Abstract
Sequential conversion of estradiol-17β to its biologically active catecholestradiols 2-hydroxyestradiol (2-OHE2) and 4-hydroxyestradiol (4-OHE2) contributes importantly to its angiogenic effects on uterine artery endothelial cells derived from pregnant (P-UAECs), but not nonpregnant (NP-UAECs) ewes via estrogen receptor-independent mechanism. Because catecholestradiols and catecholamines exhibit structural similarities and have high affinity for α- and β-adrenergic receptors (ARs), we investigated if the endothelial α- or β-ARs mediate catecholestradiols-induced proliferation of P-UAECs and whether catecholamines alter these responses. Western analyses revealed expression of specific AR subtypes in NP-UAECs and P-UAECs including α2-, β2- and β3-ARs; not α1- and β1-ARs. Levels of β2-ARs and β3-ARs were unaltered by pregnancy; whereas α2-ARs were decreased. Norepinephrine and epinephrine increased P-UAEC, but not NP-UAEC proliferation and these effects were suppressed by propranolol (β-AR blocker) but not phentolamine (α-AR blocker). Catecholamines combinations with 2-OHE2 or 4-OHE2 enhanced P-UAEC mitogenesis. Catecholestradiol-induced P-UAECs proliferation was also inhibited by propranolol but not phentolamine. β2-AR and β3-AR antagonists (ICI 118,551and SR 59230A respectively) abrogated the mitogenic effects of both 2-OHE2 and 4-OHE2. Stimulation of β2-ARs and β3-ARs using Formoterol and BRL37344 dose-dependently stimulated P-UAEC proliferation which was abrogated by ICI 118,551and SR 59230A, respectively. Proliferation effects of both catecholamines and catecholestradiols were only observed in P-UAEC (not NP-UAEC) and were mediated via β2-ARs and β3-ARs. We demonstrate for the first time convergence of the endothelial AR and estrogenic systems in the regulating endothelial proliferation, thus providing a distinct evolutionary advantage for modulating uterine perfusion during stressful pregnancies.
Keywords: catecholamines, catecholestradiols, adrenergic receptors, endothelial proliferation
Introduction
Estradiol-17β (E2β) has physiologic/pathophysiologic effects on the cardiovascular system via diverse mechanisms including local conversion to catecholestradiols 2-hydroxyestradiol (2-OHE2) and 4-hydroxyestradiol (4-OHE2 by cytochrome P450s (CYP450s).1,2 Catecholestradiols improve endothelial function and alleviate hypertension in ZSF1 rats; animal model for hypertension and metabolic syndrome.2,3 We reported that E2β, 2-OHE2 and 4-OHE2 increase proliferation in ovine uterine artery endothelial cells derived from pregnant (P-UAECs) but not the nonpregnant ewes (NP-UAECs).4 Unlike E2β, 2-OHE2 and 4-OHE2 induced P-UAEC proliferation were not blocked by ICI-182,780 demonstrating that catecholestradiols induce P-UAEC proliferation was estrogen receptor (ER)-independent.4
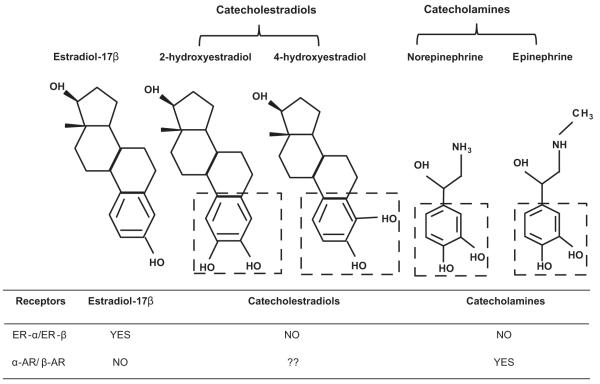
Catecholestradiols exhibit close structural similarities (Figure 1) and functional interactions with the norepinephrine and epinephrine.5 Owing to the shared phenolic A ring “catechol” moiety, catecholestradiols interact directly with catecholamine responses, a property not shared by E2β.5 Catecholestradiols compete with high affinity for binding to neuroendocrine enzymes and α-adrenergic receptors (ARs) and β-ARs in hypothalamus, anterior pituitary, corpus striatum and liver 5,6,7,8,9,10. 3D structural and functional analyses demonstrate that catecholamines “catechol” moiety is functionally important in AR activation.11,12 Thus, it is conceivable that the ER-independent mitogenic effects of 2-OHE2 and 4-OHE2 on the uterine endothelium may be mediated by α-ARs or β-ARs and they may directly interact with the catecholamines which endogenously activate ARs. There are no reports on the potential role of an endothelial AR system in catecholestradiol-induced proliferation.
Figure 1.
Adding one OH group modifies the estrogenic phenolic A ring forming “catechol” moieties potentially making it an “AR-mimetic” agent for inducing ER-independent angiogenesis. The boxes outline shared phenolic A ring “catechol” moiety between 2-hydroxyestradiol, 4-hydroxyestradiol, norepinephrine and epinephrine. ?? indicates hypotheses tested.
