Abstract
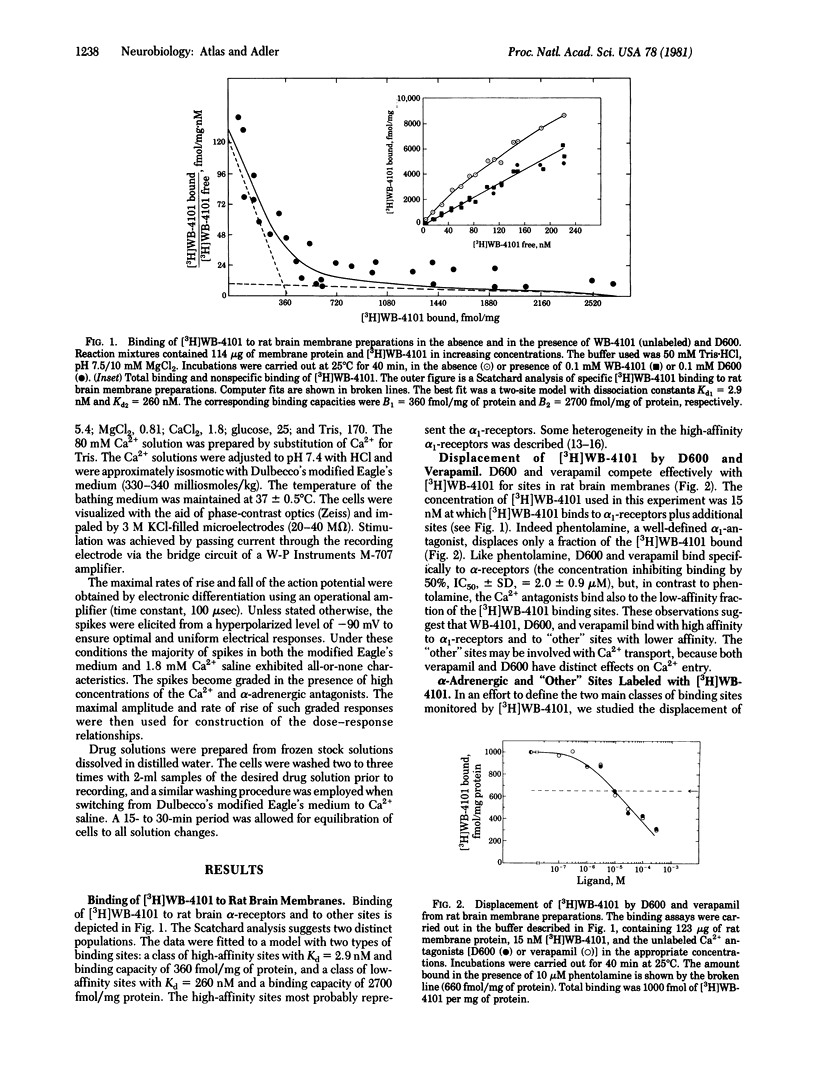
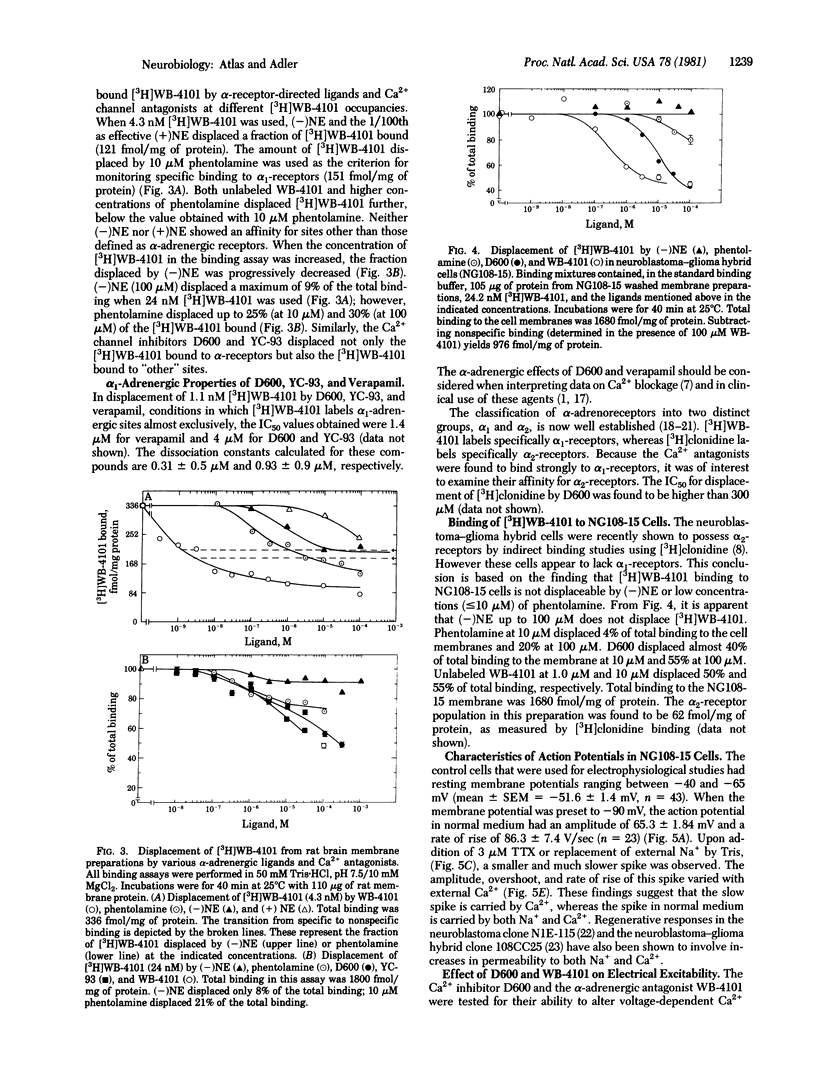
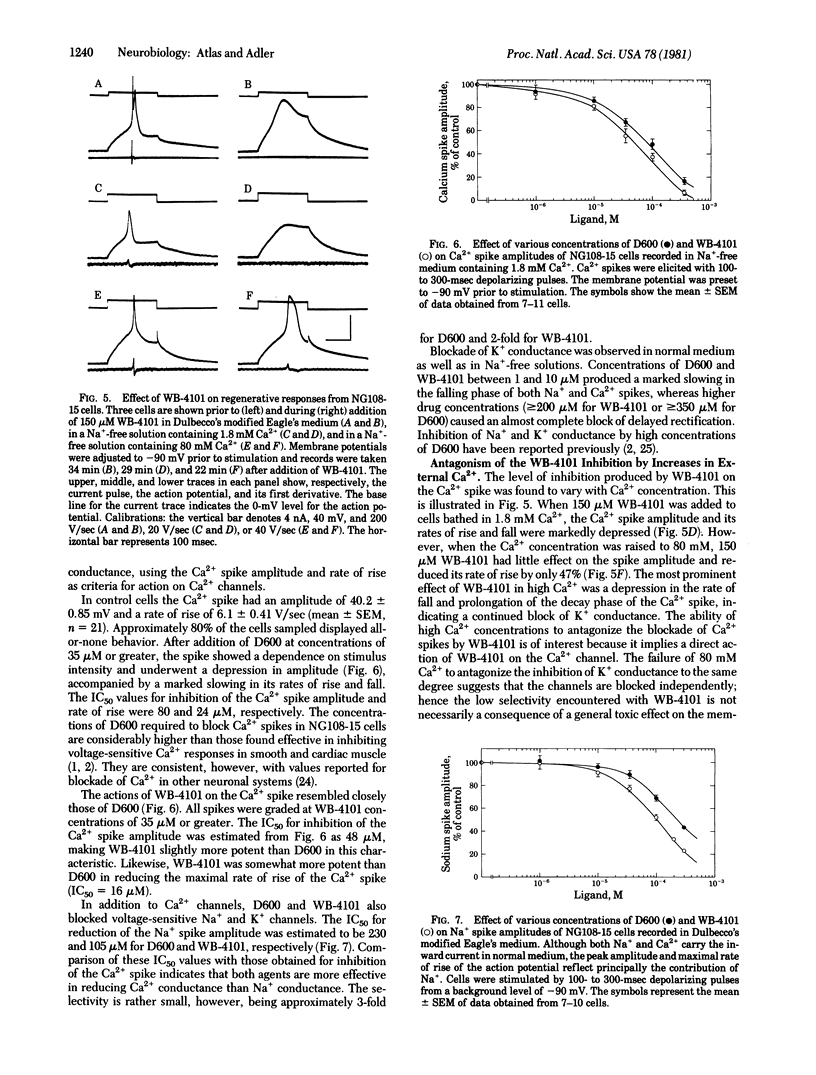
The effects of various organic Ca2+ channel inhibitors were investigated on the binding of the alpha 1-antagonist 3H-labeled 2-[(2',6'-dimethoxyphenoxyethyl)aminomethyl]-1,4-benzodioxane ([3H]WB-4101) to membranes from rat brain and neuroblastoma-glioma hybrid cells (NG108-15). As found by monitoring binding of [3H]WB-41-1, the Ca2+ channel inhibitors methoxyverapamil (D600), verapamil, and the nifedipine analogue YC-93 bind to two different sites in rat brain: a high-affinity site (dissociation constant Kd = 2.9 nM and binding capacity B = 360 fmol/mg of protein) and a low-affinity site (Kd = 260 nM and B = 2700 fmol/mg of protein). In NG108-15 cells, where no alpha 1 receptors were detected with [3H]WB-4101, the Ca2+ antagonists were found to bind to nonadrenergic sites in the membrane with a capacity B = 976 fmol/mg of protein. The binding of Ca2+ antagonists to [3H]WB-41-1 sites led to the investigation of WB-4101 as a Ca2+ inhibitor by electrophysiological techniques. WB-4101 depressed the amplitude and reduced the rate of rise of the CA2+ spike with an affinity slightly greater than that observed for D600. The concentration for 50% inhibition of the Ca2+ spike amplitude was 48 microM for WB-4101 and 80 microM for D600. The WB-4101-induced blockade of the Ca2+ spike was antagonized by high Ca2+ concentrations, indicating a common site for Ca2+ and the alpha-antagonist. D600 and WB-4101 also inhibited voltage-dependent Na+ and K+ conductances. The results suggest that Ca2+ channels can account for a fraction of the sites labeled with [3H]WB-4101 in membrane preparations from brain and NG108-15 cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew W. S., Moore A. C., Levinson S. R., Raftery M. A. Identification of a large molecular weight peptide associated with a tetrodotoxin binding protein from the electroplax of Electrophorus electricus. Biochem Biophys Res Commun. 1980 Feb 12;92(3):860–866. doi: 10.1016/0006-291x(80)90782-2. [DOI] [PubMed] [Google Scholar]
- Beneski D. A., Catterall W. A. Covalent labeling of protein components of the sodium channel with a photoactivable derivative of scorpion toxin. Proc Natl Acad Sci U S A. 1980 Jan;77(1):639–643. doi: 10.1073/pnas.77.1.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelsen S., Pettinger W. A. A functional basis for classification of alpha-adrenergic receptors. Life Sci. 1977 Sep 1;21(5):595–606. doi: 10.1016/0024-3205(77)90066-2. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Goldman D. E. Competitive action of calcium and procaine on lobster axon. A study of the mechanism of action of certain local anesthetics. J Gen Physiol. 1966 May;49(5):1043–1063. doi: 10.1085/jgp.49.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhurst A. S., Whittaker M. L., Ehlert F. J. Interactions of D600 (methoxyverapamil) and local anesthetics with rat brain alpha-adrenergic and muscarinic receptors. Biochem Pharmacol. 1980 Feb;29(2):155–162. doi: 10.1016/0006-2952(80)90323-8. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Galper J. B., Catterall W. A. Inhibition of sodium channels by D600. Mol Pharmacol. 1979 Jan;15(1):174–178. [PubMed] [Google Scholar]
- Guicheney P., Garay R. P., Levy-Marchal C., Meyer P. Biochemical evidence for presynaptic and postsynaptic alpha-adrenoceptors in rat heart membranes: positive homotropic cooperativity of presynaptic binding. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6285–6289. doi: 10.1073/pnas.75.12.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S. Ca spike. Adv Biophys. 1973;4:71–102. [PubMed] [Google Scholar]
