Abstract
Organophosphate (OP)-based pesticides have been used extensively for decades, and as a result, they have become almost ubiquitous in our environment. There is clinical and animal evidence to suggest that chronic exposures to OPs can lead to cognitive dysfunction and other neurological abnormalities, although the mechanism for these effects is unknown. We previously reported that repeated, subthreshold exposures (defined as doses not associated with signs of acute toxicity) to the commonly used OP chlorpyrifos (CPF) resulted in protracted impairments in the performance of attention and memory-related tasks in rodents as well as deficits in axonal transport ex vivo (in the sciatic nerve). Here, we investigated the effects of CPF and its active metabolite CPF oxon (CPO) on the dynamics and movement of mitochondria in rat primary cortical neurons using time-lapse imaging techniques. Exposure to CPF (1.0–20.0 μM) or CPO (5.0 nM–20.0 μM) for 1 or 24 h resulted in a concentration-dependent increase in mitochondrial length, a decrease in mitochondrial number (indicative of increased fusion events), and a decrease in their movement in axons. The changes occurred at concentrations of CPF and CPO that did not inhibit acetylcholinesterase activity (the commonly cited mechanism of acute OP toxicity), and they were not blocked by cholinergic receptor antagonists. Furthermore, the changes did not seem to be associated with direct (OP-related) effects on mitochondrial viability or function (i.e., mitochondrial membrane potential or ATP production). The results suggest that an underlying mechanism of organophosphate-based deficits in cognitive function might involve alterations in mitochondrial dynamics and/or their transport in axons.
Introduction
The highly toxic organophosphate (OP) class of chemicals comprises many of the agricultural, industrial, and residential insecticides that are used worldwide. The acute toxicity of OPs has been studied extensively and is believed to result from irreversible inhibition of the enzyme acetylcholinesterase and consequent elevations in synaptic acetylcholine levels (reviewed in Ecobichon, 1996). However, there is substantial evidence that this mechanism cannot alone account for the wide range of deleterious effects associated with OPs (Pope, 1999), especially when the exposure level is subthreshold for producing acute toxicity. There is clinical evidence that this type of exposure can result in prolonged deficits in attention and other domains of cognition (Brown and Brix, 1998; Ray and Richards, 2001), although few prospective studies have addressed this subject.
Several (primarily indirect) observations in older studies suggested that OPs might interfere with axonal transport and, as a result, produce toxic effects that were unrelated to direct effects on acetylcholinesterase. For example, it was observed that OPs that produce delayed neurotoxicity (at high doses) cause accumulations of tubulovesicular profiles within axons before degeneration, a pathology that is consistent with the stagnation of membrane traffic (Abou-Donia and Lapadula, 1990). More recently, we provided direct evidence that repeated subthreshold exposures to OPs can lead to deficits in axonal transport. In particular, both anterograde and retrograde transport of vesicles in the sciatic nerves (ex vivo) of rats was significantly reduced after a 14-day exposure period to the commonly used OP, chlorpyrifos (O,O-diethyl O-[3,5,6,-trichloro-2-pyridyl] phosphorothionate) (CPF), and these deficits persisted throughout a 14-day washout period (Terry et al., 2003). Later, time course studies indicated that a significant reduction in axonal transport occurred within 10 h of a single exposure of 18 mg/kg s.c. CPF (Terry et al., 2007). In vitro studies in our laboratories (and our collaborator's laboratories) also indicated that OPs can disrupt kinesin-driven movement, covalently modify tubulin, and inhibit microtubule formation, i.e., factors that may contribute to the observed impairments in axonal transport (Gearhart et al., 2007; Prendergast et al., 2007; Grigoryan et al., 2008).
In this study, we tested the hypothesis that OP-related disruptions in axonal transport could affect the movement of an essential cargo, the mitochondrion. It is noteworthy that a mutually dependent relationship seems to exist between mitochondrial dynamics (e.g., placement, morphology, and function) and axonal transport. Specifically, axonal transport requires intact motor proteins moving along cytoskeletal networks (i.e., ATP-dependent processes) that depend on proper mitochondrial placement. Without the appropriate placement of mitochondria, ATP availability and buffering of intracellular Ca2+ is compromised, resulting in the impairment of a variety of processes (including axonal transport), which can result in catastrophic effects on neuronal function (Chang and Reynolds, 2006). Given the relatively recent development of fluorescent markers for imaging mitochondria (e.g., MitoTracker) in neuronal culture and the importance of cortical neurons to cognitive function, we chose to use cultured primary cortical neurons as a model system for studying CPF-related effects on mitochondrial dynamics and their transport in axons.
Materials and Methods
Cell Culture.
Instructions for culturing neurons were obtained from (Poindron et al., 2005) with modifications. In brief, the cortex from postnatal day 0 Sprague-Dawley pups was removed, placed in dissecting medium + trypsin (500 ml of Hanks' balanced salt solution, 2.5 g of glucose, 2.4 g of HEPES, 3.5 g of sucrose, pH 7.3–7.4), sterile-filtered, stored at 4°C, and incubated at 37°C in a water bath with gentle shaking for 25 min. The reaction was halted by adding 5 ml of culture medium with serum [500 ml of RPMI 1640 medium, 25 ml of fetal bovine serum, 50 ml of horse serum (heat-inactivated), 1.25 ml of 100 U/ml penicillin, and 100 μg/ml streptomycin]. The tissue was centrifuged for 5 min at 1000g, and the supernatant was removed and replaced with 1 ml of culture medium with serum. Mechanical dissociation was performed, and the dissociated neurons were filtered (BD Falcon 352340; BD Biosciences, Franklin Lakes, NJ) into a sterile tube and centrifuged for 5 min at 1000g. The neuronal pellet was resuspended in 1 ml of serum culture medium, and the cells were counted and added to poly-l-lysine-coated plates at the desired concentration. After a 1-h incubation at 37°C, 5% CO2, the serum medium was removed and replaced with serum free medium (500 ml of neurobasal media, 2% B27 supplement (Invitrogen, Carlsbad, CA), 300 μl of 100 U/ml penicillin, 100 μg/ml streptomycin, 75 μl of l-glutamine), sterile-filtered, and stored at 4°C.
Drugs and Chemicals.
