Abstract
Marijuana abuse is very prominent among pregnant women. Although marijuana cannabinoids have been shown to exert immunosuppression in adults, virtually nothing is known about the effects of marijuana use during pregnancy on the developing immune system of the fetus and during postnatal life. We noted that murine fetal thymus expressed high levels of the cannabinoid receptors CB1 and CB2. Moreover, perinatal exposure to Δ9-tetrahydrocannabinol (THC) had a profound effect on the fetus as evidenced by a decrease in thymic cellularity on gestational days 16, 17, and 18 and postgestational day 1 and marked alterations in T cell subpopulations. These outcomes were reversed by CB1/CB2 antagonists, suggesting that THC-mediated these effects through cannabinoid receptors. Thymic atrophy induced in the fetus correlated with caspase-dependent apoptosis in thymocytes. Thymic atrophy was the result of direct action of THC and not based on maternal factors inasmuch as THC was able to induce T cell apoptosis in vitro in fetal thymic organ cultures. It is noteworthy that perinatal exposure to THC also had a profound effect on the immune response during postnatal life. Peripheral T cells from such mice showed decreased proliferative response to T cell mitogen as well as both T cell and antibody response to HIV-1 p17/p24/gp120 antigens. Together, our data demonstrate for the first time that perinatal exposure to THC triggers profound T cell dysfunction, thereby suggesting that the offspring of marijuana abusers who have been exposed to THC in utero may be at a higher risk of exhibiting immune dysfunction and contracting infectious diseases including HIV.
Introduction
Marijuana, or Cannabis sativa, is one of the most commonly used drugs of abuse worldwide (Berdyshev, 2000). In particular, it is the illegal drug of choice among pregnant women in the United States (Hurd et al., 2005). In addition, marijuana has been used for its medicinal properties for centuries (Voth and Schwartz, 1997). Cannabinoids, the major ingredients in marijuana, including Δ9-tetrahydrocannabinol (THC), the major psychoactive component, have been suggested as therapeutic agents in the treatment of ailments ranging from intraocular pressure caused by glaucoma to multiple sclerosis and certain types of cancers (Voth and Schwartz, 1997; Berdyshev, 2000). The ability of marijuana and THC to alleviate nausea and loss of appetite in patients with AIDS and during chemotherapy in patients with cancer (Beal et al., 1995; Schwartz et al., 1997) has led some to suggest their medicinal use to relieve morning sickness in pregnant women (Westfall et al., 2006). However, studies from our laboratory and elsewhere have shown that THC and other cannabinoids can trigger immunosuppression by inducing apoptosis in lymphoid organs such as the thymus and spleen (McKallip et al., 2002b). Other mechanisms of immunosuppression elicited by cannabinoids include induction of Foxp3+ T regulatory cells and myeloid-derived suppressor cells (Hegde et al., 2008, 2010), as well as alterations in the cytokine profiles (Berdyshev, 2000), which may render the host more susceptible to infections and cancer (Cabral and Dove Pettit, 1998; McKallip et al., 2005).
These findings suggest that the use of marijuana or cannabinoids during pregnancy, whether for recreational or medicinal purposes, might put the unborn child at a higher risk for developing immune dysfunction and susceptibility to infections. Maternal use of marijuana during pregnancy has been linked with impaired fetal growth (Hurd et al., 2005) and lower birth weight (Zuckerman et al., 1989; Hurd et al., 2005). In addition, studies in rodents have shown that perinatal exposure to THC may affect fetal brain development and therefore alter behavioral responses of the offspring later in life (Bonnin et al., 1995; Vela et al., 1995). A few studies even suggested that children exposed to marijuana in utero may be at a higher risk of developing certain cancers, such as neuroblastoma (Bluhm et al., 2006) and leukemia (Robison et al., 1989), although those findings are still somewhat controversial. Nonetheless, virtually nothing is known about the consequences of perinatal exposure to THC on the offspring's immune system.
In addition, several reports suggest that the use of marijuana and other cannabinoids may lead to a higher risk of contracting HIV, both directly and indirectly. Indirectly, the “high” experienced by marijuana abusers makes them more likely to adopt risky behavior such as having unprotected sex with HIV-positive individuals (Brodbeck et al., 2006; Drumright et al., 2006). In addition, studies performed using severe combined immunodeficient mice implanted with human peripheral blood lymphocytes show that THC both induces immunosuppression and increases HIV replication (Roth et al., 2005), suggesting a more direct link between cannabinoid use and HIV infection. Clearly, more research is needed to determine the consequences of perinatal exposure to cannabinoids on the immune system of the unborn offspring, as well as the risk that the progeny faces contracting diseases such as HIV.
In the current study, we demonstrate that perinatal exposure to THC can alter T cell development and functions in the neonatal and postnatal stages of life, which in turn could potentially have a major impact on susceptibility to infections as well as other immune disorders.
Materials and Methods
Mice.
Timed pregnant C57BL/6 mice were purchased from the National Cancer Institute, National Institutes of Health (Frederick, MD) and maintained on a 12-h light/dark lighting schedule in our animal facility. All animal experiments were conducted following standard procedures and after obtaining approval from the Institutional Animal Care and Use Committee of University of South Carolina.
Reagents.
THC was obtained from the National Institute of Drug Abuse (Rockville, MD) and initially dissolved in dimethyl sulfoxide (DMSO; Sigma, St Louis, MO) to a concentration of 20 mM and stored at −20°C. For in vivo experiments, THC was further diluted in warm phosphate-buffered saline (PBS). For in vitro experiments, THC was further diluted in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 5% fetal calf serum. The CB1 antagonist SR141716A [5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide; Rimonabant] was obtained from sanofi-aventis (Bridgewater, NJ), and the CB2 antagonist AM630 [1-[2-(morpholin-4-yl)ethyl]-2-methyl-3-(4-methoxybenzoyl)-6-iodoindole] was purchased from Tocris Bioscience (Ellisville, MO). Both compounds were dissolved in DMSO.
Detection of CB1 and CB2 Expression by RT-PCR.
Fetal thymi were harvested on gestational day (GD) 16, and single cell suspensions were prepared and erythrocytes were lysed. RNA was then isolated using the TRIzol method according to the manufacturer's protocol (Invitrogen). Because CB1 and CB2 are encoded by single exons, we included a DNase digestion step to limit contamination from genomic DNA. cDNA was prepared using the iScript cDNA synthesis kit (BioRad Laboratories, Hercules, CA). CB1 and CB2 were amplified using the following primer sequences from 5′ to 3′: CB1 forward primer, GAGTGTTGGGGCCCTTGTGTAAAT and reverse primer, GTGGTATCTGCAAGGCCGTCTAAG; CB2 forward primer, AGCGGCTGACAAATGACA and reverse primer, GCGCCGGGAGGACAGGATAATA, for product sizes of 252 and 253 base pairs, respectively. 18S RNA was used as a control and amplified with the following primers, from 5′ to 3′: forward primer, GCCCGAGCCGCCTGGAATAC and reverse primer, CCGGCGGGTCATGGGAATAAC, for a product size of 299 base pairs. After holding the PCRs at 95°C for 1 min, PCR was carried out using the following parameters: 95°C for 10 s, 58°C for 20 s, and 72°C for 45 s for 35 cycles followed by a final minute at 72°C, in a GeneAmp 9700 (Applied Biosystems, Foster City, CA). The resulting PCR products were separated on a 1% agarose gel.
Acute In Vivo Exposure to THC.
Pregnant mice were treated intraperitoneally with 20 or 50 mg/kg b.wt. THC or vehicle on GD16 because this corresponds to the time of T cell receptor rearrangement, appearance of CD8 single-positive (SP) T cells, and expression of characteristic markers such as major histocompatibility complex antigens on thymic stromal cells (Kingston et al., 1984; Haars et al., 1986; Pardoll et al., 1987). We used two pregnant mice each for vehicle or THC treatment groups. Each pregnant mouse had an average of 10 pups that were pooled separately and analyzed. In some experiments, the mothers were sacrificed on GD17 or GD18, and pups were harvested. In other experiments, the mothers were allowed to deliver the pups, and we used pups on postnatal day (PD) 1 or at 2 weeks of age.
