Abstract
It is well known that ethanol modulates the function of the Cys loop ligand-gated ion channels, which include the inhibitory glycine receptors (GlyRs). Previous studies have consistently shown that transmembrane and extracellular sites are essential for ethanol actions in GlyRs. In addition, recent evidence has shown that the ethanol modulation of GlyRs is also affected by G protein activation through Gβγ subunits. However, more specific roles of G protein α subunits on ethanol actions are unknown. Here, we show that the allosteric effect of ethanol on the human α1 GlyR is selectively enhanced by the expression of Gαs Q-L. For example, constitutively active Gαs, but not Gαq or Gαi, was able to displace the alcohol sensitivity of GlyRs toward low millimolar concentrations (17 ± 4 versus 48 ± 5% at 100 mM). Experiments under conditions that increased cAMP and protein kinase A (PKA)-mediated signaling, on the contrary, did not produce the same enhancement in sensitivity, suggesting that the Gαs Q-L effect was not dependent on cAMP/PKA-dependent signaling. On the other hand, the effect of Gαs Q-L was blocked by a Gβγ scavenger (9 ± 3% of control). Furthermore, two mutant receptors previously shown to have impaired interactions with Gβγ were not affected by Gαs Q-L, suggesting that Gβγ is needed for enhancing ethanol sensitivity. These results support the conclusion that activated Gαs can facilitate the Gβγ interaction with GlyRs in presence of ethanol, independent of increases in cAMP signaling. Thus, these data indicate that the activated form of Gαs is able to positively influence the effect of ethanol on a type of inhibitory receptor important for motor control, pain, and respiration.
Introduction
Ethanol is the most widely abused drug. Its consumption at intoxicating doses produces major modifications in motor, sensorial, and cognitive functions. The underlying mechanisms probably involve a wide variety of cellular effectors. A large body of evidence has demonstrated that ethanol can allosterically modulate the activity of several ligand-gated ion channels (LGICs), including members of the Cys loop family, composed of nicotinic acetylcholine, serotonin, GABA (GABAAR) and glycine (GlyR) receptors (for review, see Perkins et al., 2010). Because these receptors mediate fast synaptic transmission in the mammalian central nervous system, the effects of ethanol on these membrane proteins might largely explain the strong alterations on human behavior after excessive drinking.
Inhibitory GlyRs are critical for the control of neuronal network excitability through a selective increase in Cl− ion conductance, which is able to hyperpolarize the cell membrane (Aguayo et al., 2004; Lynch, 2004). GlyRs are composed of five subunits in a pentameric quaternary structure arranged around a central pore. Each subunit possesses four transmembrane (TM) domains and a large intracellular loop between TM3 and TM4 responsible for intracellular signal transduction modulation (Lynch, 2004). Previous studies in different cell types have consistently demonstrated that millimolar concentrations of ethanol can modulate the glycine-activated current (Aguayo et al., 1996; Mihic et al., 1997; Eggers et al., 2000; Lynch, 2004; Crawford et al., 2007; Perkins et al., 2010), although the molecular mechanisms involved are still not completely understood. Nevertheless, based on mutagenesis studies, it has been proposed that specific amino acids in the TM2–TM3 domains form discrete binding sites for ethanol, which also bind general anesthetics (Mihic et al., 1997; Harris et al., 2008). In addition, a residue in the extracellular domain was reported to contribute to ethanol potentiation of GlyRs (Perkins et al., 2010), possibly by linking ligand binding to channel opening (Yévenes et al., 2010) or by configuring an ethanol acceptor site (Crawford et al., 2007). Furthermore, other studies determined that the molecular volume and hydrophobicity of S267 in TM2 also contributed to GlyR ethanol sensitivity (Yamakura et al., 1999) and alcohol binding (Mascia et al., 2000). On the other hand, it was also shown that ethanol modulates ion channel activity through modifications of intracellular signal transduction pathways. For instance, the sensitivity of GlyR and GABAA to ethanol was affected by G protein activation and protein kinases (Aguayo et al., 1996; Freund and Palmer, 1997; Mascia et al., 1998; Jiang and Ye, 2003; Zhu and Ye, 2005; Qi et al., 2007). Of interest, it was reported that ethanol can indeed affect specific intracellular transduction pathways (Yao et al., 2002; Morrow et al., 2004; Ron and Jurd, 2005). More recent studies have shown that the ethanol-mediated potentiation of GlyRs was affected by a molecular interaction between intracellular residues in the receptor with G protein βγ heterodimers (Yévenes et al., 2006, 2008).
G protein-coupled receptors (GPCRs) are transmembrane proteins that mediate most intracellular actions through pathways involving activation of G proteins. After GPCR activation by ligands, heterotrimeric G proteins modulate the activity of many effectors, cycling between an inactive GDP-bound and an active GTP-bound conformation (Hamm, 1998; Oldham and Hamm, 2008). This critical cycle allows the fine tuning of protein-protein interactions between Gα-GTP and Gβγ with their targets. Despite the critical importance of the active Gα subunits for G protein-mediated signaling, their relevance for ethanol effects on LGICs has not been directly investigated.
Previous studies have examined the role of signal transduction pathways on the sensitivity of GlyRs and GABAARs to ethanol using intracellular application of G protein or kinase modulators via the patch pipette. Although these approaches have generated valuable information, the outcomes are restricted by the concentration and the specificity of the selected modulators. As an alternate approach to characterize the potential role of Gα subunits on the ethanol sensitivity of GlyRs, in the present study we used constitutively active mutant forms of three main Gα subunits, which are denominated Gα Q-L mutants due to a glutamine to leucine mutation in the GTP-binding site. The Gαs Q-L, for example, is a constitutively active mutant capable of generating high levels of cAMP through the stimulation of adenylyl cyclase in the absence of GPCR activation (Masters et al., 1989). Thus, these mutants can be used to investigate the role of a specific signal transduction pathway on a given effector, without potential nonselective interferences that might arise when more chemically based approaches are used.
