Abstract
Survival and quality of life in head and neck cancer are directly linked to the size of the primary tumor at first detection. In order to achieve substantial gain at these issues, both, primary prevention and secondary prevention, which is early detection of malignant lesions at a small size, have to be improved. So far, there is not only a lack in the necessary infrastructure not only in Germany, but rather worldwide, but additionally the techniques developed so far for early detection have a significance and specificity too low as to warrant safe implementation for screening programs. However, the advancements recently achieved in endoscopy and in quantitative analysis of hypocellular specimens open new perspectives for secondary prevention. Chromoendoscopy and narrow band imaging (NBI) pinpoint suspicious lesions more easily, confocal endomicroscopy and optical coherence tomography obtain optical sections through those lesions, and hyperspectral imaging classifies lesions according to characteristic spectral signatures. These techniques therefore obtain optical biopsies. Once a “bloody” biopsy has been taken, the plethora of parameters that can be quantified objectively has been increased and could be the basis for an objective and quantitative classification of epithelial lesions (multiparametric cytometry, quantitative histology). Finally, cytomics and proteomics approaches, and lab-on-the-chip technology might help to identify patients at high-risk. Sensitivity and specificity of these approaches have to be validated, yet, and some techniques have to be adapted for the specific conditions for early detection of head and neck cancer. On this background it has to be stated that it is still a long way to go until a population based screening for head and neck cancer is available. The recent results of screening for cancer of the prostate and breast highlight the difficulties implemented in such a task.
Keywords: quantitative histology, human cytome project, hyperspectral imaging, optical coherence tomography
1 Introduction
Recent data of the Robert Koch-Institute show an increase of newly diagnosed cancer in Germany to 425,000 cases in 2002 equaling 8% or 30,000 more cases than 2000. About 220,000 patients have died of cancer in 2002 [1]. The DRG-statistic 2006 shows that 82,401 patients have been treated as in-patients with ICD-codes C00–C14, C30–C33, C76, and C77 in German hospitals [2]. This sums up all cases from initial staging to curative treatment and palliative care.
This highlights that oncological prevention is a task for the entire society. The aim of prevention is to reduce the number of patients affected by cancer or succumbing to cancer too early. This concept is based on primary prevention (preventing the initiation of cancer) and on secondary prevention (early detection of cancer). An estimate of 50% might be prevented or cured by primary and secondary prevention [1]. However, the value of prevention is under discussion and only 1% of the resources of the general health insurance in Germany are spent on prevention [3]. It certainly is wrong that prevention in principle is always safer and cheaper than treatment [4]. Implementing screening and prevention without thorough validation into population based programs is problematic, and the only screening program introduced so far (for breast cancer) is discussed contrarily [5]. Screening mammography for women aged 50 to 69 years reduces mortality of this age group by 15%: out of 2,000 women at this age 1 woman is saved from breast cancer death in a 10 years period; but 10 women get overdiagnosis or overtherapy, and 20% of women get at least 1 false-positive report within 10 years [6]. Most relevant are false-negative cases, such called interval-carcinoma which go undetected for a long time since they were non-pathological at screening. However, this phenomenon is hard to pinpoint in numbers. For other malignancies which are under discussion for screening (malignant melanoma, colon cancer) there are no hard facts on negative side effects. On top, there is no generally accepted histological classification for epithelial neoplastic lesions, as it can also be observed in malignant melanoma where even experts hardly come to the same histological diagnosis [7].
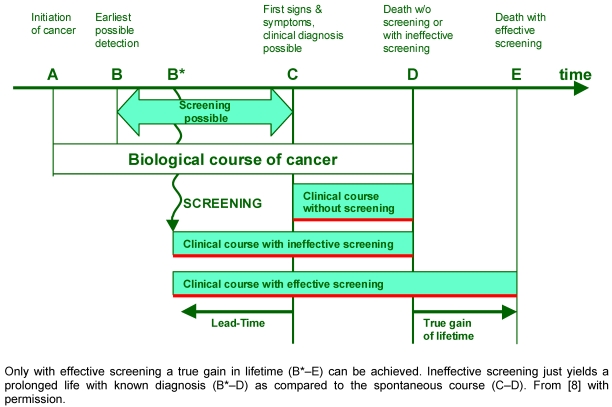
Reacting to the unsatisfied situation in Germany members of the working group “Krebsepidemiologie” of the German Society of Epidemiology have published a paper together with the German Cochrane Centre [8]. They conclude that Germany completely lacks a sufficient infrastructure for conducting and evaluating of screening programs with the only exemption of breast cancer screening. In that case for the first time a specific test is applied centrally and the process quality is monitored. The most important aim of any screening ultimately is a reduction of mortality without screening side effects. In contrast of present “opportunistic” screening the targeted population of a true screening is healthy and has no symptoms yet. The goals for such a screening are: 1. reduction of incidence; 2. reduction of mortality; 3. reduction of overall mortality; and 4. improvement of quality of life. Ineffective screening just allows making the correct diagnosis earlier without altering the course of the disease. True net gain in lifetime can only be achieved by shifting death to later periods of life yielding to an overall longer life with known diagnosis (Figure 1 (Fig. 1)). However, even optimal screening programs bear the risk of overdiagnosis and -therapy: minimal invasive and in situ carcinomas are detected and treated which would have never been clinically relevant in the patient’s lifetime.
Figure 1. Effect of screening on lifetime with cancer.
In addition, the public discussion focuses on the cost factor. According to the Fritz-Beske-Institute the overall cost for the health system will increase from 218 billiards today to 270 billiards Euro by the year of 2050 only due to the increase of expected life time. The cost per year per inhabitant in professional productive age for the treatment of cancer will therefore increase from 172.- € in 2008 to 280.- € in 2050. This calculation does not include extra costs for innovative diagnostics and therapeutics. But just due to a lack of competent health care professionals a hidden rationalization will occur [9]. This will yield to a health care collapse [10].
Up to 30% of cancer is estimated to be associated with avoidable risk factors (smoking, alcohol, occupational toxics, but also obesity, loss of mobility) [1]; nevertheless, so far the positive influence of a “healthy” lifestyle on cardiovascular disease, colon and breast cancer could not be proven [11], [12], [13]. Substitution with specific nutritients which seem to be sensible at first sight turned out to be devastating on a closer look: addition of calcium and vitamin D did not reduce the number of fractures but instead increase the number of nephroliths [14], and the “preventive” addition of estrogens and gestagens in postmenopausal women lead to significant additional health problems and massive health costs [15]. This underlines the necessary thorough evaluation of any screening program to prove that there is a positive overall cost-risk-ratio. The evidence must be clearly and objectively documented, and there must not be a negative consequence for not attending a program.
As early as 1984 Steiner showed that patient groups at high risk for head and neck cancer can be defined, that an adequate screening method is available [16], that endoscopy can be combined with cytology [17] albeit at slightly higher costs [18]. However, an anachronistic paradox still holds true unchanged: although cervix cancer has lower incidence and mortality in that anatomical location exfoliative cytology and PAP-staining are part of the routine screening but an equivalent approach for head and neck cancer is still absent. A colposcopic PAP-swab seems to be more readily accepted than a look into the oral cavity [19]. Indeed there is no high acceptance for screening of head and neck cancer throughout the population: even if directly invited in written form only 30–60% take part in screening; especially smokers have a low compliance [20], [21].
This phenomenon can be explained by the concept of cognitive dissonance [22], [23], [24]. According to that concept every individual tries to avoid non-congruent cognitive elements in order to reduce cognitive dissonance. Cognitive dissonance for example is produced by the clash of the attitude “I like smoking” with the fact “Smoking causes cancer”. In order to reduce this intraindividual dissonance smokers fade the uncomfortable information out, thinking that it is not relevant for them and not realizing the warnings printed on the cigarette packs [25]. Cognitive dissonance has direct influence on the attitude and the acceptance of screening schemes [26].
On the national level screening for head and neck cancer has not been included into the national plan on “Early Detection of Cancer” of the Deutsche Krebshilfe following a national hearing in 2005 [27]. Therefore it can be claimed a great success to have head and neck cancer included into the Nationale Onkologische Präventionskonferenz 2007 in Essen [28]. According to the Robert-Koch-Institute the number of new cases of oral and pharyngeal cancer in 2006 is 7,800 and 2,600 patients, and 2,800 and 450 for laryngeal cancer (male and female, resp.) [29]. Including the cases of cancer of nose and paranasal sinuses, salivary glands, and other rare cases altogether 18–20,000 new head and neck cancer cases per year can be estimated in Germany. The DRG-statistics of the Statistisches Bundesamt details the in-patient cases in 2006 [2]: there have been 39,080 cases of cancer of the lips, oral cavity, and pharynx (C00–C11, C14), 9,296 cases of cancer of the hypopharynx (C12, C13), and 15,108 cases of cancer of the larynx and trachea (C32, C33). These cases have been associated with a mean stay as an in-patient of 8–10 days [2]. It is estimated that worldwide 615,000 new cases of head and neck cancer occur per year with a strong male preponderance (10–15:1). However, there are strong regional differences in some anatomical sublocalisations [30].
2 Risk factors
In order to prevent cancer or to detect it early potential and known risk factors have to be evaluated. In general and also for head and neck cancer the socioeconomic status of the patient has impact on his or her treatment. A retrospective analysis based in Georgia, USA, showed that the insurance status had significant influence on the survival of patients with head and neck cancer [31]. The observed delay time in making the diagnosis is mainly due to patient factors but there are different gaps reported depending on the anatomical sublocalisation ranging from 5 to 19 weeks [32], [33]. In oral cavity and pharyngeal cancer the delay was worst in non-smokers, large tumors, and singles [34].
2.1 Smoking
Tobacco smoking is a known risk factor for head and neck cancer. The Heidelberger case-control-study showed that >30 pack years increase the risk for head and neck cancer by the factor 4.8 [35], and >60 pack years by the factor 23.4 [36]. This information is relevant in the context of a study about smoking habits of the young in Germany: for those aged 12–17 years the percentage of smokers had a historical maximum in 1970 with 30%, decreased to 20% in 1993, had another rise until 1997 to 28%, and from then on showed a decrease to recent 18% of which each 9% assign themselves as occasional and permanent smokers, respectively [37]. In addition, the novel trend of shisha-smoking has to be mentioned, which 14% name to be consumed at least once per month without assigning it as smoking nor as dangerous.
2.2 Alcohol
Alcohol is another known risk factor for head and neck cancer. The odds ratio ranges from 9.4 for 75 g ethanol per day [35] and 11.7 for >100 g ethanol per day [38]. There is a controversy about a hypothesized influence of the kind of alcohol consumed (wine vs. liquor etc.). However, there is a gender dependence: in females a consumption of >30 g ethanol per day increases the relative risk for head and neck cancer to 29; 40% of female head and neck cancer patients consumed >30 g ethanol per day [39]. In this group the amount of ethanol was lowest in cancer of the lips and highest in hypopharyngeal cancer [40].
Again, the attitude of the prospective new cancer patients, i.e. those now aged 12–17 years, has to be taken into account. Unfortunately, in this context the headlines of the rainbow press have to be verified: from 2004 to 2007 the total amount of ethanol consumed per week by this age group raised from 60 g to 71 g (male) and from 27 g to 29 g (female). Most alarming, such called binge-drinking (consuming more than 5 alcoholic drinks in one day) which is taken as an indicator for risky alcohol attitude has increased overall from 23% to 25%, focusing on 16–17 years old from 51% to shocking 63% [41].
Finally, the combination of both, smoking and drinking, has the highest propagating effect on head and neck cancer. This effect is over-additive and dose dependent. Cancer of the oral cavity and the larynx had the highest consumption of nicotine, and cancer of the oropharynx and the larynx had the highest consumption of ethanol [36]. The combined consumption of >75 g ethanol per day and >30 pack years increase the odds ratio to 92 [35]. As an additional risk factor at least for cancer of the oral cavity poor oral hygiene and dental status (>20 missing teeth) has been documented (odds ratio 5.3 and 3.4 respectively) [42].
2.3 Occupational toxics
Prof. Maier and his team have detailed various occupational poisons and showed the relative risk to develop head and neck cancer associated with them: asbestos 8.7, cement 12.9, tar 6.6, and dyes/paints/solvents 2.3 (larynx) and 3.6 (oral cavity). The highest relative risk is achieved by the combination of these poisons with nicotine and ethanol [35], [43], [44], [45]. Another risk factor is the exposition to polycyclic aromates which increases the odds ratio for laryngeal cancer to 5.2 [46]. In conclusion, the group of blue-collar workers with additional high consumption of nicotine and ethanol can be identified as a high risk group for head and neck cancer.
A special condition is the sinunasal adenocarcinoma. In this case, the exposition to inhalable dust of hard woods such as oak and beech carries a high risk for developing this cancer. The time delay is up to 40 years [47]. In Germany, it is a recognized occupational risk and accepted as occupational disease (BK 4203) with some 30 new cases per year (among some 70,000 employers). The odds ratio for joiners is 2.96. A concentration of 3.5 mg/m³ is definitively dangerous [48] showing a strong correlation especially for the histological subtype of intestinal type [49].
2.4 HPV-Infection
Although there was a general stagnation or even slight decrease in the overall incidence worldwide for head and neck cancer in parallel to the changing smoking habits, there was a marked increase for the incidence of oral cavity, tongue, and oropharyngeal cancer in never-smokers never-drinkers [50]. In this group, young men with tonsil cancer, young women with tongue cancer, and elderly women with cheek cancer are over-represented [51]. Several risk factors have been identified: >50% were serological positive for HPV16, 45% were passive smokers, 24% had occupational toxics in their history, and 30% had gastroesophageal reflux [51]. Together with other viral infections (HIV, HPV, HSV) there indeed is a plethora of risk factors in this group [52], [53].
The recent discussion focuses on the role of HPV16 in never-smokers never-drinkers [54]. zur Hausen is Nobel laureate for having established the concept of oncogenic viral infection [55]. First reports of HPV-positive cancer of the oropharynx and larynx date back to 1985 and 1987 [56], [57]. In 2006, in Sweden a marked percentage increase of tonsil cancer positive for HPV among all head and neck cancer cases from 1970 to 2000 was reported based on PCR on DNA-extracts from archived material [58]. Now, the presence of HPV-infection is recognized as a risk factor on its own for oropharyngeal cancer [59]. HPV-positive tonsil cancer can be rated as a clinical entity on its own [60], having a better prognosis than HPV-negative cancer [61]. A metaanalysis of 60 publications showed the prevalence for HPV-infection to be 25.9% for all head and neck cancer, highest in oropharynx (35.6%) and larynx (24.0%) and oral cavity cancer (23.5%). In oropharynx in 87% of positive cases are HPV16, whereas in hypopharynx and larynx a higher proportion of HPV18 is seen (17% vs. 38%) [62]. This is the basis for the discussion about vaccinating young males against HPV, too.
2.5 Chemoprevention
The great hopes initially put into the concept of chemoprevention have not hold true. It was just shown generally that the intake of 80 g per day of vegetables and fruit reduces the relative risk for head and neck cancer t0 0.91 and therefore can be assigned protective [63]. However, there is no single chemically identified substance yielding a preventive effect: 13-cis-retinoid acid did not influence the rate of secondary malignancies, recurrence, nor disease-free survival [64], and vitamine A and N-acetylcystein alone or in combination for 2 years had no influence on overall survival, disease-free survival, nor recurrence [65].
3 Precursor lesions
Any attempt of early detection of cancer implies a concept about how cancer arises in general. As early as 1890 Hansemann observed asymmetrical nuclear divisions in human cancer and discussed their relevance in the cancerogenesis [66]. This idea was reintroduced 25 years later by Boveri who was the first to describe chromosomes [67] and realized that a multipolare spindle apparatus yields to a misalignment and maldivision of chromosomes [68]. (For a detailed review of this historic background please refer to [69]). The development of stochiometric DNA-staining by Feulgen [70] and the construction of microscopes with quantitative densitometers [71] later on allowed to obtain the underlying quantitative data for these phenomena. As early as 1956 DNA-aneuploidy in malignant tumours was documented [72]. Casperson was then first to normalize the DNA-ploidy of cells from premalignant lesions using leukocytes as internal standard [73]. These analyses were repeated more recently in detail in breast cancer showing an inverse correlation of histological differentiation and the number of interphase nuclei with DNA-aneuploidy (>4.5c) [74].
Beginning in the 1980s a confronting alternative theory on the development of cancer has been developed. According to that theory cancer is caused by a stepwise activation of oncogenes and deactivation of cancer suppressor genes [75]. This construction is supported by the observation of the hereditary non-polypoid colon carcinoma (HNPCC) and of familial adenomatosis polyposis (FAP); however, these cases account for not more than 5% of all colon cancers, most cases are not hereditary but sporadic. Actually, objective analyses and observations in human cancers are in contrast to this concept: about 50% of all known carcinogens are not mutagenic [76], oncogenes are not clonally expressed so that part of the cancer cells in a given malignancy lack them [77], many oncogenes are not expressed in many cancer cells, and instead of hypothesized 4–7 mutations [78] rather thousands of genes are mutated in cancer cells [79]. Spontaneous mutations, however, are extremely rare (10-6 per mitosis) [80] yielding to just ONE in 1010–1028 human beings developing cancer taking the postulated 4–7 mutations as baseline! The supposed mutation of a “mutator-gene” can be found only in few cancers [81] and typically in late states [82]. Above of that, the hereditary “mutator-gene” which is postulated for Xeroderma pigmentosum does not lead to an increase of non-skin cancer in respective individuals [83].
These contradictions of this concept in itself recently have increased the interest in the prior hypothesis that aneuploidy is rather the cause but not the consequence of cancer [84], [85]. Duesberg has developed a convincing concept describing carcinogenesis as a chain reaction of aneuploidisation [86], [87].
As a matter of fact, a “precursor” lesion is defined by the clinical context. The WHO just states: “Precursor lesions are defined as altered epithelium with a high likelihood to develop into cancer”. Clinically in general leukoplakia, erythroplakia, and chronic inflammation are seen, histologically represented as dysplasia and atypia. However, what exactly a dysplasia is seems to be highly variable according to Barnes and coworkers: “The terminology of these precursor lesions, however, is still evolving and no single classification has been universally accepted” [88]. Histopathologically a number of histological and cytological criteria are described leading to the diagnosis “dysplasia”. In fact, also these criteria do allow neither a clear separation of hyperplasia on the one hand and early dysplasia on the other hand nor a separation of several grades of dysplasia. The only sure fact is that cancer can develop from any grade of dysplasia and can even arise from completely intact mucosa.
The corresponding theoretical concept for precursor lesions is represented by the term “intraepithelial neoplasia” (IEN) for non-invasive mucosal lesions with genetic alterations, loss of control of cellular functions, and phenotypic characteristics of invasive cancer, summing up in a lesion with high likelihood to progress into an invasive cancer [89].