CPF and CPO were obtained from ChemService (PS-674, MET-674B; West Chester, PA). CPF was dissolved in 0.5% dimethyl sulfoxide and used immediately. CPO was dissolved in methanol (80 mM) and stored at −80°C until needed. The final concentrations of dimethyl sulfoxide and methanol that were used in the cell cultures (for vehicle and OP exposures) were 0.01%. Atropine (A0257; Invitrogen) and mecamylamine (M9020; Sigma-Aldrich, St. Louis, MO) were dissolved in water for immediate use.
Measurement of Mitochondrial Axonal Transport and Morphology.
Neurons were grown on eight-well 1.5 borosilicate glass bottom chamber slides (155409; Nalge Nunc International, Rochester, NY). CPF (0, 1, 5, 10, 20 μM) or CPO (0, 0.005, 1, 5, 10, 20 μM) was added to the medium for 1 or 24 h. After the desired exposure period, the neurons were fluorescently tagged with 50 nM MitoTracker CMXRos (Invitrogen) and placed in phenol red-free neurobasal media. The chamber slides were placed on the confocal microscope (Deltavision, Deconvolution Olympus IX71; Olympus, Bothell, WA) in a Precision Control Weather Station (37°C, 5% CO2) for 5 min to equilibrate before imaging. Images of axonal mitochondria were captured every second for 8 s or for 5 min using a 60×, 1.42 numerical aperture objective (SoftWoRx; Applied Precision, Issaquah, WA). The images were compressed into audio video interleaved (AVI) animation files using NIH ImageJ (http://rsb.info.nih.gov/ij/). Mitochondrial length and number were measured within the axon (>100 μm from the cell body) from the first still frame, and the number of moving mitochondria were counted over five sequential frames using the Image J LSM reader. The specific criteria for mitochondrial movement was based on previously published literature and on our own observations (Ligon and Steward, 2000; Kaasik et al., 2007). The working definition (i.e., criteria) for mitochondrial movement was as follows: 1) mitochondrion must move in the same direction for a minimum of three of five total frames observed, and 2) mitochondrial leading and trailing edges must move in the same direction. The results were expressed as the mean number of mitochondria per micrometer, the mean mitochondrial length within the region of interest, and the mean number of mitochondria moving per micrometer (each factor was expressed as a percentage of vehicle control levels).
AChE Inhibition Assays.
Neurons were grown on clear 96-well plates (Nalge Nunc or Thermo Fisher Scientific, Waltham, MA) and treated with CPF or CPO (≥1 and ≥0.005 μM, respectively) for 24 h. AChE inhibition was determined as described previously (Prendergast et al., 2007). In brief, 5,5-dithio-bis(2-nitrobenzoic acid) and acetylthiocholine iodide were added to the wells and allowed to equilibrate for 1 min. Plates were then loaded into the Beckman Coulter DTX 880 multimodal detector (Beckman Coulter, Fullerton, CA), and absorbance at 412 nm was measured every 2 min for 16 min. The rate of AChE activity was then calculated for each time point of measurement using the formula (Δ absorbance/min)/(1.36 × 104).
Mecamylamine and Atropine Coincubation Experiments.
The method described above for assessing mitochondrial axonal transport, length, and number was also used to determine the effects of coincubation of CPF or CPO with the nicotinic antagonist mecamlyamine or the muscarinic antagonist atropine. Specifically, cortical neurons were coincubated with 1.0, 3.0, or 10 μM mecamylamine or 1.0, 10.0, or 50.0 μM atropine with 1.0 μM CPF or 0.005 μM CPO for 24 h.
Effects of CPF and CPO on Mitochondrial Membrane Potential, ATP Synthesis, and Superoxide Production.
Neurons were grown on 96-well plates (Nalge Nunc International and Thermo Fisher Scientific) and 50-mm glass coverslips (Fisher 22050238) and treated with 0 to 500 μM CPF or CPO for 24 h. The medium containing vehicle, CPF or CPO, was removed, and the neurons were incubated with the DePsipher (Trevigen, Gaithersburg, MD) reagent to determine whether mitochondrial membrane potential (ΔΨm) was altered by the OPs. The neurons were imaged using the Leica SP2-scanning confocal microscope and imaged using a 63×, 1.4 numerical aperture objective. A 485-nm excitation filter and 535- and 590-nm emission filters were used to measure green and red fluorescence. Quantification was performed using the photon-counting multimode plate reader (Mithras LB940; Berthold Technologies GmbH, Wildbad, Germany) (for more details, see Hollins et al., 2009). The ratio of green to red fluorescence was assessed, and OP-related effects were expressed as a percentage of the control ratios. To determine whether OP-related changes in superoxide production were present, MitoSOX Red (Invitrogen) kits were used to according to the manufacturer's instructions. MitoSox-derived fluorescence was measured using the plate reader described above at 515 nm/590 nm (excitation/emission) and expressed as a percentage of control. To determine whether changes in ATP production were associated with exposure to the OPs, a bioluminescent somatic cell assay kit (FLASC; Invitrogen) was used according to the manufacturer's instructions. ATP production was determined using the microplate fluorescence reader (FLx800 and KC Junior software; BioTek Instruments, Inc., Winooski, VT) and expressed a percentage of control. In these later experiments, valinomycin, a potassium ionophore known to deplete ATP levels at micromolar concentrations, and antimycin A, a mitochondrial respiratory chain complex III inhibitor known to elevate superoxide levels, were used as positive controls.
Statistics.
Comparisons between treatments were made using analysis of variance followed by the Student-Newman-Keuls method for post hoc analysis (SigmaStat 2.03; SPSS Inc., Chicago, IL) when appropriate. Statistical significance was assessed using an α level of 0.05. Data are shown as the mean ± S.E.M. p values reflect differences between treatments unless stated otherwise.
