Abstract
Islet amyloid polypeptide, (IAPP, Amylin), is responsible for islet amyloid formation in type 2 diabetes and IAPP induced toxicity is believed to contribute to the loss of β-cell mass associated with the late stages of type 2 diabetes. Islet amyloid formation may also play a role in graft failure after transplantation. IAPP is produced as a prohormone, proIAPP, and processed in the secretory granules of the pancreatic beta cells. Partially processed forms of proIAPP are found in amyloid deposits; most notably, a 48 residue intermediate, proIAPP1–48, which includes the N-terminal pro extension, but which has been properly processed at the C-terminus. Incomplete processing may plays a role in islet amyloid formation by promoting interactions with sulfated proteoglycans of the extracellular matrix which, in turn, promote amyloid formation. We show that acid fuchsin (3-(1-(4-Amino-3-methyl-5-sulphonatophenyl)-1-(4-amino-3-sulphonatophenyl) methylene) cyclohexa-1,4-dienesulphonic acid), a simple sulfonated triphenyl methyl derivative, is a potent inhibitor of amyloid formation by the proIAPP1–48 intermediate. The more complicated triphenyl methane derivative fast green FCF, {ethyl-[4-[[4-[ethyl -[(3-sulfophenyl) methyl] amino] phenyl]-(4-hydroxy-2- sulfophenyl) methylidene]-1-cyclohexa-2,5-dienylidene]-[(3-sulfophenyl) methyl] azanium}, also inhibits amyloid formation by IAPP and the proIAPP processing intermediate. Both compounds inhibit amyloid formation by mixtures of the proIAPP intermediate and the model glycosaminoglycan (GAG) heparan sulfate. Acid fuchsin also inhibits GAG mediated amyloid formation by mature IAPP. The ability to inhibit amyloid formation is not simply due to the compounds being sulfonated, since the sulfonated inhibitor of Aβ amyloid tramprosate, is not an inhibitor of amyloid formation by proIAPP1–48.
Keywords: IAPP, Amylin, proIAPP, Amyloid, Prohormone, Glycosaminoglycan
Introduction
Amyloid formation and aberrant protein aggregation plays a role in a range of human diseases including type 2 diabetes, Parkinson’s disease and Alzheimer’s disease1–2. Human islet amyloid polypeptide, (IAPP also known as amylin), is the major protein component of the pancreatic islet amyloid associated with type 2 diabetes (Figure 1)3–10. Amyloid fibrils formed by IAPP and/or intermediates produced during their formation are toxic to cultured islet β-cells, suggesting that IAPP induced toxicity may contribute to the pathology of type 2 diabetes5–7; 11–16. There is also increasing evidence linking IAPP amyloid formation with graft failure in islet cell transplantation17–20
Figure 1.
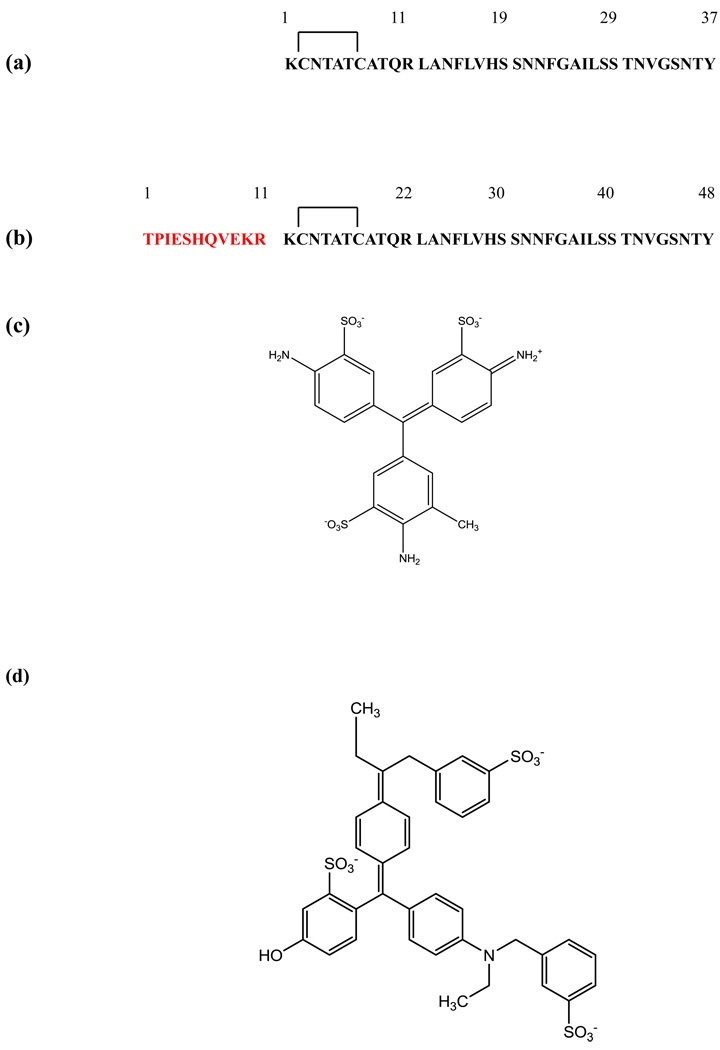
(a) The primary sequence of mature human IAPP. The peptide contains a disulfide bridge between Cys-2 and Cys-7 and has an amidated C-terminus. (b) The primary sequence of the proIAPP1–48 processing intermediate. The sequence is numbered beginning at the first residue of the intermediate. The N-terminal extension is depicted in red and the sequence of the mature hormone in black. (c) The structure of acid fuchsin. (d) The structure of fast green FCF.
IAPP is stored in the pancreatic β-cells in the same secretory granules as insulin, processed in parallel with insulin and secreted in response to the same stimuli8; 15–16; 21–22. IAPP is produced as a 89 residue preprohormone, preproIAPP. Cleavage of the signal sequence generates a 67 residue pro-form, denoted as proIAPP. ProIAPP is further processed to yield the mature 37 residue hormone by the enzymes prohormone convertase 2 and 1/3 which cleave proIAPP at the N-terminus and the C-terminus respectively. Cleavage at the C-terminus is followed by a series of steps leading to an amidated C-terminus15; 23–26. Immunohistochemical studies of islet amyloid have demonstrated the presence of a processing intermediate which corresponds to the first 48 residues of proIAPP (proIAPP1–48) that contains the N-terminal prosequence, but not the C-terminal prosequence (Figure 1)27–31. Abnormal processing of proIAPP has been suggested to play an important role in islet amyloid formation and increased levels of incomplete processing have been proposed to correlate with cell death27–28; 32.
In one model of islet amyloid formation, incomplete processing of proIAPP leads to formation of the proIAPP1–48 intermediate which can interact with the glycosaminoglycan (GAG) component of the heparan sulfate proteoglycans (HSPGs) of the basement membrane. This leads to amyloid formation by generating a high local concentration of an amyloidogenic polypeptide33–35. The amyloid fibrils thus formed act as a seed to induce additional partially processed IAPP and mature IAPP to form amyloid. HSPGs are well known to be associated with in vivo amyloid deposits, including islet amyloid36–43. The model makes several predictions; first, proIAPP1–48 is predicted to bind to HSPG’s and second, the binding is predicted to enhance the rate of amyloid formation. The amyloid thus formed is also predicted to seed amyloid fibril formation by fully processed mature IAPP. Verchere and cowokers have shown that proIAPP1–48 binds to HSPGs and the binding site was subsequently localized via peptide fragment studies33–34. Our recent in vitro experiments have provided proof of principle evidence that HSPGs promote amyloid formation by the proIAPP1–48 intermediate and have also shown that the amyloid fibrils formed by the interaction of proIAPP1–48 with GAGs can seed amyloid formation by mature IAPP35.
