Abstract
Malformations of the middle ear are classified as minor and major malformations. Minor malformations appear with regular external auditory canal, tympanic membrane and aerated middle ear space. The conducting hearing loss is due to fixation or interruption of the ossicular chain. The treatment is surgical, following the rules of ossiculoplasty and stapes surgery. In major malformations (congenital aural atresia) there is no external auditory canal and a deformed or missing pinna. The mastoid and the middle ear space may be underdevelopped, the ossicular chain is dysplastic. Surgical therapy is possible in patients with good aeration of the temporal bone, existing windows, a near normal positioned facial nerve and a mobile ossicular chain. Plastic and reconstructive surgery of the pinna should proceed the reconstruction of the external auditory canal and middle ear. In cases of good prognosis unilateral aural atresia can be approached already in childhood. In patients with high risk of surgical failure, bone anchored hearing aids are the treatment of choice. Recent reports of implantable hearing devices may be discussed as an alternative treatment for selected patients.
Keywords: middle ear malformation, congenital oral atresia, surgical treatment, bone anchored hearing aid, implantable hearing aid
1 Introduction
The rehabilitation of a sense organ’s function is one of the noblest medical tasks. In middle ear malformations normaly the inner ear is intact, the malfunction is due to mechanical problems. According to this the treatment of middle ear malformations always has to be surgical. The minor malformation is treated with established methods of middle ear surgery. Major malformation surgery is the most difficult level in middle ear surgery. Kiesselbach [1] is considered to be the first who seriously attempted atresia treatment. The patient in his 1882 published case report ended up with a facial paresis, 60 years before introduction of micro-surgical techniques into temporal bone surgery by Moritz, Wullstein and Zöllner. The simple but difficult question, most of the patients are infants, is to recognize if the malformed ear is a hearing ear. Today brainstem audiometry may answer this question at a much higher level of certainty. Crucial for indication in atresia surgery and for favourable results is the high resolution computertomography (CT). Out of the CT-scans developped grading systems help to make the results more predictable. Thus inadequate expectations can be avoided. In cases of unfavourable anatomy where classical surgery is not possible, bone-anchored hearing aids (BAHA) are an established alternative. Recent developments using implantable hearing aids show promising results for further improvement.
2 Remarks to developmental history
The normal and pathologic embryologic development is not of major importance for the therapy of middle ear malformations. But there are some aspects of the ontogenetic development, that may be of some interest.
2.1 Development of the aurical and the middle ear
Malformations of the outer and middle ear and malformations of the inner ear are not regularly associated, because of the different time of embryogenesis. Nevertheless one third of patients with middle ear malformations are considered to have a inner ear malformation as well [2], [3]. The auricle is built out of 6 hillocks. The hillocks 1-3 are dedicated to the first branchial arch (mandibular arch) and the hillocks 2-6 to the second branchial arch (hyoid arch). In 1837 Reichert [4] studied in pig embryos that Meckel’s cartilage out of the first branchial arch at the proximal end develops the cartilaginous annex of the malleus. In reptiles and birds this part develops not towards the malleus but the joint bone of the lower jaw (articulare). This bone connects with the bone of the upper jaw (quadratum). In mammals these two bones of the primary mandibular joint (articulare, quadratum) are missing. Inspite of this two new ossicles exist, malleus and incus, instead of the columella of the sauropsidae. From these studies Reichert and Gaupp [5] developped the theory of homology of primary mandibular joint and sound conducting apparatus. According to this the malleus matches the articulare, the incus the quadratum. Both ossicles originate from the first branchial arch (mandubular arch). The stapes is then originating from the hyoid arch (2. branchial arch, Reichert’s cartilage) and matches the columella auris of the sauropsidae. The unusual shape of the stapes with two crura arrives from the development around the stapedial artery.
2.2 The objection of Otto against the classical developmental theory
The theory of Reichert and Gaupp has been mistrusted again and again. According to Otto [6] there is no obvious explanation why the already good functioning columella of the sauropsidae should be replaced. There is also no hint how the columella between tympanic membrane and stapes was replaced by quadratum and articulare without any functional deficiency. Otto supposes that all 3 ossicles derive from the second branchial arch. Analogously he assumes the border between mandibular and hyoid arch between the first and second inspite between the third and sixth hillock. Ear clefts, appendices of the ear up to doubling of the pinna seem to support this objection [7].
3 Taxonomy of middle ear malformations from a surgeon’s sight
Marx [8] developped one of the first systematics of malformations of the outer and middle ear. Altmanns [9] taxonomy of 3 groups based on the study of temporal bones. He differentiated minor, medium and major malformations. From a clinical standpoint a classification in minor and major malformations is established [10], [11], [12], some authors advice further subclassification [13], [14].
3.1 Minor middle ear malformation
Minor middle ear malformations appear with a regular outer ear canal and regular tympanic membrane. In some cases the ear canal is more narrow as usual and the size of the tympanic membrane reduced. Malformed is the ossicular chain, mostly the stapes, fixed as congenital stapes ankylosis [15]. There is a broad variety of ossicular dysplasia, bony bridges with malleus and incus fixation, fusion of malleus and incus or disruption of the ossicular chain like aplasia of the long process of the incus [16].
3.2 Major middle ear malformation (congenital aural atresia)
The most typical finding in major malformations is the near total stenosis or the absence of the outer ear canal therefore the term congenital aural atresia (CAA). In most cases there is a combination with malformations of the auricle of different grade, this may not correlate with the grade of middle ear malformation [17]. The absence of the outer ear canal results in approximation of the temporal mandibular joint towards the mastoid (Figure 1 (Fig. 1)) [11], [17]. The os tympanicum may be developped rudimental or totally absent. Laterally to the more or less aerated middle ear space lies the so called atresia plate, a bony condensation product. In animal studies tissues of different origin have been recognized to build up the atresia plate (os tympanicum, squama of the temporal bone, hyperplastic labyrinthine capsule) [18]. There is a wide range of pneumatization of the mastoid (Figure 2 (Fig. 2)), from extensive aeration of the middle ear and mastoid to totally absence of a pneumatized space. Typically the ossicles are dysplastic, malleus and incus form a typical conglomerate. In most cases a normal and mobile stapes exists. As a rule the handle of the malleus is fixed. The round window is the most constant structure even in extensive malformations. Cremers and coworkers [13], [19] suggested to distinguish a group of malformations with atresia of the medial part of the ear canal only. Many authors advise to seperate severe malformations that should be excluded from reconstructive surgery [17], [19], [20], [21].
Figure 1. Regular temporal bone (a), temporal bone with congenital aural atresia (b). The missing and underdevelopped os tympanicum leads to direct attachment of the temporal mandibular joint towards the mastoid.
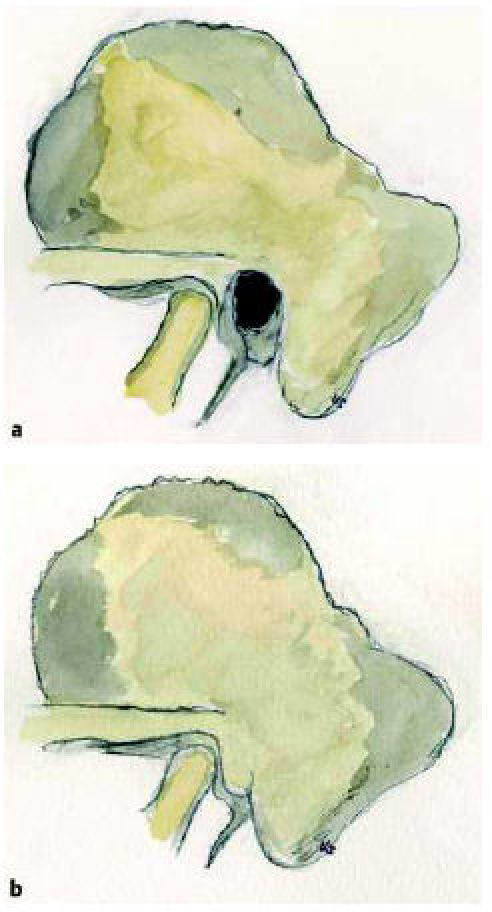
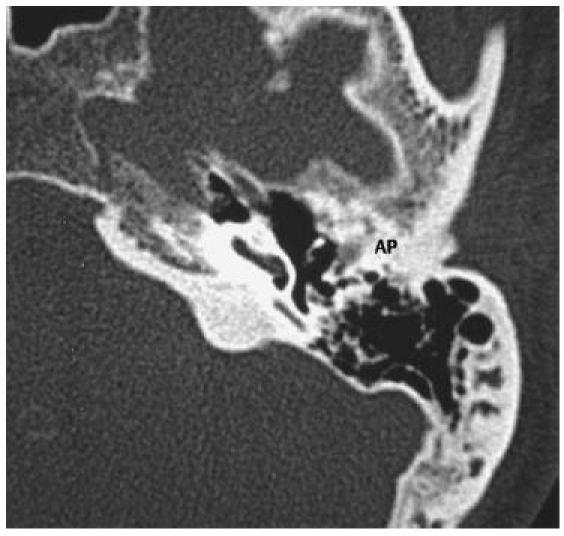
Figure 2. CT-scan of the temporal bone. Good aeration. Exposed malleus-incus-conglomerate. AP: atresia plate. (Thanks to Prof. Dr. med. E. Hofmann, director of the department for diagnostic and interventional neuroradiology, Klinikum Fulda gAG).