Results
CPF Disrupts Mitochondrial Axonal Transport and Morphology
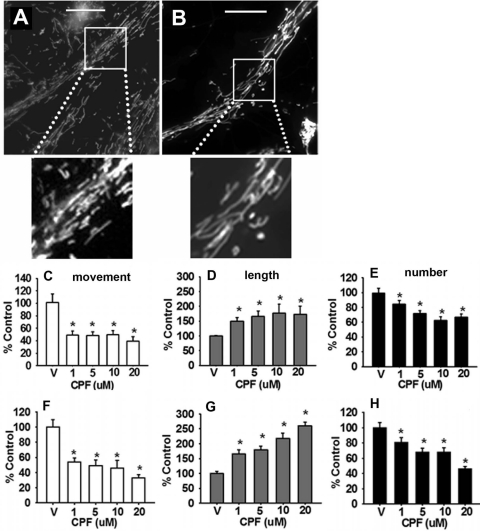
Representative images of cortical neurons exposed to vehicle or 1.0 μM CPF for 24 h are provided in Fig. 1,A and B, respectively. The images show a significant CPF-related increase in the number of elongated mitochondria. Representative time lapse images of single axons exposed to vehicle or CPF 1.0 μM are provided in Supplemental Movie Files 1 and 2, respectively. In the live cell-imaging movies, a clear (CPF-related) decrease in the movement of mitochondria is observed relative to control levels. Quantitative analysis revealed that, after 24 h of exposure to CPF, there was a significant (p < 0.05) decrease in mitochondrial axonal transport associated with all of the doses (1.0–20 μM) that were evaluated (i.e., down to 40–50% control levels; Fig. 1C). The reduction in transport was accompanied by a dose-dependent increase in mitochondrial length (up to ∼170% control) and decrease in mitochondrial number (down to ∼60% control; see Fig. 1, D and E, respectively), suggestive of an increase in mitochondrial fusion versus fission events. Subsequent experiments were conducted to determine whether the deficits in transport and changes in morphology occurred more acutely (1 h). At this time point (see Fig. 1, F, G, and H), more robust changes in axonal transport (decreases down to ∼30% control) and mitochondrial dynamics (i.e., increases in mitochondrial length up to ∼250% control and decreases in mitochondrial number down to ∼45% control) were observed. It is also important to note that at both the 1- and 24-h time point, the changes in mitochondrial length seemed to be more robust than the decreases in mitochondrial number, suggesting that mitochondrial elongation may begin before the induction of mitochondrial fusion.
Fig. 1.
CPF disrupts mitochondrial transport and alters mitochondrial dynamics in cortical neurons. Representative images of cultures exposed to vehicle or 1.0 μM CPF for 24 h are provided in A and B, respectively. Scale bar, 100 μm. CPF exposure for 24 h was associated with a dose-dependent decrease in axonal transport (mean number of mitochondria moving per micrometer) (C), an increase in mitochondrial length (mean mitochondrial length within the region of interest) (D), a decrease in mitochondrial number (mean number of mitochondria per micrometer) (E). CPF exposure for 1 h was also associated with a dose-dependent decrease in mitochondrial movement (F), an increase in mitochondrial length (G), and a decrease in mitochondrial number (H). Data are expressed as the percentage of control ± S.E.M. *, significantly different (p < 0.05) from control.
CPF Metabolite CPO Disrupts Mitochondrial Axonal Transport and Morphology
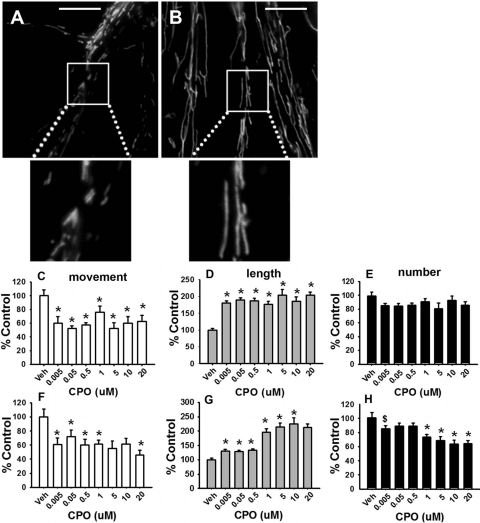
It has been suggested that the majority of CPF activity (as a cholinesterase inhibitor-based insecticide) is expressed via its conversion to CPO; therefore, it was important to determine whether changes in mitochondrial dynamics and transport occurred in the presence of CPO. Representative images of cortical neurons exposed to vehicle or 0.005 μM CPO for 24 h are provided in Fig. 2, A and B, respectively. The images (similar to the case of CPF) show a significant CPO-related increase in the number of elongated mitochondria. Representative time lapse images of single axons exposed to vehicle or 0.005 μM CPF are provided in Supplemental Movie Files 3 and 4, respectively. In the live cell-imaging movies, a clear (CPO-related) decrease in the movement of mitochondria was observed relative to control levels. Quantitative analysis revealed that 24 h of exposure to CPO (similar to CPF) resulted in a decrease in mitochondrial axonal transport (by as much as ∼40%; Fig. 2C). Figure 2D shows an increase in mitochondrial length (up to ∼200% control); however, the decreases in mitochondrial number (Fig. 2E) were less pronounced (compared with CPF), with a maximal decrease of approximately 20% (dose effect, p < 0.054). At the 1-h time point, there were also significant deficits in axonal transport (down to ∼40% control; Fig. 2F) and an increase in mitochondrial length (up to 225% control; Fig. 2G). The CPO-related decrease in mitochondrial number observed at the 1-h time point (down to ∼65% control; see Fig. 2H) was greater than that observed at the 24-h time point.
Fig. 2.
CPO disrupts mitochondrial transport and alters mitochondrial dynamics in cortical neurons. Representative images of cultures exposed to vehicle or 0.005 μM CPO for 24 h are provided in A and B, respectively. Scale bar, 100 μm. CPO exposure of 24 h was associated with a decrease in axonal transport (mean number of mitochondria moving per micrometer) (C), an increase in mitochondrial length (mean mitochondrial length within the region of interest) (D), and a nearly significant (dose effect p < 0.054) decrease in mitochondrial number (mean number of mitochondria per micrometer) (E). CPO exposure for 1 h was also associated with a decrease in mitochondrial movement (F), an increase in mitochondrial length (G); and a decrease in mitochondrial number (H). Data are presented as the percentage of control ± S.E.M. *, significantly different (p < 0.05) from control; $, p < 0.09.
