Abstract
Two calcium-stimulated protein kinase activities (ATP:protein phosphotransferase, EC 2.7.1.37) that phosphorylate protein I, a specific synaptic protein, have been identified in homogenates of rat brain. One of these is found in both the particulate and cytosolic fractions and phosphorylates a region of protein I that is phosphorylated in intact synaptosomes in response to calcium but not to cyclic AMP. The stimulation by calcium of the particulate enzyme and of the partially purified cytosolic enzyme requires the addition of calmodulin. It is not yet known whether the particulate and cytosolic enzymes are related. A second calcium-stimulated protein I kinase is found only in the cytosol and phosphorylates a region of protein I that is phosphorylated in intact synaptosomes in response to either calcium or cyclic AMP. The calcium stimulation of this latter kinase is probably mediated by calmodulin, judging from its inhibition by low concentrations of trifluoperazine. Both of the calcium-stimulated protein I kinases are more highly concentrated in brain than in other tissues. The two cytosolic kinases are distinguishable from each other and from myosin light chain kinase and phosphorylase b kinase by their substrate specificities and their chromatographic behavior on DEAE-cellulose.
Full text
PDF


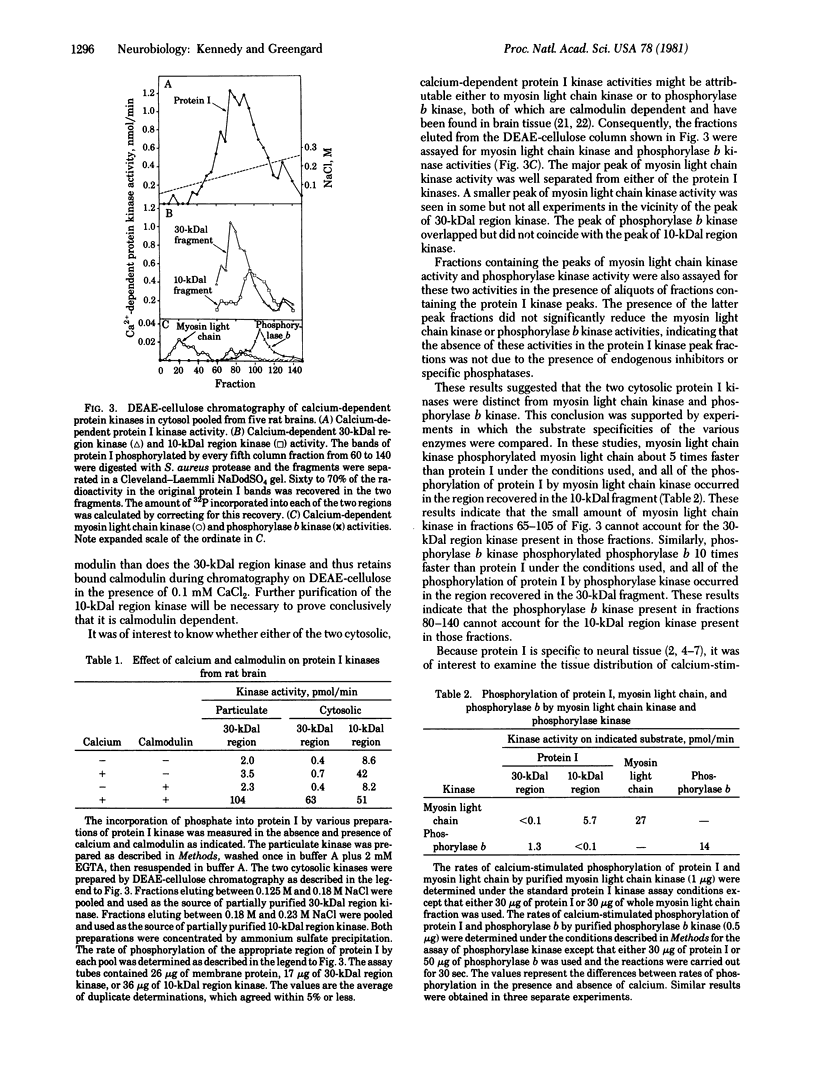

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom F. E., Ueda T., Battenberg E., Greengard P. Immunocytochemical localization, in synapses, of protein I, an endogenous substrate for protein kinases in mammalian brain. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5982–5986. doi: 10.1073/pnas.76.11.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen P., Burchell A., Foulkes J. G., Cohen P. T., Vanaman T. C., Nairn C. Identification of the Ca2+-dependent modulator protein as the fourth subunit of rabbit skeletal muscle phosphorylase kinase. FEBS Lett. 1978 Aug 15;92(2):287–293. doi: 10.1016/0014-5793(78)80772-8. [DOI] [PubMed] [Google Scholar]
- Dabrowska R., Hartshorne D. J. A Ca2+-and modulator-dependent myosin light chain kinase from non-muscle cells. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1352–1359. doi: 10.1016/0006-291x(78)91152-x. [DOI] [PubMed] [Google Scholar]
- Dabrowska R., Sherry J. M., Aromatorio D. K., Hartshorne D. J. Modulator protein as a component of the myosin light chain kinase from chicken gizzard. Biochemistry. 1978 Jan 24;17(2):253–258. doi: 10.1021/bi00595a010. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Ueda T., Bloom F. E., Battenberg E., Greengard P. Widespread distribution of protein I in the central and peripheral nervous systems. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5977–5981. doi: 10.1073/pnas.76.11.5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Greengard P. Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5402–5406. doi: 10.1073/pnas.76.10.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Krueger B. K., Forn J., Greengard P. Depolarization-induced phosphorylation of specific proteins, mediated by calcium ion influx, in rat brain synaptosomes. J Biol Chem. 1977 Apr 25;252(8):2764–2773. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ozawa E. Activation of phosphorylase kinase from brain by small amounts of calcium ion. J Neurochem. 1973 May;20(5):1487–1488. doi: 10.1111/j.1471-4159.1973.tb00263.x. [DOI] [PubMed] [Google Scholar]