The development of inhibitors of amyloid formation is an active area of research, both because of their possible therapeutic applications, and because they can serve as reagents to probe mechanisms of toxicity and pathways of amyloid assembly44–49. There are a number of reported inhibitors of amyloid formation by IAPP, but to the best of our knowledge, there have been no reported amyloid inhibitors designed to target amyloid formation by proIAPP intermediates or GAG mediated amyloid formation by proIAPP processing intermediates or mature fully processed IAPP47–48; 50–56.
The simple sulfonated triphenylmethyl derivative, acid fuchsin, (3-(1-(4-Amino-3-methyl-5-sulphonatophenyl)-1-(4-amino-3-sulphonatophenyl) methylene) cyclohexa-1,4-dienesulphonic acid) is an inhibitor of amyloid formation by mature IAPP in the absence of GAGs but its ability to inhibit amyloid formation by proIAPP processing intermediates or GAG mediated amyloid formation has not been examined55. Here we show that acid fuchsin inhibits amyloid formation by proIAPP processing intermediates and by mixtures of proIAPP and heparan sulfate as well as by mixtures of mature fully processed IAPP and heparan sulfate (Figure 1). We also report that a second sulfonated triphenyl derivative, fast green FCF, {ethyl-[4-[[4-[ethyl -[(3-sulfophenyl) methyl] amino] phenyl]-(4-hydroxy-2- sulfophenyl) methylidene]-1-cyclohexa-2,5-dienylidene]-[(3-sulfophenyl) methyl] azanium}, is an effective inhibitor of amyloid formation by IAPP and proIAPP processing intermediates, and inhibits GAG mediated amyloid formation.
Results and Discussion
Acid fuchsin is a potent inhibitor of amyloid formation by proIAPP1–48
The sequence of proIAPP1–48 and mature IAPP are shown in Figure-1. The 11 additional residues found in proIAPP1–48 reduce its amyloidogencity relative to mature IAPP by decreasing the overall hydrophobicity and increasing the net charge relative to mature IAPP, but they significantly increase the ability to bind GAGs33–35. The structure of acid fuchsin and fast green FCF are displayed in Figure 1 and are built off a triphenylmethane core. Each ring in acid fuchsin is sulfonated and has an amino substituant, while one of the three rings has a methyl substituant. The sulphonates are likely important for electrostatic interactions with the positively charged IAPP55. The structure of fast green FCF is more complex, but it is based on the same triphenylmethane core and it is sulfonated.
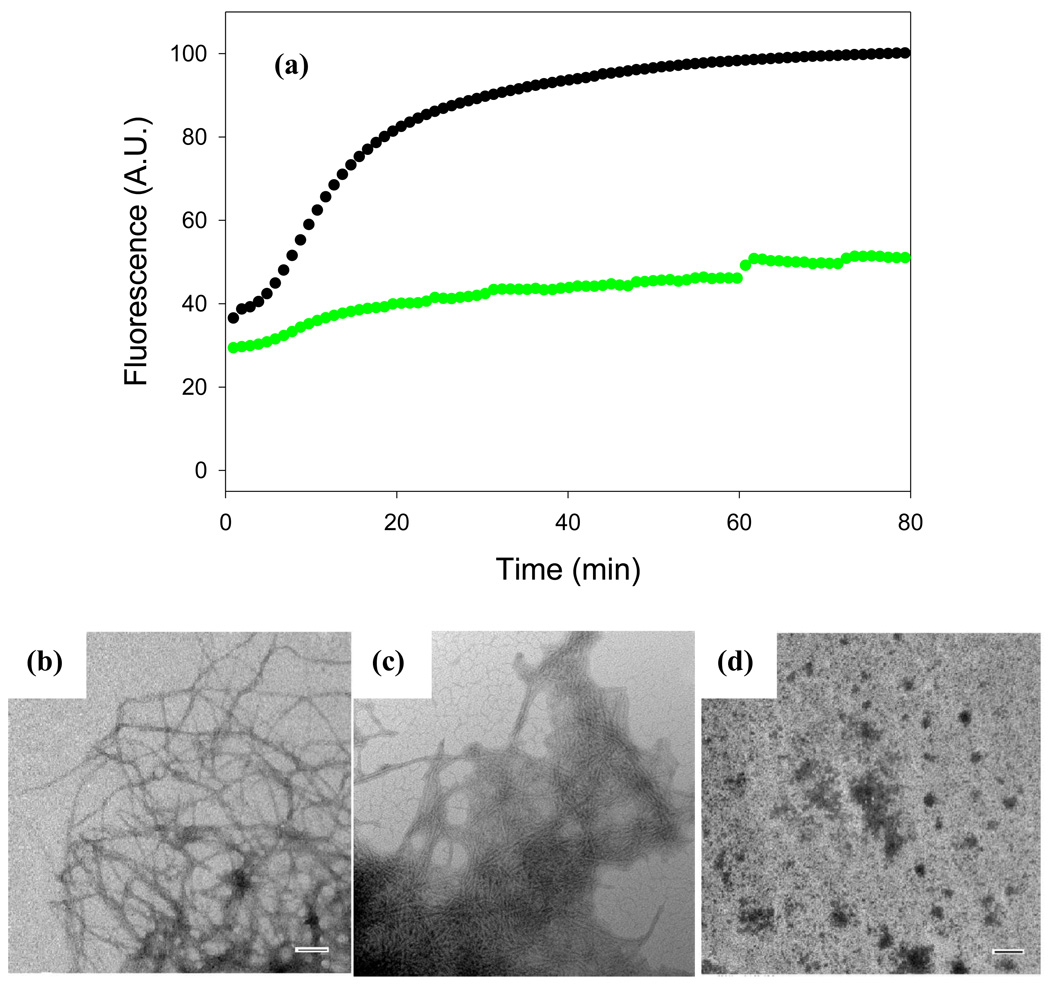
Figure 2 displays the results of a kinetic experiment in which the rate of amyloid formation by proIAPP1–48 was measured in the presence and in the absence of acid fuchsin using fluorescence detected thioflavin-T binding assays. Thioflavin-T is a widely used small molecule probe of amyloid formation. The fluorescent quantum yield of the dye increases significantly when it binds to amyloid fibrils, although the exact mode of binding is not known57. In the absence of acid fuchsin, proIAPP1–48 displays the classic sigmoidal curve expected for amyloid formation. ProIAPP1–48 is less amyloidgenic than mature IAPP in homogeneous solution and the lag time is longer for proIAPP1–48 than for mature IAPP35; 58. No detectable thioflavin-T binding is observed at a 1:1 ratio of proIAPP1–48 to inhibitor, consistent with the prevention of fibril formation. It is important to independently confirm the results of thioflavin-T binding assays, since they can sometimes give false positives or false negatives59. Therefore, CD and TEM studies were conducted. TEM images of proIAPP1–48 display numerous amyloid fibrils in the absence of acid fuchsin, but no fibrils are detectable in the presence of inhibitor (Figure 2). The CD spectrum of proIAPP1–48 at the end of reaction has an intense β-sheet signal in the absence of acid fuchsin, which is not detected when acid fuchsin is present (Supplementary Data). The CD and TEM studies confirm the conclusions of the thioflavin-T assays and provide further evidence that the compound effectively inhibits amyloid formation by proIAPP1–48.
Figure 2. Acid fuchsin inhibits amyloid formation by proIAPP1–48.
(a) Time course of amyloid fibril formation monitored by thioflavin-T binding; proIAPP1–48 in the absence of inhibitor (black), proIAPP1–48 plus an equimolar amount of acid fuchsin (red). (b) TEM image of the amyloid fibrils formed by proIAPP1–48 in the absence of acid fuchsin. Samples were removed 80 minutes after the start of the reaction. (c) TEM image of a sample of the acid fuchsin proIAPP1–48 mixture collected 80 minutes after the initiation of the reaction. Scale bars represent 100 nm. The same solutions were used for the kinetic studies and for the TEM samples. The pH of the solutions was 7.4. The solutions contained 2% HFIP by volume, 20mM Tris-HCl, 25µM thioflavin-T, 32µM peptide and were continually stirred at 25°C.