3.3 Malformation syndromes and middle ear malformation
Minor and major malformations appear as isolated entities or associate with malformation syndromes [22], [23], [24]. In western countries a major malformation can be expected in 1:8000 till 1:10,000 births [3], [25]. 20-30% are double-sided [26]. In 7% of patients with congenital aural atresia a malformation syndrome will be recognized. A common complex is the oculoauriculovertebral dysplasia (OAV dysplasia) [27]. This complex includes the craniofacial microsomia and the Goldenhar’s syndrome. These syndromes appear as asymmetrical facial malformation. Distinguished from these are symmetrical malformation syndromes like the mandibulofacial dysostosis (Franceschetti-Zwahlen-Treacher-Collins syndrome). Hypoplasia of the mandible and zygoma, antimongoloid eye position and anomalies of the lid are common [28], [29]. Similar symptomes are shown with the acrofacial (otomandibular) dysostosis, the Nager’s syndrome excluding the ocular symptomes typical for Treacher-Collins. In craniofacial dysostosis (Crouzon) or Apert’s syndrome malformations of the outer ear canal or the middle ear are not uncommon. Syndromes including malformations of the spine like Klippel-Feil or Wildervanck are associated with middle ear malformations as well. Malformation syndromes of exogenic causes are the rubella embryopathy, but the middle ear and ear canal are not regularly affected. An embryopathy induced by Thalidomide medication during pregnancy caused numerous malformations in the late 1950s and early 1960s. These included numerous malformations of the middle and outer ear, depending on the time point of medical treatment during pregnancy. Dysplasias of the OAV complex are regularly associated with major malformations. In other syndromes there is a wider variety from more mild to more servere appearance. In principle all cases of aural dysplasia need a pediatric and genetic assessment regarding a malformation complex.
4 Irregular courses and malformations of the facial nerve and blood vessels in the temporal bone
4.1 Facial nerve and middle ear malformations
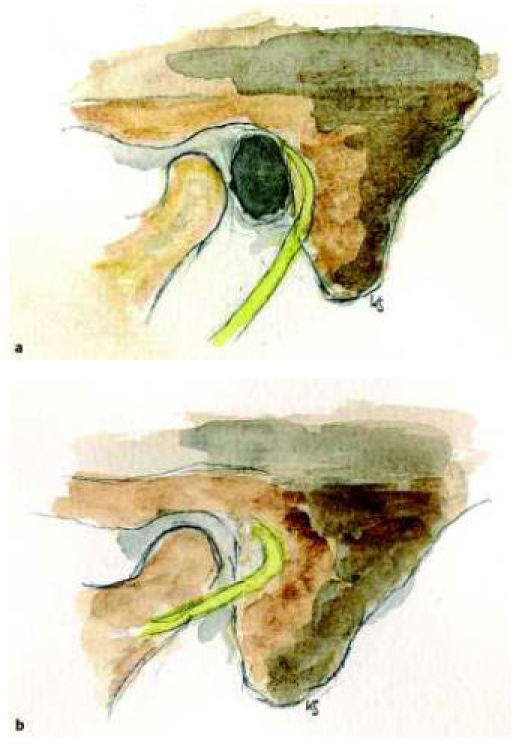
Of all cranial nerves the course of the facial nerve is most unusual. The Fallopian canal is the longest run of a cranial nerve through the bony skull base. Characteristic are two 90 degree turns of the nerve first at the geniculate ganglion and second from the transversal tympanic part to the vertical mastoid part. These anatomical characteristics are due to embryonic developments of the temporal bone, but also postfetal changes like the development of the mastoid process. The lack of development of the os tympanicum leads to an anterior positioning of the nerve in the mastoid part. The stylomastoid foramen may be very superficial behind the glenoid fossa [30], [31], a situation similar in infancy with the mastoid still hypoplastic. As well as in the surgical therapy of the infants’ antritis in these malformed situations the facial nerve is jeopardized already during skin incision (Figure 3 (Fig. 3)). The types of facial nerve malformations are numerous. Dehiscences of the Fallopian canal in the middle ear around the oval window are not unusual even in the normal temporal bone. An anterior positioned nerve at the oval niche is still a variation. Real malformations are free running nerves over the oval window niche or splitting of the nerve in the temporal bone. This may be especially a problem in the surgery of minor malformations [32]. If the nerve runs over the oval window, a promontory fenestration is the surgery of choice. The facial nerve running over the round window is not unusual [3], [33], besides anterior positioning of the nerve as well dorsal positioning is described. Elongations in the area of the second knee have the high risk of nerve injury. It is of common thought, that the Fallopian canal runs always medially of the malleus-incus-conglomerate. Lang and Hack [34] studied regular temporal bones and noticed a course of the nerve lateral to the anulus in 4%. It is reported that abnormal courses of the facial nerve are more often in malformations without meatal atresia [3]. To avoid surprises intraoperatively an exact analysis of preoperative CT-scans is necessary. An electrophysiologic monitoring of the facial nerve is helpful to reduce the risk. There are several synopses of abnormal courses of the facial nerve [12], [32], [35], [36], [37]. In rare cases transposition of the nerve intraoperatively may be an option to position a prosthesis in the oval window niche [38].
Figure 3. Course of the facial nerve in the regular (a) and malformed (b) temporal bone.
4.2 Vessel anomalies in middle ear malformations
Vessel anomalies, mainly abnormal courses, are variants also in the regular middle ear. A high located jugular bulb or an anterior positioned sigmoid sinus are rare, but not unusual. In the narrow situation of a malformed temporal bone a surgical procedure may be more difficult or even impossible. Rare is a free running carotid artery. The vessel is already jeopardized in the none malformed middle ear performing paracentesis. The exact analysis of the CT-scans again is helpful to avoid intraoperative surprises.
A real malformation is the persisting stapedial artery [39]. During tympanoscopy because of conducting hearing loss it has been seen in 1:1000 up to 1:4000 cases. A strong stapedial artery may be important for the cerebral circulation. Neurological disorders after clipping in these cases have been reported [40]. According to Schuknecht [39] a persisting stapedial artery exists in a frequency of 1:5000 up to 1:10,000.
5 Preoperative diagnostics in middle ear malformations
5.1 History, physical examination
Malformations of the pinna always have to be a reason for further examinations. Lack of hearing in newborns is more and more recognized due to screening projects. A complete ENT, phoniatric-pedaudiologic and pediatric examination is necessary. The history includes the family, birth and pregnancy history. In cases of desire of children a genetical council of the parents is necessary. Affected adults have to be informed about the risks of malformations regarding own children.
5.2 Audiological diagnostics
Depending on the age of the patient there are several studies possible. Pure tone audiogram, speech audiogram, reaction audiometry, behavioral audiometry, free field studies and bone conduction brainstem evoked response audiometry [41]. In infants and toddlers a pedaudiological testbattery including behavioral studies, bone conduction BERA and early fitting of bone conducting hearing aids in connection with early support is necessary. In basic questions regarding unilateral atresia as well as minor malformations the tuning fork tests according to Weber and Rinne are helpful to verify unclear findings. To recognize hearing in newborns and infants always was a difficult question. Using the brainstem audiometry, this question is now more certain to be answered.
5.3 Imaging and prognosting indices
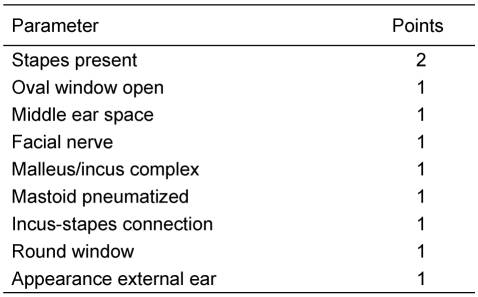
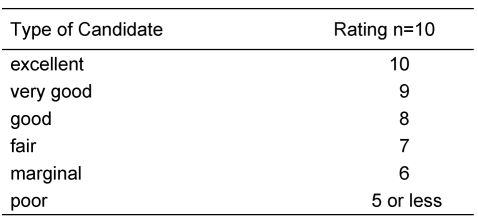
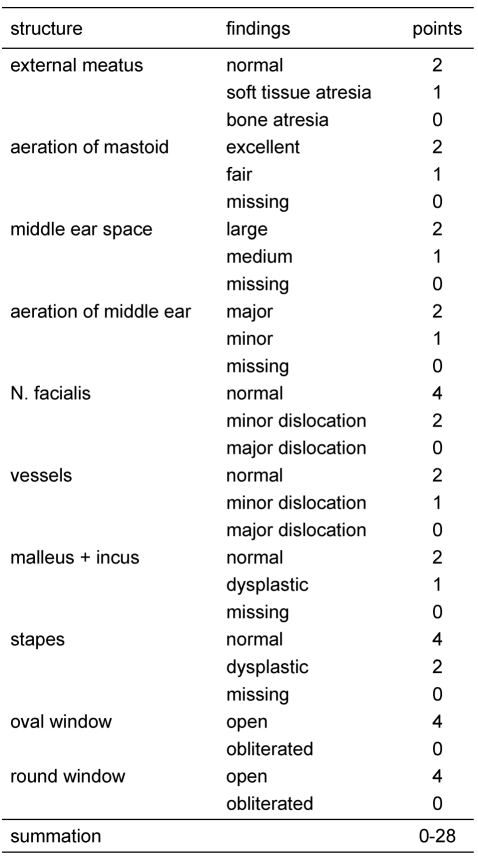
In case of a regular figured outer ear canal and a normal tympanic membrane a Schüller’s view may be sufficient. This classical X-ray gives necessary hints like pneumatization of the mastoid, position of the sigmoid sinus and the dura. But also in cases of minor malformations a high resolution CT-scan of the temporal bone should be considered as many additional informations like configuration of the ossicles and the course of the facial nerve are provided. In major malformations the high resolution computed tomography of the temporal bone is obligatory. The computed tomography is the method of choice in preoperative estimation of atresia surgery [42], [43], [44], [45], [46]. Jahrsdoerfer (Table 1 (Tab. 1), Table 2 (Tab. 2)) [46] and Siegert (Table 3 (Tab. 3)) [47] developped prognostic indices from the study of CT-scans. Jahrsdoerfer’s prognostic system consists at a maximum of 10 points. Of major importance is an existing stapes. If there is a minimum of 7 points the prognosis should be reasonable. Siegert developped a more deversified system with a maximum of 28 points. High numbers (4 each) are assigned to an open oval or round window. Surgical treatment options are adviced at a minimal number of 15 for the bilateral and 20 for the unilateral atresia. A CT-scan of the temporal bone is only indicated when there is an actual consequence for treatment [48]. Because of exposure to radiation should be avoided, there is no indication at all to perform a CT-scan in an infant, if a surgical procedure is planned years later. Exeptions are a cholesteatoma or complications following an acute or chronic otitis media. To complete imaging magnetic resonance tomography is advisable. Thus additional information regarding inner ear canal and intracranial situations especially in patients with syndromes can be achieved.
Table 1. Grading System (Jahrsdoerfer [46]).
Table 2. Prognostic Rating Scale in Congenital Aural Atresia (Jahrsdoerfer [46]).
