Abstract
In terms of pathophysiology, an anatomically narrow airway is a predisposing factor for obstruction of the upper respiratory tract. The correlation between the nasopharyngeal airway and the craniofacial structures is discussed in this context. Thus a mutual interaction between the pharynx and the mandibular position was demonstrated, whereby the transverse dimension of the nasopharynx was significantly larger in patients with prognathism than in patients with retrognathism. The influence of chronic obstruction of the nasal airway on craniofacial development was also discussed. The form-and-function interaction, which ought to explain the causal relationship between nasal obstruction and craniofacial growth, appears to be of a multifactorial rather than a one-dimensional, linear nature. It is not disputed, however, that expanding the maxilla improves not only nasal volume and nasal flow, but also the subjective sensation of patients, although it is not possible to make a prognostic statement about the extent of this improvement because of the differing reactions of individuals. Orthodontic appliances for advancing the mandible can also be successfully used in the treatment of mild obstructive sleep apnea syndrome. This treatment method should be considered particularly for patients who are unwilling to undergo or cannot tolerate CPAP (continuous positive airway pressure) treatment.
Keywords: craniofacial development, nasopharyngeal airway; adenoids, rapid maxillary expansion (RME); obstructive sleep apnea syndrome (OSAS); mandibular advancement device (MAD)
1 Introduction
As well as hypertrophic adenoids and tonsils, chronic and allergic rhinitis, environmental irritants, infections, congenital nasal deformities, nasal trauma, polyps and tumors, another predisposing factor for obstruction of the upper respiratory tracts can be an excessively narrow airway. In this respect, reference is made in the literature to the importance of jaw malpositions and jaw anomalies, to changes in airway morphology and respiratory problems [1], [2]. In the opposite direction, the influence of an obstruction of the airways on development of the stomatognathic system was postulated and the theory of the functional matrix was put forward, which describes the influence of the surrounding structures on dentofacial development [3].
2 Correlation between nasopharyngeal cavity and craniofacial structures
Owing to the close relationship between the pharynx and the dentofacial structures, a mutual interaction has long been assumed and studies on the subject have been performed [4], [5]. However, the results of these studies were based on two-dimensional measurements of the airway. In a study by Alves et al. [6] published in 2008, the upper airway in patients with retrognathism (syn. distoclusion, mandibular retraction) or prognathism (syn. mesioclusion, mandibular protrusion) with physiological breathing was measured three-dimensionally for the first time with computed tomograms. The results showed that the majority of measurements of the airway were not influenced by the type of malocclusion. However, there were significant differences between patients with retrognathism and prognathism in respect of the transverse dimension of the nasopharynx. This was significantly decreased in patients with distoclusion. Furthermore, there were gender-specific differences. For instance, the retroglossal width and height of the posterior nasal cavity were larger in male patients with distoclusion than in the females, while the retropalatal and retroglossal volumes were more extensive in male patients with mesioclusion. The results of this study, however, did not rule out the relevance of an airway obstruction on facial growth and vice versa. While Freitas et al. [7] in their 2D study found no correlation between obstructions of the upper airway and the frequency of malocclusions, Jospeh et al. [8] assumed that skeletal factors, such as a retrognathic maxilla, can lead to narrowing of the anteroposterior dimensions of the airway. Furthermore the study showed that hyperdivergent (syn. vertical, dolichofacial) growth of the facial cranium or excessively vertical growth of the maxilla can result in narrowing of the anteroposterior dimensions of the airway. These measurements were not considered in the above-mentioned 3D study.
An important role in securing the pharyngeal airway is also attributed to the hyoid and its musculature. Various studies have demonstrated that changes in the hyoid position can result in changes to the mandibular position. For instance Battagel et al. [9] reported that in patients with mandibular retrognathism, there was a posterior position of the hyoid associated with narrowing of the upper airway. Allhaija and Al-Khateeb [10] also found a significant correlation between jaw relation, hyoid position and width of the pharyngeal cavity. Studies on the influence of surgical advancement or setback of the mandible on the hyoid position and the pharyngeal airway showed that mandibular advancement resulted in forward displacement of the hyoid with widening of the minimum pharyngeal airway [11], whereas the opposite was true in the case of surgical mandibular setback [12].
3 Correlation between breathing and craniofacial structures
It is a general assumption that nasorespiratory function has an effect on dentofacial development. In particular, it has been assumed that chronic obstruction of the nasal airway causes mouth breathing, which in turn influences the tongue position and the position of the mandible. If this happens during growth it results in the development of what has been called “adenoid facies” [13].
The term “adenoid facies” dates back to Linder-Aronson (1970) [14] and describes the sequelae of hypertrophic adenoids for the face and its growth (Figure 1a (Fig. 1)). This complex includes the following characteristic symptoms: open-mouth posture, mouth breathing, hypotonia, narrow pharyngeal airway, narrow base in the area of the ala of the nose, short nasal floor, increased anterior and particularly increased lower facial height, steep angle of the mandible, narrow maxilla, high palate, anterior tongue position, frequently mandibular retrognathism and retroclined mandibular incisors. This development was explained by changes in muscular balance. The mouth breathing results in a low tongue position and an imbalance between the forces from the cheeks and tongue compared with healthy children. This furthermore causes a low mandibular position and extended head posture with the above-mentioned dental and skeletal sequelae [13], [14], [15]. Similar craniofacial consequences were also found in the case of tonsillar hypertrophy and in patients with “long face syndrome” [16] (Figure 1b (Fig. 1)). This term describes the excessively enlarged vertical dimension of the face, which is caused either by a greatly enlarged angle of the mandible or by posterior maxillary dentoalveolar excess [17], [18] (Figure 2a–b (Fig. 2)). During a critical review by O’Ryan et al. [19], however, no definite correlation could be demonstrated between obstructive nasorespiratory function and the development of adenoid facies or long face syndrome. In the study by Fields et al. [20], patients with long face syndrome had significantly less nasal air flow than the normal control group, but their respiratory volume and smallest nasal cross-sectional area were similar. Thus the form-and-function interaction, which ought to explain the causal relationship between nasal obstruction and craniofacial growth, appears to be of a multifactorial nature [21].