CPF/CPO-Related Inhibition of AChE Is Not Necessarily Responsible for the Deficits in Axonal Transport and Changes in Mitochondrial Dynamics
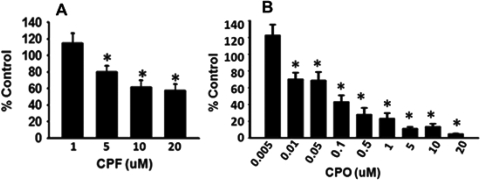
Subsequent experiments were conducted to determine whether the OP-related effects on mitochondrial length, number, and axonal transport required the inhibition of AChE. After 24 h of exposure to CPF, inhibition of AChE was observed at the 5.0, 10.0, and 20.0 μM concentration (Fig. 3A). However, there was no significant (p = 0.899) inhibition of AChE at 1.0 μM CPF (a concentration that did result in significant deficits in axonal transport and changes in mitochondrial dynamics, see above). As expected, 24-h exposure to CPO resulted in a more pronounced AChE inhibition from 0.01 to 20 μM; however, no AChE inhibition was detected after exposure to 0.005 μM CPO (Fig. 3B), again, a concentration that did result in significant deficits in axonal transport and changes in mitochondrial dynamics.
Fig. 3.
Concentration-dependent effects of CPF/CPO on AChE activity. Cortical neurons were exposed to various concentrations of CPF and CPO for 24 h. The results indicate a concentration-dependent decrease in AChE activity after exposure to 5 to 20 μM CPF (A) and 0.01 to 20 μM CPO. B, at concentrations of 1.0 μM CPF or 0.005 μM CPO (i.e., concentrations associated with alterations in axonal transport and altered mitochondrial dynamics), no AChE inhibition was detected. Data are presented as mean AChE inhibition (% control) ± S.E.M. *, significantly different (p < 0.05) from control.
CPF/CPO-Related Alterations in Mitochondrial Dynamics and Axonal Transport Are Not Necessarily Dependent on Direct or Indirect Actions at Cholinergic Receptors
Although the experiments described above indicated that the effects of CPF and CPO on mitochondria did not depend on the inhibition of AChE, a second (complimentary) set of experiments was conducted to determine whether elevated synaptic acetylcholine at its receptors (related to CPF or CPO exposure) or direct effects of the OPs at cholinergic receptors might contribute to the mitochondrial changes. In this series of experiments, mecamylamine and atropine (nicotinic and muscarinic acetylcholine receptor antagonists, respectively) were coincubated with CPF and CPO, and the effects on axonal transport and mitochondrial dynamics were assessed. The concentrations of mecamylamine and atropine were based on previously published neuronal culture studies (Heppner and Fiekers, 1992; Slotkin et al., 2007; Ueda et al., 2008).
Mecamylamine.
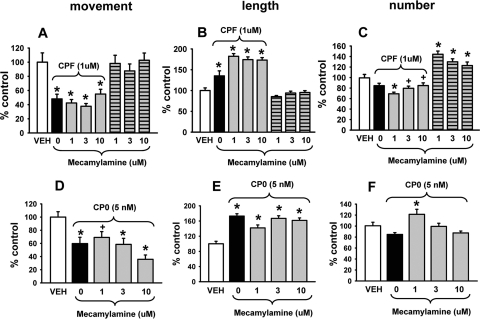
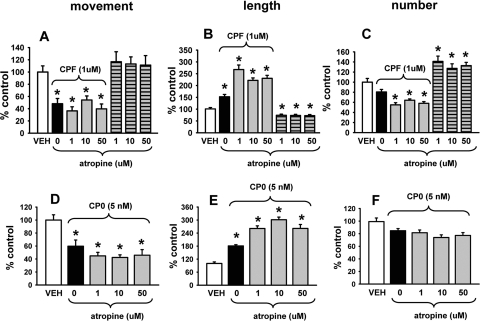
Three concentrations of mecamylamine (1.0, 3.0, or 10.0 μM) were evaluated separately and coincubated with 1.0 μM CPF or 0.005 μM CPO for 24 h. As indicated in Fig. 4, the axonal transport deficits (Fig. 4, A and D) and increased mitochondrial length (Fig. 4, B and E) induced by CPF and CPO, respectively, persisted in the presence of mecamylamine. The nonsignificant decreases in mitochondrial number associated with CPF (Fig. 4C) also were not antagonized, whereas the effects on the CPO-related response (Fig. 4F) were less clear. Mecamylamine alone was not associated with significant effects on mitochondrial transport or length (see Fig. 4, A and B, right; however, it was associated with significant increases in number (see Fig. 4C, right).
Fig. 4.
The nicotinic acetylcholine receptor antagonist mecamylamine does not block the effects of CPF or CPO on mitochondrial transport or dynamics. Various concentrations of mecamylamine were coincubated with 1.0 μM CPF or 0.005 μM CPO for 24 h. The results show that the axonal transport deficits (A and D) and increased mitochondrial length (B and E) induced by CPF and CPO, respectively, persisted in the presence of mecamylamine. The nonsignificant decreases in mitochondrial number associated with CPF (C) were also not antagonized, whereas the effects on the CPO-related effect on mitochondrial number (F) were less clear. Mecamylamine alone was not associated with significant effects on mitochondrial transport or length (A and B, right); however, it was associated with significant increases in number (C, right). Data are presented as mean (% control) ± S.E.M. *, significantly different (p < 0.05) from control; +, p < 0.09 versus control.
Atropine.
Three concentrations of atropine (1.0, 10.0, or 50.0 μM) were evaluated in the next set of experiments and coincubated with 1.0 μM CPF or 0.005 μM CPO for 24 h. As indicated in Fig. 5, the axonal transport deficits (Fig. 5, A and D) and increased mitochondrial length (Fig. 5, B and E) induced by CPF and CPO, respectively, also persisted in the presence of atropine. The nonsignificant decreases in mitochondrial number associated with CPF and CPO (Fig. 5, C and F, respectively) were also not antagonized by atropine; in fact, they were increased further. Atropine alone was not associated with significant effects on mitochondrial movement (Fig. 5A); however, it was associated with a decrease in the length and an increase in the number of mitochondria (Fig. 5, B and C).
Fig. 5.
The muscarinic acetylcholine receptor antagonist atropine does not block the effects of CPF or CPO on mitochondrial transport or dynamics. Various concentrations of atropine were coincubated with 1.0 μM CPF or 0.005 μM CPO for 24 h. The results show that the axonal transport deficits (A and D) and increased mitochondrial length (B and E) induced by CPF and CPO, respectively, persisted (and increased even further) in the presence of atropine. The nonsignificant decreases in mitochondrial number associated with CPF and CPO (C and F, respectively) were also not antagonized by atropine. Atropine alone was not associated with significant effects on mitochondrial transport (A, right); however, it was associated with significant decreases in length and increases in number (B and C, right). Data are presented as mean (% control) ± S.E.M. *, significantly different (p < 0.05) from control.