This study shows that active Gαs, possibly found during stressful conditions or under the influence of β2-adrenergic pharmacotherapy, can selectively enhance the ethanol sensitivity of GlyRs through a Gβγ-linked mechanism. Thus, our results show that activated Gαs enhances the sensitivity of GlyRs to ethanol and suggests that other targets could also be affected in the same fashion by this drug of abuse.
Materials and Methods
cDNA Constructs.
The cDNA encoding human α1 glycine receptor subunits in the pCIS2 vector was obtained from N. L. Harrison (Columbia University, New York, NY). Mutant 316–320A and 385–386A GlyRs were described previously (Yévenes et al., 2006). Activated Gαs, Gαi2, and Gαq in pcDNA3.1 vectors (Invitrogen, Carlsbad, CA) were purchased from Missouri S&T cDNA Resource Center (Rolla, MO). The mutant Q87/R196 PKA catalytic α subunit (PKA Cα Q-R) cDNA was kindly provided by Dr. Stanley McKnight (University of Washington, Seattle, WA) and has been described previously (Orellana and McKnight, 1992; Orellana et al., 1993). To monitor optimal kinase expression in our experiments, a hexahistidine-tagged version of this mutant PKA in the pcDNA3.1 vector (Invitrogen) was designed and constructed. The EYFP-ct-GRK2 expression vector was described previously (Kammermeier and Ikeda, 1999).
Cell Culture and Transfection.
HEK293 cells were cultured using standard methodologies. HEK293 cells were transfected using Lipofectamine 2000 (Invitrogen) with 2 μg of DNA for each plasmid studied per well. Expression of GFP was used as a marker of positively transfected cells and recordings were made after 18 to 36 h.
Electrophysiology.
Whole-cell recordings were performed as described previously (Yévenes et al., 2008). A holding potential of −60 mV was used. Patch electrodes were filled with 140 mM CsCl, 10 mM BAPTA, 10 mM HEPES, 4 mM MgCl2, 2 mM ATP, and 0.5 mM GTP (pH 7.4). The external solution contained 150 mM NaCl, 10 mM KCl, 2.0 mM CaCl2, 1.0 mM MgCl2, 10 mM HEPES, and 10 mM glucose (pH 7.4). The amplitude of the glycine current was assayed using a brief pulse (1–2 s) of glycine. The modulation of the glycine current by ethanol (Sigma-Aldrich, St. Louis, MO) was assayed using a pulse of 30 μM glycine coapplied with ethanol in each condition studied, without any preapplication. A brief pulse of 1 mM glycine was applied at the end of the recording period to test whether the maximal current amplitude remained stable after the recording (≥5 min). If the amplitude changed by more than 10%, the cell was discarded. To reduce the expression variability, a bicistronic vector carrying the Gαs Q-L gene together with enhanced GFP (pIRES2-Gαs Q-L) was used in most of the experiments. Otherwise, all the additional receptors and intracellular proteins were transfected at a 5:1 ratio related to the GlyR amount.
Measurement of Relative Changes in cAMP Levels.
To investigate the relative amounts of cAMP under our experimental conditions, we used the dual luciferase reporter assay system (Promega, Madison, WI) following the manufacturer's protocol (Romo et al., 2008). In brief, HEK293 cells were grown in 24-well plates (80% confluent) and transfected with the plasmids pCRE-Luc and pRL-SV40 for cAMP-dependent firefly and Renilla reniformis luciferase activity, respectively. Expression of R. reniformis luciferase provides an internal control for transfection to normalize the cAMP-dependent firefly luciferase expression. In a set of experiments, a plasmid encoding human β2-adrenergic receptor in the pCDNA3.1 vector (Invitrogen) was incorporated. For all the control reactions, the GlyR encoding plasmid was also transfected to mimic the cellular conditions attained in cells used to do the electrophysiological recordings. One day after transfection, firefly and R. reniformis luciferase activity were measured sequentially from a single lysate in a Wallac 1420 VICTOR3 Luminometer (PerkinElmer Life and Analytical Sciences, Waltham, MA) using the dual luciferase assay system (Promega). Each single experiment was performed in triplicate, whereas each dataset was reproduced at least three times.
Immunofluorescence.
HEK293 cells were first fixed with 4% paraformaldehyde (0.1 M phosphate buffer, pH 7.4) and were then permeabilized (0.3% Triton X-100) and blocked (10% normal horse serum). Then, all-night incubation with polyclonal Gαs (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and polyclonal hexahistidine antibodies (His-Tag; US Biological, Swampscott, MA) was performed. Epitope visualization was performed by incubating the sample with two secondary antibodies conjugated to Cy3 (1:600; Jackson ImmunoResearch Laboratories Inc., West Grove, PA). Finally, the cells were coverslipped using Fluorescence Mounting Medium (Dako North America, Inc., Carpinteria, CA) and were chosen randomly for imaging using a Nikon confocal microscopy (TE2000; Nikon, Melville, NY).
Data Analysis.
Statistical analyses were performed using ANOVA and are expressed as arithmetic mean ± S.E.M.; values of P < 0.05 were considered statistically significant. In all the figures, the data points plotted as control and Gαs Q-L represent the pooled average of all the individual experiments. These data points were obtained from experiments performed in parallel for every condition. No significant differences were found between the averages obtained under the same experimental conditions (e.g., control or Gαs Q-L) from different batches of cells. For all the statistical analysis and plots, Origin 6.0 (OriginLab Corp., Northampton, MA) software was used.
Results
Effects of Constitutively Active G Protein α Subunits on Ethanol Sensitivity of Wild-Type α1 Glycine Receptors.
