and crusting, especially during the first six months after surgery. The discontinuation of the airway between the nose and the subglottis and thus the lower respiratory resistance cause negative effects for the pulmonary physiology. Pulmonary function tests in laryngectomised patients show significantly lower results compared to reference values. Further there is a negative correlation between airway problems and psycho-social aspects such as tiredness, sleeping problems, quality of voice, feeling of anxiety, and depressions [52], [53], [54], [55]. Pulmonary function tests after laryngectomy indicate bacterial infections that displace in downward regions and increasing airway obstruction [56]. Investigations have revealed that even “only” tracheostomized patients suffer more frequently from infections of the lower airways [57], [58]. In order to substitute the nasal function frequently stoma filters are applied as head and moisture exchangers (HME). Their function is to retain the exhaled water vapor and its reinsertion for inhalation. Water vapor condenses at the HME surface and warms the filter up. The inhaled air is tempered and moistened while the loss of fluid and the temperature gradient between external air and tracheal air is reduced. Another advantage is the filtering of small dust particles. The respiratory resistance increased by the stoma filter has a favourable impact on the pulmonary physiology and the exhalation volume. The regular application of stoma filters is also verifiably beneficial for the physical and psychosocial consequences of tracheostomized and laryngectomized patients [59], [60]. Regular inhalations – ideally warm and damp inhalations – additionally reduce the desiccation of the tracheal mucosa. Beside those evident advantages of attachable filters, they are on the other hand an additional risk factor because expectorated secretion may obstruct them so that breathing is no longer possible. So a regular change of the filter must be imperatively observed. A regular moistening of the air by use of according humidifiers or inhalations further reduces the desiccation of the tracheal mucosa.
Tracheostomy cannulas made of plastic or silver are cleaned by means of a special brush under current water, if needed with soap or a mild rinsing agent. Disinfection is not necessary if the cannula is used by the same patients afterwards. Of course tracheostomy cannulas in intensive care units have to be changed under sterile conditions. They are replaced by new ones at any change.
The most frequent complications at the tracheostoma are infections, mainly caused by saliva or tracheo-bronchial secretion running over the skin. For prophylaxis regular tracheostomy cleaning should be performed. Also the oral space should be cleaned regularly from secretions, if needed. Tracheostoma compresses must be changed regularly; metal compresses should not be used in cases of running saliva. The skin around a reddened trachestoma may be treated with zinc ointment. In patients undergoing radiation therapy, dexpanthenol ointment is recommended. Additionally a small ointment tamponade may be wrapped around the tracheostomy cannula. Granulations at the tracheostoma often occur because of mechanic irritations caused by rigid and inflexible material, by the sieve of the voice cannula, or by a too narrow stoma. After remedy of the irritation those granulations should be removed in order to interrupt the circle of mechanic irritations. In cases of very narrow tracheostoma or a tracheostoma with severe tendency for collapse the surgical revision with creation of an epithelised stoma is indicated [42], [61].
1.9 Perspectives
Although a variety of cannulas made of most different materials is nowadays provided, the development must be promoted. One main problem is that the respiratory physiology is changed in that way that the air does not enter through the nose tempered, moistened, and cleaned but directly into the trachea which leads to crusts in the trachea or even in the cannula. Such a situation requires frequent cannula change. A focus of research should be placed on the further development of the cannula surfaces for example to create a lotus effect by nanostructure allowing dripping of the secretions. The design of the cannula must correspond to the physiological conditions. The partly still existing cannulas shaped as a quadrant do not correspond to the anatomic circumstances (steep front of the trachea behind the tracheostoma). Besides, the applied material plays in important role: preferably material should be used that adjusts to the anatomic situation of the trachea at body heat, that does not chafe, and that is nonetheless sufficiently stable. In high-risk patients or patients with an infected tracheostoma the possibility of surface coating with drugs may be important in the future.
2 Voice prostheses and their application
As the application and the function of voice prostheses are directly associated with the loss of the larynx by laryngectomy, first the intervention will be described together with the possibilities of rehabilitation of a voice that may be used for communication. Afterwards the current voice prostheses will be discussed.
2.1 History
In 1873, Christan Albert Theodor Billroth performed the first laryngectomy in Vienna, Austria. The patient was a 36 year-old RE teacher who suffered from laryngeal cancer. He first underwent laryngeal fissure with removal of parts of the tumor, which did not lead to the expected success. Four months after laryngectomy, tumor recurrence was diagnosed; three months later the patient died of his tumor disease [62].
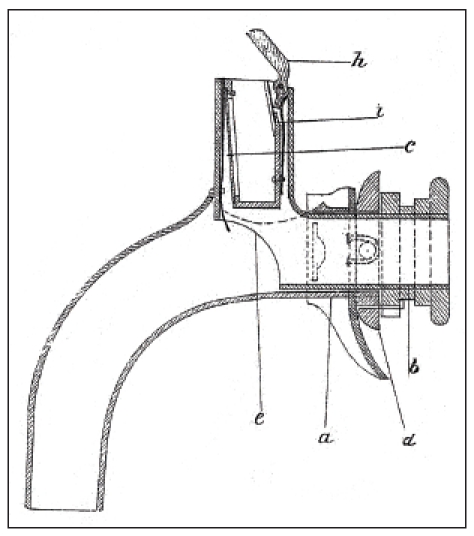
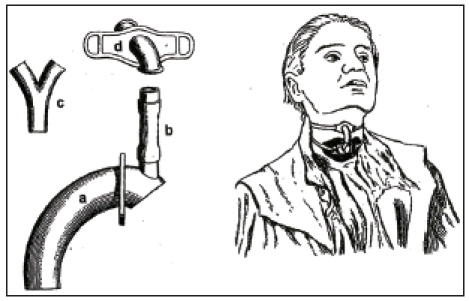
Czerny had performed animal experimental work showing successful laryngectomy in dogs. Together with the instrument maker Leiter, he constructed a voice device that was inserted in four dogs after removal of the larynx. Those artificial larynxes allowed “harmonic barking” creating the voice via metal voice platelet made of a reed pipe [63]. At the occasion of the annual meeting of the German Society of Surgery in 1874, Gussenbauer reported about the first successful extirpation of a larynx by Billroth and at the same time he presented an artificial larynx [62] (Figure 11 (Fig. 11)). This artificial larynx consisted of a tracheal, a pharyngeal, and a phonation cannula and an artificial epiglottis. Phonation occurred via a vibratory metal platelet in the phonation cannula. The insertion of the artificial larynx was difficult. The resulting voice had a strange sound. In this context it must be mentioned that the pioneers at that time tried to preserve the epiglottis when removing the larynx so that the inserted pharyngeal cannula ended directly below the epiglottis. So it was not really voice rehabilitation by means of an artificial larynx because during the meals it had to be removed, further the voice stopped because of saliva and secretion obstruction in the area of the phonation tube. The development of such artificial larynxes with pharyngeal and phonation occurred at a time when total laryngeal extirpation was not associated with complete separation of the respiratory tract and the digestive tract. This last mentioned method was introduced later by Gluck and Sörensen [64].
Figure 11. First artificial larynx according to Gussenbauer (taken from [62]), longitudinal section, with tracheostomy can¬nula (a), pharyngeal cannula (b), and the phonation can¬nula in upward direction (c). The turnable ring (d) serves for fixation of the pharyngeal to the tracheal cannula. Through the opening (e), a connection to the trachea is created. The epiglottis (h) is kept open by means of a spring (i).
In the years after the first removal of a larynx, voice rehabilitation consisted mostly in the concept of artificial larynx that was meant to replace the removed larynx as tone generator. Further efforts for voice rehabilitation after laryngectomy were characterized by two principles. With a gain in knowledge of the voice generation, researchers tried to find another body’s own tone generator to replace the removed larynx. Another development was based on the operative creation of a tracheo-esophageal fistula in order to lead the air into the esophagus for phonation during exhalation with closed tracheostoma so that the whole pulmonary volume was used. Also this method turned out to have its limitations so that technical devices were developed, socalled voice prostheses that are placed into a fistula. The last mentioned method became prevalent and will be the focus of this paper. In 2007, Reutter published a detailed history of voice rehabilitation after total laryngectomy [65]. In 2005, Koscielny gave an extensive summary of reconstructive procedures after laryngectomy [66].
2.1.1 Development of artificial larynges
Gussenbauer himself improved his artificial larynx [62] that was modified and further developed by Foulis in 1878 [67] and Victor von Bruns in 1879 [62], [68].
In 1892, Hochenegg described a phonation apparatus where air was lead through a tube via the nose into the posterior parts of the oral cavity in order to speak. In the tube a speech whistle was located. This phonation apparatus worked by means of bellows [69] (Figure 12 (Fig. 12)). Gottstein created a speech gadget in 1900 by leading the exhaled air into the oral cavity. The tone was created by a metal tongue whistle. By means of this speech gadget a laryngectomized railroad worker was able to speak with loud and monotone voice [70]. In a similar way, Sudeck constructed an artificial larynx in 1910 [71] (Figure 13 (Fig. 13)). Themistokles Gluck created a phonation gadget in 1910 consisting of a wooden box where a battery-powered electromotor ran the bellows. Thus a tone could be made in a whistle that was lead transnasally into the oral and pharyngeal space for articulation [72].
Figure 12. Speech apparatus with bellow according to Hochenegg (taken from [70]), 1892.
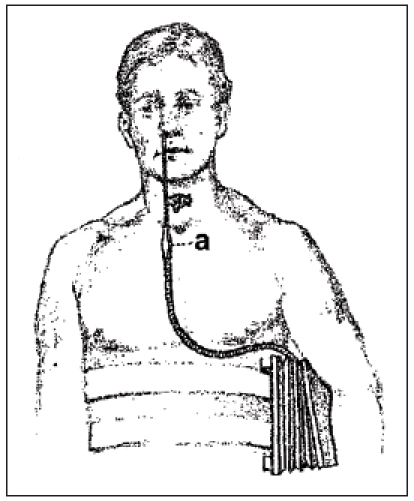
Figure 13. Artificial larynx according to Sudeck (taken from [72]), 1910.
In 1936, Iglauer reported about an artificial larynx with a whistle construction for creation of an elementary tone, also in this context with the basic principle of connection between the trachea and the hypopharynx. It was remarkable that the tone pitch could be regulated individually by means of a setscrew [73]. Those selected examples must be sufficient to show the principle of artificial larynges with integrated tone creation by vibratory material.
2.1.2 Electro-mechanical speaking aids
Having achieved a better knowledge of the physiology of phonation the next step was to replace the tone generator inserted in the artificial larynx by an external module which caused the air in the oral and pharyngeal space to vibrate so that the sound could be issued by the articulation organs. In 1942, Gilbert M Wright from Los Angeles developed an electro-larynx. It was an electronically powered vibrator with battery of accumulator supply. Approaching the device to the soft parts of the neck, the air of the oral cavity and the pharynx was caused to vibrate. This so-called electro-larynx, also called sonovox, was used in a Walt Disney film for voices of toys, e.g. a train [74]. Besides, also oral speaking aids could be found. Lowry [72] created a generator where the impulses caused by a tone generator were lead into the oral cavity via a tube (Figure 14a (Fig. 14)). In 1959, Ticchioni constructed a speech whistle. Hereby the external tone generator was hidden in a tobacco pipe dummy [75]. Today, electro-mechanical speaking aids are applied transcervically (Figure 14b (Fig. 14)).
Figure 14. (a) oral speaking aid (taken from [76]) in 1957. (b) modern speaking aid “Servox digital” produced by Servona (Troisdorf, Germany).
2.1.3 Esophagus or ructus voice
The method of esophagus or ructus voice was introduced for the first time by Gutzmann in 1908 [76], [77]. In 1919, Seemann [78], [79] coined the term of esophagus voice. Meanwhile it was clear that it was possible to substitute the removed tone generator of the larynx by the esophagus entrance that is muscle controlled. The principle of voice rehabilitation consists of the procedure that the air is either injected into the esophagus or swallowed and then pointedly exhaled. Hereby the opening of the esophageal mouth causes vibration and similar to the situation in the larynx a primary sound is caused. By means of the articulation organs in the vocal tract tones are formed using the central nervous system.
2.1.4 Surgical tracheo-eophageal shunts
About 30% of the patients are not able to learn the esophagus voice.
So it is not surprising that efforts were made to create a surgical connection between the trachea and the esophagus in order to use the above-mentioned voice generation mechanism applying the whole pulmonary volume. Those reflexions lead to the surgical construction of a tracheo-esophageal shunt that on the one hand allows the air to enter with low resistance during exhalation and articulation with closed tracheostoma, that on the other hand had to be tight for food intake, especially drinking, so that aspiration into the trachea and thus into the lung was avoided.