In contrast, the terms leukoplakia and erythroplakia are clinically defined and have no place in pathological diagnoses [90]. Leukoplakia was first used by Schwimmer in 1877 [91] and nowadays is defined by the WHO as a “white patch or plaque that cannot be characterized clinically or pathologically as any other disease“ [92]. Erythroplakia was first used by Queyrat in 1911 [93] and in analogy is defined by the WHO as a “red patch of the mucous membrane which does not represent some specific or nonspecific inflammatory lesion“ [94]. All erythroplakias presumably will show histological signs of dysplasia, in 51% invasive carcinoma has been observed and in 40% carcinoma in situ or “severe” dysplasia have been seen; as a consequence, erythroplakias even more than leukoplakias should trigger an intensive diagnostic evaluation [95]. In any case, in mixed lesions the eurythroplakia component has to be included into an incisional biopsy [90].
The problem of severe intra- and interobserver variability in histological and cytological diagnoses is increasingly realized and discussed [95], [96]. The influence of this variability on screening schemes was estimated [97]. For head and neck cancer the interobserver agreement in judging such called oral pre-malignant lesions has been evaluated. Overall only a good agreement could be achieved (κW=0.59) with further reduction in cases with accompanying inflammation (κW=–0.10) although histological instead of cytological specimens were evaluated; in addition, also the anatomical site and the way of biopsy (incision vs. punch) showed some influence on the agreement. Taking bivariate rating (carcinoma and carcinoma in situ versus less severe changes) did not improve agreement (κW=0.39). Only in non-smokers a very good agreement was achieved (κW=0.71) [98], [99]. Similar results were obtained in laryngeal lesions (κW=0.32), bivariate comparison yielding κW=0.52 [100].
In the meantime it has been realized that these problems are immanent to the histopathological analysis itself according to Lessells et al.: “Histopathological diagnosis is not carried out in an algorithmic process. Individual pathologists have a highly trained visual cortex, such that within a few seconds of looking at a slide a number of conclusions have been drawn and a diagnosis (at least provisional) made. Obviously, individual pathologists are programmed in slightly different ways, accounting for the individual variation seen in a linear spectrum of abnormality such as dysplasia. Even if strict criteria were applied, it is unlikely that any improvement would be more than marginal” [101]. This highlights the effect of screening is influenced by reproducible and correct diagnoses. Taking the variance of histology and even more cytology into account it is first of all up to the clinician to identify suspicious lesions. It is the clinician who has to decide on which lesion can be observed and which has to be biopsied as it is the case in the esophagus [102].
4 Present state of early detection
The above notes lead to the conclusion that a true screening for head and neck cancer cannot be achieved within the near future. In the following the different attempts for secondary prevention, i.e. the early detection of invasive carcinoma and its precursors, will be outlined according to the anatomical subsites.
4.1 Oral cavity and oropharynx
The oral cavity and the oropharynx are ideal for early detection due to their good accessibility for inspection. First considerations about early detection and screening were published in 1969, 1974, and 1978 [103], [104], [105]. Nevertheless, up to date there is no consensus guideline for screening of premalignant oral lesions [90]. Since the 5-year-survival correlates directly to the state at first diagnosis, early detection not only could improve the incidence but also the survival. The continuing input into patient information and the ongoing education of community based physicians and dentists are equally important [106], [107].
In a recent review by the Cochrane Collaboration [108] only one study from India where oral cavity cancer is more frequent [109] had sufficient quality. But due to methodological deficits (e.g. no data about costs and risks) also this study did not give evidence pro or con for screening for oral cavity and oropharynx cancer by inspection with or without optical tools. Nevertheless, these tools are outlined in the following chapters.
4.1.1 Chromogen-aided visual inspection
For a long time it has been recognized that altered mucosa shows a staining behavior with exogenous dyes different from that of normal mucosa. One of the early dyes is toluidine-blue which was first described in 1952 for its use as intravital dye [110]. The exact mechanism is still not understood. Several studies have evaluated its clinical use but the high rate of false-positive lesions has shown its limited use [111], [112]. Other dyes such as Bengal-Rose are little better even when intensity of the staining is compared with a standardized table [113].
Using pre-incubation of the mucosa with 1% acetic acid the ViziLite™ system tries to improve the detection of altered mucosa by blue light (490–510 nm). However, independent studies in contrary show that neither the number of detected lesions is increased nor the correlation to histology improved [114]. Some investigators rated the reflections of the blue light as disturbing [115]. This system also has a high false-positive rate leading to a number too high for routine use in screening and prompting an unacceptable number of unnecessary biopsies [116].
In conclusion, all assays based on exogenous chromogens rely on a high clinical experience of the investigator [117].
4.1.2 Autofluorescence
The phenomenon of autofluorescence is another tissue feature recognized for some time [118] and first described as a useful tool during bronchoscopy in 1962 [119]. Since then autofluorecence has been established as an integral part throughout the entire upper aerodigestive tract [120], [121], [122], [123]. Sensitivity and specificity for the discrimination of normal mucosa and cancer has been quoted to be 91% and 86%, both better than for examination with white light (75% and 43%, respectively).
Autofluorescence can be modified by applying 5-aminolevulinic acid (5-ALA) topically or systemically. While altered mucosa shows a loss of autofluorescence, it exhibits a gain of 5-ALA-induced fluorescence. The specific accumulation of 5-ALA in tumor cells was first described in 1966 [124]. The resulting protoporphyrin IX-fluorescence is used to detect cancer. Since the cells first have to metabolize the 5-ALA there has to be a time gap of 1.5–3 hours until the investigation is possible [125], [126]. For this technique, a sensitivity of 99% and a specificity of 60% are quoted.
4.1.3 Molecular markers
Cells form altered mucosa can be analyzed extensively by molecular genetic assays. Loss of heterocygocity (LOH) can be used as a marker for genetic changes: LOH at 9p and 3q has been found in dysplasias, and an additional LOH at 4q, 8p, 11q, and 17p has been associated with an increased risk for the development of cancer [127], [128].
Another genetic change is an infection with HPV. In that context HPV16-infection in non-smokers non-drinkers plays a special role. HPV16 infection is associated with a higher risk of head and neck cancer in both, smokers and non-smokers. The proportion of tumor specimens containing the genome of the virus, however, varies between neglectible and 70% [59], [61], [129]. A study comparing HPV-status in biopsies and exfoliative cytology from the same patient showed no correlation between the both: 90% of patients HPV-positive in the biopsy where negative in the exfoliative cytology [130].
The analysis of cytological parameters is given an increasing importance in the context of early detection of oral cavity and oropharyngeal cancer. Their value will be further increase by including the analysis of quantitative parameters [131] (see 5.1).
4.2 Larynx
Classical screening for laryngeal cancer is based on the subjective analysis of conventional indirect laryngoscopy with or without magnification [16]. Initially “optical amplifiers” were used such as the topical application of toluidine blue [132]. This dye, however, was last mentioned for topical application in the larynx in 1982; in that study, the comparison with histology in 272 cases showed a sensitivity of 91% and a specificity of 52% [133].
4.2.1 Autofluorescence
The application of autofluorescence in the larynx was reported first in 1995 [134]. Autofluorescence has been shown to improve both, sensitivity and specificity, in rigid and flexible endoscopy for the delineation of the borders of altered mucosa (to 97% and 84–92%, respectively). The presence of inflammation, scaring, and hyperkeratosis has been identified as limitations of this technique leading to false-positive and false-negative results [121], [135], [136], [137], [138].
The use of 5-ALA has also been evaluated for laryngoscopy. Again, a gain in 5-ALA induced fluorescence has been observed in laryngeal cancer [125], [139]. A first trial of topical 5-ALA for photodynamic therapy in a pilot study, however, has been disappointing [140].
4.3 Hypopharynx
Since early symptoms in hypopharyngeal carcinoma are vague or absent and since prognosis depends on size at first presentation as in any other region, early detection of cancer of the hypopharynx would be especially effective [141], [142], [143]. In 2006, 9,296 patients have been treated as in-patients in Germany with the diagnoses C12 or C13 (cancer of the pririform recessus or cancer of the hypopharynx; for comparison: C32 – cancer of the larynx: 14,656 cases) according to the DRG-statistics of the Statistisches Bundesamt [2].
The odds to detect hypopharyngeal cancer at an early stage are best for patients who already had a carcinoma of the upper aerodigestive tract or of the esophagus in their history. This risk group has a substantial benefit from routine endoscopies even if no symptoms are present. The proportion of second primaries of the hypopharynx in stage I + II versus stage III + IV can be dramatically increased: from 22:78 without routine endoscopy to 90:10 with routine endoscopy. As a consequence, radical therapy with larynx preservation was possible in 79.4% with routine endoscopy as compared to 45.5% without routine endoscopy [144].
4.4 Salivary glands
Tumors of the salivary glands have an especially broad histological spectrum. Accordingly, the complex classification has been revised several times [88]. For this reason the data for some entities are insufficient. The overall incidence for malignant salivary gland tumors is 0.4–2.6/100,000. The most common malignant salivary gland tumor seems to be the mucoepidermoid carcinoma [~50% of cases), adenoidcystic carcinoma raging second. The incidence increases with age culminating around the age of 70. Males have a slight preponderance [145].
A known risk factor for malignant salivary gland tumors is ionizing radiation (by the atom bomb [146] or by radiotherapy [147] or by J131 e.g. as part of treatment for thyroid disease [148]). Electromagnetic radiation of mobile phones has no demonstrable negative effect [149]. Known occupational risk factors are nickel, chrome, asbestos, and cement dust, and ingredients used in rubber production [150], [151], [152]. Also hair dressers were shown to be at increased risk [153]. Exceptionally there is no increased risk for malignant salivary gland tumors associated with smoking and drinking [154].
As a consequence there is only a small population at risk that can be defined as a target group for screening. In 2006, there have been 2,457 patients treated as in-patients in Germany with the diagnosis C07 or C08 (cancer of the parotid gland or of other salivary glands of the head) according to the DRG-statistics of the Statistisches Bundesamt [2].
4.4.1 Fine-needle aspiration biopsies
Fine-needle aspiration biopsy (FNAB) has been long established as part of the diagnostic work-up for salivary gland tumors and can in principle be used as a tool for screening schemes [155], [156], [157], [158], [159]. These studies report false-positive and false-negative results in 1–14% of cases. The correct diagnosis was made in 81–98% of cases. Schröder et al. have evaluated the use of FNABs in the work-up of parotid gland tumors in 2000 [160]. They did not see seeding of tumor cells along the biopsy canal leading to satellite metastases or any other relevant complication. Retrospectively, only 284 specimens out of 336 had been sufficient for pathology due to the presence of tissue fragments; with these 284 specimens, sensitivity was 93.1% and specificity was 99.2%. Those cases where only single cells but no tissue fragments were obtained by FNAB were scored “inadequate”. Obviously, conventional “cyto”pathology is rather some kind of “microhisto”pathology. For true cytopathology, i.e. the analysis of single cells, quantitative and objective assays are available (see 5.1).
4.4.2 Ploidy
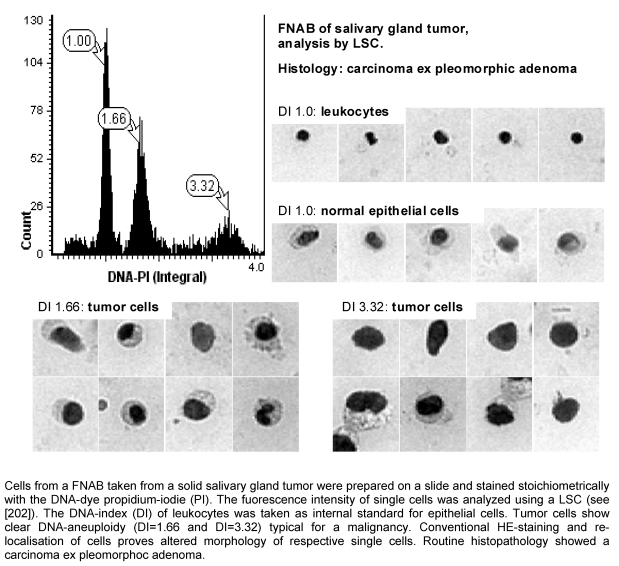
Up to recently the DNA-ploidy could only be determined from freshly resected tumor tissue. Using flow cytometry DNA-aneuploidy could be found in 24–28% of the malignant tumors, and all histologically benign tumors were DNA-diploid [161], [162]. The drawback of this method is that the tumor first has to be resected before tissue can be taken in order to obtain single cells for flow cytometry. Consequently, this test cannot be used for screening or preoperative analysis. As a matter of fact, however, the analysis of DNA-ploidy has been shown to be beneficial as part of the diagnostic work-up of salivary gland tumors. For example, this test was able to identify a carcinoma ex pleomorphic adenoma that was undetected by routine histology [163].
4.5 Second primaries and recurrences
The concept of field cancerisation was first published by Slaughter et al. in 1953 following their observation that head and neck carcinomas in general have a rather lateral spread as compared to their vertical dimension and that there is always surrounding mucosa which also shows alterations to some extent [164]. This is also highlighted by anecdotic reports of synchronous multiple carcinomas of the same anatomical sublocalization [165]. Recently, the respective molecular changes have been shown [166]. The common criteria for a “second primary” have been defined in 1932 [167]: tumors have to have a clear, but different malignant histopathological signature, and a metastasis has to be excluded. Rather arbitrary a lateral gap between the two lesions of 2.5 cm and a time interval of 3 years has been defined. In any case, patients with a newly diagnosed head and neck cancer or a positive history are at high risk to develop a second primary and should be thoroughly investigated as well initially at first presentation and during follow-up in order to detect a second primary in the entire upper aerodigestive tract. The rate of second primaries ranges from 9 to 19%; 41–46% are synchronous and 54–59% are metachronous [168], [169], [170], [171]. Using panendoscopy up to 85% of synchronous and 67% of metachronous secondary primaries can be detected [172].
Whereas recurrence occurs within the first 3 years, second primaries show an unchanged incidence beyond the 5th year [168]. For laryngeal cancer, a population based study on 20,074 patients showed a second primary in 3,533 cases (=17.6%); the cumulative risk was 26% at 10 years and 47% at 20 years. Treatment with radiation therapy increased the risk to develop a second primary especially in those patients who survived the 5th year [173]. In general, a second primary is correlated with a poor survival: the median survival is approximately 25 months [174].
The paradoxical late adverse effect of radiation therapy in terms of inducting a second primary has been first described by H. Glanz as early as 1976 [175]. Since then, the cancerogenic effect of low ionizing radiation doses has been well documented [147], [173], [176]. On that background, there is an additional risk to develop a second primary for patients with a head and neck cancer: the cancerogenic effect of radiation therapy. Especially patients with a low stage first primary should benefit from intensive follow-up since they have good chances for curative treatment of the second primary, too. Extensive surgery with reconstruction by microvascular reanastomised flaps had no influence on the risk to develop a second primary [177].
4.5.1 Endoscopy
Repeated endoscopy both, as rigid hypopharyngo-laryngo-esophagoscopy or as flexible videoesophagoscopy, are a standard that can be offered to patients with head and neck cancer and with esophageal cancer [178], [179], [180]. The highest benefit is seen for patients with a small first primary: in T1-T2 carcinoma of the oral cavity, the time gap from the end of the initial therapy to the detection of a second primary was beyond 60 months in 23% of cases [181]. The survival time was significantly increased in patients who underwent routine endoscopies as compared to patients with disease-triggered endoscopies from 32 months to 58 months [182].
4.5.2 “Tumormarker”
In a review on circulating tumor makers of head and neck cancer in 1994, not a single marker or a combination of several markers had a level of sensitivity or specificity high enough to justify its use as a “tumor marker”. If at all some use for monitoring of therapy seemed helpful [183]. This judgment was unchanged by two later reports which included molecular parameters [184], [185]. Out of the proteins Cyfra21-1, SCC- and TPS-antigen (squamous cell carcinoma antigen and tissue-polypeptide specific antigen, resp.) Cyfra21-1 seems to be adequate to detect a recurrence or a second primary (sensitivity 96%, specificity 87%) [186], [187], [188]. Indeed, the failure to define a single parameter or a combination of markers predictive for head and neck cancer is due to the fact that this group of malignancies has a wide spectrum of molecular genetic changes represented by the diverse histopathologic findings [189].
4.5.3 PET-CT
Since its introduction as fused PET-CT into oncology in general [190] there have been several studies for head and neck cancer as part of the initial staging [191], [192] and during follow-up for the detection of recurrence and second primaries [193], [194], [195]. In general, these studies just compare PET-CT with other imaging modalities. Only rarely the result of PET or PET-CT is compared to the histopathological diagnosis or the clinical course of untreated PET-CT-positive lesions is evaluated, e.g. if these lesions lead to locoregional recurrence. For example, there was a consensus of PET-CT and histopathology in only 9 out of 16 neck dissections [196]. Another study compared PET, PET-CT, CT, and MR and showed that PET-CT had a better accuracy than CT/MR in detecting primaries and metastases (98% vs. 86% and 92% vs. 85%, respectively) [197] but still there were false-positive and false-negative results [198].
5 Perspectives
In summary there are three areas that have the potential to yield an improvement of the early detection of head and neck cancer in the near future: 1. improved analysis of cytological specimens, 2. novel approaches to identify tumor markers, and 3. improved investigation techniques for the detection of altered mucosa.
5.1 Cytodiagnostic
There is a renaissance of cytodiagnostic assays in the head and neck region recently [131]. This is due to the novel insights into the development of cancer [86], [87], [199], to better minimal-invasive approaches to obtain specimens, and to improved optical (detection) systems.
In general cytopathology can be improved by the use of cytometric techniques as this has been advocated as “intensified cytodiagnostic” by Hanson et al. in 1989 [200] and lined out by Böcking et al. in 2004 [201]. Since the specimen in general is analyzed on a slide as a solid carrier these techniques are summarized as slide-based cytometry (SBC) [202].
A major focus is set on the determination of the DNA-ploidy of the tumor cells. Shortly after patenting the world’s first flow cytometer by Partec in Münster, Germany (Impulscytophotometer IPC 11) [203] the analysis of DNA-content of single cells was established [204]. The first application for head and neck cancer was published in 1972 [205]. In parallel, the analysis of DNA-ploidy by image cytometry was developed and applied to head and neck cancer, too [206]. This lead to the identification of DNA-ploidy as a prognostic parameter on its own for head and neck cancer: most tumors are DNA-aneuploid, and patients with a DNA-diploid tumor have a better prognosis for both, locoregional metastases and 5-year-survival rate [207], [208], [209], [210], [211], [212], [213].
Unlike flow cytometry which needs larger amounts of cells obtained by tissue specimens, for SBC minimal (hypocellular) specimens suffice (such as FNABs or exfoliative cytologies). The DNA-ploidy can be determined by image cytometry after Feulgen staining [214], [215] or by laser scanning cytometry after fluorescent staining [216], [217], [218] (Figure 2 (Fig. 2)), both methods being adequate [219]. The sensitivity and specificity for detecting a head and neck cancer are 79–96% and 93–100%, respectively. In addition to the DNA-ploidy, other markers can be analyzed [220]. The analysis of DNA-ploidy allows to detect a carcinoma months before it can be identified by histopathology [221]. SBC allows to reduce the proportion of “inadequate” specimens for cytological analysis. This technology can be applied throughout the entire upper aerodigestive tract even in areas not accessible for conventional swabs: esophagus and hypopharynx can be screened in a cursorical manner by using sponge that is swallowed by the patient [222].