Acid fuchsin inhibits GAG promoted amyloid formation by proIAPP1–48
The N-terminal region of proIAPP promotes binding to GAGs, and GAGs such as heparan sulfate have been shown to catalyzed amyloid formation by proIAPP1–4835. Thus, we tested the effects of acid fuchsin on amyloid formation by mixtures of proIAPP1–48 and heparan sulfate. Mixtures of proIAPP1–48 and heparan sulfate form amyloid very quickly under the conditions of our studies (Figure 3). The proIAPP1–48 heparan sulfate mixture has a lag phase of only 6 minutes under the conditions of these experiments, while proIAPP1–48 in the absence of GAG has a lag phase on the order of 25 minutes. The proIAPP1–48 heparan sulfate complex has higher initial fluorescence intensity than proIAPP1–48 alone, probably because heparan sulfate leads to the rapid formation of a partially ordered structure which can bind thioflavin-T35. Control experiments show that heparan sulfate alone does not lead to enhanced thioflavin-T fluorescence. In the presence of an equimolar amount of acid fuchsin, the proIAPP1–48 heparan sulfate mixture shows only a modest total increase in thioflavin-T fluorescence intensity during an 80 minute kinetic run and the final thioflavin-T intensity is only 50% of that observed for proIAPP1–48 and heparan sulfate in the absence of inhibitor. The effects of acid fuchsin are more pronounced when the compound is added in 10 fold molar excess. In this case, acid fuchsin abolishes amyloid formation by the proIAPP1–48 heparan sulfate mixture. The ability of acid fuchsin to inhibit heparan sulfate catalyzed amyloid formation by proIAPP1–48 was confirmed by TEM (Figure 3, Supplementary Data). In the absence of inhibitor, a dense network of amyloid fibrils was observed for the sample of proIAPP1–48 plus heparan sulfate at the end of the reaction. Addition of acid fuchsin at a 1:1 ratio results in much thinner and less prevalent fibrils, while the 10:1 mixture (acid fuchsin in 10 fold excess) was devoid of fibrils. Thinning of fibers has been observed in the presence of other inhibitors, although the molecular basis of the effect is not understood47,53. Consistent with the TEM results, CD spectroscopy confirmed some β-sheet formation for the 1:1 mixture of inhibitor with proIAPP1–48 heparan sulfate (Supplementary Data). A 10:1 ratio of inhibitor to peptide could not be tested by CD or thioflavin-T fluorescence because the high absorbance of this concentration of acid fuchsin interfered with the CD measurement and led to strong inner filter effects in fluorescence measurements.
Figure 3. Acid fuchsin inhibits amyloid formation by a mixture of proIAPP1–48 and heparan sulfate.
(a) Time course of amyloid fibril formation monitored by thioflavin-T binding: proIAPP1–48 with heparan sulfate in the absence of inhibitor (black); a 1:1 mixture of acid fuchsin with proIAPP1–48/heparan sulfate (green). (b) TEM image of the amyloid fibrils formed by the proIAPP1–48/heparan sulfate mixture in the absence of acid fuchsin. Samples were removed 80 minutes after the start of the reaction. (c) TEM image of a sample of the 1:1 mixture of acid fuchsin with proIAPP1–48/heparan sulfate collected at 80 minutes after the initiation of the reaction. (d) TEM image of a sample of the 10:1 mixture of acid fuchsin with proIAPP1–48/heparan sulfate collected at 80 minutes after the initiation of the reaction. Scale bars represent 100 nm. The same solutions were used for the kinetic studies and for the TEM samples. The pH of the solutions was 7.4. The solutions contained 2% HFIP by volume, 20mM Tris-HCl, 25µM thioflavin-T, 32µM peptide and were continually stirred at 25°C.
Acid fuchsin inhibits amyloid formation by mature IAPP in the presence of GAGs
Important early work has shown that interaction with proteoglycans and GAGs enhances amyloid formation by mature human IAPP37–38. No data is available on the effects of GAGs and proteoglycans on the kinetics of amyloid formation of IAPP, but the extent of amyloid formation has been shown to be enhanced and the effects are believed to be related to the ability of human IAPP to aggregate since no effect was observed with rat IAPP which is known not to form amyloid37–38. Thus we also studied the ability of acid fuchsin to inhibit amyloid formation by mature IAPP in the presence of heparan sulfate. Figure 4 displays fluorescence monitored thioflavin-T binding experiments. Mature IAPP rapidly form amyloid under these conditions. Addition of heparan sulfate leads to effects which are similar to those observed with proIAPP1–48. Addition of acid fuchsin has a notable effect on the kinetic curves. The 1:1 ratio of IAPP heparan sulfate plus acid fuchsin behaved similarly to the 1:1 ratio of proIAPP1–48 heparan sulfate plus acid fuchsin. The compounds effects on GAG mediated amyloid formation mature IAPP were confirmed by TEM measurements. Dense mats of amyloid fibrils are observed for the mixture of IAPP in the absence of acid fuchsin (Figure 4b). Fewer fibrils are detected in the presence of acid fuchsin (Figure 4c). A ten fold excess of the compound is even more effective. The absorbance of the acid fuchsin prevents CD and fluorescence studies at this ratio, but no amyloid fibrils were detected in the TEM images (Figure 4d).
Figure 4. Acid fuchsin inhibits amyloid formation by a mixture of IAPP and heparan sulfate.
(a) Time course of amyloid fibril formation monitored by thioflavin-T binding: IAPP with heparan sulfate in the absence of inhibitor (black); a 1:1 mixture of acid fuchsin with IAPP/heparan sulfate (green). (b) TEM image of the amyloid fibrils formed by the IAPP heparan sulfate mixture in the absence of acid fuchsin. Samples were removed 60 minutes after the start of the reaction. (c) TEM image of a sample of the 1:1 mixture of acid fuchsin with IAPP/heparan sulfate collected at 60 minutes after the initiation of the reaction. (d) TEM image of a sample of the 10:1 mixture of acid fuchsin with IAPP/heparan sulfate collected at 60 minutes after the initiation of the reaction. Scale bars represent 100 nm. The same solutions were used for the kinetic studies and for the TEM samples. The pH of the solutions was 7.4. The solutions contained 2% HFIP by volume, 20mM Tris-HCl, 25µM thioflavin-T, 32µM peptide and were continually stirred at 25°C.
Fast green FCF inhibits amyloid formation by mature IAPP and proIAPP1–48, but is less effective than acid fuchsin
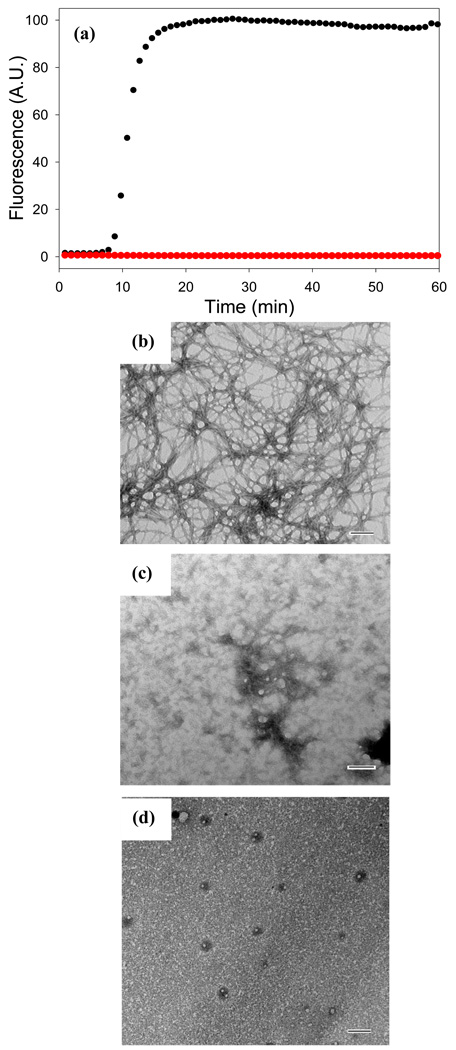
We first examined the ability of fast green FCF to inhibit amyloid formation by mature IAPP. No thioflavin-T fluorescence is observed when fast green FCF is present at a 1:1 ratio of compound to IAPP (Figure 5). TEM images recorded at the end of the kinetic experiment reveal narrow, short, bent structures which differ from classic amyloid fibrils on some portions of the grid and more typical fibrils on others (Figure 5, Supplementary Data). CD spectroscopy reveals that the material contains β-sheet structure (Supplementary Data). We also examined the effects of fast green FCF when it is added in a 10 fold excess relative to IAPP. Inner filter effects prevent thioflavin-T experiments and CD spectra can not be recorded because of the high absorbance of the sample, but no fibrils are observed in the TEM images collected after 60 minutes. The data indicates that fast green FCF does inhibit amyloid formation by mature IAPP, but is less effective than acid fuchsin. Acid fuchsin has been shown to have significant effects even at substoichiometric ratios of compound to mature IAPP and no fibrils like aggregates were detected in a 1:1 sample of acid fuchsin with mature IAPP and CD spectroscopy indicated no significant β-sheet structure formation55.