- Payne M. E., Soderling T. R. Calmodulin-dependent glycogen synthase kinase. J Biol Chem. 1980 Sep 10;255(17):8054–8056. [PubMed] [Google Scholar]
- Schulman H., Greengard P. Ca2+-dependent protein phosphorylation system in membranes from various tissues, and its activation by "calcium-dependent regulator". Proc Natl Acad Sci U S A. 1978 Nov;75(11):5432–5436. doi: 10.1073/pnas.75.11.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H., Greengard P. Stimulation of brain membrane protein phosphorylation by calcium and an endogenous heat-stable protein. Nature. 1978 Feb 2;271(5644):478–479. doi: 10.1038/271478a0. [DOI] [PubMed] [Google Scholar]
- Sieghart W., Forn J., Greengard P. Ca2+ and cyclic AMP regulate phosphorylation of same two membrane-associated proteins specific to nerve tissue. Proc Natl Acad Sci U S A. 1979 May;76(5):2475–2479. doi: 10.1073/pnas.76.5.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Greengard P. Adenosine 3':5'-monophosphate-regulated phosphoprotein system of neuronal membranes. I. Solubilization, purification, and some properties of an endogenous phosphoprotein. J Biol Chem. 1977 Jul 25;252(14):5155–5163. [PubMed] [Google Scholar]
- Ueda T., Greengard P., Berzins K., Cohen R. S., Blomberg F., Grab D. J., Siekevitz P. Subcellular distribution in cerebral cortex of two proteins phosphorylated by a cAMP-dependent protein kinase. J Cell Biol. 1979 Nov;83(2 Pt 1):308–319. doi: 10.1083/jcb.83.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D., Gonzalez C., Calkins D., Fischer E. H. Krebs EG: Purification and characterization of a protein inhibitor of adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1977–1985. [PubMed] [Google Scholar]
- Walter U., Lohmann S. M., Sieghart W., Greengard P. Identification of the cyclic AMP-dependent protein kinase responsible for endogenous phosphorylation of substrate proteins in synaptic membrane fraction from rat brain. J Biol Chem. 1979 Dec 10;254(23):12235–12239. [PubMed] [Google Scholar]
- Watterson D. M., Harrelson W. G., Jr, Keller P. M., Sharief F., Vanaman T. C. Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. J Biol Chem. 1976 Aug 10;251(15):4501–4513. [PubMed] [Google Scholar]
- Yagi K., Yazawa M., Kakiuchi S., Ohshima M., Uenishi K. Identification of an activator protein for myosin light chain kinase as the Ca2+-dependent modulator protein. J Biol Chem. 1978 Mar 10;253(5):1338–1340. [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. Evidence for three distinct forms of calmodulin-dependent protein kinases from rat brain. FEBS Lett. 1980 Jul 28;116(2):141–144. doi: 10.1016/0014-5793(80)80628-4. [DOI] [PubMed] [Google Scholar]