Abstract
Fatty acid-induced lipotoxicity plays a critical role in the pathogenesis of nonalcoholic liver disease. Saturated fatty acids and unsaturated fatty acids have differential effects on cell death and steatosis, but the mechanisms responsible for these differences are not known. Using cultured HepG2 cells and primary mouse hepatocytes, we found that unsaturated and saturated fatty acids differentially regulate autophagy and apoptosis. The unsaturated fatty acid, oleic acid, promoted the formation of triglyceride-enriched lipid droplets and induced autophagy but had a minimal effect on apoptosis. In contrast, the saturated fatty acid, palmitic acid, was poorly converted into triglyceride-enriched lipid droplets, suppressed autophagy, and significantly induced apoptosis. Subsequent studies revealed that palmitic acid-induced apoptosis suppressed autophagy by inducing caspase-dependent Beclin 1 cleavage, indicating cross-talk between apoptosis and autophagy. Moreover, our data suggest that the formation of triglyceride-enriched lipid droplets and induction of autophagy are protective mechanisms against fatty acid-induced lipotoxicity. In line with our in vitro findings, we found that high-fat diet-induced hepatic steatosis was associated with autophagy in the mouse liver. Potential modulation of autophagy may be a novel approach that has therapeutic benefits for obesity-induced steatosis and liver injury.
Introduction
Lipotoxicity refers to cellular toxicity in the presence of excessive free fatty acids (Malhi and Gores, 2008). Fatty acid-induced lipotoxicity in hepatocytes plays an essential role in the pathogenesis of nonalcoholic fatty liver disease (Malhi and Gores, 2008; Neuschwander-Tetri, 2010). Fatty acids are chemically classified as saturated and unsaturated (monounsaturated and polyunsaturated), and their structure affects their biological functions. Palmitic acid (PA), a saturated fatty acid, and oleic acid (OA), a monounsaturated fatty acid, are two of the most abundant fatty acids present in the diet and in serum (Baylin et al., 2002). Saturated and unsaturated fatty acids differentially regulate apoptosis in various experimental systems in which saturated fatty acids are the more toxic lipid species (Listenberger et al., 2003; Ricchi et al., 2009). Although the mechanisms underlying the cytotoxicity of fatty acids are largely unknown, it has been suggested that the conversion of fatty acids to triglyceride (TG) may reduce the cytotoxicity. For example, PA is poorly converted to TG and more toxic, whereas OA is readily converted to TG and less toxic (Listenberger et al., 2003). Another possibility is that different fatty acids may differ in their potential to activate endogenous cellular protective pathways. However, this has not been explored in detail.
Macroautophagy (referred to as autophagy hereafter) is a major intracellular degradation system. Autophagy is usually activated in response to the deprivation of nutrients or growth factors (Kuma et al., 2004). Autophagy also plays a role in the pathogenesis of a number of human diseases, including obesity and steatosis (Singh et al., 2009a; Zhang et al., 2009). To date, more than 30 Atg (autophagy) genes that participate in autophagy or autophagy-related processes have been defined (Klionsky et al., 2003; Mizushima, 2010). Mammalian microtubule-associated protein 1 light chain 3 (LC3), which is a homolog of yeast Atg8, is widely used as a marker to monitor the autophagy process. After its synthesis, LC3 is rapidly cleaved by Atg4 (an autophagy protein that has protein protease activity), and the cleaved form remains in the cytosol (called LC3-I) (Li et al., 2011b). Upon autophagy induction, LC3 is conjugated with phosphatidylethanolamine (PE), which is mediated by Atg7 (an E1-like protein) and the Atg12-Atg5-Atg16 complex (a complex that has E3-like activity). The conjugated form (called LC3-II) targets the autophagosomal membrane. LC3-II has been shown to have a membrane-tethering function and may play a role in the elongation and closure of the autophagosome membrane. The changes in LC3-II in the presence and absence of lysosomal inhibitors such as chloroquine (CQ) or bafilomycin A1 (BAF) have been widely used as an autophagic flux assay (Rubinsztein et al., 2009; Mizushima et al., 2010; Ni et al., 2011).
Regulation of autophagosome formation is rather complicated in that it also involves numerous intracellular mediators such as the Beclin 1-Vps34 PI3 kinase complex (He and Levine, 2010). Of note, Bcl2/xL suppresses autophagy by directly interacting with Beclin 1, whereas other BH3 domain-only Bcl-2 family proteins such as Bad promote autophagy by disrupting the Bcl2/xL interaction and releasing Beclin 1 (He and Levine, 2010). Of interest, recent data suggest that there is cross-talk between apoptosis and autophagy (Fimia and Piacentini, 2010). During apoptosis, Beclin 1 is cleaved by activated caspases, resulting in the inhibition of autophagy (Djavaheri-Mergny et al., 2010; Luo and Rubinsztein, 2010).