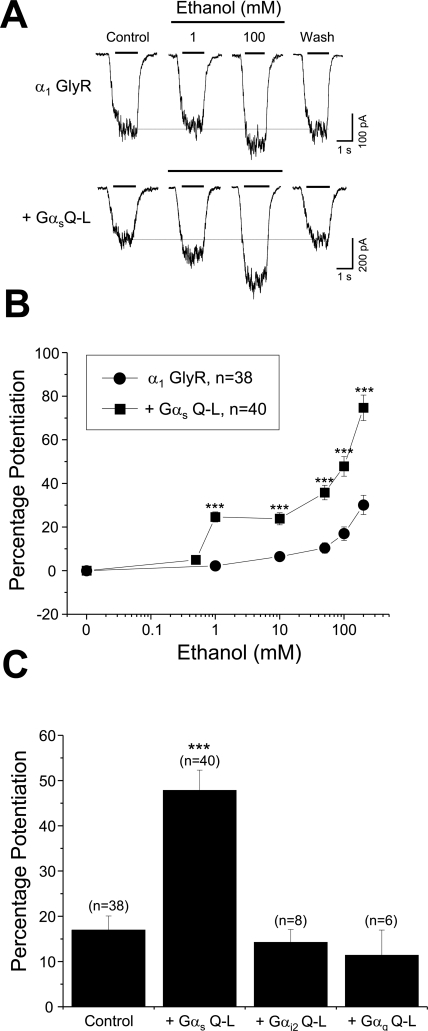
The Gαs pathway and PKA phosphorylation have been previously implicated in ethanol effects on GlyRs (Aguayo et al., 1996); therefore, we began our study by analyzing the ethanol sensitivity of GlyRs after the overexpression of the Gαs Q-L mutant. In agreement with previous results in control HEK cells (Aguayo et al., 1996; Ye et al., 1998), the glycine (∼EC50, 30 μM)-activated current elicited by activation of α1 GlyRs was consistently potentiated by ethanol, displaying a threshold effect at a concentration between 1 and 10 mM (Fig. 1, A–C). For instance, whereas 1 mM ethanol did not produce a significant effect (2 ± 2%) on the current amplitude, 100 mM potentiated the glycine-evoked current above 17 ± 4% at EC50. Of interest, the ethanol sensitivity was significantly enhanced after the expression of Gαs Q-L (Fig. 1, A and B). The current traces show that after QL overexpression, the application of 1 mM ethanol already was able to potentiate the current amplitude. The graph illustrates that this low concentration of ethanol increased the current amplitude by 25 ± 3%, reaching near 48 ± 5% potentiation with 100 mM (Fig. 1B). The data also show that under this condition the threshold for the ethanol effect was reduced to approximately 0.5 mM ethanol. To examine whether the Gαs Q-L effects correspond to a phenomenon specific for the Gαs, we performed experiments expressing the constitutive active mutants Gαi2 Q-L and Gαq Q-L. Contrary to Gαs Q-L, none of these activated Gα mutants modified the effect of 100 mM ethanol in GlyRs (Fig. 1, A–C), indicating that activated Gαs selectively increases the sensitivity of GlyR to alcohol. In other experiments, we found that both homomeric α1 and α1β heteromeric GlyRs were significantly potentiated by ethanol when Gαs Q-L was overexpressed in the cells. For example, the data in Fig. 2A show that active Gαs significantly enhanced the ethanol sensitivity in both receptor configurations (P < 0.001, ANOVA).
Fig. 1.
Constitutively active Gαs enhances the sensitivity of human α1 GlyRs to ethanol. A, current traces obtained in transfected HEK cells expressing wild-type α1 GlyRs with and without Gαs Q-L in the presence of different ethanol concentrations. B, concentration-response curves to ethanol (0.5–200 mM) in control (●, n = 38) and Gαs Q-L-expressing cells (■, n = 40). C, graph summarizing the potentiation induced by 100 mM ethanol of the normalized glycine-evoked current elicited in cells cotransfected with either Gαi2 Q-L (n = 8) or Gαq Q-L (n = 6) mutants. Note that only Gαs Q-L enhanced the ethanol sensitivity. All data are presented as means ± S.E.M. The data points plotted as control and Gαs Q-L represent the pooled average of all the individual experiments. ***, differences were significant at P < 0.001, ANOVA.
Fig. 2.
Gαs Q-L overexpression in HEK 293 cells enhances the ethanol sensitivity of homomeric and heteromeric GlyRs. A, bar graph summarizing the potentiation elicited by 100 mM ethanol of the normalized glycine-evoked current elicited in homomeric α1 (■) or α1β heteromeric (▩) GlyRs cotransfected with Gαs Q-L in parallel experiments. ***, differences were significant at P < 0.001, ANOVA. B, data obtained in the absence (circles) or presence of Gαs Q-L (■), Gαi2 Q-L (♦), or Gαq Q-L ( ) show normal receptor activation. C, glycine concentration-response curves for heteromeric α1β GlyRs in the absence (●) or presence of Gαs Q-L (♦). D, confocal microscopy images show HEK293 cells transfected with GFP and Gαs Q-L stained with a polyclonal antibody against Gαs (top panel, red). The bottom panels show endogenous Gαs expression in nontransfected cells. Scale bar, 10 μm (top panel); 50 μm (bottom panel).
) show normal receptor activation. C, glycine concentration-response curves for heteromeric α1β GlyRs in the absence (●) or presence of Gαs Q-L (♦). D, confocal microscopy images show HEK293 cells transfected with GFP and Gαs Q-L stained with a polyclonal antibody against Gαs (top panel, red). The bottom panels show endogenous Gαs expression in nontransfected cells. Scale bar, 10 μm (top panel); 50 μm (bottom panel).
