Abstract
The neuronal acceptor SNARE complex that functions as the receptor for synaptic vesicle docking and fusion at the presynaptic membrane is composed of the single-span transmembrane protein syntaxin-1A and the palmitoylated soluble protein SNAP-25. Previously we explored interactions that promote the formation of syntaxin-1A clusters in membranes. Cholesterol activates clustering in native and model membranes, and its depletion in neuroendocrine cells results in a homogeneous distribution of the protein. However, as little as 1 mol% phosphatidylinositol-4,5-bisphosphate (PI-4,5-P2) or 20 mol% phosphatidylserine was found to disperse syntaxin-1A clusters (Murray, D. H., and Tamm, L. K. (2009) Biochemistry 48, 4617-4625). Strong evidence suggests that syntaxin-1A and its synaptic vesicle cognate synaptobrevin both interact directly with PI-4,5-P2 and that this interaction activates fusion. However, the molecular details of this interaction and its relationship to the partial dispersion of syntaxin-1A clusters remain largely unexplored. Hence, we mutated the polybasic juxtamembrane motif of syntaxin-1A and found several residues that partially or fully abrogate the electrostatic interaction with PI-4,5-P2. We further show that even in the presence of physiological concentrations of phosphatidylserine, the PI-4,5-P2-syntaxin interaction is sufficiently strong to disrupt syntaxin-1A clustering. The stereochemistry of PI-4,5-P2 is not critical for this interaction as other polyphosphoinositides have similar effects. Forming an acceptor SNARE complex between syntaxin-1A and SNAP-25 reduces, but does not abrogate cholesterol/PI-4,5-P2-controlled cluster formation. Potential consequences of these interactions on synaptic vesicle fusion are discussed.
Keywords: syntaxin, cholesterol, phosphatidylinositol, membrane domain, membrane electrostatics
The neuronal integral plasma membrane protein syntaxin-1A, along with SNAP-251, forms the acceptor complex for the synaptic vesicle membrane protein synaptobrevin. In calcium-triggered membrane fusion in neurons, these proteins contribute SNARE motifs to form a four-helix bundle known as the core complex (1). SNARE recognition is thought to be responsible for docking synaptic vesicles to the presynaptic membrane and the energy gained from the assembly and folding of the SNARE core complex is believed to drive the membrane fusion reaction (2). Following fusion, the SNAREs are in a four-helical bundle with both the syntaxin and synaptobrevin SNARE motifs continuing as helices to the transmembrane domains in the plasma membrane (3). For recycling of the SNAREs, this highly-stable cis-complex is disassembled by NSF and α-SNAP (2). The disassembly of the complex is followed by synaptobrevin recycling back to the synaptic vesicles via clathrin-mediated endocytosis (4). Concurrently, Syx redistributes in the plasma membrane, achieving an equilibrium of monomers and large oligomers with about 85% of Syx assembling into nanoclusters of 70-90 subunits, with the remainder in a monomeric state (5). The plasma membrane-associated protein SNAP-25 colocalizes with syntaxin at over 90% in chromaffin cells, indicating a co-segregation of these components of the acceptor complex within the membrane (6). Although there is ample evidence that Syx and SNAP-25 form the active acceptor complex for synaptic vesicle binding, the dynamics and equilibrium of the syntaxin-SNAP-25 association are not well understood.
The oligomeric state of syntaxin is dependent on the cholesterol concentration in several cell types (7-10). Along with cholesterol, residues in the N-terminal segment of the SNARE motif are critical for clustering (11). Cholesterol-dependent clustering of syntaxin has also been observed in reconstituted model membranes in vitro (12). Based on these studies, it is thought that the oligomeric state of syntaxin depends on a balance of the interactions mediated by membrane cholesterol concentration, self-affinity through the SNARE motif, and possibly interactions between the polybasic juxtamembrane domain and anionic lipids.
A variety of membrane trafficking processes are regulated by polyphosphoinositides (13, 14). Among the most important consequences of plasma membrane PI-4,5-P2 misregulation in vivo is the inhibition of SNARE-mediated exocytosis in both mice and primary cultured neurons, and defects in synaptic vesicle recycling due to reduced clathrin-mediated endocytosis (13, 15, 16). Furthermore, mutations in PI kinases and phosphatases responsible for generating PI-4,5-P2 and other PIPs are genetically linked to bipolar disorder in humans, in addition to PI misregulation in other syndromes (15). Interactions between SNAREs and the highly negatively charged PIPs have an important role in membrane fusion upstream of the fusion event, thus likely in the membrane organization and/or formation of the acceptor SNARE complex (17-19). Besides the afore-mentioned clusters of Syx, PI-4,5-P2 also exists in plasma membranes in clusters at densities of 6 mol% of the total lipid or greater. These PI-4,5-P2 clusters colocalize with ~30% of docked dense-core vesicles at the plasma membrane (20). Despite these elegant cellular studies clarifying the activating role of PIPs in exocytosis, the mechanism and role of specific PIP-SNARE interactions are still only poorly understood. In an effort to shed more light on these interactions, we previously showed that PI-4,5-P2 interacts directly with syntaxin-1A and that this interaction is electrostatically controlled (12).
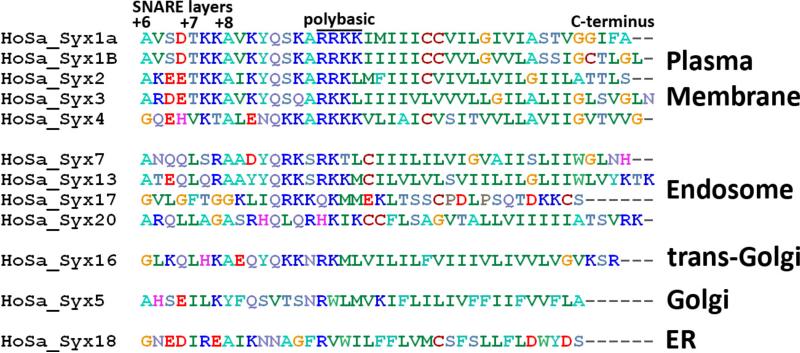
The SNARE motif in syntaxin is connected to the transmembrane domain by a highly conserved ten-residue juxtamembrane region that contains a total of six basic residues (Figure 1). An additional two lysines occur between the +7 and +8 heptad repeat layers at the C-terminal end of the SNARE motif. Indeed, these residues were identified as potential effectors of fusion. For example, a mutation of the RRKK motif in the juxtamembrane region (residues 262-265 of syntaxin-1A) to all alanines results in a fusion defect that manifests as fewer fusion events, and a greater duration and a decreased diameter of the fusion pore (17). A separate study, in which upstream lysines 252 and 253 and lysines 264 and 265 of the RRKK motif were replaced with alanines, leaving only arginines 262 and 263 unperturbed, revealed a decrease of fusion compared with wild-type when liposomes containing PI-4,5-P2 (charge -3 to -4), but not liposomes containing POPS (charge -1) were used in an in vitro fusion assay (20). The yeast syntaxin-1A homologue sso1p similarly required the polybasic region for fusion in vivo (21). However, intriguingly, a mutation of the six basic juxtamembrane residues of yeast Syx to alanine was permitted in fusion assays with liposomes containing 15% POPS in POPC, with fusion decreased to only ~84% of wild-type.
