Abstract
Reconstructive and aesthetic surgery of the auricle is one of the most challenging and diverse tasks in plastic head and neck surgery. Injuries, defects and malformations require multiple different techniques, some of which are standardized, other situations require huge experience and artistic creativity. It is a specialty that will never become monotone.
Keywords: auricular reconstruction, auricular trauma, auricular defects, auricular malformations
1 Introduction
The constructive and reconstructive surgery of the auricle is an important field of head and neck surgery. The tremendous variation of auricular forms requires many different techniques and extensive experiences in plastic and reconstructive surgery.
Abnormalities of the auricle might lead to disfigurement with severe psychosocial impact on quality of life.
2 Basics
2.1 Anatomy
The external ear includes the auricle and the external ear canal.
The anterior side of the auricle shows a typical relief. Except for the lobule the form of the auricle is determined by elastic cartilage being 1-3 mm thick [37].
The anterior skin is fixed to the perichondrium without subcutaneous tissue in-between being almost immobile. It is 0.8-1.2 mm thick. In contrast the posterior side has a subcutaneous layer of 1.2-3 mm which leads to a certain mobility.
The blood supply of the auricle is variable and dominated by branches of the superficial temporal and posterior auricular vessels.
Innervation of the auricle is due to branches of N. auricularis magnus, N. auriculotemporalis and N. occipitalis minor.
The auricular muscles are relatively unimportant and small. Two muscles might be detected intraoperatively: M. auricularis posterior and M. auricularis superior. In addition, the auricle is stabilized by one posterior and two anterior ligaments [37].
The lymphatic fluid of the auricle is drained into the superficial and deep neck nodes, into the parotid gland and towards the submandibular nodes and in addition to the postauricular and mastoid lymph nodes.
2.2 Anthropometry
The size and position of the auricle as well as its relief is of utmost importance for corrective and reconstructive auricular surgery. The knowledge of these data in relation age, sex and body stature are necessary for the individual surgical planning [25].
The length of the auricle depends on body stature as well as age. On average the auricle reaches 85% of its final length by the age of 6 and 90% with 9. Later during life the length of the auricle increases only slowly – mainly due to changes of the soft tissue of the lobule which is more or less a lobule chalasis instead or real growth.
The width of the auricle also depends on body stature and age, but reaches 95% of its final value already by the age of 6.
In contrast the auricular projection, i.e. its width seen from a strictly anterior perspective, is almost constant throughout life. On average the ear projection is 20±4 mm. Its normal range is between 12 and 28 mm. These data are important for the indication and planning of otopexia.
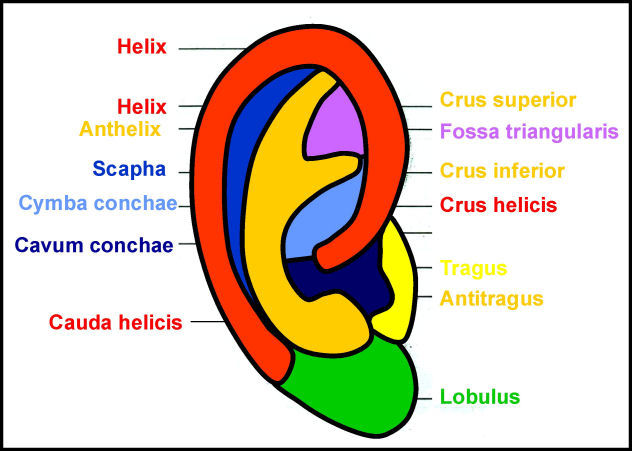
2.3 Aesthetic units of the auricle
The aesthetic units of the auricle are shown in Figure 1 (Fig. 1). In all reconstructive procedures they should be respected. Incisions should be placed along their borders and not through them as along as possible.
Figure 1. Aesthetic subunits of the auricle.
3 Trauma of the auricle
3.1 Classification
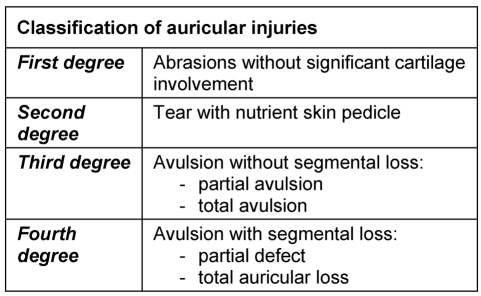
For clinical aspects Weerda's classification into four grades [37] has been proven to be very useful (Table 1 (Tab. 1)).
Table 1. Classification of auricular trauma according to Weerda [37].
3.1.1 Superficial trauma (Grade 1)
The wounds are rinsed and the wound edges adapted and carefully sutured. Small defects are closed with small flaps.
3.1.2 Skin trauma with sufficient blood supply (Grade 2)
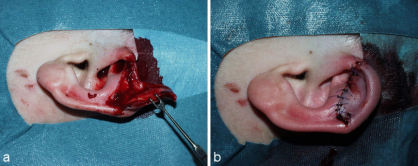
In these cases primary readaptation is the best choice. The partially amputated part of the auricle, its cartilage as well as its skin, is readapted with thin sutures (4-0 – 6-0 / Figure 2 (Fig. 2)).
Figure 2. Pedicled partial amputation of the auricle (Grade II). a: situation preoperative; b: situation after primary reconstruction.
3.1.3 Amputation of the auricle – with existing auricle (Grade 3)
Readaptation of a completely amputated auricle is rarely successful. Therefore multi-staged pocket methods have been suggested. They have in common, that the skin of the amputated auricle is in part or completely removed from the cartilage. The cartilage itself is then stored in a well vascularized pocket, either in the ear region [8], [17], [16] or remote (abdominal [9], [18], supraclavicular [31], cervical [6]). Other authors suggest repositioning of the cartilage and covering it with local skin flaps [11], with platysma [2] or with a temporoparietal flap [4].
Another, relatively successful technique has been described by Baudet [3]. He removes the posterior skin of the amputated auricle, creates little windows into the cartilage in the triangular fossa, scaphoid fold and cavum and replants it into its original position. He leaves the anterior skin intact and sutures it to the mastoid skin. After some months the auricle is elevated and the retroauricular sulcus reconstructed with a skin graft.
Nevertheless all these methods are only successful in about 40% [37]. In addition the scars might lead to some distortion of the elastic cartilage so that the aesthetic results are not very satisfactory in most cases.
The best option is the replantation with microvascular reanastomosis, but due to the small size of the auricular vessels and the trauma to the whole auricular region this can only be accomplished in exceptional cases [37].
3.1.4 Amputation of the auricle without existing auricle (Grade 4)
Severe injuries to the auricular region with loss of the pinna have to be covered by local skin flaps or free skin grafts. After some months the auricle can then be reconstructed with the techniques described below.