- Hoffman B. B., Lefkowitz R. J. An assay for alpha-adrenergic receptor subtypes using [3H]dihydroergocryptine. Biochem Pharmacol. 1980 Feb;29(3):452–454. doi: 10.1016/0006-2952(80)90528-6. [DOI] [PubMed] [Google Scholar]
- Kapur H., Mottram D. R. A comparative study on the pre- and post-synaptic alpha blocking activity of a series of benzodioxanes. Biochem Pharmacol. 1978;27(14):1879–1880. doi: 10.1016/0006-2952(78)90036-9. [DOI] [PubMed] [Google Scholar]
- Kapur H., Rouot B., Snyder S. H. Binding to alpha-adrenergic receptors: differential pharmacological potencies and binding affinities of benzodioxanes. Eur J Pharmacol. 1979 Aug 15;57(4):317–328. doi: 10.1016/0014-2999(79)90494-1. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Multiple effects of calcium antagonists on plateau currents in cardiac Purkinje fibers. J Gen Physiol. 1975 Aug;66(2):169–192. doi: 10.1085/jgp.66.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of catecholamine release. Biochem Pharmacol. 1974 Jul 1;23(13):1793–1800. doi: 10.1016/0006-2952(74)90187-7. [DOI] [PubMed] [Google Scholar]
- McGee R., Jr, Schneider J. E. Inhibition of high affinity synaptosomal uptake systems by verapamil. Mol Pharmacol. 1979 Nov;16(3):877–885. [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. The calcium action potential and a prolonged calcium dependent after-hyperpolarization in mouse neuroblastoma cells. J Physiol. 1979 Jul;292:297–306. doi: 10.1113/jphysiol.1979.sp012851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachshen D. A., Blaustein M. P. The effects of some organic "calcium antagonists" on calcium influx in presynaptic nerve terminals. Mol Pharmacol. 1979 Sep;16(2):576–586. [PubMed] [Google Scholar]