Figure 1.
Sequence alignment of the juxtamembrane domain regions of human syntaxins. The alignment (generated in BioEdit (44) using a database of SNAREs (26)) reveals a conserved polybasic region that is most pronounced in family members that are targeted to the plasma membrane. The final layers of the four-helical bundle making up the SNARE core complex and the entire transmembrane domains concluding with the C-termini are also shown flanking the juxtamembrane regions.
Synaptobrevin has a similar juxtamembrane region, with five basic residues in the ten-residue linker between the SNARE motif and the transmembrane domain, plus an additional lysine between the +7 and +8 heptad repeat layers. This region also interacts with anionic lipids in a manner that facilitates fusion (22). Binding studies of a soluble peptide comprising this region to anionic lipid model membranes provides evidence for a strong electrostatic component of the interaction. A near 50% decrease in granule secretion was observed as a consequence of replacing two of these basic with acidic residues (22). Taken together, experimental evidence suggests that the juxtamembrane domains of both SNARE fusion partners influence and perhaps even control membrane fusion, and that this influence is likely through interaction with acidic lipids in either of the two fusing membranes.
We sought to delineate the molecular mechanism that is responsible for Syx-PIP interactions and to define the determinants of the related clustering of Syx in membranes. Single and cumulative mutations of the RRKK motif of the Syx juxtamembrane domain to alanines gradually abrogates the dispersive effect of anionic lipids on Syx clusters and identifies the residues that are most important for this interaction. General electrostatics dominate these interactions and there is a lack of stereochemical specificity for different phosphate headgroup positions in the mammalian PIP repertoire. Together, these results explain the role of these signaling lipids in the lateral organization of syntaxin in model membranes, i.e., a role that they very likely also play in plasma membranes.
MATERIALS AND METHODS
Protein expression and purification
Syntaxin-1A residues 183-288 with an additional C-terminal cysteine and SNAP-25 were cloned in the pET28 expression vector. The deletion of the regulatory Habc domain did not significantly affect the clustering behavior of Syx in lipid model membranes as was noted previously (12). The proteins were expressed and purified as previously described with minor modification (12). All mutagenesis was performed using Quickchange Site-Directed mutagenesis kit (Qiagen, Hilden, Germany). Briefly, syntaxin was affinity purified on nickel resin in denaturing conditions in the presence of 5% wt/vol sodium cholate. Following extensive on column washing with buffer containing 20 mM HEPES, pH 7.4, 500 mM NaCl, 5% sodium cholate, 8M urea, 10% glycerol and 30 to 50 mM imidazole, the protein was eluted in the presence of the same buffer also containing 10 mM EDTA and 400 mM imidazole. The sample was next dialyzed against 20 mM HEPES, pH 7.4, 0.5 M NaCl, 1.5% CHAPS and 2 M urea. Thrombin was added at ~0.03 mg/mL to cleave the His-tag. The protein was dialyzed to buffer containing 0.5 M urea, then buffer lacking denaturant. Thrombin was removed by benzamidine agarose. All buffers contained 1 mM DTT. Protein concentrations were determined by Bradford assay and by UV absorbance.
Fluorescent Labeling
To fluorescently label syntaxin, samples were freshly incubated with 2-5 mM DTT for one hour at room temperature, followed by the removal of DTT by desalting against 2 mM tris-(2-carboxyethyl)-phosphine (PD-10, Invitrogen). The protein was immediately incubated in 2-10 fold molar excess Alexa Fluor 647 maleimide (Invitrogen, Carlsbad, CA) for 1 hour at room temperature. No change in efficiency was observed between 2 and 10 fold molar excess of fluorophore. Free label was removed by size-exclusion chromatography followed by dialysis. Labeling efficiencies between 55 and 100% were determined by dye absorbance using manufacturer's extinction coefficients and protein concentration by Bradford assay.
Lipids
The following materials were purchased and used without further purification: POPC, POPS, PI, PI-3,4-P2, PI-3,5-P2, PI-4,5-P2, PI-3,4,5-P2, all with dioleoyl chains, and brain PI-4,5-P2 (Avanti Polar Lipids, Alabaster, AL). Cholesterol (Sigma-Aldrich, St. Louis, MO).
Reconstitution of syntaxin in liposomes
Proteoliposomes were produced by first mixing appropriate ratios of POPC, POPS, PIPs and cholesterol in chloroform. The mixtures were dried under a stream of nitrogen and desiccated under vacuum for several hours. For vesicles containing PIPs, drying under nitrogen took place at 37 degrees C. Beginning with dried lipid films, as prepared above, appropriate amounts of protein in buffer containing 1.5 % CHAPS or 5 % sodium cholate (wt/vol) were added and incubated for 1–2 hrs to solubilize the lipids and to form mixed protein/lipid/detergent micelles. Samples were diluted threefold and dialyzed extensively with ~1 g/L SM-2 BioBeads (Biorad, Hercules, CA). All buffers contained 1 mM DTT. Syntaxin was found to be oriented ~90 % right-side-out with the C-termini facing the lumen of the liposomes. This topology was determined by trypsin digestion and subsequent SDS/PAGE. We also confirmed by absorbance and fluorescence spectroscopy of TX-100 dissolved proteoliposomes that all proteins (mutants) reconstituted into lipid bilayers with similar efficiencies and that large differences of reconstitution efficiency cannot be a potential source of increased fluorescence self-quenching.
Fluorescence Spectroscopy
All fluorescence experiments were performed essentially as described (12) and at 25 °C (unless otherwise indicated) using a Fluorolog-3 spectrofluorimeter (HORIBA Jobin-Yvon, Edison, NJ). Self-quenching experiments with Alexa Fluor 647-labeled protein were carried out with excitation at 650 nm and emission scans between 655 and 750 nm. When SNAP-25 was included, incubation was for more than 1 hour at 25 °C. Five nm slits were used in the excitation and emission paths in all experiments. Peak fluorescence emission intensities were evaluated in all experiments. In some cases, we also used integrated intensities and found the results to be quantitatively very similar to those using peak intensities. The results of at least four independent experiments were averaged in all self-quenching experiments. Error bars represent single standard deviations.