3.2 Bite injuries
Bite injuries have a relatively high risk of becoming infected. Therefore intensive wound rinsing and cleaning is mandatory. Devitalized wound edges are carefully excised. The remaining skin is then readapted. Systemic antibiotic treatment is also necessary.
4 Reconstruction of auricular defects
4.1 Classification
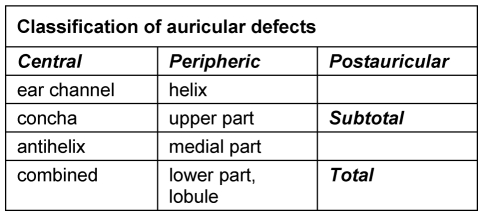
Important criteria for surgical planning are the localization, size and depth of the defect (Table 2 (Tab. 2)).
Table 2. Classification of auricular defects according to Weerda and Siegert [36].
4.1.1 Central defects
Defects of the skin of the conchal cavum can be covered with a full thickness skin graft as long as the perichondrium is intact. If not, the cartilage should be removed and the skin graft sutured onto the well vascularized connective tissue below the cartilage.
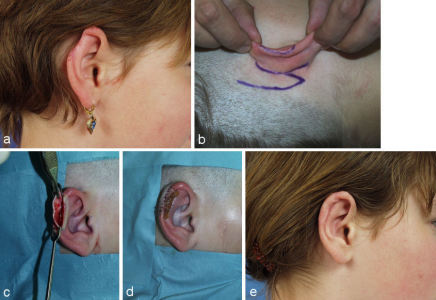
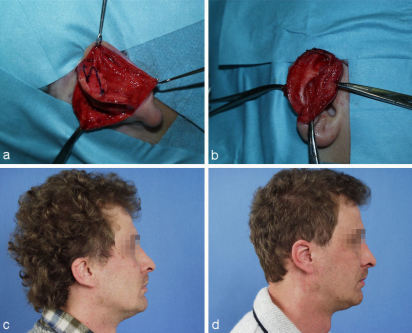
Deeper defects of the conchal cavum and the antihelix are covered with a retroauricular pedicled flap. Its pedicle can be severed after 2-3 weeks. Alternatively a subcutaneous pedicled island flap from the retroauricular sulcus can be used (Figure 3 (Fig. 3)).
Figure 3. Reconstruction of a central defect with a cranially pedicled retroauricular island flap. a: tumor; b: marked cranially pedicled retroauricular island flap; c: transposed island flap; d: result after some months.
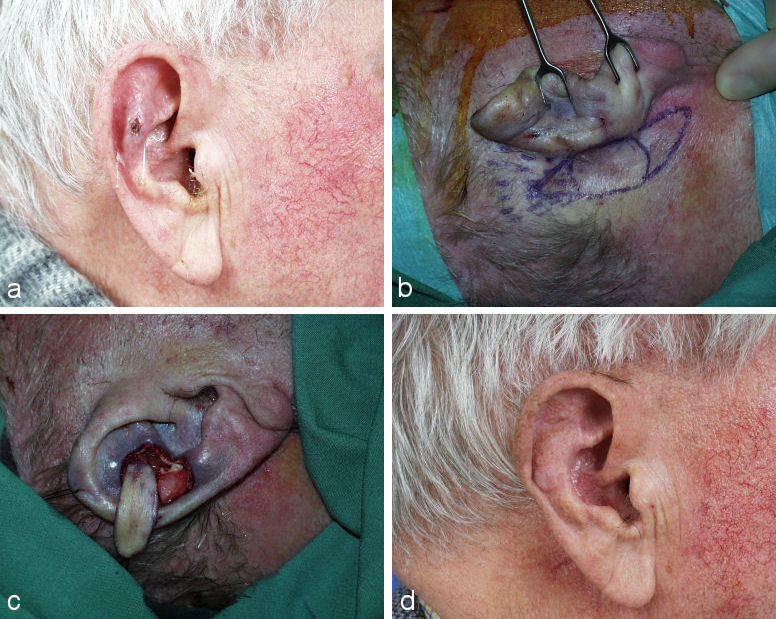
Extended central defects including the antihelix might need large bipedicled flaps from the neck and a framework reconstruction with rib cartilage (see below) (Figure 4 (Fig. 4)).
Figure 4. Reconstruction of a large central defect with a bilobed flap (Surgeon Weerda). a: defect; b: partially mobilized flap; c: partially transposed flap, antihelix reconstructed with rib cartilage; d: result after some months.
4.1.2 Peripheral defects
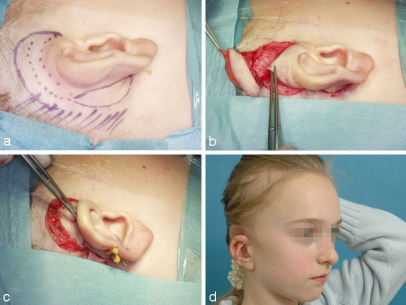
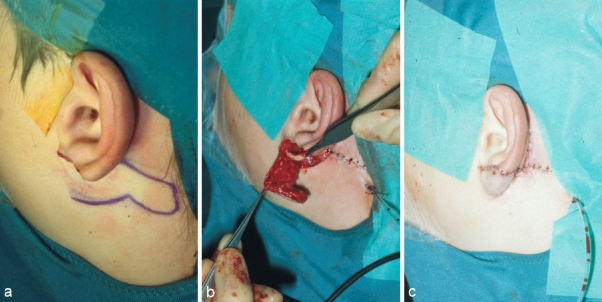
Small defects of the helical rim can be reconstructed with transposition flaps (Figure 5 (Fig. 5)). Defects that include several millimeters of cartilage need a cartilaginous support. Up to a length of about 2.5 cm it can be harvested from the ipsilateral conchal cavum through the same incision so that there is almost no additional morbidity for harvesting the cartilage.
Figure 5. Reconstruction of a peripheral defect of the upper third with a transposition flap and cartilage graft from the cavum. a: defect; b: outlined flap; c: prepared cartilage graft; d: situation at the end of the 1st operation; e: result after some months after severing the pedicle.
The pedicle of the transposition flap can be severed after about 3 weeks. Its edges have to be worked into the helical rim very meticulous to give an almost invisible scar.
A satisfying one step reconstruction can be performed with a modification of Gersuny’s sliding flap [13], [1]. It results in a slightly reduced auricular length, but since all aesthetic units are preserved differences in ear length of up to 15% between both auricles are hardly recognized in daily life. To maintain a sufficient blood supply the chondrocutaneous sliding flap is mobilized as a two layer flap with the postauricular skin untouched.
