Abstract
The cynomolgus monkey is widely used as a primate model in preclinical studies because of its evolutionary closeness to humans. Despite their importance in drug metabolism, the content of each cytochrome P450 (P450) enzyme has not been systematically determined in cynomolgus monkey livers. In this study, liver microsomes of 27 cynomolgus monkeys were analyzed by immunoblotting using selective P450 antibodies. The specificity of each antibody was confirmed by analyzing the cross-reactivity against 19 CYP1–3 subfamily enzymes using recombinant proteins. CYP2A, CYP2B6, CYP2C9/19, CYP2C76, CYP2D, CYP2E, CYP3A4, and CYP3A5 were detected in all 27 animals. In contrast, CYP1A, CYP1D, and CYP2J were below detectable levels in all liver samples. The average content of each P450 showed that among the P450s analyzed CYP3A (3A4 and 3A5) was the most abundant (40% of total immunoquantified P450), followed by CYP2A (25%), CYP2C (14%), CYP2B6 (13%), CYP2E1 (11%), and CYP2D (3%). No apparent sex differences were found for any P450. Interanimal variations ranged from 2.6-fold (CYP3A) to 11-fold (CYP2C9/19), and most P450s (CYP2A, CYP2D, CYP2E, CYP3A4, and CYP3A5) varied 3- to 4-fold. To examine the correlations of P450 content with enzyme activities, metabolic assays were performed in 27 cynomolgus monkey livers using 7-ethoxyresorufin, coumarin, pentoxyresorufin, flurbiprofen, bufuralol, dextromethorphan, and midazolam. CYP2D and CYP3A4 contents were significantly correlated with typical reactions of human CYP2D (bufuralol 1′-hydroxylation and dextromethorphan O-deethylation) and CYP3A (midazolam 1′-hydroxylation and 4-hydroxylation). The results presented in this study provide useful information for drug metabolism studies using cynomolgus monkeys.
Introduction
Cytochromes P450 (P450s) are a gene superfamily comprised of a large number of genes, 57 functional genes and 58 pseudogenes in humans (Nelson et al., 2004). P450s, especially the CYP1–3 family enzymes, play important roles in the metabolism of a variety of drugs and are responsible for approximately 80% of oxidative metabolism (Wilkinson, 2005). The major P450s involved in drug metabolism have been quantified in 60 human livers by immunoblotting (Shimada et al., 1994). That study found that CYP3A was most abundant in total hepatic P450 content, followed by CYP2C, CYP1A2, CYP2E1, CYP2A6, CYP2D6, and CYP2B6. A similar study conducted in human small intestine found that CYP3A was most abundant, followed by CYP2C, CYP2J2, and CYP2D6 (Paine et al., 2006). These studies provided useful information for understanding drug biotransformation in humans.
Cynomolgus monkey (Macaca fascicularis) is a primate species widely used in drug metabolism studies. More than 20 P450s have been identified in cynomolgus monkey, and these enzymes are highly identical to orthologous human P450s (Uno et al., 2011a). The only exception is CYP2C76 that is not orthologous to any human P450 and is expressed as a functional drug-metabolizing enzyme in liver (Uno et al., 2010a). In cynomolgus monkey liver, other CYP2C genes encoding functional drug-metabolizing enzymes are also expressed, including CYP2C8, CYP2C9, and CYP2C19 (Uno et al., 2006). In this article, cynomolgus P450s are designated as recommended by the P450 Nomenclature Committee (http://drnelson.uthsc.edu/cytochromeP450.html) (Uno et al., 2011a). The cynomolgus CYP3A subfamily includes CYP3A4 and CYP3A5, which are predominantly expressed in liver (Uno et al., 2007a) and encode enzymes involved in the metabolism of human CYP3A substrates, such as midazolam and nifedipine (Iwasaki et al., 2010; Uno et al., 2010c). Likewise, other cynomolgus P450 subfamilies including CYP1A (CYP1A1 and CYP1A2), CYP2A (CYP2A23, CYP2A24, and CYP2A26), CYP2B (CYP2B6), CYP2D (CYP2D17 and CYP2D44), and CYP2E (CYP2E1), are also predominantly expressed in liver and encode the proteins that metabolize the substrates of orthologous human P450s (Uno et al., 2007a, 2009b, 2010d, 2011d; Uehara et al., 2010). Cynomolgus CYP1D1 is orthologous to human CYP1D1P and is expressed in liver at a comparable level to CYP1A1, but is much more abundant than CYP1A2 (Uno et al., 2011d). Cynomolgus CYP2J2 is preferentially expressed in liver, along with kidney and jejunum (Uno et al., 2007a), although its function remains to be characterized.
Despite the importance of cynomolgus monkey in drug metabolism studies, the expression of the major P450 enzymes has not been systematically examined in cynomolgus monkey liver. In this study, the major P450s were measured in liver microsomes of 27 cynomolgus monkeys by immunoblotting using selective antibodies. Analyzed P450s included CYP1A(1/2), CYP1D1, CYP2A(23/24/26), CYP2B6, CYP2C9/19, CYP2C76, CYP2D(17/44), CYP2E1, CYP2J2, CYP3A4, and CYP3A5. The specificity of the antibodies was assessed using the recombinant proteins of 19 cynomolgus P450s. The specific contents of these P450s were calculated and presented as mean values and interanimal variations.
Materials and Methods
Chemicals and Materials.
Polyclonal anti-human CYP1A1, anti-human CYP2A6, anti-human CYP2E1, anti-human CYP2C9, anti-human CYP2D6, and anti-human CYP3A4 antibodies were purchased from Nosan Corporation (Yokohama, Japan), and polyclonal anti-human CYP2B6 and anti-human CYP3A5 antibodies were purchased from BD Gentest (Woburn, MA). Polyclonal anti-human CYP2J2 and anti-cynomolgus CYP2C76 antibodies were prepared as described previously (King et al., 2002; Uno et al., 2006). The secondary antibodies (donkey anti-goat and sheep anti-rabbit horseradish peroxidase-conjugated IgGs) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and SurModics, Inc. (Eden Prairie, MN), respectively. Chemicals and reagents for the polyacrylamide gels, including SDS, bis/acrylamide (37.5:1), ammonium persulfate, and TEMED were purchased from Bio-Rad Laboratories (Hercules, CA). All other chemicals were of analytical grade from Sigma-Aldrich (St. Louis, MO), unless otherwise specified.
Animals, Tissues, and Microsomal Preparation.