In addition to autophagy acting as a cell survival mechanism against cell death, emerging evidence suggests that it also regulates lipid homeostasis. Studies from autophagy gene (Atg7) adipose tissue-specific knockout mice reveal that autophagy may regulate adipose mass and differentiation in mice (Singh et al., 2009b; Zhang et al., 2009). These knockout mice are generally lean with decreased white adipose mass, an increased number of mitochondria, and enhanced insulin sensitivity (Singh et al., 2009b; Zhang et al., 2009). In contrast, liver-specific Atg7 knockout mice have an increased number of lipid droplets in hepatocytes (Singh et al., 2009a). Further experimental evidence suggests that autophagy may help to remove excess lipid droplets in hepatocytes, a process termed lipophagy (Singh et al., 2009a). We also recently reported that induction of autophagy by rapamycin significantly attenuates alcohol-induced steatosis in mice (Ding et al., 2010a). Although it seems that autophagy may attenuate steatosis, how fatty acids modulate autophagy remains largely unknown. It is also not known whether saturated or unsaturated fatty acids play different roles in autophagy. Whereas some recent studies reported that fatty acids may induce autophagy in INS-1 β-cells, no autophagic flux assays have been conducted in these studies, and thus it is unclear whether there would be an increase in autophagic activity in these scenarios (Choi et al., 2009; Komiya et al., 2010).
In the present study, we determined the effects of saturated and unsaturated fatty acids on autophagy and apoptosis in hepatocytes.
Materials and Methods
Reagents.
Antibodies used in this study were cleaved caspase-3, phosphorylated 70-kDa ribosomal protein S6 kinase-1 [p70S6K (T389], total p70S6K, phosphorylated translational initiation factor 4E binding protein-1 [4EBP1 (S65)], total-4EBP1, and perilipin from Cell Signaling Technology (Danvers, MA), β-actin from Sigma-Aldrich (St. Louis, MO), and horseradish peroxidase-labeled secondary antibodies from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA). The rabbit polyclonal anti-LC3B antibody was made using a peptide representing the NH2-terminal 14 amino acids of human LC3B and an additional cysteine (PSEKTFKQRRTFEQC) as described previously (Ding et al., 2009). The following chemicals were all from Sigma-Aldrich: delipidated bovine serum albumin (BSA), OA (unsaturated fatty acid), PA (saturated fatty acid), 3-methyladenine (3-MA), CQ, rapamycin, N-acetylcysteine (NAC), tumor necrosis factor-α (TNF-α), and Z-VAD-fmk. The fluorescence probes tetramethylrhodamine methyl ester (TMRM), Bodipy 493/503, propidium iodide, and Hoechst 33342 were all from Invitrogen (Carlsbad, CA).
Cell Culture and Treatment.
HepG2 cells, a human hepatoma cell line that was obtained from American Type Cell Culture (Manassas, VA), were cultured in Dulbecco's modified Eagle's medium with 10% fetal calf serum and other standard supplements. All cultures were maintained in a 37°C incubator with 5% CO2. All cell culture materials were obtained from Invitrogen. OA-BSA and PA-BSA conjugates were prepared as described previously (Choi et al., 2009). In brief, a 20 mM solution of OA or PA in 0.01 N NaOH was incubated at 70°C for 30 min, and fatty acid soaps were then complexed with 5% BSA in PBS at a 7:1 molar ratio of fatty acid to BSA. The OA-BSA or PA-BSA conjugate was administered to the cultured cells. BSA was used as a vehicle control.
Mice and Experimental Diets.
Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were fed either a control diet (Harlan Teklad 8604; Dyets Inc., Bethlehem, PA) or a Western diet (diet 100244; Dyets Inc.), which provides approximately 40% of calories from milk fat, for 3 months. All studies were approved by the University of Kansas Medical Center Animal Care and Use Committee and comply with National Institutes of Health guidelines. Mice were fasted overnight before sample collection. Total liver lysates were prepared using radioimmunoprecipitation assay buffer [1% NP40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl (lauryl) sulfate] with fresh protease inhibitors. Preparation of liver sections for histology and hematoxylin and eosin staining were performed as described previously (Luyendyk et al., 2010).