Broad defects of the helical rim can be reconstructed with a sliding U-flap. It might be supported by a cartilaginous graft as described before.
If the defects are large a three step reconstruction with rib cartilage is necessary (Figure 6 (Fig. 6)). In the first step cartilage from the 7th and 8th rib is harvested and the framework constructed. Therefore a template has been made from the contralateral side preoperatively. It should be about 3 mm smaller than the auricle because it is draped with the local skin that adds to the total length of the auricle these 3 mm. The framework is then transplanted to the auricular region and fixed to the remnants of the original cartilage.
Figure 6. 3-step reconstruction of a larger peripheral defect with rib cartilage. a: defect; b: partial auricular framework; c: result some months after first stage; d: final result after elevation of the auricle (2nd step) and minor corrections (3rd step).
In the 2nd step about 6 months later the framework with its surrounding connective tissue is elevated and the retroauricular sulcus constructed. The elevated auricle is stabilized with an additional piece of rib cartilage which is covered with a flap either of the superficial temporal fascia or connective retroauricular tissue. It is covered with a full thickness skin graft.
A 3rd step might be needed for some minor refinements.
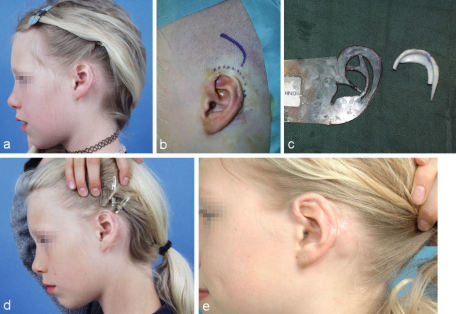
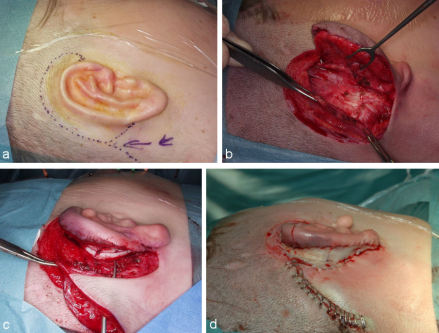
Defects of the lobule are best reconstructed with a folded transposition flap described by Gavello (1907), quoted by Weerda [37] (Figure 7 (Fig. 7)). To keep the shape of the reconstructed lobule stable it should also be supported by a cartilaginous strut harvested from the conchal cavum.
Figure 7. Reconstruction of the lobule after total amputation with a Gavello (1907) transposition flap. a: defect of the lobule with planned flap; b: elevated flap with cartilaginous support; c: situation at end of operation.
4.1.3 Subtotal and total defects
The technique and result of complete reconstruction of the auricle depends on the quality of the tissue in the auricular region. Scarred skin is relatively immobile so that it is difficult to work out a nice auricular relief.
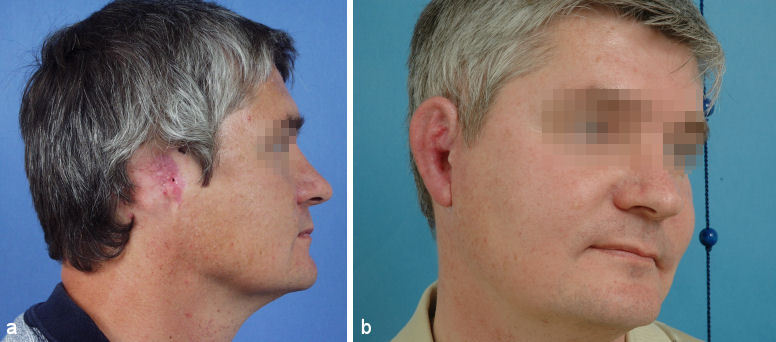
If the scars are small or mild the auricle can be reconstructed with the technique used for severe microtia (Figure 8 (Fig. 8); see below).
Figure 8. Multi-staged auricular reconstruction after total amputation with mild scarring of the retroauricular skin using rib cartilage and local skin. a: preoperative situation; b: result after 3 steps.
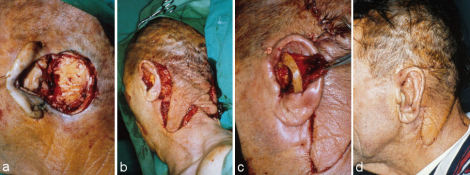
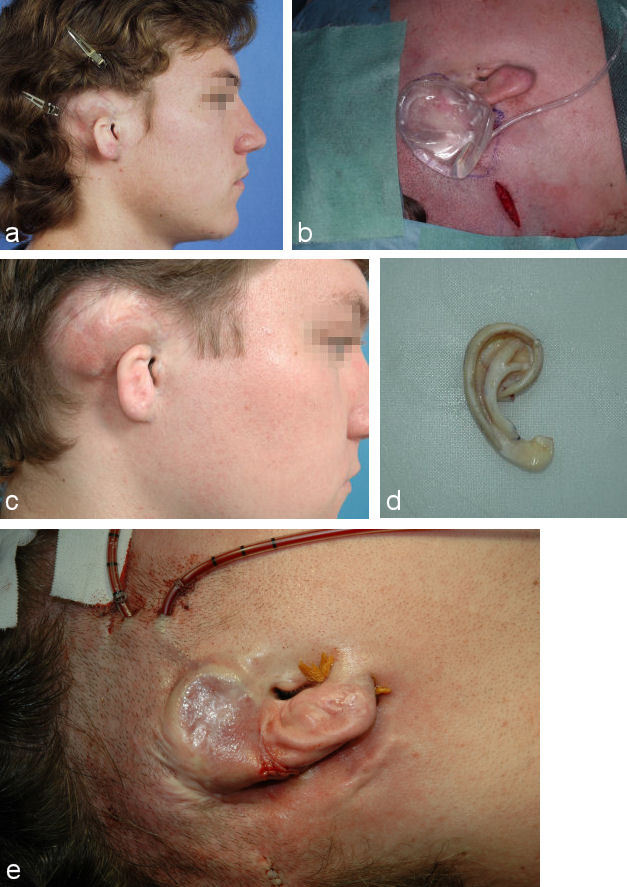
If the scarring is severe other options have to be chosen. In certain cases pretreatment with skin expansion can be used, but its long duration and risk of exposure has to be taken into consideration (Figure 9 (Fig. 9)). Two weeks after implantation filling of the balloon can begin. It has to be repeated for 8-12 weeks on a weekly basis.