Liver samples were collected from 27 cynomolgus monkeys (14 males and 13 females from Indochina or Indonesia, 4–9 years of age). This study was reviewed and approved by the Institutional Animal Care and Use Committee at Shin Nippon Biomedical Laboratories, Ltd. (Kainan, Japan). Each liver sample was homogenized in a 9-fold volume of 0.25 M Tris-buffer sucrose solution, pH 7.4, under ice-cold conditions, followed by centrifugation at 9000g for 30 min at 4°C. The resultant supernatants were centrifuged at 105,000g for 1 h at 4°C, and the microsomal pellets were resuspended in 0.25 M Tris-buffer sucrose solution, pH 7.4. Protein concentrations of the prepared microsomes were measured by the Bradford method using Bio-Rad Protein Assay Kit (Bio-Rad Laboratories) with serum albumin as the standard.
Heterologous Expression of P450s in Escherichia coli.
The recombinant proteins of 19 cynomolgus P450s (CYP1A1, CYP1A2, CYP1D1, CYP2A23, CYP2A24, CYP2A26, CYP2B6, CYP2C18, CYP2C8, CYP2C9, CYP2C19, CYP2C76, CYP2D17, CYP2D44, CYP2E1, CYP2J2, CYP3A4, CYP3A5, or CYP3A43) were expressed in E. coli, and membrane preparations were performed as described previously (Uno et al., 2006, 2007a, 2009b, 2010b, 2011d; Uehara et al., 2010). For expression of cynomolgus CYP2J2 recombinant protein, the N-terminus modification was conducted by polymerase chain reaction with the forward and reverse primers, 5′-GGAATTCCATATGGCTCTGTTATTAGCAGTTTTTGCGGCTGCCCTCTGGG-3′ and 5′-GCTCTAGAGCAAAATCACACCCGAGGAAC-3′, respectively. The NdeI and XbaI sites (underlined) in the forward and reverse primers, respectively, were used for subcloning of polymerase chain reaction products into pCW vectors that contained human NADPH-P450 reductase cDNA. The content of each P450 protein in the membrane preparation was determined by Fe2+ · CO versus Fe2+ difference spectra as described previously (Omura and Sato, 1964).
Immunoblotting.
To measure the expression of P450 proteins in cynomolgus monkey liver, immunoblotting was performed as described previously (Uno et al., 2006). In brief, specificity of each antibody was assessed using recombinant proteins (1.0 pmol each) of the 19 cynomolgus P450 proteins, which were fractionated in 10% SDS polyacrylamide gels and transferred to Hybond-P filters (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). The filters were immunoblotted with primary antibody (1:200–1:100,000), including polyclonal anti-human CYP1A1, anti-human CYP2A6, anti-human CYP2B6, anti-human CYP2C9, anti-cynomolgus CYP2C76, anti-human CYP2D6, anti-human CYP2E1, anti-human CYP2J2, anti-human CYP3A4, and anti-human CYP3A5 antibodies. The filters were then immunoblotted with secondary antibody (1:5000), and developed using an enhanced chemiluminescence Western blotting detection reagent (GE Healthcare) and autoradiography. The developed films were scanned with a desktop scanner, and the optical density of the bands was quantified using Image J software (National Institutes of Health, Bethesda, MD). Standard curves for quantification were generated using the recombinant P450. For CYP2A, CYP2C9/19, and CYP2D, the recombinant protein of CYP2A23, CYP2C9, and CYP2D17 was used. Pilot experiments for each antibody and five representative liver samples were conducted and it was decided to load 5 μg of microsomal proteins in gels to keep the densities of the protein bands within the linear range of the standard curves. Limits of detection are provided in Table 1. Each liver sample was analyzed in duplicate with each P450 antibody. The amount of each P450 protein per lane was calculated relative to the standard curve and was divided by the amount of total protein loaded to determine specific content.
TABLE 1.
Individual P450 contents in cynomolgus monkey liver
| P450 | P450 Content (Mean ± S.D.) |
Range | Detection Limit | ||
|---|---|---|---|---|---|
| Total | Male | Female | |||
| pmol/mg | |||||
| Total P450a | 724 ± 192 | 771 ± 156 | 674 ± 220 | 275–1141 | N.A. |
| CYP1A | BDL | BDL | BDL | BDL | 0.01 |
| CYP1D | BDL | BDL | BDL | BDL | 0.01 |
| CYP2A | 26 ± 7.2 | 24 ± 6.7 | 28 ± 7.2 | 14–41 | 0.025 |
| CYP2B6 | 14 ± 6.5 | 14 ± 6.3 | 14 ± 6.9 | 3.3–26 | 0.025 |
| CYP2C9/19 | 11 ± 3.8 | 9.8 ± 4.6 | 12 ± 2.4 | 1.5–16 | 0.01 |
| CYP2C76 | 4.3 ± 2.0 | 4.3 ± 1.7 | 4.3 ± 2.4 | 1.4–8.5 | 0.01 |
| CYP2D | 3.2 ± 0.7 | 3.3 ± 0.6 | 3.1 ± 0.8 | 1.4–4.6 | 0.00025 |
| CYP2E1 | 12 ± 2.7 | 12 ± 3.1 | 11 ± 2.2 | 5.5–17 | 0.01 |
| CYP2J2 | BDL | BDL | BDL | BDL | 0.01 |
| CYP3A4 | 27 ± 5.3 | 29 ± 2.5 | 26 ± 6.9 | 10–34 | 0.01 |
| CYP3A5 | 9.0 ± 3.1 | 10 ± 3.5 | 7.9 ± 2.3 | 4.8–21 | 0.01 |
| CYP3A4 + 3A5 | 36 ± 6.3 | 39 ± 4.6 | 34 ± 6.9 | 19–49 | N.A. |
| Totalb | 106 ± 14 | 106 ± 10 | 106 ± 18 | 81–130 | N.A. |
N.A., not available; BDL, below detection limit.
Spectrally determined P450.
Sum of the immunoquantified P450s.
Enzyme Assays.
Drug-metabolizing enzyme activities were measured using typical human P450 substrates (bufuralol, coumarin, dextromethorphan, 7-ethoxyresorufin, midazolam, pentoxyresorufin, and progesterone) as described previously (Yamazaki and Shimada, 1997; Yamazaki et al., 2002; Emoto et al., 2009). In brief, each mixture (0.20 ml) contained liver microsomes (20 μg of protein), an NADPH-generating system (0.25 mM NADP+, 2.5 mM glucose 6-phosphate, and 0.25 unit/ml glucose 6-phosphate dehydrogenase), and substrate (20 μM bufuralol, 10 μM coumarin, 200 μM dextromethorphan, 10 μM 7-ethoxyresorufin, 100 μM midazolam, 10 μM pentoxyresorufin, or 100 μM progesterone) in 50 to 100 mM potassium phosphate buffer, pH 7.4. After incubation at 37°C for 10 min, reactions were terminated by adding 0.40 ml of ice-cold methanol, 10 μl of 60% perchloric acid, or 1.5 ml of ethyl acetate. After centrifugation at 1500g for 10 min, the supernatant or extract was analyzed by reverse-phase high-performance liquid chromatography with a fluorescence or UV detector. Metabolic assays using diclofenac and testosterone as substrates were carried out as described previously (Nakanishi et al., 2011). To estimate a correlation between drug-metabolizing enzyme activities and P450 amounts, linear regression analysis was performed using Origin7.5J software (OriginLab Corp., Northampton, MA).