CPF/CPO-Related Alterations in Mitochondrial Dynamics and Axonal Transport Do Not Seem to Be Associated with Direct (OP-Related) Effects on Mitochondrial Viability, Function, or Superoxide Formation
Mitochondrial Membrane Potential.
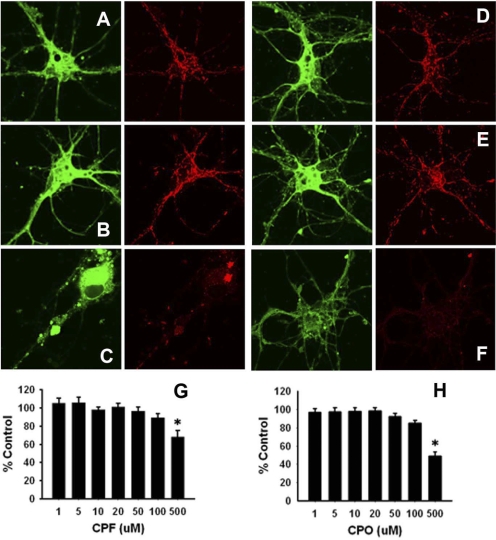
OP-related effects on ΔΨm were assessed via the DePsipher kit, which uses a unique lipophilic cationic dye to indicate loss of mitochondrial membrane potential. The dye readily enters neuronal cells and exists as a monomer in the cytoplasm (emission peak 527 nm; green). However, in the presence of healthy mitochondria (with an intact ΔΨm), the dye accumulates and aggregates (emission peak 590 nm; red). In apoptotic cells, the mitochondrial membrane potential collapses, and the DePsipher reagent cannot accumulate within the mitochondria. In these cells, DePsipher remains in the cytoplasm as a green fluorescent monomeric form. Apoptotic cells, showing primarily green fluorescence, are easily differentiated from healthy cells, which also show red fluorescence. In the current study, the ratio of green to red fluorescence was assessed, and OP-related effects were expressed as a percentage of the control ratios. Representative images after 24-h exposure to vehicle, 20 or 500 μM CPF (Fig. 6, A–C, respectively) and 20 or 500 μM CPO (Fig. 6, D, E, and F, respectively), are provided. Dose-effect relationships for CPF and CPO are presented in Fig. 6, G and H, respectively. After 24-h exposure to 1 to 20 μM CPF/CPO (concentrations relevant to the deficits in axonal transport and changes in mitochondria dynamics described above), there was no evidence of a significant change in ΔΨm. As expected, significant compromise of ΔΨm was observed at concentrations of the OPs (500 μM) that are known to be cytotoxic (p = 0.003 and p = 0.002 for CPF and CPO, respectively).
Fig. 6.
Concentrations of CPF and CPO that alter mitochondrial transport and dynamics do not compromise ΔΨm. Cortical neurons were exposed to various concentrations of CPF or CPO for 24 h and analyzed via the DePsipher assay. A and B show representative images of vehicle and 20 μM CPF exposures, respectively. D and E show representative images of vehicle and 20 μM exposures to CPO, respectively. C and F show representative images of 500 μM exposures to CPF and CPO, respectively. G and H show the dose-effect relationships for CPF and CPO, respectively. Green images on the left (emission peak 527 nm) indicate the monomeric form of the DePsipher reagent in the cytoplasm of the neuron. Red images on the right (emission peak 590 nm) indicate the accumulation and aggregation of the reagent in healthy mitochondria (i.e., with an intact ΔΨm). Thus, 20 μM CPF and CPO were not associated with a compromise in ΔΨm, whereas the lack of red labeling in the cells exposed to 500 μM CPF or CPO indicates a compromise of the mitochondrial ΔΨm. For quantitative comparisons, the ratio of green to red fluorescence was assessed and OP-related effects were expressed as a percentage of the control ratios (i.e., as mean % control ± S.E.M.). *, significantly different (p < 0.05) from control.
ATP Synthesis.
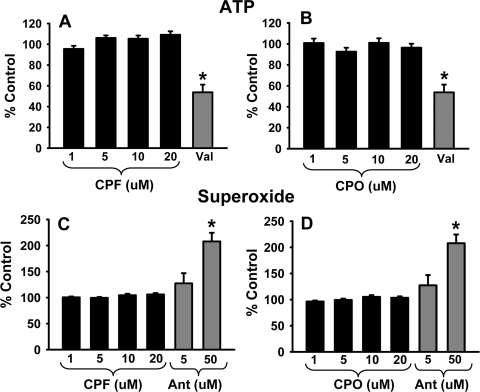
Given the importance of ATP synthesis for motor protein-dependent axonal transport and general neuronal function, ATP production was also assessed after 24-h exposure to 1 to 20 μM CPF and CPO via a bioluminescent somatic cell assay kit (FLASC; Invitrogen). The results indicate that there was no significant reduction in ATP production after exposure to the parent compound (CPF) or active metabolite (CPO) (p = 0.116–0.291 and p = 0.203, respectively) (Fig. 7, A and B). ATP production was significantly impaired (p < 0.05 vs. vehicle), however, by positive control compound, valinomycin (5 μM).
Fig. 7.
Exposure to CPF and CPO does not significantly alter ATP synthesis or increase superoxide levels. Cortical neurons were incubated with various concentrations of CPF or CPO up to 20 μM or the positive control compound valinomycin (VAL), 5 μM for 24 h. ATP production was determined with a bioluminescent somatic cell assay kit (FLASC; Invitrogen). Exposure to CPF (A) or CPO (B), respectively, did not impair ATP production. ATP production was impaired by valinomycin. In subsequent experiments, cortical neurons were incubated with various concentrations of CPF or CPO up to 20 μM or the positive control compound antimycin A (Ant) and 5 or 50 μM for 24 h. Superoxide production was subsequently determined with MitoSOX Red kits. Exposure to CPF (C) or CPO (D), respectively, did not elevate superoxide levels. Superoxide levels were significantly (p < 0.05) elevated by the higher concentration of antimycin A. Data are presented as mean (% control) ± S.E.M. *, significantly different (p < 0.05) from control.
Superoxide Production.