Figure 2. Slide-based quantitative cytometry.
In other areas the cytological work-up has been already updated for its clinical use to a much better extent than in ENT. Screening for cervical cancer and its precursors for example in many schemes includes a variant of SBC, such called liquid-based cytology: the specimen is obtained as a suspension and dispersed on a microscopic glass slide in a special centrifuge and analyzed automatically [223]. This approach has been shown to be superior to conventional swabs where cells tend to lay in clusters of several layers and show substantial variation in fixation and staining [224], [225]. In the follow-up of urological tumors, a quantitative cytological analysis of specimens obtained from voided urine using fluorescence-in-situ-hybridization has been established [226].
SBC is leading to an objective and quantitative histopathological analysis even avoiding staining with exogenous dyes at all (see 5.3.4) [227], [228], [229], [230].
5.2 Proteomics
The transition of a cell from normal to cancer includes significant changes in the expression of cellular proteins. This includes the cancerous cell itself, but affects also the surrounding normal cells of blood vessels, of the connective tissue and infiltrating inflammatory cells. This yields to a change in the proteins circulating in the serum throughout the body. Proteomics intends to identify single biomarkers or clusters of protein changes (signatures) which allow to detect the presence or recurrence of a cancer. It aims to help in screening, initial staging, and follow-up, too. Proteomic based biomarkers are thought to open the door to personalized medicine in near future [231]. Using a plethora of different assays biomarkers are targeted from the DNA via RNA to protein expression on cell, tissue, and plasma level (cDNA array, oligonucleotide arrays, reverse-phase proteomic arrays, and tissue arrays) [231]. For head and neck cancer, there have been applications developed on protein level using surface enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS) [232] and evaluated [142], [233], [234]. A SELDI-TOF-MS-based classification had a sensitivity of 82–92% and a specificity of 76–90%. As long as biomarkers are proteins they can be bound by antibodies. Novel techniques allow to bind a set of different antibodies to a glass slide [235]; this approach has already been applied for the identification of novel biomarkers of breast cancer analyzing synchronously 387 proteins on a single array [236]. Flow cytometry can also be used for the simultaneous analysis of several blood-bound proteins, e.g. cytokines [237], [238].
A fusion of different novel detection assays on the one hand and of minimal specimen requirements on the other hand is targeted by the “lab-on-the-chip”-technology. These applications could finally be less expensive than present detection systems and are reviewed excellently by Ziober et al. [239]. Taking saliva as the specimen, applications cover the entire spectrum of oncology, from screening via initial staging to detection if minimal-residual disease or recurrence.
The major problem of proteomics actually is to extract that part of information out of the vast amount of data which is relevant for the respective application. Therefore, following Virchow’s concept of Cellularpathologie putting the cell as the smallest unit of life [240] the Human Cytome Project was initiated [241]. So far, the bottom-up concept of integrating single bits of information into one working concept has not been successful: looking at protein chemistry, it took more than 30 years of research but still there is no way to predict the 3D-structure of proteins based solely on their amino acid sequence. Instead, proteins are synthesized and then their 3D-structure is analyzed. One has to keep in mind that this is a problem of just 23 variables (i.e., amino acids). Obviously it will take some time until one can construct the cellular phenotype after analyzing the up to 20,000 genes and their products as it is tried in the Human Genome Project. Besides the fact that there are novel members detected continuously, not all “rules” of interaction between the 20,000 variables are understood and even novel “regulatory boards” are discovered (e.g., siRNA). Respecting these frustrating attempts, the Human Cytome Project is working top-down: taking the known phenotype represented by the cell (normal vs. ill) the obtained cell-based information is classified in a learning set and then applied to a test set [242], [243]. This allows to predict the clinical course (i.e. survival) of colon cancer with almost 100% accuracy [244] and to identify those patients with acute myeloic leukemia (AML) who need maximal therapy (i.e. stem cell transplantation) even before any therapy has been initiated [245]. This approach has also great potential in drug discovery [246], [247].
For screening, at present those assays have the biggest potential that allow to globally determine the individual risk of cancer with a simple test. First, there are analyses of saliva detecting its genotoxic potential (AMES-test) [248] or the (hyper)methylation of specific sequences within the genome [249]. Second, there are cytological analyses of cells contained in the saliva in order to detect global changes of the genome such as micronuclei or DNA-ploidy [250], [251]. In addition, peripheral blood leukocytes can be analyzed for their susceptibility to cancerogenes (using the bleomycin-test [252] or the COMET-assays [253], [254], [255], [256], [257]). These tests could be used for a pre-selection in a population-based screening.
5.3 Optimized visualization
The assays discussed in 5.1 and 5.2 yield to identify individuals with a (so far undetected) head and neck cancer. Most assays have the major drawback that they are not able to pinpoint the exact location of the cancer in the wide field of the upper aerodigestive tract. In the following some endoscopical approaches will be discussed that deal exactly with this problem: to highlight areas within the mucosa with altered architecture or even single cancer cells.
5.3.1 Chromoendoscopy
For a long time it has been recognized that mucosa with an altered structure shows a different behavior when stained with exogenous dyes as compared to normal mucosa. In 1835 the French physician Jean Guillame Lugol described the iodine-kaliumiodine-solution named after him. A first report about its use during an esophagoscopy dates as its latest back to 1966 [258] but it almost certainly was widely used earlier than that. Recently it is re-discovered by colleagues from surgical and medical departments for endoscopy [178], [180], [259] although it was widely practiced in many ENT-departments as standard procedure according to personal communication of the author with senior members of the society. Lugol’s staining has a sensitivity of 96% and a specificity of 63% in identifying highly dysplastic lesions of the esophagus [260]. Due to its minimal technical requirements, its safe use, and the low prize justify its use for the investigation of the esophagus by ENT-doctors, too. However, its application in the oral cavity, the oro- and hypopharynx and the larynx leads to dysesthesia and even pain, and there have been warning comments about aspiration [261], so that it should not be applied for the investigation of these anatomical regions.
The potential of autofluorescence alone or in combination with 5-ALA-induced PPIX-fluorescence have already been discussed in 4.1.2 and 4.2.1. Sensitivity and specificity for the discrimination of normal mucosa and cancer by autofluorescence are quoted as 91% and 86%, respectively, by 5-ALA-induced PPIX fluorescence as 99% and 60%, respectively, and therefore are better than with white light (75% and 43%).
5.3.2 Optical coherence tomography
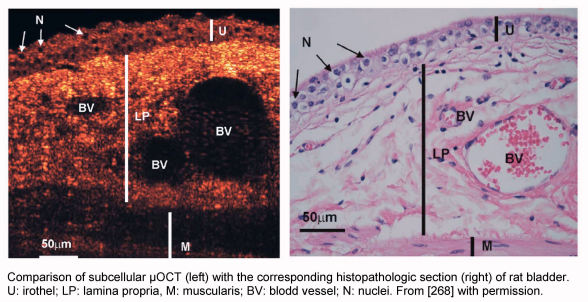
In optical coherence tomography (OCT) light with long wavelength (>1,300 nm) is used similar to ultrasound in order to obtain sectional views or 3D-reconstructions of the mucosa. Similar to applications at the retina [262] the reflected light is used to imagine thickness and structure of the mucosa with a lateral resolution of 10 µm and a depth of penetration of 2 mm. So far applications for laryngeal mucosa have been published; especially in non-exophytic lesions with smooth surface the mucosal thickness and the basal membrane can be analyzed [263], [264], [265]. Further technical modification could allow to integrate this technology into conventional endoscopes [266], [267]. Recently there has been the development of a µOCT that has a resolution down to the subcellular level showing a very good correlation with conventional histological sections [268] (Figure 3 (Fig. 3)). OCT still has to be evaluated for its clinical use.
Figure 3. Optical coherence tomography.
This also applies to narrow band imaging (NBI). This technology also makes use of the reflected light which is passed through adequate filters limiting the wavelengths to “narrow bands” (i.e. 400–430 nm, 430–460 nm, and 485–515 nm). This allows to better visualize the microvasculature within the mucosa and in deeper layers [261]. This technique has already been integrated into conventional endoscopes and can be used for the detection of superficial mucosal changes in the oral cavity and the oro- and hypopharynx [269].
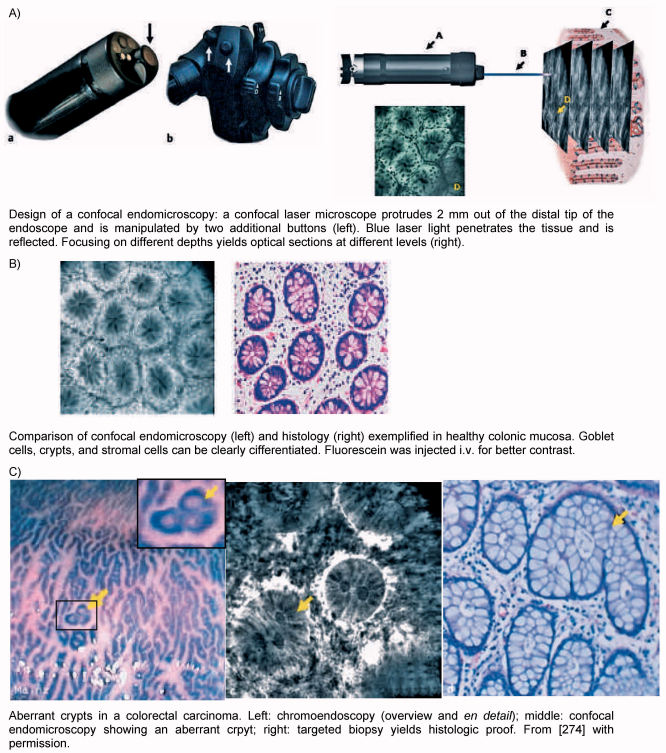
5.3.3 Confocal endomicroscopy
Another step to in-vivo-histology is offered by confocal laser scanning microscopy (cLSM). This technology yields a lateral resolution of 0.5–1 µm, an axial resolution of 3–5 µm, and a maximal depth of 200–500 µm [270]. This allows to gain most of the information that is obtained by conventional routine histopathology in order to analyze tissue and to discriminate benign from malignant lesions: size and configuration of nuclei, morphology of the chromatin, prominent nucleoli, mitoses, and the ratio nucleus:plasma. Based on microfabrication [271] the tip of conventional endoscopes can be fitted with a miniaturized cLSM-objective and to mount it to 50–100,000 optical fibers; this allows confocal endomicroscopy [272]. First applications have been described for colon cancer highlighting the optical properties impressively [273], [274] (Figure 4 (Fig. 4)). Shortly after that first applications for laryngeal endoscopy have been published [275]: the resected mucosa in this paper actually has been studied using conventional upright confocal laser scanning microscopy. However, in principle the step to laryngeal confocal endoscopy has been made.
Figure 4. Confocal endomicroscopy.
In conclusion, these techniques (optical coherence tomography, confocal endomicroscopy) will yield bloodless, optical in-vivo sections. However, as long as there are no automatic algorithms analyzing the images there will be a reduced information obtained by these sections just as it is the case with routine histopathology: the sections have to be assessed by the observer’s eye and his or her brain behind them. At least they are looking at living cells in action which is not the case in pathology.
5.3.4 Hyperspectral imaging
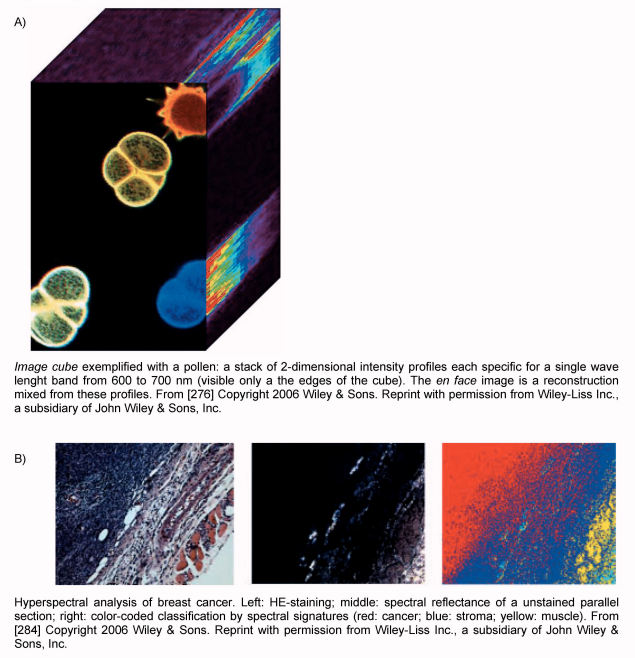
The cellular function can be better assessed by analyzing the electromagnetic spectrum. This approach has been used by geoscienticts when analyzing and classifying satellite images. “Spectral imaging” is defined as analyzing the entire spectrum from infrared to ultraviolet for every single pixel of an image. This generates an “image cube”, i.e. a stack of 2-dimensional intensity maps for single wavelengths [276] (Figure 5 (Fig. 5)). This allows to identify optical signatures specific for malignant cells. The term “spectral histochemistry” was coined 1998 [277] and points out the possibility to analyze tissue non-invasively: any altered cellular function yields to a change of the contents of the cellular and extracellular constituents. This in turn changes the spectrum of the reflected light. The potential of this technology has been described first for the diagnosis of malignant melanoma [278], [279]. Another very practical application is the determination of the Hb-content non-invasively spectroscopically by analyzing the conjunctiva within 1 second [280].
Figure 5. Hyperspectral imaging.
On tissue sections hyperspectral imaging allows to quantitatively analyze the expression of a receptor [281]. This can be achieved even without the use of any exogenous dyes [282]. A pilot study of the use of hyperspectral imaging in mucosal lesions of the oral cavity showed a sensitivity and a specificity of 96% for the in-vivo detection of normal versus dys- or neoplastic mucosa [283]. The group of Dan Farkas showed that the combination of different optical techniques [reflectance, fluorescence, scattering, 2-photon-excitation) yield even better sensitivity and specificity [284]. They have developed a suitable hyperspectral endoscopy, too [285].
5.3.5 In vivo-cytometry & molecular imaging
The ultimate analytical tool is a technology that does not take a specimen at all and avoids invasive means. Such a technology is in vivo-cytometry. Models so far have been the analysis of leukocytes in peripheral blood [286] where several modifications for murine models have been developed [287], [288], [289], [290]. In animal there have been further assays analyzing circulating tumor cells [291] and tissue analyses [292].
Novel concepts go even further in the animal model making use of the entire plethora of available technologies in order to analyze cancer cells at their different characteristics (proliferation, infiltration, metastasis) non-invasively in the living organism [293]. Genetically encoded reporter systems leading to triggered fluorescence allow to track a single cells or a cluster of cells through the body of the test animal. The number of available dyes is steadily increasing and is ever better fitting the special needs of the various applications [294], [295]. At present the aim is to tag single genome sequences and to make them work visibly in the living animal in order to better understand processes such as metastasis [296]. Another application is the fluorescence based detection of sentinel lymph nodes [297]. Instead of turning the cell to express the fluorochrome and hence light up the cells can alternatively be tagged with a fluorochrome directly; for this purpose quantum dots have proven beneficial [298]. However, some obstacles have to be taken until this can be applied to human patients; for example, quantum dots set heavy metal ions free yielding toxic effects so that they cannot applied in medicine yet.
6 Concluding remarks
We have to anticipate that most patients at risk (smokers) also in future will not attend screening programs as long as they have no symptoms. Global test that help to identify asymptomatic cancer patients using saliva specimens could however easily be used in occupational health screening of risk population (“blue collar workers”).
The most powerful tools in early detection of head and neck cancer still will be taking a thorough history concerning signs and symptoms and risk factors including previous head and neck cancer, and a close look during examination. The clinician’s eye already has some support by optical tools that guide to altered mucosal areas such as chromoendoscopy and autofluorescence. Up to now there is a lack of a tool that can be used throughout the entire upper aerodigestive tract. Hyperspectral imaging seems to be very promising in order to highlight suspicious lesions which then can be investigated by optical sectioning using optical coherence tomography or confocal endomicroscopy. These applications yield a lateral resolution in the subcellular dimension.
Pilot studies have shown that patients with detected areas of altered mucosa as well as solid tumors of salivary glands benefit from an intensified cytodiagnostic work-up: computer-based, quantitative, and objective techniques allow to analyze especially hypocellular specimens better than the naked eye. On the horizon already techniques for quantitative histology can be seen which will rapidly gain importance due to the increasing diversity of therapy strategies for a growing number of tumors (antibody-based, “targeted” therapy).
Novel technologies might in future allow to perform in vivo-cytometry and molecular imaging with revolutionizing applications for both, therapy and diagnostics, of malignancies. However, these approaches are at the test animal level at present.