RESULTS
PI-4,5-P2 is Able to Disperse Syntaxin Clusters in an Anionic Lipid Background
We showed previously that phosphatidylserine and PI-4,5-P2 each dispersed cholesterol-induced clusters of syntaxin-1A in lipid model membranes (12). However, physiological concentrations of just PS or just PI-4,5-P2 were not sufficient to completely diminish the cholesterol-dependent clustering effect. In order to investigate whether PI-4,5-P2 could further potentiate the dispersion of syntaxin clusters in model membranes containing physiological amounts of PS and cholesterol, we utilized a fluorescent self-quenching assay to observe the clustering and dispersion of Syx in more complex lipid mixtures (12).
If Syx interacts with acidic lipids by electrostatics, then that interaction should be far stronger with multivalent anionic lipids. POPS carries a net -1 charge and constitutes approximately 20% of all inner leaflet lipids of the plasma membrane (23). PI-4,5-P2 carries a net charge of -3 to -4 and occurs in the inner leaflet of plasma membranes at levels from 1-5%, although local concentrations in cells can be higher (20, 24). Thus, our strategy was to use a fluorescence quenching assay to assess dequenching by PI-4,5-P2 in liposomes containing 20% POPS and 20% cholesterol.
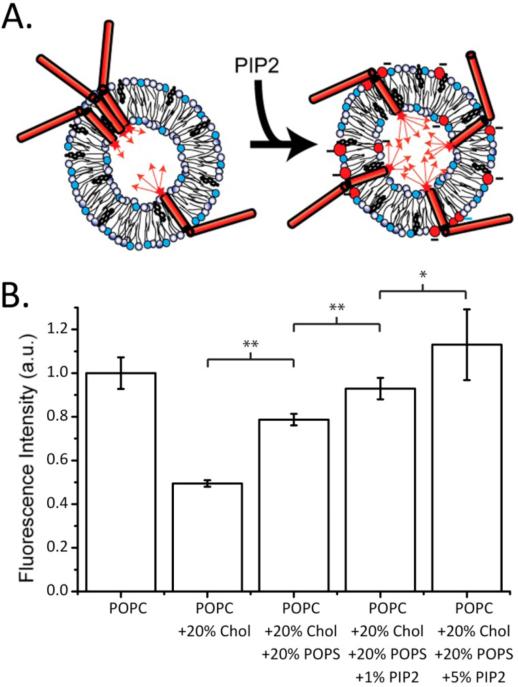
We first sought to recapitulate the cholesterol-dependent clustering of Syx (Figure 2B). In these preparations the presence of 20% cholesterol in the POPC vesicles resulted in ~50% decrease in fluorescence, which we attribute to the self-quenching of the Alexa 647 fluorophore on the C-terminus of Syx due to Syx clustering (12)2. Incorporating 20% POPS into the POPC:Chol vesicles produced a significant, though not complete, increase and recovery of the original fluorescence intensity. However, the recoveries could be further increased with the addition of 1 or 5% PI-4,5-P2 to the POPC:POPS:Chol vesicles. At 5% PI-4,5-P2, we observed a complete recovery of the original fluorescence observed in the absence of cholesterol. Thus, relatively high physiological local PI-4,5-P2 concentrations (20) are sufficient to completely disperse cholesterol-induced Syx clusters. This result is illustrated in Figure 2A.
Figure 2.
Cholesterol-dependent clustering of syntaxin-1A in lipid model membranes is relieved by the anionic lipids POPS and PI-4,5-P2 as measured by self-quenching of Alexa 647-labeled syntaxin. A. Cartoon model of syntaxin clustering and the resulting self-quenching that is reduced in the presence of anionic lipids. B. Scaled fluorescence intensities of labeled syntaxin reconstituted into liposomes of different lipid composition at protein-to-lipid ratios of 1:1000. The data represent averages of four or more independent experiments with error bars indicating single standard deviations. Paired bars are statistically significantly different at confidence levels of p<0.05 (*) and p<0.01(**).
Syntaxin Has No Specificity for the Phosphate Position on the Inositol Headgroup
We reported previously that Syx interacts with PI-4,5-P2 in an electrostatic manner (12). To further test if the interaction is purely electrostatic or also contains a stereo-specific chemical component, we employed the self-quenching assay to determine the fraction of fluorescence recovery for four different synthetic PIPs and, as a control, unphosphorylated PI. Since the length and degree of acyl chain saturation of phospholipids is known to result in differences in membrane packing and hence protein interactions (25), we compared the effects of the different PIPs with identical chain compositions, namely oleoyl chains in the sn-1 and sn-2 positions. Thus, these lipids should all have comparable phase and chain packing properties.
Like PS, PI is singly negatively charged. Including 1% of either of these lipids had no effect on the self-quenched fluorescence intensity of Alexa 647-labled Syx (not shown). The fluorescence in these experiments is scaled to the range given by concomitantly measured POPC:Chol and pure POPC control samples defining 0 and 1 fraction recoveries, respectively. Thus, with 1% PI in POPC:Chol vesicles we observed 0 fraction recovery (Figure 3).
Figure 3.
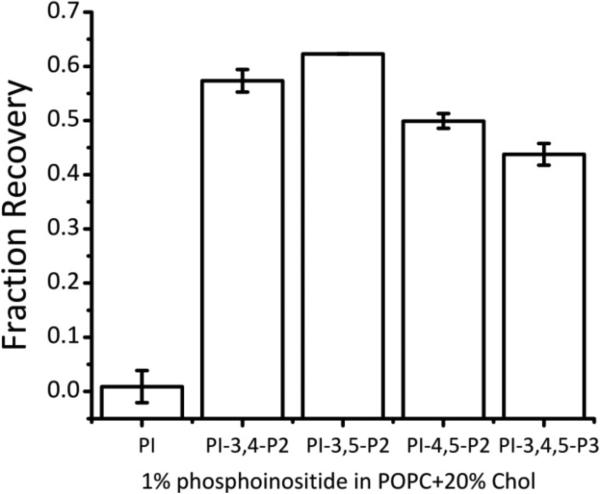
Dispersion of syntaxin-1A clusters by different polyphosphoinositides in lipid model membranes is not specific for the phosphate position on the lipid headgroup. Clustering was measured by self-quenching of labeled syntaxin in POPC:Chol (4:1) bilayers containing 1 mol% of the indicated phosphoinositide. The fluorescence is scaled (fraction recovery) to control samples with syntaxin in POPC and POPC:Chol bilayers without phosphoinositides that were measured in each experimental series to establish 1 and 0 fraction recovery, respectively. Data represent averages of four or more independent experiments with error bars indicating single standard deviations.