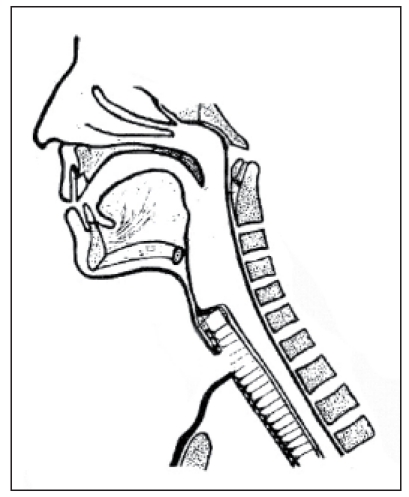
Already in 1927, Joseph C. Beck [74] from Chicago reported about a laryngectomized patient who refused the artificial larynx that was commonly applied at that time and created a fistula in the hypopharynx above the tracheostoma by means of a heated ice pick. So he could phonate when closing the tracheostoma during exhalation. In 1932, M.R. Guttman [80] reported about the experiment of creating a tracheo-esophageal fistula for voice rehabilitation after laryngectomy by means of needle electrodes. The success of this method was only short-term because frequently spontaneous closure occurred. In 1958, Conly [81] described the design of a shunt between the trachea and the esophagus. Its stability should have been achieved by inserting esophageal mucosa or venous wall (jugular vein) as tube insertion. This method was not generally accepted. In 1960, Asai [82] created a shunt by using a tube-shaped voice fistula from inverted neck skin. In 1970, Mozolewski described a mucoplastic vocal fistula [83]. In 1973, Staffieri [84] (Figure 15 (Fig. 15)) reported about a shunt surgery where a slotted neoglottis was created over the preserved stump of the cricoid cartilage.
Figure 15. Voice fistula according to Staffieri (taken from [85]).
Those surgical techniques often lead to aspiration of saliva or food and to stenosing or obstruction of the voice fistula.
With introduction of the microvascular transplantation technique, more extended defects after tumor resection in the head and neck may be covered. This technique allows a permanent connection between the hypopharynx and the trachea with autologous tissue. In 1984, Ehrenberger [85] (Figure 16 (Fig. 16)) described a jejunum speech siphon. Hereby, a speech siphon was formed by a microvascular pediculated jejunum transplantation that was fixed at the loop of the digastric muscle. In this context it must be stated critically that the surgical intervention in the abdominal cavity represents and additional stress for the patient with possible postoperative complications. In 1990, Hagen [86] (Figure 17 (Fig. 17)) described a type of laryngoplasty where a vascular pediculated skin transplantation is taken from the forearm of the patient. This skin transplantation is then connected to the cervical vessels and formed to a tube while the skin epithelium is put inwards. In cranial direction, the tube is closed by a lid of concha cartilage, similar to the epiglottis. Also in this context the high efforts must be mentioned. In 1994, Maier and Weidauer [87] (Figure 18 (Fig. 18)) reported about a tube-shaped voice fistula which was created from pharyngeal mucosa and a fascia flap.
Figure 16. Jejunum voice siphon according to Ehrenberger, modified according to Remmert, 1984 (taken from [89]).
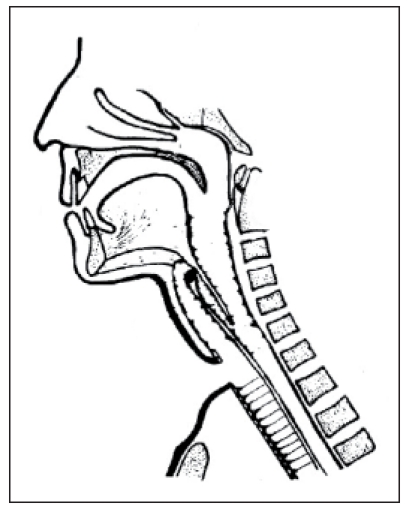
Figure 17. Laryngoplasty according to Hagen, 1990 (taken from [89]).
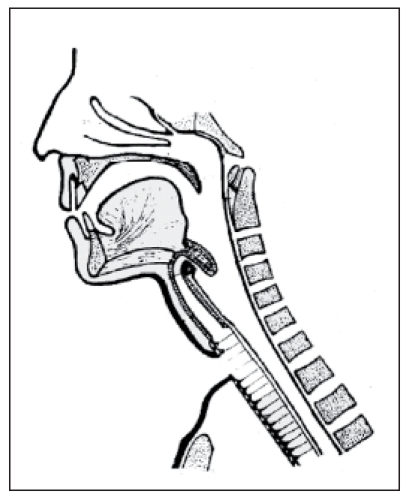
Figure 18. Voice fistula according to Maier and Weidauer, 1994 (taken from [88]).
In summary, the described methods of surgical construction of a voice fistula did not find general application. The reasons for this may be the additional surgical effort and a possible impairment of the radical nature of the tumor intervention by the creation of the fistula. Important problems occur after application of microvascular pediculated transplantations such as a jejunum loop or a forearm flap in cases of unsuccessful vascular anastomosis.
So it is comprehensible that intensive research is seen to find other solutions for voice rehabilitation after laryngectomy.
2.1.5 Voice prostheses
The efforts preliminarily ended up with the development of the so-called voice prostheses, representing tracheo-esophagel shunt valves. The principle of those speaking aids is that after tracheo-esophageal puncture a plastic valve is inserted in the created shunt. The construction of those valves consists of a cylindric tube with an open part in the trachea and a valve on the esophageal side. The term of “voice prosthesis” is not exact because the valve does not represent a tone generator but only allows the passage of the exhaled air into the hypopharynx or the esophagus with closed tracheostoma and at the end of exhalation it is packed. The same speaking mechanism occurs as in the case of esophageal voice. The only difference is that the whole volume of the lung can be used and thus the voice is more powerful and fluent. Furthermore the high pulmonary volume allows a longer vocalization. Although those voice prostheses gained their importance only since the beginning of the 1980ies, there had already been efforts in this context to substitute the voice after laryngectomy.
In 1900, Taptas produced an artificial voice apparatus that uses the nowadays applied principle the commonly used shunt valves. The air is lead into a pharyngeal fistula via a tracheostomy cannula and a connection tube. Thus an external tracheo-hypopharyngeal shunt was created. Phonation was possible by closing the tracheostoma with a finger during exhalation [88], [89] (Figure 19 (Fig. 19)).
Figure 19. Taptas speaking device, 1900 (taken from [90]).
In 1974, Edwards created a fistula way above the tracheal opening in posterior downward direction. The inserted tracheostomy cannula was connected to the fistula tube. For phonation, the opening of the tracheostomy cannula had to be closed manually. It must be mentioned that the air injection system already existed [90].
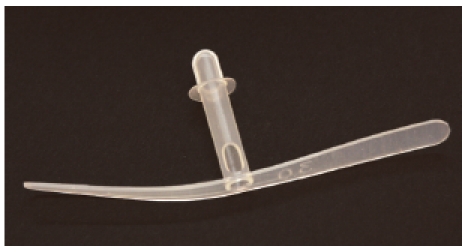
In 1972, Erwin Mozolewski from Stettin [91] (Figure 20 (Fig. 20)) was the first to present a shunt valve that was manufactured from polyvinyl chloride. The shunt valve had an outside diameter of 5.2 mm and an inside diameter of 4 mm. It was made in arcuate or straight shape. The author of this contribution could himself see the manufacturing of those voice prostheses in Stettin with most simple technical devices and their proper functioning in the 1970ies. Mozolewski’s idea was not wide-spread because it was published in a Polish journal and the technological possibilities in Poland at that time were limited so that the industrial production was not promoted. The voice prosthesis developed by Blom-Singer (USA) was first found on the market in 1979 [92] (Figure 21 (Fig. 21)).
Figure 20. Voice prosthesis according to Mozolewski, 1972 (taken from [92]).
Figure 21. Voice prosthesis according to Blom-Singer, 1979, model “duckbill”.
In 1980, a valvular mechanism was created to replace the slit valve which consisted of a thin silicone plate fixed at a silicone ring. This valve flap worked according the principle of a door hinge. This prosthesis of the second generation was called “low pressure voice prosthesis”. In 1993, Blom developed a new method of anterograde replacement technique of the voice prosthesis by means of a gelatine capsule. Before insertion, this capsule is placed over the voice prosthesis and inserted with a special metal case into the esophageal fistula. In the esophagus lumen the gelatine decomposes rapidly and the inner flange unfolds (Table 2 (Tab. 2)). Until 1995, all Blom-Singer prostheses were mere replacement prostheses. Only afterwards, the first long-term prostheses were provided. The voice prostheses that are currently sold are described in chapter 1.3.
Table 2. Taptas speaking device, 1900 (taken from [90]).
In 1982, Nijdam and co-workers from Groningen published a report about their voice prostheses. This voice prosthesis consisted of a cylinder with two flanges while also here the slit valve was placed into the esophagus [93] (Figure 22 (Fig. 22)). At the beginning of the 1990ies, the Groningen voice prostheses were further developed to low pressure prostheses [94]. In 1981, the American Panje published his voice button shunt prosthesis. It consisted of a cylinder with two flanges and a cross-shaped slit four-wing clap served valve [95].
Figure 22. Nijdam voice prosthesis, 1982.
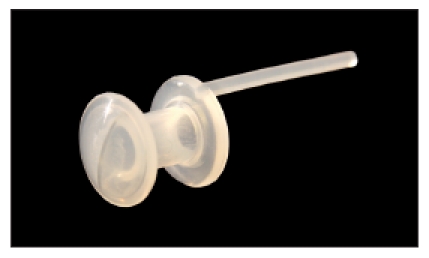
In 1986, Herrmann published a shunt valve that he had developed [96] (Figure 23 (Fig. 23)). It mainly consisted of a cylindric tube with two flanges, which was more or less curved. In the esophagus, the valve itself is formed by a slit mechanism. To increase the stability, an inner metal reinforcement is found in the curve. Shunt valves according to Henley-Cohn [97], the Bonelli voice prosthesis [98], the voice prosthesis according to Staffieri [99] as well as the voice prosthesis according to Traissac in 1986 [100] did not become accepted. In 1988, the so-called Provox I voice prosthesis was sold, it had been developed by Hilgers in the Netherlands [101]. Presenting the most commonly used voice prostheses, the focus will be placed on the voice prostheses Provox 1 and Provox 2 developed by him because those systems are internationally wide-spread together with the Blom-Singer voice prostheses (Table 2 (Tab. 2)).
Figure 23. Herrmann voice prosthesis, 1986.
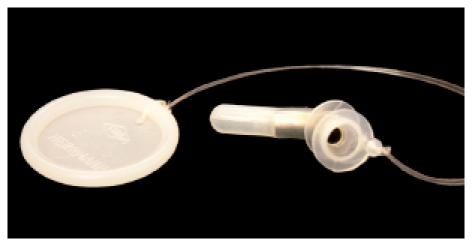
2.2 Indications for the application of voice prostheses, surgical preconditions
Voice rehabilitation is part of the integral rehabilitation of laryngectomized patients. It plays an important role because the patients sense the loss of their voices by laryngectomy as most severe. Preoperatively, the information of the patient about all consequences is essential. A clinic advisor of the association of laryngectomized patients should be involved as well as the relatives and a social worker. Only if the patient is fully conscious of the consequences of surgery, laryngectomy can be performed. Already in this preoperative information, the possibility of voice rehabilitation must be mentioned. If no contraindications occur, primary voice rehabilitation by means of voice prosthesis should be performed intraoperatively. The primary insertion of Provox 2 prosthesis is wide-spread. Whenever possible, we apply a placeholder during surgery for voice prosthesis according to ESKA-Herrmann because it has a smaller outside diameter. After removal of the voice prosthesis of this type, we have never seen any persisting fistula. Already during surgery it is important to create optimal anatomic preconditions for an unproblematic functioning of the voice prosthesis. According to Hilgers [101], the medial part of the sternocleidomastoid muscle are detached from the clavicula in order to later create a plane locating surface for a tracheostoma ring. Further median myotomy should be performed in every case in order to minimize the pressure in the pharyngo-esophageal transitory segment postoperatively. A medium-size, stable and tension-free tracheostoma also contributes to the success of voice rehabilitation. The voice prosthesis itself should be inserted about 0.5–1 cm away from the upper skinmucosa edge. Twelve days after surgery, the placeholder is removed, the ESKA-Herrmann voice prosthesis is inserted and the first exercises may start. Generally they are successful so that the patient may leave the protecting area of the hospital with a voice. However, the patient should also be confronted with the possibility of learning the esophageal voice. Our objective is always to train the patient to develop a powerful esophagus voice. When this method is mastered, the patient may decide for one of the methods. Frequently, the patients require removal of the voice prosthesis because it causes a foreign body sensation. There are different factors that contraindicate the application of shunt valves, for example the missing patient’s consent, mental or physical impairment regarding the handling of a shunt valve, also advanced cardio-vascular risks and obstructive pulmonary diseases may require that the pulmonary pressure needed vocalization cannot or must not be created [102]. When the patient was not primarily provided with voice prosthesis and learning of the esophagus voice was not possible, secondary voice prosthesis may be applied in selected cases. Some authors state that also previous irradiation with more than 70 Gy represent a contraindication [103].