The effect of overexpression of the active forms of Gα on the sensitivity of GlyR to glycine was studied because the potentiation caused by ethanol was previously shown to be dependent on the concentration of glycine (Aguayo et al., 1996; Ye et al., 1998). The data from these experiments showed that Gαs Q-L did not alter the apparent affinity of GlyR, suggesting that the effects of ethanol were not related to changes on receptor affinity for the agonist (Fig. 2, B and C). For instance, the calculated EC50 values were not significantly different compared with the controls [α1 GlyRs = 30 ± 2 μM (n = 18), Gαs Q-L = 33 ± 1 μM (n = 16), Gαi2 Q-L = 30 ± 1 μM (n = 7), and Gαq Q-L = 28 ± 3 μM (n = 6)]. In addition, the current records showed that the time course of the response was not affected by the overexpression of Gαs Q-L. The normalized amplitude of the glycine-activated currents in control cells expressing only α1 GlyRs or in cells cotransfected with Gαs Q-L was very stable over 15 min of recording [α1 GlyRs = 97 ± 1% (n = 9) and Gαs Q-L = 102 ± 4% (n = 7)]. All these results indicate that the properties of GlyR were unchanged by overexpression of Gαs Q-L. Using confocal microscopy, we found high levels of Gαs expression in cells transfected with the plasmids, assuring that the cells that were GFP-positive did indeed have high levels of activated Gα (Fig. 2D).
Chronic Increases in cAMP Levels or PKA Activity Did Not Alter the Sensitivity of GlyRs to Ethanol.
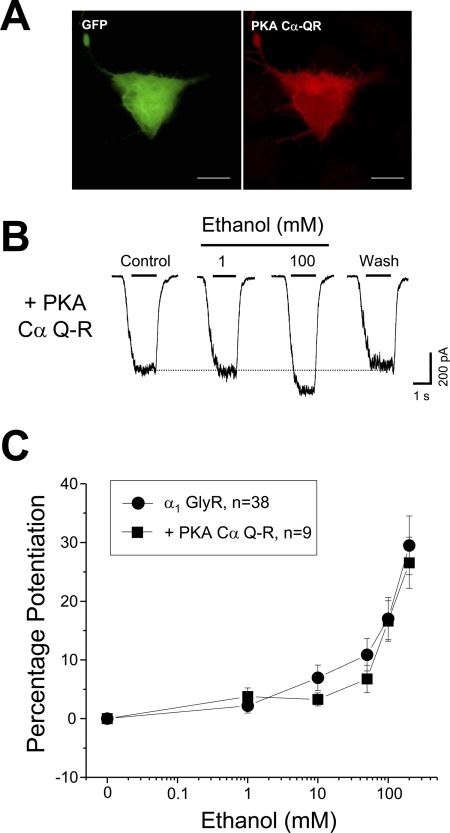
The expression of Gαs Q-L generates a sustained chronic increase in cAMP levels in the absence of GPCR activation (Masters et al., 1989). Thus, we thought that the increase in ethanol sensitivity of GlyRs could result from a sustained activation of PKA, a known GlyR intracellular modulator (Song and Huang, 1990; Lynch, 2004). Therefore, to explore the potential role of cAMP and PKA on the ethanol sensitivity of GlyRs, we decided to perform electrophysiological recordings in cells with high levels of cAMP mimicking the cellular conditions generated by Gαs Q-L expression. Before performing the physiological experiment, we confirmed that Gαs Q-L indeed increased the cellular content of cAMP (Masters et al., 1989). Results obtained using a dual luciferase reporter system showed that the HEK293 cells displayed high relative cAMP levels (46 ± 7-fold over control) after expression of β2-adrenergic receptors and chronic stimulation with the agonist isoproterenol (50 μM), which were not significantly different from the enhancement obtained with Gαs Q-L (Fig. 3A). Electrophysiological analysis of α1 GlyRs expressed in cells under this high chronic cAMP condition showed that the EC50 for glycine was unchanged (36 ± 2 μM, n = 7). Likewise, the sensitivity of the GlyR to ethanol was not altered (Fig. 3, B and C), indicating that high levels of cAMP did not increase the ethanol sensitivity like that found with GαsQL. Nevertheless, to obtain an independent confirmation for the latter result, we directly examined the PKA role on GlyR ethanol sensitivity using overexpression of the catalytic PKA α subunit (which has been used to promote cAMP/PKA-dependent events (Mellon et al., 1989). Here, the PKA Cα mutant, denominated PKA Cα Q-R, which has two mutations that confer a higher constitutive activity and resistance for the regulatory PKA subunit inhibition, was overexpressed (Orellana and McKnight, 1992; Orellana et al., 1993). We first checked the kinase overexpression using a hexahistidine-tagged PKA Cα Q-R followed by immunocytochemistry. These experiments showed good levels of protein kinase expression after the cells were cotransfected with enhanced GFP (Fig. 4A). Similar to the results with chronic activation of cAMP (Fig. 3), the electrophysiological experiments showed that the ethanol potentiation of the glycine-evoked currents was not modified by PKA Cα Q-R expression (Fig. 4, B and C). Additional experiments showed that the EC50 for glycine was unchanged (32 ± 2 μM, n = 8).
Fig. 3.
Ethanol effects on GlyRs in cells with high cAMP levels. A, HEK293 cells were transfected with either β2-adrenergic receptors (β2-AR) or Gαs Q-L, and the increase in cAMP was detected with the reporters pCRE-Luc and pRL-SV40 as described under Materials and Methods. The bars are the means ± S.E.M. from four experiments. B, examples of whole-cell recordings from α1 GlyRs in the presence or absence of ethanol in cells stimulated by overexpression of β2-adrenergic receptors in the presence of isoproterenol (10 μM, 18–36 h). C, concentration-response curves for ethanol (1–200 mM) in controls (●) and after stimulation of β2-AR with isoproterenol (■). Differences were not significant. ISO, isoproterenol.
Fig. 4.