When 1% of PI-3,4-P2 or PI-3,5-P2 were incorporated into the POPC:Chol vesicles, similar fraction recoveries of about 0.5 to 0.6 were observed as with PI-4,5-P2 (Figure 3). Thus, the configuration and stereochemistry of phosphate attachment to the PI headgroup did not seem to have an influence on the interaction and dispersing power of PIP2s on Syx. Surprisingly, including 1% of the tris-phosphoinositide PI-3,4,5-P3 into POPC:Chol vesicles also produced ~0.5 fraction recovery, i.e. no more than the recovery observed with the bis-phosphoinositides. The reason for this result is not entirely clear, but may be attributed to a shifted pKa, or electrostatic or chemical shielding of the third phosphate group. Regardless, the PIP3 concentration used here is greater than the amount present in plasma membranes at any time (13). Therefore, and considering the much higher concentrations of PIP2s in cells, it is unlikely that PIP3s have any regulatory power over the degree of clustering of Syx in plasma membranes.
The Polybasic Juxtamembrane Region of Syntaxin is Responsible for the Interaction with PI-4,5-P2
Since previous results clearly demonstrated a role for the juxtamembrane domain of Syx in fusion, we wanted to know whether this domain is also responsible for the previously described electrostatic interactions with anionic lipids (12). A sequence analysis of known human syntaxin family members shows that the polybasic motif is most prevalent in the members that are targeted to the plasma membrane, which includes syntaxin-1A studied here and syntaxin 4, which functions in glucose transport (26, 27) (Figure 1). The positively charged region is least prevalent in syntaxins that are involved early in cellular membrane trafficking pathways, i.e. in syntaxin 18 of the endoplasmic reticulum or syntaxin 5 of the Golgi (28, 29). Interestingly, the most positively charged syntaxins localize to cellular membranes with the highest negative charge density and the most cholesterol (23, 30). In syntaxins that are localized to the plasma membrane, two arginines in positions 262 and 263 and a lysine in position 264 are highly conserved in mammals. A further lysine is also frequently found in position 265 in plasma membrane syntaxins.
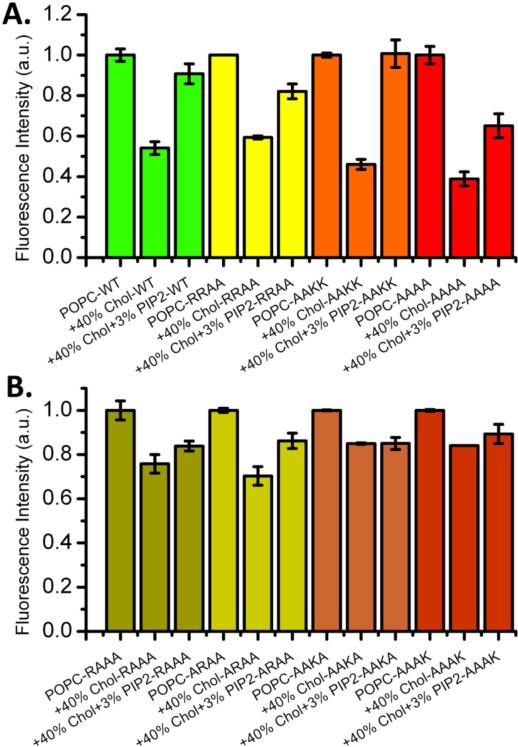
Lam et al. showed previously by nitrocellulose dot blot analysis that various PIPs and phosphatidic acid interact with syntaxin-1A (17). To ask whether these four basic residues constitute a binding site for PI-4,5-P2 also in a lipid bilayer setting, we prepared an Alexa Fluor 647 labeled mutant of Syx, in which all four basic residues were replaced with alanines. We call this mutant Syx-AAAA. We chose to use 40% Chol and 3% PI-4,5-P2 in these clustering and unclustering experiments to optimize the dynamic range for all experiments in this series with a range of mutants that will be described below. Figure 4A shows that 3% PI-4,5-P2 recovered clustering of wild-type Syx to 90% (green bars), but the recovery of clustering of Syx-AAAA was only 60% (red bars). We next asked whether the two arginines or the two lysines were mostly responsible for this effect. Mutants Syx-RRAA (two lysines replaced with alanines) and Syx-AAKK (two arginines replaced with alanines) were prepared to answer this question. Figure 4A shows that the arginine replacements had no effect (orange bars), but the lysine replacements caused a partial defect in the ability of PI-4,5-P2 to uncluster Syx (yellow bars).
Figure 4.
Role of individual basic residues of polybasic juxtamembrane motif in dispersion of cholesterol-induced syntaxin-1A clusters by binding of PI-4,5-P2. For each mutant, fluorescence self-quenching experiments were performed in POPC, POPC:Chol (3:2), and POPC:Chol (3:2) plus 3 mol% PI-4,5-P2. A. Experiments with wild-type, and RRAA, AAKK, AAAA mutants. B. Experiments with single basic residue mutants RAAA, ARAA, AAKA, and AAAK. The two lysines seem mostly responsible for the cluster dispersion interaction with PI-4,5-P2 although additional cumulative effects of all basic residues in this region are also observed. Protein-to lipid ratios were 1:1000 and measurements represent averages of four or more experiments with error bars indicating single standard deviations.
In order to test if specific individual residues were responsible for the electrostatic interaction, we prepared mutants retaining only one of each of the polybasic residues in the RRKK motif, with the remainder mutated to alanines. The specific mutants are Syx-RAAA, Syx-ARAA, Syx-AAKA, and Syx-AAAK. We observed that these mutants did not cluster in the presence of cholesterol to the same extent as wild-type or the previously described mutants, resulting in a limited dynamic range for analysis of the dispersion by PI-4,5-P2. Due to this limited dynamic range, the single-mutant syntaxins recovered only very slightly, with none of the mutants capable of a clear strong interaction with PI-4,5-P2 and none of them more effective than another (Figure 4B).