We hypothesized that catecholestradiols stimulate P-UAECs proliferation via α-ARs and/or β-ARs and that catecholamines which directly activate these ARs will alter these responses. We investigated: 1)NP-UAEC versus P-UAEC subtype specific expression of α1-, α2-, β1-, β2- and β3-ARs; 2) whether norepinephrine and epinephrine stimulate NP-UAEC versus P-UAEC proliferation and the interactive effects of catecholamines and catecholestradiol in mitogenesis; 3) whether catecholestradiols and catecholamines stimulate P-UAEC proliferation via α-ARs or β-ARs; 5) if ARs exhibit subtype specificity in catecholestradiol-induced P-UAEC proliferation; and 6) subtype specific pharmacological activation of ARs on P-UAEC proliferation. We report for the first time specific β2-AR and β3-AR subtype-mediated mechanisms for the novel convergence of the endothelial AR system with estrogen metabolites in regulating P-UAEC not NP-UAEC proliferation. We discovered in P-UAECs specific a co-dependence via conserved catechol phenolic moieties (derivatives of both E2β and tyrosine) of a unique β-AR coupled system. This may provide for a distinct gestational evolutionary advantage by modulating uterine angiogenesis to help/maintain fetal developmental well-being during periods of repeated bouts of stress releases of catecholamines as seen during “fight or flight” responses.
Methods
For complete details, please see http://hyper.ahajournals.org.
Cell Preparation and Culture
Protocols were approved by the University of Wisconsin-Madison Animal Care Committee.4,13 NP-UAECs and P-UAECs were isolated and cultured from nonpregnant (n=6) and late gestation (n=6) ewes.4 At passage 4 (~70%) confluence, UAECs were lysed for Western Analyses or transferred to 96-well plates for experimental treatments.
Western Analyses
Western Analyses were performed 4 using rabbit anti-α1-AR, anti-α2-AR, anti-β1-AR, anti-β2-AR, or anti-β3-AR antibodies (1:500) and secondary antibodies (1:2000). β-actin/GAPDH used as loading controls.
5-Bromodeoxyuridine Cell Proliferation Assays
5-Bromodeoxyuridine assay was performed and validated as previously described. 4
Experimental Treatments
Proliferation experiments were performed in quadruplicates and replicated in ≥ four preparations. NP-UAECs and P-UAECs in 96-well plates were serum starved (24 hours) and washed in endothelial basal media (EBM) and medium was replaced with EBM containing 0, 0.1, 1, 10 or 100 nmol/L norepinephrine or epinephrine (24 hours). Additional P-UAEC studies investigating interactions were performed by combination treatments (0.1 nmol/L) of 2-OHE2 or 4-OHE2 with norepinephrine or epinephrine. α-ARs and/or β-ARs were blocked nonselectively by pretreating P-UAECs (10 μmol/L; 1 hour) with phentolamine (α-AR blocker) or propranolol (β-AR blocker) propranolol followed by norepinephrine, epinephrine or 2-OHE2 or 4-OHE2 (0.1 nmol/L; 24 hours). Based on Western analyses of specific AR subtypes, we conducted AR subtype specific blockade using (10 μmol/L; 1 hour) yohimbine (α2-AR), ICI 118,551(β2-AR) and SR 59230A (β3-AR) followed by catecholestradiol treatments (0.1 nmol/L; 24 hours). We validated AR-subtype specific inhibition for P-UAEC mitogenic responses (0, 0.1, 1, 10, 100 nmol/L ) using specific β2-AR agonist Formoterol and β3-AR agonist BRL 37344. ICI 118,551 and SR 59230A (1 μmol/L) effects respectively on Formoterol and BRL 37344 (100 nmol/L) for specificity validation of β2-AR and β3-AR agonists in P-UAECs. For antagonists/agonist specificities, please see http://hyper.ahajournals.org.
Statistical Analysis
Data as Means ± SEM are presented as a fold of untreated control. Overall group differences were determined by one-way or two-way ANOVA (SigmaPlot 11 Statistical Software) followed by post hoc multiple pairwise comparison Student-Newman-Keuls/Bonferroni tests. Level of significance was established a priori at P<0.05.
Results
P-UAECs Express Distinct Adrenergic Receptor Subtypes
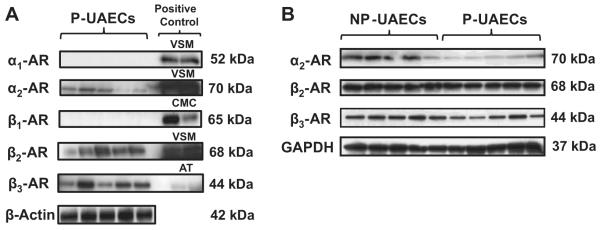
Western immunoblotting showed α2-AR, β2-AR and β3-AR not α1-AR or β1-AR subtypes in P-UAECs (Figure 2A). Positive controls show protein expressions of α1-ARs, α2-ARs, β1-ARs, β2-ARs and β3-ARs in vascular smooth muscle cells (VSM), left ventricular cardiomyocytes (CMC) and adipose tissue (AT), thus validating the absence of α1-ARs or β1-ARs. Western analyses and densitometric analyses (data not shown) showed the equal expression of β2-ARs and β3-ARs between NP-UAECs and P-UAECs; however, α2-ARs were higher in NP-UAECs versus P-UAECs.
Figure 2. AR subtypes in UAECs.
(A) Western blots demonstrating expression of α2-ARs, β2-ARs and β3-ARs; but not α1-ARs or β1-ARs subtypes in P-UAECs. Positive control lanes are vascular smooth muscle cells (VSM), left ventricle cardiomyocytes (CMC) and adipose tissue (AT). (B) Expression α2-ARs, β2-ARs and β3-ARs in NP-UAECs versus P-UAECs. Blots are representative of two independent experiments from individual UAEC cell lines.