Results
Specificity of the P450 Antibodies.
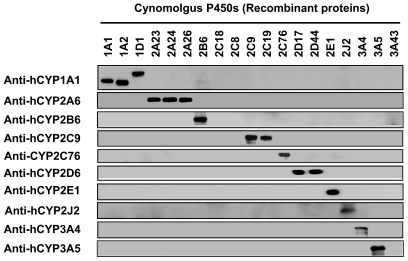
Because most antibodies used were originally raised against human P450 proteins, the specificity of each P450 antibody was assessed by Western blotting using the recombinant proteins of 19 cynomolgus P450s. CYP2A23/24/26, CYP2B6, CYP2C9/19, CYP2C76, CYP2D17/44, CYP2E1, CYP2J2, CYP3A4, and CYP3A5 were selectively detected by the antibodies used (Fig. 1). Anti-human CYP1A1 antibody reacted with CYP1A1/2 and CYP1D1 of cynomolgus monkey, but the size differences allowed the specific detection of these P450s. Cynomolgus CYP2C18, CYP2C8, and CYP3A43 were not reacted with any antibody used.
Fig. 1.
Immunoblots demonstrating specificity of anti-P450 antibodies. To assess the specificity of the antibodies, the recombinant proteins of cynomolgus P450s (0.1 pmol of P450/lane) were immunoblotted using anti-human (h) P450 antibody or anti-cynomolgus CYP2C76 antibody.
P450 Content in Cynomolgus Monkey Liver.
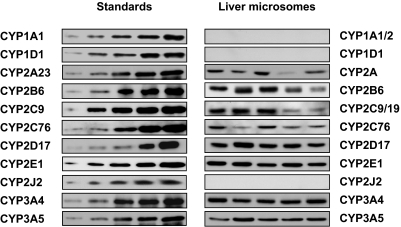
P450 expression was measured in the livers of 27 cynomolgus monkeys by immunoblotting using the selective antibodies. The blots of the standard curves and the five representative liver samples are shown in Fig. 2. Among the 11 P450 antibodies used, 9 detected P450 proteins in all liver samples, whereas no bands were observed in any of these liver samples using the anti-human CYP1A1 or CYP2J2 antibody (Fig. 2). Therefore, cynomolgus CYP2A23/24/26, CYP2B6, CYP2C9/19, CYP2C76, CYP2D17/44, CYP2E1, CYP3A4, and CYP3A5 proteins were quantified in 27 liver samples. Among these P450s, CYP3A4 content averaged 27 pmol/mg protein, ranging from 10 to 34 pmol/mg protein, and was the highest of all of the P450s examined in cynomolgus monkey liver (Table 1). CYP3A5 content averaged 9.0 pmol/mg protein, ranging from 4.8 to 21 pmol/mg protein (Table 1). Hence, total CYP3A (CYP3A4 and CYP3A5) content was 36 pmol/mg protein, ranging from 19 to 49 pmol/mg protein, making CYP3A the most abundant subfamily in cynomolgus monkey livers. This was followed by CYP2A, CYP2B6, CYP2E1, CYP2C9/19, CYP2C76, and CYP2D, which averaged 26, 14, 12, 11, 4.3, and 3.2 pmol/mg protein, respectively (Table 1). P450 content was not substantially different (>1.5-fold) between males and females.
Fig. 2.
Immunoblotting of cynomolgus monkey liver microsomal proteins. The amounts of recombinant proteins used as standards ranged from 0.01 to 0.1, 0.025 to 0.125, 0.025 to 0.3, 0.025 to 0.15, 0.01 to 0.1, 0.01 to 0.1, 0.00025 to 0.005, 0.01 to 0.1, 0.01 to 0.1, 0.01 to 0.25, and 0.01 to 0.25 pmol for cynomolgus CYP1A1, CYP1D1, CYP2A23, CYP2B6, CYP2C9, CYP2C76, CYP2D17, CYP2E1, CYP2J2, CYP3A4, and CYP3A5, respectively. Liver microsomes (5 μg/lane) were analyzed for each animal. The results of five animals are shown as representative examples.
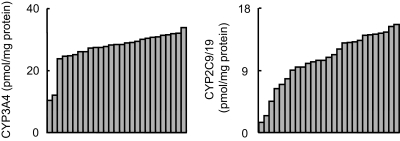
The content of most P450 proteins varied 3- to 4-fold in the animals analyzed, including CYP2A, CYP2D, CYP2E1, CYP3A4, and CYP3A5 (Table 1). The variations were even less for total CYP3A content (2.6-fold). The larger interanimal variations were observed for CYP2B6 (7.9-fold), CYP2C9/19 (11-fold), and CYP2C76 (6.3-fold) (Table 1). The differences in the interanimal variations were remarkable between CYP3A4 and CYP2C9/19, which showed the smallest and largest degree of variations in 27 animals, respectively (Fig. 3). Because of the interanimal variations, CYP2A was the most abundant P450 in three animals, whereas CYP3A was the most abundant in the rest of the animals. Likewise, CYP2C9/19 was more abundant than CYP2C76 in most animals, but CYP2C76 was more abundant than CYP2C9/19 in two animals. The CYP3A4 amount varied 3.3-fold in 27 animals, among which two animals expressed CYP3A4 approximately 2-fold less than others (Fig. 3). When these two animals were excluded, the amount of CYP3A4 varied only 1.4-fold in the 25 animals.
Fig. 3.
Variations of hepatic P450 content in 27 cynomolgus monkeys. The amounts of CYP2C9/19 and CYP3A4 immunoquantified in 27 cynomolgus monkey livers are shown. Among 27 liver samples, the CYP2C9/19 amount varied the most, whereas the CYP3A4 amount varied the least.
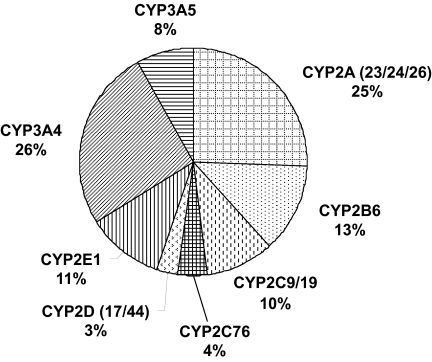
Total immunoquantified P450s averaged 106 pmol/mg protein, ranging from 81 to 130 pmol/mg protein, and were lower than spectrally determined P450s. Using this value, the content of each P450, expressed as a percentage of total P450s, ranged from 23 to 44, 11 to 34, 14 to 42, 4 to 24, 5 to 17, 2 to 16, 5 to 18, 2 to 8, and 2 to 5%, for CYP3A, CYP3A4, CYP2A, CYP2B6, CYP2E1, CYP2C9/19, CYP3A5, CYP2C76, and CYP2D, respectively, and the average values generally followed this trend (Fig. 4). These results indicated that CYP3A is the most abundant subfamily in cynomolgus monkey liver.