- Peroutka S. J., Greenberg D. A., U'Prichard D. C., Snyder S. H. Regional variations in alpha adrenergic receptor interactions of [3H]-dihydroergokryptine in calf brain: implications for a two-site model of alpha receptor function. Mol Pharmacol. 1978 May;14(3):403–412. [PubMed] [Google Scholar]
- Ray R., Morrow C. S., Catterall W. A. Binding of scorpion toxin to receptor sites associated with voltage-sensitive sodium channels in synaptic nerve ending particles. J Biol Chem. 1978 Oct 25;253(20):7307–7313. [PubMed] [Google Scholar]
- Rehavi M., Yavetz B., Ramot O., Sokolovsky M. Regional heterogeneity of two high affinity binding sites for 3H-WB-4101 in mouse brain. Life Sci. 1980 Feb 25;26(8):615–621. doi: 10.1016/0024-3205(80)90237-4. [DOI] [PubMed] [Google Scholar]
- Reiser G., Heumann R., Kemper W., Lautenschlager E., Hamprecht B. Influence of cations on the electrical activity of neuroblastoma X glioma hybrid cells. Brain Res. 1977 Jul 22;130(3):495–504. doi: 10.1016/0006-8993(77)90111-1. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B., Strichartz G. R. A new method for labelling saxitoxin and its binding to non-myelinated fibres of the rabbit vagus, lobster walking leg, and garfish olfactory nerves. J Physiol. 1976 Oct;261(2):477–494. doi: 10.1113/jphysiol.1976.sp011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol S. L., Nirenberg M. Regulation of adenylate cyclase of neuroblastoma x glioma hybrid cells by alpha-adrenergic receptors. I. Inhibition of adenylate cyclase mediated by alpha receptors. J Biol Chem. 1979 Mar 25;254(6):1913–1920. [PubMed] [Google Scholar]