Catecholamines Increase P-UAEC but not NP-UAEC Proliferation and Increase Catecholestradiol-Induced of P-UAECs Proliferation
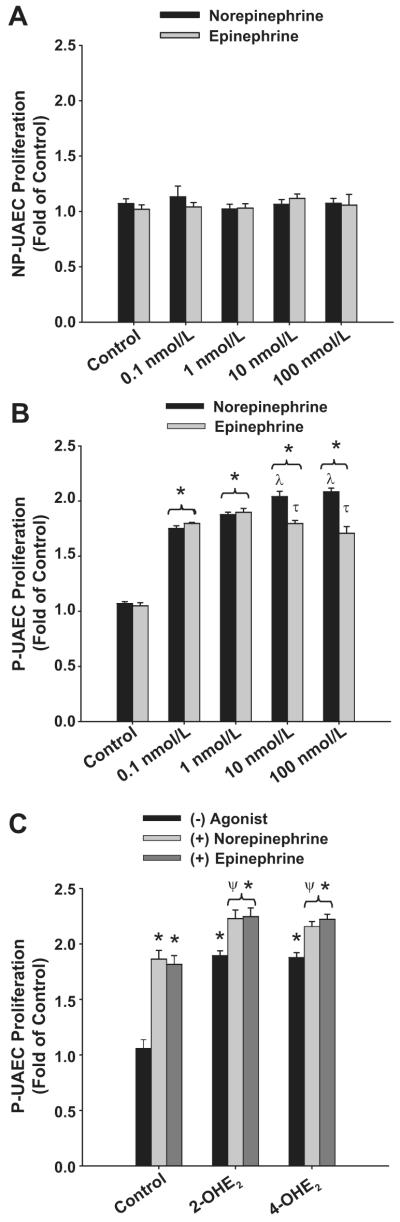
Neither norepinephrine nor epinephrine stimulated in NP-UAECs proliferation (Figure 3A). Concentration-dependent norepinephrine responses were observed in P-UAEC (Figure 3B) with maximum proliferation (2.08 ± 0.03 fold) observed at 100 nmol/L. At a low physiologic concentrations (0.1 nmol/L) norepinephrine significantly elevated proliferation (1.78 ± 0.028 fold). The highest P-UAEC proliferative responses to epinephrine were 1.89 ± 0.035 fold observed at 1 nmol/L; however, at 10 and 100 nmol/L, there was no further increase in proliferation to epinephrine and this was slightly less than that was observed with norepinephrine.
Figure 3. Catecholamines stimulate of P-UAECs but not NP-UAECs Proliferation and Augement Catecholestradiol-induced P-UAEC Proliferation.
(A) Concentration response of NP-UAECs to 0, 0.1, 1, 10 and 100 nmol/L norepinephrine and epinephrine (two-way ANOVA; concentration x group; F8,45 = 0.306, P = 0.960; n = 6). (B) Concentration response of P-UAECs to 0, 0.1, 1, 10 and 100 nmol/L norepinephrine and epinephrine (two-way ANOVA; concentration x group; F8,45 = 10.52, P < 0.001; n = 6). (C) Combinations of 0.1 nmol/L norepinephrine or epinephrine with either 2-OHE2 or 4-OHE2 augmented P-UAEC proliferation responses (two-way ANOVA; group x agonist; F4,27 = 3.73, P = 0.015; n = 4) *Increase vs. control. λ Increase vs. 0.1 and 1 nmol/L. τ norepinephrine > epinephrine. ψ Increase vs. catecholestradiols or catecholamines alone
We then determined their interactive effects using combination treatments (0.1 nmol/L) of norepinephrine or epinephrine with 2-OHE2 or 4-OHE2 (Figure 3C). At this dose, the magnitude of P-UAEC proliferative responses to catecholamines were similar to catechoestradiols (1.86± 0.02-, vs. 1.81± 0.02-fold, respectively). Combination treatments with norepinephrine or epinephrine with either 2-OHE2 or 4-OHE2 further increased P-UAEC proliferation versus either catecholamine or catecholestradiol treatments alone (P < 0.05). Proliferative responses for combination treatments of norepinephrine or epinephrine with either 2-OHE2 (2.22 ± 0.07- and 2.24±0.07-fold, respectively) or 4-OHE2 (2.15±0.07- and 2.23±0.07-fold, respectively).
β-ARs but not α-ARs Mediate Catecholamine and Catecholestradiol-Induced P-UARC Proliferation
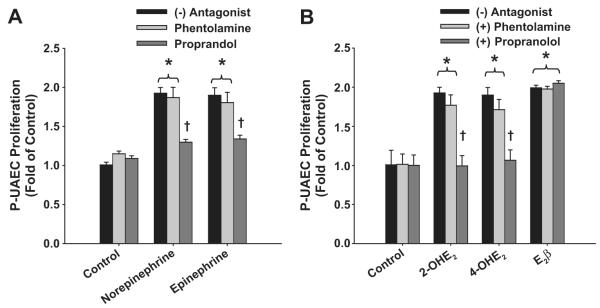
Phentolamine and propranolol antagonism (10μmol/L) of P-UAEC proliferation using both 0.1 (Figure 4A) and 100 nmol/L (not shown) doses of catecholamines yielded identical results. Neither antagonist alone altered basal P-UAEC proliferation. Increases in proliferation seen with norepinephrine or epinephrine were unaltered (P >0.05) by nonselective inhibition of α-ARs using phentolamine. In contrast, nonselective blockade of β-ARs using propranolol completely abrogated catecholamine mediated P-UAEC proliferation (P < 0.05).