Fig. 4.
The cynomolgus monkey hepatic P450 pie. For each P450, mean expression values are expressed as percentages of the total immunoquantified P450 content.
Drug-Metabolizing Enzyme Activities.
To assess the correlation between quantified P450 amount and drug-metabolizing enzyme activities, enzyme activities were measured using liver microsomes of 27 cynomolgus monkeys. We examined bufuralol 1′-hydroxylation, coumarin 7-hydroxylation, dextromethorphan N- and O-deethylation, diclofenac 4′-hydroxylation, 7-ethoxyresorufin O-deethylation, midazolam 1′- and 4-hydroxylation, pentoxyresorufin O-deethylation, progesterone 6β-hydroxylation, and testosterone 2α-, 6β-, 16α-, and 16β-hydroxylation. The correlation coefficients indicated that among the P450s analyzed CYP2D was highly correlated with bufuralol 1′-hydroxylation and dextromethorphan O-deethylation, whereas CYP3A and CYP3A4 were highly correlated with midazolam 1′-hydroxylation, midazolam 4-hydroxylation, and testosterone 6β-hydroxylation (Table 2). Significant correlation coefficients were also observed for CYP3A4 (dextromethorphan N-deethylation and progesterone 6β-hydroxylation), CYP2B6 (testosterone 16β-hydroxylation), and CYP2C9/19 (diclofenac 4-hydroxylation) (Table 2). Other occasional correlations were found for CYP2A (7-ethoxyresorufin O-deethylation), CYP2B6 (midazolam 1′- and 4-hydroxylation), CYP2C9/19 (midazolam 4-hydroxylation), CYP2C76 (bufuralol 1′-hydroxylation, 7-ethoxyresorufin O-deethylation, and pentoxyresorufin O-deethylation), CYP2D (coumarin 7-hydroxylation), and CYP3A5 (bufuralol 1′-hydroxylation and testosterone 2α- and 16α-hydroxylation) (Table 2). No correlation was found for CYP2E1.
TABLE 2.
Correlation coefficients (r) between P450 amount and drug-metabolizing enzyme activities in cynomolgus monkey livers
Metabolic activities were measured as described under Materials and Methods. Values are mean ± S.D. for 27 cynomolgus monkeys.
| Activity | Total P450 | 2A | 2B6 | 2C9/19 | 2C76 | 2D | 2E1 | 3A4 | 3A5 | 3A | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| nmol/min/mg protein | |||||||||||
| Bufuralol 1′-hydroxylation | 0.79 ± 0.26 | 0.29 | 0.25 | −0.12 | 0.15 | 0.43* | 0.65*** | −0.09 | −0.37 | 0.42* | −0.10 |
| Coumarin 7-hydroxylation | 0.097 ± 0.061 | 0.15 | 0.14 | −0.11 | −0.15 | 0.12 | 0.44* | 0.08 | −0.26 | −0.02 | −0.22 |
| Dextromethorphan N-deethylation | 0.12 ± 0.047 | 0.11 | 0.37 | −0.18 | −0.31 | 0.01 | 0.08 | 0.13 | 0.46** | 0.02 | 0.20 |
| Dextromethorphan O-deethylation | 0.63 ± 0.52 | 0.18 | 0.31 | 0.08 | −0.01 | 0.33 | 0.66*** | 0.10 | −0.51 | 0.16 | −0.35 |
| Diclofenac 4-hydroxylation | 0.13 ± 0.033 | 0.30 | 0.02 | −0.18 | 0.38* | 0.16 | 0.12 | 0.07 | 0.22 | 0.31 | 0.34 |
| 7-Ethoxyresorufin O-deethylation | 0.092 ± 0.056 | 0.06 | 0.47* | −0.01 | −0.22 | 0.39* | 0.04 | −0.22 | −0.25 | −0.21 | −0.31 |
| Midazolam 1′-hydroxylation | 1.78 ± 0.56 | 0.44* | 0.08 | 0.43* | 0.32 | 0.28 | 0.11 | −0.16 | 0.51** | 0.22 | 0.53** |
| Midazolam 4-hydroxylation | 1.33 ± 0.41 | 0.40* | 0.12 | 0.44* | 0.45* | 0.33 | −0.05 | −0.25 | 0.69*** | 0.07 | 0.61*** |
| Pentoxyresorufin O-deethylation | 0.0031 ± 0.00092 | 0.43* | 0.32 | 0.05 | 0.11 | 0.39* | −0.15 | −0.11 | 0.32 | −0.03 | 0.25 |
| Progesterone 6β-hydroxylation | 3.01 ± 0.94 | 0.32 | 0.29 | 0.16 | 0.11 | 0.16 | −0.16 | 0.02 | 0.51** | 0.04 | 0.44* |
| Testosterone 2α-hydroxylation | 0.16 ± 0.027 | 0.01 | −0.02 | 0.08 | 0.02 | −0.14 | 0.06 | 0.02 | 0.07 | 0.43* | 0.27 |
| Testosterone 6β-hydroxylation | 4.87 ± 1.16 | 0.31 | 0.22 | 0.07 | 0.07 | 0.11 | −0.08 | −0.06 | 0.64*** | 0.06 | 0.56** |
| Testosterone 16α-hydroxylation | 0.11 ± 0.074 | 0.12 | −0.01 | −0.39 | −0.07 | 0.05 | 0.22 | −0.07 | −0.35 | 0.50** | −0.04 |
| Testosterone 16β-hydroxylation | 0.29 ± 0.070 | 0.29 | 0.13 | 0.42* | −0.02 | 0.23 | 0.23 | −0.08 | 0.25 | 0 | 0.21 |
Statistical significance was determined based on the P value (probability that r is zero) of the linear regression: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Discussion
In this study, the amount of P450 proteins was immunoquantified individually in 27 cynomolgus monkey livers using anti-P450 antibodies with their specificities confirmed on cynomolgus P450 proteins. The P450 enzymes were selected based on gene and protein expression results that have been reported previously. We have analyzed the macaque genome to identify and characterize cynomolgus P450s orthologous to human P450s that are relevant to drug metabolism in the CYP1–3 family (Uno et al., 2011a), and all of the P450 proteins that were expressed at detectable levels by immunoblotting (Fig. 2) were quantified in this study.