Table 3. Siegert’s grading system [45]. Prognostic rating based on CT-scan findings.
5.4 Assessment of prognostic indices
Jahrsdoerfer’s index with a maximum of 10 points is quite restricted, Siegert’s index with a maximum of 28 points more extensive. In both systems it is questionable how an assignment of points can be used vicarious. A major problem is a missing round window. The tendency to obliterate is high in an artificial window. The opening of the inner ear bears also a servere risk of hearing loss. An unusual course of the facial nerve may complicate atresia surgery. Overall in our mind 4 anatomical conditions as requirements for a successful atresia surgery should be detectable: a regular stapes, a regular round window, a sufficient aerated middle ear space and a regular course of the facial nerve (Table 4 (Tab. 4)). The preoperative considerations have also to include the personal expection, the family and social surrounding and the psychological situation of the patient.
Table 4. Basic conditions for atresia surgery.
6 Primary steps and urgent indications for surgical treatment
6.1 Primary steps
First aim is to provide sufficient hearing to the child for a normal speech and social development. The necessary early steps have to be as soon as the diagnosis hearing impairment based on a middle ear malformation is assessed.
The middle ear malformation is resulting in a conducting hearing loss in complete atresia showing a maximum conductive block of 50 to 60 dB. Surgical therapy is not possible yet, so imaging like computertomography is not necessary. Exeptions are malformations with life-threatening extend like big defects of the skull base, cholesteatomas, infections and endocranial complications.
The necessary early treatment in congenital aural atresia is a bone conducting hearing aid. Thus it is possible to reduce the conductive hearing loss under the level of 30 dB to provide hearing for a regular speech development. These conductive hearing aids used inconvenient fixation devices. Alternatively nowadays elastic ribbons are available with individual colors and ornaments. The hearing aid can be placed on different sites of the skull, that bears more comfort for the little patient.
6.2 Indications for urgent surgery
The rules for surgical treatment in complications are not different to the regular configurated temporal bone. A condition that needs surgery is the cholesteatoma. There are congenital cholesteatomas, but also cholesteatomas resulting from debris retention in narrow auditory canals [14]. The frequency of primary congenital cholesteatomas is reported to be 2-5% [49], [50]. A formation of cells (epidermoidformation), that is recognized during the 33rd week of gestation is considered the origin of congenital cholesteatoma. After the 33rd week this formation disappears [51]. This type of cholesteatoma should not be more frequent in malformed middle ears than those with regular anatomy. Of more importance are the mentioned cholesteatomas resulting from congenital subtotal stenosis. Narrowings less than 2 mm are seen to predestine for this type of lesion. An early sanation is recommended [52].
7 Therapy of middle ear malformations
7.1 Classical operative techniques
7.1.1 Surgery for minor malformations
The definition of a minor malformation is a regular outer ear canal and meatus as well as a regular pneumatized middle ear space [53]. Typically an isolated malformation of the ossicles is recognized. Excellent pneumatization due to regular function of middle ear mucosa and Eustachian tube is the basis of good prognosis for surgical therapy. The most common situation is the congenital stapes fixation. The surgical technique follows the classical stapes surgery in otosclerosis. The approach is endaural [54], [55], followed by partial stapedectomy, removing of the posterior third of the foot plate and fixing the prosthesis at the long process of the incus. A higher risk of a Gusher in the malformed temporal bone has to be considered. A wide open cochlear aqueduct may be a hint for that condition [55]. Good results can be achieved using titanium prosthesis. This material seems to be of advantage compared to gold or platinum teflon. In cases of a facial nerve running over the oval niche a promontory fenestration should be performed. Under certain circumstances a transposition of the nerve, a rather difficult technique, may be an option [38]. The contact of the prosthesis with the free running nerve should be avoided. Some patients report of unusual and disturbing sensations. In cases of incus fixation it may be necessary to remove the incus. Then a malleovestibulopexy is necessary. A special stapes prosthesis adapted by Häusler [56] is available for these cases. The risk for perforation of the tympanic membrane seems to be low for this ribbon like clips of titanium prosthesis. Other types of ossicular malformations are treated according to the findings. Bony bridges, disruptions of the chain, missing of the long process of the incus, all problems that can be solved using classic methods of ossiculoplasty [55], [57], [58]. While the stapes fixation has a good prognosis, the results in complex malformations may be unsatisfactory [59], [60].
Indication and time in treatment of minor middle ear malformations
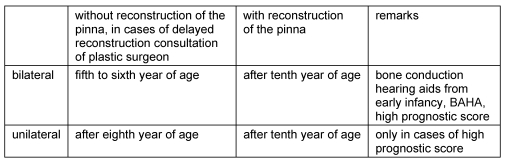
Double-sided malformations should be treated in the fifth to sixth year of age, before starting school. A minor malformation with normal hearing on the contralateral ear in many cases is recognized not before eight or ten. Because there is no actual consequence especially in cases of minor conductive hearing loss, one should wait till age 12 (Table 5 (Tab. 5)).
Table 5. Indications for surgery in minor malformations.
7.1.2 Surgery in major malformations
More difficult is the situation in major malformations. Besides some exceptions it is a functional problem with missing meatus and conductive hearing loss as well as an aesthetic problem due to a different degree of a malformed pinna. It is always point of discussion what should proceed, the construction of the auricle or the reconstruction of the meatus and middle ear. It is common opinion that middle ear surgery should not hinder a planned construction of the pinna [61]. Necessary skin incisions should be performed that way, that a construction of the auricle should not be disturbed by skin incisions. The remnant of the pinna always is positioned to far caudal and anterior. A shifting posterior superior is necessary, scars will result, making auricular reconstruction more difficult. For these reasons some authors refuse middle ear surgery if pinna construction is planned, but not yet performed [61].
Indication and time for treatment of bilateral atresia
Patients with bilateral congenital atresia should undergo surgery just before starting school in the fifth or sixth year of age [62]. Construction of the missing pinna is not possible earlier than 9 years of age, the situation is even better after year 12 [47]. At this time there is enough rib cartilage available to construct the cartilage framework. Because of the disadvantages of classical bone conducting hearing aids alternatively in these patients a bone-anchored hearing aid (BAHA) should be considered.
Surgical issues of atresia operation
Precondition for a successfull operation is the correct indication resulting from the exact analysis of the CT-scans [42]. Helpful are prognostic indices as proposed by Jahrsdoerfer and Siegert or our own suggestion. These indices may be helpful, but can provide certain hints only. The pneumatization may be of high prognostic value, but it seems not to be the overall crucial factor for a good hearing result [63]. Nevertheless under similar conditions the better aerated temporal bone will be choosen for surgery. Half of the patients are no candidates for atresia surgery after extensive diagnostic. The missing development of the os tympanicum implements the approach of the temporomandibular joint towards the mastoid. Thus the anatomical place where the outer ear canal should be positioned is missing. The middle ear space will be covered by the temporal mandibular joint [64]. The new created meatus has to be build more or less crooked around the temporal mandibular joint towards the middle ear space. If these anatomical situations are not recognized the middle ear space with the malleus-incus-conglomerate will be missed and the surgeon goes straight to the facial nerve and the labyrinth (Figure 4 (Fig. 4)) [41].
Figure 4. The positioning of the new meatus has to consider the special anatomical situation of the missing os tympanicum. The meatus runs anterior partly behind the temporomandibular joint (a). If it is positioned perpenticular the middle ear space is missed with the risk of facial nerve and labyrinthine injury.
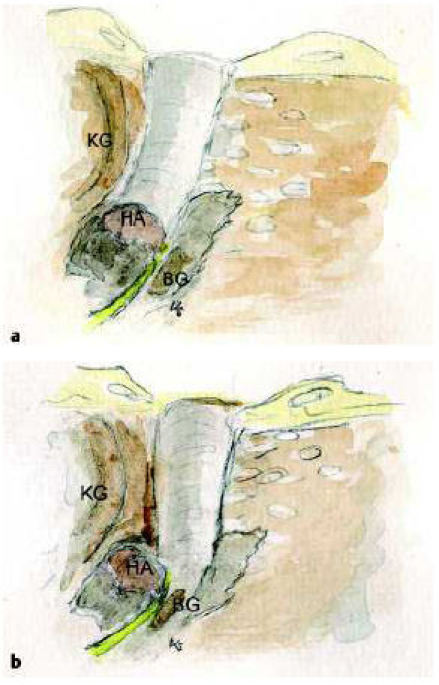
KG: temporomandibular joint, BG: lateral semicircular canal, HA: malleus-incus-conglomerat
Most feared postoperative problems are a wet meatus with continuous secretion and restenosis. Major series present 30-40% of cases showing these complications [21], [65], [66].
Preconditions for atresia surgery
Temporal bone surgery even under normal circumstances is highly challenging microsurgery. In the situation of a malformed temporal bone it is even more difficult. Great experience in temporal bone and middle ear surgery is the precondition. Of major interest for the patient is the function of the facial nerve. The risk for the nerve has to be reduced to a minimum under all circumstances. Elektrophysiological monitoring of the nerve is mandatory. It can help to identify the nerve under difficult situation. But it should not be used as a tool to find the nerve. There is no necessity to use a navigation system. Navigation should be considered like monitoring [67]. Surgery is save following landmarks.
Indication and time of treatment in unilateral atresia
Unilateral atresia in normal hearing of the opposite side is a special situation. Because of common problems like restenosis, recurrent inflammation and unsatisfactory audiological results many authors refused to do any surgery in unilateral cases at all [14], [17], [68], [69], [70]. Other authors recommended surgery after the patient is of full age and self-dependent [11], [71], [72], also our opinion in former publications regarding this topic [12]. Considering the criteria for choosing patients consequently a more differentiated judgment may be possible [73], [74], [75]. Selection of patients using prognostic systems and more developped techniques [76] can help to reduce the risk for unsatisfactory results [77]. As we suggest, atresia surgery should follow the construction of the pinna. The patient then being at least 12 years of age may better understand the situation and decide together with parental support.
The value of bilateral hearing is seen more and more important for many cognitive abilities [78], [79], [80]. A binaural hearing rehabilitation through classical surgery or an hearing aid should be achieved (Table 6 (Tab. 6)) [74].
Table 6. Indications for surgery in major malformations.