Figure 4. β-ARs, but not α-ARs, mediate Catecholamine and Catecholestradiol-induced P-UAEC Proliferation.
(A) Effects of phentolamine or propranolol on P-UAECs proliferation to 0.1 nmol/L norepinephrine or epinephrine. Phentolamine had no effect; whereas propranolol completely abrogated catecholamine-induced P-UAEC proliferation (two-way ANOVA; antagonist x group; F8,45 = 9.12, P < 0.001; n = 4). (B) Effects of phentolamine or propranolol on P-UAECs proliferative responses to 2-OHE2, 4-OHE2 or E2β (0.1 nmol/L). Phentolamine had no effect; whereas propranolol completely abrogated 2-OHE2- and 4-OHE2-, but not E2β-induced P-UAECs proliferative responses (two-way ANOVA; antagonist x group; F6,36 = 7.88, P < 0.001; n = 4). *Increase vs. untreated. † Complete inhibition.
Confirming our previous report,4 the magnitude of proliferation of P-UAECs in responses to 2-OHE2 (1.89±0.02-fold), and 4-OHE2 (1.88±0.02-fold) were similar (Figure 4B). Increases in P-UAEC proliferation seen with 0.1 nmol/L 2-OHE2 and 4-OHE2 were unaltered (P >0.05) by nonselective inhibition of α-ARs using phentolamine. In contrast, nonselective blockade of β-ARs using propranolol completely abrogated catecholestradiol-mediated P-UAEC proliferation (P < 0.05). To determine the putative role of adrenergic G-protein coupled receptors in E2β-induced P-UAEC proliferation, we evaluated these α-AR and β-AR antagonists on E2β-induced proliferation of P-UAECs. The E2β-induced rise (0.1 nmol/L) in P-UAEC proliferations was not altered (P >0.05) by either phentolamine or propranolol.
β2-ARs and to a Lesser Extent β3-ARs Mediate Catecholestradiol-Induced P-UAEC Proliferation
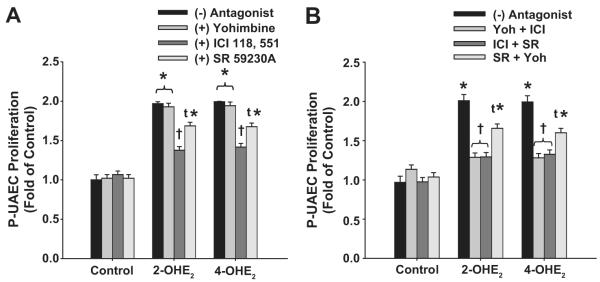
We then evaluated subtype specific α-AR and β-AR inhibition (10 μmol/L) on the P-UAEC proliferative responses to catecholestradiols. None of the inhibitors alone altered basal control P-UAEC proliferation (Figure 5A). Inhibition of α2-AR subtype with Yohimbine did not inhibit 2-OHE2 and 4-OHE2-induced P-UAEC proliferation. In contrast, the selective antagonists of β2-AR (ICI 118,551) or β3-AR (SR 59230A) respectively either blocked (P < 0.01) or only partially attenuated (P<0.05) the proliferation induced by 0.1 nmol/L 2-OHE2 and 4-OHE2. .
Figure 5. β2-ARs and to a Lesser Extent β3-ARs Mediate Catecholestradiol-induced P-UAEC Proliferation.
(A) Effects of yohimbine, ICI 118,551 or SR59230A on P-UAEC proliferation to 2-OHE2 or 4-OHE2 (0.1 nmol/L). Yohimbine had no effect; whereas ICI 118,551 attenuated and SR59230A partially inhibited catecholestradiol-mediated P-UAEC proliferation (two-way ANOVA; antagonist x group; F8,33= 7.871, P < 0.001; n = 4). (B) Effects of yohimbine, ICI 118,551, and SR59230A combinations on P-UAEC proliferative responses to catecholestradiols. ICI 118,551 in all combinations completely blocked P-UAEC proliferation responses to catecholestradiols (two-way ANOVA; antagonist combination x group; F8,33 = 9.551, P < 0.001; n = 4). *Increase vs. untreated. † Complete inhibition. τ Partial inhibition.
We further evaluated additive effects of AR subtypes and putative AR heterodimerization in regulating catecholestradiol-mediated P-UAEC proliferation (Figure 5B). Combination of ICI 118,551 with either yohimbine or SR 59230A inhibited catecholestradiol-induced proliferation of P-UAECs demonstrating primary involvement of β2-ARs. In contrast combination of SR59230A and yohimbine only partially decreased catecholestradiol-induced P-UAEC proliferation demonstrating only partial β3-AR subtype involvement. These combination inhibitor studies neither support dimerization nor significant cross talk between these AR subtypes.