Lipid peroxidation (related to elevated superoxide levels) has been observed after exposure to high levels of CPF; therefore, we investigated whether there were elevated levels of mitochondrial superoxide after exposure to 1 to 20 μM CPF and CPO using MitoSOX Red kits. The results, shown in Fig. 7, C and D, indicate no significant increase in superoxide production after 24-h exposure to CPF or the active metabolite CPO compared with vehicle controls (p = 0.130 and 0.236, respectively). However, superoxide levels were significantly increased by the higher concentration of positive control compound antimycin A (50 μM).
Discussion
The initial goal of the experiments described in this report was to determine whether the impairments of axonal transport that we had observed previously in the peripheral nerves of rats exposed to CPF would be observed in CNS neurons. We chose to analyze mitochondrial movement in cultured neurons for these experiments for several reasons: 1) mitochondria are easy to identify, especially with the availability of novel fluorescent markers such as MitoTracker; 2) they, like other organelles and vesicles, move along microtubules via motors of the kinesin family and cytoplasmic dynein; 3) their transport is known to be modulated in response to physiological signals; and 4) they are of fundamental importance to normal neuronal functions, including aerobic metabolism, calcium homeostasis, and apoptotic processes (see Hollenbeck and Saxton, 2005 for review). During these experiments, our first (surprising) observation was the distinct alterations in morphology (i.e., elongations) of the mitochondria in neurons exposed to CPF and CPO (discussed further below). Nevertheless, we were able (using the defined criteria for mitochondrial movement described under Materials and Methods) to clearly detect decreases in mitochondrial movement in the OP-treated versus vehicle-treated neurons (i.e., decreases to approximately 40 and 60% control for CPF and CPO, respectively, at the lowest concentrations that were evaluated). The mechanism for these effects on mitochondrial movement are unclear but may be related to the same factors that affect the movement of vesicles. Previous work by us (and our collaborators) suggested that alterations in axonal transport of vesicles after OP exposure might be the result of covalent modifications of specific tyrosine residues located near GTP binding sites or within regions of protofilament-protofilament interactions, thus inhibiting microtubule formation (Prendergast et al., 2007; Grigoryan et al., 2008). In addition, coincubation of kinesin (anterograde motor protein) with CPF and CPO was shown to disrupt kinesin-dependent transportation along microtubules (Gearhart et al., 2007). The effects of OPs on dyneins/dynactin (retrograde motor proteins) have not been investigated to date; however, it is well documented that disruptions of kinesins or dyneins/dynactin can impair bidirectional transport (Martin et al., 1999).
The changes in mitochondrial transport noted above may also be related to the altered mitochondrial morphology (i.e., elongation) associated with CPF and CPO. As indicated under Results, in neurons exposed to either CPF or CPO, we observed a relatively dramatic (concentration-dependent) increase in mitochondrial length (e.g., as high as 350% of control in CPO-treated neurons) and a decrease in mitochondrial number. The mechanism of these distinct morphological changes is unclear but could involve OP-related effects on key fusion and/or fission proteins such as mfn2/opa1 or drp1. It is interesting to note that the fusion protein mfn2 has been shown to interact with Miro (an essential member of the complex that links mitochondria to kinesin motor proteins) to assist with bidirectional axonal transport of mitochondria (Russo et al., 2009). More recently, Misko et al. (2010) determined that disruption of mfn2 can selectively alter mitochondrial transport/distribution (a suggested mechanism of peripheral axon degeneration in Charcot-Marie-Tooth disease, Cartoni and Martinou, 2009). Further studies will be necessary to determine whether CPF and/or CPO have direct effects on these crucial fusion/fission proteins or, conversely, whether OP-related axonal transport deficits actually promote mitochondrial fusion in some manner.
It is noteworthy that deficits in axonal transport (particularly of mitochondria) have been implicated in several neurodegenerative illnesses, including Alzheimer's disease, Parkinson's disease, Huntington's disease, and peripheral neuropathies (Pigino et al., 2003; Trushina et al., 2004; Chang and Reynolds, 2006; Misko et al., 2010). From a pathophysiological standpoint, it is important to note that axonal transport deficits and a decreased ability of mitochondria to meet the spatial and local transient demands of the cell can compromise neuronal function and promote programmed cell death (Chang and Reynolds, 2006; Chen and Chan, 2006; Iijima-Ando et al., 2009). Although no causal connections can be made at this time, it also is interesting to note (given the results of our experiments) that occupational exposures to OP-pesticides have recently been associated with an increased risk for developing Alzheimer's disease (Hayden et al., 2010).
Subsequent to the evaluations of the CPF and CPO-related effects on mitochondrial transport and morphology, a series of experiments were conducted to determine whether the OP-related effects required the inhibition of AChE and/or if they might be related to indirect or direct effects of the OPs at cholinergic receptors. Direct effects of CPF/CPO at both muscarinic and nicotinic receptors have been described previously (Huff et al., 1994; Katz et al., 1997). From these experiments, we concluded that the mitochondrial changes occurred at concentrations of CPF and CPO that did not inhibit acetylcholinesterase activity (i.e., 1.0 μM and 5.0 nM, respectively) and, further, that they were not blocked by either a nicotinic or muscarinic receptor antagonist. It is important to note that both the nicotinic antagonist mecamylamine and the muscarinic antagonist atropine (when administered alone) increased the number of mitochondria above control levels, indicating that cholinergic receptor antagonism might promote the process of fission in some manner.
Additional experiments were conducted to determine whether the OPs might have direct effects on mitochondrial viability or function as would be suggested by alterations in ΔΨm or ATP production. The results indicated that ΔΨm and ATP production in CPF- and CPO-treated neurons were not altered significantly at physiologically relevant concentrations, (i.e., for subthreshold dosing, see Terry et al., 2007), suggesting that direct mitochondrial toxicity or impairments in the ability of mitochondria to generate ATP were not likely to be responsible for the OP-related effects on mitochondrial dynamics or transport. However, given our observations of OP-related increases in elongated mitochondria, it should be noted that mitochondrial fusion and the formation of mitochondrial networks have been described as prosurvival mechanisms whereby ΔΨm and ATP production are maintained under stressful conditions, even when Bax has translocated to the mitochondrion (Lee et al., 2004; Tondera et al., 2009). In contrast, impaired mitochondrial fusion can result in the loss of ΔΨm and a reduction in oxidative phosphorylation (OXPHOS) (Olichon et al., 2003; Chen et al., 2005). It has been suggested that ATP production via OXPHOS results in the production of highly networked mitochondria, whereas glycolysis-dependent ATP production leads to the generation of more spherical mitochondria, although this has yet to be established in neuronal cells (Plecitá-Hlavatá et al., 2008). In the experiments reported here, we did not differentiate between OXPHOS- and glycolysis-related ATP production; however, our results would support the hypotheses that the concentrations of OPs evaluated did not compromise ΔΨm or OXPHOS-dependent ATP production or that they drove mitochondrial fusion to maintain OXPHOS-dependent ATP and preserve mitochondrial function.