Surgical techniques
In principle two surgical approaches are possible [19], [20], [64], [65], [70], [81], [82]. One is the approach via the pneumanized mastoid. Other surgeons are in favour for the direct approach via the atresia plate towards the antrum [3], [17], [30], [54], [62], [76], [83], [84], [85], [86], [87], [88], [89], [90]. Fenestration surgery according to the development in stapes surgery is abandoned [21].
Transmastoid approach
The first step is a classical mastoidectomy followed by identification and exposure of the middle fossa dura and the sigmoid sinus. Using the dura of the middle fossa as landmark, the antrum is located. Anterior limitation is the temporomandibular joint. The glenoid fossa bone should stay intact, but exposure of a small soft tissue area of the temporomandibular joint is without consequences. The atresia plate will be burred away and the ossicles will be exposed. Typically it is a conglomerate of malleus and incus. Touching the ossicles with the burr has to be avoided [91]. Otherwise there is a high risk of permanent inner ear damage. If the deformed ossicular chain appears with good movement, it will be left in place and the reconstructed tympanic membrane will be positioned upon it. For epithelialization of the outer ear canal split thickness skin grafts are used, additionally pediceled skin flaps [64].
The advantage of this transmastoid approach is the good anatomical overview. Sigmoid sinus and dura of the middle fossa are two distinctive anatomical landmarks [92], but the resulting cavities with opended mastoid cells have the tendency for permanent inflammation and drainage.
Direct approach through the atresia plate
Because of unsatisfactory results using the transmastoid approach more and more the direct approach via the atresia plate was suggested [3], [31], [46], [93], [94], [95]. If a construction of the external ear is planned, this should precede middle ear surgery. The approach is retroauricular. In cases of preceding middle ear surgery to the reconstruction of the pinna it is mandatory necessary to coordinate the procedure with the plastic surgeon. In these cases an anterior incision may be necessary. The cribriform area dorsal to the root of the zygoma will be exposed. This is part of the so called atresia plate. The most important surgical landmarks are the dura of the middle fossa and anteriorly the temporomandibular joint. The direction of drilling has to consider the crooked way into the middle ear space as mentioned before. Otherwise there is the risk for the facial nerve or the labyrinthine capsule [3], [46], [61]. An extensive opening of the mastoid cells should be avoided. In anatomical unclear situations the surgeon should not hesitate to perform a transmastoid approach as described above [88], [92]. If the direction of drilling is correct, the malleus-incus-conglomerate will be found (Figure 5 (Fig. 5)) [96]. Normally the facial nerve runs deeper. An exact analysis of the CT-scans regarding these questions is necessary. The possibility of a lateral course of the Fallopian canal has been mentioned [34]. The facial nerve imposes as a whitish structure, typically with small accompanying blood vessels. The bone around the ossicles will be thinned out with the diamond burr, so the remaining bone remnants can be removed with the curette [55]. The malleus handle regularly is found to be fixed to the bony wall and has to be freed during this procedure. Using the curette minimizes the risk of inner ear damage. An intact and mobile chain should be saved if possible [3], [54], [97], [98]. The high resolution CT-scan already may show the continuity. So an exact exposition of the stapes and the oval niche with the risk of dislocation of the malleus-incus-complex is not necessary. The bone of the neomeatus should be drilled under the level of the malleus-incus-complex. This helps to avoid postoperative blunting. Materials for reconstruction of the tympanic membrane are temporalis fascia and perichondrium, but the latter in atresia cases not always available to the necessary amount. Favourable regarding stability is cartilage with good audiological results [99]. Palisade techniques and cartilage perichondrium islands are very feasible, also as the so called crowncork technique [100]. Epithelialization of the neomeatus is provided using split thickness skin graft of 0.2 mm from the inside of the upper arm for example. The split thickness skin is cut such way, that the epithelium covers the tympanic membrane like pieces of a cake (Figure 6 (Fig. 6)). Opened cells should be covered with cartilage to hinder the ingrowth of epithelium. Because of the tendency to shrink it is recommended to create the diameter of the new meatus twice as a regular one. To avoid restenosis as additional technique bone sutures according to Zühlke [101] are applied [54]. Silicon stents, rubber foam and gelatin foam soaked with antibiotic solutions are used as packing. Recommendations, how long a packing should stay, starts from 1 week [95] to 3 weeks or even longer [54]. The use of silicon sheets should help to reduce postoperative granulation.
Figure 5. Malleus-incus-conglomerate. If possible the ossicles should be used for reconstruction.
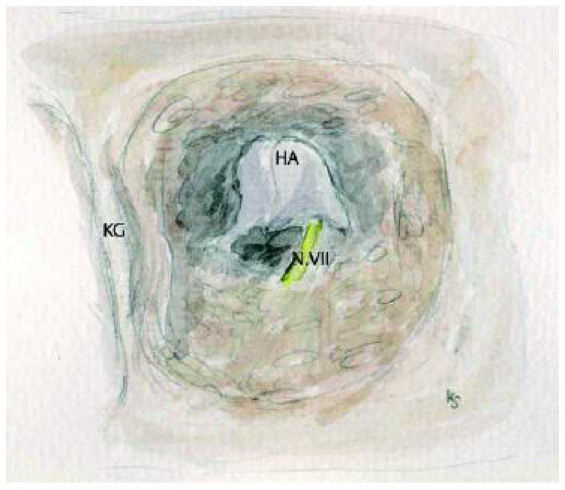
KG: temporomandibular joint, HA: malleus-incus-conglomerate
Figure 6. Split thickness skin graft of the meatus.

The lateralized tympanic membrane (technique of Helms)
Restenosis, a wet meatus and the lateralization of the tympanic membrane is considered as the main reasons for failures in atresia surgery [82]. These problems caused Helms to suggest a different technique [102]. The lateralized tympanic membrane in reptiles was adapted for atresia surgery. In a first step like in the classical technique a direct approach towards the middle ear space is performed. In opposite to create a meatus, a chain reconstruction using an extremely long middle ear prosthesis will be performed (Figure 7 (Fig. 7)). First glasionomeric cement and steel wire were used. Later a special titanium wire prosthesis was developped. The materials for the tympanic membrane are cartilage, fascia, perichondrium and split thickness skin grafts. The audiological results are similar to those of classical techniques [54]. Because of the missing meatus the use of a classical hearing aid is not possible. Many patients can not arrange with the aesthetic deficit of a missing meatus especially in single sided atresia an important aspect.
Figure 7. Lateralized tympanic membrane, technique according to Helms [102].
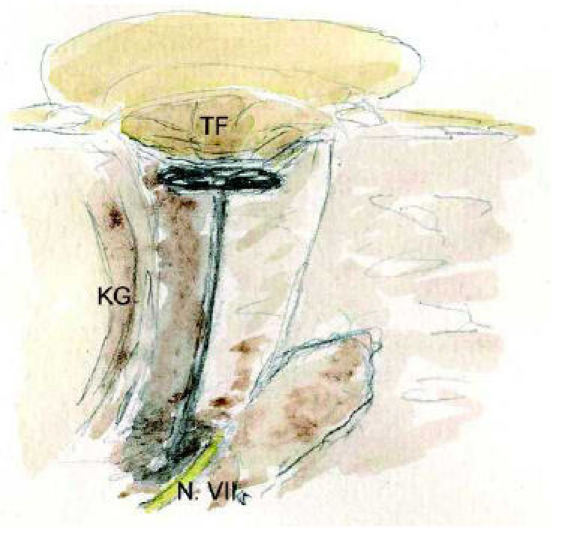
TF: tympanic membrane, KG: temporomandibular joint
Combined technique for reconstruction of the outer ear and the middle ear (technique of Siegert)
Basis for a successfull reconstruction in congenital aural atresia is a good coordination of plastic reconstruction and functional middle ear surgery. Siegert consequently had these principles in mind when he developped his technique of a combined reconstruction of pinna, meatus and middle ear [47], [48]. In the first surgical step not only the cartilage framework of the pinna is build, but at the same time the tympanic membrane and the meatus is formed from rib cartilage using silicon stents. This will be kept in a skin pocket at the thorax for at least 6 months. In the second step the pinna reconstruction, the opening of the middle ear according previous described techniques and the positioning of the preformed cartilaginous meatus will be performed. After at least 6 months in a third step the meatus will be epithelialized with split thickness skin. The technique in particular will be described by Siegert in his own paper, therefor the basic principles only will be outlined.
Siegert [48] reports of quite less stenosis and better epithelialization than in the classical technique. One reason is probably the stability of the meatus built of rib cartilage. Favourable functional results support this approach. The aesthetic aspect was already mentioned describing the technique of Helms. Many patients wish especially in unilateral atresia a „normal“ meatus, the hearing results for them are of second range.
7.1.3 Results of middle ear malformation surgery
Whereas the audiological results in minor malformation surgery are favourable and similar to otosclerosis surgery, the results in congenital aural atresia are far from this. 30 dB conducting hearing loss with regular inner ear function may be considered a good result, but this can not be achieved in all patients. Choosing consequently patients that are eligible for reconstructive surgery may meliorate the results. The development of scaling systems (Jahrsdoerfer, Siegert, own suggestion) seemed to help improvement. 8 points out of 10 in Jahrsdoerfers grading system should bear a 80% probability of a conductive hearing loss of less than 25 dB. In cases of 7 points the probability should be 70%.
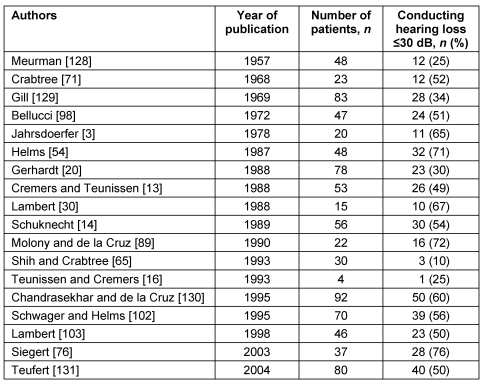
Table 7 (Tab. 7) gives an overview of publications regarding atresia surgery.
Table 7. Surgical results in major middle ear malformations.
A deterioration on the long run has been mentioned by Lambert [103]. One third of the patients need revision surgery. Including revision operations 50% had a conductive hearing loss of less than 25 dB and two-thirds of less than 30 dB. Classical middle ear surgery therefore has to match with new hearing devices that have to be discussed as real alternatives.