Stimulation of β2- and β3-ARs Promote P-UAEC Proliferation
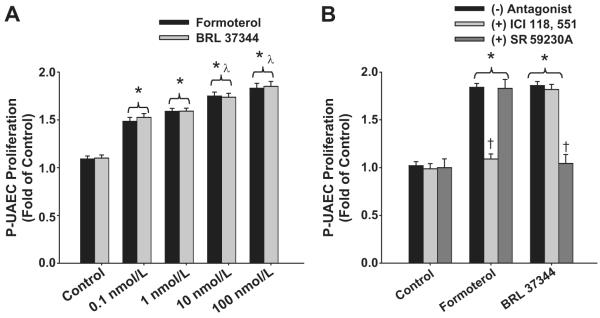
To further evaluate β2-ARs vs. β3-ARs, we tested the actions of specific β-AR agonists. Both of the subtype specific β2-AR (Formoterol) and β3-AR agonist (BRL 37344) produced concentration-dependent and similar P-UAEC proliferative responses (Figure 6A). Formoterol and BRL 37344 (100 nmol/L) produced maximal P-UAEC proliferations of 1.89 ± 0.07- and 1.90 ± 0.07-fold respectively. We then validated specificities of these agonists on their respective ARs (Figure 6B) P-UAEC proliferation by Formoterol was completely attenuated by β2-AR (ICI 118,551) but not β3-AR (SR 59230A) antagonist. β3-AR antagonist completely abrogated responses by BRL 37344, not by Formoterol.
Figure 6. Stimulation of β2- and β3-ARs promotes P-UAEC proliferation.
(A) Concentration response of P-UAECs to 0, 0.1, 1, 10 and 100 nmol/L of β2- and β3-AR agonists Formoterol and BRL 37344 (two-way ANOVA; concentration x group; F4,30 = 3.01, P < 0.001; n = 6). (B) Effects of ICI 118, 551 or SR59230A on P-UAEC proliferative responses to Formoterol and BRL 37344 (two-way ANOVA; antagonist x group; F8,45 = 20.53, P < 0.001; n = 6). *Increase vs. untreated. λ Increase vs. 0.1 and 1 nmol/L. † Complete inhibition.
Discussion
Recently we reported that unlike their parent substrate hormone E2β, 2-OHE2 and 4-OHE2 do not stimulate P-UAEC proliferation via classical ERs.4 Herein we hypothesized that catecholestradiols mediate P-UAEC proliferation via either α-ARs or β-ARs and that the catecholamines will modify/interact with these proliferative effects. We describe the first report of a complete and coupled AR system in P-UAECs (not NP-UAECs) that are responsible for catecholestradiol- and catecholamine-mediated proliferation, a critical process for angiogenic-mediated uterine perfusion during gestation. These data provide a novel previously not described model by which estrogen metabolites function as potential circulating β-AR mimetic agonists. Therefore, modifying phenolic A ring of estrogens to a “catechol” moieties generate an endogenous β-mimetic agent with angiogenic and possibly other cardiovascular capabilities. Our key findings are that: 1) in NP-UAECs and P-UAECs there are distinct AR subtypes expressed including α2-ARs, β2-ARs and β3-ARs but only in P-UAECs do norepinephrine and epinephrine increase proliferation; 2) catecholamines play a complementary and a conserved role to 2-OHE2 and 4-OHE2 by acting as positive modulators of P-UAEC proliferation; 3) neither catecholestradiols nor catecholamines induce P-UAEC proliferation α-ARs, but rather solely via β-ARs; 4) 2-OHE2 and 4-OHE2 modulate P-UAEC proliferation primarily via β2-ARs and to a lesser-extent via β3-ARs; and 5) pharmacologic agonists for either β2-ARs or β3-ARs specifically stimulate P-UAEC proliferation suggesting similar coupling mechanisms and/or signaling convergence.
We report, for the first time, in vitro expressions of several specific AR subtypes α2-ARs, β2-ARs, and β3-ARs in NP-UAECs and P-UAECs, findings consistent with reports demonstrating distinct individual AR subtypes in endothelia of aorta, choroid, placenta, femoral artery and retina.14,15,16,17,18,19 When compared to NP-UAECs, β2-AR and β3-AR expressions were unaltered by pregnancy status, whereas α2-ARs were reduced. It is unclear whether co-expression of different specific ARs within the same endothelial cells represents unappreciated signaling complexity or just simply a functional redundancy. Using high throughput proteomic analyses of P-UAECs, we observed that β2-ARs are abundantly localized in the P-UAEC caveolae domain, a “hub” for compartmentalizing signal transduction for regulation of multiple functions (Ramadoss and Magness, unpublished data, [2011]). Therefore, demonstration of specific AR expression relative to the subcellular localization of α2, β2 and β3-ARs in NP-UAECs versus P-UAECs needs to be determined. This may fulfill distinct physiologic and pathophysiologic functional significance for expression relative to localization of multiple AR subtypes in endothelium. .
Since ARs are present on the endothelium, they are undoubtedly exposed to circulating endogenous norepinephrine and epinephrine released from the adrenal medulla. Normal physiologic circulating catecholamine concentrations are 1-2 nmol/L 20,21,22 and increase dramatically in pathologic cardiovascular conditions and during “fight or flight” stress responses. Hence, we demonstrated that even at a low physiologic concentration (0.1 nmol/L) of both norepinephrine and epinephrine significantly increases P-UAEC, not NP-UAEC, proliferation suggesting that catecholamines indeed may play roles in regulating physiologic angiogenesis during gestation. Consistent with these finding, catecholamines augment in vivo angiogenesis in dopamine β-hydroxylase knockout mice deficient in plasma catecholamines.23 Confirming our recent report, a low physiologic concentration (0.1 nmol/L) of 2-OHE2 and 4-OHE2 stimulate P-UAEC proliferation.4 We report herein for the first time that catecholamine and catecholestradiol combinations induced significantly higher P-UAEC proliferation. We further demonstrate for the first time that both catecholamines and catechoestradiols individually elevate P-UAEC proliferation only via β-ARs suggesting that functional β-ARs are likely important for regulating physiologic and/or pathologic angiogenesis during gestation. These data therefore demonstrate that catecholamines play a complementary and even an additive role to 2-OHE2 and 4-OHE2 as positive β-AR-mediated modulators of physiologic angiogenesis. These data also implied that catecholamines and catechoestradiols should exhibit similar AR-subtype-specific signaling pathways to induce P-UAEC proliferation. Catecholestradiols have been previously shown to competitively bind to AR subtypes in rat cerebral cortex, striatum, and anterior pituitary as well as to guinea-pig hypothalamic membranes.9,10 Therefore, our data show that “catechol” moieties of catecholestradiols and catecholamines are very important for the binding and activation of β-ARs signaling.