Cell Preparation.
We harvested and pooled the thymi from the fetuses or pups from each mother in each treatment group on GD17, GD18, and PD1 because of low yield of thymocytes. On an average, 10 pups were obtained from each mother. Single cell suspensions were then prepared using a laboratory homogenizer (Stomacher; Tekmar, Cincinnati, OH). Contaminating erythrocytes were lysed by suspending the cells in 3 ml of red blood cell lysing buffer (Sigma-Aldrich). After two washes in complete RPMI 1640 medium, the cellularity was determined using the trypan blue dye exclusion method. The data were expressed as mean viable thymic cellularity per fetus/pup. This was calculated by dividing the total number of cells in each pooled sample by the total number of fetuses/pups used.
Subchronic In Vivo Exposure to THC.
Pregnant mice (two per group) were treated intraperitoneally with 25 mg/kg THC or vehicle on GD16 and 10 mg/kg THC or vehicle every day thereafter until they delivered the pups (for a total of four injections or 55 mg/kg of THC). One week after the pups were born, the thymi and spleens were harvested and made into single cell suspension. Contaminating red blood cells were lysed, and cellularity was determined as described above.
Detection of THC-Induced Apoptosis In Vivo.
Thymocytes obtained from the fetuses of THC- and vehicle-treated pregnant mice were cultured in vitro in 96-well flat-bottomed plates (106 cells/well in 0.2 ml of medium) overnight at 37°C because this method prevents apoptotic cells from being engulfed by phagocytic cells and therefore allows a better detection of apoptosis. The cells were then harvested, washed twice with PBS, fixed at room temperature with 100 μl of 4% paraformaldehyde for 30 min, and subsequently stained for apoptosis using the TUNEL method.
Blockade of THC-Induced Apoptosis In Vivo Using CB1 and CB2 Inhibitors.
Pregnant mice (two per group) were treated intraperitoneally with 20 mg/kg of the CB1 antagonist SR141716A or 40 mg/kg of the CB2 antagonist AM630 on GD16. After 1 h, the mice received 50 mg/kg of THC or vehicle intraperitoneally. On GD17, the mothers were sacrificed, and the fetuses were harvested and pooled from each mother. The thymocytes were prepared and cultured overnight at 37°C as described above. The cells were then harvested, fixed, and stained for apoptosis using the TUNEL method.
Fetal Thymic Organ Cultures.
FTOCs were prepared by the standard procedure. In brief, thymic lobes were aseptically removed from 16-day-old fetuses from several pregnant mice. Up to six randomly chosen thymic lobes were placed on each nitrocellulose filter with 45-μm pore size (Millipore Corporation, Billerica, MA), and each filter was set in a 24-well plate culture well with 300 μl of complete RPMI 1640 medium supplemented with 5% FBS, 2 mM l-glutamine, 100 μM nonessential amino acids, 1 mM sodium pyruvate, 50 μM β-mercaptoethanol, 100 U/ml penicillin, and 0.1 mg/ ml streptomycin. Three wells were used for each treatment. THC was added to a final concentration of 5, 10, or 20 μM. Control lobes were cultured in medium containing vehicle (DMSO). The thymic lobes were incubated at 37°C and 6% CO2 in a water-saturated atmosphere on day 0. Cultures were fed on day 3 by transferring the filters to new wells containing fresh medium with THC or DMSO. On day 6, thymic lobes from each well were harvested and pooled, and single cell suspensions were prepared. After lysis of the erythrocytes and two washes, the cell viability was determined using trypan blue exclusion. The cells were then analyzed for apoptosis using the TUNEL method.
Detection of Apoptosis Using the TUNEL Method.
Apoptotic events were quantified at the single cell level using the TUNEL method (Roche Diagnostics, Indianapolis, IN). In brief, cells were washed with PBS twice and permeabilized on ice for 2 min using 100 μl of 0.1% Triton X-100 in 0.1% sodium citrate. After two additional washes, the cells were incubated with 25 μl of TUNEL reaction composed of fluorescein isothiocyanate (FITC)-dUTP and terminal deoxynucleotidyl transferase at 37°C with 5% CO2. After 1 h, the cells were washed two more times and resuspended in PBS, and fluorescence was determined by flow cytometry.
Assessment of THC-Induced Activation of Caspase-3 and/or Caspase-7.
Fetal thymocytes harvested from THC- and vehicle-treated mothers were cultured in vitro in 96-well flat-bottomed plates (106 cells/well in 0.1 ml of medium) overnight at 37°C. The induction of apoptosis was then detected using the Apo-ONE homogeneous caspase-3/7 assay (Promega, Madison, WI) according to the manufacturer's recommendation. In brief, 100 μl of a 1:100 substrate/buffer solution was added to the plate. The plate was then shaken for 30 s and incubated at room temperature for at least 30 min before reading the results on a Wallac Victor2 instrument (PerkinElmer Life and Analytical Sciences, Waltham, MA).
Detection of Phenotypic Markers on Thymocytes.
Thymocytes (106) derived from pups of THC- and vehicle-treated mothers were washed with PBS and incubated with anti-CD16/CD32 mAb on ice for 10 min to block the Fc receptors. The cells were subsequently washed and double-stained for CD4 and CD8, using FITC-conjugated anti-CD4 mAb and R-phycoerythrin (PE)-conjugated anti-CD8 mAb (BD Pharmingen, San Diego, CA), by incubating on ice for 30 min. The cells were then analyzed using a flow cytometer. Absolute numbers for each marker were calculated based on total cell numbers determined by trypan blue exclusion, and frequency of each cell population as determined by flow cytometry.
Effect of Perinatal Exposure to THC on the Proliferative Response of Splenocytes and Thymocytes to Mitogens.
Splenocytes and thymocytes collected from pups born to vehicle- or THC-treated mothers (5 × 105 in 100 μl) were cultured in 96-well plates with complete RPMI 1640 medium containing 10% FBS, 2 mM l-glutamine, 50 μM β-mercaptoethanol, 100 U/ml penicillin, and 0.1 mg/ml streptomycin and either left unstimulated or stimulated with 2 μg/ml concanavalin A (Con A; Sigma-Aldrich), LPS (5 μg/ml), or 5 μg/ml anti-CD3 mAbs for 48 h. Eight hours before the end of the assay, the cells were pulsed with 2 μCi of [3H]thymidine (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). DNA synthesis was determined by β-scintillation counting.
Effect of Perinatal Exposure to THC on Postnatal (at Adult Stage) Immune Response to HIV-1 p17/p24/gp120.
Pregnant mice were treated intraperitoneally with 50 mg/kg THC or vehicle on GD16 as described above. Five weeks after the pups were born, they were injected with 5 μg of HIV-1 p17/p24/gp120 (Prospec Technogene, East Brunswick, NJ) emulsified in complete Freund's adjuvant (CFA) in each rear footpad. One week later, the mice were sacrificed, and draining popliteal lymph nodes (LNs) were harvested and made into a single cell suspension. The viable cellularity was determined using the trypan blue dye exclusion method. The LN cells were then plated and left unstimulated or restimulated in vitro with 25 μg/ml p17/p24/gp120 for 72 h. Eight hours before the end of the assay, the cells were pulsed with 2 μCi of [3H]thymidine. DNA synthesis was determined by β-scintillation counting.
Detection of HIV-1 p17/p24/gp120-Specific Abs by ELISA in Mice Exposed Prenatally to THC.
Pregnant mice were treated intraperitoneally with 50 mg/kg THC or vehicle on GD16. Five weeks after the pups were born, they were injected with 5 μg of HIV-1 p17/p24/gp120 emulsified in CFA in each rear footpad. One week later, serum samples were screened for Abs using ELISA. High binding (2HB) Immulon microtiter plates (VWR, West Chester, PA) were coated with 50 μl of HIV-1 p17/p24/gp120 peptide (200 ng/ml) in 100 mM NaHCO3 coating buffer, pH 9.6, for 2 h at room temperature and incubated overnight at 4°C. After blocking for 1 h with 2% bovine serum albumin, serum samples diluted in 1% bovine serum albumin and 0.05% Tween 20 (100 μl/well) were then added and incubated at room temperature for 2 h. p17/p24/gp120-specific Abs were then detected by incubating them with horseradish peroxidase-conjugated anti-mouse IgG and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (Sigma-Aldrich). The colorimetric change was measured at 405 nm on a Wallac Victor2 instrument.