References
- 1.Essener Erklärung, Nationale Onkologische Präventionskonferenz NOP. Essen: 2007. Available from: http://www.praevention-krebs.de/downloads/essener_erklaerung.pdf. [Google Scholar]
- 2.Statistisches Bundesamt. DRG Statistik 2007. Wiesbaden: Statistisches Bundesamt; 2009. Available from: https://www-ec.destatis.de/csp/shop/sfg/bpm.html.cms.cBroker.cls?cmspath=struktur,vollanzeige.csp&ID=1023219 [letzter Zugriff am 20.12.2007] [Google Scholar]
- 3.Ahrens HJ. Perspektiven der Prävention in Deutschland aus der Sicht der GKV. In: Kirch W, Badura B, editors. Prävention. Berlin, Heidelberg: Springer; 2006. pp. 41–53. [Google Scholar]
- 4.Sackett DL. The arrogance of preventive medicine. CMAJ. 2002;167:263–264. [PMC free article] [PubMed] [Google Scholar]
- 5.Mühlhauser I. Ist Vorbeugen besser als Heilen? Dtsch Ärztebl. 2007;104(25):A1804–A1807. doi: 10.1016/j.zgesun.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Gøtzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database of Systematic Reviews. 2001;(4):CD001877. doi: 10.1002/14651858.CD001877.pub2. Available from: http://dx.doi.org/10.1002/14651858.CD001877.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Farmer ER, Gonin R, Hanna MP. Discordance in the histopathologic diagnosis of melanoma and melanocytic nevi between expert pathologists. Hum Pathol. 1996;27:528–531. doi: 10.1016/S0046-8177(96)90157-4. Available from: http://dx.doi.org/10.1016/S0046-8177(96)90157-4. [DOI] [PubMed] [Google Scholar]
- 8.Giersiepen K, Hense HW, Klug SJ, Antes G, Zeeb H. Entwicklung, Durchführung und Evaluation von Programmen zur Krebsfrüherkennung - ein Positionspapier. ZaeFQ. 2007;101:43–49. doi: 10.1016/j.zgesun.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Beske F, Becker E, Krauss C, Katalinic A, Pritzkuleit R. Gesundheitsversorgung 2050. Kiel: Schmidt & Klaunig; 2007. [Google Scholar]
- 10.Rabatta S. Sorge vor dem Gesundheitsgau. Dtsch Ärztebl. 2007;104(39):A2624. [Google Scholar]
- 11.Howard BV, Manson JE, Stefanick ML, Beresford SA, Frank G, Jones B, Rodabough RJ, Snetselaar L, Thomson C, Tinker L, Vitolins M, Prentice R. Low-fat dietary pattern and weight change over 7 years: the Women's Health Initiative Dietary Modification Trial. JAMA. 2006;295(1):39–49. doi: 10.1001/jama.295.1.39. Available from: http://dx.doi.org/10.1001/jama.295.1.39. [DOI] [PubMed] [Google Scholar]
- 12.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, Kuller LH, LaCroix AZ, Langer RD, Lasser NL, Lewis CE, Limacher MC, Margolis KL, Mysiw WJ, Ockene JK, Parker LM, Perri MG, Phillips L, Prentice RL, Robbins J, Rossouw JE, Sarto GE, Schatz IJ, Snetselaar LG, Stevens VJ, Tinker LF, Trevisan M, Vitolins MZ, Anderson GL, Assaf AR, Bassford T, Beresford SA, Black HR, Brunner RL, Brzyski RG, Caan B, Chlebowski RT, Gass M, Granek I, Greenland P, Hays J, Heber D, Heiss G, Hendrix SL, Hubbell FA, Johnson KC, Kotchen JM. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):655–666. doi: 10.1001/jama.295.6.655. Available from: http://dx.doi.org/10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 13.Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, Paskett E, Phillips L, Robbins J, Rossouw JE, Sarto GE, Shikany JM, Stefanick ML, Thomson CA, Van Horn L, Vitolins MZ, Wactawski-Wende J, Wallace RB, Wassertheil-Smoller S, Whitlock E, Yano K, Adams-Campbell L, Anderson GL, Assaf AR, Beresford SA, Black HR, Brunner RL, Brzyski RG, Ford L, Gass M, Hays J, Heber D, Heiss G, Hendrix SL, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Kotchen JM, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Henderson MM. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):629–642. doi: 10.1001/jama.295.6.629. Available from: http://dx.doi.org/10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 14.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O'Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D Women's HealthInitiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–683. doi: 10.1056/NEJMoa055218. Available from: http://dx.doi.org/10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 15.Petitti D. Commentary: Hormone replacement therapy and coronary heart disease: four lessons. Int J Epidemiol. 2004;33:461–463. doi: 10.1093/ije/dyh192. Available from: http://dx.doi.org/10.1093/ije/dyh192. [DOI] [PubMed] [Google Scholar]
- 16.Steiner W. Krebsprävention und Früherkennung im oberen Aerodigestivtrakt - Ergebnisse und Konsequenzen zweier Erlanger Feldstudien. [Cancer prevention and early detection in the upper aero-digestive tract. Results and follow-up from 2 Erlangen field studies]. Fortschr Med. 1984;102:529–533. (Ger). [PubMed] [Google Scholar]
- 17.Steiner W. Krebsfrüherkennung im Bereich der oberen Luft- und Speisewege. HNO. 1993;41:360–7 & 497. [PubMed] [Google Scholar]
- 18.Ambrosch P. Screeninguntersuchungen zur Früherkennung von Karzinomen der oberen Luft- und Speisewege. HNO. 1996;44:609–611. [PubMed] [Google Scholar]
- 19.Smart RS. Screening for cancer of the aerodigestive tract. Cancer. 1993;72:1061–1065. doi: 10.1002/1097-0142(19930801)72:3+<1061::AID-CNCR2820721320>3.0.CO;2-1. Available from: http://dx.doi.org/10.1002/1097-0142(19930801)72:3+<1061::AID-CNCR2820721320>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Barra S, Barón AE, Barzan L, Caruso G, Veronesi A, Talamini R, Comoretto R, Franceschi S. Patients compliance in an early detection program for upper aero-digestive tract tumours in north-eastern Italy. Soz Praeventivmed. 1990;35:159–163. doi: 10.1007/BF01359480. Available from: http://dx.doi.org/10.1007/BF01359480. [DOI] [PubMed] [Google Scholar]
- 21.Talamini R, Barzan L, Franceschi S, Caruso G, Gasparin A, Comoretto R. Determinant in compliance with an early detection programme for cancer of the head and neck in north-eastern Italy. Eur J Cancer B Oral Oncol. 1994;30B:415–518. doi: 10.1016/0964-1955(94)90022-1. Available from: http://dx.doi.org/10.1016/0964-1955(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 22.Festinger L. A theory of cognitive dissonance. Evanston: Row, Peterson; 1957. [Google Scholar]
- 23.Brehm JW. Increasing cognitive dissonance by a fait accompli. J Abnorm Psychol. 1959;58(3):379–382. doi: 10.1037/h0047791. Available from: http://dx.doi.org/10.1037/h0047791. [DOI] [PubMed] [Google Scholar]
- 24.Case DO, Andrews JE, Johnson JD, Allard SL. Avoiding versus seeking: the relationship of information seeking to avoidance, blunting, coping, dissonance, and related concepts. J Med Libr Assoc. 2005;93(3):353–362. [PMC free article] [PubMed] [Google Scholar]
- 25.McMaster C, Lee C. Cognitive dissonance in tobacco smokers. Addict Behav. 1991;16(5):349–353. doi: 10.1016/0306-4603(91)90028-G. Available from: http://dx.doi.org/10.1016/0306-4603(91)90028-G. [DOI] [PubMed] [Google Scholar]
- 26.Steckelberg A, Kasper J, Redegeld M, Mühlhauser I. Risk information--barrier to informed choice? A focus group study. Soz Praventivmed. 2004;49(6):375–380. doi: 10.1007/s00038-004-3153-4. Available from: http://dx.doi.org/10.1007/s00038-004-3153-4. [DOI] [PubMed] [Google Scholar]
- 27.Deutsche Krebshilfe. Sachstandserhebung "Krebs-Früherkennung 2005". Bonn: Deutsche Krebshilfe; 2007. Available from: http://www.krebshilfe.de/fileadmin/Inhalte/Downloads/PDFs/Sachstandsbericht-KFU.pdf. [Google Scholar]
- 28.Krebsgesellschaft NRW. Nationale Onkologische Präventionskonferenz. Düsseldorf: Krebsgesellschaft NRW; 2007. Available from: http://www.praevention-krebs.de/index.php [letzter Zugriff am 20.11.2009] [Google Scholar]
- 29.Gesellschaft der epidemiologischen Krebsregister in Deutschland GEKID; Robert Koch Institut. Krebs in Deutschland. 5. Auflage. Saarbrücken: Gesellschaft der epidemiologischen Krebsregister in Deutschland; 2006. Available from: http://www.krebsregister.saarland.de/publikationen/PDF/KiD_2006_Internet-Version.pdf [letzter Zugriff am 20.11.2009] [Google Scholar]
- 30.Stewart BW, Kleihues P, editors. WHO World Cancer Report 2003. Lyon: IARC Press; 2003. [Google Scholar]
- 31.Gourin CG, Podolksy RH. Racial disparities in patients with head and neck squamous cell carcinoma. Laryngoscope. 2006;116:1093–1106. doi: 10.1097/01.mlg.0000224939.61503.83. Available from: http://dx.doi.org/10.1097/01.mlg.0000224939.61503.83. [DOI] [PubMed] [Google Scholar]
- 32.Dost P, Talanow DD, Kaiser S, Hirche H, Jahnke K. Zum Zeitintervall zwischen Symptom- und Behandlungsbeginn bei Kopf- und Halstumoren. [The time span between symptom onset and starting treatment in head and neck tumors]. HNO. 1996;44:492–496. doi: 10.1007/s001060050043. (Ger). [DOI] [PubMed] [Google Scholar]
- 33.Koscielny S, Wagner C, Beleites E. Untersuchungen zum Intervall zwischen Erstsymptom und Behandlungsbeginn bei Patienten mit Kopf-Hals-Tumoren. [Interval between initial symptoms and first treatment in patients with head-neck tumors]. HNO. 1999;47:551–555. doi: 10.1007/s001060050423. (Ger). [DOI] [PubMed] [Google Scholar]
- 34.Pitiphat W, Diehl SR, Laskaris G, Cartsos V, Douglass CW, Zavras AI. Factors associated with delay in the diagnosis of oral cancer. J Dent Res. 2002;81:192–197. doi: 10.1177/154405910208100310. Available from: http://dx.doi.org/10.1177/154405910208100310. [DOI] [PubMed] [Google Scholar]
- 35.Maier H, Sennewald E, editors. Risikofaktoren für Plattenepithelkarzinome im Kopf-Hals-Bereich - Ergebnisse der Heidelberger Fallkontrollstudien. Sankt Augustin: HVBG; 1994. [Google Scholar]
- 36.Maier H, Dietz A, Gewelke U, Heller WD, Weidauer H. Tobacco and alcohol and the risk of head and neck cancer. Clin Invest. 1992;70:320–327. doi: 10.1007/BF00184668. Available from: http://dx.doi.org/10.1007/BF00184668. [DOI] [PubMed] [Google Scholar]
- 37.Bundeszentrale für gesundheitliche Aufklärung. Förderung des Nichtrauchens bei Jugendlichen 2007. Köln: Bundeszentrale für gesundheitliche Aufklärung; 2007. Available from: http://www.ginko-ev.de/download/Foerderung_Nichtrauchen_2007.pdf [letzter Zugriff am 20.12.2007] [Google Scholar]
- 38.Grønbæk M, Becker U, Johansen D, Tønnesen H, Jensen G, Sørensen TIA. Population based cohort study of the association between alcohol intake and cancer of the upper digestive tract. BMJ. 1998;317:844–848. doi: 10.1136/bmj.317.7162.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maier H, Tisch M, Conradt C. Pötschke-Langer M. Alkoholgenuß und Krebs des oberen Aerodigestivtraktes bei Frauen. Dtsch Med Wochenschr. 1999;124:851–854. doi: 10.1055/s-2007-1024430. Available from: http://dx.doi.org/10.1055/s-2007-1024430. [DOI] [PubMed] [Google Scholar]
- 40.Luce D, Guenel P, Leclerce A, Brugere J, Point D, Rodriguez J. Alcohol and tobacco consumption in cancer of the mouth, pharynx, and larynx: a study of 316 female patients. Laryngoscope. 1988;98:313–316. doi: 10.1288/00005537-198803000-00015. Available from: http://dx.doi.org/10.1288/00005537-198803000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Bundeszentrale für gesundheitliche Aufklärung. Alkoholkonsum der Jugendlichen in Deutschland 2004-2007. Köln: Bundeszentrale für gesundheitliche Aufklärung; 2007. Available from: http://www.bmg.bund.de/cln_151/SharedDocs/Downloads/DE/Neu/Alkohol__Alkoholkonsum-jugendliche,templateId=raw,property=publicationFile.pdf/Alkohol_Alkoholkonsum-Jugendliche.pdf [letzter Zugriff am 20.11.2009] [Google Scholar]
- 42.Rosenquist K, Wennerberg J, Schildt EB, Bladström A, Hansson BG, Andersson G. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Oto-Laryngologica. 2005;125:1327–1336. doi: 10.1080/00016480510012273. Available from: http://dx.doi.org/10.1080/00016480510012273. [DOI] [PubMed] [Google Scholar]
- 43.Maier H, Tisch M, Enderle G, Dietz A, Weidauer H. Berufliche Exposition gegenüber Farben, Lacken und Lösungsmitteln und Krebsrisiko im Bereich des oberen Aerodigestivtraktes. HNO. 1997;45:905–908. doi: 10.1007/s001060050172. [DOI] [PubMed] [Google Scholar]
- 44.Maier H, Tisch M, Dietz A, Conradt C. Arbeiter in der Bauindustrie - eine Höchstrisikogruppe für Krebserkrankungen des oberen Atmungs- und Verdauungstraktes? HNO. 1999;47:730–736. doi: 10.1007/s001060050453. [DOI] [PubMed] [Google Scholar]
- 45.Maier H, Tisch M. Beruf und Krebs im Kopf-Hals-Bereich. HNO. 1999;47:1025–1037. doi: 10.1007/s001060050487. [DOI] [PubMed] [Google Scholar]
- 46.Becher H, Ramroth H, Ahrens W, Risch A, Schmezer P, Dietz A. Occupation, exposure to polycyclic aromatic hydrocarbons and laryngeal cancer risk. Int J Cancer. 2005;116:451–457. doi: 10.1002/ijc.21049. Available from: http://dx.doi.org/10.1002/ijc.21049. [DOI] [PubMed] [Google Scholar]
- 47.Wills JH. Nasal carcinomas in wood workers: a review. J Occup Med. 1982;24:526–530. [PubMed] [Google Scholar]
- 48.Pesch B, Pierl CB, Gebel M, Gross I, Becker D, Johnen G, Rihs HP, Donhuijsen K, Lepentsiotis V, Meier M, Schulze J, Brüning T. Occupational risks for adenocarcinoma. Occup Environ Med. 2008;65:191–196. doi: 10.1136/oem.2007.033886. Available from: http://dx.doi.org/10.1136/oem.2007.033886. [DOI] [PubMed] [Google Scholar]
- 49.Donhuijsen K, Hattenberger S, Schroeder HG. Sinunasale Karzinome nach Holzstaubbelastung - morphologisches Spektrum an 160 Fällen. Pathologe. 2004;25:14–20. doi: 10.1007/s00292-003-0668-z. Available from: http://dx.doi.org/10.1007/s00292-003-0668-z. [DOI] [PubMed] [Google Scholar]
- 50.Shiboski CH, Schmidt BL, Jordan RCK. Tongue and tonsil carcinoma. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. Available from: http://dx.doi.org/10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 51.Dahlstrom RK, Little JA, Zafereo ME, Margaret Lung M, Wie Q, Sturgis EM. Squamous cell carcinoma of the head and neck in never smoker-never drinkers: a descriptive epidemiologic study. Head Neck. 2008;30:75–84. doi: 10.1002/hed.20664. Available from: http://dx.doi.org/10.1002/hed.20664. [DOI] [PubMed] [Google Scholar]
- 52.Wight R, Paleri V, Arullendran P. Current theories for the development of nonsmoking and nondrinking laryngeal carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2003;11:73–77. doi: 10.1097/00020840-200304000-00002. Available from: http://dx.doi.org/10.1097/00020840-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 53.León X, Rinaldo A, Saffiotti U, Ferlito A. Laryngeal cancer in non-smoking and non-drinking patients. Acta Otolaryngol. 2004;124:664–669. doi: 10.1080/00016480410017008. Available from: http://dx.doi.org/10.1080/00016480410017008. [DOI] [PubMed] [Google Scholar]
- 54.Dahlstrom RK, Adler-Storthz K, Etzel CJ, Liu Z, Dillon L, El-Naggar AK, Spitz MR, Schiller JT, Wie Q, Sturgis EM. Human papillomavirus type 16 infection and squamous cell carcinoma of the head and neck in never-smokers: a matched pair analysis. Clin Cancer Res. 2003;9:2620–2626. [PubMed] [Google Scholar]
- 55.zur Hausen H. Papillomaviruses and cancer: from basic science to clinical application. Nature Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. Available from: http://dx.doi.org/10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 56.Löning T, Ikenberg H, Becker J, Gissmann L, Hoepfer I, zur Hausen H. Analysis of oral papillomas, leukoplakias, and invasive carcinomas for human papilloma virus type related DNA. J Invest Dermatol. 1985;84:417–420. doi: 10.1111/1523-1747.ep12265517. Available from: http://dx.doi.org/10.1111/1523-1747.ep12265517. [DOI] [PubMed] [Google Scholar]
- 57.Syrjänen S, Syrjänen K, Mäntyjärvi R, Collan Y, Kärjä J. Human papillomavirus DNA in squamous cell carcinomas of the larynx demonstrated by in situ DNA hybridization. ORL J Otorhinol Relat Spec. 1987;49:175–186. doi: 10.1159/000275933. [DOI] [PubMed] [Google Scholar]
- 58.Hammarstedt L, Lindquist D, Dahlstrand D, Romanitan M, Onelöv (Dahlgren) L, Joneberg J, Creson N, Lindholm J, Ye W, Dalianis T, Munck-Wikland E. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623. doi: 10.1002/ijc.22177. Available from: http://dx.doi.org/10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 59.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. Available from: http://dx.doi.org/10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 60.Klussmann JP, Weissenborn SJ, Wieland U, Dries V, Kolligs J, Jungehuelsing M, Eckel HE, Dienes HP, Pfister HJ, Fuchs PG. Prevalence, distribution, and viral load of human papillomavirus 16 DNA in tonsillar carcinomas. Cancer. 2001;92:2875–2884. doi: 10.1002/1097-0142(20011201)92:11<2875::AID-CNCR10130>3.0.CO;2-7. Available from: http://dx.doi.org/10.1002/1097-0142(20011201)92:11<2875::AID-CNCR10130>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 61.Ritchie JM, Smith EM, Summersgill KF, Hoffman HT, Wang D, Klussmann JP, Turek LP, Haugen TH. Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer. 2003;104:336–344. doi: 10.1002/ijc.10960. Available from: http://dx.doi.org/10.1002/ijc.10960. [DOI] [PubMed] [Google Scholar]