The lack of alteration of P-UAEC proliferation when the nonspecific α-AR antagonist phentolamine and α2-AR specific blocker yohimbine was used show that α2-ARs that were reduced by pregnancy do not play a role in catecholestradiol-induced angiogenesis in P-UAECs. There are no reports showing a role of α2-ARs regulating endothelial cell proliferation. However, α2-ARs have been closely associated with nitric oxide signaling in endothelial cells 24, suggesting functional relevance of α2-AR expression in endothelial-mediated vasodilatation.
Consistent with our novel findings that propranolol abrogated 2-OHE2 and 4-OHE2–induce P-UAEC proliferation, are reports showing that stimulation of β-ARs by various pharmacokinetic compounds stimulate proliferation of endothelial cells.15,19,25 Classically, β-ARs are prototypical G-protein coupled receptors triggering diverse signaling cascades through α, β and γ G-protein subunits, adenylate cyclase, intracellular cAMP and protein kinase A and C.26 However, new layers of complexity in signaling suggest that β-AR activation can induce a myriad of cellular responses via p38 and p42/44 mitogen-activated protein kinases independent of adenylate cyclase, cAMP and protein kinase A and C. 27,28,29,30 Therefore signal transduction studies are needed to further elucidate the potential differences in β-AR-mediated molecular mechanism of action of the catecholestradiol versus catecholamines in endothelial cells.
The current observation that subtype specific β2-AR antagonist ICI 118,551 abolished P-UAEC proliferation stimulated by 2-OHE2 and 4-OHE2 suggests β2-AR coupling whereas the partial inhibition by β3-AR blocker SR 59230A also implies potential involvement of β3-ARs. In contrast, both the specific β2-AR (Formoterol) and β3-AR (BRL 37344) agonists equally induced P-UAEC proliferation which were specifically blocked by their specific antagonists (Figure 6), suggesting that both β-ARs may regulate these proliferative effects. Thus, the partial inhibitory effects of SR 59230A on catecholestradiol responses (Figure 5A) do not point to a lack of potency or effective concentration since a similar concentration of SR 59230A induced significant abrogation of β3-ARs in response to BRL 37344. Activation of either β2-AR and/or β3-AR have been shown to play a role in endothelial cell proliferation from human umbilical vein, retina and bovine aorta.14,15,16,18. However, ours is the first report to demonstrate that β2-AR and β3-AR mediate the catecholestradiol-induced proliferation of endothelial cells. P-UAECs express similar levels of β2-ARs and β3-ARs compared to NP-UAECs, demonstrating that the AR-mediated effects are not dependent on expression levels, but rather on other gestational-programming factors at the level of signaling. These data therefore provide a broader understanding of the mechanism of action of catecholestradiols in endothelial cell proliferation. Importantly, these results also point to the potential relevance of previously unappreciated complexities of estrogen signaling in the cardiovascular system via interactions of steroid metabolites and endothelial AR system.
Overall, the present study indicates that actions of catecholestradiols and catecholamines via endothelial ARs represent an evolutionary conserved and highly versatile signaling mechanism for regulating endothelial proliferation. During gestation angiogenic processes are to a great extent responsible for the dramatic 30-50 fold-rises in uterine blood flow, such that by term the uterine vascular bed receives nearly 20% of the also greatly expanded cardiac output and blood volume. 31,32 Furthermore, maternal uterine perfusion is maintained 1-2 fold in excess of the needs of the parallel, but separate, fetoplacental circulation.33 We previously suggested that during an acute gestational “flight or fight” response when catecholamines are greatly elevated -far in excess of the efficacious levels utilized herein- cardiac output redistributes away from the uterine vascular bed (α-AR-mediated) to the muscles and other tissues (β-AR-mediate) for survival of the mother and her fetus, thus providing a distinct short term survival advantage for placental mammals.34,35
Perspectives
The current study sheds new light on the existence of a previously unrecognized two ligand system for a single AR family representing a mechanism by which the same physiological regulators of the “flight or fight” responses that protect the mother during a state of acute but repeated physiologic stress will indeed act as an angiogenic switch to subsequently induce maintenance in uterine relative to fetoplacental blood flows. This provides for a marked evolutionary advantage of maintaining delivery of oxygen and nutrients through the uteroplacental circulation thus protecting the growing fetus from subsequent stress-induced profound reductions in uterine blood flow. These data also uncover novel complexities of estrogen signaling in the cardiovascular system via ARs and necessitates the further investigation of estrogen metabolites such as catecholestradiols in the vascular system which do not signal via the classical estrogen receptors.