Figure 1. a) Patient with “adenoid facies” (open lip posture, mouth breathing, hypotonia), b) Patient with “long face syndrome” (excessively increased vertical dimension owing to greatly enlarged angle of the mandible).
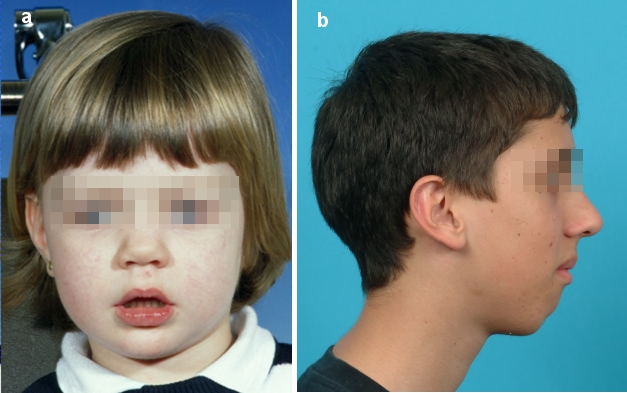
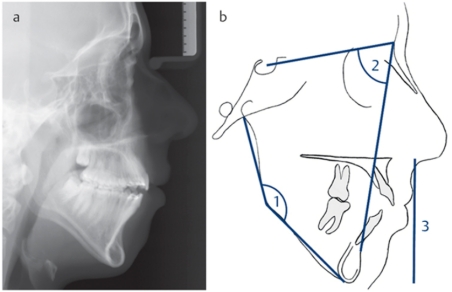
Figure 2. Patient with “long face syndrome”. a) lateral cephalogram, b) cephalometric variations: 1) increased angle of the mandible, 2) retrognathic mandible, 3) increased lower facial height.
In the study by Oulis et al. [22], the incidence of a lateral crossbite and oral habits was investigated in 120 children with hypertrophic adenoids with or without enlarged tonsils. The results showed that 47% of the children had a lateral crossbite. The crossbite was particularly pronounced in children with severe airway obstruction, especially children with simultaneous hypertrophy of adenoids and tonsils. As most of the children with a crossbite had no history of thumb or finger sucking, the obstruction of the upper airway was held responsible for the genesis of the narrow maxilla. In a recently published study by Souki et al. [23], the prevalence of tooth and jaw malpositions was investigated in 401 children with mouth breathing. The children were aged 2–12 years. An adenoidal and/or tonsillar obstruction was found in 71.8% of the children, allergic rhinitis in 18%. There was non-obstructive mouth breathing in 9.5%. A posterior crossbite was diagnosed in nearly 30% of the children in their deciduous or mixed dentition and 48% in the permanent dentition. Thus the prevalence was increased in comparison with the overall population.
The extent to which chronic mouth breathing also influences the position and growth direction of the mandible in children with adenoid hypertrophy or adenoidal and tonsillar hypertrophy was investigated by Sousa et al. [24]. The results of this study revealed no significant correlation, so that the influence of mouth breathing on mandibular growth could not be confirmed.
3.1 Effects of adenoidectomy on craniofacial growth pattern
Following adenoidectomy, accelerated growth of the mandible was observed, with reduction of the mandibular plane angle. Increased growth of the ascending ramus of the mandible and the condyloid process were assumed to be the cause [25]. This was explained by a change in tongue position and hence auto-rotation of the mandible [14]. Overall, however, there was great variability in the reaction pattern. Despite weakening of the vertical growth pattern, the anterior facial height remained longer than in the healthy control group. By contrast, the inclination of the maxilla did not alter after adenoidectomy [26], [27].
4 Transverse maxillary deficiency
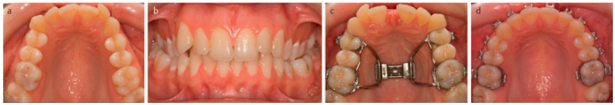
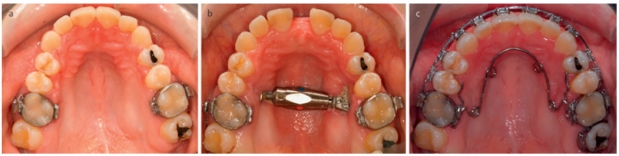
A narrow maxilla is one of the most common maxillary deformities (Figure 3a (Fig. 3)). Patients with transverse maxillary deficiency usually display a unilateral or bilateral posterior crossbite in occlusion (Figure 3b (Fig. 3)). The distance between the lateral walls of the nasal cavity and the nasal septum in these patients is often reduced, so that resistance of nasal air flow is increased [28]. In principle, there are four techniques for expanding excessively narrow maxillae: (a) orthodontic expansion e.g. by plate therapy or using a quad-helix appliance, (b) rapid maxillary expansion (RME) using a hyrax screw or palatal distractor, (c) surgically assisted orthodontic maxillary expansion and (d) transverse segmental osteotomy. Orthodontic expansion and – in more severe forms – rapid maxillary expansion are effective methods during childhood and adolescence (Figure 3c–d (Fig. 3)). When longitudinal growth has finished and bone maturity is almost complete, the intermaxillary suture is largely closed, which means resistance to orthodontic expansion is increased and a combined orthodontic and surgical approach is required to avoid complications such as fractures of the alveolar process (Figure 4a–c (Fig. 4)). For this purpose, maxillary osteotomies along the zones of skeletal resistance are performed [29], [30].
