Abstract
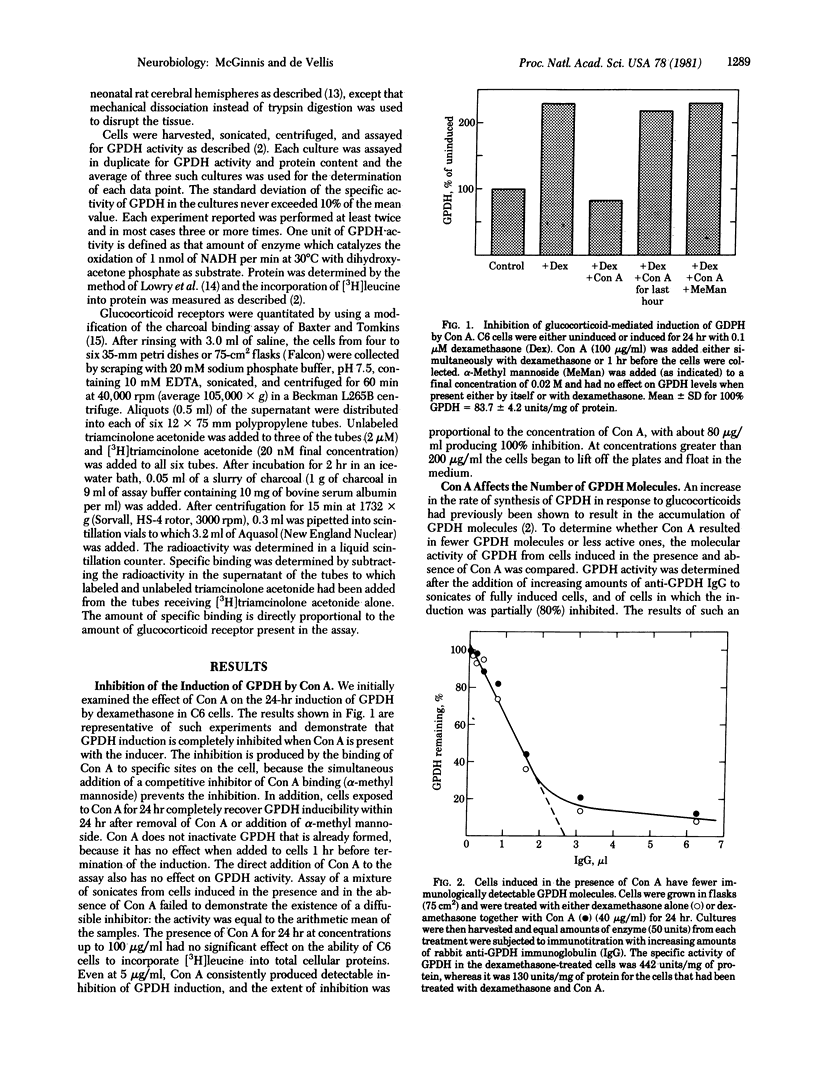
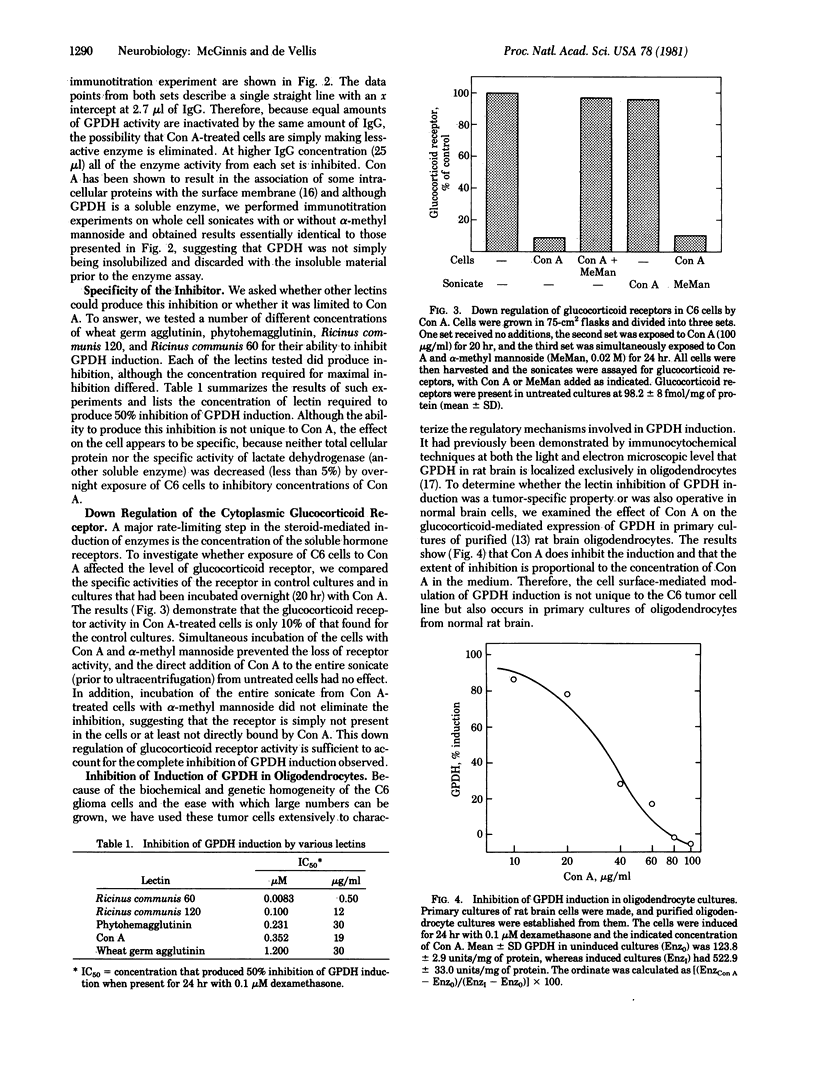
The concentration of glycerol-3-phosphate dehydrogenase (GPDH; sn-glycerol-3-phosphate:NAD+ 2-oxidoreductase, EC 1.1.1.8) had previously been determined to be regulated by glucocorticoids in rat brain cells in vivo and in cell culture. We now demonstrate that concanavalin A (Con A) can inhibit the induction of GPDH in dose-dependent manner in C6 rat glioma cells and in primary cultures of rat brain oligodendrocytes. Con A is not cytotoxic, because its effect can be prevented or reversed by α-methyl mannoside. The inhibition specifically prevents the appearance of new molecules of GPDH, although Con A does not significantly inhibit protein synthesis in these cells, nor does it affect the activity of another soluble enzyme, lactate dehydrogenase. The ability to block enzyme induction is not limited to Con A, because other lectins also inhibit induction, with Ricinus communis agglutinin 60 being the most potent (50% inhibition of induction at 0.0083 μM) and wheat germ agglutinin being the least potent (50% inhibition of induction at 1.2 μM). The molecular mechanism by which Con A inhibits GPDH induction appears to be by the “down regulation” of the cytoplasmic glucocorticoid receptors, because exposure to Con A results in the loss of more than 90% of the receptor activity. Con A does not inhibit the receptor assay and no direct interaction between the receptor and Con A could be demonstrated. This down regulation is not tumor cell specific and appears to be a general phenomenon, because it occurs in normal oligodendrocytes and even in normal astrocytes (a cell type in which the gene for GPDH is not expressed). The down regulation of glucocorticoid receptors in normal brain cells suggests two important corollaries. First, it demonstrates the existence of a rate-limiting step controlling the glucocorticoid-dependent gene expression in brain cells and possibly represents a regulatory site common to all glucocorticoid target cells. Second, it suggests that the response to glucocorticoids of oligodendrocytes and astrocytes can be regulated in vivo by cell surface contact with endogenous lectins, neighboring cells, or both.