PKA activity did not alter the ethanol sensitivity of GlyRs. A, the images show transfected HEK293 cells stained with antibodies against the hexahistidine epitope (red) that recognize tagged PKA Cα Q-R. GFP expression is shown in green (scale bar, 10 μm). B, glycine-activated responses in the presence of ethanol recorded in cells that expressed the constitutively active PKA Cα Q-R. C, concentration-response curves for ethanol (1–200 mM) in control (circles) and PKA Cα Q-R-expressing cells (■). Differences were not significant.
Taken together, these results indicate that increases in cAMP and PKA activity are not involved in the regulation of ethanol effects on GlyRs, suggesting that the enhancement of alcohol sensitivity promoted by Gαs Q-L expression could be explained by other mechanisms that do not involve cAMP- or PKA-related signaling.
G Protein βγ Subunits Mediate the Gαs Q-L Increase in GlyR Sensitivity to Ethanol.
The previous data suggest that constitutively active mutant Gαs increases the sensitivity of GlyRs to ethanol by cAMP- and PKA-independent mechanisms. Thus, it is possible to suggest that the increase in sensitivity to ethanol produced by Gαs Q-L is via a membrane-delimited mechanism, independent of a diffusible second messenger and dependent on the Gβγ dimer (Yévenes et al., 2008; Guzman et al., 2009). Thus, we examined whether the latter pathway is involved in the shift on alcohol sensitivity promoted by Gαs Q-L. A commonly used strategy to explore the participation of Gβγ is the overexpression of “Gβγ sequesters,” which are high-affinity proteins that bind the heterodimer and block their effects in a variety of cellular effectors (Ikeda, 1996; Kammermeier and Ikeda, 1999). Previous studies have identified the carboxyl-terminal region of the β-adrenergic receptor kinases (ct-β-adrenergic kinase or ct-G protein receptor kinase) as a specific sequester of Gβγ (Daaka et al., 1997; Kammermeier and Ikeda, 1999). Therefore, we studied the ethanol sensitivity of GlyRs after coexpression of Gαs Q-L and ct-GRK2. Of note, the Gβγ sequester significantly inhibited the effect of this active form of Gαs on ethanol sensitivity (Fig. 5, A and B). The potentiation of the glycine-evoked current with 100 mM ethanol was 9 ± 3% (n = 14) in the ct-GRK2-expressing cells, which was lower but not significantly different from the control condition (17 ± 3%, n = 38). These results suggest the presence of a Gβγ-driven, rather than a cAMP-PKA-mediated mechanism explaining the effect of active Gαs on ethanol sensitivity. Thus, we examined whether Gαs Q-L was able to enhance ethanol potentiation of two mutated GlyRs, which contained selective intracellular mutations in key basic residues that are critical for Gβγ modulation and ethanol effects (Yévenes et al., 2006, 2008). These mutated GlyRs were previously denominated 316–320A and 385–386A due to consecutive alanine substitutions in the corresponding residues 316RFRRK320 and 385KK386 of the human wild-type GlyR (Sadtler et al., 2003). The Gαs Q-L expression in HEK cells did not modify the ethanol sensitivity of these two mutant GlyRs (Fig. 5, C and D), supporting the idea that the mechanism by which Gαs Q-L enhances the ethanol potentiation in GlyRs requires the interaction of free Gβγ dimers with the large intracellular loop of the ion channel.
Fig. 5.
The increase in ethanol sensitivity produced by activated Gαs depends on βγ subunits. A, whole-cell recordings obtained in HEK293 cells that coexpressed Gαs Q-L and the Gβγ sequester ct-GRK2. Current traces during the application of 1 and 100 mM ethanol are shown. B, concentration response curves for ethanol (1–200 mM) show that the shift in the ethanol sensitivity promoted by Gαs Q-L (■) is blocked by expression of the Gβγ sequester (●). Differences were significant (***, P < 0.001, ANOVA). C, traces are Cl− currents induced in two α1 GlyRs with intracellular mutations in cells overexpressing Gαs Q-L during the application of different ethanol concentrations. D, graph shows that the expression of the constitutively active Gαs did not modify the ethanol sensitivity of either 316–320A (■) or 385–386A (●) mutated GlyRs with respect to cells expressing Gαs Q-L (○ and □). Differences were not significant.
Discussion
In the present study, we found that overexpression of an activated form of Gαs shifted the sensitivity of the GlyR toward lower concentrations of ethanol. This effect was blocked by reducing the activity of Gβγ, suggesting that activated Gαs increases the availability of the dimer to interact with GlyRs.
Effects of Ethanol on G Protein Activation.
Most ubiquitous cellular signal transduction pathways are associated to stimulation of GPCRs and subsequent activation of heterotrimeric G proteins (Hamm, 1998; Oldham and Hamm, 2008). Previous studies have shown that ethanol alters several cellular functions through modifications in G protein-associated signaling. For instance, it was reported that pharmacological ethanol concentrations (10–100 mM) affected Gαs- and Gαq-mediated intracellular pathways, causing changes in PKA and protein kinase C activities, respectively (Gonzales et al., 1986; Bode and Molinoff, 1988; Hoffman and Tabakoff, 1990; Yao et al., 2002; Morrow et al., 2004; Ron and Jurd, 2005). These findings are relevant because G proteins control diverse functions, including cell division, differentiation, and, in the case of the central nervous system, excitability regulation, synaptic transmission, and disease. Thus, it seems possible that ethanol might affect a number of cellular functions by altering G proteins. One of these functions can be associated with ion channels responsible for the control of neuronal excitability. In the present study, therefore, we used constitutively active mutant forms of Gα subunits to study specific G protein pathways. These mutant proteins are unable to hydrolyze GTP, causing, in the case of Gαs Q-L, an elevation in intracellular cAMP through a sustained stimulation of adenylyl cyclase in the absence of GPCR activation (Masters et al., 1989), as shown in Fig. 3A. In addition, because the GTP-bound Gα has a low affinity for Gβγ, it is expected that the dimer will be available to interact with distinct effectors, such as ion channels (i.e., GlyRs). The present data support the latter possibility.