2.3 Types of voice prostheses (material, use, valve mechanisms, methods of insertion, duration)
The first prostheses developed by Blom-Singer were replacement prostheses that the patient had to change in daily intervals. They were soon developed and received a second flange for a firmer hold in the tracheo-esophageal fistula. The technical data of the commonly used voice prostheses are summarized in Table 2 (Tab. 2).
The curved voice prostheses presented by Herrmann was as well conceived as replacement prosthesis. It had two flanges the secured a safe hold in the fistula. However, in
reality, only very few patients could change their voice prostheses without help. The particularity of the curved ESKA-Herrmann voice prosthesis was a safety thread with a large plate that should avoid aspiration of the voice prosthesis into the tracheo-bronchial system during expectoration. We only observed very few avoided aspirations, but often the safety system represented an obstacle when the clothes were changed so that the voice prosthesis was accidentally removed.
The voice prosthesis described by Mozolewski in 1972 was manufactured and consisted of hard polyvinyl chloride synthetic material (Figure 20 (Fig. 20)). It was not appropriate for long-term application. The nowadays commonly applied voice prostheses are made of high-quality silicone that adjusts easily to the existing anatomic structures. Karschay and Leunisse [104], [105] described differences in the composition of silicone which might have an impact on the flow conditions of the air, the
durability, and the microbial settlement. The Phonax voice prosthesis recently presented by Heimomed Company, Germany, consists of a synthetic material that does not contain silicone.
Another specially manufactures device that is very expensive it the Provox Activalve produced by Atos Medical. It was developed for patients who need frequent change of the voice prosthesis in intervals of less than 8 weeks because of a leak, or who suffer from such massive air inhalation into the gastro-intestinal tract that air exits in uncontrolled manner orally or anally. The voice prosthesis has a magnet-fixed valve that avoids uncontrolled air intake, further it contains parts of teflon that impede fungal infestation [106], [107].
The outside diameter of the different voice prostheses varies between 5.5 mm (ESKA-Herrmann prosthesis) and 7.5 mm (Provox-2 voice prosthesis of ATOS Medical). Large lumina or outside diameters allow air flow with low resistance, but they also bear the risk of persisting fistula after removal of the voice prosthesis due to different reasons (Table 2 (Tab. 2)). The durability of the prosthesis depends on an important factor which is the valve of the voice prosthesis. The simplest technology is creating a slit on the esophageal side of the prosthesis tube (voice prosthesis according to Herrmann, curved or straight, Groningen voice prosthesis). Exhaling and phonation dilates the valve lips in case of closed tracheostoma; at the end of the phase they close again due to the elasticity of the silicone and the cohesiveness. The voice prostheses Provox 1 and 2 (ATOS Medical), Blom-Singer (In Health), ADEVA High Flow (ESKA Medical), and the Phonax voice prosthesis have valves working as a door hinge (Table 1 (Tab. 1)). One particularity is the voice prosthesis Voicemaster produced by TRACOE Company that has a ball valve.
It is optimal when voice prostheses can be inserted easily and safely into the tracheoesophageal fistula. In this context, the difference must be made between anterograde and retrograde procedures. The first mentioned method means that the voice prosthesis is inserted in anterior-posterior direction via an auxiliary means over the tracheostoma. Hereby, insertion sticks have turned out to be useful; they are moved into the lumen of the voice prosthesis (ESKA-Herrmann voice prosthesis, Phonax voice prosthesis). A special insertion capsule is used by ATOS Medical for Provox-2 and Activalve voice prostheses. After positioning the voice prosthesis in the capsule, it is inserted into the tracheoesophageal fistula. This elegant procedure must however be trained. Blom-Singer use as inserter a gelatine capsule that decomposes after a short time in the esophagus and releases the esophageal flange for adjustment (Table 2 (Tab. 2)).
A traction system of the TRACOE voice prostheses allows by means of temporary reduction of the diameter the insertion which also requires some training. Another procedure that is very useful is the retrograde peroral insertion of the voice prosthesis. This procedure was developed by ATOS Medical for Provox-1 voice prostheses. A guide wire made of hard synthetic material is conducted through the tracheo-esophageal fistula via the oral cavity to the outside. The voice prosthesis is attached at the guide wire so that the voice prosthesis may be inserted in the shunt via the oral cavity and the pharynx. This procedure has not proven itself successful for the routine because it is not comfortable for the patient and the physician, but it may be a good solution when anterograde procedures fail.
The duration of the voice prosthesis is an important characteristic for its quality and it is an economic factor that must not be underestimated. Schäfer et al. described a median duration of stay of the Provox 1 voice prosthesis of 224 days and of the Provox 2 voice prosthesis of 96 days [108]. Lequeux et al. observed durations of 303 days of the Provox 1 voice prosthesis compared to 144 days of the Provox 2 voice prosthesis. Sens revealed a median duration of 143 days of the Provox 1 and of 90 days of the Provox 2 voice prothesis in the patient population of Jena, Germany. In the patient population of Hamburg, Germany, Hummel could show a median du-ration of 201 days of the Provox 1 and a median duration of 95 days of the Provox 2 voice prosthesis [109]. Hilgers et al. reported about a median duration of the Provox voice prostheses of 141 days [110], [111]. Schäfer et al. [108] evaluated the durations of 172 Blom-Singer voice prostheses and found a median duration of 107 days.
2.4 Microbial settlement of the voice prostheses
The usage time of voice prostheses is mostly limited to three or four months because of different reasons [112]. A relevant factor is the development of a bacterial or fungal biofilm at the surface of the prostheses [113]. The germs do not only stay at the surface but also grow into the depth and degrade the silicone [114]. The results are crusting and leakiness of the valve with increasing breathing pressure and following function failure so that a prosthesis replacement becomes necessary. The development of biofilms is influenced by several exogenous factors such as the food composition and endogenous factors such as the quantity and quality of the saliva for example after radio-chemo-therapy [115].
Because of the anatomic location and function of voice prostheses with different contamination pathways, a series of different germs was found at the surface: up to 700 different types of bacteria and fungi that exist physiologically in the human oral cavity may play a role [116]. The contact of the esophageal side with food represents an additional germ exposition. Further, the tracheostoma is closed with the fingers for phonation so that germs of the hands pass into the area of the voice prosthesis [117].
Microbiological examinations of the biofilms revealed differences in different patients and their prostheses. A predominance of certain germs could be detected. Among the bacteria, the staphylococci, streptococci, and enterococci prevailed. Staphylococci are an important part of the physiological skin flora and may found in almost every voice prosthesis [114]. Regarding fungus infections, nearly always the different candida species are present. The dominating ones are C. albicans, C. glabrata, C. krusei, and C. tropicalis [118].
The microbiological settlement of the surface of the voice prosthesis starts immediately after insertion of the prosthesis and is a natural process. Already within a few days, all predominant germs settle at the surface with subsequent maturing and growth of the biofilm [119]. Hereby, different bacteria as for example staphylococcus aureus positively influence the settlement of single candida species [120].
In the course of the time, a balance of the flora is achieved while dynamism may be observed in different species. Regarding the different models of the prostheses, almost the same bacteria and fungi may occur isolated at the surfaces so that the described contamination pathways mainly determine the settlement. This microbial settlement at the surface of the voice prostheses occurs regularly and limits the time of usage of the prostheses. However, voice prostheses should be replaced only in case of functional loss. If fever occurs without knowing the genesis, the voice prosthesis should be replaced even if it still works in order to exclude a focus.
2.5 Efficiency of voice prostheses in voice rehabilitation
After laryngectomy, the patients do no longer dispose of their pulmonary volume of about 3000 ml for phonation because of the exhalation via tracheostoma. Applying the esophageal voice, the patient can regurgitate the swallowed air only with a volume of about 80 ml through the esophagus and use it for phonation. The important advantage of a mechanism via voice prosthesis in comparison to the esophageal voice is that almost the whole pulmonary volume can be used for phonation instead of only 80 ml [102].
The tone issued by the esophageal voice or the voice prosthesis is made by vibrations of mucosal folds in the pharyngeal space. Physically it is no real tone or sound but only a noise with non-periodic sound wave production. Filtering or modulation of the noises in the vocal tract leads to an adaptation (formant creation) so that the esophageal voice has a natural sound in comparison to electronic speaking aids. Measurements of the different formants in speakers with esophageal voice, voice prostheses, or other surgical shunt interventions with pharyngeal voice showed that the formant spectrum only varies insignificantly from the one of healthy speakers. Those changes may be explained by the surgically caused deformation of the vocal tract after laryngectomy or the different fundamental tones (laryngeal tone, pharyngeal tone) [121], [122].
The median pitch of the voice in normally phonating adults is between 98 and 131 Hz in men and 196–262 Hz in women while the growth acceleration tends to a decrease of the median vocal pitch [123], [124].
In comparison to this, the sound created in the pharyngeal tube of laryngectomized patients issues with an unphysiologically deep frequency; its basic frequency for the esophageal voice is given with 50 Hz and 110 Hz in the literature. Evaluations on the middle pitch of the voice in patients with voice prosthesis showed a similar frequency range between 80 and 110 Hz [124], [125], [126], [127].
Those basic frequencies have a broad distribution. Probably they are independent from the rehabilitations procedure, also because the actual sound generator in the area of the esophageal opening is identical in cases of the same laryngectomy technique [128].
For the quality of the substitute voice the holding period of the sound is a significant criterion. According to Böhme, the average maximum phonation lasts about 15–25 seconds in females and to 25–35 seconds in males [128]. Because of the low air volume at disposition for phonation, the holding period of the sound in cases of ructus voice amounts to only 1.6 seconds. Speakers with voice prostheses achieve a significantly longer maximum phonation that lasts about 10.5 seconds; however, in comparison to healthy individuals it is still clearly reduced [129]. This is mainly due to the fact that a higher air flow is necessary for phonation [130].
Regarding the mean speaking velocity, patients with voice prosthesis achieve an average of 150 syllables per minute and are thus rather close to the tempo of healthy speakers than patients with esophageal substitute voice. Even the average number of syllables per inhalation is clearly better in patients with voice prostheses with about 16 compared to 5.7 in patients with ructus voice [131], [132].
In healthy test persons, the volume of the speech for common speech amounts to a mean of 62.5 dB, between low (average of 50 dB) and as loud as possible (100 dB). So there is a dynamic area of about 50 dB [131]. Measurements of the sound pressure level performed by Kischk and Gross revealed minimum, mean, and maximum values of 50-60-71 dB and a dynamic of 21 dB. The values of the esophageal voice were only insignificantly below so that no important difference could be found in both substitute voices regarding the loudness. The vocal range of the prosthesis voice amounted to 5.4 halftones on the average; the ructus voice achieved a range of 4.6 halftones [126].
There are numerous studies comparing the quality of the voice of the different mechanisms of the substitute voices. It is unanimously agreed that the quality of the voice with voice prosthesis is most similar to a normal voice. In second place ranges the esophageal voice, in last place electro-acoustic speaking aids [133], [134].
The majority of the patients already achieve a practicable voice with voice prostheses during their hospital stay [109]. The substitute voice achieved by means of voice prostheses seems to be more melodic, dynamic, and individual to the listener compared to electronic speaking aids or ructus voice and the success rate of about 65% in long-term users it is very high [102].
For the better function of the voice prosthesis it is thus decisive to use the greater, controllable air volume of the lung that does not only cause a clearly longer holding period of the sound but also a greater loudness and dynamic of the voice. Finally this means also a significantly better understanding [135].
The telephone test after laryngectomy developed by Zenner and Pfrang allows comparing the comprehensibility of the different substitute voices in a rather simple way [136]. Own evaluations confirm the clear superiority of the voice prostheses in comparison to the esophageal voice and the electro-larynx [129], [137].
Especially for female laryngectomized patients the basic noise created in the pharynx represents an additional stigmatisation because of the deep frequencies. With about 120 Hz the basic frequency of female patients with voice prostheses is nearly equal to the one of healthy male people [128].