Statistical Analysis.
All statistical analyses were performed using Prism 4 (GraphPad Software Inc., San Diego, CA). One-way and two-way ANOVAs were carried out to compare experimental groups with controls as detailed in the figure legends. Two-tailed unpaired t test was also used where appropriate.
Results
Thymocytes from GD16 Fetuses Express CB1 and CB2 mRNA.
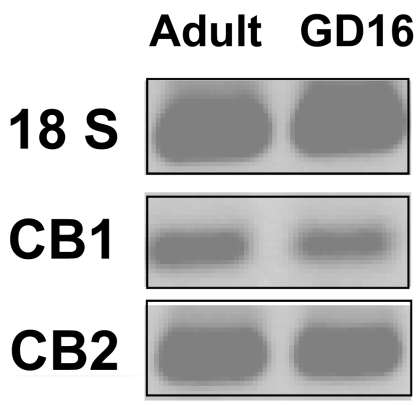
To investigate whether perinatal exposure to THC affects the fetal thymus, we first examined whether GD16 fetal thymocytes exhibit the cannabinoid receptors CB1 and CB2. To this end, we performed RT-PCR using RNA extracted from thymocytes of GD16 fetuses and RNA from adult thymocytes for comparison. As shown in Fig. 1, we found a similar pattern of expression of CB1 and CB2 in fetal thymocytes compared with adult thymocytes, with CB2 being expressed at much higher levels than CB1.
Fig. 1.
Fetal thymocytes express cannabinoid receptors. Fetal thymocytes were harvested on GD16. RNA was extracted and used for RT-PCR to check the expression of CB1 and CB2. The amplicons were run on a 1% agarose gel and visualized with ethidium bromide. 18S was used as an internal control. RNA from adult thymocytes was used as a positive control for CB1 and CB2 expression.
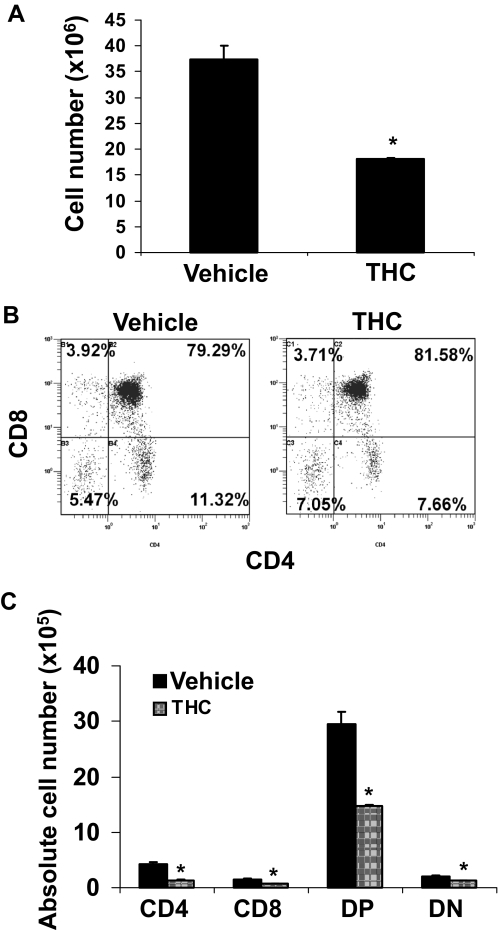
Acute Perinatal Exposure to THC Induces Apoptosis and Alterations in T Cell Subsets of the Fetal Thymus.
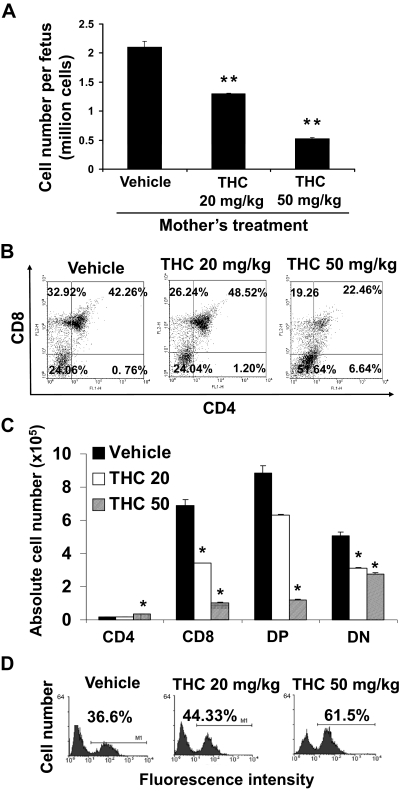
To determine whether THC has an influence on thymic development, we injected pregnant C57BL/6 mice intraperitoneally with THC (20 or 50 mg/kg) or vehicle on GD16. Analysis of the fetal thymi on GD17 revealed a dose-dependent decrease in thymic cellularity (Fig. 2A) indicative of thymic atrophy. In addition, THC treatment led to a decrease in the percentages of SP CD8+ T cells at both doses and double-positive (DP) T cells only at the higher dose (Fig. 2B). Moreover, we noted an increase in the proportions of SP CD4+ T cells and double-negative (DN) T cells at higher doses of THC (Fig. 2B). When we enumerated the absolute numbers of various T cell subpopulations (Fig. 2C), we found that all subsets were decreased after THC exposure in a dose-dependent manner except the SP CD4+ T cells.
Fig. 2.
Perinatal exposure to THC alters fetal thymic development. Groups of two C57BL/6 pregnant mice (n = 2) were injected on GD16 with THC (20 or 50 mg/kg) or vehicle. On GD17, the thymi from the fetuses were harvested. Thymi of fetuses from each pregnant mouse (average 10) were pooled separately for analysis. A, thymic cellularity was determined by trypan blue dye exclusion. The data represent the mean thymic cellularity per fetus ± S.E.M., p = 0.0062, one-way ANOVA. **, statistically significant difference from vehicle control (p < 0.01). B, the thymocytes were double-stained with FITC-anti-CD4 and PE-anti-CD8 mAbs and analyzed by flow cytometry. Representative dot plots are shown where the percentage of cells in each subset is depicted on each dot plot. C, absolute numbers of cells for each subset per fetus are shown and expressed as mean ± S.E.M. *, statistically significant difference (p < 0.05) in the mean cellularity of THC-exposed thymocytes compared with the vehicle control. D, the thymocytes were analyzed for apoptosis using the TUNEL method followed by flow cytometric analysis as described under Materials and Methods. The percentage of apoptotic cells is depicted in each histogram.
Analysis of the samples by the TUNEL method revealed a dose-dependent increase in the level of apoptosis detected in the fetal thymocytes upon exposure to THC (Fig. 2D). At lower doses of THC, the percentage of apoptosis was modest, which could be because the cells that undergo apoptosis in vivo are rapidly phagocytosed and cleared (Kamath et al., 1997). It has to be noted that thymocytes from vehicle-treated animals show significant levels of apoptosis. This is not surprising because thymocytes, which comprise a majority of immature double-negative T cells, spontaneously undergo apoptosis when they are cultured in vitro (Kamath et al., 1997).
Together, these data suggested that acute perinatal exposure to THC affects T cell development in the thymus of the fetus. In addition, these data suggested that THC may mediate its effect, at least in part, through induction of apoptosis.