Figure 3. Patient with transverse mandibular deficiency and primary anterior crowding. a) Panoramic tomograph of maxilla pretreatment, b) Postero-anterior radiograph pretreatment with bilateral crossbite in occlusion, c) Treatment with maxillary expansion appliance: marked increase in the transverse width of the maxilla, particularly visible from formation of the diastema between the central incisors, d) status following removal of the expansion appliance and before the end of orthodontic fine adjustment.
Figure 4. Adult patient with transverse maxillary deficiency and high palate. a) panoramic tomograph of maxilla pretreatment, b) status following surgically assisted maxillary expansion with a transpalatal distractor and formation of a medial diastema, c) status following removal of the distractor shortly before the end of the orthodontic shaping of the maxilla.
By contrast, purely surgical segmental osteotomy to correct a narrow maxilla is rare and is usually only undertaken in conjunction with other surgical corrections of the maxilla [31]. The skeletal effect of rapid maxillary expansion on the upper jaw is, however, age-dependent. If it is performed with the aid of a tooth-supported hydrax screw in the permanent dentition when the palatine suture is still open, the orthopedic effect versus the dental effect is roughly equivalent to one third of screw activation, whereas the effect is more than 50% in the deciduous and mixed dentition [32].
4.1 Influence of maxillary expansion on nasal cavity and breathing
Numerous studies have radiographically investigated the changes to the nasal cavity after maxillary expansion on the basis of posterior-anterior cephalometry [33], [34], [35]. Anatomically speaking, widening of the nasal cavity, especially the nasal floor, close to the palatal suture was found. Depending on the patients’ age and treatment method, the increase varied from 1.06 mm to 3.47 mm between the studies. Furthermore it was shown that posterior expansion of the maxilla also has a positive influence on the function of the nasopharyngeal cavity [36]. Studies using rhinomanometry and acoustic rhinometry before and after expansion revealed that the nasal volume and minimum cross-sectional area (MCA) of the nose increased, whereas nasal resistance decreased [37], [38], [39]. Questionnaires on the patients’ subjective assessment of their breathing showed that more than 50% felt an improvement following maxillary expansion [38]. However, the degree of reduction of nasal air resistance cannot be predicted because there is a wide variation in the individual response to maxillary expansion [40], [28].
In a recently published study by Monini et al. [41] on the effect of rapid maxillary expansion in children aged under 12 years, enlargement of the posterior airway was demonstrated as well as a significant improvement in nasal air flow. The authors concluded that maxillary expansion plays an important role not only in correction of maxillary narrowness, but also in the treatment of severe constrictions of the nasopharyngeal cavity. In patients with obstructive sleep apnea syndrome (OSAS), the improved nasal flow following maxillary expansion thus led to lower subatmospheric inspiratory pressure, which in turn reduced the pharyngeal collapse [42]. This situation was explained by the fact that the altered tongue position within the enlarged oral cavity reduced the retroglossal obstruction.
However, it is a matter of some debates how much maxillary expansion also influences the type of breathing. For instance, Warren et al. [43] stated that increased nasal air flow is not enough to achieve nasal breathing because other factors, such as nasal concha hyperplasia, nasal polyps, adenoidal hypertrophy and septal deviation, are responsible for mouth breathing. Consequently, the authors did not consider it justified to perform maxillary expansion solely to increase nasal breathing capacity in cases of mouth breathing.
5 Obstructive sleep apnoea syndrome (OSAS)
During sleep, muscle activity is decreased and the resistance of the upper airways increased [44]. The result is that the reduction of muscle tone can lead to OSAS in children with hypertrophic lymphatic tissue or another abnormality of the upper airways. Apart from adenotonsillar hypertrophy, other risk factors for OSAS in children are overweight, neuromuscular disorders and craniofacial anomalies.
It has furthermore been reported that sleep disturbances have an influence on the endocrine system, especially on the secretion of growth hormones [45], [46], [47]. Children with OSAS and hypertrophy of the lymphatic tissue showed disturbed somatic growth because of abnormal nocturnal secretion of growth hormone (GH) [48]. Following adenotonsillectomy, a significant increase in the serum levels of GH mediators, such as insulin-like growth factor I (IGF I) and its binding protein, was found [48], [49]. Thereafter somatic growth returned to normal or even caught up [48], [49].
Overall, similar craniofacial characteristics were found in children with OSAS to those in children with “adenoid facies” [50]. To investigate the extent to which OSAS alters the morphology of the jaws, the orthodontic models from children with OSAS were compared with those of children with a history of snoring and a group of healthy children [51]. In comparison with the control children, the OSAS children had a significantly enlarged overjet (horizontal overlap of upper over lower incisors), a reduced vertical overbite (vertical overlap) with a higher incidence of an open bite and a narrower upper and shorter lower dental arch. Both the OSAS children and the children with nocturnal snoring had distoclusion more commonly than the control group. The number of children with mandibular crowding and an anterior open bite rose with the increasing obstructive apnea-hypopnea index. The effects of the increased resistance of the upper airways on jaw morphology were explained by the long-term repercussions of the altered head, mandibular and tongue posture adopted in order to ensure an adequate airway during sleep [51].