Figure 5. Fast green FCF inhibits amyloid formation by IAPP.
(a) The effects of fast green FCF on the kinetics of mature IAPP monitored by thioflavin-T fluorescence: IAPP alone (black); a mixture of fast green FCF and IAPP at a ratio of 1:1 (red). (b) TEM image of a sample of IAPP in the absence of inhibitor. (c) TEM image of a 1:1 mixture of fast green FCF and IAPP. (d) TEM image of a 10:1 mixture of fast green FCF and IAPP. Aliquots were removed from solution 60 minutes after the reactions were started. All samples were examined at pH 7.4. Scale bars represent 100 nm. Solutions contained 20 mM Tris-HCl buffer, 25 µM thioflavin-T, 32 µM peptide 2% HFIP, and were continually stirred at 25°C.
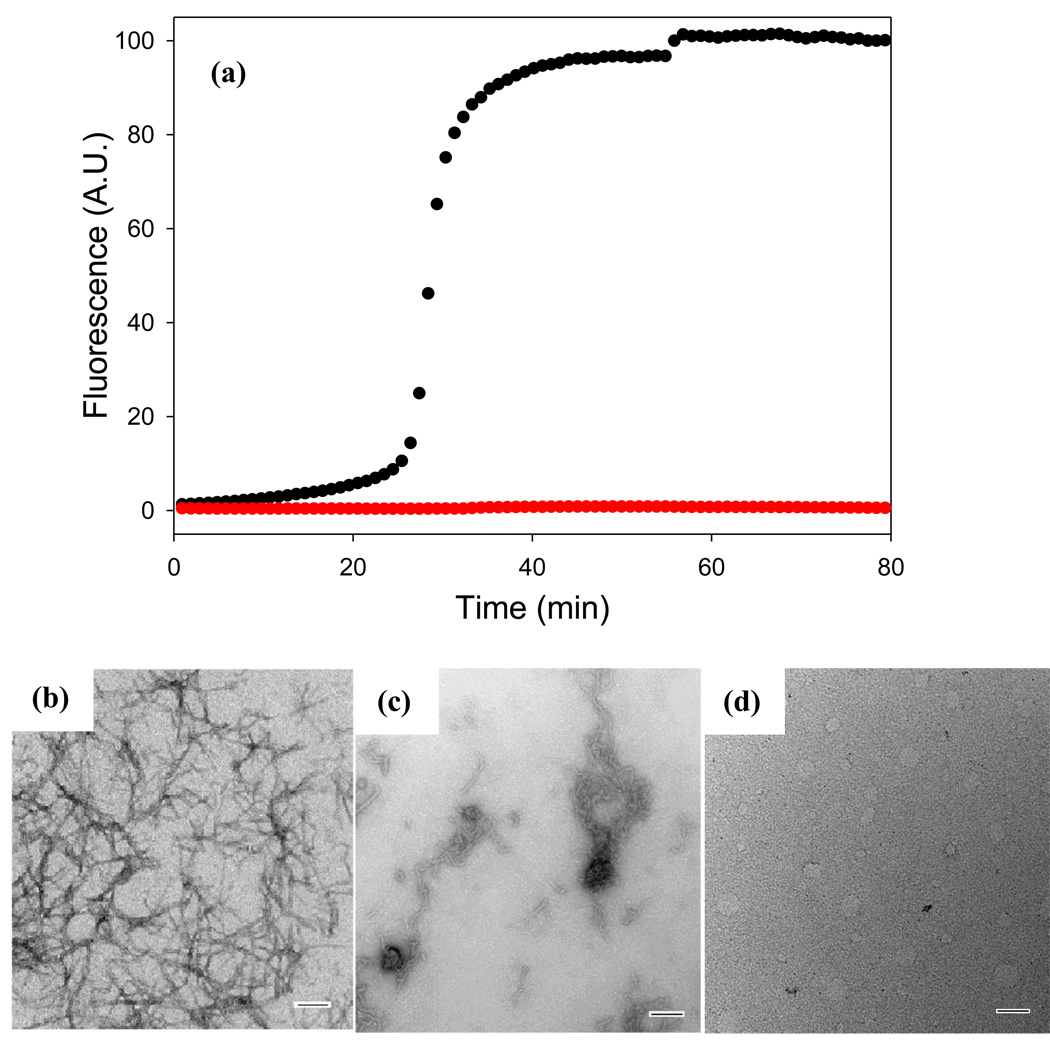
Fast green FCF also inhibits amyloid formation by the proIAPP1–48 intermediate, but again, less effectively than does acid fuchsin. Figure 6 displays the thioflavin-T monitored kinetics of proIAPP1–48 amyloid formation in the presence and in the absence of fast green FCF. The sample of proIAPP1–48 without fast green FCF shows a typical sigmoidal kinetic curve and dense mats of fibrils are observed by TEM. No significant thioflavin-T fluorescence is observed for the 1:1 mixture of fast green FCF with proIAPP1–48 and the TEM images recorded from samples removed at the end of the kinetic run, (80 minutes), are very different from those observed in the absence of inhibitor. Small, bent, elongated structures are detected in most images, while some show more fibril like objects (Figure 6, Supplementary Data). The CD spectrum of the 1:1 mixture of compound with proIAPP1–48 reveals significant β-structure (Supplementary Data). Experiments were also conducted with a 10 fold excess of fast green FCF. Again, the high absorbance of the sample prevents thioflavin-T studies and CD measurements, but TEM images recorded after 80 minutes of incubation were devoid of fibril like structures (Figure 6, Supplementary Data). Thus, the results obtained with proIAPP1–48 are similar to those obtained with mature IAPP; fast green FCF is an inhibitor of amyloid formation, but is less effective than acid fuchsin. It is also worth noting that the studies with fast green FCF provide another example of the difficulty of relying only on thioflavin-T based assays since the thioflavin-T studies would suggest, erroneously, that fast green FCF was as effective at inhibiting amyloid formation as acid fuchsin.
Figure 6. Fast green FCF inhibits amyloid formation by proIAPP1–48.
(a) The effects of fast green FCF on the kinetics of proIAPP1–48 monitored by thioflavin-T fluorescence: proIAPP1–48 alone (black); a mixture of fast green FCF and proIAPP1–48 at a ratio of 1:1 (red). (b) TEM image of proIAPP1–48 in the absence of inhibitor collected at 80 minutes after the initiation of the reaction. (c) TEM image of a mixture of fast green FCF and proIAPP1–48 at a 1:1 ratio collected at 80 minutes after the initiation of the reaction. (d) TEM image of a mixture of fast green FCF and proIAPP1–48 at a 10:1 ratio collected at 80 minutes after the initiation of the reaction. All samples were examined at pH 7.4. Scale bars represent 100 nm. Solutions contained 20 mM Tris-HCl buffer (pH 7.4), 25 µM thioflavin-T, 32 µM peptide, 2% HFIP, and were continually stirred at 25°C. The thioflavin-T curve shown and TEM images displayed for proIAPP1–48 without inhibitor are the same as shown in Figure 4.