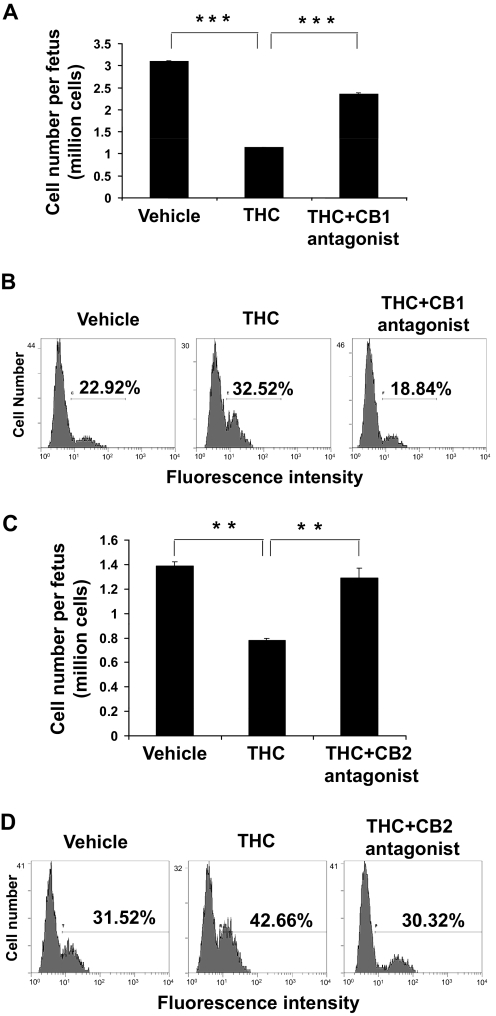
THC-Induced Apoptosis in Fetal Thymocytes Is Cannabinoid Receptor-Mediated.
Next, we investigated whether THC mediated its effects on the fetus through CB1 and CB2 receptors or nonspecific mechanisms such as maternal-derived factors. To this end, pregnant mice were pretreated with CB1 (Fig. 3, A and B) or CB2 (Fig. 3, C and D) antagonist and subsequently treated with THC (50 mg/kg) or vehicle on GD16. Analysis of the fetal thymocytes on GD17 revealed that both CB1 and CB2 antagonists were able to block, at least in part, the THC-induced fetal thymic atrophy (Fig. 3, A and C) and apoptosis (Fig. 3, B and D). Together, these data suggested that THC-induced apoptosis resulted from activation of both the CB1 and the CB2 receptors.
Fig. 3.
THC-induced apoptosis in the fetal thymus is mediated through both CB1 and CB2. On GD16, groups of two C57BL/6 pregnant mice (n = 2) were pretreated with CB1 (SR141716A) (A and B) or CB2 (AM630) (C and D) antagonist followed by injection with 50 mg/kg THC or the vehicle. A and C, on GD17, the thymi from the fetuses of each mother (average 10) were pooled and analyzed for viable cells as described in Fig. 2. The data represent the mean thymic cellularity per fetus ± S.E.M. A, ***, p < 0.0001, one-way ANOVA. C, p = 0.0062 one-way ANOVA. Asterisks indicate statistically significant difference (**, p < 0.05) in the mean cellularity in THC + antagonist group compared with THC-treated group and in the THC-treated group compared with vehicle-treated group. B and D, the thymocytes were analyzed for apoptosis using the TUNEL followed by flow cytometric analysis. The percentage of apoptotic cells is depicted in each histogram.
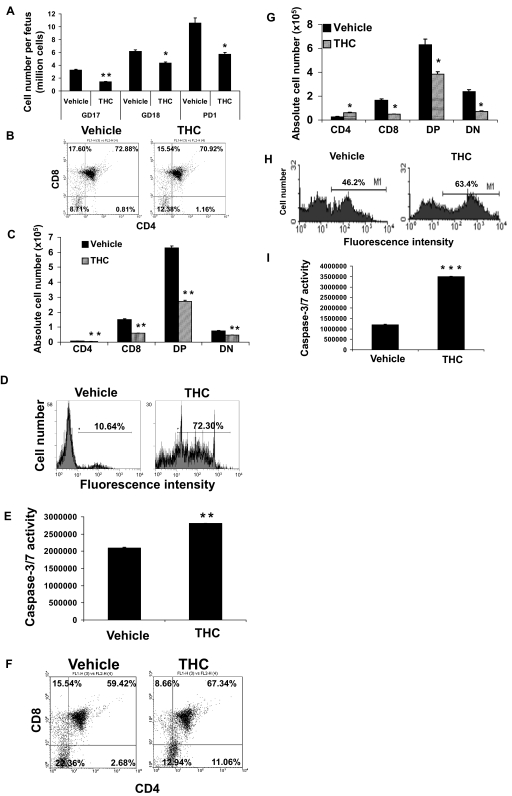
Alterations of Thymic Cellularity and T Cell Subpopulations Persist Several Days after Acute Perinatal Exposure to THC.
To determine whether the effects of THC on T cell development in the thymus were transient or whether they persisted beyond the first 24 h after exposure, pregnant mice received a single injection of THC (50 mg/kg) on GD16, and fetal thymi were harvested on GD18 and PD1. Fetuses from vehicle-treated mice showed an increase in thymic cellularity from GD17 to PD1 consistent with normal thymic development (Fig. 4A). Thymi harvested from the fetus of THC-treated mothers showed decreased cellularity at all days tested (Fig. 4A). In addition, THC-induced apoptosis was still detectable on GD18 (Fig. 4D) and PD1 (Fig. 4H) by TUNEL staining. We also noted that the THC-induced apoptosis was mediated by caspases as shown by use of the caspase-3/7 assay (Fig. 4, E and I). Although we could not detect any major changes in the percentages of cells found in each T cell subset on GD18, there were significant changes seen on PD1 (Fig. 4, B and F). However, when we investigated the absolute number of T cell subsets we noted a consistent decrease in the absolute numbers of SP CD8+, DP CD4+ CD8+, and DN T cells on both GD18 (Fig. 4C) and PD1 (Fig. 4G), similar to GD16 (Fig. 2C). Together, these data suggested that a single perinatal exposure to THC around the time of T cell development can have a profound effect on T cell development, which persists until the birth of the pups.
Fig. 4.
THC-induced thymic atrophy and alteration of T cell subsets persist until birth. Groups of two C57BL/6 pregnant mice (n = 2) were injected on GD16 with 50 mg/kg THC or vehicle. On GD17 (A), GD18 (A–E), and PD1 (A, F–I), the thymi from the fetuses or pups (average 10) from each mother were harvested and pooled. A, viable thymic cellularity was determined by trypan blue dye exclusion. The data represent the mean thymic cellularity per fetus/pup ± S.E.M. B, C, F, and G, the thymocytes were double-stained with FITC-anti-CD4 and PE-anti-CD8 mAbs and analyzed by flow cytometry. Representative dot plots are shown in B and F where the percentage of cells in each subset is depicted on each dot plot. Absolute numbers of cells found in each subset are shown in C and G. D and H, thymocytes were analyzed for apoptosis using TUNEL followed by flow cytometric analysis. The percentage of apoptotic cells is depicted in each histogram. E and I, the thymocytes were analyzed for levels of caspase-3 and caspase-7 activity as described under Materials and Methods. The results are depicted as mean ± S.E.M. A, C, E, G, and I, statistically significant difference between vehicle control and THC treatment group by two-tailed unpaired Student's t test (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001).
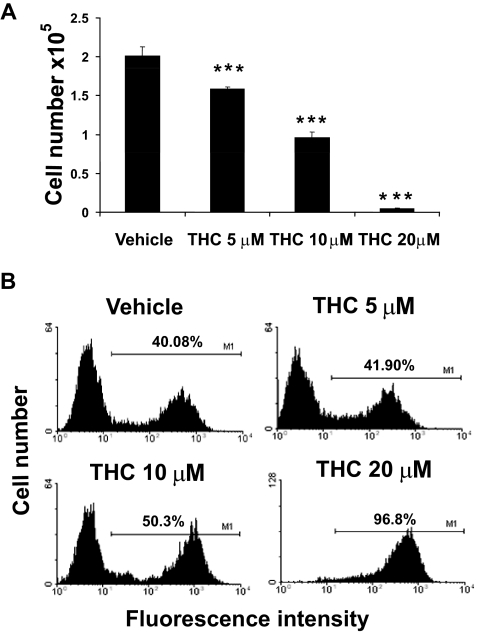
Exposure of Fetal Thymic Organ Cultures to THC Results in Decreased Cellularity and Induction of Apoptosis.