- 62.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. Available from: http://dx.doi.org/10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 63.Boeing H, Dietrich T, Hoffmann K, et al. Intake of fruits and vegetables and risk of cancer of the upper aero-digestive tract: the prospective EPIC-study. Cancer Causes Control. 2006;17:957–969. doi: 10.1007/s10552-006-0036-4. Available from: http://dx.doi.org/10.1007/s10552-006-0036-4. [DOI] [PubMed] [Google Scholar]
- 64.Perry CF, Stevens M, Rabie I, Yarker ME, Cochrane J, Perry E, Traficante R, Coman W. Chemoprevention of head and neck cancer with retinoids - a negative result. Arch Otolaryngol Head Neck Surg. 2005;131:198–203. doi: 10.1001/archotol.131.3.198. Available from: http://dx.doi.org/10.1001/archotol.131.3.198. [DOI] [PubMed] [Google Scholar]
- 65.van Zandwijk N, Dalesio O, Pastorino U, de Vries N, van Tinteren H. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. J Natl Cancer Inst. 2000;92:977–986. doi: 10.1093/jnci/92.12.977. Available from: http://dx.doi.org/10.1093/jnci/92.12.977. [DOI] [PubMed] [Google Scholar]
- 66.Hansemann D. Über asymmetrische Zelltheilung in Epithelkrebsen und deren biologische Bedeutung. Arch Pathol Anat Physiol Klin Medicin. 1890;119:299–326. doi: 10.1007/BF01882039. Available from: http://dx.doi.org/10.1007/BF01882039. [DOI] [Google Scholar]
- 67.Boveri T. Ergebnisse über die Konstitution der chromatischen Substanz des Zellkerns. Jena: Fischer; 1904. [Google Scholar]
- 68.Boveri T. Zur Frage der Entstehung maligner Tumore. Jena: Fischer; 1914. [Google Scholar]
- 69.Hardy PA, Zacharias H. Reappraisal of the Hansemann-Boveri hypothesis on the origin of tumors. Cell Biology International. 2005;29:983–992. doi: 10.1016/j.cellbi.2005.10.001. Available from: http://dx.doi.org/10.1016/j.cellbi.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 70.Feulgen R, Rosenbeck H. Mikroskopisch-chemischer Nachweis einer Nucleinsäure vom Typus der Thymonucleinsäure und die darauf beruhende elektive Färbung von Zellkernen in mikroskopischen Präparaten. Hoppe-Seyler's Zeitschr Physiol Chem. 1924;135:203–248. [Google Scholar]
- 71.Caspersson T, Lomakka G, Svensson G, Säfström R. A versatile ultramicrospectrograph for multiple-line and surface scanning high resolution measurements employing automatized data analysis. Exp Cell Res. 1955;3:40–51. [PubMed] [Google Scholar]
- 72.Atkin NB, Richards BM. Deoxyribonucleic acid in human tumours as measured by microspectrophotometry of Feulgen stain: a comparison of tumours arising at different sites. Brit J Cancer. 1956;10:769–786. doi: 10.1038/bjc.1956.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Capersson O. Quantitative cytochemical studies on normal, malignant, pre-malignant and atypical cell populations from the human uterine cervix. Acta Cytol. 1964;8:45–60. [PubMed] [Google Scholar]
- 74.Steinbeck RG, Auer GU, Zetterberg AD. Reliability and significance of DNA measurements in interphase nuclei and division figures in histological sections. Eur J Cancer. 1999;35:787–795. doi: 10.1016/S0959-8049(98)00427-4. Available from: http://dx.doi.org/10.1016/S0959-8049(98)00427-4. [DOI] [PubMed] [Google Scholar]
- 75.Pitot HC, Goldsworthy T, Moran S. The natural history of carcinogenesis: implications of experimental carcinogenesis in the genesis of human cancer. J Supramol Struct Cellul Biochem. 1981;17:133–146. doi: 10.1002/jsscb.380170204. Available from: http://dx.doi.org/10.1002/jsscb.380170204. [DOI] [PubMed] [Google Scholar]
- 76.Oshimura M, Barrett JC. Chemically induced aneuploidy in mammalian cells: mechansims and biological significance in cancer. Environ Mutagen. 1986;8:129–159. doi: 10.1002/em.2860080112. Available from: http://dx.doi.org/10.1002/em.2860080112. [DOI] [PubMed] [Google Scholar]
- 77.Walch A, Bink K, Gais P, Stangl S, Hutzer P, Aubele M, Mueller J, Hofler H, Werner M. Evaluation of c-erbB-2 overexpression and Her-2/neu gene copy number heterogeneity in Barrett's adenocarcinoma. Anal Cell Pathol. 2000;20:25–32. doi: 10.1155/2000/947249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. 1993;9:138–141. doi: 10.1016/0168-9525(93)90209-Z. Available from: http://dx.doi.org/10.1016/0168-9525(93)90209-Z. [DOI] [PubMed] [Google Scholar]
- 79.Pollack JR, Sorlie T, Perou CM, Rees CA, Jeffrey SS, Lonning PE, Tibshirani R, Botstein D, Borresen-Dale AL, Brown PO. Microarray analysis reveals a major direct role of DNA copy number alteration in the transcriptional program of human breast tumors. Proc Natl Acad Sci USA. 2002;99:12963–12968. doi: 10.1073/pnas.162471999. Available from: http://dx.doi.org/10.1073/pnas.162471999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vogel F, Motulsky AG. Human genetics: problems and approaches. New York: Springer; 1986. [Google Scholar]
- 81.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–627. doi: 10.1038/386623a0. Available from: http://dx.doi.org/10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 82.Tomlinson I, Bodmer W. Selection, the mutation rate and cancer: insuring that the tale does not wag the dog. Nature Med. 1999;5:11–12. doi: 10.1038/4687. Available from: http://dx.doi.org/10.1038/4687. [DOI] [PubMed] [Google Scholar]
- 83.Cairns J. The origin of human cancers. Nature. 1981;289:353–357. doi: 10.1038/289353a0. Available from: http://dx.doi.org/10.1038/289353a0. [DOI] [PubMed] [Google Scholar]
- 84.Stock RP, Bialy H. The sigmoidal curve of cancer. Nature Biotech. 2003;21:13–14. doi: 10.1038/nbt0103-13. Available from: http://dx.doi.org/10.1038/nbt0103-13. [DOI] [PubMed] [Google Scholar]
- 85.Meijer GA. Chromosomes and cancer, Boveri revisited. Cell Oncol. 2005;27:273–275. doi: 10.1155/2005/712434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duesberg P, Li R. Multistep carcinogenesis: a chain reaction of aneuploidizations. Cell Cycle. 2002;2:202–210. [PubMed] [Google Scholar]
- 87.Duesberg P, Li R, Fabarius A, Hehlmann R. The chromosomal basis of cancer. Cell Oncol. 2005;27:293–318. doi: 10.1155/2005/951598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barnes L, Eveson JW, Reichart P, Sidransky D, editors. WHO Classification of Tumours, Pathology and Genetics of Head and Neck Tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 89.O'Shaughnessy JA, Kelloff GJ, Gordon GB, et al. Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Clin Cancer Res. 2002;8:314–346. [PubMed] [Google Scholar]
- 90.Neville BM, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52:195–215. doi: 10.3322/canjclin.52.4.195. Available from: http://dx.doi.org/10.3322/canjclin.52.4.195. [DOI] [PubMed] [Google Scholar]
- 91.Schwimmer E. Die idiopathischen Schleimhautplaques der Mundhöhle (Leukoplakia buccalis) Arch Dermat Syph. 1877;9:570–611. [Google Scholar]
- 92.Kramer IR, Lucas RB, Pindborg, JJ, Sobin LH. WHO collaborating centre for oral precancerous lesions - definition of leukoplakia and related lesions: an aid to studies on oral precancer. Oral Surg Oral Med Oral Pathol. 1978;46:518–539. [PubMed] [Google Scholar]
- 93.Queyrat L. Erythroplasie de gland. Bull Soc Franc Derm Syph. 1911;22:378–382. [Google Scholar]
- 94.Shafer WG, Waldron CA. Erythroplakia of the oral cavity. Cancer. 1975;36:1021–1028. doi: 10.1002/1097-0142(197509)36:3<1021::AID-CNCR2820360327>3.0.CO;2-W. Available from: http://dx.doi.org/10.1002/1097-0142(197509)36:3<1021::AID-CNCR2820360327>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 95.LiVolsi VA. Can we agree to disagree? Hum Pathol. 2003;34:1081–1082. doi: 10.1053/j.humpath.2003.09.005. Available from: http://dx.doi.org/10.1053/j.humpath.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 96.Ackerman AB. Can we agree to disagree - reflections. Hum Pathol. 2004;35:643–644. doi: 10.1016/j.humpath.2003.12.001. Available from: http://dx.doi.org/10.1016/j.humpath.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 97.Foucar E. Diagnostic precision and accuracy in interpretation of specimens from cancer screening programs. Semin Diagn Pathol. 2005;22:147–155. doi: 10.1053/j.semdp.2006.02.002. Available from: http://dx.doi.org/10.1053/j.semdp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 98.Fischer DJ, Epstein JB, Morton TH, Schwartz SM. Interobserver reliability in the histopathologic diagnosis of oral pre-malignant and malignant lesions. J Oral Pathol Med. 2004;33:65–70. doi: 10.1111/j.1600-0714.2004.0037n.x. Available from: http://dx.doi.org/10.1111/j.1600-0714.2004.0037n.x. [DOI] [PubMed] [Google Scholar]
- 99.Kujan O, Khattab A, Oliver RJ, Roberts SA, Thakker N, Sloan P. Why oral histopathology suffers inter-observer variability on grading oral epithelial dysplasia: an attempt to understand the sources of variation. Oral Oncol. 2007;43:224–231. doi: 10.1016/j.oraloncology.2006.03.009. Available from: http://dx.doi.org/10.1016/j.oraloncology.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 100.McLaren KM, Burnett RA, Goodlad JR, et al. Consistency of histopathological reporting of laryngeal dysplasia - the Scottish pathology consistency group. Histopathology. 2000;37:460–463. doi: 10.1046/j.1365-2559.2000.00998.x. Available from: http://dx.doi.org/10.1046/j.1365-2559.2000.00998.x. [DOI] [PubMed] [Google Scholar]
- 101.Lessells AM, Burnett RA, Goodlad JR, et al. Comment on a recent paper and editorial on the subject of dysplasia classification. J Pathol. 2002;198:131–132. doi: 10.1002/path.1181. Available from: http://dx.doi.org/10.1002/path.1181. [DOI] [PubMed] [Google Scholar]
- 102.Montgomery E. Is there a way for pathologists to decrease interobserver variability in the diagnosis of dysplasia? Arch Pathol Lab Med. 2005;129:174–176. doi: 10.5858/2005-129-174-ITAWFP. [DOI] [PubMed] [Google Scholar]
- 103.Morgenroth K. Über die Früherkennung maligner Veränderungen der Mundschleimhaut. Zahnärztl Mitt. 1969;59:341–344. [PubMed] [Google Scholar]
- 104.Kränzl B, Sorgo E, Wutka P. Untersuchung zur Anwendbarkeit der Zytodiagnostik für die Früherkennung von Mundhöhlenkrebs an der Klinik für Mundchirurgie. Wien Klin Wochenschr. 1974;86:648–650. [PubMed] [Google Scholar]
- 105.Wächter R. Technik und Indikationen der Zytodiagnostik für die Krebsfrüherkennung. SSO Schweiz Monatsschr Zahnheilk. 1978;88:829–836. [PubMed] [Google Scholar]
- 106.McLeod NMH, Saeed NR, Ali EA. Oral cancer: Delays in referral and diagnosis persist. Br Dent J. 2005;198:681–684. doi: 10.1038/sj.bdj.4812381. Available from: http://dx.doi.org/10.1038/sj.bdj.4812381. [DOI] [PubMed] [Google Scholar]
- 107.Mignogna MD, Fedele S, Lo Russo L, Ruoppo E, Lo Muzio L. Oral and pharyngeal cancer: lack of prevention and early detection by health care providers. Eur J Cancer Prev. 2001;10:381–383. doi: 10.1097/00008469-200108000-00014. Available from: http://dx.doi.org/10.1097/00008469-200108000-00014. [DOI] [PubMed] [Google Scholar]
- 108.Kujan O, Glenny AM, Oliver RJ, Thakker N, Sloan P. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database of Systematic Reviews. 2006;(3):CD004150. doi: 10.1002/14651858.CD004150.pub2. Available from: http://dx.doi.org/10.1002/14651858.CD004150.pub2. [DOI] [PubMed] [Google Scholar]
- 109.Sankaranarayanan R, Ramadas K, Thomas G, Muwonge R, Thara S, Mathew B, Rajan B. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet. 2005;365:1927–1933. doi: 10.1016/S0140-6736(05)66658-5. Available from: http://dx.doi.org/10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 110.Dalcq A. Vital staining with toluidine blue and its metachromatic manifestations. C R Seances Soc Biol Fil. 1952;146:1408–1411. [PubMed] [Google Scholar]
- 111.Little JW. Toluidine blue staining in the detection of oral precancerous and malignant lesions. Oral Surg Oral Med Oral Pathol. 1984;57:379–382. doi: 10.1016/0030-4220(84)90154-3. Available from: http://dx.doi.org/10.1016/0030-4220(84)90154-3. [DOI] [PubMed] [Google Scholar]
- 112.Gupta A, Singh M, Ibrahim R, Mehrotra R. Utility of toluidine blue staining and brush biopsy in precancerous and cancerous oral lesions. Acta Cytol. 2007;51:788–794. doi: 10.1159/000325843. [DOI] [PubMed] [Google Scholar]
- 113.Du GF, Li CZ, Chen HZ, Chen XM, Xiao Q, Cao ZG, Shang SH, Cai X. Rose bengal staining in detection of oral precancerous and malignant lesions with colorimetric evaluation: a pilot study. Int J Cancer. 2007;120:1958–1963. doi: 10.1002/ijc.22467. Available from: http://dx.doi.org/10.1002/ijc.22467. [DOI] [PubMed] [Google Scholar]
- 114.Farah CS, McCullough MJ. A pilot case control study on the efficacy of acetic wash and chemiluminescent illumination (ViziLite®) in the visualisation of oral mucosal white lesions. Oral Oncol. 2007;43:820–824. doi: 10.1016/j.oraloncology.2006.10.005. Available from: http://dx.doi.org/10.1016/j.oraloncology.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 115.Oh ES, Laskin DM. Efficacy of the ViziLite® system in the identification of oral lesions. J Oral Maxillofac Surg. 2007;65:424–426. doi: 10.1016/j.joms.2006.10.055. Available from: http://dx.doi.org/10.1016/j.joms.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 116.Ram S, Siar CH. Chemiluminescence as a diagnostic aid in the detection of oral cancer and potentially malignant epithelial lesions. Int J Oral Maxillofac Surg. 2005;34:521–527. doi: 10.1016/j.ijom.2004.10.008. Available from: http://dx.doi.org/10.1016/j.ijom.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 117.Epstein JB, Silverman S, jr, Epstein JD, Lonky SA, Bride MA. Analysis of oral lesion biopsies identified and evaluated by visual examination, chemiluminescence and toluidine blue. Oral Oncol. 2008;44:538–544. doi: 10.1016/j.oraloncology.2007.08.011. Available from: http://dx.doi.org/10.1016/j.oraloncology.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 118.Policard A. Etude sur les aspects offerts par des tumeurs experimentales examinées a la lumière de Wood. C R Soc Biol. 1924;91:1423–1424. [Google Scholar]
- 119.Lipson RL, Baldes JE, Olsen AM. Hematoporphyrin derivative: a new aid for endoscopic detection of malignant tissue. J Thorac Cardiovasc Surg. 1962;42:623–629. [PubMed] [Google Scholar]
- 120.Delank W, Khanavkar B, Nakhosteen JA, Stoll W. A pilot study of autofluorescent endoscopy for the in vivo detection of laryngeal cancer. Laryngoscope. 2000;110:368–373. doi: 10.1097/00005537-200003000-00007. Available from: http://dx.doi.org/10.1097/00005537-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 121.Arens C, Reussner D, Woenkhaus J, Leunig A, Betz CS, Glanz H. Indirect fluorescence laryngoscopy in the diagnosis of precancerous and cancerous laryngeal lesions. Eur Arch Otorhinolaryngol. 2007;264:621–626. doi: 10.1007/s00405-007-0251-y. Available from: http://dx.doi.org/10.1007/s00405-007-0251-y. [DOI] [PubMed] [Google Scholar]
- 122.Svistun E, Alizadeh-Naderi E, El-Naggar A, Jacob R, Gillenwater A, Richards-Kortum R. Vision enhancement system for detection of oral cavity neoplasia based on autofluorescence. Head Neck. 2004;26:205–215. doi: 10.1002/hed.10381. Available from: http://dx.doi.org/10.1002/hed.10381. [DOI] [PubMed] [Google Scholar]
- 123.Zeng H, Weiss A, Cline R, MacAulay CE. Real-time endoscopic fluorescence imaging for early cancer detection in the gastrointestinal tract. Bioimaging. 1998;6:151–165. doi: 10.1002/1361-6374(199812)6:4<151::AID-BIO1>3.0.CO;2-G. Available from: http://dx.doi.org/10.1002/1361-6374(199812)6:4<151::AID-BIO1>3.0.CO;2-G. [DOI] [Google Scholar]
- 124.Rasetti L, Rubino G, Drago W. Ferrochelatase, ALA-dehydrase and ALA-synthetase activity in human tumor tissue. Minerva Med. 1966;57:2834–2837. [PubMed] [Google Scholar]
- 125.Leunig A, Betz CS, Heinrich P, Janda P, Baumgartner R. Fluoreszenzdiagnostik von Karzinomen im Mund-Rachen-Kehlkopf-Bereich nach Applikation von 5-Aminolävulinsäure. Laryngo-Rhino-Otol. 2002;81:807–814. doi: 10.1055/s-2002-35769. Available from: http://dx.doi.org/10.1055/s-2002-35769. [DOI] [PubMed] [Google Scholar]
- 126.Leunig A, Betz CS, Mehlmann M, Stepp H, Arbogast S, Grevers G, Baumgartner R. Detection of squamous cell carcinoma of the oral cavity by imaging 5-aminolevulinic acid-induced protoporphyrin IX fluorescence. Laryngoscope. 2000;110:78–83. doi: 10.1097/00005537-200001000-00015. Available from: http://dx.doi.org/10.1097/00005537-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 127.Mao L, Lee JS, Fan YH, Ro JY, Batsakis JG, Lippman S, Hittelman W, Hong WK. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nature Med. 1996;2:682–685. doi: 10.1038/nm0696-682. Available from: http://dx.doi.org/10.1038/nm0696-682. [DOI] [PubMed] [Google Scholar]
- 128.Rosin MP, Cheng X, Poh C, et al. Use of allelic loss to predict malignant risk for low-grade oral epithelial dysplasia. Clin Cancer Res. 2000;6:357–362. [PubMed] [Google Scholar]