New developments aim at a gender specific phonation by voice prostheses. Those membrane-based voice prostheses consist of two membranes that are integrated directly into the lumen of the prosthesis as voice producing elements. First trials to integrate a sound generating element into the voice prosthesis were performed by Herrmann in the 1980ies. In 1998, Hagen published experimental and first clinical results on voice prostheses with a metal tongue element for sound generation [130]. Tack et al. developed a voice prosthesis where two membranes are integrated directly into the lumen of the prosthesis in a distance of 0.28 mm as sound generators. Thus not only the clearly higher basic frequencies were similar to female voices with 234–313 Hz; further the necessary blowing pressure was lower so that phonation was possible also in patients with too low tonicity of the pharyngoesophageal segment [138], [139], [140].
2.6 Tracheostomy valves for freehand speech
Even if voice rehabilitation after total extirpation of the larynx meanwhile succeeds in a fast and high-quality way by means of voice prostheses, there are still several problems when using them. The fact that only one arm can be used functionally is a severe handicap for patients in their family lives and their leisure time, especially for professionals during their work. Further, the hands are always a high risk for germ infection impairing the deep airways. Besides, all laryngectomized people suffer from a pulmonary stress because the nasal function is omitted and cold, non-moistured, and non-cleaned air is inhaled. In order to find a solution to both problems, special tracheostoma valves had been developed. They are fixed in the trachea or on the skin or at special cannulas. A valve, generally equipped with a hinge mechanism, replaces the covering hand during exhalation and the air is lead into the hypopharynx via the voice prosthesis. Those valves have to be able to open when the patient coughs so that the secretion may be transported outside. The so-called cough lid is adjusted individually to the patient because it must only open if certain airflow is surpassed. This is effectuated by means of magnet that may be adjusted individually (Adeva Window® tracheostoma valve, ESKA; or free-hand valve, Provox) [141] (Figure 24 (Fig. 24), Figure 25 (Fig. 25)). Both valves allow the simultaneous use of a HME (head and moisture exchanger) for conditioning of the inhaled air. Further it is possible to omit unpleasant noise resulting from the closing of the valves. A tracheostomy valve that is easy to handle is the ATSV II Valve developed by Blom-Singer [142], [143] (Figure 26 (Fig. 26)). It is characterized by a separator at the anterior side so that it may be covered by the clothes. For individual adjustment to the airflow or pressure, the valve membrane can be adjusted by turning the valve case. The simultaneous use of HME is possible.
Figure 24. ADEVA Window® tracheostomy valve (ESKA Company, Lübeck,Germany).

Figure 25. Provox® Free Hands HME (Atos Medical, Hörby, Sweden).

Figure 26. Blom-Singer ATSV valve (In Health, USA).
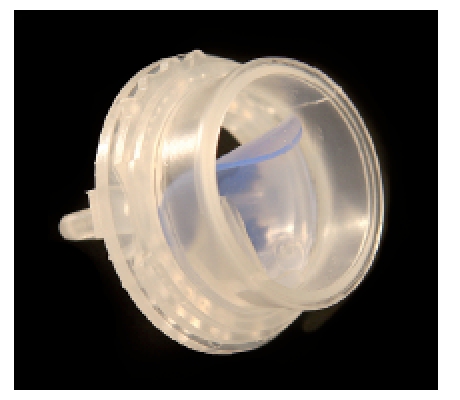
Those tracheostomay valves may be fixed on the base plate adhesive to the skin. An optimal sealing of the tracheostoma is very important in this context and required operative preconditions. If the skin surface around the tracheostoma is not completely flat, silicone sealing rings may be used.
The care of the skin around the tracheostoma needs special diligence. Special cleaning tissue is offered to degrease the skin before applying the according glue and to remove it. If the sealing necessary for free-hand speech is not achieved, the base plate can be used with a silicone ring carrying the HME valve, which facilitated the use of one hand for generating an acceptable voice. This promotes the pulmonary rehabilitation and the germy finger does not directly touch the tracheostoma. In some cases, also a short cannula or a stoma button may be used.
Another way is pursued by Herrmann who inserts a half tube into the tracheostoma and fixes the tracheostoma valve to it [142] (Figure 27 (Fig. 27)). The precondition for this procedure is creating a trachea funnel in upward direction that needs to be created by means of the special surgical technique that is not widespread.
Figure 27. ESKA Herrmann tracheostomy valve (Lübeck, Germany).

In case of expressed wish of the patient to speak in a finger-free way, the individual adjustment of a tracheal epithesis is possible that allows optimal sealing. Before prescribing such an epithesis, an application should always be written to the responsible payer because the custom-made production is very expensive.
2.7 Pros and cons of prosthesis voice in comparison to esophageal voice
The Rostock model of voice rehabilitation after laryngectomy consists of the primary application of voice prosthesis and the possibly early speech therapy in order to achieve an acceptable ructus voice. An electronic speaking aid is only applied when both procedures to not have the desired success. The discussion that is sometimes open about esophageal voice compared to voice prosthesis does not meet the individual requirements and possibilities of the patients. The patient should master both procedures in order to be able to decide. A comparison of the advantages and disadvantages of both methods will make those problems clearer.
The most significant advantage of a prosthesis voice is the fast voice rehabilitation. In cases of optimal healing course, the patient may already be able to communicate with a voice already 10–14 days after laryngectomy which encourages him for a life without larynx. Very soon he is able to communicate with his family and friends without depending on a writing tablet. The quality of the voice is superior to all substitute voices. The early training of voice generation is a good preparation to learn the ructus voice. The disadvantage for the patient is that he needs one hand for sealing the tracheostoma, so that he is functionally one-armed. If possible, a tracheostoma valve should be used to meet this need. Voice prosthesis needs continuous diligent care which is difficult for some patients. The voice prosthesis causes a dependence of a well-trained ENT specialist who is able to solve the problems with the voice prosthesis, to introduce the necessary steps in case of complications, or to change the prosthesis. Some patients (apparently more in Germany than in the Netherlands or in the USA) sense the voice prosthesis as disturbing foreign body so that they want to omit it when mastering a good esophageal voice. Especially luminous low-pressure voice prostheses bear the risk of persisting fistula after removal. The undeniable advantage of the esophageal voice is that it is a body’s own substitute voice that can be always applied without technical devices.
Additional surgical intervention is not needed so that no further complications occur [137]. Those patients do not depend on an ENT specialist and no additional care is needed. In the speaking process no hand is needed for closure of the tracheostoma so that bimanual working is possible.
On the other hand, about 30% of our patients do not learn the esophageal voice. Beside certain anatomy preconditions, strong inner and outer motivation is necessary while a significant training effort must be made by the therapist and the patient [144]. Demanding patients are not satisfied with the quality of this voice. Often tiredness is observed after a short speaking time [134]. Further some patients complain about recurrent acid reflux and a feeling of fullness [145].
Summarizing the pros and cons of both methods of voice rehabilitation, an individual solution must be found for every patient meeting his needs and requirements: both methods are equally valuable for the voice rehabilitation of laryngectomized patients.
2.8 Management of problems of the application of voice prosthesis
ENT specialists who care for patients with voice prostheses must have the special knowledge of the complications and problems of the application of voice prostheses in order to be able to act fast and correctly in the sense of their patients [146]. So the most important symptoms, origins, and necessary measures will be described.
The exit of fluids into the trachea during the drinking process, generally associated with coughing, can occur when the closure is defect, for example caused by a defective valve or perifistular leakiness. The diagnosis must be made by careful endoscopy during drinking. If the valve is leaky, fluid exits to the outside through the voice prosthesis; if the valve is intact, the fluid exits into the trachea through the fistula. The reason for valvar leakiness may be a candida settlement with closure impairment or long duration of stay with subsequent material fatigue that does not allow normal function of the valve. Further a mechanical damage of the valve may occur when it is cleaned too vehemently with the brush. In such a case, the patients should insert a plug so that he closes the voice prosthesis himself and food intake is possible. Thus he may go to the caring ENT specialist at the next possible date without any need for hurry.
The reason for perifistular leakiness is generally the dilation of the tracheo-esophageal fistula [147], [148]. This phenomenon frequently occurs in patients after irradiation leading to tissue atrophy around the tracheo-esophageal shunt. Additionally gastro-intestinal reflux or stenoses in the pharyngo-esophageal transition segment may have a negative impact. Shrinking should be tried by inserting a blockable cannula. A tobacco pouch suture is sometimes useful in cases of lying voice prosthesis; however, it is often not successful. Special voice prostheses with large diameters may be an immediate help in the sense of sealing but they are never a satisfactory durable solution. A possible measure is the insertion of a Blom-Singer prosthesis with a big so-called Trier silicone ring on the esophageal side [108]. Kress et al. reported about positive experiences of sealing periprosthetic fistulas with the custom fit voice prosthesis, which is a modified Blom-Singer voice prosthesis with a large esophageal ring [149]. Hilgers et al. recommend a 0.5 mm silicone washer on the tracheal side of the voice prosthesis as primary measure in cases of periprosthetic leakiness [150].
Another urgent measure is the application of fibrin glue, collagen [151], [152] or hyaluronic acid [153] and fat [154]. Bioplastique [155], [156] may only be applied for augmentation after compatibility tests. If the shunt is reduced after the shrinking procedure, new voice prosthesis may be inserted. Frequently, the simple shrinking or a tobacco pouch suture do not lead to a success in regionally irradiated patients [157] so that a plastic reduction of the defect must be performed [158], [159], sometimes even with the risk of closure of the fistula.
Granulations around the voice prosthesis that are often associated with a difficult phonation are generally caused by the pressure of too short voice prosthesis on the fistula wall. It should be removed electro-surgically or by means of high frequency or laser surgery. Then the width of the tracheo-esophageal shunt must be measured with following insertion of an appropriate voice prosthesis that does no longer exert pressure on the walls of the shunt.
In cases of dislocation of the prosthesis, often the phonation is aggravated. Also here the reason is often a too short voice prosthesis exerting high pressure on the fistula wall. The voice prosthesis must be removed carefully with protection of the mucosa and measured longer voice prosthesis is inserted.
Accidental removal when changing clothes may lead to an unnoticed loss of the voice prosthesis, often it occurs with Herrmann voice prostheses with safety thread (see above). Further it may happen that the valve is swallowed unnoticed in the esophagus, especially when the tracheal flanges do no longer hold because of material fatigue. After radiologic diagnosis, the spontaneous excretion of the remaining parts of the voice prosthesis may be expected. New voice prosthesis should be inserted immediately into the shunt. A loss of the prosthesis may also occur because of aspiration of the voice prosthesis into the tracheo-bronchial system, unnoticed by the patient. Apparently, laryngectomized patients loose a certain degree of mucosa sensitivity that under physiological conditions would lead to cough up the foreign body. Tracheo-bronchoscopy or thoracic x-ray may confirm aspiration of the voice prosthesis: mostly it can be well identified at the entrance of the right main bronchus and may be removed under local anesthesia. When the existing fistula is still wide enough, new voice prosthesis may be inserted with a normal inserter. A shrinked fistula, however, must be dilated by means of a bougie produced by Provox Company or Blom-Singer. If this measure is not successful, the voice prosthesis must be inserted by means of a guide-wire (ATOS Medical) applying the retrograde pulling technique. An already closed fistula must be punctured secondarily in a protective and esophagoscope-guided way and new voice prosthesis is inserted applying the pulling technique.
The reasons for a difficult phonation with regular position and good passive function of the prosthesis could be obstructing granulations in the esophagus or functional disturbances of the hypopharynx. Generally the pressure in the pharyngo-esophageal segment is too high. The diagnosis is made by esophagoscopy or insufflation trial via the voice prosthesis. Granulations are removed; in cases of too high pressure with spasm in the pharyngo-esophageal segment it may be helpful to inject botulinum [160], [161], [162], [163], [164], [165]. Moricz et al. refer to endoscopy-based balloon dilation as a non-traumatic method that can be easily performed [166]. Delank and Scheuermann recommend manufacturing an individually adjusted Herrmann voice prosthesis in order to bypass the spastic or stenotic pharyngoesophageal area [167].
If air exits when trying to close the tracheostoma manually, an optimal phonation is not possible. There may be several reasons for this problem. The patient might be not well coordinated to close the tracheostoma during exhalation or the opening is too large. In the first case, a special training may be useful. In a too large tracheostoma a stoma button can be inserted where a HME is fixed and on which the patient has to exert pressure to close the tracheostoma sufficiently. In exceptional cases, epithetics might adjust an individual tracheostoma epithesis. However, as the expenses are very high, an application should be formulated to submit to the health insurance companies. Those epitheses can also be used for insertion of a tracheostoma valve for free-hand speech.