To exclude the possibility that THC-induced apoptosis was the result of the stress or caused by maternally derived factors, we investigated the direct effects of THC on developing T cells using FTOCs. Fetal thymi were harvested on GD16 and cultured in the presence of various concentrations of THC or vehicle for 6 days with a media change on day 3. Analysis at the end of the treatment showed a dose-dependent decrease in thymic cellularity upon exposure to THC (Fig. 5A), which correlated with an increase in apoptosis, particularly at higher doses of THC (Fig. 5B). At lower doses, the apoptosis was not demonstrable, which suggested that in FTOCs the low levels of apoptosis may not be detectable because of phagocytosis of apoptotic cells, or it is also likely that THC may inhibit T cell differentiation. Together, these data suggested that the effects seen in vivo on the fetus may result from direct exposure to THC, leading to apoptosis.
Fig. 5.
Effects of THC on FTOCs. FTOCs were prepared and exposed to THC or the vehicle as described under Materials and Methods. Thymi from fetuses of each mother were plated with up to six thymic lobes/well, and at the time of harvesting lobes from each well were pooled to prepare cell suspensions. Three such wells were used for each in vitro treatment. On day 6, thymic lobes were harvested and pooled. A, cell viability was determined using the trypan blue dye exclusion method. The data represent the mean thymic cellularity per organ ± S.E.M. ***, p ≤ 0.001, statistically significant difference in the mean cellularity of THC-exposed FTOCs compared with the vehicle control by one-way ANOVA. B, the cells were analyzed for apoptosis using the TUNEL method followed by flow cytometry. The percentage of apoptotic cells is depicted on each histogram.
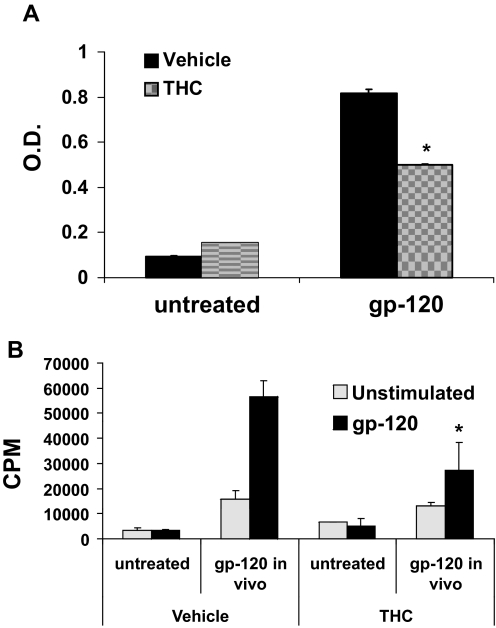
Acute Perinatal Exposure to THC Results in Decreased Immune Response to HIV-1 p17/p24/gp120.
Illegal drug abuse, including marijuana abuse, has been linked to a higher risk of contracting HIV. However, little is known about the risk faced by children exposed to marijuana in utero. To shed some light on this question, C57BL/6 pregnant mice were treated with an acute dose of THC or vehicle on GD16 and allowed to deliver their pups. Five weeks after birth, the pups were injected in each rear footpad with HIV-1 p17/p24/gp120 emulsified in CFA or left untreated (naive) and allowed to rest for 1 week. Serum and draining lymph nodes were then collected and used to measure HIV-1 p17/p24/gp120-specific antibodies and the proliferative response to restimulation with HIV-1 p17/p24/gp120 in vitro, respectively. THC-exposed animals showed lower serum titers of HIV-1 p17/p24/gp120-specific antibodies compared with animals exposed to vehicle (Fig. 6A). In addition, THC-exposed lymphocytes had a lower proliferative response to HIV-1 p17/p24/gp120 restimulation in vitro, compared with their vehicle-exposed counterpart (Fig. 6B). Together, these data suggested that perinatal exposure to THC decreases the immune response to HIV antigens.
Fig. 6.
Effect of acute perinatal exposure to THC on the immune response of postnatal 5 weeks of age to gp120. Groups of two C57BL/6 pregnant mice (n = 2) were injected with 50 mg/kg THC or vehicle on GD16. Five weeks after the pups were born, they were injected in each rear footpad with 5 μg of HIV-1 p17/p24/gp120 emulsified in CFA. After 1 week, sera (A) and draining LNs (B) were collected. A, sera were analyzed for the presence of HIV-1 p27/p24/gp120-specific IgG by ELISA. B, the draining LN cells were left unstimulated or restimulated with 25 μg/ml of HIV-1 p17/p24/gp120 for 72 h. During the final 8 h, the cells were pulsed with 2 μCi of [3H]thymidine. Thymidine incorporation was determined by β-scintillation counting. The data represent mean ± S.E.M. of triplicate cultures. *, statistically significant differences, p ≤ 0.05. A, one-way ANOVA, interaction, p = 0.0005; DMSO vehicle versus THC, p = 0.017; untreated versus gp120, p < 0.0001. B, one-way ANOVA, interaction not significant; DMSO versus THC, not significant; untreated versus gp120, p = 0.0016.
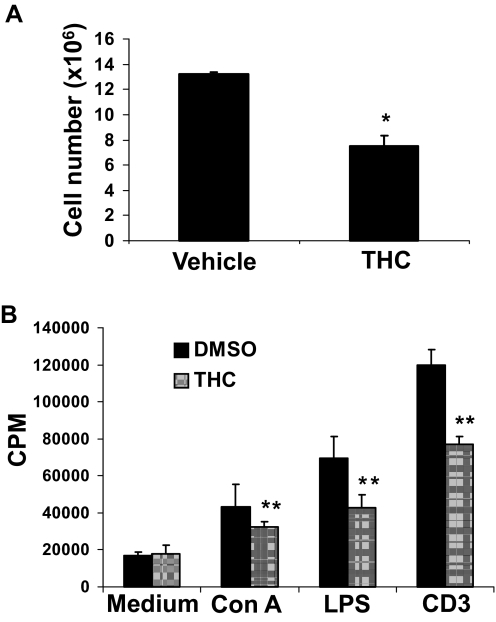
Effect of Subchronic Perinatal Exposure to THC on the Thymus and Spleen of 1-Week-Old Pups.
To this point, we noted that acute perinatal exposure to THC has a negative effect on the immune system of the progeny that can still be detected up to 5 weeks into life. However, in humans, perinatal exposure to THC is more likely to be chronic than acute. Therefore, we investigated the effects of subchronic perinatal exposure to THC on the thymus and spleen of the progeny. To this end, C57BL/6 pregnant mice were injected with 25 mg/kg THC or vehicle on GD16 and 10 mg/kg THC or the vehicle every day thereafter until the pups were born, for a total of four injections or 55 mg/kg THC. One week after the pups were born, thymi and spleens were harvested. The results showed that subchronic perinatal exposure to THC leads to thymic (Fig. 7A) and splenic (Fig. 8A) atrophy in 1-week-old animals. Although there was no marked changes in the percentage of T cell subsets upon THC treatment (Fig. 7B), there was a significant decrease in the absolute number of most T cell subsets in the thymus of THC-exposed animals (Fig. 7C). Likewise, splenocytes from THC-exposed animals showed a significant decrease in proliferative response to Con A, LPS, and anti-CD3 mAb compared with splenocytes from vehicle-exposed animals (Fig. 8B). Together, these data demonstrated that subchronic perinatal exposure to THC, like acute perinatal exposure to THC, negatively affects the immune system of the progeny.
Fig. 7.
Effect of subchronic perinatal exposure to THC on the thymus of 1-week-old pups. Groups of two C57BL/6 pregnant mice (n = 2) were injected with 25 mg/kg THC or vehicle on GD16 and 10 mg/ml THC or the vehicle every day thereafter until they delivered the pups (for a total of four injections, or 55 mg/kg THC). One week after the pups were born, the thymi were harvested and pooled for each mother (average 10 pups per mother). A, thymic cellularity was determined by the trypan blue dye exclusion method. The data represent mean thymic cellularity per pup + S.E.M. *, statistically significant difference compared with the vehicle control, p = 0.0196 by two-tailed unpaired Student's t test. B and C, the thymocytes were double-stained with FITC-anti-CD4 and PE-anti-CD8 mAbs and analyzed by flow cytometry. Representative dot plots are shown in B where the percentage of cells in each subset is depicted on each dot plot. Absolute numbers of cells found in each subset are shown in C. * denotes statistically significant difference compared with the vehicle control by two-tailed unpaired t test. CD4, p = 0.0116; CD8, p = 0.0178; DP, p = 0.0206; DN: p = 0.0362.