5.1 Importance of oral orthodontic appliances in the treatment of obstructive sleep apnea syndrome
The most common intervention for patients with OSAS is removal of the adenoids and tonsils [48], [52]. However, this procedure is limited by the surgical risks in children with comorbidities and patients without hypertrophy of the lymphatic tissue.
This is why oral orthodontic appliances (OA; or mandibular advancement device, MAD) were used to enlarge the upper airway and prevent its collapse by displacing the mandible forwards [53]. The minimum pharyngeal distance behind the soft palate and tongue improved by 1 mm and 0.8 mm respectively. Despite the smaller faces of female patients, they showed a more pronounced reaction to mandibular protrusion. A larger degree of hyoid movement was associated with a more positive airway response.
In a study by Hänggi et al. [54], the changes in the pharyngeal airway during growth in children and adolescents were measured and compared with those of a group whose distoclusion had been treated by activator headgear therapy (an oral orthodontic appliance for forward displacement of the mandible, which is anchored to the head with a metal strap and cap). The results of this study showed significant differences between the two groups before and after treatment: in the activator headgear group, the mandibular malposition decreased more markedly, there was a larger increase in the pharyngeal area, pharyngeal length and narrowest distance between tongue and posterior pharyngeal wall. These changes remained stable in a long-term comparison. The positive effect of the orthodontic treatment, however, was not explained solely by the skeletal changes but also by the impact on the soft tissue. The altered tongue position, caused by increased tone of the genioglossus muscle, or other soft tissue changes resulting from the anterior shifting of the mandible, were held to be responsible for the airway changes.
In a review by Carvalho et al. [55] from 2009, the efficacy of MADs was to be investigated in children with OSAS. The review included all the randomized and quasi-randomized controlled trials comparing all types of appliances with a placebo or no treatment in children aged ≤15 years. The primary treatment outcome was to be reduction of apnea to less than one episode per hour. Furthermore data on the following parameters were to available: dental and skeletal relationship, improvement in sleep parameters, cognitive and phonoaudiologic function, behavioral problems, dropouts and withdrawals, quality of life, side effects, tolerability and cost-effectiveness. One of the 384 original trials fulfilled the inclusion criteria with a total number of 23 patients. Although this study did not answer all the questions posed in the review, the results do favor the use of MADs in children with OSAS. Nevertheless, at present there is not sufficient evidence to state with certainty that oral orthodontic appliances can be used effectively in the treatment of children with OSAS
However, OSAS is most common in middle-aged people. The reasons are overweight, family predisposition or natural physiological changes. It was found, for instance, that the depth of the oropharyngeal airway decreases with age [56] and the soft palate gets longer and thicker [57]. In addition, lying supine and a physiological decrease in muscle tone during sleep significantly reduce the dimensions of the pharyngeal airway [58].
Thus both snorers and people with OSAS have narrower airways, reduced oropharyngeal areas and larger tongues [59]. Furthermore cephalometric analyses in OSAS patients revealed shorter and more retrognathic mandibles [60]. Studies have particularly indicated the significance of the most narrowed area of the upper airway (retropalatal and retroglossal) in the dynamics of air flow [61]. As this area is probably narrowed in middle-aged adults, the importance of therapeutically altering these dimensions was stressed [62].
In 2009 a review was published on the subject of using MADs for forward displacement of the mandible in obstructive sleep apnea compared with CPAP treatment and corrective surgery [63]. A total of 17 studies met the inclusion criteria. Compared with control appliances, MADs reduced the subjective feeling of tiredness and improved the apnea-hypopnea index. Compared with CPAP treatment, however, MAD therapy was less effective in reducing the apnea-hypopnea index. It was assumed that high nasal resistance and BMI, in particular, have an adverse effect on the efficacy of MADs [64]. CPAP therapy was more effective at improving minimum arterial oxygen saturation during sleep. However, the MADs were superior to corrective surgery on the upper airway. At present CPAP treatment therefore continues to be the therapy of choice for OSAS patients with severe symptoms and sleep disturbances. In the authors’ opinion, MADs should be reserved for patients with mild symptomatic OSAS and for patients who are unwilling to undergo or cannot tolerate CPAP treatment. According to a review by Hoffstein [65], the side effects of MADs are minor but frequent. The most common are excessive salivation, dry mouth, jaw pains and teeth discomfort. According to this study, efficacy and side effects depend particularly on the type of appliance, the degree of mandibular protrusion and vertical opening. After 30 months’ follow-up, 56–68% of the patients were still wearing the appliances. Again in this report the MAD was superior to the surgical procedure (UPPP) in reducing the apnea-hypopnea index.
Furthermore magnetic resonance imaging of the pharynx was performed to study the efficacy of MADs in OSAS [66]. Müller’s maneuver with and without MAD was performed in 13 patients. The polysomnographic analysis revealed a reduction of the apnea-hypopnea index from 19.8±14.5 to 7.2±7.4 h–1 by means of MAD. A reduction of >50% occurred in 7 patients, whereas the MAD therapy was unsuccessful in 6 patients. Five of the 7 patients who responded to the treatment had no significant pharyngeal obstruction during Müller’s maneuver when wearing the MAD, whereas they all did without the device. Conversely, there was a single velopharyngeal obstruction in 4 of the 6 patients for whom treatment failed and a combined obstruction of the velo- and glossopharynx in 2 patients during Müller’s maneuver. The results of this study suggest that the patency of the airway during Müller’s maneuver when wearing an MAD may be a suitable parameter for predicting the success of this appliance in cases of OSAS.