Fast green FCF also inhibits amyloid formation by proIAPP1–48 in the presence of heparan sulfate, but is less effective than acid fuchsin
Fast green FCF also has an effect on GAG mediated amyloid formation by proIAPP1–48. Figure 7 displays the results of a kinetic experiment involving a mixture of proIAPP1–48 and heparan sulfate conducted in the presence and absence of compound. Thioflavin-T curves are included for completeness, but as noted above, can be misleading for fast green FCF in the absence of additional data. More informative are the TEM images. The images collected of the 1:1 mixture of fast green FCF with proIAPP1–48 heparan sulfate differ from those recorded for samples which lacked the compound. More pronounced differences are observed when the compound is added in a 10 fold excess (Figure 7, Supplementary Data). Some short fibril like species are detected, but the morphology is different from the fibrils formed in the absence of fast green FCF and they are less prevalent. None-the-less, the TEM data clearly indicates that a 10 fold excess of fast green FCF, while having inhibitory effects, is less effective than acid fuchsin at inhibiting GAG mediated amyloid formation by the mixture of proIAPP1–48 heparan sulfate. CD measurements (Figure 8) show that the proIAPP1–48 heparan sulfate mixture forms β-structure in the presence of a 1:1 mixture of fast green FCF.
Figure 7. Fast green FCF inhibits amyloid formation by mixture of proIAPP1–48 and heparan sulfate.
(a) Time course of amyloid fibril formation monitored by fluorescence detected thioflavin-T binding: proIAPP1–48/heparan sulfate (black); a 1:1 molar ratio mixture of fast green and proIAPP1–48/heparan sulfate (green). (b) TEM image of proIAPP1–48/heparan sulfate in the absence of inhibitor collected at 80 minutes after the initiation of the reaction. (c) TEM image of a 1:1 molar ratio mixture of fast green and proIAPP1–48/heparan sulfate collected at 80 minutes after the initiation of the reaction. (d) TEM image of a 10:1 molar ratio mixture of fast green and proIAPP1–48/heparan sulfate collected at 80 minutes after the initiation of the reaction. The pH of the solutions was 7.4. The solutions contained 2% HFIP by volume, 20mM Tris-HCl, 25 µM thioflavin-T, 32 µM peptide and were continually stirred at 25°C. The thioflavin-T curve shown and TEM images displayed for proIAPP1–48/heparan sulfate without inhibitor are the same as shown in Figure 3.
Figure 8.
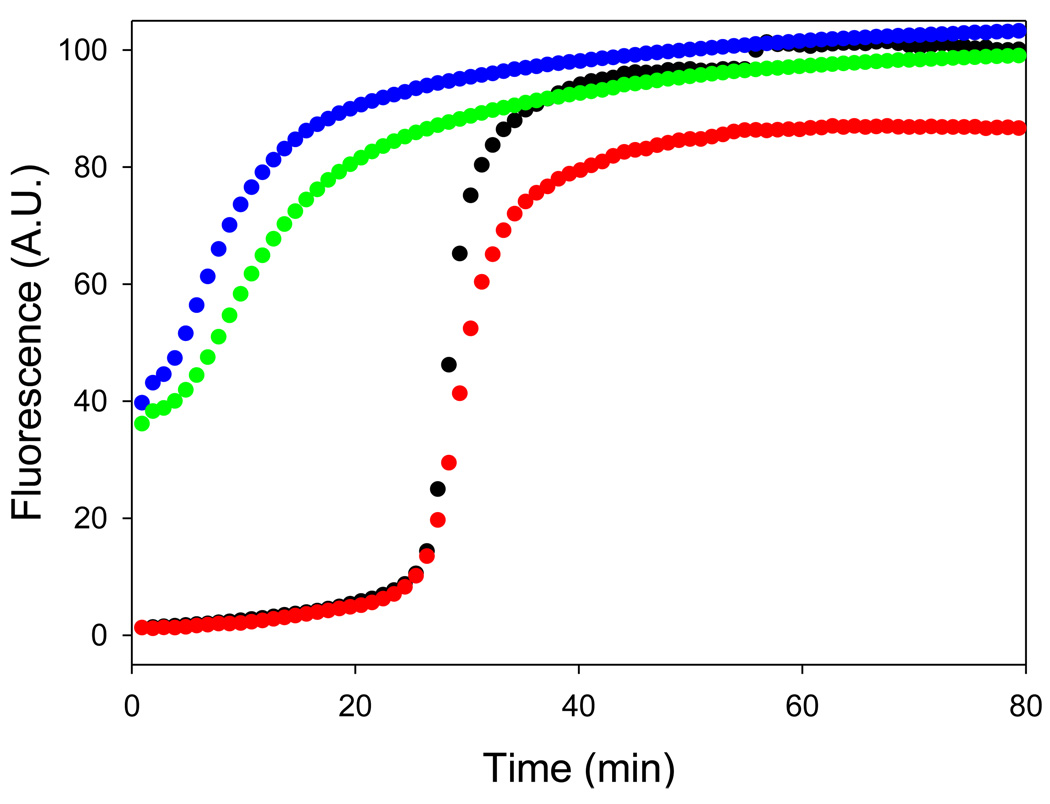
Tramprosate does not inhibit amyloid formation by proIAPP1–48 or by the proIAPP1–48/heparan sulfate mixture. Time course of amyloid fibril formation monitored by fluorescence detected thioflavin-T binding: proIAPP1–48 alone (black); a 40:1 molar ratio mixture of tramprosate and proIAPP1–48 (red); proIAPP1–48/heparan sulfate (green); a 40:1 molar ratio mixture of tramprosate and proIAPP1–48/heparan sulfate (blue). The pH of the solutions was 7.4. The solutions contained 2% HFIP by volume, 20mM Tris-HCl, 25 µM thioflavin-T, 32 µM peptide and were continually stirred at 25°C.
Conclusions
Acid fuchsin and fast green FCF are, to the best of our knowledge, the first compounds which have been shown to inhibit amyloid formation by the proIAPP1–48 processing intermediate and the first compounds demonstrated to inhibit GAG mediated amyloid formation by proIAPP processing intermediates. Acid fuchsin is the more effective inhibitor in both the presence and in the absence of GAGs. Acid fuchsin also inhibits GAG mediated amyloid formation by mature IAPP.
The ability of the compounds to inhibit amyloid formation is likely to be due in part to the presence of sulfonated groups55and electrostatic interactions with the positively charged IAPP appear to be important, but additional features are clearly required since not all sulfonated compounds are inhibitors of proIAPP1–48 amyloid formation. GAGs such as heparan sulfate, for example, clearly accelerate the process. In addition, not all sulfonated small molecules are inhibitors. For example, tramprosate, which is an inhibitor of amyloid formation by the Aβ peptide60, has no detectable effect on amyloid formation by proIAPP1–48 or on amyloid formation by mixtures of proIAPP1–48 and heparan sulfate (Figure 10).
Aromatic aromatic interactions between inhibitors and IAPP have been proposed to be important51; 56 and acid fuchsin may be an effective inhibitor because it combines an aromatic core with multiple sulfonates. The weaker effects observed with fast green FCF may reflect the different relative spacing of the sulfonates.
Materials and Methods
Peptide Synthesis and Purification
Peptides were synthesized on a 0.25 mmol scale using an applied Biosystems 433A peptide synthesizer, by 9-fluornylmethoxycarbonyl (Fmoc). Pseudoprolines were incorporated to facilitate the synthesis61. 5-(4’-fmoc-aminomethyl-3’,5-dimethoxyphenol) valeric acid (PAL-PEG) resin was used to afford an amidated C-terminal. Standard Fmoc reaction cycles were used. The first residue attached to the resin, β-branched residues, residues directly following β-branched residues and pseudoprolines were double coupled. Crude peptides were oxidized by dimethyl sulfoxide (DMSO) for 24 hours at room temperature62. The peptides were purified by reverse-phase HPLC using a Vydac C18 preparative column. Analytical HPLC was used to check the purity of the peptides. The identity of the pure peptides was confirmed by mass spectrometry using a Bruker MALDI-TOF MS (IAPP observed 3904.6, expected 3904.8; proIAPP1–48 observed 5209.5, expected 5209.9).