Fig. 8.
Effect of subchronic perinatal exposure to THC on the spleen of 1-week-old pups. Groups of two C57BL/6 pregnant mice (n = 2) were injected with 25 mg/kg THC or vehicle on GD16 and 10 mg/ml THC or the vehicle every day thereafter until they delivered the pups (for a total of four injections, or 55 mg/kg THC). One week after the pups were born, the spleens were harvested and pooled for each mother (average 10 pups per mother). A, splenic cellularity was determined by the trypan blue dye exclusion method. The data represent mean thymic cellularity per pup ± S.E.M. * denotes statistically significant difference compared with the vehicle control, p = 0.0201 by two-tailed unpaired Student's t test. B, the cells were left unstimulated or stimulated with Con A, LPS, or anti-CD3 mAb for 48 h. During the final 8 h, the cells were pulsed with 2 μCi of [3H]thymidine. Thymidine incorporation was determined by β-scintillation counting. The data represent mean ± S.E.M. of triplicate cultures. **, p ≤ 0.01 statistically significant difference. Two-way ANOVA, overall interaction not significant; medium versus stimulus, p < 0.0001, DMSO versus THC, p = 0.021.
Discussion
In this article, we demonstrated that exposure to THC in utero can have long-term consequences on the immune system of the offspring. Indeed, we found that acute perinatal exposure to THC triggered fetal thymic atrophy, via CB1 and CB2 signaling and induction of apoptosis. There were significant changes in the fetal thymic subpopulations, leaving the offspring with lower absolute numbers of DP T cells. We found that this effect was dose-dependent and could still be detected on PD1. In addition, we demonstrated that acute perinatal exposure to THC leads to decreased immune response to HIV-1 p17/p24/gp120. Finally, subchronic exposure to THC also led to impairment of the development and functions of thymus and the spleen.
Marijuana has been used recreationally for centuries, and research shows that it is the most commonly used drug of abuse in women of childbearing age worldwide (Hurd et al., 2005). Of the 4% of American women acknowledging the use of illegal drugs during pregnancy, 75% admit to using marijuana (Hurd et al., 2005). On the other hand, more and more studies are suggesting the use of marijuana and cannabinoids as therapeutic agents in the treatment of ailments ranging from multiple sclerosis and epilepsy (Voth and Schwartz, 1997; Berdyshev, 2000) to certain types of cancer (Schwartz et al., 1997; Berdyshev, 2000; McKallip et al., 2002a). In addition, the approval of oral THC by the Food and Drug Administration to treat nausea in patients with AIDS and undergoing cancer therapy (Schwartz et al., 1997) has encouraged some to suggest the use of marijuana to relieve morning sickness during pregnancy (Westfall et al., 2006). However, surveys in humans and studies in rodents suggest detrimental effects stemming from prenatal exposure to cannabinoids. Some studies report that children exposed to marijuana during pregnancy have a slower gestational growth rate (Hurd et al., 2005) and lower birth weight (Zuckerman et al., 1989; Hurd et al., 2005), as well as reduced gestational length (Fried et al., 1984; Hurd et al., 2005). In addition, perinatal exposure to THC has been shown to affect brain development, resulting in an alteration in behavioral responses, in both rodents and humans (Bonnin et al., 1995; Vela et al., 1995; de Moraes Barros et al., 2006). Still, very little is known about the effects of perinatal exposure to cannabinoids on the developing immune systems. Perinatal exposure to (6aR,10aR)- 9-(hydroxymethyl)- 6,6-dimethyl- 3-(2-methyloctan-2-yl)- 6a,7,10,10a-tetrahydrobenzo[c]chromen-1-ol (HU-210), a cannabinoid agonist, caused altered distribution of lymphocyte subpopulations in the spleen and peripheral blood of Wistar rats. In addition, there was a reduction in the T helper subpopulation in the spleen and a decrease in the rate of T helper/T cytotoxic cells in peripheral blood (del Arco et al., 2000).
Studies from our laboratory and others have shown that THC and other cannabinoids induce apoptosis and alter the proliferative response as well as effector functions of a variety of adult immune cells, such as thymic T cells (McKallip et al., 2002b), splenic B and T cells (McKallip et al., 2002b), natural killer cells (Patrini et al., 1997), macrophages (Sacerdote et al., 2000), and bone marrow-derived dendritic cells (Do et al., 2004), resulting in overall immunosuppression of the host (McKallip et al., 2002b; Do et al., 2004). Such studies suggest that cannabinoids may serve as a double-edged sword, on one hand exhibiting the potential to treat inflammatory diseases, while on the other hand, potentially increasing the susceptibility to cancer and infections (Nagarkatti et al., 2009, 2010; Rieder et al., 2010). In this article, we show that perinatal exposure to THC negatively affects the immune system of the offspring, potentially compromising its response to infections. In particular, there is some evidence linking the use of marijuana to a higher risk of contracting HIV (Roth et al., 2005). However, not much work has been done to study how maternal use of marijuana during pregnancy affects the offspring's risk of getting infected with HIV. Our studies demonstrate that animals that have been exposed to THC in utero have a lower immune response to HIV at 5 weeks of age, as evidenced by a decreased proliferative response to HIV-1 p17/p24/gp120 restimulation in vitro and lower HIV-1 p17/p24/gp120-specific antibody titer in the serum. These data suggest that perinatal exposure to THC may increase the child's risk of contracting infections such as HIV.
Maternal transmission of HIV still remains a global concern. According to the Joint United Nations Program on HIV/AIDS (UNAIDS/WHO 2003 Epidemic Update, available at http://www.unaids.org), at the end of 2003, an estimated 2.5 million children, under age 15, all over the world, were living with HIV/AIDS. In addition, approximately 500,000 children under 15 had died from HIV/AIDS or associated causes in that year alone. It is striking that >90% of all HIV-infected children acquire the virus from their mothers before or during birth or through breastfeeding. The precise mechanisms of HIV transmission and how the children's immune response fights against the HIV infection are not clear. Moreover, it is likely that intrauterine environmental factors that the offspring are exposed to during pregnancy could have a major impact on HIV transmission, immunosuppression, and consequent increased susceptibility to disease in the children. It is therefore expected that the risk of HIV transmission increases with drug abuse by the mother. Our studies indicate for the first time that prenatal exposure to marijuana THC may cause immunosuppression in the fetus and the neonate, thereby decreasing the ability of the newborn or neonate to fight against HIV infection acquired from maternal transmission.
Our initial experiments were conducted with an acute dose of THC because of the short gestation period of mice compared with humans. However, we realize that perinatal exposure is more likely to be chronic because of repeated consumption of marijuana over an extended period of time during the pregnancy. Therefore we conducted experiments in which mice were exposed to lower doses of THC every day from GD16 to the day that they delivered the pups, to better mimic a situation that may be encountered in humans. We found that even in this setting perinatal exposure to THC resulted in immunosuppression of the offspring, confirming the potential dangers of abusing marijuana during pregnancy.
In most experiments, we observed significant effects with a single dose of 20 mg/kg injected on GD16. This translates to a human equivalent dose of 60 mg/m2 (1.6 mg/kg) based on the body surface area normalization (Reagan-Shaw et al., 2008). The recommended dose of THC (dronabinol or Marinol) in cancer patients can be as high as 20 mg/m2/day (0.54 mg/kg), as an antiemetic during chemotherapy. Rats injected with 50 mg/kg body weight of THC had a serum concentration of 10 mM THC within 10 h of administration (Chan et al., 1996). In humans, levels as high as 1 mM in the plasma after recreational use of marijuana have been reported (Azorlosa et al., 1992), which can preferentially get concentrated 15- to 20-fold in some tissues (Johansson et al., 1989). Such levels of THC may cause significant impairment of immune cell function and increased susceptibility to infections. An experimental dose of 8 mg/kg in mice has been shown to significantly suppress the response to Legionella pneumophila infection (Klein et al., 2000). Overall, our results suggest that THC exposure during pregnancy at close to pharmacological or recreational dose can have significant effect and impair the developing immune system of fetus.