If phonation is suddenly no longer possible, the reason may be an obstruction of the prosthesis valve by secretion or food. Also when Herrmann voice prosthesis is turned downwards it may loose its function. Inspection or endoscopy of the voice prosthesis reveals those problems that may then be resolved (e.g. removal of the nutrition residues, turning of the prosthesis etc.). If occurred once, such an incidence may happen repeatedly because the pharyngo-esophageal segment may be so larger in this area that turning of the prosthesis is easily possible.
n a small group of patients, the prosthesis must be changed in very short intervals because of a loss of their function or leakiness of the valve based on fungus infection. Blom and Singer developed advantage tracheoesophageal voice prosthesis with modified flanges and a valve flap that is covered with 7% silver oxide in order to avoid candida community. Leder et al. and Kress et al. evaluated a longer duration of those voice prosthesis in comparison to the classic Blom-Singer voice prostheses [168], [169].
2.9 Perspectives
Voice prostheses are meanwhile accepted for the voice rehabilitation of laryngectomized patients. They improve the quality of life of those patients [170].
As the duration depends significantly on the type and quality of the superficially developing biofilm, its impact on the change of the surface of the prostheses or development of alternative material is currently in the focus. Further, efforts are made to develop electronic systems with implantable sensors in the oral cavity. Those trials, however, are still in initial stages [171].
References
- 1.Sosath J. Die geschichtliche Entwicklung der Perkutanen Dilatativen Tracheotomieverfahren im historischen Kontext (Inaug Diss) Greifswald: Ernst-Moritz-Arndt-Univ; 2006. [Google Scholar]
- 2.Pahor L. Ear, Nose and Throat in Ancient Egypt. J of Laryngol Otol. 1992;106:773–779. doi: 10.1017/s0022215100120869. [DOI] [PubMed] [Google Scholar]
- 3.Joachim H. Papyrus Ebers - das älteste Buch über Heilkunde. Berlin: de Gruyter; 1890. pp. 188–189. [Google Scholar]
- 4.Holmes G. Die Geschichte der Laryngologie von den frühesten Zeiten bis zur Gegenwart. Berlin: Hirschwald; 1887. p. 21. [Google Scholar]
- 5.Holmes G. Die Geschichte der Laryngologie von den frühesten Zeiten bis zur Gegenwart. Berlin: Hirschwald; 1887. pp. 23–24. [Google Scholar]
- 6.Aegineta (Aegina) P. Pavlvs Aegineta edidit IL. Heiberg. Pars Altera, Libri V-VII. Leipzig und Berlin: B. G. Teubner; 1924. (Corpvs Medicorvm graecorvm; IX, 2). [Google Scholar]
- 7.Kühne J. Die Entwicklung der Operationstechnik und der Instrumente in der Geschichte der Tracheotomie (Inaug Diss) Univ Bonn; 1973. p. 16. [Google Scholar]
- 8.Sercer A. 2000 Jahre Tracheotomie. Ciba-Symposium. 1962;10:83–84. [Google Scholar]
- 9.Keys TE. Die Geschichte der Chirurgischen Anaesthesie. Berlin, Heidelberg, NewYork: Springer; 1968. pp. 90–91. [Google Scholar]
- 10.Benivienius A. De abditis nonnullis ac mirandis morborum et sanationum causis liber. In: Cratander A, editor. Basileae Cap. 58. 1529. pp. 241–242. [Google Scholar]
- 11.Fabricius Hieronymus ab Aqvapendente. Opera chirvrgica. I, Cap. XLIV. N. Hoffmann, Francofvrti, I, Cap. XLIV; 1620. pp. 155–166. [Google Scholar]
- 12.Casserius I. De vocisavditvsque organis historia anatomica. Ferrara: Baldimus; 1600. pp. Lib. I, Cap. XX: 119–Lib.124 u. Tab. XXII. [Google Scholar]
- 13.Keminger K. Tracheotomie. In: Kremer K, Platzer W, editors. Hals, Gefäße. Stuttgart, New York: Georg Thieme; 1989. pp. 33–35. (Chirurgische Operationslehre; Bd. 1). [Google Scholar]
- 14.Kühne J. Die Entwicklung der Operationstechnik und der Instrumente in der Geschichte der Tracheotomie (Inaug Diss) Univ Bonn; 1973. pp. 40–42. [Google Scholar]
- 15.Schuchardt B. Zur Geschichte der Tracheotomie bei Croup und Diphtherie, besonders in Deutschland. Arch Klin Chir. 1887;36:540. [Google Scholar]
- 16.Dekkers F. Exercitationes practicae circa methodum medendi observationibus illustratae. Leidae; 1694. p. 241. [Google Scholar]
- 17.Holmes G. Die Geschichte der Laryngologie von den frühesten Zeiten bis zur Gegenwart. Berlin: Hirschwald; 1887. p. 52. [Google Scholar]
- 18.Heister L. Chirurgie. Nürnberg: Stein & Raspe; 1752. p. 557. [Google Scholar]
- 19.Martin G. Account of the Operation of Bronchotomy, as it was performed at St.Andrews. Philosophical Transactions. 1730:448–455. [Google Scholar]
- 20.Richter AG. Anfangsgründe der Wundarzneykunst. Göttingen: Dietrich; 1797. p. 226. [Google Scholar]
- 21.Drachter R, Gossmann JR. Chirurgie des Kindesalters. Leipzig: FCW Vogel; 1930. pp. 397–408. [Google Scholar]
- 22.Königlich Preussisches Statistisches Landesamt; Königlich Preussischen Statistischen Landesamt, editor. Statistisches Jahrbuch für den Preussischen Staat 1914. Elfter Jahrgang. Berlin: Selbstverlag; pp. 60–61. [Google Scholar]
- 23.Bretonneau P. Des inflammations spéciales du tissu muqueux, et en particullier de la diphthérie. Paris: 1826. [Google Scholar]
- 24.Greb J. Atlas. Erlangen: F. Enke; 1885. (Pitha F v. Billroth Th: Handbuch der allgemeinen und speciellen Chirurgie). [Google Scholar]
- 25.Byhan C, Lischke V, Westphal K. Trachetomie – Indikation und Anwendung in der Intensivmedizin. Darmstadt: Steinkopff Verlag: 2000. p. 10 ff. [Google Scholar]
- 26.Björk VO. Partial resection of the only remaining lung with the aid of respirator treatment. J Thorac Cardiovasc Surg. 1960;39:179–188. [PubMed] [Google Scholar]
- 27.Ciaglia P, Firsching R, Syniec C. Elective percutaneous dilatational tracheostomy, A new simple bedside procedure; prelimnary report. Chest. 1985;87:715–719. doi: 10.1378/chest.87.6.715. Available from: http://dx.doi.org/10.1378/chest.87.6.715. [DOI] [PubMed] [Google Scholar]
- 28.Griggs WM, Worthley LI, Gilligan JE, Thomas PD, Myburg JA. A simple percutaneous tracheostomy technique. Surg Gynecol Obstet. 1990;170:543–545. [PubMed] [Google Scholar]
- 29.Fantoni A, Ripamonti DA. Non-derivative, non-surgical tracheostomy; the translaryngeal method. Intensiv Care Med. 1997;23:386–392. doi: 10.1007/s001340050345. Available from: http://dx.doi.org/10.1007/s001340050345. [DOI] [PubMed] [Google Scholar]
- 30.Byhahn C, Lischke V, Halbig S, Scheifler G, Westphal K. Ciaglia Blue Rhino: Ein weiterentwickeltes Verfahren der perkutanen Dilatationstracheotomie. Anaesthesist. 2000;49:206. doi: 10.1007/s001010050815. Available from: http://dx.doi.org/10.1007/s001010050815. [DOI] [PubMed] [Google Scholar]
- 31.Gründling M, Kuhn SO, Nees J, Westphal K, Pavlovic D, Wendt M, Feyerherd F. PercuTwist-Dilatationstracheotomie Prospektive Evaluation an 54 konsekutiven Patienten. Anaesthesist. 2004;53:434. doi: 10.1007/s00101-004-0665-5. [DOI] [PubMed] [Google Scholar]
- 32.Graumüller S. Langzeitkomplikationen nach PDT. J Anäst Intensivbeh. 2006;3:38–42. 33. [Google Scholar]
- 33.Gerson CR, Tucker GF. Infant tracheotomy. Ann Otol Rhinol Laryngol. 1982;91:413–416. doi: 10.1177/000348948209100419. [DOI] [PubMed] [Google Scholar]
- 34.Stoll W, Joch G, Kritenbrink E. Aspekte zur Säuglings- und Kindertracheotomie. Laryngo Rhino Otol. 1987;65:63–66. doi: 10.1055/s-2007-998600. Available from: http://dx.doi.org/10.1055/s-2007-998600. [DOI] [PubMed] [Google Scholar]
- 35.Sisk EA, Kim TB, Schumacher R, Dechert R, Driver L, Ramsey AM, Lesperance MM. Tracheotomy in very low birth weight neonates: indications and outcomes. Laryngoscope. 2006;116:928–933. doi: 10.1097/01.MLG.0000214897.08822.14. Available from: http://dx.doi.org/10.1097/01.MLG.0000214897.08822.14. [DOI] [PubMed] [Google Scholar]
- 36.Graf JM, Montagnino BA, Hueckel R , McPherson ML. Pediatric tracheostomies: a recent experience from one academic center. Pediatr Crit Care Med. 2008;9:6–100. doi: 10.1097/01.PCC.0000298641.84257.53. Available from: http://dx.doi.org/10.1097/01.PCC.0000298641.84257.53. [DOI] [PubMed] [Google Scholar]
- 37.Carron JD, Derkay CS, Strope GL, Nosonchuk JE, Darrow DH. Pediatric tracheotomies: changing indications and outcomes. Laryngoscope. 2000;110:1099–1104. doi: 10.1097/00005537-200007000-00006. Available from: http://dx.doi.org/10.1097/00005537-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Rozsasi A, Neagos A, Nolte F, Riechelmann H, Rettinger G, Keck T. Kritische Analyse von Komplikationen und Wundheilungsstörungen nach Tracheotomie im Kindesalter. Laryngo Rhino Otol. 2003;82:826–832. doi: 10.1055/s-2004-814137. Available from: http://dx.doi.org/10.1055/s-2004-814137. [DOI] [PubMed] [Google Scholar]
- 39.Wood DE, Mathisen DJ. Late complications of tracheotomy. Clin Chest Med. 1991;12:597–609. [PubMed] [Google Scholar]
- 40.Waki EY, Madgy DN, Zablocki H, Belenky WM, Hotaling AJ. An analysis of the inferior based tracheal flap for pediatric tracheotomy. Int J Pediatr Otorhinolaryngol. 1993;27:47–54. doi: 10.1016/0165-5876(93)90035-2. Available from: http://dx.doi.org/10.1016/0165-5876(93)90035-2. [DOI] [PubMed] [Google Scholar]
- 41.Donelly MJ, Lacey PD, Maguire AJ. A twenty-year (1971-1990) review of tracheostomies in a major pediatric hospital. Int J Pediatr Otorhinolaryngol. 1996;35:1–9. doi: 10.1016/0165-5876(95)01255-9. Available from: http://dx.doi.org/10.1016/0165-5876(95)01255-9. [DOI] [PubMed] [Google Scholar]
- 42.Seidl RO, Nusser-Müller-Busch R. Die Trachealkanüle: Segen und Fluch. In: Nusser-Müller-Busch R, editor. Die Therapie des Facio-oralen Trakts: F.O.T.T. nach Kay Coombes. 2. Auflage . Berlin: Springer; 2007. pp. 148–168. [Google Scholar]
- 43.Knöbber D. Der tracheotomierte Patient. Berlin: Springer; 1991. [Google Scholar]
- 44.Schrader M. Tracheostoma. In: Zenner HP, editor. Praktische Therapie von HNO-Krankheiten. 2. Auflage . Stuttgart: Schattauer; 2008. pp. 61–65. [Google Scholar]
- 45.Leder SB, Ross DA, Burell MI, Sasaki CT. Tracheotomy tube occlusion status and aspiration in early postsurgical head and neck cancer patients. Dysphagia. 1998;13:167–171. doi: 10.1007/PL00009568. Available from: http://dx.doi.org/10.1007/PL00009568. [DOI] [PubMed] [Google Scholar]
- 46.Montgomery WW. T-tube tracheal stent. Arch Otolaryngol. 1965;82:320–321. doi: 10.1001/archotol.1965.00760010322023. [DOI] [PubMed] [Google Scholar]