Figure 9. Multi-staged auricular reconstruction after total amputation with severe scarring of the retroauricular skin using rib cartilage and local skin after pretreatment with skin expansion. a: preoperative situation; b: implantation of skin expander; c: situation after 10 weeks of skin expansion; d: auricular framework constructed out of rib cartilage; e: situation after implantation of the auricular framework at end of 2nd step.
After the skin has been stimulated to grow the balloon can be explanted. In the same operation rib cartilage is harvested, the framework constructed and implanted into the pouch of the expander.
Alternatively the framework can be covered with the pedicled superficial temporal fascia and a full thickness skin graft (Figure 10 (Fig. 10)).
Figure 10. Single stage auricular reconstruction with pedicled temporoparietal fascia flap (TPF-flap), auricular framework constructed out of rib cartilage and full thickness skin graft. a: preoperative situation; b: elevated temporoparietal fascia flap (TPF-flap) and auricular framework constructed out of rib cartilage; c: result several months after single stage auricular reconstruction.
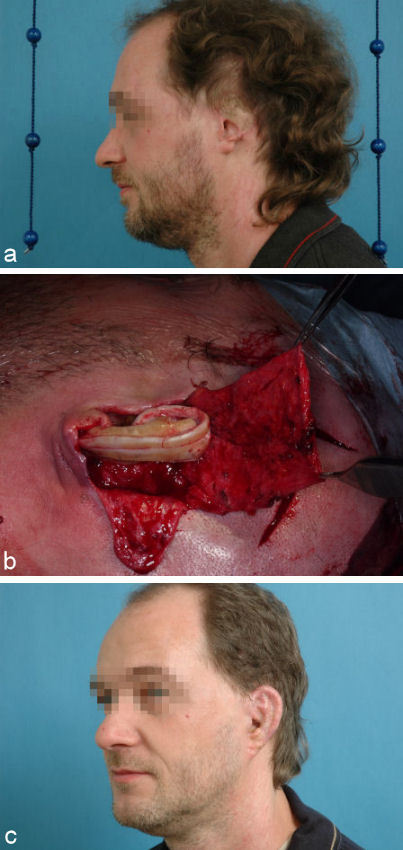
If the temporal vessels are insufficient due to the trauma of the temporal region either the superficial temporal fascia from the contralateral side or the radialis fascia can be used to cover the framework. The vessels of the fascia are then reanastomized with the facial vessels of the traumatized side.
In any case minor refinements have to be considered but should not be performed before 6 months after the last operation because it takes a relatively long time for the swelling to subside and the scars to mature.
5 Surgery of auricular dysplasia
5.1 Classification
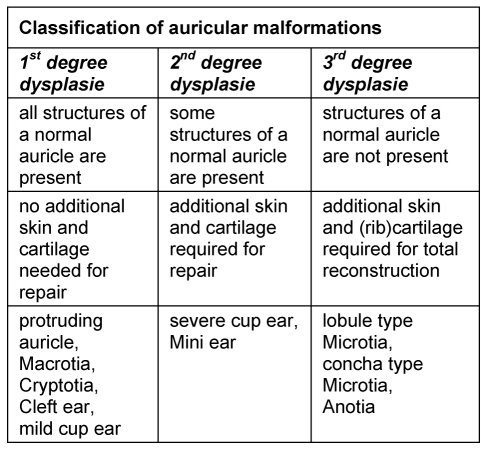
According to Marx [15] and Weerda [37] auricular dysplasia is classified into 3 grades (Table 3 (Tab. 3)).
Table 3. Classification of auricular dysplasias according to Weerda [37].
5.1.1 1st degree dysplasia
These dysplasias are only minor malformations. All structures of the auricle are present. Surgical correction means realignment of the given tissue without the necessity for grafts.
5.1.1.1 Protruding ears
Since protruding ears are the most common malformation of the head their treatment is presented in detail in an extra chapter of this book.
5.1.1.2 Macrotia
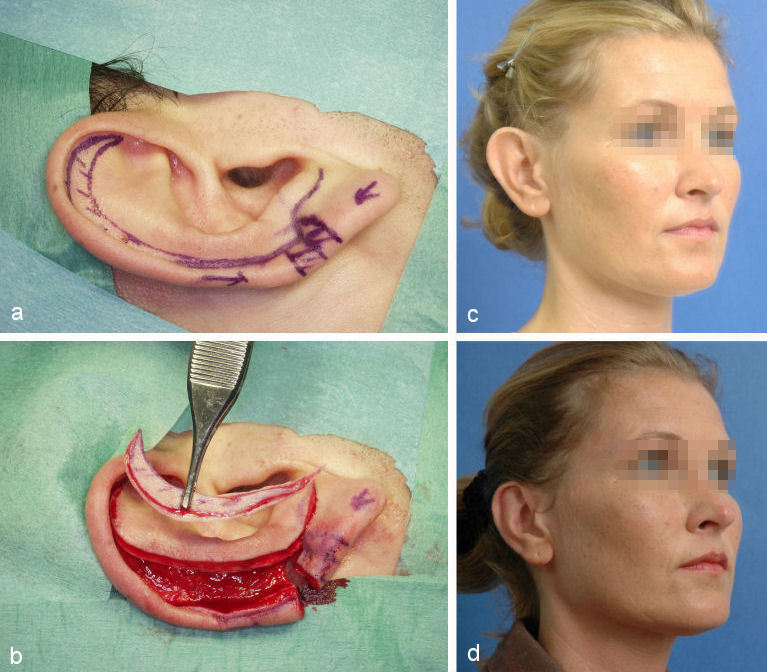
Macrotia denotes an auricle that is too large with regard to the patient’s body stature. Often it is combined with abnormal protrusion. Since its predominant feature is the hypertrophic scapha the method of choice is the modified Gersuny-technique (see above, Figure 11 (Fig. 11)).
Figure 11. Correction of macrotia with modified Gersuny-technique. a: crescent-shaped marking of the area to be resected; b: situation after crescent-shaped excision of the scaphoid fold; c: preoperative situation; d: postoperative situation.
5.1.1.3 Cryptotia
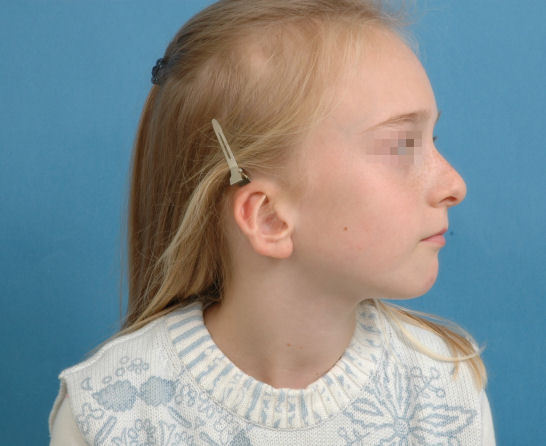
Cryptotia (Figure 12 (Fig. 12)) is relatively rare in Europe, but more common in Japan. It is characterized by the upper helical rim being present, but hidden under the scalp. The goal of its surgical correction is to increase projection of the upper part of the auricle and to construct the upper sulcus. This technique is similar to the 2nd step of total auricular reconstruction.