Supplementary Material
Acknowledgements
Timothy J Morschauser, Mayra B Pastore, Mary Y Sun, Jason L Austin, Gladys E Lopez, Terrance M Phernetton and Cindy L Goss
Sources of Funding: NIH: HL49210, HD38843, HL87144 (RR Magness), AA19446 (J Ramadoss), R25-GM083252 (ML Carnes) and T32-HD041921-07 (IM Bird).
Footnotes
Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubey RK, Tofovic SP, Jackson EK. Cardiovascular pharmacology of estradiol metabolites. J. Pharmacol. Exp. Ther. 2004;308:403–409. doi: 10.1124/jpet.103.058057. [DOI] [PubMed] [Google Scholar]
- 3.Tofovic SP, Dubey RK, Jackson EK. 2-Hydroxyestradiol attenuates the development of obesity, metabolic syndrome, and vascular and renal dysfunction in obese ZSF1 rats. J. Pharmacol. Exp. Ther. 2001;299:973–977. [PubMed] [Google Scholar]
- 4.Jobe SO, Ramadoss J, Koch JM, Jiang Y, Zheng J, Magness RR. Estradiol-17β and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites stimulate proliferation in uterine artery endothelial cells: Role of estrogen receptor-alpha versus estrogen receptor-beta. Hypertension. 2010;55:1005–1011. doi: 10.1161/HYPERTENSIONAHA.109.146399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ball P, Knuppen R, Haupt M, Breuer H. Interactions between estrogens and catechol amines III. Studies on the methylation of catechol estrogens, catechol amines and other catechols by the catechol-o-methyltransferase of human liver. J. Clin. Endocrinol. Metab. 1972;34:736–746. doi: 10.1210/jcem-34-4-736. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd T, Weisz J. Direct inhibition of tyrosine hydroxylase activity by catechol estrogens. J Biol Chem. 1978;253:4841–4843. [PubMed] [Google Scholar]
- 7.Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): Biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 8.Foreman MM, Porter JC. Effects of catechol estrogens and catecholamines on hypothalamic and corpus striatal tyrosine hydroxylase activity. J Neurochem. 1980;34:1175–1183. doi: 10.1111/j.1471-4159.1980.tb09957.x. [DOI] [PubMed] [Google Scholar]
- 9.Paden CM, McEwen BS, Fishman J, Snyder L, DeGroff V. Competition by estrogens for catecholamine receptor binding in vitro. J Neurochem. 1982;39:512–520. doi: 10.1111/j.1471-4159.1982.tb03974.x. [DOI] [PubMed] [Google Scholar]
- 10.Etchegoyen GS, Cardinali DP, Perez AE, Tamayo J, Perez-Palacios G. Binding and effects of catecholestrogens on adenylate cyclase activity, and adrenoceptors, benzodiazepine and GABA receptors in guinea-pig hypothalamic membranes. European Journal of Pharmacology. 1986;129:1–10. doi: 10.1016/0014-2999(86)90329-8. [DOI] [PubMed] [Google Scholar]
- 11.Freddolino PL, Kalani MY, Vaidehi N, Floriano WB, Hall SE, Trabanino RJ, Kam VWT, Goddard WA., III Predicted 3D structure for the human β2 adrenergic receptor and its binding site for agonists and antagonists. Proc Natl Acad Sci. 2004;101:2736–2741. doi: 10.1073/pnas.0308751101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolb P, Rosenbaum DM, Irwin JJ, Fung JJ, Kobilka BK, Shoichet BK. Structure-based discovery of β2-adrenergic receptor ligands. Proc Natl Acad Sci. 2009;106:6843–6848. doi: 10.1073/pnas.0812657106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bird IM, Sullivan JA, Di T, Cale JM, Zhang L, Zheng J, Magness RR. Pregnancy-dependent changes in cell signaling underlie changes in differential control of vasodilator production in uterine artery endothelial cells. Endocrinology. 2000;141:1107–1117. doi: 10.1210/endo.141.3.7367. [DOI] [PubMed] [Google Scholar]
- 14.Sherline P, Mascardo R. Catecholamines are mitogenic in 3T3 and bovine aortic endothelial cells. J. Clin. Invest. 1984;74:483. doi: 10.1172/JCI111445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sexl V, Mancusi G, Baumgartner-Parzer S, Schütz W, Freissmuth M. Stimulation of human umbilical vein endothelial cell proliferation by A2-adenosine and beta 2-adrenoceptors. Br J Pharmacol. 1995;114:1577–1586. doi: 10.1111/j.1476-5381.1995.tb14942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferro A, Queen LR, Priest RM, Xu B, Ritter JM, Poston L, Ward JPT. Activation of nitric oxide synthase by β2-adrenoceptors in human umbilical vein endothelium in vitro. Br J Pharmacol. 1999;126:1872–1880. doi: 10.1038/sj.bjp.0702512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu B, Li J, Gao L, Ferro A. Nitric oxide-dependent vasodilatation of rabbit femoral artery by beta(2)-adrenergic stimulation or cyclic AMP elevation in vivo. Br J Pharmacol. 2000 Mar;129:969–974. doi: 10.1038/sj.bjp.0703155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinle JJ, Booz GW, Meininger CJ, Day JN, Granger HJ. Beta 3-adrenergic receptors regulate retinal endothelial cell migration and proliferation. J Biol Chem. 2003;278:20681–20686. doi: 10.1074/jbc.M300368200. [DOI] [PubMed] [Google Scholar]