As shown by average immunoquantified P450 values in cynomolgus monkey liver, CYP3A4 represented the most abundant P450, making CYP3A (CYP3A4 plus CYP3A5) the most abundant P450 subfamily in this species. In human liver, CYP3A is also the most abundant P450 subfamily and constitutes approximately 40% of total immunoquantified P450 content (Shimada et al., 1994), similar to that of cynomolgus monkey (35%) as shown in this study. Moreover, CYP3A4 and total CYP3A content varied 3.3- and 2.6-fold, respectively, and these contents (CYP3A4 and total CYP3A) varied even less (1.4-fold) when two animals showing low expression were excluded. In contrast, human CYP3A4 content seems to vary nearly 60-fold (Wrighton et al., 1990; Mimura et al., 1993; Stevens et al., 1993; Shimada et al., 1994), and the variation is approximately 6-fold even when the outliers from the data set are excluded. Therefore, the variation of hepatic CYP3A4 content is much smaller in cynomolgus monkeys than in humans.
In humans, the variation in CYP3A4 hepatic content seems to be accounted for by regulatory factors, including pregnane X receptor, but less likely by genetic variants (Stevens, 2006). Cynomolgus CYP3A4 is predominantly expressed in liver (Uno et al., 2007a) and can be substantially induced by P450 inducer such as rifampicin via pregnane X receptor, similar to human CYP3A4 (Kim et al., 2010), suggesting that cynomolgus monkeys and humans share transcriptional regulatory mechanisms for CYP3A4. Moreover, cynomolgus CYP3A4 metabolizes various human CYP3A4 substrates (e.g., midazolam, nifedipine, and dexamethasone), but not the substrates largely metabolized by other P450 subfamily enzymes (Iwasaki et al., 2010). This is further supported by high correlation coefficients observed between CYP3A4 content and catalytic activities for human CYP3A substrates, such as midazolam, indicating the similar substrate selectivity of CYP3A4 in cynomolgus monkeys and human. Based on similarities in hepatic content and substrate selectivity of CYP3A4, regulatory mechanism for CYP3A4, and small interanimal variations of cynomolgus CYP3A4, cynomolgus monkey would be a suitable animal species to investigate a CYP3A-dependent drug metabolism in liver.
CYP2A was the second most abundant P450 subfamily in cynomolgus monkey liver, representing 25% of total immunoquantified P450 content. In human liver, CYP2A6 represents 6% (14 pmol/mg protein) of total immunoquantified P450 content, less than that (26 pmol/mg protein) of cynomolgus monkey. In human liver, CYP2A6 is the major CYP2A expressed, whereas CYP2A23, CYP2A24, and CYP2A26 are expressed in cynomolgus monkey liver and metabolize the human CYP2A substrate coumarin (Uehara et al., 2010). A larger number of CYP2A enzymes and their predominant expression in liver might partly account for more abundant CYP2A proteins and the higher rate of coumarin 7-hydroxylation in cynomolgus monkey liver than in human liver (Sharer et al., 1995; Bogaards et al., 2000). The abundance of CYP2A protein suggests the possible critical role of CYP2A for drug metabolism in cynomolgus monkey liver.
CYP2C was the third most abundant P450 subfamily in cynomolgus monkey liver, representing 14% of total immunoquantified P450s, including CYP2C9/19 (10%) and CYP2C76 (4%). The CYP2C subfamily represents the second most abundant P450 subfamily in human liver, constituting 25% of total immunoquantified P450s (Shimada et al., 1994). In liver, human CYP2C content (60 pmol/mg protein) is more abundant than cynomolgus CYP2C content (15 pmol/mg protein). Less CYP2C protein might partly account for the lower rate of tolbutamide 4-hydroxylation (Weaver et al., 1999; Turpeinen et al., 2007), which cynomolgus CYP2C9 and CYP2C76 catalyze (Uno et al., 2006, 2007b). In this study, CYP2C8 was not analyzed, because specific antibody for cynomolgus CYP2C8 was not available. CYP2C8 is one of the major functional CYP2C enzymes in human liver. CYP2C8 content, if quantified, would provide more accurate CYP2C content in cynomolgus monkey liver.
CYP2C76 protein content (4%) was less than CYP2C9/19 protein content (10%) in cynomolgus monkey liver. A previous study showed that CYP2C76 mRNA was most abundantly expressed in cynomolgus monkey liver among the major CYP2C mRNAs (Uno et al., 2006). In this study, CYP2C76 was more abundant in CYP2C9/19 in only 2 of the 27 animals analyzed. This discrepancy is most likely accounted for by the interanimal variations in expression of CYP2C9/19 and CYP2C76 proteins, which varied 11- and 6.3-fold, respectively, in this study. Moreover, the content of CYP2C9 and CYP2C19 might also vary among animals, although their contents were not measured separately in this study. Therefore, the most abundant CYP2C enzyme might be different in each animal.
Cynomolgus CYP2B6, the only CYP2B enzyme in cynomolgus monkey, represented 13% of total immunoquantified P450s in liver. In human liver, previous reports indicated that CYP2B6 amount was 1 pmol/mg protein, constituting <1% of total P450 content (Shimada et al., 1994). However, studies using selective antibodies demonstrated that mean CYP2B6 content in human liver was higher, ranging from 2 to 82 pmol/mg protein (Stresser and Kupfer, 1999) and 0.7 to 71 pmol/mg protein (Ekins et al., 1998), making CYP2B6 content 6% of total hepatic P450 content (Stresser and Kupfer, 1999). In human liver, a large variation has been observed in CYP2B6 content. CYP2B6 content varied 108-fold (Shimada et al., 1994) and 100-fold (Ekins et al., 1998), representing the largest interindividual variations among the P450s analyzed (Shimada et al., 1994). In this study, relatively large interanimal differences (7.9-fold) among the P450s analyzed were observed in CYP2B6 content of cynomolgus monkey liver. These interindividual variations might account for variation in a CYP2B6-dependent drug metabolism in cynomolgus monkey as well as human.
Cynomolgus CYP2E1, the only cynomolgus CYP2E enzyme, represented 11% of total immunoquantified P450s in liver. In human, CYP2E1 content is 22 pmol/mg protein, representing 9% of total immunoquantified P450s (Shimada et al., 1994). Less CYP2E1 content (12 pmol/mg protein) of cynomolgus monkey might account for the lower rate of aniline p-hydroxylation (metabolized by human CYP2E1) in cynomolgus monkey liver than in human liver (Shimada et al., 1997).