Figure 12. Cryptotia.
An interesting alternative to using a full thickness skin graft is the application of a cranially pedicled island flap transposed into the new sulcus (Figure 13 (Fig. 13)).
Figure 13. Correction of cryptotia. a: planning of the reconstruction using a thin skin flap and a subcutaneous pedicled sliding flap; b: skin flap (below) and elevated sliding flap and pedicled sliding flap (above); c: situation at end of operation; d: result after several months.
5.1.1.4 Minor cup ear deformity
The name cup ear deformity is used for a helical rim that “hangs over“ the scaphoid fold. The superior crus might be hypoplastic whereas the inferior crus is normal in most cases.
If the helical overhang is very minor it can simply be resected, but this is rare.
The technique we typically choose for its correction is the one described by Tanzer [32]. It is an open approach exposing the whole malformed cartilage via a so-called “auricular degloving procedure”. The malformed cartilage is then corrected by a Z-plasty constructing a harmonious helical rim (Figure 14 (Fig. 14)). This procedure might be combined with an anthelixplasty.
Figure 14. Correction of cup ear deformity by Tanzer-technique (1975). a: exposure of deformity via an „Auricular Degloving“ and planning the Z-plasty in the area of the malformed helical rim; b: situation after remodeling the cartilage; c: preoperative situation; d: result after several months.
5.1.1.5 Coloboma
Clefts or colobomas of the auricle are rare. They can be found in the area between the helical rim and the lobule. It can be corrected with a Z-plasty.
5.1.1.6 Appendages
Auricular appendages are relatively frequent, but small in most cases, but they can be as large as a second auricle. They are excised and the wounds are closed meticulously.
5.1.1.7 Preauricular cysts and fistulas
Preauricular cysts and fistulas are mostly superficial and short, but can be very long going all the way to the skull base or run adjacent to the facial nerve. Meticulous excision taking care to avoid injury to the facial nerve is the method of choice. Intraoperative nerv monitoring might be helpful.
5.1.2 2nd degree dysplasia
These dysplasias are moderate malformation. Some parts of the auricle are normal, others have to be reconstructed. Typical examples are:
5.1.2.1 Severe cup ear deformity
In severe cup ear deformity the auricle is smaller than normal. In addition many auricles show a certain amount of dystopia mostly into ventro-caudal direction.
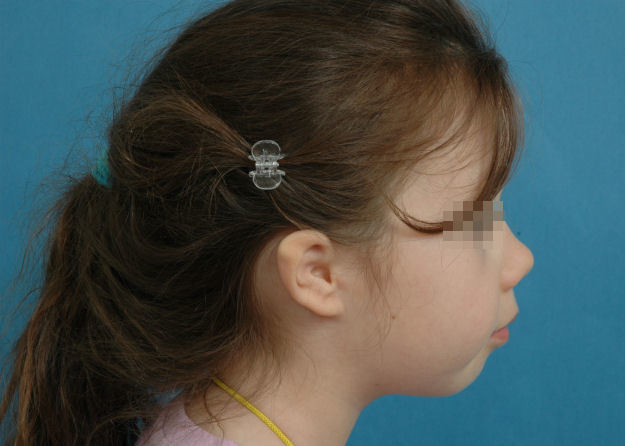
5.1.2.2 Mini-ear
The mini-ear is smaller than normal (Figure 15 (Fig. 15)). Its structures can be almost normal or its relief might also be malformed.
Figure 15. Mini-ear.
5.1.3 3rd degree dysplasia
In 3rd degree dysplasia hardly any normal structures of the auricle are present. They can be sub classified into lobule-type (Figure 16 (Fig. 16)), concha-type (Figure 17 (Fig. 17)) and anotia (Figure 18 (Fig. 18)). In most cases they are combined with congenital auricular atresia and in about 10% they are part of a syndrome, e.g. OAV-dysplasia or Franceschetti-Syndrome. In addition 18% of our patients have partial or total congenital facial paralysis. Therefore our rehabilitation concept goes beyond the reconstruction of the auricle and includes treatment of hearing disorder, facial paralysis and skeletal anomalies.
Figure 16. Lobule-type 3rd degree microtia.
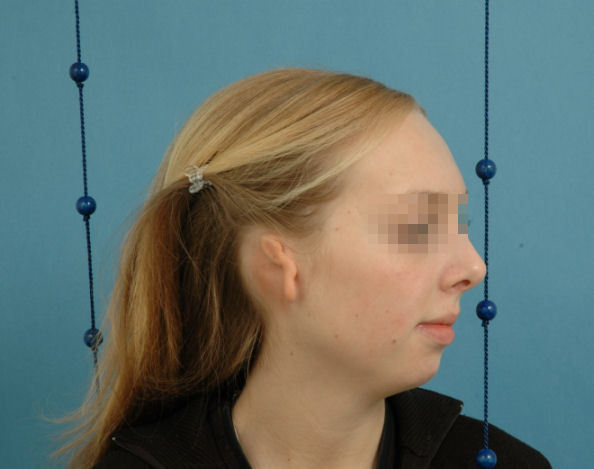
Figure 17. Concha-type 3rd degree microtia.
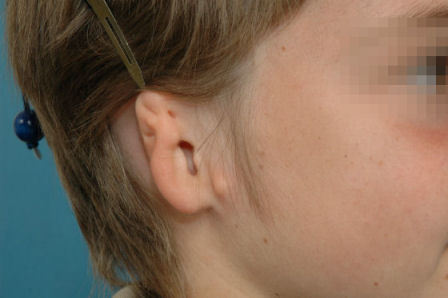
Figure 18. Anotia.
We start reconstruction by the age of about 10 for two reasons:
For a complete framework we need sufficient and plenty of rib cartilage. By this age most children have a thoracic circumference of at least 63 cm. This is necessary to get enough cartilage of rib 6-9.
By this age they should be mature enough to understand the procedure and appreciate its benefits as well weight them against its morbidity. In this sense the doctor-patient relationship gets a different quality compared to treating little children.
Our surgical treatment is very standardized for the majority of cases. It includes 3 operative steps mainly based on the technique described by Nagata [20], [21], [22], [23], which we have further modified over the years.