References
- 1.McNamara JA. Influence of respiratory pattern on craniofacial growth. Angle Orthod. 1981;51:269–300. doi: 10.1043/0003-3219(1981)051<0269:IORPOC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Sosa FA, Graber TM, Muller TP. Postpharyngeal lymphoid tissue in Angle Class I and Class II malocclusions. Am J Orthod. 1982;81:299–309. doi: 10.1016/0002-9416(82)90216-0. Available from: http://dx.doi.org/10.1016/0002-9416(82)90216-0. [DOI] [PubMed] [Google Scholar]
- 3.Moss M. The functional matrix: vistas in orthodontics. Philadelphia: Lea & Febinger; 1962. p. 85. [Google Scholar]
- 4.Linder-Aronson S. Adenoids. Their effect on mode of breathing and nasal airflow and their relationship to characteristics of the facial skeleton and the denition. A biometric, rhino-manometric and cephalometro-radiographic study on children with and without adenoids. Acta Otolaryngol Suppl. 1970;265:1–132. [PubMed] [Google Scholar]
- 5.Solow B, Siersbaek-Nielsen S, Greve E. Airway adequacy, head posture, and craniofacial morphology. Am J Orthod. 1984;86:214–223. doi: 10.1016/0002-9416(84)90373-7. Available from: http://dx.doi.org/10.1016/0002-9416(84)90373-7. [DOI] [PubMed] [Google Scholar]
- 6.Alves PV, Zhao L, O'Gara M, Patel PK, Bolognese AM. Three-dimensional cephalometric study of upper airway space in skeletal class II and III healthy patients. J Craniofac Surg. 2008;19:1497–1507. doi: 10.1097/SCS.0b013e31818972ef. Available from: http://dx.doi.org/10.1097/SCS.0b013e31818972ef. [DOI] [PubMed] [Google Scholar]
- 7.de Freitas MR, Alcazar NM, Janson G, de Freitas KM, Henriques JF. Upper and lower pharyngeal airways in subjects with Class I and Class II malocclusions and different growth patterns. Am J Orthod Dentofacial Orthop. 2006;130:742–745. doi: 10.1016/j.ajodo.2005.01.033. Available from: http://dx.doi.org/10.1016/j.ajodo.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Joseph AA, Elbaum J, Cisneros GJ, Eisig SB. A cephalometric comparative study of the soft tissue airway dimensions in persons with hyperdivergent and normodivergent facial patterns. J Oral Maxillofac Surg. 1998;56:135–139, discussion 139. doi: 10.1016/S0278-2391(98)90850-3. Available from: http://dx.doi.org/10.1016/S0278-2391(98)90850-3. [DOI] [PubMed] [Google Scholar]
- 9.Battagel JM, Johal A, L'Estrange PR, Croft CB, Kotecha B. Changes in airway and hyoid position in response to mandibular protrusion in subjects with obstructive sleep apnoea (OSA) Eur J Orthod. 1999;21:363–376. doi: 10.1093/ejo/21.4.363. Available from: http://dx.doi.org/10.1093/ejo/21.4.363. [DOI] [PubMed] [Google Scholar]
- 10.Abu Allhaija ES, Al-Khateeb SN. Uvulo-glosso-pharyngeal dimensions in different anteroposterior skeletal patterns. Angle Orthod. 2005;75:1012–1018. doi: 10.1043/0003-3219(2005)75[1012:UDIDAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Achilleos S, Krogstad O, Lyberg T. Surgical mandibular advancement and changes in uvuloglossopharyngeal morphology and head posture: a short- and long-term cephalometric study in males. Eur J Orthod. 2000;22:367–381. doi: 10.1093/ejo/22.4.367. Available from: http://dx.doi.org/10.1093/ejo/22.4.367. [DOI] [PubMed] [Google Scholar]
- 12.Achilleos S, Krogstad O, Lyberg T. Surgical mandibular setback and changes in uvuloglossopharyngeal morphology and head posture: a short- and long-term cephalometric study in males. Eur J Orthod. 2000;22:383–394. doi: 10.1093/ejo/22.4.383. Available from: http://dx.doi.org/10.1093/ejo/22.4.383. [DOI] [PubMed] [Google Scholar]
- 13.Solow B, Kreiborg S. Soft-tissue stretching: a possible control factor in craniofacial morphogenesis. Scand J Dent Res. 1977;85:505–507. doi: 10.1111/j.1600-0722.1977.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 14.Linder-Aronson S. Respiratory function in relation to facial morphology and the dentition. Br J Orthod. 1979;6:59–71. doi: 10.1179/bjo.6.2.59. [DOI] [PubMed] [Google Scholar]
- 15.Solow B, Siersbaek-Nielsen S, Greve E. Airway adequacy, head posture, and craniofacial morphology. Am J Orthod. 1984;86:214–223. doi: 10.1016/0002-9416(84)90373-7. Available from: http://dx.doi.org/10.1016/0002-9416(84)90373-7. [DOI] [PubMed] [Google Scholar]
- 16.Behlfelt K, Linder-Aronson S, Neander P. Posture of the head, the hyoid bone, and the tongue in children with and without enlarged tonsils. Eur J Orthod. 1990;12:458–467. doi: 10.1093/ejo/12.4.458. [DOI] [PubMed] [Google Scholar]
- 17.Björk A, Skieller V. Contrasting mandibular growth and facial development in long face syndrome, juvenile rheumatoid polyarthritis, and mandibulofacial dysostosis. J Craniofac Genet Dev Biol Suppl. 1985;1:127–138. [PubMed] [Google Scholar]