Additional experiments were conducted to determine the potential contributions of superoxide formation to the aforementioned (OP-related) effects on mitochondria. It is relatively well accepted that OPs can elicit oxidative stress and DNA damage to cells (particularly at high concentrations, see Soltaninejad and Abdollahi, 2009). In addition, Slotkin et al. (2005) have shown that CPF can evoke lipid peroxidation in the developing rat brain at concentrations that only cause mild signs of systemic toxicity. Moreover, Crumpton et al. (2000) observed a concentration-dependent increase in reactive oxygen species formation in response to CPF exposure in PC12 cells (commonly used as a neuronal model). We did not detect significant increases in the production of superoxide in this study (at the concentrations of CPF and CPO we evaluated); however, we cannot completely rule out the possibility that low levels of superoxide (i.e., below our limits of detection) could be produced over time in neurons exposed to OPs potentially leading to alterations of mitochondrial dynamics.
There are some potential limitations to this study that should be discussed. As noted in the Introduction, one reason that we chose to use cultured primary neurons as a model system for studying CPF-related effects was the relatively recent introduction of fluorescent markers such as MitoTracker. This marker made it easy to identify, microscopically analyze, and monitor the movement (using time-lapse imaging techniques) of an important organelle that is fundamental to the physiology of neurons. Neuronal culture systems offer additional advantages, such as the ability to test multiple drugs and drug concentrations (for dose-effect evaluations) in a high throughput manner. However, limitations of cell culture experiments include the artificial two dimensional environments where natural neuronal (afferent and efferent) networks are not developed and the glial support system is absent. In the current study, we chose OP concentrations that approximated levels we detected in rodents that showed persistent cognition-related abnormalities as well as neurochemical alterations ex vivo. However, it is unclear how well such concentrations accurately model conditions in the brain where multiple factors are different such as blood flow, supportive cell networks, and so on. In addition, given the “embryonic” nature of the culture system used, it is important to exercise caution when extrapolating the current findings to those observed in adult animals or humans. It is noteworthy that there is a large database of literature on the deleterious neurodevelopmental effects of OPs, and our results could have important ramifications in this context.
In conclusion, the results of this study can be summarized as follows: in cultured cortical neurons, exposure to the commonly used OP insecticide CPF and its oxidative metabolite CPO resulted in a concentration-dependent decrease in the transport of mitochondria in axons, an increase in mitochondrial length, and a decrease in mitochondrial number (indicative of increased fusion versus fission events). The neuronal changes occurred at concentrations of CPF and CPO that did not inhibit acetylcholinesterase activity, they were not blocked by cholinergic receptor antagonists, and they did not seem to be associated with directly toxic effects on mitochondria (as would be suggested by diminished ATP production, alterations in mitochondrial membrane potential, or elevations in superoxide production). The results suggest that an underlying mechanism of organophosphate-based deficits in cognition and other neurological functions might involve alterations in mitochondrial dynamics and/or their transport in axons.
Supplementary Material
Acknowledgments
We thank Ashley Davis for administrative assistance in preparing this article.
This work was supported by the National Institutes of Health National Institute of Environmental Health Sciences [Grant ES012241].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.184762.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- OP
- organophosphate
- AChE
- acetylcholinesterase
- CPF
- chlorpyrifos
- CPO
- chlorpyrifos oxon
- ΔΨm
- mitochondrial membrane potential
- OXPHOS
- oxidative phosphorylation.
Authorship Contributions
Participated in research design: Middlemore-Risher, Adam, Lambert, and Terry.
Conducted experiments: Middlemore-Risher and Adam.
Performed data analysis: Middlemore-Risher.
Wrote or contributed to the writing of the manuscript: Middlemore-Risher and Terry.
References
- Abou-Donia MB, Lapadula DM. (1990) Mechanisms of organophosphorus ester-induced delayed neurotoxicity: type I and type II. Annu Rev Pharmacol Toxicol 30:405–440 [DOI] [PubMed] [Google Scholar]
- Brown MA, Brix KA. (1998) Review of health consequences from high-, intermediate- and low-level exposure to organophosphorus nerve agents. J Appl Toxicol 18:393–408 [DOI] [PubMed] [Google Scholar]
- Cartoni R, Martinou JC. (2009) Role of mitofusin 2 mutations in the physiopathology of Charcot-Marie-Tooth disease type 2A. Exp Neurol 218:268–273 [DOI] [PubMed] [Google Scholar]
- Chang DT, Reynolds IJ. (2006) Mitochondrial trafficking and morphology in healthy and injured neurons. Prog Neurobiol 80:241–268 [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC. (2006) Critical dependence of neurons on mitochondrial dynamics. Curr Opin Cell Biol 18:453–459 [DOI] [PubMed] [Google Scholar]
- Chen H, Chomyn A, Chan DC. (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280:26185–26192 [DOI] [PubMed] [Google Scholar]
- Crumpton TL, Seidler FJ, Slotkin TA. (2000) Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos? Brain Res Dev Brain Res 121:189–195 [DOI] [PubMed] [Google Scholar]
- Ecobichon DJ. (1996) Toxic effects of pesticides, in Casarett & Doull's Toxicology (Klaassen CD, Amdur MO, Doull J. eds.) pp 643–689 McGraw-Hill, New York [Google Scholar]
- Gearhart DA, Sickles DW, Buccafusco JJ, Prendergast MA, Terry AV., Jr (2007) Chlorpyrifos, chlorpyrifos-oxon, and diisopropylfluorophosphate inhibit kinesin-dependent microtubule motility. Toxicol Appl Pharmacol 218:20–29 [DOI] [PubMed] [Google Scholar]