GFP-LC3 Adenovirus Infection.
Adenovirus expressing GFP-LC3B (human; Ad-GFP-LC3) was used as described previously (Ding et al., 2009). HepG2 cells (105/well in a 12-well plate with microscopic cover glasses; Thermo Fisher Scientific, Waltham, MA) were infected with adenoviral GFP-LC3 (100 viral particles/cell) in Dulbecco's modified Eagle's medium overnight followed by treatment with BSA or BSA-conjugated fatty acids.
Fluorescence Microscopy.
For fluorescence microscopy, cells were cultured in 12-well plates with microscope cover glasses. After designated treatments, cells were fixed with 4% paraformaldehyde in PBS. All the cellular images were obtained using an inverted Nikon Eclipse 200 fluorescence microscope. For quantification of autophagic cells, GFP-LC3 punctated dots were determined from triplicates by counting a total of more than 60 cells. For the intracellular lipid droplets, cells were stained with Bodipy 493/503 (0.1 μM) for 15 min at room temperature before the analysis. Apoptotic cell death was determined by nuclear staining with Hoechst 33342 (5 μg/ml) for fragmented and condensed nuclei. For mitochondria membrane potential, live cells were stained with TMRM (50 nM) for 15 min followed by fluorescence microscopy.
Electron Microscopy.
Liver tissue or HepG2 cells were fixed with 2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4), followed by 1% OsO4. After dehydration, thin sections were cut and stained with uranyl acetate and lead citrate. Digital images were obtained using a JEM 1016CX electron microscope. Random images were chosen, and the number of typical autophagosome and autolysosomes from each cell section were counted from more than 30 cells.
Immunoblot Analysis.
Cells were washed in PBS, and cell pellets were lysed in radioimmunoprecipitation assay buffer. Then 30 μg of protein were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were incubated with the indicated primary and secondary antibodies and developed with SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific). Densitometry analysis was performed using ImageJ software (National Institutes of Health, Bethesda, MD). The relative levels of LC3-II in each group were normalized to their loading control.
Caspase-3 Activity Assay.
Caspase-3 activity was determined as we described previously (Ding et al., 2004). In brief, caspase-3 activities were measured using 30 μg of proteins and 20 μM concentrations of fluorescent substrates (Ac-DEVD-AFC; Enzo Life Sciences. Inc., Farmingdale, NY). The fluorescence signals were detected by a fluorometer (GENios; Tecan, Durham, NC) at excitation and emission wavelengths of 400 and 510 nm, respectively.
TG Analysis.
The level of TG was determined as described previously (Wobser et al., 2009). After treatment, cells were briefly washed with PBS and scraped off with a cell scraper. After centrifugation, the cell pellets were resuspended in 200 μl of ice-cold lysis buffer (18 mM Tris-HCl, 300 mM mannitol, and 50 mM EGTA, pH 7.6, with protease inhibitors) followed by sonication with a microtip. Then 10 μl of cell extracts were taken out for the protein assay. The rest of the cell extracts were further mixed with 3 ml of chloroform-methanol mix (2:1) and incubated for 1 h at room temperature with occasional shaking to extract the lipid. Afterward, 0.4 ml of KCl (0.15 M) was added and mixed by vortex. These mixtures were centrifuged for 5 min at 3000g, and the lower lipid phase was collected and dried by a vacuum at room temperature. The lipids were further dissolved in 20 μl of isopropanol with 10% Triton X-100. Lipid analysis was performed following the manufacturer's instruction with a colorimetric assay kit (Sigma-Aldrich). The protein amount was determined with a protein assay kit (Thermo Fisher Scientific).
Statistical Analysis.
Results are given as mean ± S.E.M. One-way ANOVA and the Scheffé post hoc test were used for multiple comparisons and Student's t test was used for comparison of two matched groups using SPSS 13.0. Statistical significance was P < 0.05.
Results
Oleic Acid Induces Autophagy in HepG2 Cells.