- 129.Ha PK, Pai SI, Westra WH, Gillison ML, Tong BC, Sidransky D, Califano JA. Real-time quantitative PCR demonstrates low prevalence of human papillomavirus type 16 in premalignant and malignant lesions of the oral cavity. Clin Cancer Res. 2002;8:1203–1209. [PubMed] [Google Scholar]
- 130.Herrero R, Castellsagué X, Pawlita M, et al. Human papillomavirus and oral cancer: the international agency for research on cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 131.Hullmann M, Reichert T, Dahse R, von Eggeling F, Pistner H, Kosmehl H, Driemel O. Orale Zytologie - historische Entwicklung, aktueller Stand und Ausblick. Mund Kiefer Gesichts Chir. 2007;11:1–9. doi: 10.1007/s10006-006-0041-5. Available from: http://dx.doi.org/10.1007/s10006-006-0041-5. [DOI] [PubMed] [Google Scholar]
- 132.Koburg E, Steinbach E. Toluidinblau-Vitalfärbung zur Früherkennung und Abgrenzung maligner Kehlkopftumoren. Eur Arch Otorhinolaryngol. 1969;194:320–323. doi: 10.1007/BF02594485. Available from: http://dx.doi.org/10.1007/BF02594485. [DOI] [PubMed] [Google Scholar]
- 133.Olofsson J. Laryngeal carcinoma: problems in diagnosis and classification. J Otolaryngol. 1982;11:167–177. [PubMed] [Google Scholar]
- 134.Harries ML, Lam S, Macaulay C, Qu JA, Palcic B. Diagnostic imaging of the larynx: autofluorescence of laryngeal tumors using the helium-cadmium laser. J Laryngol Otol. 1995;109:108–110. doi: 10.1017/S002221510012941X. Available from: http://dx.doi.org/10.1017/S002221510012941X. [DOI] [PubMed] [Google Scholar]
- 135.Arens C, Malzahn K, Dias O, Andrea M, Glanz H. Endoskopische bildgebende Verfahren in der Diagnostik des Kehlkopfkarzinoms und seiner Vorstufen. Laryngorhinootolgie. 1999;78:685–691. doi: 10.1055/s-1999-8775. Available from: http://dx.doi.org/10.1055/s-1999-8775. [DOI] [PubMed] [Google Scholar]
- 136.Žargi M, Fajdiga I, Šmid L. Autofluorescence imaging in the diagnosis of laryngeal cancer. Eur Arch Otorhinolaryngol. 2000;257:17–23. doi: 10.1007/PL00007506. Available from: http://dx.doi.org/10.1007/PL00007506. [DOI] [PubMed] [Google Scholar]
- 137.Malzahn K, Dreyer T, Glanz H, Arens C. Autofluorescence endoscopy in the diagnosis of early laryngeal cancer and its precursor lesions. Laryngoscope. 2002;112:488–493. doi: 10.1097/00005537-200203000-00015. Available from: http://dx.doi.org/10.1097/00005537-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 138.Arens C, Dreyer T, Glanz H, Malzahn K. Indirect autofluorescence laryngoscopy in the diagnosis of laryngeal cancer and its precursor lesions. Eur Arch Otorhinolaryngol. 2004;261:71–76. doi: 10.1007/s00405-003-0653-4. Available from: http://dx.doi.org/10.1007/s00405-003-0653-4. [DOI] [PubMed] [Google Scholar]
- 139.Mehlmann M, Betz CS, Stepp H, Arbogast S, Baumgartner R, Grevers G, Leunig A. Fluorescence staining of laryngeal neoplasms after topical application of 5-aminolevulinic acid: preliminary results. Lasers Surg Med. 1999;25:414–420. doi: 10.1002/(SICI)1096-9101(1999)25:5<414::AID-LSM8>3.0.CO;2-E. Available from: http://dx.doi.org/10.1002/(SICI)1096-9101(1999)25:5<414::AID-LSM8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 140.Franco RA., jr Aminolevulinic acid 585 nm pulsed dye laser photodynamic treatment of laryngeal keratosis with atypia. Otolaryngol Head Neck Surg. 2007;136:882–887. doi: 10.1016/j.otohns.2007.01.026. Available from: http://dx.doi.org/10.1016/j.otohns.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 141.Hoffman HAT, Karnell LH, Shah JP, Ariyan S, Brown GS, Fee WE, Glass AG, Goepfert H, Ossoff RH, Fremgen AM. Hypopharyngeal cancer patient care evaluation. Laryngoscope. 1997;107:1005–1017. doi: 10.1097/00005537-199708000-00001. Available from: http://dx.doi.org/10.1097/00005537-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 142.Zhou L, Cheng L, Tao L, Jia X, Lu Y, Liao P. Detection of hypopharyngeal squamous cell carcinoma using serum proteomics. Acta Oto-Laryngologica. 2006;126:853–860. doi: 10.1097/00005537-199708000-00001. Available from: http://dx.doi.org/10.1097/00005537-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 143.Eckel HE, Staar S, Volling P, Sittel C, Damm M, Jungehülsing M. Surgical treatment for hypopharynx carcinoma: feasibility, mortality, and results. Otolaryngol Head Neck Surg. 2001;124:561–569. doi: 10.1067/mhn.2001.115060. Available from: http://dx.doi.org/10.1067/mhn.2001.115060. [DOI] [PubMed] [Google Scholar]
- 144.Watanabe A, Hosokawa M, Taniguchi M, Tsujie H, Sasaki S. Impact of endoscopic screening on early detection of hypopharyngeal cancer. Head Neck. 2006;28:350–354. doi: 10.1002/hed.20336. Available from: http://dx.doi.org/10.1002/hed.20336. [DOI] [PubMed] [Google Scholar]
- 145.Pinkston JA, Cole P. Incidence rates of salivary gland tumors: results from a population-based study. Otolaryngol Head Neck Surg. 1999;120:834–840. doi: 10.1016/S0194-5998(99)70323-2. Available from: http://dx.doi.org/10.1016/S0194-5998(99)70323-2. [DOI] [PubMed] [Google Scholar]
- 146.Belsky JL, Takeichi N, Yamamoto T, Cihak RW, Hirose F, Ezaki H, Inoue S, Blot WJ. Salivary gland neoplasms following atomic radiation: additional cases and reanalysis of combined data in a fixed population, 1957-1970. Cancer. 1975;35:555–559. doi: 10.1002/1097-0142(197502)35:2<555::AID-CNCR2820350240>3.0.CO;2-G. Available from: http://dx.doi.org/10.1002/1097-0142(197502)35:2<555::AID-CNCR2820350240>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 147.Mihailescu D, Shore-Freedman E, Mukani S, Lubin J, Ron E, Schneider AB. Multiple neoplasms in an irradiated cohort: pattern of occurrence and relationship to thyroid cancer outcome. J Clin Endocrinol Metab. 2002;87:3236–3241. doi: 10.1210/jc.87.7.3236. Available from: http://dx.doi.org/10.1210/jc.87.7.3236. [DOI] [PubMed] [Google Scholar]
- 148.Hoffman DA, McConahey WM, Fraumeni JF, jr, Kurland LT. Cancer incidence following treatment of hyperthyroidism. Int J Epidemiol. 1982;11:218–224. doi: 10.1093/ije/11.3.218. Available from: http://dx.doi.org/10.1093/ije/11.3.218. [DOI] [PubMed] [Google Scholar]
- 149.Auvinen A, Hietanen M, Luukkonen R, Koskela RS. Brain tumors and salivary gland cancers among cellular telephone users. Epidemiology. 2002;13:356–359. doi: 10.1097/00001648-200205000-00018. Available from: http://dx.doi.org/10.1097/00001648-200205000-00018. [DOI] [PubMed] [Google Scholar]
- 150.Horn-Ross PL, Ljung BM, Morrow M. Environmental factors and the risk of salivary gland cancer. Epidemiology. 1997;8:414–419. doi: 10.1097/00001648-199707000-00011. Available from: http://dx.doi.org/10.1097/00001648-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 151.Milham S., jr Cancer mortality pattern associated with exposure to metals. Ann N Y Acad Sci. 1976;271:243–249. doi: 10.1111/j.1749-6632.1976.tb23118.x. Available from: http://dx.doi.org/10.1111/j.1749-6632.1976.tb23118.x. [DOI] [PubMed] [Google Scholar]
- 152.Dietz A, Barme B, Gewelke U, Sennewald E, Heller WD, Maier H. Zur Epidemiologie der Parotistumoren - eine Fallkontrollstudie. HNO. 1993;41:83–90. [PubMed] [Google Scholar]
- 153.Swanson GM, Burns PB. Cancers of the salivary gland: workplace risks among women and men. Ann Epidemiol. 1997;7:369–374. doi: 10.1016/S1047-2797(97)00041-0. Available from: http://dx.doi.org/10.1016/S1047-2797(97)00041-0. [DOI] [PubMed] [Google Scholar]
- 154.Muscat JE, Wynder EL. A case/control study of risk factors for major salivary gland cancer. Otolaryngol Head Neck Surg. 1998;118:195–198. doi: 10.1016/S0194-5998(98)80013-2. Available from: http://dx.doi.org/10.1016/S0194-5998(98)80013-2. [DOI] [PubMed] [Google Scholar]
- 155.Droese M, Haubrich J, Tute M. Stellenwert der Punktionszytologie in der Diagnostik der Speicheldrüsentumoren. Schweiz Med Wochenschr. 1978;108:933–935. [PubMed] [Google Scholar]
- 156.Knapp I, Mann W, Wachter W. Stellenwert der ultraschallgesteuerten Feinnadelbiopsie in der Diagnostik unklarer Halstumoren. Laryngo Rhino Otol. 1989;68:683–689. doi: 10.1055/s-2007-998430. Available from: http://dx.doi.org/10.1055/s-2007-998430. [DOI] [PubMed] [Google Scholar]
- 157.Siegert R, Schrader B, Barreton G. Die ultraschallgeführte Feinnadelpunktion pathologischer Raumforderungen im Kopf-Halsbereich. HNO. 1990;38:287–291. [PubMed] [Google Scholar]
- 158.Schoengen A, Binder T, Krause HR, Stussak G, Zeelen U. Der Wert der Feinnadelaspirationszytologie bei tumorverdächtigen Speicheldrüsenschwellungen. HNO. 1995;43:239–243. [PubMed] [Google Scholar]
- 159.Zbären P, Schar C, Hotz MA, Loosli H. Value of fine-needle aspiration cytology of parotid gland masses. Laryngoscope. 2001;111:1989–1992. doi: 10.1097/00005537-200111000-00023. Available from: http://dx.doi.org/10.1097/00005537-200111000-00023. [DOI] [PubMed] [Google Scholar]
- 160.Schröder U, Eckel HE, Rasche V, Arnold G, Ortmann M, Stennert E. Wertigkeit der Feinnadelpunktionszytologie bei Neoplasien der Glandula parotis. HNO. 2000;48:421–429. doi: 10.1007/s001060050592. [DOI] [PubMed] [Google Scholar]
- 161.Driemel O, Kraft K, Hemmer J. DNA-Ploidie und proliferative Aktivität bei Speicheldrüsentumoren. Mund Kiefer Gesichts Chir. 2007;11:139–144. doi: 10.1007/s10006-007-0060-x. Available from: http://dx.doi.org/10.1007/s10006-007-0060-x. [DOI] [PubMed] [Google Scholar]
- 162.Driemel O, Kraft K, Hemmer J. Flow cytometric S-phase fraction contributes to diagnosis of diploid malignant salivary gland tumours. Int J Oral Maxillofac Surg. 2006;35:947–950. doi: 10.1016/j.ijom.2006.03.025. Available from: http://dx.doi.org/10.1016/j.ijom.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 163.Driemel O, Maier H, Kraft K, Haase S, Hemmer J. Flow cytometric DNA ploidy in salivary gland tumors. Oncol Rep. 2005;13:161–165. [PubMed] [Google Scholar]
- 164.Slaughter DP, Southwick HW, Smejkal W. "Field cancerization" in oral stratified squamous epithelium - clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::AID-CNCR2820060515>3.0.CO;2-Q. Available from: http://dx.doi.org/10.1002/1097-0142(195309)6:5<963::AID-CNCR2820060515>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 165.Hao SP. Six primary squamous cell carcinomas of the head and neck. Auris Nasus Larynx. 1998;25:101–104. doi: 10.1016/S0385-8146(97)00015-1. Available from: http://dx.doi.org/10.1016/S0385-8146(97)00015-1. [DOI] [PubMed] [Google Scholar]
- 166.Braakhuis BJM, Brakenhoff RH, Leemans CR. Second field tumors: a new opportunity for cancer prevention? Oncologist. 2005;10:493–500. doi: 10.1634/theoncologist.10-7-493. Available from: http://dx.doi.org/10.1634/theoncologist.10-7-493. [DOI] [PubMed] [Google Scholar]
- 167.Warren S, Gates O. Multiple primary malignant tumors. A survey of the literature and a statistical study. Am J Cancer. 1932;16:1358–1414. [Google Scholar]
- 168.Shikhani AH, Matanoski GM, Jones MM, Kashima HK, Johns ME. Multiple primary malignancies in head and neck cancer. Arch Otolaryngol Head Neck Surg. 1986;112:1172–1179. doi: 10.1001/archotol.1986.03780110048006. [DOI] [PubMed] [Google Scholar]
- 169.Shaha AR, Hoover EL, Mitrani M, Marti JR, Krespi YP. Synchronicity, multicentricity, and metachronicity of head and neck cancer. Head Neck Surg. 1988;10:225–228. doi: 10.1002/j.1930-2398.1988.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 170.Panosetti E, Luboinski B, Mamelle G, Richard JM. Multiple synchronous and metachronous cancers of the upper aerodigestive tract: a nine-year study. Laryngoscope. 1989;99:1267–1273. doi: 10.1288/00005537-198912000-00011. Available from: http://dx.doi.org/10.1288/00005537-198912000-00011. [DOI] [PubMed] [Google Scholar]
- 171.Schwartz LH, Ozsahin M, Zhang GN, Touboul E, de Vafaire F, Andolenko P, Lacau-Saint-Guily J, Lnugier A, Schlienger M. Synchronous and metachronous head and neck carcinomas. Cancer. 1994;74:1933–1938. doi: 10.1002/1097-0142(19941001)74:7<1933::AID-CNCR2820740718>3.0.CO;2-X. Available from: http://dx.doi.org/10.1002/1097-0142(19941001)74:7<1933::AID-CNCR2820740718>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 172.Jäckel MC, Martin A, Steiner W. Lokale und regionäre Rezidive von laserchirurgisch behandelten Plattenepithelkarzinomen des oberen Aerodigestivtrakts - Häufigkeit, Latenz und Prognose sowie Einfluss der initialen Tumorparameter. HNO. 2007;55:1001–1008. doi: 10.1007/s00106-007-1590-0. [DOI] [PubMed] [Google Scholar]
- 173.Gao X, Fisher SG, Mohideen N, Emami B. Second primary cancers in patients with laryngeal cancer: a population-based study. Int J Radio Oncol Biol Phys. 2003;56:427–435. doi: 10.1016/S0360-3016(02)04613-8. Available from: http://dx.doi.org/10.1016/S0360-3016(02)04613-8. [DOI] [PubMed] [Google Scholar]
- 174.Gleich LL, Ryzenman J, Gluckman JL, Wilson KM, Barrett WL, Redmond KP. Recurrent advanced (T3 or T4) head and neck squamous cell carcinoma: is salvage possible? Arch Otolaryngol Head Neck Surg. 2004;130:35–38. doi: 10.1001/archotol.130.1.35. Available from: http://dx.doi.org/10.1001/archotol.130.1.35. [DOI] [PubMed] [Google Scholar]
- 175.Glanz H. Late recurrence of radiation induced cancer of the larynx. Clin Otolaryngol Allied Sci. 1976;1:123–129. doi: 10.1111/j.1365-2273.1976.tb00863.x. Available from: http://dx.doi.org/10.1111/j.1365-2273.1976.tb00863.x. [DOI] [PubMed] [Google Scholar]
- 176.Martin G, Glanz H, Kleinsasser O. Ionisierende Strahlen und Kehlkopfkrebse. Laryngo Rhino Otol. 1979;58:187–195. [PubMed] [Google Scholar]
- 177.Roostaeian J, Suh JD, Sercarz JA, Abemayor E, Lee JT, Blackwell KE. Factors affecting cancer recurrence after microvascular flap reconstruction of the head and neck. Laryngoscope. 2005;115:1391–1394. doi: 10.1097/01.MLG.0000166706.61652.15. Available from: http://dx.doi.org/10.1097/01.MLG.0000166706.61652.15. [DOI] [PubMed] [Google Scholar]
- 178.Scherübl H, Steinberg J, Schwertner C, Mir-Salim P, Stölzel U, de Villiers EM. "Field cancerization" im oberen Aerodigestivtrakt - Überwachungsempfehlungen für Risikopersonen. HNO. 2008;56:603–608. doi: 10.1007/s00106-007-1616-7. [DOI] [PubMed] [Google Scholar]
- 179.Watanabe A, Hosokawa M, Taniguchi M, Sasaki S. Periodic pharyngolaryngoscopy detects early head and neck cancer and improves survival in esophageal cancer. Ann Thorac Surg. 2003;76:1699–1705. doi: 10.1016/S0003-4975(03)00957-3. Available from: http://dx.doi.org/10.1016/S0003-4975(03)00957-3. [DOI] [PubMed] [Google Scholar]
- 180.Möschler O, Spahn TW, Middelberg-Bisping C, Grosse-Thie W, Christoph B, Kloeppel G, Mueller MK. Chromoendoscopy is a valuable tool for screening of high-risk patients with head and neck cancer for early detection of esophageal cancer. Digestion. 2006;73:160–166. doi: 10.1159/000094523. Available from: http://dx.doi.org/10.1159/000094523. [DOI] [PubMed] [Google Scholar]
- 181.Merkx MAW, van Gulick JJM, Marres HAM, Kaanders JHAM, Bruaset J, Verbeek A, de Wilde PCM. Effectiveness of routine follow-up of patients treated for T1-2 N0 oral squamous cell carcinomas of the floor of mouth and tongue. Head Neck. 2006;28:1–7. doi: 10.1002/hed.20296. Available from: http://dx.doi.org/10.1002/hed.20296. [DOI] [PubMed] [Google Scholar]
- 182.de Visscher AV, Manni JJ. Routine long-term follow-up in patients treated with curative intent for squamous cell carcinoma of the larynx, pharynx, and oral cavity - does it make sense? Arch Otolaryngol Head Neck Surg. 1994;120:934–939. doi: 10.1001/archotol.1994.01880330022005. [DOI] [PubMed] [Google Scholar]
- 183.Rassekh CH, Johnson JT, Eibling DE. Circulating markers in squamous cell carcinoma of the head and neck: a review. Eur J Cancer B Oral Oncol. 1994;30B:23–28. doi: 10.1016/0964-1955(94)90046-9. Available from: http://dx.doi.org/10.1016/0964-1955(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 184.Tannapfel A, Weber A. Tumor markers in squamous cell carcinoma of the head and neck: clinical effectiveness and prognostic value. Eur Arch Otorhinolaryngol. 2001;258:83–88. doi: 10.1007/s004050000303. Available from: http://dx.doi.org/10.1007/s004050000303. [DOI] [PubMed] [Google Scholar]