- Grigoryan H, Schopfer LM, Thompson CM, Terry AV, Masson P, Lockridge O. (2008) Mass spectrometry identifies covalent binding of soman, sarin, chlorpyrifos oxon, diisopropyl fluorophosphate, and FP-biotin to tyrosines on tubulin: a potential mechanism of long term toxicity by organophosphorus agents. Chem Biol Interact 175:180–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden KM, Norton MC, Darcey D, Ostbye T, Zandi PP, Breitner JC, Welsh-Bohmer KA, and Cache County Study Investigators (2010) Occupational exposure to pesticides increases the risk of incident AD: the Cache County study. Neurology 74:1524–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner TJ, Fiekers JF. (1992) Comparison of atropine pre- and post-treatment in ganglion neurons exposed to soman. Brain Res Bull 28:849–852 [DOI] [PubMed] [Google Scholar]
- Hollins B, Kuravi S, Digby GJ, Lambert NA. (2009) The C terminus of GRK3 indicates rapid dissociation of G protein heterotrimers. Cell Signal 21:1015–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. (2005) The axonal transport of mitochondria. J Cell Sci 118:5411–5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff RA, Corcoran JJ, Anderson JK, Abou-Donia MB. (1994) Chlorpyrifos oxon binds directly to muscarinic receptors and inhibits cAMP accumulation in rat striatum. J Pharmacol Exp Ther 269:329–335 [PubMed] [Google Scholar]
- Iijima-Ando K, Hearn SA, Shenton C, Gatt A, Zhao L, Iijima K. (2009) Mitochondrial mislocalization underlies Abeta42-induced neuronal dysfunction in a Drosophila model of Alzheimer's disease. PLoS One 4:e8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasik A, Safiulina D, Choubey V, Kuum M, Zharkovsky A, Veksler V. (2007) Mitochondrial swelling impairs the transport of organelles in cerebellar granule neurons. J Biol Chem 282:32821–32826 [DOI] [PubMed] [Google Scholar]
- Katz EJ, Cortes VI, Eldefrawi ME, Eldefrawi AT. (1997) Chlorpyrifos, parathion, and their oxons bind to and desensitize a nicotinic acetylcholine receptor: relevance to their toxicities. Toxicol Appl Pharmacol. October; 146:227–236 [DOI] [PubMed] [Google Scholar]
- Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. (2004) Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 15:5001–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon LA, Steward O. (2000) Movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol 427:340–350 [DOI] [PubMed] [Google Scholar]
- Martin M, Iyadurai SJ, Gassman A, Gindhart JG, Jr, Hays TS, Saxton WM. (1999) Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol Biol Cell 10:3717–3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH. (2010) Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci 30:4232–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G. (2003) Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem 278:7743–7746 [DOI] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Pelsman A, Mattson MP, Brady ST, Busciglio J. (2003) Alzheimer's presenilin 1 mutations impair kinesin-based axonal transport. J Neurosci 23:4499–4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plecitá-Hlavatá L, Lessard M, Santorová J, Bewersdorf J, Jezek P. (2008) Mitochondrial oxidative phosphorylation and energetic status are reflected by morphology of mitochondrial network in INS-1E and HEP-G2 cells viewed by 4Pi microscopy. Biochim Biophys Acta 1777:834–846 [DOI] [PubMed] [Google Scholar]
- Poindron P, Piguet P, Forster E. (2005) New methods for culturing cells from nervous tissues, in BioValley Monograph, Vol. 1, pp 12–22, Karger, Basel, Switzerland [Google Scholar]
- Pope CN. (1999) Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Health B Crit Rev 2:161–181 [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Self RL, Smith KJ, Ghayoumi L, Mullins MM, Butler TR, Buccafusco JJ, Gearhart DA, Terry AV., Jr (2007) Microtubule-associated targets in chlorpyrifos oxon hippocampal neurotoxicity. Neuroscience 146:330–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray DE, Richards PG. (2001) The potential for toxic effects of chronic, low-dose exposure to organophosphates. Toxicol Lett 120:343–351 [DOI] [PubMed] [Google Scholar]
- Russo GJ, Louie K, Wellington A, Macleod GT, Hu F, Panchumarthi S, Zinsmaier KE. (2009) Drosophila Miro is required for both anterograde and retrograde axonal mitochondrial transport. J Neurosci 29:5443–5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Oliver CA, Seidler FJ. (2005) Critical periods for the role of oxidative stress in the developmental neurotoxicity of chlorpyrifos and terbutaline, alone or in combination. Brain Res Dev Brain Res 157:172–180 [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Seidler FJ. (2007) Ameliorating the developmental neurotoxicity of chlorpyrifos: a mechanisms-based approach in PC12 cells. Environ Health Perspect 115:1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltaninejad K, Abdollahi M. (2009) Current opinion on the science of organophosphate pesticides and toxic stress: a systematic review. Med Sci Monit 15:RA75–RA90 [PubMed] [Google Scholar]
- Terry AV, Jr, Gearhart DA, Beck WD, Jr, Truan JN, Middlemore ML, Williamson LN, Bartlett MG, Prendergast MA, Sickles DW, Buccafusco JJ. (2007) Chronic, intermittent exposure to chlorpyrifos in rats: protracted effects on axonal transport, neurotrophin receptors, cholinergic markers, and information processing. J Pharmacol Exp Ther 322:1117–1128 [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Stone JD, Buccafusco JJ, Sickles DW, Sood A, Prendergast MA. (2003) Repeated exposures to subthreshold doses of chlorpyrifos in rats: hippocampal damage, impaired axonal transport, and deficits in spatial learning. J Pharmacol Exp Ther 305:375–384 [DOI] [PubMed] [Google Scholar]
- Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, et al. (2009) SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J 28:1589–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trushina E, Dyer RB, Badger JD, 2nd, Ure D, Eide L, Tran DD, Vrieze BT, Legendre-Guillemin V, McPherson PS, Mandavilli BS, et al. (2004) Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Mol Cell Biol 24:8195–8209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M, Iida Y, Kitamura Y, Kawashima H, Ogawa M, Magata Y, Saji H. (2008) 5-Iodo-A-85380, a specific ligand for alpha 4 beta 2 nicotinic acetylcholine receptors, prevents glutamate neurotoxicity in rat cortical cultured neurons. Brain Res 1199:46–52 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.