CYP2D represented 3% of total immunoquantified P450 content in cynomolgus monkey liver. Likewise, human CYP2D6, orthologous to cynomolgus CYP2D17/44, constitutes 4% of total immunoquantified P450 content in liver (Shimada et al., 1994). The previous studies showed that the rate of the reactions catalyzed by CYP2D enzymes (i.e., bufuralol 1′-hydroxylation, dextromethorphan O-demethylation) was higher in cynomolgus monkey liver than in human liver (Sharer et al., 1995; Weaver et al., 1999). CYP2D17/44 content was 3.2 pmol/mg protein, similar to that of human CYP2D6 (5 pmol/mg protein) (Shimada et al., 1994). Thus, the higher rate of CYP2D-dependent reaction in cynomolgus monkey liver might be partly caused by the faster rate of cynomolgus CYP2D enzyme, as shown previously (Mankowski et al., 1999; Uno et al., 2010d). In human, CYP2D6, involved in the metabolism of approximately 25% of known drugs in the market, is highly polymorphic, leading to interindividual variations in response to drugs that are metabolized by CYP2D6 (Ingelman-Sundberg, 2005). Genetic polymorphisms have been also identified in cynomolgus P450 genes (Uno et al., 2009c, 2010c). Genetic polymorphisms in CYP2D genes, if any, might account for the higher rate of the CYP2D-dependent reaction in some animals.
In this study, CYP1A1/2 by the anti-human CYP1A1 antibody, was not detected in monkey liver (< 0.01 pmol/mg protein). In contrast, CYP1A2 is abundantly expressed and constitutes 18% of total immunoquantified P450 content in human liver (Shimada et al., 1994). Previous studies also reported that the proteins that reacted with anti-CYP1A antibody were not detected or barely detected in untreated cynomolgus monkey liver, but highly induced by P450 inducers such as β-naphthoflavone, 3-methylcholanthrene, and 2,3,7,8-tetrachlorodibenzo-p-dioxin (Edwards et al., 1994; Bullock et al., 1995; Sadrieh and Snyderwine, 1995). CYP1A1 mRNA is highly induced in cynomolgus monkey hepatocyte culture by the P450 inducer omeprazole (Nishimura et al., 2007; Ise et al., 2011), suggesting that CYP1A is induced to a sufficient level to play a functional role, upon exposure to exogenous compounds. Likewise, CYP2J2 was not detected in cynomolgus monkey liver using anti-human CYP2J2 antibody (< 0.01 pmol/mg protein). CYP2J2 mRNA is expressed in cynomolgus monkey liver (Uno et al., 2007a). The anti-human CYP2J2 antibody used might not be sensitive enough to detect CYP2J2 expression in cynomolgus monkey liver.
In this study, total immunoquantified P450s represented 15% of spectrally determined P450 content in 27 cynomolgus monkeys, lower than human liver where 72% of spectrally determined P450 content are total immunoquantified P450s (Shimada et al., 1994). This raises the possibility that other enzymes such as CYP4A and CYP4F enzymes are abundantly expressed in cynomolgus monkey liver. Indeed, CYP4A and CYP4F mRNAs are predominantly expressed in cynomolgus monkey liver (Uno et al., 2007a), and CYP4F enzymes are involved in the metabolism of drugs in cynomolgus monkey small intestine (Hashizume et al., 2001; Nishimuta et al., 2011). It is of great interest to measure the CYP4 family enzymes in cynomolgus monkey liver.
The correlation of P450 enzyme amounts to enzyme activities showed relatively high correlations for CYP2D with bufuralol 1′-hydroxylation and dextromethorphan O-deethylation, which are catalyzed by cynomolgus CYP2D (Uno et al., 2010d). Likewise, CYP3A4 was highly correlated with midazolam 1′-hydroxylation and midazolam 4-hydroxylation, which are catalyzed by cynomolgus CYP3A4 (Iwasaki et al., 2010). Other significant correlations found also coincided well with previous studies: testosterone 16β-hydroxylation by CYP2B6 (Uno et al., 2009b), diclofenac 4-hydroxylation by CYP2C9/19 (Uno et al., 2011c), bufuralol 1′-hydroxylation and 7-ethoxyresorufin O-deethylation by CYP2C76 (Uno et al., 2011b), dextromethorphan N-deethylation by CYP3A4 (Iwasaki et al., 2010), and bufuralol 1′-hydroxylation by CYP3A5 (Iwasaki et al., 2010). In contrast, CYP2A content was not well correlated with coumarin 7-hydroxylation, which is catalyzed by cynomolgus CYP2A (Uno et al., 2007a; Uehara et al., 2010). A previous study showed that cynomolgus CYP2A23, CYP2A24, and CYP2A26 catalyzed coumarin 7-hydroxylation, but the efficiency varied between the enzymes (Uehara et al., 2010). Because the content of each CYP2A enzyme might vary in animal livers, the apparent low correlation of cynomolgus CYP2A with coumarin 7-hydroxylation might be accounted for by the variable amount of each CYP2A enzyme in the animal livers analyzed.
Significant correlation coefficients between metabolic activity and P450 content were generally smaller in cynomolgus monkeys than in humans. For example, in cynomolgus monkeys, correlation coefficients were 0.65 between CYP2D content and bufuralol 1′-hydroxylation and 0.64 between CYP3A4 content and testosterone 6β-hydroxylation, which were 0.80 and 0.81 in humans, respectively (Shimada et al., 1994). Lower correlation coefficients in cynomolgus monkeys can be simply attributable to the fact that these substrates were selected for human P450s, not cynomolgus P450s. In addition, the involvement of other P450s in these reactions might also account for lower correlation coefficients in cynomolgus monkeys; bufuralol 1′-hydroxylation is also catalyzed by CYP2C76 (Uno et al., 2011b) and CYP3A5 (Iwasaki et al., 2010). CYP2C76, not orthologous to any human P450, is partly responsible for differences in pitavastatin metabolism between cynomolgus monkeys and humans (Uno et al., 2010a). This information needs to be carefully considered when conducting drug metabolism studies using cynomolgus monkeys.
In summary, immunoquantification of P450 enzymes revealed that CYP3A was the most abundant P450 subfamily in cynomolgus monkey liver, similar to human liver, representing 35% of total immunoquantified P450 content, followed by CYP2A (25%), CYP2C (14%), CYP2B6 (13%), CYP2E1 (11%), and CYP2D (3%). Interanimal variations were observed, generally 3- to 4-fold for most P450s including CYP3A4. This degree of variation is much less than that in human. The results provide essential information for better understanding drug metabolism in cynomolgus monkey and estimating the contribution of the P450 enzymes for the metabolism of drugs in development.
Acknowledgments
We thank Dr. Ryoichi Nagata, Dr. Koichiro Fukuzaki, Masahiro Utoh, and Dr. Chika Nakamura for supporting this work and Patrick Gray for reviewing the manuscript.
This research was supported, in part, by the Intramural Research Program of the National Institutes of Health National Institute of Environmental Health Sciences.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.185009.
- P450
- cytochrome P450
- TEMED
- N,N,N′,N′-tetramethylethylenediamine.
Authorship Contributions
Participated in research design: Yamazaki and Uno.
Conducted experiments: Uehara, Murayama, and Nakanishi.