- 47.Phillips PS, Kubba H, Hartley BE, Albert DM. The use of the Montgomery T-tube in difficult paediatric airways. Int J Pediatr Otorhinolaryngol. 2006;70:39–44. doi: 10.1016/j.ijporl.2005.05.008. Available from: http://dx.doi.org/10.1016/j.ijporl.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Wahidi MM, Ernst A. The Montgomery T-tube tracheal stent. Clin Chest Med. 2003;24:437–443. doi: 10.1016/S0272-5231(03)00042-X. Available from: http://dx.doi.org/10.1016/S0272-5231(03)00042-X. [DOI] [PubMed] [Google Scholar]
- 49.Eavey RD, Shannon DC, Montgomery SK. Customizing tracheotomy tubes. Laryngoscope. 1985;11:1407–1408. doi: 10.1288/00005537-198511000-00022. [DOI] [PubMed] [Google Scholar]
- 50.Becker HD. Derzeitige Möglichkeiten und Grenzen der bronchoskopischen tracheobronchialen Schienung. Pneumol. 1994;48:182–190. [PubMed] [Google Scholar]
- 51.Winklmaier U, Wüst K, Wallner F. Evaluation des Aspirationsschutzes blockbarer Trachealkanülen. HNO. 2005;53:1057–1062. doi: 10.1007/s00106-005-1263-9. [DOI] [PubMed] [Google Scholar]
- 52.Ackerstaff AH, Hilgers FJ. Die Folgen der totalen Kehlkopfentfernung unter besonderer Beachtung der Rehabilitaion der Stimme und der unteren Luftwege. HNO. 1997;45:97–104. doi: 10.1007/s001060050098. [DOI] [PubMed] [Google Scholar]
- 53.Ackerstaff AH, Hilgers FJ, Balm AJ, Van Zandwijk N. Long-term pulmonary function after total laryngectomy. Clin Otolaryngol Allied Sci. 1995;20:547–551. doi: 10.1111/j.1365-2273.1995.tb01599.x. Available from: http://dx.doi.org/10.1111/j.1365-2273.1995.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 54.Ackerstaff AH, Hilgers FJ, Meeuwis CA, Knegt PP, Weenink C. Pulmonary function pre- and post-total laryngectomy. Clin Otolaryngol Allied Sci. 1999;24:491–494. doi: 10.1046/j.1365-2273.1999.00298.x. Available from: http://dx.doi.org/10.1046/j.1365-2273.1999.00298.x. [DOI] [PubMed] [Google Scholar]
- 55.Hess MM, Schwenk RA, Frank W, Loddenkemper R. Pulmonary function after total laryngectomy. Laryngoscope. 1999;109:988–994. doi: 10.1097/00005537-199906000-00027. Available from: http://dx.doi.org/10.1097/00005537-199906000-00027. [DOI] [PubMed] [Google Scholar]
- 56.Todisco T, Mauritzi M, Paludetti G, Dottorini M, Merante F. Laryngeal Cancer: Long- term follow-up of respiratory functions after laryngectomy. Resp. 1984;45:303–315. doi: 10.1159/000194635. [DOI] [PubMed] [Google Scholar]
- 57.Kelsey MC, Mitchell CA, Griffin M. Prevalence of lower respiratory tract infections in hospitalized patients in the United Kingdom and Eire - Results from the Second National Prevalence Survey. J Hosp Infect. 2000;46:12–22. doi: 10.1053/jhin.2000.0775. Available from: http://dx.doi.org/10.1053/jhin.2000.0775. [DOI] [PubMed] [Google Scholar]
- 58.Harlid R, Andersson G, Frostell CG, et al. Respiratory tract colonization and infection in patients with chronic tracheostomy. Am J Resp Crit Care Med. 1996;154:124–129. doi: 10.1164/ajrccm.154.1.8680667. [DOI] [PubMed] [Google Scholar]
- 59.Ackerstaff AH, Hilgers FJM, Aaronson NK, Balm AJM, van Zandwijk N. Improvements in respiratory and psychosocial functioning following total laryngectomy by the use of a heat and moisture exchanger. Ann Otol Rhinol Laryngol. 1993;102:878–883. doi: 10.1177/000348949310201111. [DOI] [PubMed] [Google Scholar]
- 60.Jones AS, Young PE, Hanafi ZB, Makura ZG, Fenton JE, Hughes JP. A study of the effect of a resistive heat moisture exchanger (Trachinaze) on pulmonary function and blood gas tensions in patients who have undergone a laryngectomy: a randomized control trial of 50 patients studied over a 6-month period. Head Neck. 2003;25:361–367. doi: 10.1002/hed.10264. Available from: http://dx.doi.org/10.1002/hed.10264. [DOI] [PubMed] [Google Scholar]
- 61.Graumüller S, Dommerich St, Mach H, Eich H. Spätkomplikationen und Nachsorge nach Tracheotomie unter besonderer Berücksichtigung der Punktionstracheotomie in der neurologischen Frührehabilitation. Neurol Rehabil. 2002;8:122–127. [Google Scholar]
- 62.Gussenbauer C. Über die erste durch Th. Billroth am Menschen ausgeführte Kehlkopf- Exstirpation. Arch Klin Chir. 1874;15:478–479. [Google Scholar]
- 63.Czerny V. Versuche über Kehlkopfexstirpation. Wien Med Wschr. 1870;20:557–561. [Google Scholar]
- 64.Werner E, Gerabek BD, Hagge G, Keil W, Wegner, et al. Enzyklopädie Medizingeschichte. Berlin: Walter de Gruyter; 2004. [Google Scholar]
- 65.Reutter S. Prothetische Stimmrehabilitation nach totaler Kehlkopfentfernung - eine historische Abhandlung seit Billroth (1873) [Inaug Diss] Univ Ulm; 2007. pp. Prothetische Stimmrehabilitation nach totaler Kehlkopfentfernung –Pro eine historische Abhandlung seit Billroth (1873) [Inaug Diss]. [Google Scholar]
- 66.Koscielny S. Wiederherstellende Verfahren nach Kehlkopf-Totalexstirpation. Laryngo Rhino Otol. 2005;84(Suppl 1):221–227. doi: 10.1055/s-2005-861128. Available from: http://dx.doi.org/10.1055/s-2005-861128. [DOI] [PubMed] [Google Scholar]
- 67.Foulis D. Exstirpation of the Larynx. Lancet. 1878;1:118–120. doi: 10.1016/S0140-6736(02)43154-6. Available from: http://dx.doi.org/10.1016/S0140-6736(02)43154-6. [DOI] [Google Scholar]
- 68.Kapff F. Über die Exstirpation des Kehlkopfes [Inaug Diss] Univ Tübingen; 1879. [Google Scholar]
- 69.Hochenegg J. Totale Kehlkopf-Exstirpation und Resection des Oesophagus wegen Carcinoma laryngis. Oesophagoplastik. Ein neuer Sprechapparat. Wien Klin Wschr. 1892;8:123–127. [Google Scholar]
- 70.Gottstein G. Pseudostimme nach Totalexstirpation des Larynx. Arch Klin Chir. 1900;62:126–146. [Google Scholar]
- 71.Sudeck P. Sprechkanüle nach totaler Kehlkopfexstirpation, 1-3 Sonderdruck aus. :Dtsch Z Chir 1910. [Google Scholar]
- 72.Lowry LD. Artificial Larynges: A review and development of a prototype self-contained intraoral artificial Larynx. Laryngoscope. 1981;91:1332–1335. doi: 10.1288/00005537-198108000-00016. [DOI] [PubMed] [Google Scholar]
- 73.Iglauer S. Artificial Larynx, with patient demonstrating its use. Ann Otol. 1936;45:1176– 1177. [Google Scholar]
- 74.Mason M. The Rehabilitation of Patients following surgical removal of the Larynx. J Laryngol Otol. 1950;64:759–770. doi: 10.1017/S0022215100012767. Available from: http://dx.doi.org/10.1017/S0022215100012767. [DOI] [PubMed] [Google Scholar]
- 75.Ticchioni R. US-Patentnr. No. 2868876. Jan 13, 1959. [Google Scholar]
- 76.Wendler J. 75 Jahre Phoniatrie. Festschrift zu Ehren von Herrmann Gutzmann. Wiss Z der Humboldt Univ Berlin. 1980 [Google Scholar]
- 77.Seidner W, Wendler J. Gedanken an den Begründer der Phoniatrie Herrmann Gutzmann. HNO aktuell. 2005;13:37–40. [Google Scholar]
- 78.Seeman M. 1919. Speech without a Larynx. J Laryngol Otol. 1925;40:789–792. [Google Scholar]
- 79.Seeman M. Rehabilitation of Laryngectomized Subjects. Acta Oto Laryngol. 1967;64:235–241. doi: 10.3109/00016486709139111. Available from: http://dx.doi.org/10.3109/00016486709139111. [DOI] [PubMed] [Google Scholar]
- 80.Guttman MR. Rehabilitation of the voice in laryngotomized patients. Arch Otolaryngol. 1932;15:478–479. [Google Scholar]
- 81.Conley JJ, De Amesti F, Pierce MK. A new surgical technique for the vocal rehabilitation of the laryngectomized patient. Ann Oto Rhinol Laryngol. 1958;67:655–664. doi: 10.1177/000348945806700306. [DOI] [PubMed] [Google Scholar]
- 82.Asai R. Laryngoplasty. J Jap Broncho Esophagol Soc. 1960;12:1–3. [Google Scholar]
- 83.Mozolewski E, et al. Studies on formation of Pseudoglottis after surgical rehabilitation of voice and speech. Otol Pol. 1972;26:663. [Google Scholar]
- 84.Staffieri M. Funktionelle totale Laryngektomie. Chirurgische Technik, Indikationen und Resultate einer eigenen Technik zur Glottisplastik mit Wiederherstellung der Stimme. Mschr Ohrenheilk Laryngorhinol. 1973;107:77–89. [PubMed] [Google Scholar]
- 85.Ehrenberger K, Wicke W, Piza H, Roka R, Gras IM, Swoboda H. Jejunal grafts for reconstructing a phonatory neoglottis in laryngectomized patients. Arch Otorhinolaryngol. 1985;242:217–223. doi: 10.1007/BF00454424. Available from: http://dx.doi.org/10.1007/BF00454424. [DOI] [PubMed] [Google Scholar]
- 86.Hagen R. Stimmrehabilitation nach totaler Laryngektomie in der Bundesrepublik Deutschland. Eine aktuelle Bestandsaufnahme. HNO. 1990;38:417–420. [PubMed] [Google Scholar]
- 87.Maier H, Weidauer H. Chirurgische Stimmrehabilitation nach Laryngektomie durch eine Modifikation des Verfahrens nach Asai. HNO. 1994;42:99–103. [PubMed] [Google Scholar]
- 88.Hagen R. Operative Verfahren zur Wiederherstellung des Sprechvermögens nach totaler Laryngektomie. Sprache Stimme Gehör. 1997;21:7–12. [Google Scholar]
- 89.Taptas N. Un cas de laryngectomie totale pour sarcome: Larynx artificiel externe. Annales des maladies de l`oreille, du larynx, du nez et du pharynx. 1900;26:37–45. [Google Scholar]
- 90.Edwards N. Post-laryngectomy vocal rehabilitation: Preliminary report of a one-stage method using expired air and a valve posthesis. J Laryngol Otol. 1974;88:905–918. doi: 10.1017/s0022215100079561. [DOI] [PubMed] [Google Scholar]
- 91.Mozolewski E. Surgical rehabilitation of voice and speech after laryngectomy. Otol Pol. 1972;26:654. [PubMed] [Google Scholar]
- 92.Blom ED. Evolution of tracheoesophageal voice prostheses. In: Blom ED, Singer MI, Hamaker RC, editors. Tracheoesophageal voice restoration following total laryngectomy. San Diego: Singular Publishing Group; 1998. pp. 1–8. [Google Scholar]
- 93.Nijdam HF, Anuyas AA, Schutte HK, Leever H. Arch Otorhinolaryngol. 1982;237:27–33. doi: 10.1007/BF00453713. Available from: http://dx.doi.org/10.1007/BF00453713. [DOI] [Google Scholar]
- 94.Medin Instrumenten B. V. Voice Prosthesis: The "Groningen" Prosthesis. The "Groningen" low resistance Prosthesis. Herstellerinformation. Groningeen/Niederlande: E. N. T. Instruments and supplies, Mein Instrumenten B. V.; o. J.. [Google Scholar]
- 95.Panje WR. Prostethic vocal rehabilitation following Laryngectomy. The voice button. Ann Otol Rhinol Laryngol. 1981;90:503–505. doi: 10.1177/000348948109000204. [DOI] [PubMed] [Google Scholar]
- 96.Herrmann JF, Koss W. Experience with ESKA-Herrmann tracheostoma valve. In: Herrmann JF, editor. Speech restoration via voice prosthesis. Berlin: Springer; 1986. pp. 185–186. [Google Scholar]