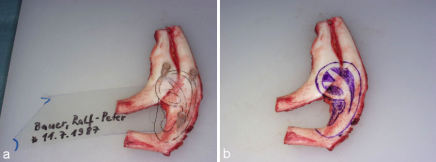
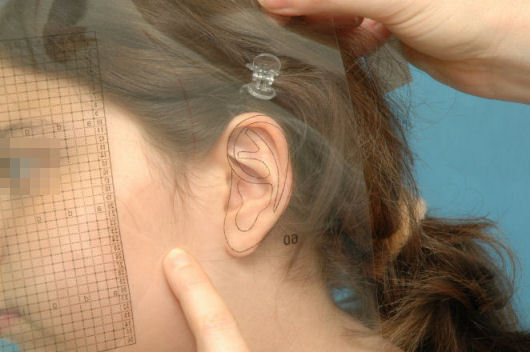
On the day before surgery we make a template according to the contralateral healthy side in unilateral cases and according to general anthropometric data in bilateral cases (Figure 19 (Fig. 19)). The planed position of the new auricle is than marked onto the skin.
Figure 19. Template of the auricle.
5.1.3.1 1st step
Generally we work with 2 teams. One removes cartilage from the ipsilateral ribs 6-9 (Figure 20 (Fig. 20)). In male patient the incision is above rib 7. In female patients we have described a special T-shaped incision 2 cm below the submammarian fold, that is excised in the second step and the skin transposed cranially, so that the final scar lies almost invisible exactly in the submammarian fold [14].
Figure 20. Intact perichondrium after harvesting rib cartilage.
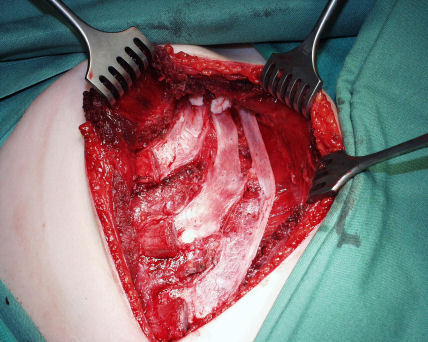
After removal of the cartilage the ribs are reconstructed by suturing resorbable nets (Vicryl®) onto the left in place inner perichondrium. These are filled with the otherwise unusable left over, little pieces of rib cartilage that are collected throughout the carving procedure (Figure 21 (Fig. 21)) [26]. We have been using this technique regularly since more that 7 years and could show, that stable regeneration of the ribs develop. On palpation the regenerated ribs are similar to the original or contralateral ribs.
Figure 21. Reconstruction of the ribs with an absorbable net filled with left over pieces of cartilage fixed onto the perichondrium.
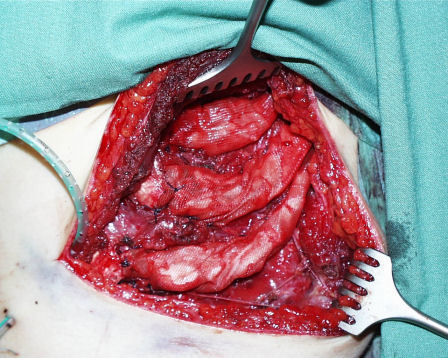
The 2nd team creates a skin pocket in the auricular region and removes the malformed auricular cartilage. This team also carves and constructs the framework according to Nagata’s suggestions (Figure 22 (Fig. 22), Figure 23 (Fig. 23)). It is then transplanted to the planned position. The skin is draped over it and pulled into the relief with 2 suction drains (Figure 24 (Fig. 24)).
Figure 22. Planning the base plate of the auricular framework on the ribs 7 and 8. a: planning with the template; b: planning on the rib cartilage.
Figure 23. Auricular framework.
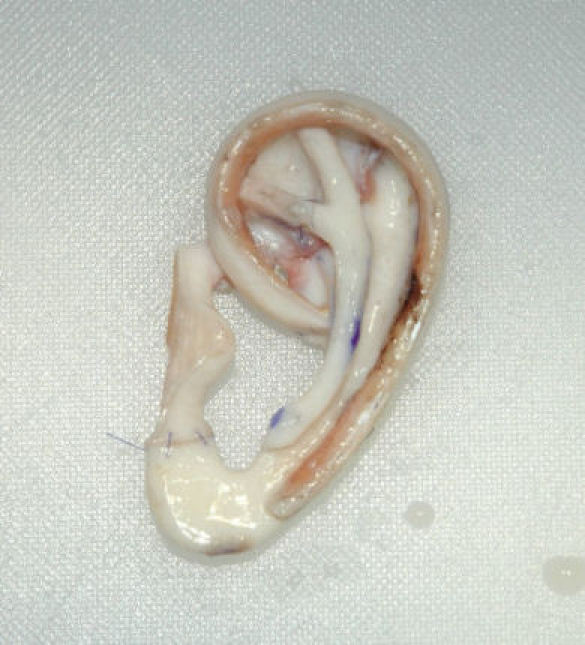
Figure 24. Situation at end of 1st step.
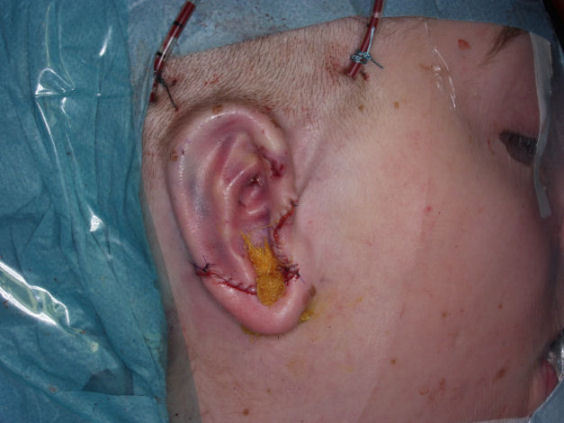
If middle ear reconstruction is feasible an ear canal is fabricated made out of little pieces of rib cartilage around a Silastic cylinder. In addition the tympanic membrane is built out of the elastic malformed cartilage positioned into a special mold [30].
5.1.3.2 2nd step
Approximately 6 months after the first step auricular reconstruction is continued with the second step. It includes the elevation of the auricle and in appropriate cases also the so-called atresia operation, which is the creation of an external ear canal, tympanic membrane and creation of the sound conducting apparatus.
The incision is made 1 cm distant to the helical rim. The skin is elevated as a very thin flap leaving the hair follicles in place. Care is taking not to expose the cartilage. The whole framework remains covered with connective tissue. A highly vascularized, posteriorly pedicled flap of superficial temporalis fascia or SMAS is mobilized. After carving a crescent-shaped piece of cartilage used as a buttress for the elevation and sutured to the back of the base plate it covers the cartilage and is the base for the full thickness skin graft that covers the created retroauricular fold. The skin graft is harvested from the rib region. It includes the scar from rib harvesting, so that no additional scar is created.