7.1.4 Revision surgery
By nature reoperations are more difficult than any primary surgery. An exact analysis of the situation and possible reasons for a failure can help to assess the chances for a revision operation. A narrow meatus with permanent secretion indicates revision surgery. In cases of continuing conductive hearing loss in a good aerated middle ear a further surgical attempt is promising. In these cases always it is advisable to talk about technical devices as alternatives.
7.1.5 Postsurgical treatment
The new meatus needs an intensive postoperative local treatment. Subtile covering the split thickness skin graft of the ear canal with silicon sheets and leaving the meatal packing for 4 weeks will reduce granulation production and restenosis. Granulations will be removed regularly and a local treatment with corticoid ointment will follow. It is important to keep the ear canal dry. Dye solutions and the use of a hairdryer may be helpful. In the beginning short time controls are necessary. Because of lack of cooperation, this postoperative treatment may be very difficult in children. If not possible, this may be a contraindication for a surgical treatment.
7.2 Bone-anchored hearing aids (BAHA)
The development of bone-anchored, active hearing implants changed the treatment of major conductive hearing losses, that have no or little chances of improvement, dramatically [104], [105], [106]. Bone conducting hearing aids are of major importance in early treatment of binaural middle ear malformations. The limitation for bone-anchored hearing aids is a sensory neural hearing loss of maximum 40 dB. Almost all of the patients with congenital aural atresia appear with normal or near normal inner ear function.
Brånemark [107] implemented the expression osseointegration for the implant bone connection. The bone anchor penetrates the outer skin, thus here is a region of higher risk of inflammation. The irritation of the skin, and this is not due to the material, can also lead to an unusual thickening of the cutis. To avoid infection around the osseointegrated screw, the skin has to be hairless. This can be achieved using split thickness skin (0.20 mm) or an extremely thinning of the skin flap. A second important point is the local care. This has to be as important as regular dental care. Thus inflammation of the skin can be reduced to a minimum. According to Federspil [108] the age limit is about 2 ½ years. The thickness of the bone is various. Children under the age of 2 were measured between 1.2 and 4.2 mm, in the age between 2 and 5 years 3.0 to 6.1 mm [109], [110]. From 3 mm on it is possible to fix the anchor screw. It is suggested in small children with a thickness of bone between 2.5 and 3 mm to use the 4 mm screws versus the 3 mm. Using Goretex® foil a positional bone growth can be induced [108]. In children the two step procedure is suggested, that means to fix the anchor 4 to 6 months later, after osseointegration. According to the schedule of delayed pinna reconstruction, the placement of the bone-anchored hearing aid should be far away enough from the place of aurical. The distance should be at least 5 cm [48]. The BAHA-results are favourable. In a series of 70 children all patients could achieve 30 dB or better in pure tone audiogram [111]. It should be mentioned also that the system of osseointegrated screws is the method of choice to fix auricular epitheses [106].
In bone-anchored hearing aids as well there is the question, should the supply be uni- or bilateral. The bilateral supply is of importance for all types of hearing rehabilitation from reconstructive middle ear surgery to cochlear implant. This topic was already discussed in the chapter of unilateral atresia. The unilateral bone-anchored hearing aid stimulates both inner ears, but it has been shown, that binaural supply provides better speech understanding and more important better spacial orientation, even it is a pseudo stereo hearing only.
Quality of life studies showed good results for BAHA-supplied patients with congenital atresia [112].
Indication for BAHA-supply
Because of definitive surgical rehabilitation including aurical reconstruction not earlier than 10 years of age bone-anchored hearing aids are indicated alternatively to classical bone conducting hearing aids. Because of the postponed pinna reconstruction it is necessary to have good rehabilitation for these important years of learning.
In cases where surgical middle ear reconstruction is not possible, a permanent supply with bone-anchored hearing aids [113] is a possible treatment. Unfavourable are the conditions for classical atresia surgery regarding patients with Franceschetti-Zwahlen-Treacher-Collins syndrome [29], [114]. Some authors mention that these patients should not be candidates for atresia surgery at all [11]. For these and similar cases the bone-anchored hearing aid is the alternative to surgery [55], [108].
7.3 Implantable hearing aids and middle ear malformation
Full- and partial implantable hearing aids were developped for improvement in sensory neural hearing loss. The common principle of the different systems is the coupling of a swinging element toward the ossicular chain. In many cases of congenital aural atresia the ossicular chain is malformed but mobile, still possible to fix a swinger. There are several implantable hearing systems on the market, that are feasible. The systems MET® (semiimplantable) and Fimos® (fullimplantable) from Otologics use an electromechanical transducer. Coupling and adjustment towards an intact chain demands high precision with intraoperative calibration. In a mobile chain it can be used in the malformed middle ear [115], [116]. St. Croix Medical provides the system Envoy™. It uses the eardrum as microphone, the chain has to be interrupted and the stapes is stimulated using a piezoelectric element [117]. The direct system of Soundtec activates the chain via a magnet, that is stimulated from an external conductor from a behind or in ear hearing aid [118]. A pinna and a meatus is necessary, but both is missing in atresia patients. Recently Cochlear presented the system DACS (Direct Acoustic Cochlear Stimulation). This device is intended to treat mixed hearing loss, it is semiimplantable and has a transcutaneous connection towards an active and passive stapes prosthesis [119]. Problems with a transcutaneous connection are known from BAHA. Because it is necessary to open the vestibule, the risks for inner ear function have to be considered. The Vibrant® Soundbridge® system provided by MED-EL seems to be feasible for the treatment of major middle ear malformations. Since 1996 more than 2000 devices have been implanted in regular middle ears. The advantage of this system, the implanted part has no extern sensitive electronical or mechanical part. The coupling of the processor is magnetic transcutaneous, it can be programmed and changed at any time. In fully implantable systems difficult, changing of batteries in this system is unproblematic. The Floating Mass Transducer (FMT) fixed to the long process of the incus in the regular middle ear is the stimulating device. In a similar way the FMT can be connected to the malformed but mobile chain. The treatment of first patients is reported by several study groups (Streitberger [120], Wollenberg and Frenzel [121], Beltrame [122]).
Coupling of the Floating Mass Transducer at the round window
Spindel and coworkers [123] reported of studies with acoustic stimulation at the round window. A magnet was connected to the round window and stimulated through an inductor. The successfull coupling was proven with brainstam audiometry. Hüttenbrink [124] used a tube filled with water for acoustic stimulation of the cochlea via the round window. Colletti [125], [126] was the first who reported the use of the Floating Mass Transducers directly at the round window niche. His patients had surgically untreatable mixed hearing losses. The round window being one of the most constant anatomical structures, this technique seems to be very feasible to treat patients with congenital aural atresia. The FMT will be covered with fascia and placed in the round window niche (Figure 8 (Fig. 8)). An increasing adhesion and scarring postoperatively may even improve postoperative results. The coupling at the malformed chain as well as the direct stimulation via the round window the conductive hearing loss may be reduced to less than 20 dB in the main speech aerea [121].
Figure 8. Positioning of the Floating Mass Transducer (FMT) in the round window niche.
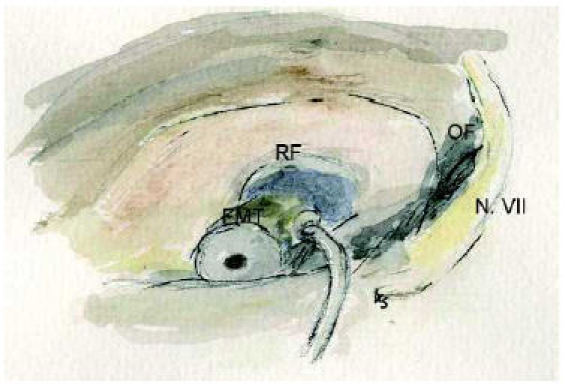
RF: round window niche, OF: oval window
Till now there is only a small number of patients treated with the Vibrant® Soundbridge® system. But the published data open new possibilities for hearing rehabilitation with implantable hearing systems. Critical to remark that many patients, that would benefit from this type of middle ear implant may not have the necessary space for implantation. Especially the aberrant course of the facial nerve may limit the use of these devices.
7.4 Cochlear implant and middle ear malformation
The cochlear implant (CI) is the therapeutic option for deaf ears or types of high grade sensorineural hearing loss. If there is an association with middle ear malformations, the CI is indicated. But many of these patients have complex disturbances, mental retarding etc., so a meticulous, interdisciplinary consideration and consultation is necessary.
8 Future prospects
The treatment of middle ear malformations changed within the last decades. Increasing diagnostic possibilities, better qualitiy of imaging e.g. and high resolution computertomography of the temporal bone help to improve preoperative prognostic assessment. The risk of failure may be reduced, thus the rate of frustrating results. Patients that are no candidates for surgery can be supplied with bone-anchored hearing aids. The BAHA is despite a known complication rate the alternative to surgery. The spectrum in treatment possibilities has now expanded with middle ear implantable hearing aids. First clinical results are promising. Till today there are only few cases with short followup. But it can be assumed that in future the implantable hearing devices will be of more and more importance in the therapy of middle ear malformations.
Notes
Dedicated to Prof. Dr. med. Dr. h.c. Jan Helms on the occasion of his seventieth birthday.