- 97.Henley-Cohn JL. "How I do it" - head and neck: a targeted problem and its solution. New technique for insertion of laryngeal prosthesis. Laryngoscope. 1981;91:1957–1959. doi: 10.1288/00005537-198111000-00020. Available from: http://dx.doi.org/10.1288/00005537-198111000-00020. [DOI] [PubMed] [Google Scholar]
- 98.Bonelli L, Aluffi E, Aversa SA. Shunt phonatoire avec deglutition normal. Riv Ital Otorinolarignol Audiol Foniatr. 1982;2:96–10. [Google Scholar]
- 99.van Weissenbruch R, Albers FWJ. Voice rehabilitation after total laryngectomy. Acta Oto Rhino Laryngol Belg. 1992;46:221–246. [PubMed] [Google Scholar]
- 100.Traissac L, Lecoq M, Petit J, Devars F, Ignatow I, Nizou JY, Benkirane M, Mourot F. The phoniatric results of our voice postheses. In: Herrmann JF, editor. Speech restoration via voice prosthesis. Berlin: Springer; 1986. pp. 37–42. [Google Scholar]
- 101.Hilgers FJM. Strömungsleichte Alternative aus Amsterdam: Die Provox-Stimmprothese. Med Rep. 1993:8.3. [Google Scholar]
- 102.Schultz-Coulon HJ. Die Stimmprothese. Sprache Stimme Gehör. 1997;21:13–18. [Google Scholar]
- 103.Hilgers FJM, Balm AJM, Gregor RT. Stimmrehabilitation nach Laryngektomie mit der Provox Stimmprothese: Chirurgische und technische Aspekte. HNO. 1995;43:197–201. [PubMed] [Google Scholar]
- 104.Karschay P. In Vitro Experiments Using Valve Prostheses. In: Herrmann JF, editor. Speech restoration via voice prosthesis. Berlin: Springer; 1986. pp. 63–68. [Google Scholar]
- 105.Leunisse C, van Weissenbruch R, Busscher HJ, van der Mei HC, Dijk F, Albers FWJ. Biofilm Formation and Design Features of Indwelling Silicone Rubber Tracheoesophageal Voice Prostheses - An Electron Microscopical Study. J Biomed Mater Res. 2011;58:556–563. doi: 10.1002/jbm.1054. Available from: http://dx.doi.org/10.1002/jbm.1054. [DOI] [PubMed] [Google Scholar]
- 106.Hilgers FJ, Ackerstaff AH, Balm AJ, van den Brekel MW, Bing Tanl, Persson JO. A new problem-solving indwelling voice prosthesis, eliminating the need for frequent Candida- and "underpressure" - related replacements: Provox Activalve. Acta Otolaryngol. 2003;123:972–979. doi: 10.1080/00016480310015371. Available from: http://dx.doi.org/10.1080/00016480310015371. [DOI] [PubMed] [Google Scholar]
- 107.Soolsma J, van den Brekel MW, Ackerstaff AH, Balm AJ, Tan B, Hilgers FJ. Long-term results of Provox Activalve, solving the problem of frequent candida- and "underpressure"related voice prosthesis replacements. Laryngoscope. 2008;118:252–257. doi: 10.1097/MLG.0b013e318159ebde. Available from: http://dx.doi.org/10.1097/MLG.0b013e318159ebde. [DOI] [PubMed] [Google Scholar]
- 108.Schäfer P, Klutzke N, Schwerdtfeger FP. Prothetische Stimmrehabilitation nach Laryngektomie. Laryngorhinootol. 2001;80:677–681. doi: 10.1055/s-2001-18276. [DOI] [PubMed] [Google Scholar]
- 109.Hummel J. Stimmprothesen in der sprachlichen und sozialen Rehabilitation nach Laryngektomie. Vergleich der Provox® mit der Provox 2® Stimmprothese [Inaug Diss] Univ Hamburg; 1999. [Google Scholar]
- 110.Hilgers FJM, Balm AJM. Long term results after total laryngectomy with low-resistence, indwelling Provox voice prosthesis system. Clin Otolaryngol. 1993;18:517–523. doi: 10.1111/j.1365-2273.1993.tb00627.x. Available from: http://dx.doi.org/10.1111/j.1365-2273.1993.tb00627.x. [DOI] [PubMed] [Google Scholar]
- 111.Hilgers FJM, Balm AJM, Gregor RT. Stimmrehabilitation nach Laryngektomie mit der Provox®-Stimmprothese. Chirurgische und technische Aspekte Teil 2. HNO. 1995;43:261–267. [PubMed] [Google Scholar]
- 112.Op de Coul BMR, Hilgers FJM, Balm AJM, Tan IB, van den Hoogen FJA, van Tinteren HA. Decade of Postlaryngectomy Vocal Rehabilitation in 318 Patients. Arch Otolaryngol Head Neck Surg. 2000;126:1320–1328. doi: 10.1001/archotol.126.11.1320. [DOI] [PubMed] [Google Scholar]
- 113.Mahieu HF, van Saene HKF, Rosingh HJ, Schutte HK. Candida Vegetations on Silicone Voice Prostheses. Arch Otolaryngol Head Neck Surg. 1986;112:321–325. doi: 10.1001/archotol.1986.03780030085017. [DOI] [PubMed] [Google Scholar]
- 114.Elving GJ, van der Mei HC, Busscher HJ, van Weissenbruch R, Albers FWJ. Air-Flow Resistances of Silicone Rubber Voice Prostheses after Formation of Bacterial and Fungal Biofilms. J Biomed Mater Res. 2001;58:421–426. doi: 10.1002/jbm.1037. Available from: http://dx.doi.org/10.1002/jbm.1037. [DOI] [PubMed] [Google Scholar]
- 115.Free RH, van der Mei HC, Elving GJ, van Weissenbruch R, Albers FWJ, Busscher HJ. Influence of the Provox Flush, Blowing and Imitated Coughing on Voice Prosthetic Biofilms In Vitro. Acta Otolaryngol. 2003;123:547–551. doi: 10.1080/0036554021000028118. Available from: http://dx.doi.org/10.1080/0036554021000028118. [DOI] [PubMed] [Google Scholar]
- 116.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the Normal Bacterial Flora of the Oral Cavity. J Clin Microbio. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. Available from: http://dx.doi.org/10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bauters TGM, Moerman M, Pini G, Vermeersch H, Nelis HJ. Colonization of a voice prosthesis by Cyptococcus neoformans. Med Mycol. 2001;39:379–381. doi: 10.1080/mmy.39.4.379.381. [DOI] [PubMed] [Google Scholar]
- 118.Elving GJ, van der Mei HC, Busscher HJ, van Weissenbruch R, Albers FWJ. Influence of different combinations of bacteria and yeasts in voice prosthesis biofilms on air flow resistance. Antonie van Leeuwenhoek. 2003;83:45–56. doi: 10.1023/A:1022952712257. Available from: http://dx.doi.org/10.1023/A:1022952712257. [DOI] [PubMed] [Google Scholar]
- 119.Ramage G, Vande Walle K, Wickes BL, López-Ribot JL. Characteristics of biofilm formation by Candida albicans. Rev Iberoam Micol. 2001;18:163–170. [PubMed] [Google Scholar]
- 120.Elving G, van der Mei HC, Busscher HJ, van Weissenbruch R, Albers FWJ. Comparison of the microbial composition of voice prosthesis biofilms from patients requiring frequent versus infrequent replacement. Ann Otol Rhinol Laryngol. 2002;111:200–203. doi: 10.1177/000348940211100302. [DOI] [PubMed] [Google Scholar]
- 121.Hagen R. Zur Stimmrehabilitation nach totaler Laryngektomie [Habil Univ] Würzburg: 1991. [Google Scholar]
- 122.Weinberg B. Readings in speech following total laryngectomy. Baltimore: Univ Park Press; 1980. [Google Scholar]
- 123.Böhme G. Sprach-, Sprech-, Stimm-und Schluckstörungen. Band 1: Klinik. 4. Auflage. München, Jena: Urban & Fischer; 2003. Stimmstörungen; pp. 153–285. [Google Scholar]
- 124.Pahn J. Musikerziehung und Phoniatrie. Musik in der Schule. Teil 1 und 2. 1976. [Google Scholar]
- 125.Sopk J, Wey W, Faust H. Zur Klinik der Ösophagusersatzstimme. HNO. 1977;25:433–435. [PubMed] [Google Scholar]
- 126.Kischk BT, Gross M. Vergleichende Beurteilung der Ersatzstimme nach Laryngektomie. HNO. 1995;43:304–310. [PubMed] [Google Scholar]
- 127.Robbins J, Fisher HB, Blom EC, Singer MI. A comparative acoustic study of normal, esophageal and tracheoesophageal speech production. J Speech Hear Disord. 1984;49(2):202–210. doi: 10.1044/jshd.4902.202. [DOI] [PubMed] [Google Scholar]
- 128.Böhm F. Die Stimmrehabilitation nach totaler Laryngektomie bei Kehlkopfkarzinomen mit besonderer Berücksichtigung von Stimmprothesen im Zeitraum von 1986 bis 1997 [Inaug Diss] Univ Rostock; 2000. [Google Scholar]
- 129.Dommerich S, Kramp B, Böhm F. Funktionelle Ergebnisse von Stimmprothesen und Ösophagusersatzstimme bei laryngektomierten Patienten. In: Gross M, Kruse E, editors. Aktuelle phoniatrisch-pädaudiologische Aspekte 2003/2004. Band 11. Videel; 2003. pp. 178–182. Available from: http://www.egms.de/en/meetings/dgpp2003/03dgpp001.shtml. [Google Scholar]
- 130.Hagen R, Berning K, Korn M, Schön F. Stimmprothesen mit tonerzeugendem Metallzungen-Element – experimentelle und erste klinische Ergebnisse. Laryngo Rhino Otol. 1998;77:312–321. doi: 10.1055/s-2007-996980. Available from: http://dx.doi.org/10.1055/s-2007-996980. [DOI] [PubMed] [Google Scholar]
- 131.Pindzola RH, Cain BH. Duration and frequency characteristics of tracheoesophageal speech. Ann Otol Rhinol Laryngol. 1989;98:960–964. doi: 10.1177/000348948909801208. [DOI] [PubMed] [Google Scholar]
- 132.Max L, Steurs W, de Bruyn W. Vocal capacities in esophageal and tracheoesophageal speakers. Laryngoscope. 1996;106:93–96. doi: 10.1097/00005537-199601000-00018. Available from: http://dx.doi.org/10.1097/00005537-199601000-00018. [DOI] [PubMed] [Google Scholar]
- 133.Daniilidis I, Nikolaou A, Markou C, Kotsani A. Stimmrehabilitation nach totaler Laryngektomie. Stimmprothese oder Ösophagus-Ersatzstimme? Laryngorhinootologie. 1998;77:89–92. doi: 10.1055/s-2007-996939. Available from: http://dx.doi.org/10.1055/s-2007-996939. [DOI] [PubMed] [Google Scholar]
- 134.de Maddalena H, Pfrang H, Schohe R, Zenner HP. Sprachverständlichkeit und psychosoziale Anpassung bei verschiedenen Stimmrehabilitationsmethoden nach Laryngektomie. Laryngol Rhinol Otol. 1991;70:562–567. doi: 10.1055/s-2007-998098. Available from: http://dx.doi.org/10.1055/s-2007-998098. [DOI] [PubMed] [Google Scholar]
- 135.Ackerstaff AH, Hilgers FJM, Aaronxon NK, Balm AJM. Communication, functional disorders and lifestyle changes after total laryngectomy. Clin Otolaryngol. 1994;19:295–300. doi: 10.1111/j.1365-2273.1994.tb01234.x. Available from: http://dx.doi.org/10.1111/j.1365-2273.1994.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 136.Zenner HP, Pfrang H. Ein einfacher Sprachverständlichkeitstest zur Beurteilung der Stimmrehabilitation des Laryngektomierten. Laryngo Rhino Otol. 1986;65:271–276. doi: 10.1055/s-2007-1007966. Available from: http://dx.doi.org/10.1055/s-2007-1007966. [DOI] [PubMed] [Google Scholar]
- 137.Kramp B, Böhm F, Fischer AL. Speech rehabilitation using a voice prostheses following laryngectomy. Otolaryngol Pol. 2000;54:697–701. [PubMed] [Google Scholar]
- 138.van der Torn M, van Gogh CDL, Verdonck-de Leeuw IM, Festen JM, Verkerke GJ, Mahieu HF. Assessment of a laryngeal speech using a sound-producing voice prosthesis in relation to sex and pharyngoesophageal segment tonicity. Head Neck. 2006;28:400–412. doi: 10.1002/hed.20355. Available from: http://dx.doi.org/10.1002/hed.20355. [DOI] [PubMed] [Google Scholar]