The Syntaxin/SNAP-25 Acceptor SNARE Complex is Less Clustered in Cholesterol-Containing Membranes than Syntaxin Alone
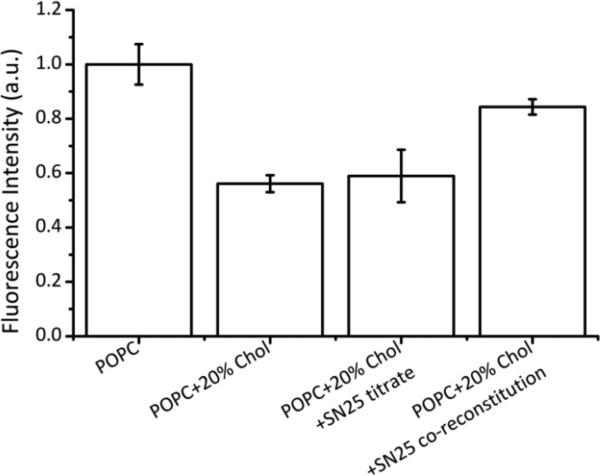
Although there is now ample evidence that Syx clusters in plasma and model membranes, little is known about how this clustering is affected by Syx forming an acceptor SNARE complex with SNAP-25. Clusters of Syx are thought to be relatively densely packed with their SNARE motifs interacting and standing upright on the membrane (5). Since SNAP-25 contributes an additional two helices to the acceptor SNARE complex, one might expect that SNAP-25 could have an effect on the Syx clustering density in membranes. To test this hypothesis, we prepared Syx/SNAP-25 complexes in membranes by two different methods, namely by titration of SNAP-25 to pre-reconstituted Syx in proteoliposomes, or by first forming complexes and then reconstituting the pre-formed complexes into proteoliposomes by dialytic detergent removal.
As controls, we first tested whether SNAP-25 would have an effect on the Alexa 647-labeled Syx fluorescence in POPC vesicles, i.e. in the absence of Syx clustering. We observed no change in fluorescence intensity by titration or co-reconstitution of SNAP-25 in proteoliposomes without cholesterol (data not shown). When SNAP-25 was titrated up to a 10-fold excess over pre-reconstituted Syx in POPC:Chol vesicles, we observed no significant change in fluorescence intensity (Figure 5). However, when Syx and SNAP-25 were first assembled into a complex (10-fold excess of SNAP-25 over Syx to minimize formation of a 2:1 complex) before reconstitution into POPC:Chol vesicles, we observed a significant increase in fluorescence intensity compared to Syx alone in the same kind of vesicles (Figure 5). The simplest explanation for this result is that SNAP-25 does not bind to clustered Syx in cholesterol-containing liposomes and is not able to disrupt such clusters after they have formed – a result that we also confirmed in supported bilayers (Domanska, Murray and Tamm, unpublished results). However, when SNAP-25/Syx complexes are formed before reconstitution into model membranes, SNAP-25 prevents the formation of high order Syx clusters by chemically shielding the open SNARE motif that is thought to contribute to cluster formation (11) and/or by sterically hindering the formation of laterally dense Syx aggregates.
Figure 5.
SNAP-25 partially disrupts clustering of syntaxin-1A in cholesterol-containing lipid model membranes. Disruption is only observed when SNAP-25 is co-reconstituted as a complex with syntaxin. Titration of SNAP-25 up to 10-fold excess over syntaxin to preformed syntaxin clusters in cholesterol-containing bilayers has no effect, i.e. is unable to disrupt these clusters. Mean scaled fluorescence intensities of labeled syntaxin at protein:lipid ratios of 1:1000 are shown for each lipid composition and SNAP-25 condition.
Syntaxin Clustering is Decreased but not Abolished at Higher Temperatures
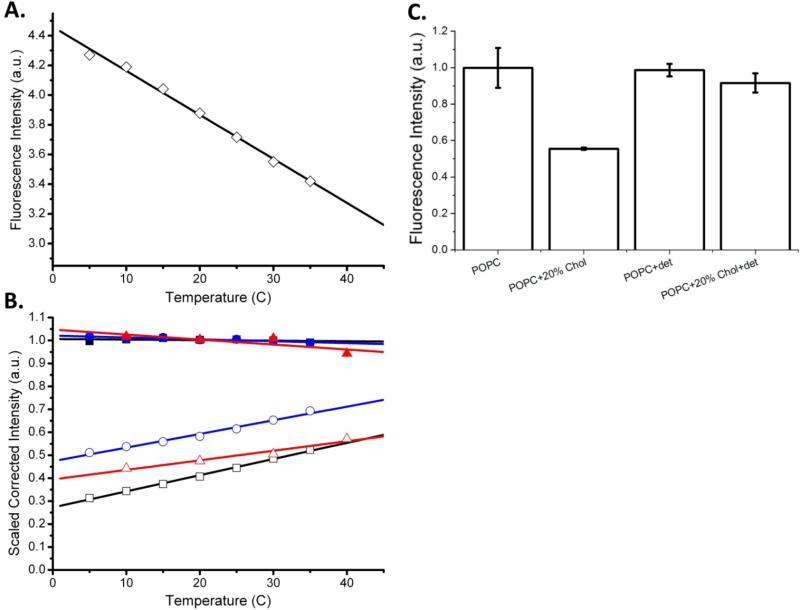
All experiments described so far were performed at 25 °C. We wanted to know if the clustering of Syx persists at higher temperatures, for example at the body temperature of 37 °C, which is often used in membrane fusion assays. It is well known that fluorescence emission of many fluorophores decreases as the temperature is increased due to molecular dynamics and increased solvent relaxation (31, 32). Therefore, to establish a baseline of temperature effects intrinsic to the photophysics of our probe, we first measured the fluorescence of Alexa 647-labeled Syx in Triton X-100. The sample was diluted to a concentration producing a similar fluorescence intensity as that of Syx in vesicles (approximately 1 μM). The fluorescence decreased linearly as the temperature increased, with a total decrease of approximately 20% over the 5 to 35 °C interval (Figure 6A). We then used this curve as a standard to normalize fluorescence intensities obtained from Syx reconstituted into liposomes.
Figure 6.
The clustering of syntaxin-1A in cholesterol-containing lipid model membranes decreases with increasing temperature as revealed by relief of self-quenching of fluorescently labeled syntaxin. A. Fluorescence intensities of 1 μM Alexa 647-labeled syntaxin in 2% v/v Triton X-100 as a function of temperature. B. Scaled fluorescence intensities of labeled syntaxin reconstituted into either POPC (closed symbols) or POPC:Chol (open symbols) bilayers at protein-to-lipid ratios of 1:1000. Three representative experiments are shown for each lipid composition. The data are normalized with the values in POPC bilayers at 20 °C set to one and corrected with the fluorophor temperature coefficient determined from graph A as described in the text. C. Mean scaled fluorescence intensities of labeled syntaxin in different environments from three or more experiments as indicated. 2% v/v Triton X-100 was added to the samples labeled with “det”.