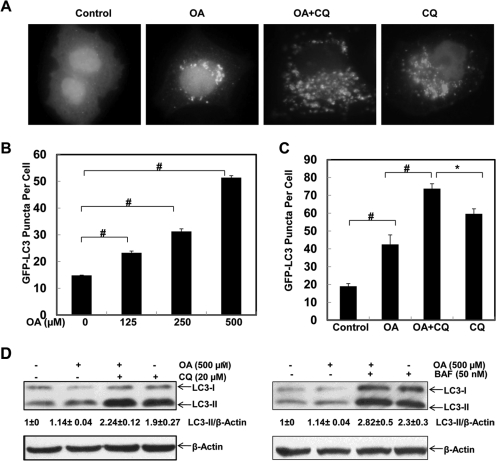
Because it has been reported that the biological functions of saturated and unsaturated fatty acids are different (Listenberger et al., 2003), we first determined the effect of oleic acid, an unsaturated fatty acid that is most abundant in the diet, serum, and liver tissue (Baylin et al., 2002; Xu et al., 2011), on autophagy in HepG2 cells (a human hepatoma cell line) expressing GFP-LC3 by infecting them with adenovirus GFP-LC3. GFP-LC3 behaves in a manner similar to that of endogenous LC3 and has been widely used to monitor autophagy (Hosokawa et al., 2006). GFP-LC3 is located in the cytosol and displays a diffuse pattern. Upon autophagy induction, GFP-LC3 translocates to the preautophagosome and autophagosome membranes, displaying a punctated pattern after its conjugation with PE (Hosokawa et al., 2006; Suzuki and Ohsumi, 2010). The number of GFP-LC3 puncta can be quantified and represent the number of autophagosomes and autolysosomes. To assess whether OA, a monounsaturated fatty acid, would affect autophagy, we first examined the changes in the GFP-LC3 pattern in OA-treated HepG2 cells. HepG2 cells were infected with an adenovirus GFP-LC3 and treated with OA in the presence or absence of CQ, which inhibits lysosomal degradation by increasing lysosomal pH (Ni et al., 2011). Compared with the vehicle-treated cells, the number of GFP-LC3 puncta increased in OA-treated cells in a concentration-dependent manner and further increased in the presence of CQ (Fig. 1, A–C), suggesting that OA induces autophagy in HepG2 cells. In addition to the changes in GFP-LC3 puncta, we also determined the autophagic flux by examining the changes in endogenous LC3-II in the presence of lysosomal inhibitors. We found that the LC3-II levels were not affected by OA treatment. Treatment with either CQ or BAF (Fig. 1D), which inhibit lysosomal functions by either increasing lysosomal pH or suppressing vacuolar type H+-ATPase (Klionsky et al., 2008), increased levels of LC3-II. Although OA cotreatment tended to increase LC3-II levels in the presence of CQ, this difference did not achieve statistical significance. Taken together, these data indicate that OA induces autophagy in HepG2 cells.
Fig. 1.
OA induces autophagy in HepG2 cells. HepG2 cells were first infected with Ad-GFP-LC3 (100 viral particles/cell) overnight and then treated with vehicle control (5% BSA), OA (500 μM), OA plus CQ (20 μM) or CQ (20 μM) alone, or various concentrations of OA (0, 125, 250, and 500 μM) for 6 h followed by fluorescence microscopy. A, representative GFP-LC3 images. B and C, number of GFP-LC3 dots per cell. Data are presented as the mean ± S.E. from three independent experiments by counting more than 20 cells in each individual experiment. *, p < 0.05; #, p < 0.01 (one-way ANOVA with the Scheffé post hoc test). D, HepG2 cells were treated with vehicle control (5% BSA), OA (500 μM), OA plus CQ (20 μM), CQ (20 μM) alone, OA plus Baf (50 nM), or Baf (50 nM) alone for 6 h. Total cell lysates were subjected to immunoblot analysis with anti-LC3 and anti-β-actin antibodies. Densitometry analysis for the expression level of LC3-II was performed using ImageJ software, which was further normalized with its loading control (β-actin). Digital data are presented as the ratio of LC3-II of the vehicle control (mean ± S.E.) from at least three independent experiments.
Palmitic Acid Does Not Activate Autophagy in HepG2 Cells.