References
- 1.Kiesselbach W. Versuch zur Anlegung eines äußeren Gehörganges bei angeborener Missbildung beider Ohrmuscheln mit Fehlen der äußeren Gehörgänge. Arch Ohrenh Leipzig. 1882;19:127–131. [Google Scholar]
- 2.Patterson ME, Linthicum FH., Jr Congenital hearing impairment. Otolaryngol Clin North Am. 1970;3:201–219. [PubMed] [Google Scholar]
- 3.Jahrsdoerfer RA. Congenital atresia of the ear. Laryngoscope. 1978;88(9 Pt 3 Suppl 13):1–48. [PubMed] [Google Scholar]
- 4.Reichert C. Über die Visceralbögen der Wirbeltiere im Allgemeinen und deren Metamorphosen bei den Vögeln und Säugetieren. Arch Anat Physiol wiss Med Berlin. 1837:120–222. [Google Scholar]
- 5.Gaupp E. Die Reichertsche Theorie (Hammer-, Amboss- und Kieferfrage) Arch Anat. 1912;Suppl I:416. [Google Scholar]
- 6.Otto HD. An error in the Reichert-Gaupp theory. A contribution to onto- and phylogenesis of the temporomandibular joint and ear ossicles in mammals. Anat Anz. 1984;155(1-5):223–238. [PubMed] [Google Scholar]
- 7.Sauter R, Villavicencio E, Schwager K. Doppelanlage der Ohrmuschel, eine seltene Kiemenbogen-Fehlbildung. Laryngo-Rhino-Otol. 2006;85:657–660. doi: 10.1055/s-2006-924987. [DOI] [PubMed] [Google Scholar]
- 8.Marx H. Beitrag zur Morphologie und Genese der Mittelohrmissbildungen mit Gehörgangsatresie. HNO. 1922;1:230–246. [Google Scholar]
- 9.Altmann F. Missbildungen des Ohres. In: Berendes J, Link R, Zöllner F, editors. Ohr. Band III. Stuttgart, New York: Thieme; 1965. pp. 643–667. [Google Scholar]
- 10.Portmann M, Bebear JP, Le-Grignou P. Les agénésies de l'oreille: étude analytique. A propos de 150 ans. Ann Otolaryngol Chir Cervicofac. 1983;100:403–407. [PubMed] [Google Scholar]
- 11.Hildmann H, Rauchfuß A, Hildmann A. Indikation und chirurgische Behandlung der großen Mittelohrfehlbildung. HNO. 1992;40:232–235. [PubMed] [Google Scholar]
- 12.Helms J, Schwager K. Oto-Rhino-Laryngologie in Klinik und Praxis. Band 1: Ohr. In: Helms J, editor. Klinik des Mittelohres. Mittelohrmissbildungen. Stuttgart: Thieme; 1994. pp. 545–563. [Google Scholar]
- 13.Cremers CW, Teunissen E, Marres EH. Classification of congenital aural atresia and results of reconstructive surgery. Adv Otorhinolaryngol. 1988;40:9–14. doi: 10.1159/000415666. [DOI] [PubMed] [Google Scholar]
- 14.Schuknecht HF. Congenital aural atresia. Laryngoscope. 1989;99:908–917. doi: 10.1288/00005537-198909000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Gerhardt HJ, Otto HD. Steigbügelmissbildungen. Acta Otolaryng. 1970;70:35–44. doi: 10.3109/00016487009181856. [DOI] [PubMed] [Google Scholar]
- 16.Teunissen E, Cremers CW. Surgery for congenital anomalies of the middle ear with mobile stapes. Eur Arch Otorhinolaryngol. 1993;250(6):327–331. doi: 10.1007/BF00188380. [DOI] [PubMed] [Google Scholar]
- 17.Bellucci RJ. Congenital aural malformations: diagnosis and treatment. Otolaryngol Clin North Am. 1981;14(1):95–124. [PubMed] [Google Scholar]
- 18.Weidenbecher M. Missbildungen des Felsenbeins und der angrenzenden Schädelbasis. Arch Oto-Rhino-Laryngology Suppl I. 1988;2-62. [PubMed] [Google Scholar]
- 19.Cremers CW, Oudenhoven JM, Marres EH. Congenital aural atresia. A new subclassification and surgical management. Clin Otolaryngol Allied Sci. 1984;9(2):119–127. doi: 10.1111/j.1365-2273.1984.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 20.Gerhardt HJ. One hundred and seventy-five surgically treated malformations of the external and middle ear: findings and results. Auris Nasus Larynx. 1988;15(2):81–87. doi: 10.1016/s0385-8146(88)80012-9. [DOI] [PubMed] [Google Scholar]
- 21.Glasscock ME, 3rd, Schwaber MK, Nissen AJ, Jackson CG. Management of congenital ear malformations. Ann Otol Rhinol Laryngol. 1983;92(5 Pt 1):504–509. doi: 10.1177/000348948309200519. [DOI] [PubMed] [Google Scholar]
- 22.Leiber B, Olbrich G. Die klinischen Syndrome. 7. Aufl. München: Urban & Schwarzenberg; 1990. [Google Scholar]
- 23.Naumann HH. Differentialdiagnostik in der Hals-Nasen-Ohren-Heilkunde. Stuttgart, New York: Thieme; 1990. [Google Scholar]
- 24.Grevers G. Syndrome. In: Helms J, editor. Oto-Rhinolaryngologie in Klinik und Praxis. Band 1: Ohr. Stuttgart: Thieme; 1994. pp. 914–920. [Google Scholar]
- 25.Mündnich K, Terrahe K. Missbildungen des Ohres. In: Berendes J, Link R, Zöllner F, editors. Ohr I. Band 5. Stuttgart: Thieme; 1979. pp. 1–49. [Google Scholar]
- 26.Chang SO, Min YG, Kim CS, Koh TY. Surgical management of congenital aural atresia. Laryngoscope. 1994;104(5 Pt 1):606–611. doi: 10.1002/lary.5541040514. [DOI] [PubMed] [Google Scholar]
- 27.Gorlin RJ, Pindborg JJ, Cohen MM. Syndromes of the head and neck. 2nd ed. New York: Mc Graw Hill Book; 1976. Oculoauriculovertebral dysplasia; pp. 546–552. [Google Scholar]
- 28.Phelps PD, Poswillo D, Lloyd GA. The ear deformities in mandibulofacial dysostosis (Treacher Collins syndrome) Clin Otolaryngol Allied Sci. 1981;6(1):15–28. doi: 10.1111/j.1365-2273.1981.tb01782.x. [DOI] [PubMed] [Google Scholar]
- 29.Jahrsdoerfer RA, Aguilar EA, Yeakley JW, Cole RR. Treacher Collins syndrome: an otologic challenge. Ann Otol Rhinol Laryngol. 1989;98(10):807–812. doi: 10.1177/000348948909801011. [DOI] [PubMed] [Google Scholar]
- 30.Lambert PR. Major congenital ear malformations: surgical management and results. Ann Otol Rhinol Laryngol. 1988;97(6 Pt 1):641–649. doi: 10.1177/000348948809700612. [DOI] [PubMed] [Google Scholar]
- 31.Jahrsdoerfer R. Congenital malformations of the ear. Analysis of 94 operations. Ann Otol Rhinol Laryngol. 1980;89(4 Pt 1):348–352. doi: 10.1177/000348948008900410. [DOI] [PubMed] [Google Scholar]
- 32.Jahrsdoerfer RA. The facial nerve in congenital middle ear malformations. Laryngoscope. 1981;91(8):1217–1225. doi: 10.1288/00005537-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Bellucci RJ. Ear Related Developmental Anomalies of Branchial Clefts and Arches I and II. Trans Pa Acad Opththalmol Otolaryngol. 1977;30:27–33. [PubMed] [Google Scholar]
- 34.Lang J, Hack Ch. Über Lage und Lagevariationen der Kanalsysteme im Os temporale. HNO. 1985;33:279–284. [PubMed] [Google Scholar]
- 35.Helms J. The Cranial Nerves. Berlin: Springer; 1981. Variations of the course of the facial nerve in the middle ear and mastoid. [Google Scholar]
- 36.Nager GT, Proctor B. The facial canal: normal anatomy, variations and anomalies. II. Anatomical variations and anomalies involving the facial canal. Ann Otol Rhinol Laryngol. 1982;97(Suppl):45–61. [PubMed] [Google Scholar]
- 37.Sataloff RT. Embryology and Anomalies of the Facial Nerve and Their Surgical Implications. New York: Raven Press; 1991. [Google Scholar]
- 38.Jahrsdoerfer RA. Transposition of the facial nerve in congenital aural atresia. Am J Otol. 1995;16(3):290–294. [PubMed] [Google Scholar]
- 39.Schuknecht HF. Gavin Livingstone Memorial Lecture. Anatomical variants and anomalies of surgical significance. J Laryngol Otol. 1971;85(12):1238–1241. [PubMed] [Google Scholar]
- 40.Hogg ID, Stephens CB, Arnold GE. Theoretical anomalies of the stapedial artery. Ann Otol Rhinol Laryngol. 1972;81:860–870. doi: 10.1177/000348947208100619. [DOI] [PubMed] [Google Scholar]
- 41.Jahrsdoerfer RA, Hall JW., 3rd Congenital malformations of the ear. Am J Otol. 1986;7(4):267–269. [PubMed] [Google Scholar]
- 42.Jahrsdoerfer RA, Yeakley JW, Hall JW, 3rd, Robbins KT, Gray LC. High-resolution CT scanning and auditory brain stem response in congenital aural atresia: patient selection and surgical correlation. Otolaryngol Head Neck Surg. 1985;93(3):292–298. doi: 10.1177/019459988509300303. [DOI] [PubMed] [Google Scholar]
- 43.Mehra YN, Dubey SP, Mann SB, Suri S. Correlation between high-resolution computed tomography and surgical findings in congenital aural atresia. Arch Otolaryngol Head Neck Surg. 1988;114:137–141. doi: 10.1001/archotol.1988.01860140035016. [DOI] [PubMed] [Google Scholar]
- 44.Andrews JC, Anzai Y, Mankovich NJ, Favilli M, Lufkin RB, Jabour B. Three-dimensional CT scan reconstruction for the assessment of congenital aural atresia. Am J Otol. 1992;13(3):236–240. [PubMed] [Google Scholar]
- 45.Siegert R, Weerda H, Mayer T, Bruckmann H. Hochauflösende Computertomographie fehlgebildeter Mittelohren. Laryngo-Rhino-Otol. 1996;75:187–194. doi: 10.1055/s-2007-997561. [DOI] [PubMed] [Google Scholar]
- 46.Jahrsdoerfer RA, Yeakley JW, Aguilar EA, Cole RR, Gray LC. Grading system for the selection of patients with congenital aural atresia. Am J Otol. 1992;13(1):6–12. [PubMed] [Google Scholar]