Keywords: lectins, induction of glycerol phosphate dehydrogenase, C6 rat glioma cells, oligodendrocytes, astrocytes
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash J. F., Singer S. J. Concanavalin-A-induced transmembrane linkage of concanavalin A surface receptors to intracellular myosin-containing filaments. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4575–4579. doi: 10.1073/pnas.73.12.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. D., Tomkins G. M. Specific cytoplasmic glucocorticoid hormone receptors in hepatoma tissue culture cells. Proc Natl Acad Sci U S A. 1971 May;68(5):932–937. doi: 10.1073/pnas.68.5.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968 Jul 26;161(3839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Harwood J. P., Aguilera G., Dufau M. L. Hormonal regulation of peptide receptors and target cell responses. Nature. 1979 Jul 12;280(5718):109–116. doi: 10.1038/280109a0. [DOI] [PubMed] [Google Scholar]
- Ciment G., de Vellis J. Cellular interactions uncouple beta-adrenergic receptors from adenylate cyclase. Science. 1978 Nov 17;202(4369):765–768. doi: 10.1126/science.213832. [DOI] [PubMed] [Google Scholar]
- Frazier W., Glaser L. Surface components and cell recognition. Annu Rev Biochem. 1979;48:491–523. doi: 10.1146/annurev.bi.48.070179.002423. [DOI] [PubMed] [Google Scholar]
- Geiger B., Singer S. J. The participation of alpha-actinin in the capping of cell membrane components. Cell. 1979 Jan;16(1):213–222. doi: 10.1016/0092-8674(79)90202-2. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan T. V., Thompson E. B. Effect of concanavalin A on tyrosine aminotransferase in rat hepatoma tissue culture cells. Rapid reversible inactivation of soluble enzyme. J Biol Chem. 1977 Apr 25;252(8):2717–2725. [PubMed] [Google Scholar]
- Hoffmann M. K. Antigen-specific induction and regulation of antibody synthesis in cultures of human peripheral blood mononuclear cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1139–1143. doi: 10.1073/pnas.77.2.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D. Hormone receptor interactions at the cell membrane. Pharmacol Rev. 1978 Dec;30(4):393–410. [PubMed] [Google Scholar]
- Hollenberg M. D. Hormone receptor interactions at the cell membrane. Pharmacol Rev. 1978 Dec;30(4):393–410. [PubMed] [Google Scholar]
- Kozak L. P. Coaggregation with tumor cells inhibits expression by cerebellar cells of the adult isozyme locus, Gdc-1. Dev Biol. 1979 Feb;68(2):407–421. doi: 10.1016/0012-1606(79)90214-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leveille P. J., McGinnis J. F., Maxwell D. S., de Vellis J. Immunocytochemical localization of glycerol-3-phosphate dehydrogenase in rat oligodendrocytes. Brain Res. 1980 Sep 8;196(2):287–305. doi: 10.1016/0006-8993(80)90397-2. [DOI] [PubMed] [Google Scholar]
- Linser P., Moscona A. A. Induction of glutamine synthetase in embryonic neural retina: localization in Müller fibers and dependence on cell interactions. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6476–6480. doi: 10.1073/pnas.76.12.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis J. F., de Vellis J. Glucocorticoid regulation in rat brain cell cultures. Hydrocortisone increases the rate of synthesis of glycerol phosphate dehydrogenase in C6 glioma cells. J Biol Chem. 1978 Dec 10;253(23):8493–8492. [PubMed] [Google Scholar]
- Painter R. G., White A. Effect of concanavalin A on expression of cell surface sialyltransferase activity of mouse thymocytes. Proc Natl Acad Sci U S A. 1976 Mar;73(3):837–841. doi: 10.1073/pnas.73.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Weiser M. M., La Mont J. T., Isselbacher K. J. Galactosyltransferase and concanavalin A agglutination of cells. Proc Natl Acad Sci U S A. 1974 Mar;71(3):904–908. doi: 10.1073/pnas.71.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]