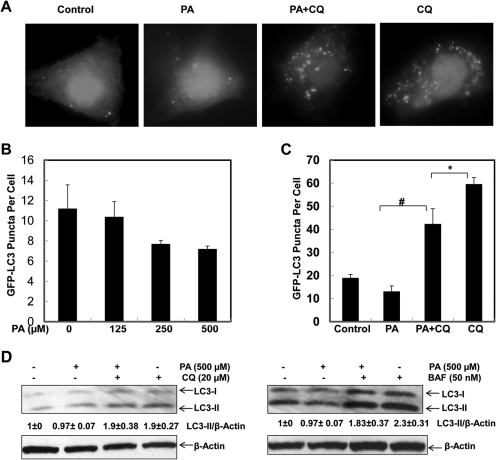
We next determined the effects of PA, a saturated fatty acid that is the most abundant in the diet and in serum and liver tissue (Baylin et al., 2002; Xu et al., 2011) on autophagy induction in HepG2 cells. In contrast to OA, PA did not increase autophagy in HepG2 cells as examined by a series of autophagic flux assays including GFP-LC3 puncta (Fig. 2, A–C) and the changes in endogenous LC3-II in the presence or absence of CQ or BAF (Fig. 2D). PA treatment alone slightly decreased the number of GFP-LC3 puncta at various concentrations, although this did not achieve a statistic difference. However, the number of GFP-LC3 puncta after PA treatment in the presence of CQ was significantly lower than that after CQ treatment alone (Fig. 2, A–C). Likewise, PA alone did not alter the level of endogenous LC3-II. Moreover, PA cotreatment did not significantly affect the levels of LC3-II in cells cotreated with CQ or BAF (Fig. 2D). Similar results were found in OA- or PA-treated primary cultured mouse hepatocytes (Supplemental Fig. 1). These results suggest that unlike OA, PA does not activate autophagy in either HepG2 hepatoma cells or normal hepatocytes.
Fig. 2.
PA fails to induce autophagy in HepG2 cells. HepG2 cells were first infected with Ad-GFP-LC3 overnight and then treated with vehicle control (5% BSA), PA (500 μM), PA plus CQ (20 μM), CQ alone, or various concentrations of PA (0, 125, 250, and 500 μM) for 6 h followed by fluorescence microscopy. A, representative GFP-LC3 images. B and C, number of GFP-LC3 dots per cell. Data are presented as the mean ± S.E. from three independent experiments by counting more than 20 cells in each individual experiment. *, p < 0.05; #, p < 0.01 (one-way ANOVA with the Scheffé post hoc test). D, HepG2 cells were treated with vehicle control (5% BSA), PA (500 μM), PA plus CQ (20 μM), CQ (20 μM) alone, PA plus Baf (50 nM), or Baf (50 nM) alone for 6 h. Total cell lysates were subjected to immunoblot analysis with anti-LC3 and anti-β-actin antibodies. Densitometry analysis for the expression level of LC3-II was performed using ImageJ software, which was further normalized with its loading control (β-actin). Digital data are presented as the ratio of LC3-II of the vehicle control (mean ± S.E.) from at least three independent experiments.
Although OA (18:1) and PA (16:0) represent extreme unsaturated and saturated fatty acids, there is a possibility that their differential effects on autophagy could also be due to the different carbon length in addition to their saturating status. We thus determined the effect of another unsaturated fatty acid, palmitoleate (16:1), which has the same carbon length as PA, on autophagy. We found that palmitoleate induces autophagy in HepG2 cells on the basis of the autophagic flux assay, either assessing for the GFP-LC3 puncta formation or the levels of LC3-II changes (Supplemental Fig. 2). These results suggest that the saturate status rather than the carbon length contribute to the differential effects of saturated and unsaturated fatty acids on autophagy.
Oleic Acid but Not Palmitic Acid Increases the Number of Autophagosomes and Neutral Lipid Storage.
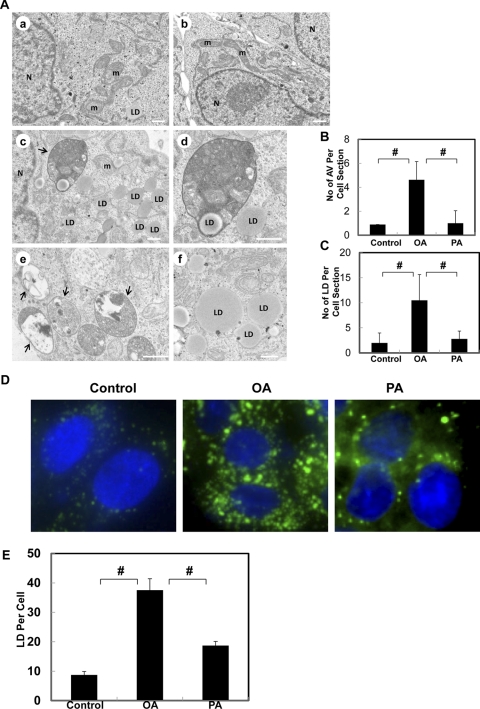
To further confirm the differential roles of OA and PA on autophagy, we performed EM studies on OA- and PA-treated HepG2 cells. OA treatment significantly increased the number of double-membrane autophagosomes (AV), and most of them had enveloped cytosolic contents (Fig. 3A, e, arrows) and lipid droplets (LD) (Fig. 3A, c and d, arrow). In addition to increasing the number of AV, OA treatment also increased the number of LD, which are featured as phospholipid monolayer membrane structures with electron lucent content (Fig. 3A, c, d, and f). In contrast, AV and LD are barely detectable in BSA-treated control (Fig. 3A, a) or PA-treated cells (Fig. 3, A, b, B, and C). Fluorescence microscopy studies using the fluorescent dye Bodipy493/503 for neutral lipids further confirmed that OA increased the number of LD significantly more than PA although PA also slightly increased LD numbers in HepG2 cells (Fig. 3, D and E). Similar results were found in primary mouse hepatocytes (Supplemental Fig. 3). These data indicate that OA but not PA increases the number of autophagosomes in HepG2 cells. Moreover, OA also increases more LD than PA in HepG2 cells and normal hepatocytes.