Contributed new reagents or analytic tools: Zeldin.
Performed data analysis: Uehara, Murayama, and Uno.
Wrote or contributed to the writing of the manuscript: Uehara, Zeldin, Yamazaki, and Uno.
References
- Bogaards JJ, Bertrand M, Jackson P, Oudshoorn MJ, Weaver RJ, van Bladeren PJ, Walther B. (2000) Determining the best animal model for human cytochrome P450 activities: a comparison of mouse, rat, rabbit, dog, micropig, monkey and man. Xenobiotica 30:1131–1152 [DOI] [PubMed] [Google Scholar]
- Bullock P, Pearce R, Draper A, Podval J, Bracken W, Veltman J, Thomas P, Parkinson A. (1995) Induction of liver microsomal cytochrome P450 in cynomolgus monkeys. Drug Metab Dispos 23:736–748 [PubMed] [Google Scholar]
- Edwards RJ, Murray BP, Murray S, Schulz T, Neubert D, Gant TW, Thorgeirsson SS, Boobis AR, Davies DS. (1994) Contribution of CYP1A1 and CYP1A2 to the activation of heterocyclic amines in monkeys and human. Carcinogenesis 15:829–836 [DOI] [PubMed] [Google Scholar]
- Ekins S, Vandenbranden M, Ring BJ, Gillespie JS, Yang TJ, Gelboin HV, Wrighton SA. (1998) Further characterization of the expression in liver and catalytic activity of CYP2B6. J Pharmacol Exp Ther 286:1253–1259 [PubMed] [Google Scholar]
- Emoto C, Murayama N, Wakiya S, Yamazaki H. (2009) Effects of histidine-tag on recombinant human cytochrome P450 3A5 catalytic activity in reconstitution systems. Drug Metab Lett 3:207–211 [DOI] [PubMed] [Google Scholar]
- Hashizume T, Mise M, Matsumoto S, Terauchi Y, Fujii T, Imaoka S, Funae Y, Kamataki T, Miyazaki H. (2001) A novel cytochrome P450 enzyme responsible for the metabolism of ebastine in monkey small intestine. Drug Metab Dispos 29:798–805 [PubMed] [Google Scholar]
- Ingelman-Sundberg M. (2005) Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J 5:6–13 [DOI] [PubMed] [Google Scholar]
- Ise R, Uehara S, Akiyama H, Kondo S, Iwasaki K, Nagata R, Nobumasa H, Yamazaki H, Uno Y. (2011) A newly developed DNA microarray is useful to assess induction of cytochromes p450 in the cynomolgus monkey. Drug Metab Pharmacokinet 26:228–235 [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Murayama N, Koizumi R, Uno Y, Yamazaki H. (2010) Comparison of cytochrome P450 3A enzymes in cynomolgus monkeys and humans. Drug Metab Pharmacokinet 25:388–391 [DOI] [PubMed] [Google Scholar]
- Kim S, Dinchuk JE, Anthony MN, Orcutt T, Zoeckler ME, Sauer MB, Mosure KW, Vuppugalla R, Grace JE, Jr, Simmermacher J, et al. (2010) Evaluation of cynomolgus monkey pregnane X receptor, primary hepatocyte, and in vivo pharmacokinetic changes in predicting human CYP3A4 induction. Drug Metab Dispos 38:16–24 [DOI] [PubMed] [Google Scholar]
- King LM, Ma J, Srettabunjong S, Graves J, Bradbury JA, Li L, Spiecker M, Liao JK, Mohrenweiser H, Zeldin DC. (2002) Cloning of CYP2J2 gene and identification of functional polymorphisms. Mol Pharmacol 61:840–852 [DOI] [PubMed] [Google Scholar]
- Mankowski DC, Laddison KJ, Christopherson PA, Ekins S, Tweedie DJ, Lawton MP. (1999) Molecular cloning, expression, and characterization of CYP2D17 from cynomolgus monkey liver. Arch Biochem Biophys 372:189–196 [DOI] [PubMed] [Google Scholar]
- Mimura M, Baba T, Yamazaki H, Ohmori S, Inui Y, Gonzalez FJ, Guengerich FP, Shimada T. (1993) Characterization of cytochrome P-450 2B6 in human liver microsomes. Drug Metab Dispos 21:1048–1056 [PubMed] [Google Scholar]
- Nakanishi Y, Matsushita A, Matsuno K, Iwasaki K, Utoh M, Nakamura C, Uno Y. (2011) Regional distribution of drug-metabolizing enzyme activities in the liver and small intestine of cynomolgus monkeys. Drug Metab Pharmacokinet 26:288–294 [DOI] [PubMed] [Google Scholar]
- Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. (2004) Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 14:1–18 [DOI] [PubMed] [Google Scholar]
- Nishimura M, Koeda A, Suganuma Y, Suzuki E, Shimizu T, Nakayama M, Satoh T, Narimatsu S, Naito S. (2007) Comparison of inducibility of CYP1A and CYP3A mRNAs by prototypical inducers in primary cultures of human, cynomolgus monkey, and rat hepatocytes. Drug Metab Pharmacokinet 22:178–186 [DOI] [PubMed] [Google Scholar]
- Nishimuta H, Sato K, Mizuki Y, Yabuki M, Komuro S. (2011) Species differences in intestinal metabolic activities of cytochrome P450 isoforms between cynomolgus monkeys and humans. Drug Metab Pharmacokinet 26:300–306 [DOI] [PubMed] [Google Scholar]
- Omura T, Sato R. (1964) The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem 239:2370–2378 [PubMed] [Google Scholar]
- Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. (2006) The human intestinal cytochrome P450 “pie”. Drug Metab Dispos 34:880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadrieh N, Snyderwine EG. (1995) Cytochromes P450 in cynomolgus monkeys mutagenically activate 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) but not 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx). Carcinogenesis 16:1549–1555 [DOI] [PubMed] [Google Scholar]
- Sharer JE, Shipley LA, Vandenbranden MR, Binkley SN, Wrighton SA. (1995) Comparisons of phase I and phase II in vitro hepatic enzyme activities of human, dog, rhesus monkey, and cynomolgus monkey. Drug Metab Dispos 23:1231–1241 [PubMed] [Google Scholar]
- Shimada T, Mimura M, Inoue K, Nakamura S, Oda H, Ohmori S, Yamazaki H. (1997) Cytochrome P450-dependent drug oxidation activities in liver microsomes of various animal species including rats, guinea pigs, dogs, monkeys, and humans. Arch Toxicol 71:401–408 [DOI] [PubMed] [Google Scholar]
- Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. (1994) Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther 270:414–423 [PubMed] [Google Scholar]
- Stevens JC. (2006) New perspectives on the impact of cytochrome P450 3A expression for pediatric pharmacology. Drug Discov Today 11:440–445 [DOI] [PubMed] [Google Scholar]
- Stevens JC, Shipley LA, Cashman JR, Vandenbranden M, Wrighton SA. (1993) Comparison of human and rhesus monkey in vitro phase I and phase II hepatic drug metabolism activities. Drug Metab Dispos 21:753–760 [PubMed] [Google Scholar]
- Stresser DM, Kupfer D. (1999) Monospecific antipeptide antibody to cytochrome P-450 2B6. Drug Metab Dispos 27:517–525 [PubMed] [Google Scholar]
- Turpeinen M, Ghiciuc C, Opritoui M, Tursas L, Pelkonen O, Pasanen M. (2007) Predictive value of animal models for human cytochrome P450 (CYP)-mediated metabolism: a comparative study in vitro. Xenobiotica 37:1367–1377 [DOI] [PubMed] [Google Scholar]
- Uehara S, Murayama N, Yamazaki H, Uno Y. (2010) A novel CYP2A26 identified in cynomolgus monkey liver metabolizes coumarin. Xenobiotica 40:621–629 [DOI] [PubMed] [Google Scholar]
- Uno Y, Fujino H, Iwasaki K, Utoh M. (2010a) Macaque CYP2C76 encodes cytochrome P450 enzyme not orthologous to any human isozymes. Curr Drug Metab 11:142–152 [DOI] [PubMed] [Google Scholar]
- Uno Y, Fujino H, Kito G, Kamataki T, Nagata R. (2006) CYP2C76, a novel cytochrome P450 in cynomolgus monkey, is a major CYP2C in liver, metabolizing tolbutamide and testosterone. Mol Pharmacol 70:477–486 [DOI] [PubMed] [Google Scholar]
- Uno Y, Hosaka S, Matsuno K, Nakamura C, Kito G, Kamataki T, Nagata R. (2007a) Characterization of cynomolgus monkey cytochrome P450 (CYP) cDNAs: is CYP2C76 the only monkey-specific CYP gene responsible for species differences in drug metabolism? Arch Biochem Biophys 466:98–105 [DOI] [PubMed] [Google Scholar]
- Uno Y, Iwasaki K, Yamazaki H, Nelson DR. (2011a) Macaque cytochromes P450: nomenclature, transcript, gene, genomic structure, and function. Drug Metab Rev 43:346–361 [DOI] [PubMed] [Google Scholar]
- Uno Y, Kumano T, Kito G, Nagata R, Kamataki T, Fujino H. (2007b) CYP2C76-mediated species difference in drug metabolism: a comparison of pitavastatin metabolism between monkeys and humans. Xenobiotica 37:30–43 [DOI] [PubMed] [Google Scholar]
- Uno Y, Matsuno K, Murayama N, Nakamura C, Yamazaki H. (2011b) Metabolism of P450 probe substrates by cynomolgus monkey CYP2C76. Basic Clin Pharmacol Toxicol 109:315–318 [DOI] [PubMed] [Google Scholar]
- Uno Y, Matsuno K, Nakamura C, Utoh M, Yamazaki H. (2009a) Cloning, expression, and characterization of CYP3A43 cDNA in cynomolgus macaque (Macaca fascicularis). Drug Metab Lett 3:228–233 [DOI] [PubMed] [Google Scholar]
- Uno Y, Matsuno K, Nakamura C, Utoh M, Yamazaki H. (2009b) Identification and characterization of CYP2B6 cDNA in cynomolgus macaques (Macaca fascicularis). J Vet Med Sci 71:1653–1656 [DOI] [PubMed] [Google Scholar]
- Uno Y, Matsuno K, Nakamura C, Utoh M, Yamazaki H. (2010b) Identification and characterization of CYP2C18 in the cynomolgus macaque (Macaca fascicularis). J Vet Med Sci 72:225–228 [DOI] [PubMed] [Google Scholar]
- Uno Y, Matsushita A, Osada N, Uehara S, Kohara S, Nagata R, Fukuzaki K, Utoh M, Murayama N, Yamazaki H. (2010c) Genetic variants of CYP3A4 and CYP3A5 in cynomolgus and rhesus macaques. Drug Metab Dispos 38:209–214 [DOI] [PubMed] [Google Scholar]
- Uno Y, Sakuraba H, Uehara S, Kumano T, Matsuno K, Nakamura C, Kito G, Kamataki T, Nagata R. (2009c) A null allele impairs function of CYP2C76 gene in cynomolgus monkeys: a possible genetic tool for generation of a better animal model in drug metabolism. Drug Metab Dispos 37:14–17 [DOI] [PubMed] [Google Scholar]
- Uno Y, Uehara S, Kohara S, Iwasaki K, Nagata R, Fukuzaki K, Utoh M, Murayama N, Yamazaki H. (2011c) Newly identified CYP2C93 is a functional enzyme in rhesus monkey, but not in cynomolgus monkey. PLoS One 6:e16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno Y, Uehara S, Kohara S, Murayama N, Yamazaki H. (2010d) Cynomolgus monkey CYP2D44 newly identified in liver, metabolizes bufuralol and dextromethorphan. Drug Metab Dispos 38:1486–1492 [DOI] [PubMed] [Google Scholar]
- Uno Y, Uehara S, Murayama N, Yamazaki H. (2011d) CYP1D1, pseudogenized in human, is expressed and encodes a functional drug-metabolizing enzyme in cynomolgus monkey. Biochem Pharmacol 81:442–450 [DOI] [PubMed] [Google Scholar]
- Weaver RJ, Dickins M, Burke MD. (1999) A comparison of basal and induced hepatic microsomal cytochrome P450 monooxygenase activities in the cynomolgus monkey (Macaca fascicularis) and man. Xenobiotica 29:467–482 [DOI] [PubMed] [Google Scholar]
- Wilkinson GR. (2005) Drug metabolism and variability among patients in drug response. N Engl J Med 352:2211–2221 [DOI] [PubMed] [Google Scholar]
- Wrighton SA, Brian WR, Sari MA, Iwasaki M, Guengerich FP, Raucy JL, Molowa DT, Vandenbranden M. (1990) Studies on the expression and metabolic capabilities of human liver cytochrome P450IIIA5 (HLp3). Mol Pharmacol 38:207–213 [PubMed] [Google Scholar]
- Yamazaki H, Nakamura M, Komatsu T, Ohyama K, Hatanaka N, Asahi S, Shimada N, Guengerich FP, Shimada T, Nakajima M, et al. (2002) Roles of NADPH-P450 reductase and apo- and holo-cytochrome b5 on xenobiotic oxidations catalyzed by 12 recombinant human cytochrome P450s expressed in membranes of Escherichia coli. Protein Expr Purif 24:329–337 [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Shimada T. (1997) Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes. Arch Biochem Biophys 346:161–169 [DOI] [PubMed] [Google Scholar]