- 185.Brown JJ, Xu H, Nishitani J, Mohammed H, Osborne R, Teklehaimanot S, Gill G, Liu X. Potential biomarkers for head and neck squamous cell carcinoma. Laryngoscope. 2003;113:393–400. doi: 10.1097/00005537-200303000-00001. Available from: http://dx.doi.org/10.1097/00005537-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 186.Nagler RM, Barak M, Peled M, Ben-Aryeh H, Filatov M, Laufer D. Early diagnosis and treatment monitoring roles of tumor markers Cyfra 21-1 and TPS in oral squamous cell carcinoma. Cancer. 1999;85:1018–1025. doi: 10.1002/(SICI)1097-0142(19990301)85:5<1018::AID-CNCR2>3.0.CO;2-R. Available from: http://dx.doi.org/10.1002/(SICI)1097-0142(19990301)85:5<1018::AID-CNCR2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 187.Doweck I, Barak M, Uri N, Greenberg E. The prognostic value of the tumour marker Cyfra 21-1 in carcinoma of head and neck and its role in early detection of recurrent disease. Br J Cancer. 2000;83:1696–1701. doi: 10.1054/bjoc.2000.1502. Available from: http://dx.doi.org/10.1054/bjoc.2000.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Arlt A, Luckhaupt H, Hildmann H. Diagnostik von Kopf-Hals-Karzinom-Rezidiven mit dem Tumormarker SSC-Antigen. Laryngo Rhino Otol. 2000;79:207–212. doi: 10.1055/s-2000-8796. Available from: http://dx.doi.org/10.1055/s-2000-8796. [DOI] [PubMed] [Google Scholar]
- 189.Welkoborsky HJ, Bernauer HS, Riazimand HS, Jacob R, Mann WJ, Hinni ML. Patterns of chromosomal aberrations in metastasizing and nonmetastasizing squamous cell carcinomas of the oropharynx and hypopharynx. Ann Otol Rhinol Laryngol. 2000;109:401–410. doi: 10.1177/000348940010900411. [DOI] [PubMed] [Google Scholar]
- 190.Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, Jerin J, Young J, Byars L, Nutt R. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–1379. [PubMed] [Google Scholar]
- 191.Gordin A, Daitzchman M, Doweck I, Yefremov N, Golz A, Keidar Z, Bar-Shalom R, Kuten A, Israel O. Fluorodeoxyglucose-positron emission tomography/computed tomography imaging in patients with carcinoma of the larynx: diagnostic accuracy and impact on clinical management. Laryngoscope. 2006;116:273–278. doi: 10.1097/01.mlg.0000197930.93582.32. Available from: http://dx.doi.org/10.1097/01.mlg.0000197930.93582.32. [DOI] [PubMed] [Google Scholar]
- 192.Brouwer J, Senft A, de Bree R, Comans EF, Golding RP, Castelijns JA, Hoekstra OS, Leemans CR. Screening for distant metastases in patients with head and neck cancer: is there a role for (18)FDG-PET? Oral Oncol. 2006;42:275–280. doi: 10.1016/j.oraloncology.2005.07.009. Available from: http://dx.doi.org/10.1016/j.oraloncology.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 193.Schöder H, Yeung HWD, Gonen M, Kraus D, Larson SM. Head and neck cancer: clinical usefulness and accuracy of PET/CT image fusion. Radiology. 2004;231:65–72. doi: 10.1148/radiol.2311030271. Available from: http://dx.doi.org/10.1148/radiol.2311030271. [DOI] [PubMed] [Google Scholar]
- 194.Basu D, Siegel BA, McDonald DJ, Nussenbaum B. Detection of occult bone metastases from head and neck squamous cell carcinoma - impact of positron emission tomography-computed tomography with fluorodeoxyglucose F 18. Arch Otolaryngl Head Neck Surg. 2007;133:801–805. doi: 10.1001/archotol.133.8.801. Available from: http://dx.doi.org/10.1001/archotol.133.8.801. [DOI] [PubMed] [Google Scholar]
- 195.Fakhry N, Lussato D, Jacob T, Giorgi R, Giovanni A, Zanart M. Comparison between PET and PET/CT in recurrent head and neck cancer and clinical implications. Eur Arch Otorhinolaryngol. 2007;264:531–538. doi: 10.1007/s00405-006-0225-5. Available from: http://dx.doi.org/10.1007/s00405-006-0225-5. [DOI] [PubMed] [Google Scholar]
- 196.Ha PK, Hdeib A, Goldenberg D, et al. The role of positron emission tomography and computed tomography fusion in the management of early-stage and advanced-stage primary head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:12–16. doi: 10.1001/archotol.132.1.12. Available from: http://dx.doi.org/10.1001/archotol.132.1.12. [DOI] [PubMed] [Google Scholar]
- 197.Roh JL, Yeo NK, Kim JS, Cho KJ, Choi SH, Nam SY, Kim SY. Utility of 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography and positron emission tomography/computed tomography imaging in the preoperative staging of head and neck squamous cell carcinoma. Oral Oncol. 2007;43:887–893. doi: 10.1016/j.oraloncology.2006.10.011. Available from: http://dx.doi.org/10.1016/j.oraloncology.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 198.Ritoe SC, Bergman H, Krabbe PFM, Kaanders JHAM, van den Hoogen FJA, Verbeek ALM, Marres HAM. Cancer recurrence after total laryngectomy: treatment options, survival, and complications. Head Neck. 2006;28:383–388. doi: 10.1002/hed.20350. Available from: http://dx.doi.org/10.1002/hed.20350. [DOI] [PubMed] [Google Scholar]
- 199.Weaver BAA, Cleveland DW. Does aneuploidy cause cancer? Curr Opin Cell Biol. 2006;18:658–667. doi: 10.1016/j.ceb.2006.10.002. Available from: http://dx.doi.org/10.1016/j.ceb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 200.Hanson J, Bruchmüller W, Nischwitz AS. Krebsvorstufen und die Früherkennung von Pharynx- und Larynx-Karzinomen. Arch Geschwulstforschung. 1989;59:99–105. [PubMed] [Google Scholar]
- 201.Böcking A, Stockhausen J, Meyer-Ebrecht D. Towards a single cell cancer diagnosis - multimodal and monocellular measurements of markers and morphology (5M) Cell Oncol. 2004;26:73–79. doi: 10.1155/2004/792424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tárnok A, Gerstner A. Clinical applications of laser-scanning cytometry. Cytometry (Clin Cytometry) 2002;50:133–143. doi: 10.1002/cyto.10099. [DOI] [PubMed] [Google Scholar]
- 203.Dittrich W, Göhde W. Automatisches Meß- und Zählgerät für die Teilcheneiner Dispersion. Deutsches Patentamt; 1971. Available from: http://www.uni-muenster.de/imperia/md/content/wwu/afo/afoab2009/leitthemen/patente/de1815352a1_g__hde.pdf. [Google Scholar]
- 204.Göhde W, Dittrich W. Simultane Impulsfluorimetrie des DNS- und Proteingehaltes von Tumorzellen. Z Anal Chem. 1970;252:328–330. doi: 10.1007/BF00571362. Available from: http://dx.doi.org/10.1007/BF00571362. [DOI] [Google Scholar]
- 205.Kraus H, Bremke U, Wedding C. Ultraschnelle Messungen der DNS-Fluoreszenz an normalen Geweben, Entzündungen und Tumoren von Kopf und Hals. Archiv klin. exp. Ohren-, Nasen-, und Kehlkopfheilk. 1972;2(Bd. 202.) [PubMed] [Google Scholar]
- 206.Holm LE. Cellular DNA amount of squamous cell carcinomas of the head and neck region in relation to prognosis. Laryngoscope. 1982;92:1064–1069. doi: 10.1288/00005537-198209000-00018. Available from: http://dx.doi.org/10.1288/00005537-198209000-00018. [DOI] [PubMed] [Google Scholar]
- 207.Wang XL, Chen RM, Wang ZS, Chen WL, Zuo LF. Flow cytometric DNA-ploidy as a prognostic indicator in oral squamous cell carcinoma. Chin Med J. 1990;103:572–575. [PubMed] [Google Scholar]
- 208.Hemmer J, Kreidler J. Flow cytometric DNA ploidy analysis of squamous cell carcinoma of the oral cavity - comparison with clinical staging and histologic grading. Cancer. 1990;66:317–320. doi: 10.1002/1097-0142(19900715)66:2<317::AID-CNCR2820660220>3.0.CO;2-X. Available from: http://dx.doi.org/10.1002/1097-0142(19900715)66:2<317::AID-CNCR2820660220>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 209.Bueno PR, Gias LN, Delgado RG, Cebollada JD, Díaz González FJ. Tumor DNA content as a prognostic indicator in squamous cell carcinoma of the oral cavity and tongue base. Head Neck. 1998;20:232–239. doi: 10.1002/(SICI)1097-0347(199805)20:3<232::AID-HED8>3.0.CO;2-1. Available from: http://dx.doi.org/10.1002/(SICI)1097-0347(199805)20:3<232::AID-HED8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 210.Hemmer J, Kraft K, Kreidler J. The significance of DNA flow cytometry in predicting survival and delayed clinical manifestation of occult lymph node metastasis to the untreated neck in patients with oral squamous cell carcinoma. J Cranio Maxillofac Surg. 1998;26:405–410. doi: 10.1016/S1010-5182(98)80076-0. Available from: http://dx.doi.org/10.1016/S1010-5182(98)80076-0. [DOI] [PubMed] [Google Scholar]
- 211.Ruá S, Comino A, Fruttero A, Cera G, Semeria C, Lanzillotty L, Boffetta P. Relationship between histologic features, DNA flow cytometry, and clinical behavior of squamous cell carcinomas of the larynx. Cancer. 1991;67:141–149. doi: 10.1002/1097-0142(19910101)67:1<141::AID-CNCR2820670125>3.0.CO;2-E. Available from: http://dx.doi.org/10.1002/1097-0142(19910101)67:1<141::AID-CNCR2820670125>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 212.Walter MA, Peters GE, Peiper SC. Predicting radioresistance in early glottic squamous carcinoma by DNA content. Ann Otol Rhinol Laryngol. 1991;100:523–526. doi: 10.1177/000348949110000701. [DOI] [PubMed] [Google Scholar]
- 213.Golusinski W, Olofsson J, Szmeja Z, Biczysko W, Krygier-Stoja?owska A, Kulczynski B. A comprehensive analysis of selected diagnostic methods with respect to their usefulness in evaluating the biology of neoplastic cells in patients with laryngeal cancer. Eur Arch Otorhinolaryngol. 1999;256:306–311. doi: 10.1007/s004050050252. Available from: http://dx.doi.org/10.1007/s004050050252. [DOI] [PubMed] [Google Scholar]
- 214.Höfken F, Welkoborsky HJ, Jacob R, Mann W. DNA-zytometrische Untersuchungen von Mund- und Rachenspülflüssigkeit als Screening-Verfahren zur Diagnose von Malignomen der Mundhöhle und der Oropharynx. Laryngo Rhino Otol. 1995;74:678–683. doi: 10.1055/s-2007-997824. Available from: http://dx.doi.org/10.1055/s-2007-997824. [DOI] [PubMed] [Google Scholar]
- 215.Remmerbach TW, Weidenbach H, Pomjanski N, Knops K, Mathes S, Hemprich A, Böcking A. Cytologic and DNA-cytometric early diagnosis of oral cancer. Anal Cell Pathol. 2001;22:211–221. doi: 10.1155/2001/807358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gerstner AOH, Thiele A, Tárnok A, Tannapfel A, Weber A, Bootz F. Prediction of upper aerodigestive tract cancer by slide-based cytometry. Cytometry Part A. 2006;69A:582–587. doi: 10.1002/cyto.a.20316. Available from: http://dx.doi.org/10.1002/cyto.a.20316. [DOI] [PubMed] [Google Scholar]
- 217.Gerstner AOH, Müller AK, Machlitt J, Tárnok A, Tannapfel A, Weber A, Bootz F. Slide-based cytometry for predicting malignancy in solid salivary gland tumors by fine needle aspirate biopsies. Cytometry Part B (Clin. Cytometry) 2003;53B:20–25. doi: 10.1002/cyto.b.10037. Available from: http://dx.doi.org/10.1002/cyto.b.10037. [DOI] [PubMed] [Google Scholar]
- 218.Gerstner AOH, Thiele A, Tárnok A, Machlitt J, Oeken J, Tannapfel A, Weber A, Bootz F. Preoperative detection of laryngeal cancer in mucosal swabs by slide-based cytometry. Eur J Cancer. 2005;41:445–452. doi: 10.1016/j.ejca.2004.10.012. Available from: http://dx.doi.org/10.1016/j.ejca.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 219.Gerstner AOH, Machlitt J, Welkoborsky HJ, Bootz F, Tárnok A. Analysis of ploidy in hypopharyngeal cancer by laser scanning cytometry on fine needle aspirate biopsies. Anal Cell Pathol. 2003;25:51–62. doi: 10.1155/2003/971748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gerstner AOH, Gutsche M, Bücheler M, Machlitt J, Emmrich F, Sommerer F, Tárnok A, Bootz F. Eosinophilia in nasal polyposis: its objective quantification and clinical relevance. Clin Exp Allerg. 2004;34:65–70. doi: 10.1111/j.1365-2222.2004.01842.x. Available from: http://dx.doi.org/10.1111/j.1365-2222.2004.01842.x. [DOI] [PubMed] [Google Scholar]
- 221.Remmerbach TW, Weidenbach H, Hemprich A, Böcking A. Earliest detection of oral cancer using non-invasive brush biopsy including DNA-image-cytometry: report on four cases. Anal Cell Pathol. 2004;25:159–166. doi: 10.1155/2003/305151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Leoni-Parvex S, Mihaescu A, Pellanda A, Monnier P, Bosman FT. Esophageal cytology in the follow-up of patients with treated upper aerodigestive tract malignancies. Cancer. 2000;90:10–16. doi: 10.1002/(SICI)1097-0142(20000225)90:1<10::AID-CNCR2>3.0.CO;2-N. Available from: http://dx.doi.org/10.1002/(SICI)1097-0142(20000225)90:1<10::AID-CNCR2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 223.Hutchinson ML, Cassin CM, Ball HG. The efficacy of an automated preparation device for cervical cytology. Am J Clin Pathol. 1991;96:300–305. doi: 10.1093/ajcp/96.3.300. [DOI] [PubMed] [Google Scholar]
- 224.Klinkhamer PJJM, Meerding WJ, Rosier PFWM, Hanselaar AGJM. Liquid-based cervical cytology - a review of the literature with methods of evidence-based medicine. Cancer (Cancer Cytopathol) 2003;99:263–271. doi: 10.1002/cncr.11673. Available from: http://dx.doi.org/10.1002/cncr.11673. [DOI] [PubMed] [Google Scholar]
- 225.Bollmann R, Bankfalvi A, Griefingholt H, Trosic A, Speich N, Schmitt C, Bollmann N. Validity of combined cytology and human papillomavirus (HPV) genotyping with adjuvant DNA-cytometry in routine cervical screening: results from 31.031 women from the Bonn-region in West Germany. Oncol Rep. 2005;13:915–922. [PubMed] [Google Scholar]
- 226.Bollmann M, Heller H, Bánkfalvi A, Griefingholt H, Bollmann R. Quantitative molecular urinary cytology by fluorescence in situ hybridization: a tool for tailoring surveillance of patients with superficial bladder cancer? Br J Urol. 2005;95:1219–1225. doi: 10.1111/j.1464-410X.2005.05509.x. [DOI] [PubMed] [Google Scholar]
- 227.Gerstner AOH, Trumpfheller C, Racz P, Osmancik P, Tenner-Racz K, Tárnok A. Quantitative histology by multicolor slide-based cytometry. Cytometry Part A. 2004;59A:210–219. doi: 10.1002/cyto.a.20054. Available from: http://dx.doi.org/10.1002/cyto.a.20054. [DOI] [PubMed] [Google Scholar]
- 228.Rubin MA, Zerkowski MP, Camp RL, Kuefer R, Hofer MD, Chinnaiyan AM, Rimm DL. Quantitative determination of expression of the prostate cancer protein ?-Methylacyl-CoA racemase using automated quantitative analysis (AQUA) - a novel paradigm for automated and continuous biomarker measurements. Am J Pathol. 2004;164:831–840. doi: 10.1016/s0002-9440(10)63171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Taylor CR, Levenson RM. Quantification of immunohistochemistry - issues concerning methods, utility and semiquantitative assessment II. Histopathology. 2006;49:411–424. doi: 10.1111/j.1365-2559.2006.02513.x. Available from: http://dx.doi.org/10.1111/j.1365-2559.2006.02513.x. [DOI] [PubMed] [Google Scholar]
- 230.de Solórzano CO, Costes S, Callahan DE, Parvin B, Barcellos-Hoff MH. Applications of quantitative digital image analysis to breast cancer research. Microscop Res Tech. 2002;59:119–127. doi: 10.1002/jemt.10183. Available from: http://dx.doi.org/10.1002/jemt.10183. [DOI] [PubMed] [Google Scholar]
- 231.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nature Rev Cancer. 2005;5:845–856. doi: 10.1038/nrc1739. Available from: http://dx.doi.org/10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- 232.Issaq HJ, Veenstra TD, Conrads TP, Felschow D. The SELDI-TOF MS approach to proteomics: protein profiling and biomarker identification. Biochem Biophys Res Comm. 2002;292:587–592. doi: 10.1006/bbrc.2002.6678. Available from: http://dx.doi.org/10.1006/bbrc.2002.6678. [DOI] [PubMed] [Google Scholar]
- 233.Wadsworth JT, Somers KD, Stack BC, Cazares L, Malik G, Adam BL, Wright GL, Semmes OJ. Identification of patients with head and neck cancer using serum protein profiles. Arch Otolaryngol Head Neck Surg. 2004;130:98–104. doi: 10.1001/archotol.130.1.98. Available from: http://dx.doi.org/10.1001/archotol.130.1.98. [DOI] [PubMed] [Google Scholar]
- 234.Gourin CG, Xia ZS, Han Y, French AM, O'Rourke AK, Terris DJ, Adam BL. Serum protein profile analysis in patients with head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:390–397. doi: 10.1001/archotol.132.4.390. Available from: http://dx.doi.org/10.1001/archotol.132.4.390. [DOI] [PubMed] [Google Scholar]
- 235.Kopf E, Zharhary D. Antibody arrays - an emerging tool in cancer proteomics. Int J Biochem Cell Biol. 2007;39:1305–1317. doi: 10.1016/j.biocel.2007.04.029. Available from: http://dx.doi.org/10.1016/j.biocel.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 236.Hudelist G, Pacher-Zavisin M, Singer CF, Holper T, Kubista E, Schreiber M, Manavi M, Bilban M, Czerwenka K. Use of high-throughput protein array for profiling of differentially expressed proteins in normal and malignant breast tissue. Breast Cancer Res Treat. 2004;86:281–291. doi: 10.1023/B:BREA.0000036901.16346.83. Available from: http://dx.doi.org/10.1023/B:BREA.0000036901.16346.83. [DOI] [PubMed] [Google Scholar]
- 237.Hathaway B, Landsittel DP, Gooding W, Whiteside TL, Grandis JR, Siegfried JM, Bigbee WL, Ferris RL. Multiplexed analysis of serum cytokines as biomarkers in squamous cell carcinoma of head and neck patients. Laryngocope. 2005;115:522–527. doi: 10.1097/01.mlg.0000157850.16649.b8. Available from: http://dx.doi.org/10.1097/01.mlg.0000157850.16649.b8. [DOI] [PubMed] [Google Scholar]
- 238.Linkov F, Lisovich A, Yurkovetsky Z, et al. Early detection of head and neck cancer: development of a novel screening tool using multiplexed immunobead-based biomarker profiling. Cancer Epidemiol Biomarkers Prev. 2007;16:102–107. doi: 10.1158/1055-9965.EPI-06-0602. Available from: http://dx.doi.org/10.1158/1055-9965.EPI-06-0602. [DOI] [PubMed] [Google Scholar]