G Protein Activation Modifies Actions of Ethanol on Inhibitory LGICs.
Ethanol affects the function of GlyRs by sites located in extracellular, transmembrane, and intracellular domains (Lynch, 2004; Perkins et al., 2010). In addition, previous studies have reported that the actions of ethanol on ion channels are affected by cell signaling associated with G proteins. For instance, potentiation of the glycine-activated current produced by ethanol is attenuated by protein kinase C inhibitors and guanosine 5′-O-(2-thiodiphosphate) in recombinant and neuronal systems (Aguayo et al., 1996; Mascia et al., 1998; Jiang and Ye, 2003; Zhu and Ye, 2005). In addition, although the inhibition of PKA was unable to alter ethanol effects on GlyRs (Aguayo et al., 1996; Mascia et al., 1998), chronic incubation with cholera toxin, which specifically affects the Gαs pathway, strongly abolished the alcohol potentiation in spinal neurons (Aguayo et al., 1996). Furthermore, the sensitivity of GABAARs to ethanol was affected by G protein activation and protein kinases (Weiner et al., 1994; Freund and Palmer, 1997; Aguayo et al., 2002). Thus, this experimental evidence suggests that G proteins and associated kinases have critical roles in the control of ethanol effects on GlyRs and GABAARs.
In the present study, we found that activated Gαs, but not Gαq or Gαi2, subunits affect the ethanol sensitivity of human α1 GlyRs. Furthermore, the effect of activated Gαs occurred through a Gβγ-linked mechanism, which was not reproduced by increases in cAMP and PKA-associated signaling. These results are in line with our previous studies showing that ethanol actions are affected by the interaction of Gβγ with the intracellular loop of the ion channel (Yévenes et al., 2008; Guzman et al., 2009). If we take into account these observations, it appears that the activated form of Gαs shifts the ethanol sensitivity, facilitating the functional interaction between Gβγ and the GlyR. This enhanced interaction is probably produced by an increase in free Gβγ unable to bind to the excess GTP-bound Gαs. At this point, the presence of a direct interaction between activated Gαs and GlyRs emerges as a potential chance to fine-tune the Gβγ interaction in a membrane-delimited context, especially considering previous reports that show direct interactions between Gα subunits and ion channels such as nicotinic acetylcholine receptors and G protein-coupled inwardly rectifying potassium channels (Peleg et al., 2002; Fischer et al., 2005). However, our results cannot eliminate other possibilities such as the facilitation of the Gβγ-GlyR interaction as a consequence of cytoskeleton modifications induced by activated Gαs, which also is independent of cAMP and PKA (Yu et al., 2009).
Significance of the Present Findings for Ethanol Intoxication.
We found that the overexpression of a constitutively active Gαs mutant (Gαs Q-L) shifted the ethanol sensitivity of GlyRs toward lower ethanol concentrations (1–10 mM). In this study, we used a higher concentration of glycine than was used in other previous studies (Mihic et al., 1997; Ye et al., 1998; Jiang and Ye, 2003; Crawford et al., 2007; Yévenes et al., 2008), because the effect of ethanol became much more evident already at 1 to 10 mM, which is close to the concentration achieved during low to moderate alcohol intake. At the glycine concentration used in the study, the receptors should have a higher saturation with the agonist, somewhat closer to a synaptic condition. Although our results do not define a mechanism by which activated Gαs subunits modulate the sensitivity of GlyR to alcohol, they show a new role for Gαs in GlyR pharmacology, which can help to reconcile the controversial results obtained by studying GABAA receptors, which at times can be more or less sensitive to ethanol (Aguayo et al., 2002; Weiner and Valenzuela, 2006). In addition, this study confirms the role of Gβγ interaction for the ethanol sensitivity of GlyRs (Yévenes et al., 2008; Guzman et al., 2009). Because GlyR potentiation by ethanol might be related to acute intoxication, sleep obstructive apnea, ethanol intake, and possibly alcoholism (Gibson and Berger, 2000; Molander et al., 2005), these data could also be useful for the pharmacological control of ethanol effects on GlyRs in vivo. The present results show that the effects of ethanol and Gαs Q-L were similar in homomeric α1 and heteromeric α1β receptors, and this implies that the sensitivity to ethanol of both synaptic and extrasynaptic GlyRs could be enhanced by G protein activation. The significance of homomeric receptors in relation to ethanol consumption is interesting because brain regions without significant glycinergic inputs, such as the nucleus accumbens and ventral tegmental area (Molander et al., 2005; Wang et al., 2005), are extrasynaptic in nature. Although the subunit composition of these GlyRs is still not clear and despite the fact that our study only examined α1-containing GlyRs, our results suggest that G protein activation might also increase the ethanol sensitivity of other GlyR subunits (e.g., α2 and α3). Thus, the increase in neuronal inhibition resulting from the higher sensitivity of these receptors to ethanol should produce a strong reduction in excitability, shifting the toxic effects of ethanol toward lower concentrations. Future experiments designed to investigate these ideas will clarify the specific roles of activated Gαs and Gβγ on the alcohol sensitivity of GlyRs. Finally, it is possible to propose that during disorders that induce modifications in Gαs-associated pathways (i.e., stress or antiasthmatic therapy with β2 agonists) or altered levels of GTP-bound G proteins, the depressing effects of ethanol on the central nervous system might be enhanced because of the presence of higher levels of activated Gαs.
Acknowledgments
We greatly appreciate the technical assistance of Lauren Aguayo. We thank Pamela Izaurieta who helped with early patch-clamp experiments and also Dr. S. R. Ikeda (National Institutes of Health, Bethesda, MD) and Dr. Stanley McKnight (University of Washington, Seattle, WA) for providing the ct-GRK2 and PKA encoding plasmids, respectively.
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant AA15150]; and Comisión Nacional de Investigación Científica y Tecnológica [Grant AT-4040102].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.184408.