Syx proteoliposomes with and without 20% cholesterol were cooled to 5 °C, the temperature was increased in steps to 40 °C, and fluorescence intensities were determined at each temperature. When normalized to the standard curve determined in Triton X-100, no significant change in fluorescence intensity was observed for Syx in POPC vesicles with increasing temperature (solid symbols in Figure 6B). However, the relative fluorescence of Syx in POPC:Chol vesicles was significantly decreased compared to the POPC vesicles at all temperatures and a significant fluorescence increase occurred for Syx in the POPC:Chol model membranes as the temperature was raised (open symbols in Figure 6B). In order to assure that the same amounts of protein were present in all experiments, we disrupted the vesicles at the end of each experiment and solubilized all Syx by adding Triton X-100 to 2% v/v. The resulting fluorescence showed no significant difference between the POPC and POPC:Chol samples, indicating that the reconstitution process resulted in equal incorporation of protein in either lipid composition (Figure 6C).
DISCUSSION
It is well established that polyphosphoinositides are directly implicated in neuronal exocytosis and specifically in SNARE-mediated membrane fusion. However, the nature and determinants of the responsible interactions have been understood only poorly. Depending on the study, syntaxin has been proposed to be in lipid rafts, excluded from lipid rafts, or associated with special nanodomains enriched with polyphosphoinositides (19, 33, 34). Our own previous studies showed that Syx forms cholesterol-dependent clusters that can be disrupted with acidic lipids in uniform (non-raft) lipid model membranes (12). High concentrations of PS or low concentrations of PI-4,5-P2 could disrupt these cholesterol-induced clusters. Our interpretation of these effects is that Syx requires free phospholipid (e.g. POPC) to be fully solvated in the lipid bilayer. In the presence of high cholesterol there is not enough free phospholipid to fully solvate the protein because much is needed to solvate cholesterol. Similar to chaotropic salts reversibly precipitating proteins in solution, cholesterol is able to drive Syx into clusters in membranes. However, anionic lipids with a high affinity to the polybasic motif of the juxtamembrane domain of Syx will bind and thereby solvate Syx even in the presence of high cholesterol. Starting from this model, we now ask: could PI-4,5-P2 be a regulatory factor of Syx clustering even in a background of physiological concentrations of PS in a presynaptic plasma membrane? And, is the cluster disrupting power unique to PI-4,5-P2 or can other PIPs achieve the same effect?
To answer these questions, we used our previously developed reconstituted membrane model system to measure the disruption of Syx clusters by relief of fluorescence self-quenching. Our data show that 20% PS and 1% PI-4,5-P2, which are typical average lipid concentrations in the inner leaflets of plasma membranes, are each not sufficient to completely disrupt the Syx clusters in cholesterol-containing lipid model membranes. However, when combined with 20% PS, 1% and especially 5% PI-4,5-P2 were able to completely disperse the Syx clusters (Figure 2). Since plasma membranes of secretory cells contain localized patches of PI-4,5-P2 of at least this concentration (20) and since these patches have been identified to be at or near the locations of exocytosis, it is likely that PI-4,5-P2 could dissociate Syx from clusters in preparation for fusion. This function appears not to be unique to PI-4,5-P2 because other PIPs can exert the same effect on the clusters (Figure 3). Even with this being the case, PI-4,5-P2 still is the most likely Syx-liberating lipid molecule in secretory cells, because this species is the most abundant PIP in plasma membranes near secretory granules or synaptic vesicles (13). This lipid is also the PIP that is the most regulated by phosphoinositide kinases and phosphatases, which likely provide another layer of Syx cluster regulation in these specialized membranes.
Once pried loose from a cluster, Syx may associate with SNAP-25 to form an acceptor complex that is ready to receive a R-SNARE (i.e. a SNARE containing an arginine in the central heptad repeat layer of the SNARE complex, such as synaptobrevin 2 of synaptic vesicles) for docking and trans-SNARE complex formation. Syx and SNAP-25 are both non-uniformly distributed and colocalize in plasma membranes to a high degree (6, 7). Our results of Figure 5 show that SNAP-25 cannot bind to Syx in clusters, but once SNAP-25 is bound to dispersed monomeric Syx, it prevents the re-formation of Syx clusters to a large degree. Therefore, acceptor SNARE complexes consisting of Syx and SNAP-25 are likely ready for engaging in vesicle docking and fusion and are unlikely to revert back to the clustered state. These clustering/unclustering and SNAP-25 binding equilibria clearly happen at 25 °C, i.e. the temperature at which most of our experiments have been carried out, but they very likely also persist at physiological temperatures of 37 °C as demonstrated by the experiments of Figure 6.
Lipid bilayers composed of POPC, 20% cholesterol, and 20% POPS have an average charge density per lipid of –0.2. If the bilayers contain an additional 1% PI-4,5-P2, the charge density increases only marginally to –0.23. However, with 5% PI-4,5-P2 the charge density becomes –0.35, i.e. almost twice that of the PC:Chol:PS bilayers. We previously showed that fluorescently labeled PI-4,5-P2 is evenly distributed and does not separate into any kind of domain distinct from lipid background of POPC and POPC:Chol (12). An evenly distributed charge of –0.35 per lipid creates a –25 mV surface potential at ~10 Å from the membrane surface (35). However, the positive potential formed by the highly charged juxtamembrane region is expected to further attract the more negatively charged PIPs and thereby demix this lipid from the bulk lipid even in the presence of 20% PS (14). PS is unlikely to demix under these conditions (14). We thus expect PI-4,5-P2 to be enriched in the vicinity of Syx, which further contributes to the dispersion of Syx clusters even in a uniform background of 20% monovalent anionic lipid.
If we consider that the Syx SNARE motif is mostly disordered in the absence of other SNAREs, then it likely samples the environment both parallel and perpendicular to the membrane. Electron paramagnetic resonance studies favor a conformation that places the bulk of the juxtamembrane region at or below the lipid-water interface (36). After formation of a complex with SNAP-25 and synaptobrevin, the complex may assume a more perpendicular alignment (3, 37). Yet, even in the most extreme perpendicular alignment, the negative membrane surface potential would still reach all basic residues of the juxtamembrane region. Thus, complexes between Syx and PI-4,5-P2 are likely also present in the Syx/SNAP-25 acceptor complex, being thermodynamically and electrostatically favorable, and may very well persist in the post-fusion cis-SNARE complex (3). Since the calcium sensor synaptotagmin through its C2B domain also has a high affinity for PI-4,5-P2, PI-4,5-P2 may actually function as an organizing platform, bringing SNAREs and synaptotagmin together and thus helping to drive the assembly of the neuronal fusion machinery (6, 19, 20, 22, 38, 39).