- 18.Merville LC, Diner PA. Long face: new proposals for taxonomy, diagnosis, treatment. J Craniomaxillofac Surg. 1987;15:84–93. doi: 10.1016/S1010-5182(87)80024-0. Available from: http://dx.doi.org/10.1016/S1010-5182(87)80024-0. [DOI] [PubMed] [Google Scholar]
- 19.O'Ryan FS, Gallagher DM, LaBanc JP, Epker BN. The relation between nasorespiratory function and dentofacial morphology: a review. Am J Orthod. 1982;82:403–440. doi: 10.1016/0002-9416(82)90189-0. Available from: http://dx.doi.org/10.1016/0002-9416(82)90189-0. [DOI] [PubMed] [Google Scholar]
- 20.Fields HW, Warren DW, Black K, Phillips CL. Relationship between vertical dentofacial morphology and respiration in adolescents. Am J Orthod Dentofacial Orthop. 1991;99:147–154. doi: 10.1016/0889-5406(91)70117-F. Available from: http://dx.doi.org/10.1016/0889-5406(91)70117-F. [DOI] [PubMed] [Google Scholar]
- 21.Vig KW. Nasal obstruction and facial growth: the strength of evidence for clinical assumptions. Am J Orthod Dentofacial Orthop. 1998;113:603–611. doi: 10.1016/S0889-5406(98)70219-7. Available from: http://dx.doi.org/10.1016/S0889-5406(98)70219-7. [DOI] [PubMed] [Google Scholar]
- 22.Oulis CJ, Vadiakas GP, Ekonomides J, Dratsa J. The effect of hypertrophic adenoids and tonsils on the development of posterior crossbite and oral habits. J Clin Pediatr Dent. 1994;18:197–201. [PubMed] [Google Scholar]
- 23.Souki BQ, Pimenta GB, Souki MQ, Franco LP, Becker HM, Pinto JA. Prevalence of malocclusion among mouth breathing children: do expectations meet reality? Int J Pediatr Otorhinolaryngol. 2009;73:767–773. DOI. 10.1016/j.ijporl.2009.02. doi: 10.1016/j.ijporl.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Sousa JB, Anselmo-Lima WT, Valera FC, Gallego AJ, Matsumoto MA. Cephalometric assessment of the mandibular growth pattern in mouth-breathing children. Int J Pediatr Otorhinolaryngol. 2005;69:311–317. doi: 10.1016/j.ijporl.2004.10.010. Available from: http://dx.doi.org/10.1016/j.ijporl.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Kerr WJ, McWilliam JS, Linder-Aronson S. Mandibular form and position related to changed mode of breathing--a five-year longitudinal study. Angle Orthod. 1989;59:91–96. doi: 10.1043/0003-3219(1989)059<0091:MFAPRT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 26.Linder-Aronson S, Woodside DG, Lundström A. Mandibular growth direction following adenoidectomy. Am J Orthod. 1986;89:273–284. doi: 10.1016/0002-9416(86)90049-7. Available from: http://dx.doi.org/10.1016/0002-9416(86)90049-7. [DOI] [PubMed] [Google Scholar]
- 27.Woodside DG, Linder-Aronson S, Lundstrom A, McWilliam J. Mandibular and maxillary growth after changed mode of breathing. Am J Orthod Dentofacial Orthop. 1991;100:1–18. doi: 10.1016/0889-5406(91)70044-W. Available from: http://dx.doi.org/10.1016/0889-5406(91)70044-W. [DOI] [PubMed] [Google Scholar]
- 28.Hartgerink DV, Vig PS, Abbott DW. The effect of rapid maxillary expansion on nasal airway resistance. Am J Orthod Dentofacial Orthop. 1987;92:381–389. doi: 10.1016/0889-5406(87)90258-7. Available from: http://dx.doi.org/10.1016/0889-5406(87)90258-7. [DOI] [PubMed] [Google Scholar]
- 29.Bailey LJ, White RP, Jr, Proffit WR, Turvey TA. Segmental LeFort I osteotomy for management of transverse maxillary deficiency. J Oral Maxillofac Surg. 1997;55:728–731. doi: 10.1016/S0278-2391(97)90588-7. Available from: http://dx.doi.org/10.1016/S0278-2391(97)90588-7. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher V, Gallagher C, Sleeman D. Surgically assisted rapid palatal expansion for management of transverse maxillary deficiency. J Ir Dent Assoc. 2002;48:18–21. [PubMed] [Google Scholar]
- 31.Lines PA. Adult rapid maxillary expansion with corticotomy. Am J Orthod. 1975;67:44–56. doi: 10.1016/0002-9416(75)90128-1. Available from: http://dx.doi.org/10.1016/0002-9416(75)90128-1. [DOI] [PubMed] [Google Scholar]
- 32.da Silva Filho OG, Montes LA, Torelly LF. Rapid maxillary expansion in the deciduous and mixed dentition evaluated through posteroanterior cephalometric analysis. Am J Orthod Dentofacial Orthop. 1995;107:268–275. doi: 10.1016/S0889-5406(95)70142-7. Available from: http://dx.doi.org/10.1016/S0889-5406(95)70142-7. [DOI] [PubMed] [Google Scholar]
- 33.Hershey HG, Stewart BL, Warren DW. Changes in nasal airway resistance associated with rapid maxillary expansion. Am J Orthod. 1976;69:274–284. doi: 10.1016/0002-9416(76)90076-2. Available from: http://dx.doi.org/10.1016/0002-9416(76)90076-2. [DOI] [PubMed] [Google Scholar]