- LGIC
- ligand-gated ion channel
- GABAAR
- GABA receptor
- GlyR
- glycine receptor
- TM
- transmembrane
- GPCR
- G protein-coupled receptor
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- PKA
- protein kinase A
- HEK
- human embryonic kidney
- GFP
- green fluorescent protein
- ANOVA
- analysis of variance
- ct-GRK
- carboxyl-terminal G protein-coupled receptor kinase.
Authorship Contributions
Participated in research design: Yévenes, Moraga-Cid, Romo, and Aguayo.
Conducted experiments: Yévenes, Moraga-Cid, and Romo.
Performed data analysis: Yévenes, Moraga-Cid, and Romo.
Wrote or contributed to the writing of the manuscript: Yévenes and Aguayo.
References
- Aguayo LG, Peoples RW, Yeh HH, Yevenes GE. (2002) GABAA receptors as molecular sites of ethanol action. Direct or indirect actions? Curr Top Med Chem 2:869–885 [DOI] [PubMed] [Google Scholar]
- Aguayo LG, Tapia JC, Pancetti FC. (1996) Potentiation of the glycine-activated Cl− current by ethanol in cultured mouse spinal neurons. J Pharmacol Exp Ther 279:1116–1122 [PubMed] [Google Scholar]
- Aguayo LG, van Zundert B, Tapia JC, Carrasco MA, Alvarez FJ. (2004) Changes on the properties of glycine receptors during neuronal development. Brain Res Brain Res Rev 47:33–45 [DOI] [PubMed] [Google Scholar]
- Bode DC, Molinoff PB. (1988) Effects of ethanol In Vitro on the beta adrenergic receptor-coupled adenylate cyclase system. J Pharmacol Exp Ther 248:1040–1047 [PubMed] [Google Scholar]
- Crawford DK, Trudell JR, Bertaccini EJ, Li K, Davies DL, Alkana RL. (2007) Evidence that ethanol acts on a target in Loop 2 of the extracellular domain of α1 glycine receptors. J Neurochem 102:2097–2109 [DOI] [PubMed] [Google Scholar]
- Daaka Y, Pitcher JA, Richardson M, Stoffel RH, Robishaw JD, Lefkowitz RJ. (1997) Receptor and Gβγ isoform-specific interactions with G protein-coupled receptor kinases. Proc Natl Acad Sci USA 94:2180–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, O'Brien JA, Berger AJ. (2000) Developmental changes in the modulation of synaptic glycine receptors by ethanol. J Neurophysiol 84:2409–2416 [DOI] [PubMed] [Google Scholar]
- Fischer H, Liu DM, Lee A, Harries JC, Adams DJ. (2005) Selective modulation of neuronal nicotinic acetylcholine receptor channel subunits by Go-protein subunits. J Neurosci 25:3571–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund RK, Palmer MR. (1997) β-Adrenergic sensitization of γ-aminobutyric acid receptors to ethanol involves a cyclic AMP/protein kinase A second-messenger mechanism. J Pharmacol Exp Ther 280:1192–1200 [PubMed] [Google Scholar]
- Gibson IC, Berger AJ. (2000) Effect of ethanol upon respiratory-related hypoglossal nerve output of neonatal rat brain stem slices. J Neurophysiol 83:333–342 [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Theiss C, Crews FT. (1986) Effects of ethanol on stimulated inositol phospholipid hydrolysis in rat brain. J Pharmacol Exp Ther 237:92–98 [PubMed] [Google Scholar]
- Guzman L, Moraga-Cid G, Avila A, Figueroa M, Yevenes GE, Fuentealba J, Aguayo LG. (2009) Blockade of ethanol-induced potentiation of glycine receptors by a peptide that interferes with Gβγ binding. J Pharmacol Exp Ther 331:933–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm HE. (1998) The many faces of G protein signaling. J Biol Chem 273:669–672 [DOI] [PubMed] [Google Scholar]
- Harris RA, Trudell JR, Mihic SJ. (2008) Ethanol's molecular targets. Sci Signal 1: 28, re7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PL, Tabakoff B. (1990) Ethanol and guanine nucleotide binding proteins: a selective interaction. FASEB J 4:2612–2622 [DOI] [PubMed] [Google Scholar]
- Ikeda SR. (1996) Voltage-dependent modulation of N-type calcium channel by G-protein βγ subunits. Nature 380:255–258 [DOI] [PubMed] [Google Scholar]
- Jiang ZL, Ye JH. (2003) Protein kinase Cε is involved in ethanol potentiation of glycine-gated Cl− current in rat neurons of ventral tegmental area. Neuropharmacology 44:493–502 [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Ikeda SR. (1999) Expression of RGS2 alters the coupling of metabotropic glutamate receptor 1a to M-type K+ and N-type Ca2+ channels. Neuron 22:819–829 [DOI] [PubMed] [Google Scholar]
- Lynch JW. (2004) Molecular structure and function of the glycine receptor chloride channel. Physiol Rev 84:1051–1095 [DOI] [PubMed] [Google Scholar]
- Mascia MP, Trudell JR, Harris RA. (2000) Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci USA 97:9305–9310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia MP, Wick MJ, Martinez LD, Harris RA. (1998) Enhancement of glycine receptor function by ethanol: role of phosphorylation. Br J Pharmacol 125:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters SB, Miller RT, Chi MH, Chang FH, Beiderman B, Lopez NG, Bourne HR. (1989) Mutations in the GTP-binding site of Gsα alter stimulation of adenylyl cyclase. J Biol Chem 264:15467–15474 [PubMed] [Google Scholar]
- Mellon PL, Clegg CH, Correll LA, McKnight GS. (1989) Regulation of transcription by cyclic AMP-dependent protein kinase. Proc Natl Acad Sci USA 86:4887–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, et al. (1997) Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature 389:385–389 [DOI] [PubMed] [Google Scholar]