- 47.Siegert R, Weerda H. Two-step external ear canal construction in atresia as part of auricular reconstruction. Laryngoscope. 2001;111(4 Pt 1):708–714. doi: 10.1097/00005537-200104000-00026. [DOI] [PubMed] [Google Scholar]
- 48.Siegert R. Neue Wege bei der chirurgischen Behandlung der kongenitalen Gehörgangsatresie. HNO. 2004;52:275–288. doi: 10.1007/s00106-003-0983-y. [DOI] [PubMed] [Google Scholar]
- 49.House JW, Sheehy JL. Cholesteatoma with intact tympanic membrane: A report of 41 cases. Laryngoscope. 1980;90:70–76. doi: 10.1288/00005537-198001000-00008. [DOI] [PubMed] [Google Scholar]
- 50.McDonald TJ, Cody DT, Ryan RE., jr Congenital cholesteatoma of the ear. Ann Otol. 1984;93:637–640. doi: 10.1177/000348948409300619. [DOI] [PubMed] [Google Scholar]
- 51.Michaels L. An epidermoid formation in the developing middle ear: Possible source of cholesteatoma. J Otolaryngol. 1986;15:169–174. [PubMed] [Google Scholar]
- 52.Cole RR, Jahrsdoerfer RA. Congenital aural atresia. Clin Plast Surg. 1990;17(2):367–371. [PubMed] [Google Scholar]
- 53.Ombredanne M. La chirurgie des aplasies mineures. Ann Oto-laryngol. 1964;81:201–222. [PubMed] [Google Scholar]
- 54.Helms J. Ergebnisse der Mikrochirurgie bei Ohrmissbildungen. Laryngol Rhinol Otol. 1987;66:16–18. [PubMed] [Google Scholar]
- 55.Hildmann H, Sudhoff H. Middle Ear Surgery. Heidelberg: Springer; 2006. [Google Scholar]
- 56.Singh PP. Initial experience with titanium MVP clip prosthesis. 4th International Symposium on Middle Ear Mechanics in Research and Otology; 2006; Zürich. 2006. [Google Scholar]
- 57.Funasaka S. Congenital ossicular anomalies without malformations of the external ear. Arch Otorhinolaryngol. 1979;224(3-4):231–240. doi: 10.1007/BF01108781. [DOI] [PubMed] [Google Scholar]
- 58.Hashimoto S, Yamamoto Y, Satoh H, Takahashi S. Surgical treatment of 52 cases of auditory ossicular malformations. Auris Nasus Larynx. 2002;29(1):15–18. doi: 10.1016/s0385-8146(01)00125-0. [DOI] [PubMed] [Google Scholar]
- 59.Charachon R, Barthez M, Lavieille JP. Les Malformations Mineures de la Chaine Ossiculaire. Ann Oto laryngol Chir Cervicofac Paris. 1994;111:69–74. [PubMed] [Google Scholar]
- 60.Baba S, Ikezono T, Pawankar R, Yagi T. Congenital Malformations of the Middle Ear with an Intact External Ear: A Review of 38 Cases. ORL. 2004;66:74–79. doi: 10.1159/000077799. [DOI] [PubMed] [Google Scholar]
- 61.Aguilar EA, III, Jahrsdoerfer RA. The surgical repair of congenital microtia and atresia. Otolaryngol Head Neck Surg. 1988;98:600–606. doi: 10.1177/019459988809800612. [DOI] [PubMed] [Google Scholar]
- 62.Jahnke K, Schrader M. Surgery for congenital aural atresia. The Tubingen Study. Adv Otorhinolaryngol. 1988;40:1–8. doi: 10.1159/000415665. [DOI] [PubMed] [Google Scholar]
- 63.Cole RR, Jahrsdoerfer RA. The Risk of Cholesteatoma in Congenital Aural Stenosis. Laryngoscope. 1990;100:576–578. doi: 10.1288/00005537-199006000-00004. [DOI] [PubMed] [Google Scholar]
- 64.Chiossone E. Surgical management of major congenital malformations of the ear. Amer J Otol. 1985;6:237–242. [PubMed] [Google Scholar]
- 65.Shih L, Crabtree JA. Long-term surgical results for congenital aural atresia. Laryngoscope. 1993;103(10):1097–1102. doi: 10.1288/00005537-199310000-00004. [DOI] [PubMed] [Google Scholar]
- 66.Nishizaki K, Masuda Y, Karita K. Surgical management and its post-operative complications in congenital aural atresia. Acta Otolaryngol. 1999;540(Suppl):42–44. [PubMed] [Google Scholar]
- 67.Caversaccio M, Romualdez J, Baechler R, Nolte LP, Kompis M, Hausler R. Valuable use of computer-aided surgery in congenital bony aural atresia. J Laryngol Otol. 2003;117(4):241–248. doi: 10.1258/00222150360600814. [DOI] [PubMed] [Google Scholar]
- 68.Crabtree JA. Congenital Atresia: case selection, complications and prevention. Otolaryngology Clin North Am. 1982;15:755–762. [PubMed] [Google Scholar]
- 69.Lund WS. The surgery of congenital deafness. The Oxford, England series of 235 ears. Acta Oto-Rhino-Laryngologica Belgica. 1988;42:5–11. [PubMed] [Google Scholar]
- 70.Weerda H, Bockenheimer S, Trübi M. Gehörverbessernde Operationen bei Ohrmuschelmissbildungen. HNO. 1985;33:449–452. [PubMed] [Google Scholar]
- 71.Crabtree JA. Tympanoplastic Techniques in Congenital Atresia. Arch of Otolaryngol Head Neck Surg. 1968;88:89–96. doi: 10.1001/archotol.1968.00770010065012. [DOI] [PubMed] [Google Scholar]
- 72.Bauer GP, Wiet RJ, Zappia JJ. Congenital aural atresia. Laryngoscope. 1994;104(10):1219–1224. doi: 10.1288/00005537-199410000-00006. [DOI] [PubMed] [Google Scholar]
- 73.Marquet J. Congenital malformations and middle ear surgery. J roy Soc Med. 1981;74:119–128. doi: 10.1177/014107688107400207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lambert PR. Management of the Unilateral Atretic Ear. In: Pensak ML, editor. Controversies in Otolaryngology. New York, Stuttgart: Thieme; 2001. [Google Scholar]
- 75.De la Cruz A, Kesser BW. Management of the Unilateral Atretic Ear. In: Pensak ML, editor. Controversies in Otolaryngology. New York, Stuttgart: Thieme; 2001. [Google Scholar]
- 76.Siegert R. Combined reconstruction of congenital auricular Atresia and severe microtia. Laryngoscope. 2003;113(11):2021–2027. doi: 10.1097/00005537-200311000-00031. [DOI] [PubMed] [Google Scholar]
- 77.Trigg DJ, Applebaum EL. Indications for the surgical repair of unilateral aural atresia in children. Am J Otol. 1998;19(5):679–684. [PubMed] [Google Scholar]
- 78.Humes LE, Allen SK, Bess FH. Horizontal sound localization skills of unilaterally hearing-impaired children. Audiology. 1980;19:508–518. doi: 10.3109/00206098009070082. [DOI] [PubMed] [Google Scholar]
- 79.Bess FH. The unilaterally hearing-impaired child: a final comment. Ear Hearing. 1986;7:52–54. doi: 10.1097/00003446-198602000-00009. [DOI] [PubMed] [Google Scholar]
- 80.Viehweg R, Campbell RA. Localization difficulty in monaurally impaired listeners. Trans Otol Soc. 1960;48:339–350. doi: 10.1177/000348946006900223. [DOI] [PubMed] [Google Scholar]
- 81.Kemink JL, Graham MD. Surgery for congenital malformations of the ear canal and middle ear. Ear, Nose, Throat J. 1983;62:644–648. [PubMed] [Google Scholar]
- 82.De la Cruz A, Linthicum FH, Jr, Luxford WM. Congenital atresia of the external audiotry canal. Laryngoscope. 1985;95(4):421–427. [PubMed] [Google Scholar]
- 83.Jahrsdoerfer RA, Aguilar EA., III . Chirurgie der kongenitalen Ohrmissbildungen. In: Helms J, Jahrsdoerfer RA, editors. Ohr. Band 2. Stuttgart, New York: Thieme; 1996. pp. 35–66. [Google Scholar]
- 84.Gill NW. Congenital atresia of the ear. J Laryngol Otol. 1971;85:1251–1256. doi: 10.1017/s0022215100074752. [DOI] [PubMed] [Google Scholar]
- 85.Wigand ME. Das Konzept der endauralen Tympanoplastiken bei kongenitalen Atresien. Laryngol Rhinol Otol. 1975;54:148–154. [PubMed] [Google Scholar]
- 86.Schuknecht HF. Reconstructive procedures for congenital aural atresia. Arch Otolaryngol. 1975;101(3):170–172. doi: 10.1001/archotol.1975.00780320028006. [DOI] [PubMed] [Google Scholar]
- 87.Marquet J. Surgical management of the congenital ear. Acta oto-rhino-laryngol belg. 1984;38:232–237. [PubMed] [Google Scholar]
- 88.Mattox DE, Fisch U. Surgical correction of congenital atresia of the ear. Otolaryngol Head Neck Surg. 1986;94:574–577. doi: 10.1177/019459988609400607. [DOI] [PubMed] [Google Scholar]
- 89.Molony TB, De la Cruz A. Surgical approaches to congenital atresia of the external auditory canal. Otolaryngol Head Neck Surg. 1990;103:991–1001. doi: 10.1177/019459989010300618. [DOI] [PubMed] [Google Scholar]
- 90.Mattox DE, Nager GT, Levin LS. Congenital aural atresia: embryology, pathology, classification, genetics and surgical management. In: Paparella MM, Chumrick DA, Gluckman JL, Meyerhoff WL, editors. Otolaryngology. Vol. II: Otology and Neuro-Otology. Philadelphia: Saunders; 1991. p. Congenital aural atresia: embryology, pathology, classification, genetics and surgical management. [Google Scholar]
- 91.Helms J. Acoustic trauma from the bone cutting burr. Laryng otol. 1976;90:1143–1149. doi: 10.1017/s0022215100083225. [DOI] [PubMed] [Google Scholar]
- 92.Hartwick CJ, Choo DI. Management of the Unilateral Atretic Ear. In: Pensak ML, editor. Controversies in Otolaryngology. New York, Stuttgart: Thieme; 2001. [Google Scholar]
- 93.Fenner T, Wachter I, Fisch U. Atresia auris congenita, Probleme und Resultate der operativen Therapie. Akt Probl d Otorhinolaryngol. 1981:186–192. [Google Scholar]