To decrease the size of the retroauricular wound area a large transposition-rotation-flap from the neck is mobilized and transposed behind the framework. Then the remaining defect is covered with the skin graft (Figure 25 (Fig. 25)). The wound is covered with a stable bandage using multiple tapes which are not removed before one week postoperatively.
Figure 25. 2nd step of auricular reconstruction. a: planning of the incision; b: elevated framework with mobilized SMAS-flap; c: cartilaginous buttress and transposition flap; d: situation at end of 2nd step.
5.1.3.3 3rd step
In the 3rd step refinements are performed. Remnants of the malformed pinna might have to be worked into the relief in a very meticulous way. Unwanted hair on the helix in patients with a low hair line might have to be removed (Figure 26 (Fig. 26)). For epilation we predominantly use electroepilation, which still is the Gold standard in epilation. Alternatively Laser or intense pulsed light (IPL) might be used.
Figure 26. 3rd step of auricular reconstruction with refinements of the relief and hair removal by electro coagulation.
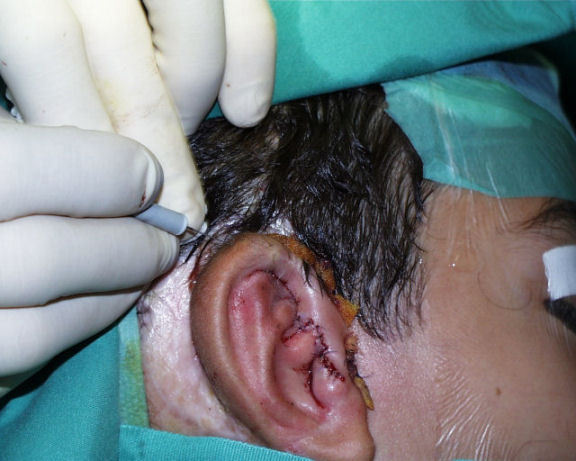
If the atresia operation has been performed in the 2nd step the implants are removed and the constructed external ear canal is covered with another full thickness skin graft.
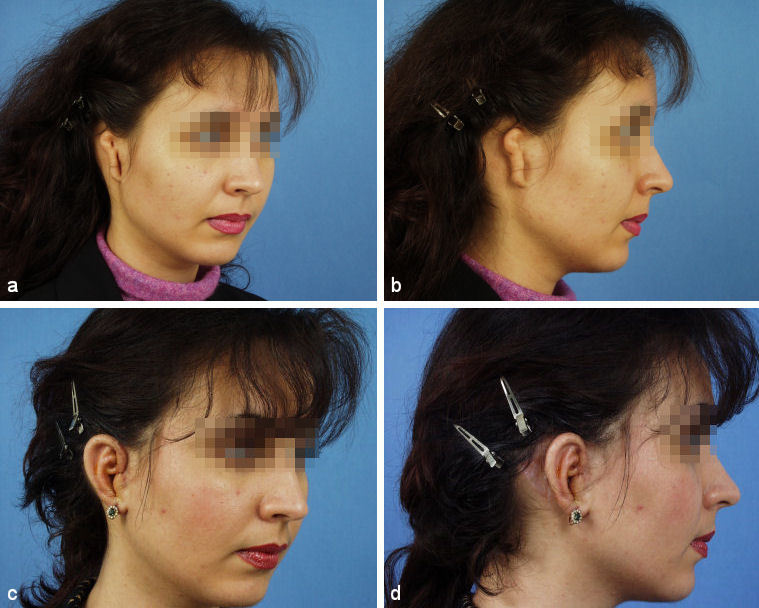
In most cases these 3 operations result in good aesthetic results (Figure 27 (Fig. 27), Figure 28 (Fig. 28)). They can mimic normality, but due to different biophysical properties of rib cartilage they can never be completely normal. In some cases additional minor refinements can be suggested and further improve the aesthetic outcome.
Figure 27. Situation at end of 3rd step. a, b: preoperative situation; c, d: postoperative situation.
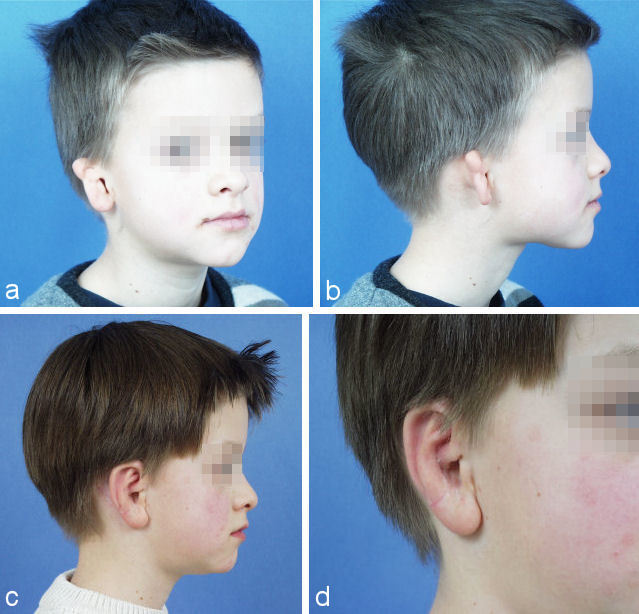
Figure 28. Result after 3 steps. a, b: preoperative situation; c, d: postoperative situation.