- Molander A, Löf E, Stomberg R, Ericson M, Söderpalm B. (2005) Involvement of accumbal glycine receptors in the regulation of voluntary ethanol intake in the rat. Alcohol Clin Exp Res 29:38–45 [DOI] [PubMed] [Google Scholar]
- Morrow AL, Ferrani-Kile K, Davis MI, Shumilla JA, Kumar S, Maldve R, Pandey SC. (2004) Ethanol effects on cell signaling mechanisms. Alcohol Clin Exp Res 28:217–227 [DOI] [PubMed] [Google Scholar]
- Oldham WM, Hamm HE. (2008) Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol 9:60–71 [DOI] [PubMed] [Google Scholar]
- Orellana SA, Amieux PS, Zhao X, McKnight GS. (1993) Mutations in the catalytic subunit of the cAMP-dependent protein kinase interfere with holoenzyme formation without disrupting inhibition by protein kinase inhibitor. J Biol Chem 268:6843–6846 [PubMed] [Google Scholar]
- Orellana SA, McKnight GS. (1992) Mutations in the catalytic subunit of cAMP-dependent protein kinase result in unregulated biological activity. Proc Natl Acad Sci USA 89:4726–4730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg S, Varon D, Ivanina T, Dessauer CW, Dascal N. (2002) Gαi controls the gating of the G protein-activated K+ channel, GIRK. Neuron 33:87–99 [DOI] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Crawford DK, Alkana RL, Davies DL. (2010) Molecular targets and mechanisms for ethanol action in glycine receptors. Pharmacol Ther 127:53–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi ZH, Song M, Wallace MJ, Wang D, Newton PM, McMahon T, Chou WH, Zhang C, Shokat KM, Messing RO. (2007) Protein kinase Cε regulates γ-aminobutyrate type A receptor sensitivity to ethanol and benzodiazepines through phosphorylation of γ2 subunits. J Biol Chem 282:33052–33063 [DOI] [PubMed] [Google Scholar]
- Romo X, Pastén P, Martínez S, Soto X, Lara P, de Arellano AR, Torrejón M, Montecino M, Hinrichs MV, Olate J. (2008) xRic-8 is a GEF for Gαs and participates in maintaining meiotic arrest in Xenopus laevis oocytes. J Cell Physiol 214:673–680 [DOI] [PubMed] [Google Scholar]
- Ron D, Jurd R. (2005) The “ups and downs” of signaling cascades in addiction. Sci STKE 309: re14. [DOI] [PubMed] [Google Scholar]
- Sadtler S, Laube B, Lashub A, Nicke A, Betz H, Schmalzing G. (2003) A basic cluster determines topology of the cytoplasmic M3–M4 loop of the glycine receptor α1 subunit. J Biol Chem 278:16782–16790 [DOI] [PubMed] [Google Scholar]
- Song YM, Huang LY. (1990) Modulation of glycine receptor chloride channels by cAMP-dependent protein kinase in spinal trigeminal neurons. Nature 348:242–245 [DOI] [PubMed] [Google Scholar]
- Wang F, Xiao C, Ye JH. (2005) Taurine activates excitatory non-synaptic glycine receptors on dopamine neurones in ventral tegmental area of young rats. J Physiol 565:503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. (2006) Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther 111:533–554 [DOI] [PubMed] [Google Scholar]
- Weiner JL, Zhang L, Carlen PL. (1994) Guanosine phosphate analogs modulate ethanol potentiation of GABAA-mediated synaptic currents in hippocampal CA1 neurons. Brain Res 665:307–310 [DOI] [PubMed] [Google Scholar]
- Yamakura T, Mihic SJ, Harris RA. (1999) Amino acid volume and hydropathy of a transmembrane site determine glycine and anesthetic sensitivity of glycine receptors. J Biol Chem 274:23006–23012 [DOI] [PubMed] [Google Scholar]
- Yao L, Arolfo MP, Dohrman DP, Jiang Z, Fan P, Fuchs S, Janak PH, Gordon AS, Diamond I. (2002) βγ Dimers mediate synergy of dopamine D2 and adenosine A2 receptor-stimulated PKA signaling and regulate ethanol consumption. Cell 109:733–743 [DOI] [PubMed] [Google Scholar]
- Ye Q, Koltchine VV, Mihic SJ, Mascia MP, Wick MJ, Finn SE, Harrison NL, Harris RA. (1998) Enhancement of glycine receptor function by ethanol is inversely correlated with molecular volume at position α267. J Biol Chem 273:3314–3319 [DOI] [PubMed] [Google Scholar]
- Yévenes GE, Moraga-Cid G, Avila A, Guzmán L, Figueroa M, Peoples RW, Aguayo LG. (2010) Molecular requirements for ethanol differential allosteric modulation of glycine receptors based on selective Gβγ modulation. J Biol Chem 285:30203–30213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yévenes GE, Moraga-Cid G, Guzmán L, Haeger S, Oliveira L, Olate J, Schmalzing G, Aguayo LG. (2006) Molecular determinants for G protein βγ modulation of ionotropic glycine receptors. J Biol Chem 281:39300–39307 [DOI] [PubMed] [Google Scholar]
- Yévenes GE, Moraga-Cid G, Peoples RW, Schmalzing G, Aguayo LG. (2008) A selective Gβγ-linked intracellular mechanism for modulation of a ligand-gated ion channel by ethanol. Proc Natl Acad Sci USA 105:20523–20528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JZ, Dave RH, Allen JA, Sarma T, Rasenick MM. (2009) Cytosolic Gαs acts as an intracellular messenger to increase microtubule dynamics and promote neurite outgrowth. J Biol Chem 284:10462–10472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Ye JH. (2005) The role of G proteins in the activity and ethanol modulation of glycine-induced currents in rat neurons freshly isolated from the ventral tegmental area. Brain Res 1033:102–108 [DOI] [PubMed] [Google Scholar]