We chose to treat the animals from GD16 onward because this time point corresponds to the initial stages of fetal T cell development, with T cell receptor rearrangement, appearance of CD8 single-positive T cells, and expression of major histocompatibility complex on the stromal cells (Kingston et al., 1984; Haars et al., 1986; Pardoll et al., 1987), and exposure to toxicants at this stage may greatly influence the development of the immune system.
THC and other cannabinoids are believed to act through two distinct mechanisms. Because of its lipophilic properties, THC was initially thought to be acting via intercalation into the cell membrane. THC has now been shown to bind and signal through at least two G protein-coupled receptors, namely CB1 and CB2, although other receptors may exist (Berdyshev, 2000; Fride et al., 2003). In this study, we found that both CB1 and CB2 antagonists could prevent, at least in part, THC-induced thymic atrophy and apoptosis, suggesting the involvement of both receptors. It is still unclear whether THC affects the pups directly or indirectly by affecting the mother or placental transport. Cannabinoids have been shown to cross the placenta. Samples from the newborns such as hair, meconium, and plasma are often used to determine whether the child was exposed to marijuana in utero (Hutchings et al., 1989; Vinner et al., 2003). Moreover, studies performed with pregnant dogs injected with radioactive THC intravenously showed that THC could be detected in the brains of both mother and offspring 30 min after the injection, suggesting that once it reaches the mother's bloodstream THC can quickly reach the fetus (Martin et al., 1977). In addition, our studies involving fetal thymic organ cultures confirmed the induction of apoptosis after exposure to THC in vitro, suggesting that perinatal exposure to THC may affect the fetal thymus directly.
The idea that perinatal insult may carry long-lasting effects is not new. A relationship between low birth weight, which is the most common measure of fetal development, and the onset of high blood pressure, diabetes, stroke, and cardiovascular diseases into adulthood, a phenomenon commonly known as the “fetal origins of adult disease” is known (Holladay, 1999). However, more and more studies are now focusing on the direct effects of perinatal exposure to toxicants on the immune system, showing that exposure during fetal development may have more severe consequences than exposure during adulthood (Holladay, 1999). Studies from our laboratory and others have shown that perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin induces thymic atrophy and alterations in thymic subsets that can still be detected several days after exposure (Holladay, 1999; Camacho et al., 2004). In addition, others have shown that mice that had been exposed to chlordane during fetal development had decreased delayed-type hypersensitivity and mixed-lymphocyte reactivity as adults (Urso and Gengozian, 1984). The current study demonstrates for the first time that marijuana abuse during pregnancy may affect the immune response of the fetus that could last into the adulthood.
Together, our studies suggest that pregnant women should be cautious about the decision to use cannabinoids whether for recreational or medicinal purposes because they could potentially have lifelong consequences for the health of their child. This is especially true if the mother is infected or at risk of being infected with HIV, inasmuch as our study suggests that perinatal exposure to THC could increase the risk of contracting the disease.
This work was supported in part by the National Institutes of Health National Institute of Environmental Health Sciences [Grants R01-ES009098, R01-ES019313]; the National Institutes of Health National Institute on Drug Abuse [Grant R01-DA016545]; and the National Institutes of Health National Center for Complementary and Alternative Medicine [Grant P01-AT003961].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.181206.
- THC
- Δ9-tetrahydrocannabinol
- CB
- cannabinoid receptor
- CFA
- complete Freund's adjuvant
- FITC
- fluorescein isothiocyanate
- FTOC
- fetal thymic organ culture
- GD
- gestational day
- mAb
- monoclonal antibody
- PD
- postnatal day
- Con A
- concanavalin A
- PE
- phycoerythrin
- TUNEL
- terminal deoxynucleotidyl transferase dUTP nick end labeling
- ANOVA
- analysis of variance
- PBS
- phosphate-buffered saline
- DMSO
- dimethyl sulfoxide
- RT-PCR
- reverse transcription-polymerase chain reaction
- SR141716A
- 5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide
- AM630
- 1-[2-(morpholin-4-yl)ethyl]-2-methyl-3-(4-methoxybenzoyl)-6-iodoindole
- LPS
- lipopolysaccharide
- LN
- lymph node
- ELISA
- enzyme-linked immunosorbent assay
- SP
- single positive
- DP
- double positive
- DN
- double negative
- HU-210
- (6aR,10aR)- 9-(hydroxymethyl)- 6,6-dimethyl- 3-(2-methyloctan-2-yl)- 6a,7,10,10a-tetrahydrobenzo[c]chromen-1-ol.
Authorship Contributions
Participated in research design: M. Nagarkatti and P. S. Nagarkatti.
Conducted experiments: Lombard.
Performed data analysis: Lombard, M. Nagarkatti, and P. S. Nagarkatti.
Wrote or contributed to the writing of the manuscript: Lombard, Hegde, M. Nagarkatti, and P. S. Nagarkatti.
References
- Azorlosa JL, Heishman SJ, Stitzer ML, Mahaffey JM. (1992) Marijuana smoking: effect of varying Δ9-tetrahydrocannabinol content and number of puffs. J Pharmacol Exp Ther 261:114–122 [PubMed] [Google Scholar]
- Beal JE, Olson R, Laubenstein L, Morales JO, Bellman P, Yangco B, Lefkowitz L, Plasse TF, Shepard KV. (1995) Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage 10:89–97 [DOI] [PubMed] [Google Scholar]
- Berdyshev EV. (2000) Cannabinoid receptors and the regulation of immune response. Chem Phys Lipids 108:169–190 [DOI] [PubMed] [Google Scholar]
- Bluhm EC, Daniels J, Pollock BH, Olshan AF, and Children's Oncology Group (United States) (2006) Maternal use of recreational drugs and neuroblastoma in offspring: a report from the Children's Oncology Group (United States). Cancer Causes Control 17:663–669 [DOI] [PubMed] [Google Scholar]
- Bonnin A, de Miguel R, Hernández ML, Ramos JA, Fernández-Ruiz JJ. (1995) The prenatal exposure to Δ9-tetrahydrocannabinol affects the gene expression and the activity of tyrosine hydroxylase during early brain development. Life Sci 56:2177–2184 [DOI] [PubMed] [Google Scholar]
- Brodbeck J, Matter M, Moggi F. (2006) Association between cannabis use and sexual risk behavior among young heterosexual adults. AIDS Behav 10:599–605 [DOI] [PubMed] [Google Scholar]
- Cabral GA, Dove Pettit DA. (1998) Drugs and immunity: cannabinoids and their role in decreased resistance to infectious disease. J Neuroimmunol 83:116–123 [DOI] [PubMed] [Google Scholar]
- Camacho IA, Nagarkatti M, Nagarkatti PS. (2004) Evidence for induction of apoptosis in T cells from murine fetal thymus following perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol Sci 78:96–106 [DOI] [PubMed] [Google Scholar]
- Chan PC, Sills RC, Braun AG, Haseman JK, Bucher JR. (1996) Toxicity and carcinogenicity of Δ9-tetrahydrocannabinol in Fischer rats and B6C3F1 mice. Fundam Appl Toxicol 30:109–117 [DOI] [PubMed] [Google Scholar]
- del Arco I, Muñoz R, Rodríguez De Fonseca F, Escudero L, Martín-Calderón JL, Navarro M, Villanúa MA. (2000) Maternal exposure to the synthetic cannabinoid HU-210: effects on the endocrine and immune systems of the adult male offspring. Neuroimmunomodulation 7:16–26 [DOI] [PubMed] [Google Scholar]
- de Moraes Barros MC, Guinsburg R, de Araújo Peres C, Mitsuhiro S, Chalem E, Laranjeira RR. (2006) Exposure to marijuana during pregnancy alters neurobehavior in the early neonatal period. J Pediatr 149:781–787 [DOI] [PubMed] [Google Scholar]