- 34.Basciftci FA, Mutlu N, Karaman AI, Malkoc S, Küçükkolbasi H. Does the timing and method of rapid maxillary expansion have an effect on the changes in nasal dimensions? Angle Orthod. 2002;72:118–123. doi: 10.1043/0003-3219(2002)072<0118:DTTAMO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 35.Ramires T, Maia RA, Barone JR. Nasal cavity changes and the respiratory standard after maxillary expansion. Braz J Otorhinolaryngol. 2008;74:763–769. doi: 10.1016/S1808-8694(15)31388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cistulli PA, Palmisano RG, Poole MD. Treatment of obstructive sleep apnea syndrome by rapid maxillary expansion. Sleep. 1998;21:831–835. doi: 10.1093/sleep/21.8.831. [DOI] [PubMed] [Google Scholar]
- 37.Doruk C, Sökücü O, Sezer H, Canbay EI. Evaluation of nasal airway resistance during rapid maxillary expansion using acoustic rhinometry. Eur J Orthod. 2004;26:397–401. doi: 10.1093/ejo/26.4.397. Available from: http://dx.doi.org/10.1093/ejo/26.4.397. [DOI] [PubMed] [Google Scholar]
- 38.Babacan H, Sokucu O, Doruk C, Ay S. Rapid maxillary expansion and surgically assisted rapid maxillary expansion effects on nasal volume. Angle Orthod. 2006;76:66–71. doi: 10.1043/0003-3219(2006)076[0066:RMEASA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira De Felippe NL, Da Silveira AC, Viana G, Kusnoto B, Smith B, Evans CA. Relationship between rapid maxillary expansion and nasal cavity size and airway resistance: short- and long-term effects. Am J Orthod Dentofacial Orthop. 2008;134:370–382. doi: 10.1016/j.ajodo.2006.10.034. Available from: http://dx.doi.org/10.1016/j.ajodo.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 40.Timms DJ. The effect of rapid maxillary expansion on nasal airway resistance. Br J Orthod. 1986;13:221–228. doi: 10.1179/bjo.13.4.221. [DOI] [PubMed] [Google Scholar]
- 41.Monini S, Malagola C, Villa MP, Tripodi C, Tarentini S, Malagnino I, Marrone V, Lazzarino AI, Barbara M. Rapid maxillary expansion for the treatment of nasal obstruction in children younger than 12 years. Arch Otolaryngol Head Neck Surg. 2009;135:22–27. doi: 10.1001/archoto.2008.521. Available from: http://dx.doi.org/10.1001/archoto.2008.521. [DOI] [PubMed] [Google Scholar]
- 42.Baik UB, Suzuki M, Ikeda K, Sugawara J, Mitani H. Relationship between cephalometric characteristics and obstructive sites in obstructive sleep apnea syndrome. Angle Orthod. 2002;72:124–134. doi: 10.1043/0003-3219(2002)072<0124:RBCCAO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 43.Warren DW, Hershey HG, Turvey TA, Hinton VA, Hairfield WM. The nasal airway following maxillary expansion. Am J Orthod Dentofacial Orthop. 1987;91:111–116. doi: 10.1016/0889-5406(87)90467-7. Available from: http://dx.doi.org/10.1016/0889-5406(87)90467-7. [DOI] [PubMed] [Google Scholar]
- 44.Worsnop C, Kay A, Kim Y, Trinder J, Pierce R. Effect of age on sleep onset-related changes in respiratory pump and upper airway muscle function. J Appl Physiol. 2000;88:1831–1839. doi: 10.1152/jappl.2000.88.5.1831. [DOI] [PubMed] [Google Scholar]
- 45.Stradling JR, Thomas G, Warley AR, Williams P, Freeland A. Effect of adenotonsillectomy on nocturnal hypoxaemia, sleep disturbance, and symptoms in snoring children. Lancet. 1990;335:249–253. doi: 10.1016/0140-6736(90)90068-G. Available from: http://dx.doi.org/10.1016/0140-6736(90)90068-G. [DOI] [PubMed] [Google Scholar]
- 46.Leach J, Olson J, Hermann J, Manning S. Polysomnographic and clinical findings in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1992;118:741–744. doi: 10.1001/archotol.1992.01880070071013. [DOI] [PubMed] [Google Scholar]
- 47.Cooper BG, White JE, Ashworth LA, Alberti KG, Gibson GJ. Hormonal and metabolic profiles in subjects with obstructive sleep apnea syndrome and the acute effects of nasal continuous positive airway pressure (CPAP) treatment. Sleep. 1995;18:172–179. [PubMed] [Google Scholar]
- 48.Nieminen P, Löppönen T, Tolonen U, Lanning P, Knip M, Löppönen H. Growth and biochemical markers of growth in children with snoring and obstructive sleep apnea. Pediatrics. 2002;109:55. doi: 10.1542/peds.109.4.e55. Available from: http://dx.doi.org/10.1542/peds.109.4.e55. [DOI] [PubMed] [Google Scholar]
- 49.Bar A, Tarasiuk A, Segev Y, Phillip M, Tal A. The effect of adenotonsillectomy on serum insulin-like growth factor-I and growth in children with obstructive sleep apnea syndrome. J Pediatr. 1999;135:76–80. doi: 10.1016/S0022-3476(99)70331-8. Available from: http://dx.doi.org/10.1016/S0022-3476(99)70331-8. [DOI] [PubMed] [Google Scholar]
- 50.Zettergren-Wijk L, Forsberg CM, Linder-Aronson S. Changes in dentofacial morphology after adeno-/tonsillectomy in young children with obstructive sleep apnoea--a 5-year follow-up study. Eur J Orthod. 2006;28:319–326. doi: 10.1093/ejo/cji119. Available from: http://dx.doi.org/10.1093/ejo/cji119. [DOI] [PubMed] [Google Scholar]