In model peptides, there is no preference for lysine or arginine to bind to negatively charged lipid bilayers (40). Moreover, the ability of the polybasic peptides to bind anionic lipids scales linearly with the number of basic residues using a series of equivalent lysine- or arginine-containing model peptides (41). This seems not to be the case for the Syx-PI-4,5-P2 interactions studied here in the context of a larger transmembrane protein. The results of Figure 4 show that lysines 264 and 265 are more effective in binding PI-4,5-P2 than arginines 262 and 263. Even if the lysines are anchored deeper in the membrane interface than the arginines (36), they may still snorkel up to about the same plane at the membrane surface as the arginines. If that is the case, lysines could be more effective interacting with the PI phosphates because their positive charge is localized on the terminal amine grouping of lysine whereas the positive charge is delocalized on the guanidinium grouping of arginine. Previously, it was shown that mutation of these arginines is responsible for a decreased binding to phosphatidic acid in nitrocellulose blots and that the Syx-AAKK mutant fails to rescue secretion (17). However, the converse mutant Syx-RRAA was not studied in this previous work. Therefore, it is not clear whether our current findings are consistent or inconsistent with these earlier studies. We also found that sequential mutation of the polybasic region showed a biphasic behavior on Syx clustering. In the absence of acidic lipids, single mutations seem more disruptive to clustering than wild-type and double mutations. Although this observation does not affect any of our conclusions, the reason for this behavior is presently unclear. Although sequence position specificity is rare in electrostatic surface interactions of peptides on membranes, it is not without precedent. For example, an arginine within SCAMP2, a protein also involved in the regulation of exocytosis, is critical, but a nearby lysine is disposable for binding of this peptide to PI-4,5-P2 (42).
In addition to generating quite strongly negative electrostatic sinks, polyphosphoinositides have an inverted-conical shape, i.e. a larger polar headgroup than hydrophobic tail cross-section. Lipids of this shape generally inhibit fusion at an early stage, namely at the hemifusion stage where lipids communicate between two membranes, but no aqueous pore is formed yet (43). Indeed PI-4,5-P2 has been observed to inhibit fusion in an in vitro liposome fusion assay (20). Thus, the presence and requirement for PI-4,5-P2 to promote calcium-dependent membrane fusion seems contradictory. A recent study describing evidence for synaptobrevin interaction with anionic lipids proposes a solution to this dilemma (22). The authors suggest that the polybasic juxtamembrane region may provide a screen for the negatively charged membrane. This charge screening may allow the vesicle to better approach the plasma membrane where the local electrostatic effects of anionic lipids, synaptobrevin, and syntaxin together may pin the two membranes in close contact. It remains to be seen how these various properties of PI-4,5-P2 balance with SNARE organization, docking, and fusion to ultimately allow SNARE complex zippering and coupling to calcium-release in neuronal exocytosis.
ACKNOWLEDGMENTS
We thank members of the Tamm laboratory and Dr. David Cafiso, University of Virginia, for many helpful discussions and for critical reading of the manuscript.
Footnotes
This work was supported by NIH grant P01 GM072694
Abbreviations: CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate; Chol, cholesterol; DTT, dithiothreitol; EDTA, ethylenediaminetetraacetic acid; HEPES, (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; POPC, 1-palmitoyl-2-oleoylphosphatidylcholine; POPS, 1-palmitoyl-2-oleoyl-phosphatidylserine; PI, phosphatidylinositol; PIP, phosphatidylinositolphosphate; PI-4,5-P2, phosphatidylinositol-4,5-bisphosphate; SNAP-25, synaptosomal-associated protein of 25 kDa; SNARE, soluble N-ethylmaleimide-sensitive factor attachment receptor; Syx, syntaxin-1A; TM, transmembrane.
We observed that the quenching may vary between 30 and 50%, dependent on the sample preparation (this work and 12). The reasons for this variability are not fully understood. These differences affect only the dynamic range of each experiment, but have no consequences on any of the results reported here. Vesicles prepared from identical lipid and fluorescently labeled protein stocks produced more precise results with relatively small error bars.
REFERENCES
- 1.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 2.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 3.Stein A, Weber G, Wahl MC, Jahn R. Helical extension of the neuronal SNARE complex into the membrane. Nature. 2009;460:525–528. doi: 10.1038/nature08156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dittman JS, Kaplan JM. Factors regulating the abundance and localization of synaptobrevin in the plasma membrane. Proc Natl Acad Sci U S A. 2006;103:11399–11404. doi: 10.1073/pnas.0600784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sieber JJ, Willig KI, Kutzner C, Gerding-Reimers C, Harke B, Donnert G, Rammner B, Eggeling C, Hell SW, Grubmuller H, Lang T. Anatomy and dynamics of a supramolecular membrane protein cluster. Science. 2007;317:1072–1076. doi: 10.1126/science.1141727. [DOI] [PubMed] [Google Scholar]
- 6.Rickman C, Meunier FA, Binz T, Davletov B. High affinity interaction of syntaxin and SNAP-25 on the plasma membrane is abolished by botulinum toxin E. J Biol Chem. 2004;279:644–651. doi: 10.1074/jbc.M310879200. [DOI] [PubMed] [Google Scholar]
- 7.Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 2001;20:2202–2213. doi: 10.1093/emboj/20.9.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Low SH, Vasanji A, Nanduri J, He M, Sharma N, Koo M, Drazba J, Weimbs T. Syntaxins 3 and 4 are concentrated in separate clusters on the plasma membrane before the establishment of cell polarity. Mol Biol Cell. 2006;17:977–989. doi: 10.1091/mbc.E05-05-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohara-Imaizumi M, Nishiwaki C, Nakamichi Y, Kikuta T, Nagai S, Nagamatsu S. Correlation of syntaxin-1 and SNAP-25 clusters with docking and fusion of insulin granules analysed by total internal reflection fluorescence microscopy. Diabetologia. 2004;47:2200–2207. doi: 10.1007/s00125-004-1579-0. [DOI] [PubMed] [Google Scholar]
- 10.Predescu SA, Predescu DN, Shimizu K, Klein IK, Malik AB. Cholesterol-dependent syntaxin-4 and SNAP-23 clustering regulates caveolar fusion with the endothelial plasma membrane. J Biol Chem. 2005;280:37130–37138. doi: 10.1074/jbc.M505659200. [DOI] [PubMed] [Google Scholar]