- 239.Ziober BL, Mauk MG, Falls EM, Chen Z, Ziober AF, Bau HH. Lab-on-a-chip for oral cancer screening and diagnosis. Head Neck. 2008;30:111–121. doi: 10.1002/hed.20680. Available from: http://dx.doi.org/10.1002/hed.20680. [DOI] [PubMed] [Google Scholar]
- 240.Virchow R. Cellularpathologie in ihrer Begründung auf physiologische und pathologische Gewebelehre - 20 Vorlesungen. Berlin: August Hirschwald; 1858. [Google Scholar]
- 241.Valet G, Leary JF, Tárnok A. Cytomics - new technologies: towards a human cytome project. Cytometry Part A. 2004;59A:167–171. doi: 10.1002/cyto.a.20047. Available from: http://dx.doi.org/10.1002/cyto.a.20047. [DOI] [PubMed] [Google Scholar]
- 242.Valet G, Valet M, Tschöpe D, Gabriel H, Rothe G, Kellerman W, Kahle H. White cell and thrombocyte disorders - standardized, self-learning flow cytometric list mode data classification with the CLASSIF1 program system. Ann N Y Acad Sci. 1993;20:233–251. doi: 10.1111/j.1749-6632.1993.tb38781.x. Available from: http://dx.doi.org/10.1111/j.1749-6632.1993.tb38781.x. [DOI] [PubMed] [Google Scholar]
- 243.Valet G. Cytomics, the human cytome project and systems biology: top-down resolution of the molecular biocomplexity of organisms by single cell analysis. Cell Prolif. 2005;38:171–174. doi: 10.1111/j.1365-2184.2005.00342.x. Available from: http://dx.doi.org/10.1111/j.1365-2184.2005.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.van Driel BEM, Valet GK, Lyon H, HansenU, Song JY, van Noorden CJF. Prognostic estimation of survival of colorectal cancer patients with the quantitative histochemical assay of G6PDH activity and the multiparameter classification program CLASSIF1. Cytometry (Comm Clin Cytometry) 1999;38:176–183. doi: 10.1002/(SICI)1097-0320(19990815)38:4<176::AID-CYTO4>3.0.CO;2-#. Available from: http://dx.doi.org/10.1002/(SICI)1097-0320(19990815)38:4<176::AID-CYTO4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 245.Valet G, Repp R, Link H, Ehninger A, Gramatzki M SHG-AML study group. Pretherapeutic identification of high-risk acute myeloid leukemia (AML) patients from immunophenotypic, cytogenetic, and clinical parameters. Cytometry Part B (Clinical Cytometry) 2003;53B:4–10. doi: 10.1002/cyto.b.10028. Available from: http://dx.doi.org/10.1002/cyto.b.10028. [DOI] [PubMed] [Google Scholar]
- 246.Valet G. Cytomics: an entry to biomedical cell systems biology. Cytometry Part A. 2005;63A:67–68. doi: 10.1002/cyto.a.20110. Available from: http://dx.doi.org/10.1002/cyto.a.20110. [DOI] [PubMed] [Google Scholar]
- 247.Valet G. Cytomics as a new potential for drug discovery. Drug Discov Today. 2006;11:785–791. doi: 10.1016/j.drudis.2006.07.003. Available from: http://dx.doi.org/10.1016/j.drudis.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 248.Bloching M, Stephan D, Agha-Mir-Salim P, Berghaus A, Lautenschläger C, Grummt T. Der Ames-Test als Biomarker. HNO. 2001;49:440–446. doi: 10.1007/s001060170094. [DOI] [PubMed] [Google Scholar]
- 249.Righini CA, de Fraipont F, Timsit JF, Faure C, Brambilla E, Reyt E, Favrot MC. Tumor-specific methylation in saliva: a promising biomarker for early detection of head and neck cancer recurrence. Clin Cancer Res. 2007;13:1179–1185. doi: 10.1158/1078-0432.CCR-06-2027. Available from: http://dx.doi.org/10.1158/1078-0432.CCR-06-2027. [DOI] [PubMed] [Google Scholar]
- 250.Bloching M, Hofmann A, Berghaus A, Lautenschläger C, Grummt T. Mikrokerne als Biomarker zum Nachweis der Feldkanzerisierung im oberen Aerodigestivtrakt. HNO. 2000;48:444–450. doi: 10.1007/s001060050595. [DOI] [PubMed] [Google Scholar]
- 251.Kamboj M, Mahajan S. Micronucleus-an upcoming marker of genotoxic damage. Clin Oral Invest. 2007;11:121–126. doi: 10.1007/s00784-006-0075-y. Available from: http://dx.doi.org/10.1007/s00784-006-0075-y. [DOI] [PubMed] [Google Scholar]
- 252.Hsu TC, Johnston DA, Cherry LM, Rmakissoon D, Schantz SP, Jessup JM, Winn RJ, Shirley L, Furlong C. Sensitivity to genotoxic effects of bleomycin in humans: possible relationship to enviromental carcinogenesis. Int J Cancer. 1989;43:403–409. doi: 10.1002/ijc.2910430310. Available from: http://dx.doi.org/10.1002/ijc.2910430310. [DOI] [PubMed] [Google Scholar]
- 253.Kleinsasser NH, Schmid K, Sassen AW, Harréus UA, Staudenmaier R, Folwaczny M, Glas J, Reichl FX. Cytotoxic and genotoxic effects of resin monomers in human salivary gland tissue and lymphocytes as assessed by the single cell microgel electrophoresis (COMET) assay. Biomaterials. 2006;27:1762–1770. doi: 10.1016/j.biomaterials.2005.09.023. Available from: http://dx.doi.org/10.1016/j.biomaterials.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 254.Kleinsasser NH, Wallner BC, Kastenbauer, Muenzenrieder RK, Harréus UA. Comparing the genotoxic sensitivities of human peripheral blood lymphocytes and mucosa cells of the upper aerodigestive tract using the Comet assay. Mutation Res. 2000;467:21–30. doi: 10.1016/s1383-5718(00)00022-x. [DOI] [PubMed] [Google Scholar]
- 255.Olive PL, Durand RE, le Riche J, Olivotto IA, Jackson SM. Gel electrophoresis of individual cells to quantify hypoxic fraction in human breast cancers. Cancer Res. 1993;53:733–736. [PubMed] [Google Scholar]
- 256.Singh NP, McCoy MT, Tice RR, Scheider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. Available from: http://dx.doi.org/10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 257.McKelvey-Martin VJ, Green MHL, Schmezer P, Pool-Zobel PL, DeMeo MP, Collins A. The single cell gel electrophoresis assay (Comet assay): a European review. Mutat Res. 1993;288:47–63. doi: 10.1016/0027-5107(93)90207-V. Available from: http://dx.doi.org/10.1016/0027-5107(93)90207-V. [DOI] [PubMed] [Google Scholar]
- 258.Voegeli R. Die Schillersche Jodprobe im Rahmen der Ösophagusdiagnostik (Vorläufige Mitteilung) Pract Oto-Rhino-Laryngol. 1966;28:230–239. [PubMed] [Google Scholar]
- 259.Connor MJ, Sharma P. Chromoendoscopy and magnification endoscopy for diagnosing esophageal cancer and dysplasia. Thorac Surg Clin. 2004;14:87–94. doi: 10.1016/S1547-4127(04)00042-8. Available from: http://dx.doi.org/10.1016/S1547-4127(04)00042-8. [DOI] [PubMed] [Google Scholar]
- 260.Dawsey SM, Fleischer DE, Wang GQ, Zhou B, Kidwell JA, Lu N, Lewin KJ, Roth MJ, Tio L, Taylor FR. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83:220–231. doi: 10.1002/(SICI)1097-0142(19980715)83:2<220::AID-CNCR4>3.0.CO;2-U. Available from: http://dx.doi.org/10.1002/(SICI)1097-0142(19980715)83:2<220::AID-CNCR4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 261.Muto M, Nakane M, Katada C, Sano Y, Ohtsu A, Esumi H, Ebihara S, Yoshida S. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer. 2004;101:1375–1381. doi: 10.1002/cncr.20482. Available from: http://dx.doi.org/10.1002/cncr.20482. [DOI] [PubMed] [Google Scholar]
- 262.Voo I, Mavrofrides EC, Puliafito CA. Clinical applications of optical coherence tomography for the diagnosis and management of macular diseases. Ophthalmol Clin North Am. 2004;17:21–31. doi: 10.1016/j.ohc.2003.12.002. Available from: http://dx.doi.org/10.1016/j.ohc.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 263.Bibas AG, Podoleanu AG, Cucu RG, Bonmarin M, Dobre GM, Ward VMM, Odell E, Boxer A, Gleeson MJ, Lackson DA. 3-D optical coherence tomography of the laryngeal mucosa. Clin Otolaryngol. 2004;29:713–720. doi: 10.1111/j.1365-2273.2004.00902.x. Available from: http://dx.doi.org/10.1111/j.1365-2273.2004.00902.x. [DOI] [PubMed] [Google Scholar]
- 264.Armstrong WB, Ridgway JM, Vokes DE, et al. Optical coherence tomography of laryngeal cancer. Laryngoscope. 2006;116:1107–1113. doi: 10.1097/01.mlg.0000217539.27432.5a. Available from: http://dx.doi.org/10.1097/01.mlg.0000217539.27432.5a. [DOI] [PubMed] [Google Scholar]
- 265.Kraft M, Lüerßen K, Lubatschowski H, Glanz H, Arens C. Technique of optical coherence tomography of the larynx during microlaryngoscopy. Laryngoscope. 2007;117:950–952. doi: 10.1097/MLG.0b013e318038166d. Available from: http://dx.doi.org/10.1097/MLG.0b013e318038166d. [DOI] [PubMed] [Google Scholar]
- 266.Xie T, Xie H, Fedder GK, Pan Y. Endoscopic optical coherence tomography with a modified microelectromechanical systems mirror for detection of bladder cancers. Appl Optics. 2003;42:6422–6426. doi: 10.1364/AO.42.006422. Available from: http://dx.doi.org/10.1364/AO.42.006422. [DOI] [PubMed] [Google Scholar]
- 267.Wang Z, Lee CS, Waltzer WC, Liu J, Xie H, Yuan Z, Pan Y. In vivo bladder imaging with microelectromechanical-systems-based endoscopic spectral domain optical coherence tomography. J Biomed Opt. 2007;12:034009. doi: 10.1117/1.2749744. Available from: http://dx.doi.org/10.1117/1.2749744. [DOI] [PubMed] [Google Scholar]
- 268.Pan YT, Wu ZL, Yuan ZJ, Wang ZG, Dub CW. Subcellular imaging of epithelium with time-lapse optical coherence tomography. J Biomed Opt. 2007;12:050504. doi: 10.1117/1.2800007. Available from: http://dx.doi.org/10.1117/1.2800007. [DOI] [PubMed] [Google Scholar]
- 269.Watanabe A, Tsujie H, Taniguchi M, Hosokawa M, Fujita M, Sasaki S. Laryngoscopic detection of pharyngeal carcinoma in situ with narrowband imaging. Laryngoscope. 2006;116:650–654. doi: 10.1097/01.mlg.0000204304.38797.34. Available from: http://dx.doi.org/10.1097/01.mlg.0000204304.38797.34. [DOI] [PubMed] [Google Scholar]
- 270.Koenig F, González S, White WM, Lein M, Rajadhyaksha M. Near-infrared confocal laser scanning microscopy of bladder tissue in vivo. Urology. 1999;53:853–857. doi: 10.1016/S0090-4295(98)00628-1. Available from: http://dx.doi.org/10.1016/S0090-4295(98)00628-1. [DOI] [PubMed] [Google Scholar]
- 271.Dickensheets DL, Kino GS. Micromachined scanning confocal optical microscope. Optics Letters. 1996;21:764–766. doi: 10.1364/OL.21.000764. Available from: http://dx.doi.org/10.1364/OL.21.000764. [DOI] [PubMed] [Google Scholar]
- 272.Koenig F, Knittel J, Stepp H. Diagnosing Cancer in Vivo. Science. 2001;292:1401–1403. doi: 10.1126/science.292.5520.1401. Available from: http://dx.doi.org/10.1126/science.292.5520.1401. [DOI] [PubMed] [Google Scholar]
- 273.Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706–713. doi: 10.1053/j.gastro.2004.06.050. Available from: http://dx.doi.org/10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 274.Hoffman A, Goetz M, Vieth M, Galle PR, Neurath MF, Kiesslich R. Confocal laser endomicroscopy: technical status and current indications. Endoscopy. 2006;38:1275–1283. doi: 10.1055/s-2006-944813. Available from: http://dx.doi.org/10.1055/s-2006-944813. [DOI] [PubMed] [Google Scholar]
- 275.Just T, Stave J, Boltze C, Wree A, Kramp B, Guthoff RF, Pau HW. Laser scanning microscopy of the human larynx mucosa: a preliminary, ex vivo study. Laryngoscope. 2006;116:1136–1141. doi: 10.1097/01.mlg.0000217529.53079.59. Available from: http://dx.doi.org/10.1097/01.mlg.0000217529.53079.59. [DOI] [PubMed] [Google Scholar]
- 276.Harris AT. Spectral mapping tools from the earth sciences applied to spectral microscopy data. Cytometry Part A. 2006;69A:872–879. doi: 10.1002/cyto.a.20309. Available from: http://dx.doi.org/10.1002/cyto.a.20309. [DOI] [PubMed] [Google Scholar]
- 277.Farkas DL, Du C, Fisher GW, Lau C, Niu W, Wachman ES, Levenson RM. Non-invasive image acquisition and advanced processing in optical bioimaging. Comput Med Imaging Graphics. 1998;22:89–102. doi: 10.1016/S0895-6111(98)00011-1. Available from: http://dx.doi.org/10.1016/S0895-6111(98)00011-1. [DOI] [PubMed] [Google Scholar]
- 278.Yang P, Farkas DL, Kirkwood JM, Abernethy JL, Edington HD, Becker D. Macroscopic spectral imaging and gene expression analysis of the early stages of melanoma. Mol Med. 1999;5:785–794. [PMC free article] [PubMed] [Google Scholar]
- 279.Farkas DL, Becker D. Applications of spectral imaging: detection and analysis of human melanoma and its precursors. Pigment Cell Res. 2001;14:2–8. doi: 10.1034/j.1600-0749.2001.140102.x. Available from: http://dx.doi.org/10.1034/j.1600-0749.2001.140102.x. [DOI] [PubMed] [Google Scholar]
- 280.McMurdy JW, Jay GD, Suner S, Trespalacios FM, Crawford GP. Diffuse reflectance spectra of the palpebral conjunctiva and its utility as a noninvasive indicator of total hemoglobin. J Biomed Opt. 2006;11:014019. doi: 10.1117/1.2167967. Available from: http://dx.doi.org/10.1117/1.2167967. [DOI] [PubMed] [Google Scholar]
- 281.Ecker RC, de Martin R, Steiner GE, Schmid JA. Application of spectral imaging microscopy in cytomics and fluorescence resonance energy transfer (FRET) analysis. Cytometry Part A. 2004;59A:172–181. doi: 10.1002/cyto.a.20053. Available from: http://dx.doi.org/10.1002/cyto.a.20053. [DOI] [PubMed] [Google Scholar]
- 282.Chung A, Wachsmann-Hogiu S, Zhao T, Xiong Y, Joseph A, Farkas DL. Advanced optical imaging requiring no contrast agents - a new armamentarium for medicine and surgery. Current Surg. 2005;62:365–370. doi: 10.1016/j.cursur.2004.12.011. Available from: http://dx.doi.org/10.1016/j.cursur.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 283.Müller MG, Valdez TA, Georgakoudi I, et al. Spectroscopic detection and evaluation of morphologic and biochemical changes in early human oral carcinoma. Cancer. 2003;97:1681–1692. doi: 10.1002/cncr.11255. Available from: http://dx.doi.org/10.1002/cncr.11255. [DOI] [PubMed] [Google Scholar]
- 284.Chung A, Karlan S, Lindsley E, Wachsmann-Hogiu S, Farkas DL. In vivo cytometry: a spectrum of possibilities. Cytometry Part A. 2006;69A:142–146. doi: 10.1002/cyto.a.20220. Available from: http://dx.doi.org/10.1002/cyto.a.20220. [DOI] [PubMed] [Google Scholar]
- 285.Lindsley E, Wachman ES, Farkas DL. The hyperspectral imaging endoscope: a new tool for in vivo cancer detection. Prog Biomed Opt Imaging. 2004;5:75–82. [Google Scholar]
- 286.Adams CL, Kobets N, Meiklejohn GR, Millington OR, Grierson AM, Rush CM, Smith KM, Garside P. Tracking lymphocytes in vivo. Arch Immunol Ther Exp. 2004;52:173–187. [PubMed] [Google Scholar]
- 287.Boutrus S, Greiner C, Hwu D, Chan M, Kuperwaser C, Lin CP, Georgakoudi I. Portable two-color in vivo flow cytometer for real-time detection of fluorescently-labeled circulating cells. J Biomed Opt. 2007;12:020507. doi: 10.1117/1.2722733. Available from: http://dx.doi.org/10.1117/1.2722733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Novak J, Puoris'haag M. Two-color, doulbe-slit in vivo flow cytometer. Opt Lett. 2007;32:2993–2995. doi: 10.1364/OL.32.002993. Available from: http://dx.doi.org/10.1364/OL.32.002993. [DOI] [PubMed] [Google Scholar]
- 289.Zharov VP, Galanzha EI, Tuchin VV. In vivo photothermal flow cytometry: imaging and detection of individual cells in blood and lymph flow. J Cell Biochem. 2006;97:916–932. doi: 10.1002/jcb.20766. Available from: http://dx.doi.org/10.1002/jcb.20766. [DOI] [PubMed] [Google Scholar]
- 290.Zharov VP, Galanzha EI, Shashkov EV, Khlebtsov NG, Tuchin VV. In vivo photoacustic flow cytometry for monitoring of circulating single cancer cells and contrast agents. Opt Lett. 2006;31:3623–3625. doi: 10.1364/OL.31.003623. Available from: http://dx.doi.org/10.1364/OL.31.003623. [DOI] [PubMed] [Google Scholar]
- 291.He W, Wang H, Hartmann LC, Cheng JX, Low PS. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. Proc Natl Acad Sci USA. 2007;104:11760–11765. doi: 10.1073/pnas.0703875104. Available from: http://dx.doi.org/10.1073/pnas.0703875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204:345–356. doi: 10.1084/jem.20061890. Available from: http://dx.doi.org/10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wessels JT, Busse AC, Mahrt J, Dullin C, Grabbe E, Mueller GA. In vivo imaging in experimental preclinical tumor research - a review. Cytometry Part A. 2007;71A:542–549. doi: 10.1002/cyto.a.20419. Available from: http://dx.doi.org/10.1002/cyto.a.20419. [DOI] [PubMed] [Google Scholar]
- 294.Levenson RM, Mansfield JR. Multispectral imaging in biology and medicine: slices of life. Cytometry Part A. 2006;69A:748–758. doi: 10.1002/cyto.a.20319. Available from: http://dx.doi.org/10.1002/cyto.a.20319. [DOI] [PubMed] [Google Scholar]
- 295.Shcherbo D, Merzlyak EM, Chepurnykh TV, et al. Bright far-red fluorescent protein for whole-body imaging. Nature Methods. 2007;4:741–746. doi: 10.1038/nmeth1083. Available from: http://dx.doi.org/10.1038/nmeth1083. [DOI] [PubMed] [Google Scholar]
- 296.Sahai E. Illuminating the metastatic process. Nature Rev Cancer. 2007;7:737–749. doi: 10.1038/nrc2229. Available from: http://dx.doi.org/10.1038/nrc2229. [DOI] [PubMed] [Google Scholar]
- 297.Kim S, Lim YT, Soltesz EG, et al. Near-infrared fluorescence type II quantum dots for sentinel lymph node mapping. Nature Biotech. 2004;22:93–97. doi: 10.1038/nbt920. Available from: http://dx.doi.org/10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotech. 2004;22:969–976. doi: 10.1038/nbt994. Available from: http://dx.doi.org/10.1038/nbt994. [DOI] [PubMed] [Google Scholar]