- 51.Pirilä-Parkkinen K, Pirttiniemi P, Nieminen P, Tolonen U, Pelttari U, Löppönen H. Dental arch morphology in children with sleep-disordered breathing. Eur J Orthod. 2009;31:160–167. doi: 10.1093/ejo/cjn061. Available from: http://dx.doi.org/10.1093/ejo/cjn061. [DOI] [PubMed] [Google Scholar]
- 52.Guilleminault C, Huang YS, Glamann C, Li K, Chan A. Adenotonsillectomy and obstructive sleep apnea in children: a prospective survey. Otolaryngol Head Neck Surg. 2007;136:169–175. doi: 10.1016/j.otohns.2006.09.021. Available from: http://dx.doi.org/10.1016/j.otohns.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 53.Battagel JM, Johal A, L'Estrange PR, Croft CB, Kotecha B. Changes in airway and hyoid position in response to mandibular protrusion in subjects with obstructive sleep apnoea (OSA) Eur J Orthod. 1999;21:363–376. doi: 10.1093/ejo/21.4.363. Available from: http://dx.doi.org/10.1093/ejo/21.4.363. [DOI] [PubMed] [Google Scholar]
- 54.Hänggi MP, Teuscher UM, Roos M, Peltomäki TA. Long-term changes in pharyngeal airway dimensions following activator-headgear and fixed appliance treatment. Eur J Orthod. 2008;30:598–605. doi: 10.1093/ejo/cjn055. Available from: http://dx.doi.org/10.1093/ejo/cjn055. [DOI] [PubMed] [Google Scholar]
- 55.Carvalho FR, Lentini-Oliveira D, Machado MA, Prado GF, Prado LB, Saconato H. Oral appliances and functional orthopaedic appliances for obstructive sleep apnoea in children. Cochrane Database Syst Rev. 2007;18:CD005520. doi: 10.1002/14651858.CD005520.pub2. [DOI] [PubMed] [Google Scholar]
- 56.Martin SE, Mathur R, Marshall I, Douglas NJ. The effect of age, sex, obesity and posture on upper airway size. Eur Respir J. 1997;10:2087–2090. doi: 10.1183/09031936.97.10092087. Available from: http://dx.doi.org/10.1183/09031936.97.10092087. [DOI] [PubMed] [Google Scholar]
- 57.Johnston CD, Richardson A. Cephalometric changes in adult pharyngeal morphology. Eur J Orthod. 1999;21:357–362. doi: 10.1093/ejo/21.4.357. Available from: http://dx.doi.org/10.1093/ejo/21.4.357. [DOI] [PubMed] [Google Scholar]
- 58.Smith AM, Battagel JM. Non-apneic snoring and the orthodontist: radiographic pharyngeal dimension changes with supine posture and mandibular protrusion. J Orthod. 2004;31:124–131. doi: 10.1179/146531204225020418. Available from: http://dx.doi.org/10.1179/146531204225020418. [DOI] [PubMed] [Google Scholar]
- 59.Battagel JM, Johal A, Kotecha B. A cephalometric comparison of subjects with snoring and obstructive sleep apnoea. Eur J Orthod. 2000;22:353–365. doi: 10.1093/ejo/22.4.353. Available from: http://dx.doi.org/10.1093/ejo/22.4.353. [DOI] [PubMed] [Google Scholar]
- 60.Lowe AA, Fleetham JA, Adachi S, Ryan CF. Cephalometric and computed tomographic predictors of obstructive sleep apnea severity. Am J Orthod Dentofacial Orthop. 1995;107:589–595. doi: 10.1016/S0889-5406(95)70101-X. Available from: http://dx.doi.org/10.1016/S0889-5406(95)70101-X. [DOI] [PubMed] [Google Scholar]
- 61.Morrison DL, Launois SH, Isono S, Feroah TR, Whitelaw WA, Remmers JE. Pharyngeal narrowing and closing pressures in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;148:606–611. doi: 10.1164/ajrccm/148.3.606. [DOI] [PubMed] [Google Scholar]
- 62.Ono T, Otsuka R, Kuroda T, Honda E, Sasaki T. Effects of head and body position on two- and three-dimensional configurations of the upper airway. J Dent Res. 2000;79:1879–1884. doi: 10.1177/00220345000790111101. Available from: http://dx.doi.org/10.1177/00220345000790111101. [DOI] [PubMed] [Google Scholar]
- 63.Lim J, Lasserson TJ, Fleetham J, Wright J. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst Rev. 2006;25:CD004435. doi: 10.1002/14651858.CD004435. [DOI] [PubMed] [Google Scholar]
- 64.Chan AS, Lee RW, Cistulli PA. Non-positive airway pressure modalities: mandibular advancement devices/positional therapy. Proc Am Thorac Soc. 2008;15(5):179–184. doi: 10.1513/pats.200707-104MG. Available from: http://dx.doi.org/10.1513/pats.200707-104MG. [DOI] [PubMed] [Google Scholar]
- 65.Hoffstein V. Review of oral appliances for treatment of sleep-disordered breathing. Sleep Breath. 2007;11:1–22. doi: 10.1007/s11325-006-0084-8. Available from: http://dx.doi.org/10.1007/s11325-006-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanner BM, Heise M, Knoben B, Machnick M, Laufer U, Kikuth R, Zidek W, Hellmich B. MRI of the pharynx and treatment efficacy of a mandibular advancement device in obstructive sleep apnoea syndrome. Eur Respir J. 2002;20:143–150. doi: 10.1183/09031936.02.00268902. Available from: http://dx.doi.org/10.1183/09031936.02.00268902. [DOI] [PubMed] [Google Scholar]