Sample Preparation
1.58 mM peptide stock solutions were prepared in 100% hexafluoroisopropanol (HFIP) and stored at 4°C. Acid fuchsin and fast green FCF were obtained from Sigma-Aldrich, lot numbers F8129 and F7252 respectively, and dissolved at 1.58 mM in 20 mM Tris-HCl (pH 7.4) buffer. Heparan sulfate was obtained from Sigma-Aldrich. A heparan sulfate stock solution was prepared in 20 mM Tris-HCl (pH 7.4) buffer at 2mg/2.2ml.
Thioflavin-T Fluorescence
All fluorescence experiments were performed with an Applied Photontechnology Fluorescence Spectrophotometer. An excitation wavelength of 450 nm and emission wavelength of 485 nm was used for the thioflavin-T studies57. The excitation and emission slits were set at 6 nm. A 1.0 cm cuvette was used and each point was averaged for 1 minute. Solutions were prepared by diluting filtered stock solution (0.45µm filter) into a Tris-HCl buffered (20mM, pH 7.4) thioflavin-T solution immediately before the measurement. The final composition of the solutions was 32 µM peptide and 25 µM thioflavin-T with or without inhibitor in 2% HFIP. For experiments which involve proIAPP1–48/heparan sulfate mixtures or IAPP/heparan sulfate mixtures, the final conditions were 32 µM peptide, 2.7 µM heparan sulfate and 25 µM thioflavin-T in 20 mM Tris-HCl buffer (pH 7.4) 2% HFIP. All solutions were stirred during the fluorescence experiments.
Transmission Electron Microscopy (TEM)
15 µL samples of peptide solution were removed at the end of the thioflavin-T monitored kinetic experiments and placed on a carbon-coated Formvar 300 mesh copper grid for 1 min and then negatively stained with saturated uranyl acetate for 1 min. The same solutions that were used for the fluorescence measurements were used so that samples could be compared under as similar conditions as possible.
Circular Dichroism Spectroscopy (CD)
CD experiments were conducted using an Applied Photophysics Chirascan circular dichroism spectrometer. Far-UV CD experiments were performed using a 0.1cm quartz cuvette. Wavelength scans were recorded at 25°C, pH 7.4 over a range of 190 to 260nm, at 1nm intervals with an averaging time of 0.5 second and are the result of 5 repeats. Background spectra were subtracted from the collected data. Samples were in pH 7.4, 2% HFIP and 20mM Tris-HCl.
Supplementary Material
Acknowledgements
This work was supported by a grant from the NIH GM078114 to D.P.R.
Abbreviations
- Aβ
the proteolytical fragment of amyloid precursor protein which is responsible for amyloid formation in Alzheimer’s disease.
- CD
circular dichroism spectroscopy
- DMSO
dimethyl sulfoxide
- Fmoc
9-fluorenylmethoxycarbonyl
- GAG
glycosaminoglycan
- HFIP
hexafluoroisopropanol
- HPLC
high performance liquid chromatography
- HSPGs
heparan sulfate proteoglycans
- IAPP
human islet amyloid polypeptide
- MALDI-TOF MS
matrix assisted laser desorption ionization-time of flight mass spectrometry
- PAL-PEG
5-(4’-Fmoc-aminomethyl-3’,5-dimethoxyphenyl) valeric acid
- PC2
subtilisin-like prohormone convertase enzyme 2
- PC(1/3)
subtilisin-like prohormone convertase enzyme 1/3
- proIAPP
pro-islet amyloid polypeptide
- proIAPP1–48
a peptide corresponding to residues 1–48 of human pro-islet amyloid polypeptide
- TEM
transmission electron microscopy
- TFA
trifluoroacetic acid.
References
- 1.Sipe JD. Amyloidosis. Crit. Rev. Clin. Lab. Sci. 1994;31:325–354. doi: 10.3109/10408369409084679. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Cell Biology of Protein Misfolding: The Examples of Alzheimer's and Parkinson's Diseases. Nat. Cell Biol. 2004;6:1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- 3.Westermark P, Wernstedt C, Wilander E, Hayden DW, Obrien TD, Johnson KH. Amyloid Fibrils in Human Insulinoma and Islets of Langerhans of the Diabetic Cat Are Derived from a Neuropeptide-Like Protein Also Present in Normal Islet Cells. Proc. Natl. Acad. Sci. U. S. A. 1987;84:3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper GJS, Willis AC, Clark A, Turner RC, Sim RB, Reid KBM. Purification and Characterization of a Peptide from Amyloid-Rich Pancreases of Type-2 Diabetic-Patients. Proc. Natl. Acad. Sci. U. S. A. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn SE, Andrikopoulos S, Verchere CB. Islet Amyloid: A Long-Recognized but Underappreciated Pathological Feature of Type 2 Diabetes. Diabetes. 1999;48:241–253. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- 6.Clark A, Lewis CE, Willis AC, Cooper GJS, Morris JF, Reid KBM, Turner RC. Islet Amyloid Formed from Diabetes-Associated Peptide May Be Pathogenic in Type-2 Diabetes. Lancet. 1987;2:231–234. doi: 10.1016/s0140-6736(87)90825-7. [DOI] [PubMed] [Google Scholar]
- 7.Hull RL, Westermark GT, Westermark P, Kahn SE. Islet Amyloid: A Critical Entity in the Pathogenesis of Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2004;89:3629–3643. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 8.Marzban L, Park K, Verchere CB. Islet Amyloid Polypeptide and Type 2 Diabetes. Exp. Gerontol. 2003;38:347–351. doi: 10.1016/s0531-5565(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 9.Westermark P, Wernstedt C, Wilander E, Sletten K. A Novel Peptide in the Calcitonin Gene Related Peptide Family as an Amyloid Fibril Protein in the Endocrine Pancreas. Biochem. Biophys. Res. Commun. 1986;140:827–831. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]
- 10.Jaikaran ETAS, Higham CE, Serpell LC, Zurdo J, Gross M, Clark A, Fraser PE. Identification of a Novel Human Islet Amyloid Polypeptide Beta-Sheet Domain and Factors Influencing Fibrillogenesis. J. Mol. Biol. 2001;308:515–525. doi: 10.1006/jmbi.2001.4593. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic-Islet Cell Toxicity of Amylin Associated with Type-2 Diabetes-Mellitus. Nature. 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 12.Clark A, Wells CA, Buly ID, Cruickshank JK, Vanhegan RI, Matthews DR, Cooper GJS, Holman RR, Turner RC. Islet Amyloid, Increased Alpha Cells, Reduced Beta Cells and Exocrine Fibrosis: Quantitative Changes in the Pancreas in Type 2 Diabetes. Diabetes Res. Clin. Pract. 1988;9:151–159. [PubMed] [Google Scholar]
- 13.Rocken C, Linke RP, Saeger W. Immunohistology of Islet Amyloid Polypeptide in Diabetes-Mellitus - Semiquantitative Studies in a Postmortem Series. Virchows Archiv a. 1992;421:339–344. doi: 10.1007/BF01660981. [DOI] [PubMed] [Google Scholar]
- 14.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-Cell Deficit and Increased Beta-Cell Apoptosis in Humans with Type 2 Diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 15.Cooper GJS. Amylin Compared with Calcitonin-Gene-Related Peptide - Structure, Biology, and Relevance to Metabolic Disease. Endocr. Rev. 1994;15:163–201. doi: 10.1210/edrv-15-2-163. [DOI] [PubMed] [Google Scholar]