References
- 1.Anita N, Buch V. Chondrocutaneous advancement flap fort he marginal defects of the ear. Plast Reconstr Surg. 1967;39:472–477. doi: 10.1097/00006534-196705000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Ariyan S, Chicarilli Z. Replantation of a totally amputated ear by means of a platysma musculocutaneous "sandwich" flap. Plast Reconstr Surg. 1986;78:385–389. doi: 10.1097/00006534-198609000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Baudet J, Tramond P, Gonmain A. A propos d'un procede original de reimplantation d'un pavillon de l'oreille totalement separe. Ann Chir Plast Esthet. 1972;17:67–72. [PubMed] [Google Scholar]
- 4.Brent B, Byrd HS. Secondary ear reconstruction with cartilage grafts covered by axial, random and free flaps of temporoparietal fascia. Plast Reconstr Surg. 1983;72:141–151. doi: 10.1097/00006534-198308000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Chongchet V. A method of anthelix reconstruction. Br J Plast Surg. 1963;16:268–272. doi: 10.1016/s0007-1226(63)80120-4. [DOI] [PubMed] [Google Scholar]
- 6.Conroy W. Letter to the editor: Salvage of an amputated ear. Plast Reconstr Surg. 1972;49:564. doi: 10.1097/00006534-197205000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Converse JM. Construction of the auricle in congenital microtia. Plast Reconstr Surg. 1963;32:425–438. doi: 10.1097/00006534-196310000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Converse JM. Reconstruction of the auricle. Part 1, Part 2. Plast Reconstr Surg. 1958;22:150–63, 230. doi: 10.1097/00006534-195808000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Conway H, Neumann CG, Gelb J, Leveridge LL, Joseph JM. Reconstruction of the external ear. Ann Surg. 1948;128:226–238. [PMC free article] [PubMed] [Google Scholar]
- 10.Crikelair GF. Another solution fort he problem of the prominent ear. Ann Surg. 1964;160:314–324. doi: 10.1097/00000658-196408000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elsahy N. Ear replantation combined with local flaps. Ann Plast Surg. 1986;77:102–111. doi: 10.1097/00000637-198608000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Feuerstein SS. Complications and revisions in otoplasty. Fac Plast Surg. 1985;2:141–151. [Google Scholar]
- 13.Gersuny R. Über einige kosmetische Operationen. Wien Med Wschr. 1903;48:2253–2257. [Google Scholar]
- 14.Magritz R, Siegert R. Die submammäre Inzision zur ausgedehnten Rippenknorpelentnahme. Laryngo Rhino Otol. 2005;84:395–397. [Google Scholar]
- 15.Marx H. Die Mißbildungen des Ohres. In: Denker-Kahler, editor. Handbuch der HNO-Heilkunde. Bd. 6/1. Berlin: Springer; 1926. [Google Scholar]
- 16.Mladick R, Carraway J. Ear reattachement by the modified pocket principle. Plast Reconstr Surg. 1973;51:584–587. doi: 10.1097/00006534-197305000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Mladick R, Horton C, Adamson J, Cohen B. The pocket principle. Plast Reconstr Surg. 1971;48:219–223. [PubMed] [Google Scholar]
- 18.Musgrave RH, Garrett WS. Management of avulsion injuries of the external ear. Plast Reconstr Surg. 1967;40:534–539. doi: 10.1097/00006534-196740060-00004. [DOI] [PubMed] [Google Scholar]
- 19.Mustarde JC. The correction of prominent ears. Using simple mattress sutures. Br J Plast Surg. 1963;16:170–176. doi: 10.1016/s0007-1226(63)80100-9. [DOI] [PubMed] [Google Scholar]
- 20.Nagata S. A new method of total reconstruction of the auricle for microtia. Plast Reconstr Surg. 1993;92:187–201. doi: 10.1097/00006534-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Nagata S. Modification of the stages in total reconstruction of the auricle. Part II: Grafting the three-dimensional costal cartilage framework for concha type microtia. Plast Reconstr Surg. 1994;93:231–242. [PubMed] [Google Scholar]
- 22.Nagata S. Modification of the stages in total reconstruction of the auricle. Part III: Grafting the three-dimensional costal cartilage framework for small concha type microtia. Plast Reconstr Surg. 1994;93:243–253. [PubMed] [Google Scholar]
- 23.Nagata S. Modification of the stages in total reconstruction of the auricle. Part IV: Ear elevation for the constructed auricle. Plast Reconstr Surg. 1994;93:254–266. [PubMed] [Google Scholar]
- 24.Salyapongse A, Lorenzo P, Suthunyarat P. Succesfull replantation of a totally severed ear. Plast Reconstr Surg. 1979;64:706–707. [PubMed] [Google Scholar]
- 25.Siegert R, Krappen S, Kaesemann L, Weerda H. Computer-assisted anthropometry of the auricle. Face. 1998;6:1–6. [Google Scholar]
- 26.Siegert R, Magritz R. Rippenrekonstruktion nach ausgedehnter Knorpelentnahme. Laryngo Rhino Otol. 2005;84:474–478. [Google Scholar]
- 27.Siegert R, Weerda H, Mayer T, Brückmann H. Hochauflösende Computertomographie fehlgebildeter Mittelohren. Laryngo Rhino Otol. 1996;75:187–194. doi: 10.1055/s-2007-997561. [DOI] [PubMed] [Google Scholar]
- 28.Siegert R. Correction of the Lobule. Facial Plastic Surgery. 2004;20:293–298. doi: 10.1055/s-2005-865388. [DOI] [PubMed] [Google Scholar]
- 29.Siegert R. Lobuluspexie durch Nahtzügelung. Laryngo Rhino Otol. 2004;83:720–725. [Google Scholar]
- 30.Siegert R. The combined reconstruction of congenital auricular atresia and severe microtia. Laryngoscope. 2003;113:2021–2029. doi: 10.1097/00005537-200311000-00031. [DOI] [PubMed] [Google Scholar]
- 31.Spira M. Early care of deformities of the auricle resulting from mechanical trauma. In: Tanzer RC, Edgerton MT, editors. Symposium on reconstruction of the auricle. X. St. Louis: CV Mosby & Co.; 1974. pp. 204–217. [Google Scholar]
- 32.Tanzer RC. The constricted and lop ear. Plast Reconstr Surg. 1975;55:406–415. [PubMed] [Google Scholar]
- 33.Walter C. Die Probleme der Rekonstruktion der Ohrmuschel. HNO. 1969;17:301–305. [PubMed] [Google Scholar]
- 34.Weerda H, Grüner R, Cannive B. Die Einheilungsrate frei transplantierter, großer "Composite grafts". Arch Otorhinolaryngol. 1986;Suppl II:129. [Google Scholar]
- 35.Weerda H, Münker G. Einzeitige Rekonstruktion von Ohrmuscheldefekten mit einem Transpositions-Rotations-Lappen. Laryngo Rhino Otol. 1981;60:312–317. [PubMed] [Google Scholar]
- 36.Weerda H, Siegert R. Classification and treatment of aquired deformities. Face. 1998;6:79–82. [Google Scholar]
- 37.Weerda H. Chirurgie der Ohrmuschel. Verletzungen, Defekte, Anomalien. Thieme; 2004. [Google Scholar]
- 38.Weerda H. Fibrinkleber in der Ohrmuschelchirurgie. In: Refferscheid M, editor. Neue Techniken in der operativen Medizin. Berlin: Springer; 1986. [Google Scholar]
- 39.Weerda H. Plastic surgery of the ear. In: Kerr AG, editor. Scott Brown's diseases of the ear, nose and throat. 5th ed. London: Butterworth; 1987. p. 3. [Google Scholar]
- 40.Weerda H. Plastisch-rekonstruktive Chirurgie im Hals-Nasen-Ohrenbereich. Hamburg-Norderstedt: Ethicon; 1980. pp. 2–13. [Google Scholar]