- Do Y, McKallip RJ, Nagarkatti M, Nagarkatti PS. (2004) Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-κB-dependent apoptosis: novel role for endogenous and exogenous cannabinoids in immunoregulation. J Immunol 173:2373–2382 [DOI] [PubMed] [Google Scholar]
- Drumright LN, Little SJ, Strathdee SA, Slymen DJ, Araneta MR, Malcarne VL, Daar ES, Gorbach PM. (2006) Unprotected anal intercourse and substance use among men who have sex with men with recent HIV infection. J Acquir Immune Defic Syndr 43:344–350 [DOI] [PubMed] [Google Scholar]
- Fride E, Foox A, Rosenberg E, Faigenboim M, Cohen V, Barda L, Blau H, Mechoulam R. (2003) Milk intake and survival in newborn cannabinoid CB1 receptor knockout mice: evidence for a “CB3” receptor. Eur J Pharmacol 461: 27–34 [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Willan A. (1984) Marijuana use during pregnancy and decreased length of gestation. Am J Obstet Gynecol 150:23–27 [DOI] [PubMed] [Google Scholar]
- Haars R, Kronenberg M, Gallatin WM, Weissman IL, Owen FL, Hood L. (1986) Rearrangement and expression of T cell antigen receptor and γ genes during thymic development. J Exp Med 164:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde VL, Hegde S, Cravatt BF, Hofseth LJ, Nagarkatti M, Nagarkatti PS. (2008) Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: involvement of regulatory T cells. Mol Pharmacol 74: 20–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde VL, Nagarkatti M, Nagarkatti PS. (2010) Cannabinoid receptor activation leads to massive mobilization of myeloid-derived suppressor cells with potent immunosuppressive properties. Eur J Immunol 40:3358–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holladay SD. (1999) Prenatal immunotoxicant exposure and postnatal autoimmune disease. Environ Health Perspect 107(Suppl 5):687–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Wang X, Anderson V, Beck O, Minkoff H, Dow-Edwards D. (2005) Marijuana impairs growth in mid-gestation fetuses. Neurotoxicol Teratol 27:221–229 [DOI] [PubMed] [Google Scholar]
- Hutchings DE, Martin BR, Gamagaris Z, Miller N, Fico T. (1989) Plasma concentrations of Δ9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sci 44:697–701 [DOI] [PubMed] [Google Scholar]
- Johansson E, Norén K, Sjövall J, Halldin MM. (1989) Determination of Δ1-tetrahydrocannabinol in human fat biopsies from marihuana users by gas chromatography-mass spectrometry. Biomed Chromatogr 3:35–38 [DOI] [PubMed] [Google Scholar]
- Kamath AB, Xu H, Nagarkatti PS, Nagarkatti M. (1997) Evidence for the induction of apoptosis in thymocytes by 2,3,7,8-tetrachlorodibenzo-p-dioxin in vivo. Toxicol Appl Pharmacol 142:367–377 [DOI] [PubMed] [Google Scholar]
- Kingston R, Jenkinson EJ, Owen JJ. (1984) Characterization of stromal cell populations in the developing thymus of normal and nude mice. Eur J Immunol 14:1052–1056 [DOI] [PubMed] [Google Scholar]
- Klein TW, Newton CA, Nakachi N, Friedman H. (2000) Δ9-Tetrahydrocannabinol treatment suppresses immunity and early IFN-γ, IL-12, and IL-12 receptor β2 responses to Legionella pneumophila infection. J Immunol 164:6461–6466 [DOI] [PubMed] [Google Scholar]
- Martin BR, Dewey WL, Harris LS, Beckner JS. (1977) 3H-Δ9-tetrahydrocannabinol distribution in pregnant dogs and their fetuses. Res Commun Chem Pathol Pharmacol 17:457–470 [PubMed] [Google Scholar]
- McKallip RJ, Lombard C, Fisher M, Martin BR, Ryu S, Grant S, Nagarkatti PS, Nagarkatti M. (2002a) Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood 100:627–634 [DOI] [PubMed] [Google Scholar]
- McKallip RJ, Lombard C, Martin BR, Nagarkatti M, Nagarkatti PS. (2002b) Δ9-Tetrahydrocannabinol-induced apoptosis in the thymus and spleen as a mechanism of immunosuppression in vitro and in vivo. J Pharmacol Exp Ther 302:451–465 [DOI] [PubMed] [Google Scholar]
- McKallip RJ, Nagarkatti M, Nagarkatti PS. (2005) Δ9-Tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J Immunol 174:3281–3289 [DOI] [PubMed] [Google Scholar]
- Nagarkatti M, Rieder SA, Hegde VL, Kanada S, Nagarkatti P. (2010) Do cannabinoids have a therapeutic role in transplantation? Trends Pharmacol Sci 31:345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M. (2009) Cannabinoids as novel anti-inflammatory drugs. Future Med Chem 1:1333–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll DM, Fowlkes BJ, Bluestone JA, Kruisbeek A, Maloy WL, Coligan JE, Schwartz RH. (1987) Differential expression of two distinct T-cell receptors during thymocyte development. Nature 326:79–81 [DOI] [PubMed] [Google Scholar]
- Patrini G, Sacerdote P, Fuzio D, Manfredi B, Parolaro D. (1997) Regulation of immune functions in rat splenocytes after acute and chronic in vivo treatment with CP-55,940, a synthetic cannabinoid compound. J Neuroimmunol 80:143–148 [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. (2008) Dose translation from animal to human studies revisited. FASEB J 22:659–661 [DOI] [PubMed] [Google Scholar]
- Rieder SA, Chauhan A, Singh U, Nagarkatti M, Nagarkatti P. (2010) Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology 215:598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison LL, Buckley JD, Daigle AE, Wells R, Benjamin D, Arthur DC, Hammond GD. (1989) Maternal drug use and risk of childhood nonlymphoblastic leukemia among offspring. An epidemiologic investigation implicating marijuana (a report from the Childrens Cancer Study Group). Cancer 63:1904–1911 [PubMed] [Google Scholar]
- Roth MD, Tashkin DP, Whittaker KM, Choi R, Baldwin GC. (2005) Tetrahydrocannabinol suppresses immune function and enhances HIV replication in the huPBL-SCID mouse. Life Sci 77:1711–1722 [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Massi P, Panerai AE, Parolaro D. (2000) In vivo and in vitro treatment with the synthetic cannabinoid CP55, 940 decreases the in vitro migration of macrophages in the rat: involvement of both CB1 and CB2 receptors. J Neuroimmunol 109:155–163 [DOI] [PubMed] [Google Scholar]
- Schwartz RH, Voth EA, Sheridan MJ. (1997) Marijuana to prevent nausea and vomiting in cancer patients: a survey of clinical oncologists. South Med J 90:167–172 [DOI] [PubMed] [Google Scholar]
- Urso P, Gengozian N. (1984) Subnormal expression of cell-mediated and humoral immune responses in progeny disposed toward a high incidence of tumors after in utero exposure to benzo[a]pyrene. J Toxicol Environ Health 14:569–584 [DOI] [PubMed] [Google Scholar]
- Vela G, Fuentes JA, Bonnin A, Fernández-Ruiz J, Ruiz-Gayo M. (1995) Perinatal exposure to Δ9-tetrahydrocannabinol (Δ9-THC) leads to changes in opioid-related behavioral patterns in rats. Brain Res 680:142–147 [DOI] [PubMed] [Google Scholar]
- Vinner E, Vignau J, Thibault D, Codaccioni X, Brassart C, Humbert L, Lhermitte M. (2003) Neonatal hair analysis contribution to establishing a gestational drug exposure profile and predicting a withdrawal syndrome. Ther Drug Monit 25:421–432 [DOI] [PubMed] [Google Scholar]
- Voth EA, Schwartz RH. (1997) Medicinal applications of Δ9-tetrahydrocannabinol and marijuana. Ann Intern Med 126:791–798 [DOI] [PubMed] [Google Scholar]
- Westfall RE, Janssen PA, Lucas P, Capler R. (2006) Survey of medicinal cannabis use among childbearing women: patterns of its use in pregnancy and retroactive self-assessment of its efficacy against ‘morning sickness’. Complement Ther Clin Pract 12:27–33 [DOI] [PubMed] [Google Scholar]
- Zuckerman B, Frank DA, Hingson R, Amaro H, Levenson SM, Kayne H, Parker S, Vinci R, Aboagye K, Fried LE. (1989) Effects of maternal marijuana and cocaine use on fetal growth. N Engl J Med 320:762–768 [DOI] [PubMed] [Google Scholar]