- 19.Steinle JJ, Zamora DO, Rosenbaum JT, Granger HJ. Beta 3-adrenergic receptors mediate choroidal endothelial cell invasion, proliferation, and cell elongation. Exp Eye Res. 2005;80:83–91. doi: 10.1016/j.exer.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Izzo JL. The sympathetic nervous system in human hypertension. In: Izzo JL, Black HR, editors. Hypertension primer. 2nd ed Lippincott Williams &Wilkins; 1999. pp. 109–112. [Google Scholar]
- 21.Zacharia LC, Jackson EK, Gillespie DG, Dubey RK. Catecholamines block 2-hydroxyestradiol-induced antimitogenesis in mesangial cells. Hypertension. 2002;39:854–859. doi: 10.1161/01.hyp.0000014502.44988.39. [DOI] [PubMed] [Google Scholar]
- 22.Dubey RK, Jackson EK, Gillespie DG, Zacharia LC, Imthurn B. Catecholamines block the antimitogenic effect of estradiol on human coronary artery smooth muscle cells. J Clin Endocrinol Metab. 2004;89:3922–3931. doi: 10.1210/jc.2004-0115. [DOI] [PubMed] [Google Scholar]
- 23.Chalothorn D, Zhang H, Clayton JA, Thomas SA, Faber JE. Catecholamines augment collateral vessel growth and angiogenesis in hindlimb ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H947–H959. doi: 10.1152/ajpheart.00952.2004. [DOI] [PubMed] [Google Scholar]
- 24.Liao JK, Homey CJ. The release of endothelium-derived relaxing factor via α2-adrenergic receptor activation is specifically mediated by Giα2. J Biol Chem. 1993;268:19528–19533. [PubMed] [Google Scholar]
- 25.Iaccarino G, Ciccarelli M, Sorriento D, Galasso G, Campanile A, Santulli G, Cipolletta E, Cerullo V, Cimini V, Altobelli GG, Piscione F, Priante O, Pastore L, Chiariello M, Salvatore F, Koch WJ, Trimarco B. Ischemic neoangiogenesis enhanced by beta2-adrenergic receptor overexpression: a novel role for the endothelial adrenergic system. Circ Res. 2005;97:1182–1189. doi: 10.1161/01.RES.0000191541.06788.bb. [DOI] [PubMed] [Google Scholar]
- 26.Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Keys JR, Rockman HA, Koch WJ, Daaka Y, Lefkowitz RJ, Stamler JS. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 27.Lindquist JM, Fredriksson JM, Rehnmark S, Cannon B, Nedergaard J. β3- and α1-Adrenegic Erk1/2 activation is Src- but not Gi-mediated in brown adipocytes. J. Biol. Chem. 2000;275:22670–22677. doi: 10.1074/jbc.M909093199. [DOI] [PubMed] [Google Scholar]
- 28.Zheng M, Zhang SJ, Zhu WZ, Ziman B, Kobilka BK, Xiao RP. β2-adrenergic receptor-induced p38 MAPK activation is mediated by protein kinase A rather than by Gi or Gβγ in adult mouse cardiomyocytes. J Biol Chem. 2000;275:40635–40640. doi: 10.1074/jbc.M006325200. [DOI] [PubMed] [Google Scholar]
- 29.Lavoie C, Mercier JF, Salahpour A, Umapathy D, Breit A, Villeneuve LR, Zhu WZ, Xiao RP, Lakatta EG, Bouvier M, Hébert TE. β1/β2-adrenergic receptor heterodimerization regulates β2-adrenergic receptor internalization and ERK signaling efficacy. J Biol Chem. 2002;277:35402–35410. doi: 10.1074/jbc.M204163200. [DOI] [PubMed] [Google Scholar]
- 30.Yin F, Wang YY, Du JH, Li C, Lu ZZ, Han C, Zhang YY. Noncanonical cAMP pathway and p38 MAPK mediate β2-adrenergic receptor-induced IL-6 production in neonatal mouse cardiac fibroblasts. J Mol Cell Cardiol. 2006;40:384–393. doi: 10.1016/j.yjmcc.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Rosenfeld CR. Distribution of cardiac output in ovine pregnancy. Am J Physiol. 1977;232:H231–H235. doi: 10.1152/ajpheart.1977.232.3.H231. [DOI] [PubMed] [Google Scholar]
- 32.Magness RR. Maternal cardiovascular and other physiologic responses to the endocrinology of pregnancy. In: Bazer F, editor. The Endocrinology of Pregnancy. Humana Press Inc; Totowa, NJ: 1998. pp. 507–539. [Google Scholar]
- 33.Morriss FH, Jr, Rosenfeld CR, Resnik R, Meschia G, Makowski EL, Battaglia FC. Growth of uterine oxygen and glucose uptakes during pregnancy in sheep. Gynecol Invest. 1974;5:230–241. doi: 10.1159/000301654. [DOI] [PubMed] [Google Scholar]
- 34.Magness RR, Rosenfeld CR. Systemic and uterine responses to alpha-adrenergic stimulation in pregnant and nonpregnant ewes. Am J Obstet Gynecol. 1986;155:897–904. doi: 10.1016/s0002-9378(86)80047-3. [DOI] [PubMed] [Google Scholar]
- 35.Magness RR, Rosenfeld CR. The role of steroid hormones in the control of uterine blood flow. In: Rosenfeld CR, editor. The uterine circulation. Vol. 10. Perinatology Press; Ithaca, N.Y: 1989. pp. 239–271. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.