- 16.Bonner-Weir S, O'Brien TD. Islets in Type 2 Diabetes: In Honor of Dr. Robert C. Turner. Diabetes. 2008;57:2899–2904. doi: 10.2337/db07-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson A, Bohman S, Borg LAH, Paulsson JF, Schultz SW, Westermark GT, Westermark P. Amyloid Deposition in Transplanted Human Pancreatic Islets: A Conceivable Cause of Their Long-Term Failure. Experimental Diabetes Research. 2008;2008 doi: 10.1155/2008/562985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westermark GT, Westermark P, Berne C, Korsgren O, Transpla NNCI. Widespread Amyloid Deposition in Transplanted Human Pancreatic Islets. New Eng J Med. 2008;359:977–979. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]
- 19.Udayasankar J, Kodama K, Hull RL, Zraika S, Aston-Mourney K, Subramanian SL, Tong J, Faulenbach MV, Vidal J, Kahn SE. Amyloid Formation Results in Recurrence of Hyperglycaemia Following Transplantation of Human Iapp Transgenic Mouse Islets. Diabetologia. 2009;52:145–153. doi: 10.1007/s00125-008-1185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potter KJ, Abedini A, Marek P, Klimek AM, Butterworth S, Driscoll M, Baker R, Nilsson MR, Warnock GL, Oberholzer J, Bertera S, Trucco M, Korbutt GS, Fraser PE, Raleigh DP, Verchere CB. Islet Amyloid Deposition Limits the Viability of Human Islet Grafts but Not Porcine Islet Grafts. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4305–4310. doi: 10.1073/pnas.0909024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutton JC. The Insulin Secretory Granule. Diabetologia. 1989;32:271–281. doi: 10.1007/BF00265542. [DOI] [PubMed] [Google Scholar]
- 22.Nishi M, Sanke T, Nagamatsu S, Bell GI, Steiner DF. Islet Amyloid Polypeptide - a New Beta-Cell Secretory Product Related to Islet Amyloid Deposits. J. Biol. Chem. 1990;265:4173–4176. [PubMed] [Google Scholar]
- 23.Marcinkiewicz M, Ramla D, Seidah NG, Chretien M. Developmental Expression of the Prohormone Convertases Pc1 and Pc2 in Mouse Pancreatic-Islets. Endocrinology. 1994;135:1651–1660. doi: 10.1210/endo.135.4.7925129. [DOI] [PubMed] [Google Scholar]
- 24.Furuta M, Yano H, Zhou A, Rouille Y, Holst JJ, Carroll R, Ravazzola M, Orci L, Furuta H, Steiner DF. Defective Prohormone Processing and Altered Pancreatic Islet Morphology in Mice Lacking Active Spc2. Proc. Natl. Acad. Sci. U. S. A. 1997;94:6646–6651. doi: 10.1073/pnas.94.13.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Xu J, Finnerty J, Furuta M, Steiner DF, Verchere CB. The Prohormone Convertase Enzyme 2 (PC2) Is Essential for Processing Pro-Islet Amyloid Polypeptide at the Nh2-Terminal Cleavage Site. Diabetes. 2001;50:534–539. doi: 10.2337/diabetes.50.3.534. [DOI] [PubMed] [Google Scholar]
- 26.Marzban L, Trigo-Gonzales G, Zhu XR, Rhodes CJ, Halban PA, Steiner DF, Verchere CB. Role of Beta-Cell Prohormone Convertase (PC) 1/3 in Processing of Pro-Islet Antyloid Polypeptide. Diabetes. 2004;53:141–148. doi: 10.2337/diabetes.53.1.141. [DOI] [PubMed] [Google Scholar]
- 27.Paulsson JF, Westermark GT. Aberrant Processing of Human Proislet Amyloid Polypeptide Results in Increased Amyloid Formation. Diabetes. 2005;54:2117–2125. doi: 10.2337/diabetes.54.7.2117. [DOI] [PubMed] [Google Scholar]
- 28.Marzban L, Rhodes CJ, Steiner DF, Haataja L, Halban PA, Verchere CB. Impaired Nh2-Terminal Processing of Human Proislet Amyloid Polypeptide by the Prohormone Convertase PC2 Leads to Amyloid Formation and Cell Death. Diabetes. 2006;55:2192–2201. doi: 10.2337/db05-1566. [DOI] [PubMed] [Google Scholar]
- 29.Paulsson JF, Andersson A, Westermark P, Westermark GT. Intracellular Amyloid-Like Deposits Contain Unprocessed Pro-Islet Amyloid Polypeptide (Proiapp) in Beta Cells of Transgenic Mice Overexpressing the Gene for Human Iapp and Transplanted Human Islets. Diabetologia. 2006;49:1237–1246. doi: 10.1007/s00125-006-0206-7. [DOI] [PubMed] [Google Scholar]
- 30.Westermark GT, Steiner DF, Gebre-Medhin S, Engstrom U, Westermark P. Proislet Amyloid Polypeptide Immunoreactivity in the Islets of Langerhans. Upsala J. Med. Sci. 2000;105:97–106. doi: 10.1517/03009734000000057. [DOI] [PubMed] [Google Scholar]
- 31.Westermark P, Engstrom U, Westermark GT, Johnson KH, Permerth J, Betsholtz C. Islet Amyloid Polypeptide (Iapp) and Pro-Iapp Immunoreactivity in Human Islets of Langerhans. Diabetes Res. Clin. Pract. 1989;7:219–226. doi: 10.1016/0168-8227(89)90008-9. [DOI] [PubMed] [Google Scholar]
- 32.Marzban L, Tomas A, Becker TC, Rosenberg L, Oberholzer J, Fraser PE, Halban PA, Verchere CB. Small Interfering Rna-Mediated Suppression of Proislet Amyloid Polypeptide Expression Inhibits Islet Amyloid Formation and Enhances Survival of Human Islets in Culture. Diabetes. 2008;57:3045–3055. doi: 10.2337/db08-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park K, Verchere CB. Identification of a Heparin Binding Domain in the N-Terminal Cleavage Site of Pro-Islet Amyloid Polypeptide - Implications for Islet Amyloid Formation. J. Biol. Chem. 2001;276:16611–16616. doi: 10.1074/jbc.M008423200. [DOI] [PubMed] [Google Scholar]
- 34.Abedini A, Tracz SM, Cho JH, Raleigh DP. Characterization of the Heparin Binding Site in the N-Terminus of Human Pro-Islet Amyloid Polypeptide: Implications for Amyloid Formation. Biochemistry. 2006;45:9228–9237. doi: 10.1021/bi0510936. [DOI] [PubMed] [Google Scholar]
- 35.Meng F, Abedini A, Song B, Raleigh DP. Amyloid Formation by Pro-Islet Amyloid Polypeptide Processing Intermediates: Examination of the Role of Protein Heparan Sulfate Interactions and Implications for Islet Amyloid Formation in Type 2 Diabetes. Biochemistry. 2007;46:12091–12099. doi: 10.1021/bi7004834. [DOI] [PubMed] [Google Scholar]
- 36.Young ID, Ailles L, Narindrasorasak S, Tan R, Kisilevsky R. Localization of the Basement-Membrane Heparan-Sulfate Proteoglycan in Islet Amyloid Deposits in Type-Ii Diabetes-Mellitus. Arch. Pathol. Lab. Med. 1992;116:951–954. [PubMed] [Google Scholar]
- 37.Watson DJ, Lander AD, Selkoe DJ. Heparin-Binding Properties of the Amyloidogenic Peptides a Beta and Amylin - Dependence on Aggregation State and Inhibition by Congo Red. J. Biol. Chem. 1997;272:31617–31624. doi: 10.1074/jbc.272.50.31617. [DOI] [PubMed] [Google Scholar]
- 38.Castillo GM, Cummings JA, Yang WH, Judge ME, Sheardown MJ, Rimvall K, Hansen JB, Snow AD. Sulfate Content and Specific Glycosaminoglycan Backbone of Perlecan Are Critical for Perlecan's Enhancement of Islet Amyloid Polypeptide (Amylin) Fibril Formation. Diabetes. 1998;47:612–620. doi: 10.2337/diabetes.47.4.612. [DOI] [PubMed] [Google Scholar]