- 139.Tack JW, Verkerke GJ, van der Houwen EB, Mahieu HF, Schutte HK. Development of a double-membrane sound generator for application in a voice-producing element for laryngetomized patients. Ann Biomed Engineer. 2006;34:1896–1907. doi: 10.1007/s10439-006-9196-3. Available from: http://dx.doi.org/10.1007/s10439-006-9196-3. [DOI] [PubMed] [Google Scholar]
- 140.Tack JW, Qiu Q, Schutte HK, Koolijman PGC, Meeuwis CA, van der Houwen EB, Mahieu HF, Verkerke GJ. Clinical evaluation of a membrane-based voice-producing element for laryngectomized woman. Head Neck. 2008;30:1156–1166. doi: 10.1002/hed.20853. Available from: http://dx.doi.org/10.1002/hed.20853. [DOI] [PubMed] [Google Scholar]
- 141.Lorenz KJ, Groll K, Ackerstaff AH, Hilgers FJM, Maier H. Hands-free speech after surgical voice rehabilitation with a Provox® voice prosthesis; experience with the Provox FreeHands HME Tracheostoma valve®system. Eur Arch ORL. 2007;264:151–157. doi: 10.1007/s00405-006-0155-2. Available from: http://dx.doi.org/10.1007/s00405-006-0155-2. [DOI] [PubMed] [Google Scholar]
- 142.Hoogen van den FJ, Meeuwis C, Oudes MJ, Janssen P, Manni JJ. The Blom-Singer tracheostoma valve as a valuable addition in the rehabilitation of the laryngectomized patient. Eur Arch Otorhinolaryngol. 1996;253:126–129. doi: 10.1007/BF00615108. [DOI] [PubMed] [Google Scholar]
- 143.Grolman W, Schouwenburg PF, de Boer MF, Knegt PP, Spoelstra HA, Meeuwis CA. First results with the Blom-Singer adjustable tracheostoma valve. J Otorhinolaryngol Relat Spec. 1995;57:165–170. doi: 10.1159/000276732. Available from: http://dx.doi.org/10.1159/000276732. [DOI] [PubMed] [Google Scholar]
- 144.Terada T, Saeki N, Toh K, Uwa N, Sagawa K, Takayasu S, Sakagami M. Voice rehabilitation with Provox 2 voice prosthesis following total laryngectomy for laryngeal and hypopharyngeal carcinoma. Auris Nas Lar. 2007;34:65–71. doi: 10.1016/j.anl.2006.09.017. Available from: http://dx.doi.org/10.1016/j.anl.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 145.Jassar P, England RJA, Stafford ND. Restoration of voice after laryngectomy. J R Soc Med. 1999;92:299–302. doi: 10.1177/014107689909200608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Malik T, Bruce I, Cherry J. Surgical complications of tracheo-oesophageal puncture and speech valves. Curr Opin Otol Haed Neck Surg. 2007;15:117–122. doi: 10.1097/MOO.0b013e3280964dc8. Available from: http://dx.doi.org/10.1097/MOO.0b013e3280964dc8. [DOI] [PubMed] [Google Scholar]
- 147.Natarajan B, Richardson MD, Irvine BW, Thomas M. The Provox voice prosthesis and Candida albicans growth: a preliminary report of clinical, mycological and scanning electron microscopic assessment. J Laryngol Otol. 1994;108:666–668. doi: 10.1017/S002221510012777X. Available from: http://dx.doi.org/10.1017/S002221510012777X. [DOI] [PubMed] [Google Scholar]
- 148.Issing WJ, Fuchshuber S, Wehner M. Indence of tracheo-oesophageal fistulas after primary voice rehabilitation with the Provox or the Eska-Herrmann voice prosthesis. Eur Arch Otorhinolaryngol. 2001;258:240–242. doi: 10.1007/s004050100352. Available from: http://dx.doi.org/10.1007/s004050100352. [DOI] [PubMed] [Google Scholar]
- 149.Kress P, Schäfer P, Schwerdtfeger FP. The custom-fit voice prosthesis, for treatment of periprothetic leakage after tracheoesophageal voice restoration. J Laryngol Otol. 2006;85:496–500. doi: 10.1055/s-2006-925081. [DOI] [PubMed] [Google Scholar]
- 150.Hilgers FJ, Soolsma J, Ackerstaff AH, Balm FJ, Tan IB, van den Brekel MW. A thin tracheal silicone washer to solve periprosthetic leakage in laryngectomies: direct results and long-term clinical effects. Laryngoscope. 2008;118:640–645. doi: 10.1097/MLG.0b013e31816067d5. Available from: http://dx.doi.org/10.1097/MLG.0b013e31816067d5. [DOI] [PubMed] [Google Scholar]
- 151.Brasnu D, Pages JC, Laccourreye O, Jouffre V, Monfrais Pfauwadel MC, Crevier Buchman L. Results of the treatment of spontaneous widening of tracheo-esophageal punctures after laryngeal implant. Ann Otolaryngol Chir Cervofac. 1994;111:456–460. [PubMed] [Google Scholar]
- 152.Hoffmann TK, Sommer P, Bier H. Collagen injection for augmentation of periprosthetic leakage after tracheo-esophageal voice restoration. J Laryngol Otol. 2006;85:556–558. doi: 10.1055/s-2006-949540. [DOI] [PubMed] [Google Scholar]
- 153.Luff DA, Izzat S, Farrington WT. Visoaugmentation as a treatment for leakage around the Provox 2 voice rehabilitation system. J Laryngol Otol. 1999;113:847–848. doi: 10.1017/S0022215100145372. Available from: http://dx.doi.org/10.1017/S0022215100145372. [DOI] [PubMed] [Google Scholar]
- 154.Komatsubara S, Hanamure Y, Suda Y, Haruta A, Kasano F, Kashima N. Fat injection to treat widening of a tracheoesophageal fistula with a voice prosthesis. Nippon Jibiinkoka Gakkai Kaiho. 2008;111:412–415. doi: 10.3950/jibiinkoka.111.412. Available from: http://dx.doi.org/10.3950/jibiinkoka.111.412. [DOI] [PubMed] [Google Scholar]
- 155.Lorincz BB, Lichtenberger G, Bihari A, Falvai J. Therapy of periprosthetical leakage with tissue augmentation using Bioplastique around the implanted voice prosthesis. Eur Arch Otorhinolaryngol. 2005;262:32–34. doi: 10.1007/s00405-004-0747-7. Available from: http://dx.doi.org/10.1007/s00405-004-0747-7. [DOI] [PubMed] [Google Scholar]
- 156.Rokade AV, Mathews J, Reddy KT. Tissue augmentation using Bioplastique as a treatment of leakage around a Provox 2 voice prosthesis. J Laryngol Otol. 2003;117:80–82. doi: 10.1258/002221503321046739. Available from: http://dx.doi.org/10.1258/002221503321046739. [DOI] [PubMed] [Google Scholar]
- 157.Jacobs K, Delaere PR, Vander Poorten VL. Submucosal purse-string suture as a treatment of leakage around the indwelling voice prosthesis. Head Neck. 2008;30:485–491. doi: 10.1002/hed.20732. Available from: http://dx.doi.org/10.1002/hed.20732. [DOI] [PubMed] [Google Scholar]
- 158.Moerman M, Vermeersch H, Heylbroeck P. A simple surgical technique for tracheoesophageal fistula closure. Eur Arch Otorhinolaryngol. 2004;261:381–385. doi: 10.1007/s00405-003-0693-9. Available from: http://dx.doi.org/10.1007/s00405-003-0693-9. [DOI] [PubMed] [Google Scholar]
- 159.Judd O, Bridger M. Failed voice restoration: closure of the tracheo-oesophageal fistula. Clin Otolaryngol. 2008;33:261–264. doi: 10.1111/j.1749-4486.2008.01689.x. Available from: http://dx.doi.org/10.1111/j.1749-4486.2008.01689.x. [DOI] [PubMed] [Google Scholar]
- 160.Blitzer A, Komisar A, Aredes S, Brin MF, Stewart C. Voice failure after tracheoesophageal puncture: managment with botulinum toxin. Otolaryngol Head Neck Surg. 1995,:668–670. doi: 10.1016/S0194-59989570002-1. [DOI] [PubMed] [Google Scholar]
- 161.Meleca RJ, Dworkin JP, Zormeier MM, Simpson ML, Shibuya T, Mathog RH. Videostroboscopy of the pharygoesophageal segment in laryngectomy patients treated with botulinum toxin. Otolaryngol Head Neck Surg. 2000,:38–43. doi: 10.1067/mhn.2000.106400. Available from: http://dx.doi.org/10.1067/mhn.2000.106400. [DOI] [PubMed] [Google Scholar]
- 162.Hamaker RC, Blom ED. Botulinum neurotoxin for pharyngeal constrictor muscle spasm in tracheoesophageal voice restoration. Laryngoscope. 2003;113:1479–1482. doi: 10.1097/00005537-200309000-00010. Available from: http://dx.doi.org/10.1097/00005537-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 163.Ramachandran K, Arunachalam PS. Hurren A, Marsh RL, Samuel PR. Botulinum toxin injection for failed tracheo-oesophageal voice in laryngectomees: the Sunderland experience. J Laryngol Otol. 2003,:544–548. doi: 10.1258/002221503322112978. [DOI] [PubMed] [Google Scholar]
- 164.Mullan GP, Lee MT, Clarke PM. Botulinum neurotoxin for management of intractable central leakage through a voice prosthesis in surgical voice restoration. J Laryngol Otol. 2006;120:789–792. doi: 10.1017/S0022215106001575. Available from: http://dx.doi.org/10.1017/S0022215106001575. [DOI] [PubMed] [Google Scholar]
- 165.Chone CT, Teixeira C, Andreollo NA, Spina AL, Barcelllos IH, Quagliato E, Crespo AN. Botulinum toxin in speech rehabilitation with voice prosthesis after total laryngectomy. Braz J Otol. 2008;74:230–234. doi: 10.1016/S1808-8694(15)31093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Móricz P, Gerlinger I, Solt J, Somogyvári K, Pytel J. Voice prosthesis insertion after endoscopic balloon-catheter dilatation in case of a stenotic hypopharyngo-oesophageal junction. Eur Arch Otol. 2007;264:1441–1445. doi: 10.1007/s00405-007-0406-x. Available from: http://dx.doi.org/10.1007/s00405-007-0406-x. [DOI] [PubMed] [Google Scholar]
- 167.Delank KW, Scheuermann K. Practical aspects of voice prosthesis use after laryngectomy. Laryngo Otol. 2008;87:160–166. doi: 10.1055/s-2007-995370. Available from: http://dx.doi.org/10.1055/s-2007-995370. [DOI] [PubMed] [Google Scholar]
- 168.Leder SB, Acton LM, Kmiecik J, Ganz C, Blom ED. Voice restoration with the advantage tracheoesophageal voice prosthesis. Otol Head Neck Surg. 2005;133:681–684. doi: 10.1016/j.otohns.2005.08.009. Available from: http://dx.doi.org/10.1016/j.otohns.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 169.Kress P, Schäfer P, Schwerdtfeger FP. Clinical use of a voice prosthesis with a flap valve containing silver oxide (Blom-Singer Advantage), biofilm formation, in-situ lifetime and indication. Laryngo Otol. 2006;85:893–896. doi: 10.1055/s-2006-925292. Available from: http://dx.doi.org/10.1055/s-2006-925292. [DOI] [PubMed] [Google Scholar]
- 170.Gerwin JM, Culton GL. Quality of life in prosthetic voice users. Otol Head Neck Surg. 2005;133:685–688. doi: 10.1016/j.otohns.2005.06.028. Available from: http://dx.doi.org/10.1016/j.otohns.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 171.Fagan MJ, Ell SR, Gilbert JM, Sarrazin E, Chapman PM. Development of a (silent) speech recognition system for patients following laryngectomy. Med Eng Phys. 2008;30:419–425. doi: 10.1016/j.medengphy.2007.05.003. Available from: http://dx.doi.org/10.1016/j.medengphy.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 172.Bruns V. Vortrag über eine von ihm ausgeführte Totalexstirpation des Kehlkopfes mit nachfolgendem Einsatz eines künstlichen Kehlkopfes. Württemberg Med Correspondenzblatt. 1878 [Google Scholar]
- 173.Herrmann IF, Koss W. Fingerfreies Sprechen nach totaler Laryngektomie: Instrumente und Techniken der chirurgischen Stimmrehabilitation. HNO. 1985;33:124–129. [PubMed] [Google Scholar]