- 94.Wullstein HL. Die operativen Aufgaben in der Basis Kranii für die HNO-Heilkunde. Teil III. HNO. 1984;32:401–412. [PubMed] [Google Scholar]
- 95.Jahrsdoerfer RA, Kesser BW. Issues on aural atresia for the facial plastic surgeon. Facial Plast Surg. 1995;11(4):274–277. doi: 10.1055/s-2008-1064543. [DOI] [PubMed] [Google Scholar]
- 96.Cole RR. The "buttock sign" in congenital aural atresia. Otolaryngol Head Neck Surg. 1993;109(1):140–141. doi: 10.1177/019459989310900126. [DOI] [PubMed] [Google Scholar]
- 97.Plester D. Congenital malformation of the middle ear. Acta Otorhinolaryngol Belg. 1971;25(6):877–884. [PubMed] [Google Scholar]
- 98.Bellucci RJ. Congenital auricular malformations. Indications, contraindications, and timing of middle ear surgery. Ann Otol Rhinol Laryngol. 1972;81(5):659–663. doi: 10.1177/000348947208100506. [DOI] [PubMed] [Google Scholar]
- 99.Zahnert T. Gestörtes Hören. Chirurgische Verfahren. Laryngo-Rhino-Otol. 2005;84:S37–S50. doi: 10.1055/s-2005-861139. [DOI] [PubMed] [Google Scholar]
- 100.Hartwein J, Leuwer RM, Kehrl W. The total reconstruction of the tympanic membrane by the "crowncork" technique. Am J Otolaryngol. 1992;13(3):172–175. doi: 10.1016/0196-0709(92)90118-d. [DOI] [PubMed] [Google Scholar]
- 101.Zühlke D. Chirurgische Behandlung der Missbildungen des Ohres. Arch Klin Exp Ohren Nasen Kehlkopfheilk. 1972;202(1):153–202. [PubMed] [Google Scholar]
- 102.Schwager K, Helms J. Mikrochirurgie großer Mittelohrfehlbildungen. Operationstechnische Überlegungen. HNO. 1995;43:427–431. [PubMed] [Google Scholar]
- 103.Lambert PR. Congenital aural atresia: stability of surgical results. Laryngoscope. 1998;108(12):1801–1805. doi: 10.1097/00005537-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 104.Abramson M, Thomas H, Kelly J, Wazen J, Linden G, Tjellström A. Clinical results with a percutaneous bone-anchored hearing aid. Congenital middle ear malformations. Laryngoscope. 1989;99:707–710. doi: 10.1288/00005537-198907000-00008. [DOI] [PubMed] [Google Scholar]
- 105.Lustig LR, Arts HA, Brackmann DE, Francis HF, Molony T, Megerian CA, Moore GF, Moore KM, Morrow T, Potsic W, Rubenstein JT, Srireddy S, Syms CA, 3rd, Takahashi G, Vernick D, Wackym PA, Niparko JK. Hearing rehabilitation using the BAHA bone-anchored hearing aid: results in 40 patients. Otol Neuroto. 2001;22(3):328–334. doi: 10.1097/00129492-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 106.Granström G, Bergström K, Tjellström A. The bone-anchored hearing aid and bone-anchored epithesis for congenital ear malformations. Otolaryngol Head Neck Surg. 1993;109(1):46–53. doi: 10.1177/019459989310900109. [DOI] [PubMed] [Google Scholar]
- 107.Brånemark PI, Breine U, Adell R, Hanson BO, Lindström J, Ohlsson A. Intra-osseous anchorage of dental prosthesis: experimental studies. Scand J Plast Reconstr Surg. 1969;3:81–100. doi: 10.3109/02844316909036699. [DOI] [PubMed] [Google Scholar]
- 108.Federspil P, Federspil PA. Knochenverankerte aktive Hörimplantate. Deutsches Ärzteblatt. 2000;10(97):609–614. [Google Scholar]
- 109.Mack KF, Müller J, Helms J. Dimensions of the Temporal Bone in Small Children in Relation to the Cochlear Implant - An Analysis of CT Scans. Adv Otorhinolaryngol Basel, Karger. 1997;52:57–59. doi: 10.1159/000059007. [DOI] [PubMed] [Google Scholar]
- 110.Linder T, Simmen D. Atresia auris congenita - wie beraten, wann behandeln? Schweiz Med Forum. 2005;5:897–903. [Google Scholar]
- 111.Stevenson DS, Proops DW, Wake MJ, Deadman MJ, Worrollo SJ, Hobson JA. Osseointegrated implants in the management of childhood ear abnormalities: the initial Birmingham experience. J Laryngol Otol. 1993;107(6):502–509. doi: 10.1017/s0022215100123576. [DOI] [PubMed] [Google Scholar]
- 112.Arunachalam PS, Kilby D, Meikle D, Davison T, Johnson IJ. Bone-anchored hearing aid quality of life assessed by Glasgow Benefit Inventory. Laryngoscope. 2001;111(7):1260–1263. doi: 10.1097/00005537-200107000-00022. [DOI] [PubMed] [Google Scholar]
- 113.Declau F, Cremers C, Van de Heyning P. Diagnosis and management strategies in congenital atresia of the external auditory canal. Study Group on Otological Malformations and Hearing Impairment. Br J Audiol. 1999;33(5):313–327. doi: 10.3109/03005369909090115. [DOI] [PubMed] [Google Scholar]
- 114.Manach Y, Perrin A, Griscelli AL, Florant A, Jaulin JF, Roulleau P. La chirurgie fonctionnelle de l'aplasie majeure de l'oreille. A propos de 109 patients. Ann Oto-laryng. 1987;104:607–614. [PubMed] [Google Scholar]
- 115.Siegert R, Müllender G, Waldmann B. Implantation von aktiven Mittelohrimplantaten bei schweren Fehlbildungen des Mittelohres mit Atresia auris congenita - Erste Erfahrungen. HNO-Informationen. 2005;84:51–231. [Google Scholar]
- 116.Mattheis S, Siegert R. First application of fully implantable hearing aids in patients with congenital auricular atresia. 4th International Symposium on Middle Ear Mechanics in Research and Otology; 2006; Zürich. 2006. [DOI] [PubMed] [Google Scholar]
- 117.Chen DA, Backous DD, Arriaga MA, Garvin R, Kobylek D, Littman T, Walgren S, Lura D. Phase 1 clinical trial results of the Envoy System: a totally implantable middle ear device for sensorineural hearing loss. Otolaryngol Head Neck Surg. 2004;131:904–916. doi: 10.1016/j.otohns.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 118.Hough JV, Matthews P, Wood MW, Dyer RK., Jr Middle ear electromagnetic semi-implantable hearing device: results of the phase II SOUNDTEC direct system clinical trial. Otol Neurotol. 2002;23:895–903. doi: 10.1097/00129492-200211000-00015. [DOI] [PubMed] [Google Scholar]
- 119.Mojallal H, Stieger C, Grasshof E, Lenarz T, Häusler R, Haller M. DACS - Ein neues implantierbares Hörsystem für mittel- bis hochgradig kombinierte Schwerhörigkeiten. 77. Jahresversammlung der Deutschen Gesellschaft HNO, Kopf- und Hals-Chirurgie. HNO-Abstractband; Mannheim. 2006. p. 109. [Google Scholar]
- 120.Streitberger C. New clinical indications for the VSB: the experience of the clinic in Meran. 8th European Symposium on Pediatric Cochlear Impl; 2006; Venice. 2006. [Google Scholar]
- 121.Wollenberg B, Beltrame M, Schönweiler R, Gehrking E, Nitsch S, Steffen A, Frenzel H. Integration des aktiven Mittelohrimplantates in die plastische Ohrmuschelrekonstruktion. HNO. 2007;55(5):349–356(8). doi: 10.1007/s00106-007-1540-x. [DOI] [PubMed] [Google Scholar]
- 122.Beltrame MA, Cumer G, Frau GN. Middle-ear implant transducer results of the coupling of the floating mass transducer (FMT) to the round window in patients with mixed hearing loss. Surgical technique and performance results. 8th European Symposium on Pediatric Cochlear Impl; 2006; Venice. 2006. [Google Scholar]
- 123.Spindel JH, Lambert PR, Ruth RA. The round window electromagnetic implantable hearing aid approach. Otolaryngol Clin North Am. 1995;28:189–205. [PubMed] [Google Scholar]
- 124.Hüttenbrink KB. Implantierbare Hörgeräte für hochgradige Schwerhörigkeit. Grundsätzliche Überlegungen zu ihrem Einsatz und ein Versuch der technischen Realisierung (Dresdner/Bochumer Modell) HNO. 1997;45:742–744. doi: 10.1007/s001060050151. [DOI] [PubMed] [Google Scholar]
- 125.Colletti V, Carner M, Sacchetto L, Colletti L, Giarbini N. Round window stimulation with the Floating Mass Transducer: A new approach for surgical failures of mixed hearing losses. IFOS; 2005; Rome. 2005. [Google Scholar]
- 126.Colletti V, Carner M, Colletti L, Sacchetto The round window approach for MED-EL VSB in difficult-to-treat middle ear problems. 4th International Symposium on Middle Ear Mechanics in Research and Otology; 2006; Zürich. 2006. [Google Scholar]
- 127.Schwager K, Helms J, Hofmann E. Ergebnisse nach Operation großer Ohrfehlbildungen. Arch Otorhinolaryngol. 1994;(Suppl II):247. [Google Scholar]
- 128.Meurman OH. Congenital Microtia and Meatal Atresia. Arch of Otolaryngol Head Neck Surg. 1957;66:443–463. doi: 10.1001/archotol.1957.03830280073008. [DOI] [PubMed] [Google Scholar]
- 129.Gill NW. Congenital atresia of the ear. Journal of Layngology and Otology. 1969;83:551–587. doi: 10.1017/s0022215100070687. [DOI] [PubMed] [Google Scholar]
- 130.Chandrasekhar SS, De la Cruz A, Garrido E. Surgery of congenital aural atresia. Am J Otol. 1995;16(6):713–717. [PubMed] [Google Scholar]
- 131.Teufert KB, De la Cruz A. Advances in congenital aural atresia surgery: effects on outcome. Otolaryngol Head Neck Surg. 2004;131(3):263–270. doi: 10.1016/j.otohns.2004.03.006. [DOI] [PubMed] [Google Scholar]