- 11.Sieber JJ, Willig KI, Heintzmann R, Hell SW, Lang T. The SNARE motif is essential for the formation of syntaxin clusters in the plasma membrane. Biophys J. 2006;90:2843–2851. doi: 10.1529/biophysj.105.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray DH, Tamm LK. Clustering of syntaxin-1A in model membranes is modulated by phosphatidylinositol 4,5-bisphosphate and cholesterol. Biochemistry. 2009;48:4617–4625. doi: 10.1021/bi9003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 15.Vicinanza M, D'Angelo G, Di Campli A, De Matteis MA. Function and dysfunction of the PI system in membrane trafficking. EMBO J. 2008;27:2457–2470. doi: 10.1038/emboj.2008.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vicinanza M, D'Angelo G, Di Campli A, De Matteis MA. Phosphoinositides as regulators of membrane trafficking in health and disease. Cell Mol Life Sci. 2008;65:2833–2841. doi: 10.1007/s00018-008-8353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam AD, Tryoen-Toth P, Tsai B, Vitale N, Stuenkel EL. SNARE-catalyzed Fusion Events Are Regulated by Syntaxin1A-Lipid Interactions. Mol Biol Cell. 2008;19:485–497. doi: 10.1091/mbc.E07-02-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Paolo G, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, Flavell R, Fitzsimonds RM, Ryan TA, De Camilli P. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431:415–422. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- 19.Aoyagi K, Sugaya T, Umeda M, Yamamoto S, Terakawa S, Takahashi M. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J Biol Chem. 2005;280:17346–17352. doi: 10.1074/jbc.M413307200. [DOI] [PubMed] [Google Scholar]
- 20.James DJ, Khodthong C, Kowalchyk JA, Martin TF. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J Cell Biol. 2008;182:355–366. doi: 10.1083/jcb.200801056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Komen JS, Bai X, Rodkey TL, Schaub J, McNew JA. The polybasic juxtamembrane region of Sso1p is required for SNARE function in vivo. Eukaryot Cell. 2005;4:2017–2028. doi: 10.1128/EC.4.12.2017-2028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams D, Vicogne J, Zaitseva I, McLaughlin S, Pessin JE. Evidence that electrostatic interactions between vesicle-associated membrane protein 2 and acidic phospholipids may modulate the fusion of transport vesicles with the plasma membrane. Mol Biol Cell. 2009;20:4910–4919. doi: 10.1091/mbc.E09-04-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 25.Huang CH. Mixed-chain phospholipids: structures and chain-melting behavior. Lipids. 2001;36:1077–1097. doi: 10.1007/s11745-001-0818-1. [DOI] [PubMed] [Google Scholar]
- 26.Kloepper TH, Kienle CN, Fasshauer D. An elaborate classification of SNARE proteins sheds light on the conservation of the eukaryotic endomembrane system. Mol Biol Cell. 2007;18:3463–3471. doi: 10.1091/mbc.E07-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grusovin J, Macaulay SL. Snares for GLUT4--mechanisms directing vesicular trafficking of GLUT4. Front Biosci. 2003;8:d620–641. doi: 10.2741/1052. [DOI] [PubMed] [Google Scholar]
- 28.Bankaitis VA, Morris AJ. Lipids and the exocytotic machinery of eukaryotic cells. Curr Opin Cell Biol. 2003;15:389–395. doi: 10.1016/s0955-0674(03)00076-0. [DOI] [PubMed] [Google Scholar]
- 29.Schuldiner M, Metz J, Schmid V, Denic V, Rakwalska M, Schmitt HD, Schwappach B, Weissman JS. The GET complex mediates insertion of tail-anchored proteins into the ER membrane. Cell. 2008;134:634–645. doi: 10.1016/j.cell.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 31.Kubin RF, Fletcher AN. Fluorescence quantum yields of some rhodamine dyes. Journal of Luminescence. 1983;27:455–462. [Google Scholar]
- 32.Sens R, Drexhage KH. Fluorescence quantum yield of oxazine and carbazine laser dyes. Journal of Luminescence. 1981;24-25:709–712. [Google Scholar]
- 33.Bacia K, Schuette CG, Kahya N, Jahn R, Schwille P. SNAREs prefer liquid-disordered over “raft” (liquid-ordered) domains when reconstituted into giant unilamellar vesicles. J Biol Chem. 2004;279:37951–37955. doi: 10.1074/jbc.M407020200. [DOI] [PubMed] [Google Scholar]
- 34.Salaun C, Gould GW, Chamberlain LH. Lipid raft association of SNARE proteins regulates exocytosis in PC12 cells. J Biol Chem. 2005;280:19449–19453. doi: 10.1074/jbc.M501923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peitzsch RM, Eisenberg M, Sharp KA, McLaughlin S. Calculations of the electrostatic potential adjacent to model phospholipid bilayers. Biophys J. 1995;68:729–738. doi: 10.1016/S0006-3495(95)80253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kweon DH, Kim CS, Shin YK. Insertion of the membrane-proximal region of the neuronal SNARE coiled coil into the membrane. J Biol Chem. 2003;278:12367–12373. doi: 10.1074/jbc.M211123200. [DOI] [PubMed] [Google Scholar]
- 37.Kiessling V, Tamm LK. Measuring distances in supported bilayers by fluorescence interference-contrast microscopy: polymer supports and SNARE proteins. Biophys J. 2003;84:408–418. doi: 10.1016/S0006-3495(03)74861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bar-On D, Gutman M, Mezer A, Ashery U, Lang T, Nachliel E. Evaluation of the heterogeneous reactivity of the syntaxin molecules on the inner leaflet of the plasma membrane. J Neurosci. 2009;29:12292–12301. doi: 10.1523/JNEUROSCI.0710-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willig KI, Rizzoli SO, Westphal V, Jahn R, Hell SW. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature. 2006;440:935–939. doi: 10.1038/nature04592. [DOI] [PubMed] [Google Scholar]
- 40.Kim J, Mosior M, Chung LA, Wu H, McLaughlin S. Binding of peptides with basic residues to membranes containing acidic phospholipids. Biophys J. 1991;60:135–148. doi: 10.1016/S0006-3495(91)82037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben-Tal N, Honig B, Peitzsch RM, Denisov G, McLaughlin S. Binding of small basic peptides to membranes containing acidic lipids: theoretical models and experimental results. Biophys J. 1996;71:561–575. doi: 10.1016/S0006-3495(96)79280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao H, Ellena J, Liu L, Szabo G, Cafiso D, Castle D. Secretory carrier membrane protein SCAMP2 and phosphatidylinositol 4,5-bisphosphate interactions in the regulation of dense core vesicle exocytosis. Biochemistry. 2007;46:10909–10920. doi: 10.1021/bi701121j. [DOI] [PubMed] [Google Scholar]
- 43.Chernomordik LV, Kozlov MM. Mechanics of membrane fusion. Nat Struct Mol Biol. 2008;15:675–683. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]