- 39.Snow AD, Bramson R, Mar H, Kisilevsky R, Hassell JR, Kimata K, Wight TN. Immuno-Identification and Localization of Heparin Sulfate Proteoglycans to Aa Amyloid Fibrils in an Experimental Mouse Model of Inflammation-Associated Amyloidosis. Clin. Res. 1989;37:A223–a223. [Google Scholar]
- 40.Inoue S. Basement Membrane and Beta Amyloid Fibrillogenesis in Alzheimer's Disease. International Review of Cytology - a Survey of Cell Biology, Vol 210. 2001;210:121–161. doi: 10.1016/s0074-7696(01)10005-7. [DOI] [PubMed] [Google Scholar]
- 41.Potter-Perigo S, Hull RL, Tsoi C, Braun KR, Andrikopoulos S, Teague J, Verchere CB, Kahn SE, Wight TN. Proteoglycans Synthesized and Secreted by Pancreatic Islet Beta-Cells Bind Amylin. Arch. Biochem. Biophys. 2003;413:182–190. doi: 10.1016/s0003-9861(03)00116-4. [DOI] [PubMed] [Google Scholar]
- 42.Hull RL, Zraika S, Udayasankar J, Kisilevsky R, Szarek WA, Wight TN, Kahn SE. Inhibition of Glycosaminoglycan Synthesis and Protein Glycosylation with Was-406 and Azaserine Result in Reduced Islet Amyloid Formation in Vitro. American Journal of Physiology-Cell Physiology. 2007;293:C1586–C1593. doi: 10.1152/ajpcell.00208.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snow AD, Wight TN. Proteoglycans in the Pathogenesis of Alzheimers-Disease and Other Amyloidoses. Neurobiology of Aging. 1989;10:481–497. doi: 10.1016/0197-4580(89)90108-5. [DOI] [PubMed] [Google Scholar]
- 44.Blazer LL, Neubig RR. Small Molecule Protein-Protein Interaction Inhibitors as Cns Therapeutic Agents: Current Progress and Future Hurdles. Neuropsychopharmacology. 2009;34:126–141. doi: 10.1038/npp.2008.151. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi T, Mihara H. Peptide and Protein Mimetics Inhibiting Amyloid Beta-Peptide Aggregation. Acc. Chem. Res. 2008;41:1309–1318. doi: 10.1021/ar8000475. [DOI] [PubMed] [Google Scholar]
- 46.Feng BY, Toyama BH, Wille H, Colby DW, Collins SR, May BCH, Prusiner SB, Weissman J, Shoichet BK. Small-Molecule Aggregates Inhibit Amyloid Polymerization. Nat. Chem. Biol. 2008;4:197–199. doi: 10.1038/nchembio.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abedini A, Meng FL, Raleigh DP. A Single-Point Mutation Converts the Highly Amyloidogenic Human Islet Amyloid Polypeptide into a Potent Fibrillization Inhibitor. J. Am. Chem. Soc. 2007;129 doi: 10.1021/ja072157y. 11300-+ [DOI] [PubMed] [Google Scholar]
- 48.Yan LM, Tatarek-Nossol M, Velkova A, Kazantzis A, Kapurniotu A. Design of a Mimic of Nonamyloidogenic and Bioactive Human Islet Amyloid Polypeptide (Iapp) as Nanomolar Affinity Inhibitor of Iapp Cytotoxic Fibrillogenesis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2046–2051. doi: 10.1073/pnas.0507471103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lansbury PT, Lashuel HA. A Century-Old Debate on Protein Aggregation and Neurodegeneration Enters the Clinic. Nature. 2006;443:774–779. doi: 10.1038/nature05290. [DOI] [PubMed] [Google Scholar]
- 50.Mishra R, Bulic B, Sellin D, Jha S, Waldmann H, Winter R. Small-Molecule Inhibitors of Islet Amyloid Polypeptide Fibril Formation. Angew. Chem. Inter. Ed. 2008;47:4679–4682. doi: 10.1002/anie.200705372. [DOI] [PubMed] [Google Scholar]
- 51.Porat Y, Abramowitz A, Gazit E. Inhibition of Amyloid Fibril Formation by Polyphenols: Structural Similarity and Aromatic Interactions as a Common Inhibition Mechanism. Chemical Biology & Drug Design. 2006;67:27–37. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 52.Mishra R, Sellin D, Radovan D, Gohlke A, Winter R. Inhibiting Islet Amyloid Polypeptide Fibril Formation by the Red Wine Compound Resveratrol. ChemBioChem. 2009;10 doi: 10.1002/cbic.200800762. 445-+ [DOI] [PubMed] [Google Scholar]
- 53.Cao P, Meng F, Abedini A, Raleigh DP. The Ability of Rodent Islet Amyloid Polypeptide to Inhibit Amyloid Formation by Human Islet Amyloid Polypeptide Has Important Implications for the Mechanism of Amyloid Formation and the Design of Inhibitors. Biochemistry. 2010;49:872–881. doi: 10.1021/bi901751b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scrocchi LA, Chen Y, Wang F, Han K, Ha K, Wu L, Fraser PE. Inhibitors of Islet Amyloid Polypeptide Fibrillogenesis, and the Treatment of Type-2 Diabetes. Lett. Pept. Sci. 2003;10:545–551. [Google Scholar]
- 55.Meng F, Abedini A, Plesner A, Middleton CT, Potter KJ, Zanni MT, Verchere CB, Raleigh DP. The Sulfated Triphenyl Methane Derivative Acid Fuchsin Is a Potent Inhibitor of Amyloid Formation by Human Islet Amyloid Polypeptide and Protects against the Toxic Effects of Amyloid Formation. J. Mol. Biol. 2010;400:555–566. doi: 10.1016/j.jmb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Porat Y, Mazor Y, Efrat S, Gazit E. Inhibition of Islet Amyloid Polypeptide Fibril Formation: A Potential Role for Heteroaromatic Interactions. Biochemistry. 2004;43:14454–14462. doi: 10.1021/bi048582a. [DOI] [PubMed] [Google Scholar]
- 57.Levine H. Thioflavine-T Interaction with Amyloid Beta-Sheet Structures. Amyloid. 1995;2:1–6. [Google Scholar]
- 58.Yonemoto IT, Kroon GJA, Dyson HJ, Balch WE, Kelly JW. Amylin Proprotein Processing Generates Progressively More Amyloidogenic Peptides That Initially Sample the Helical State. Biochemistry. 2008;47:9900–9910. doi: 10.1021/bi800828u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meng FL, Marek P, Potter KJ, Verchere CB, Raleigh DP. Rifampicin Does Not Prevent Amyloid Fibril Formation by Human Islet Amyloid Polypeptide but Does Inhibit Fibril Thioflavin-T Interactions: Implications for Mechanistic Studies Beta-Cell Death. Biochemistry. 2008;47:6016–6024. doi: 10.1021/bi702518m. [DOI] [PubMed] [Google Scholar]
- 60.Gervais F, Paquette J, Morissette C, Krzywkowski P, Yu M, Azzi M, Lacombe D, Kong XQ, Aman A, Laurin J, Szarek WA, Tremblay P. Targeting Soluble a Beta Peptide with Tramiprosate for the Treatment of Brain Amyloidosis. Neurobiology of Aging. 2007;28:537–547. doi: 10.1016/j.neurobiolaging.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 61.Abedini A, Raleigh DP. Incorporation of Pseudoproline Derivatives Allows the Facile Synthesis of Human Iapp, a Highly Amyloidogenic and Aggregation-Prone Polypeptide. Organic Letters. 2005;7:693–696. doi: 10.1021/ol047480+. [DOI] [PubMed] [Google Scholar]
- 62.Abedini A, Singh G, Raleigh DP. Recovery and Purification of Highly Aggregation-Prone Disulfide-Containing Peptides: Application to Islet Amyloid Polypeptide. Anal. Biochem. 2006;351:181–186. doi: 10.1016/j.ab.2005.11.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.