Fig. 3.
OA but not PA increases the number of autophagosomes and lipid droplets in HepG2 cells. A, HepG2 cells were treated with BSA vehicle control (a), PA (500 μM, b), or OA (500 μM, c–f) for 6 h, and the cells were further processed for EM. Arrows denote autophagosomes. N, nuclei; LD, lipid droplet; m, mitochondria. The number of autophagosomes (B) and lipid droplets (C) per cell section was determined (mean ± S.D.) from more than 30 different cells. #, p < 0.01 (one-way ANOVA with the Scheffé post hoc test). D, HepG2 cells were treated with BSA vehicle control (a), OA (500 μM, b), or PA (500 μM, c) for 6 h and fixed with 4% paraformaldehyde. The cells were further stained with Bodipy 493/503 (0.1 μM) for lipid droplets and Hoechst 33342 (0.5 μg/ml) for the nuclei followed by fluorescence microscopy. E, the number of lipid droplets per cell was quantified, and data are presented as the mean ± S.E. from at least three independent experiments. #, p < 0.01 (one-way ANOVA with the Scheffé post hoc test).
ROS but not mTOR Contributes to OA-Induced Autophagy in HepG2 Cells.
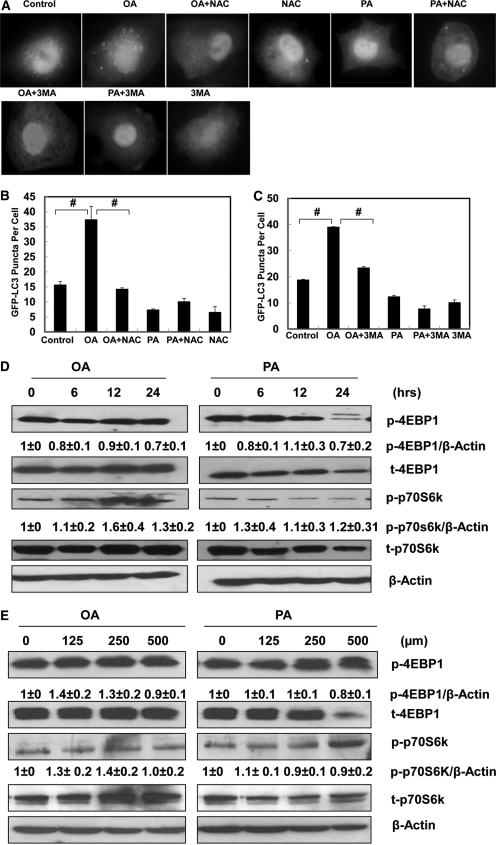
Because increased oxidative stress is often observed in human and experimental models of steatohepatitis (Chalasani et al., 2004; Seki et al., 2005), we next determined the levels of ROS production in OA- or PA-treated cells in the presence or absence of the antioxidant NAC. NAC has been widely used as an antioxidant and has been shown to block autophagy in many experimental models (Scherz-Shouval et al., 2007; Ding et al., 2010b). We found that PA significantly increased the level of ROS production in HepG2 cells compared with the control cells. OA also increased the level of ROS production but not to a degree of statistical significance. However, NAC significantly decreased the levels of ROS in both PA- and OA-treated cells (Supplemental Fig. 4). We then determined the effects of NAC on OA-induced GFP-LC3 puncta. We found that OA-induced GFP-LC3 puncta formation was significantly suppressed by NAC (Fig. 4, A and B). Because Beclin 1/class-III PI3 kinase complex is important in regulating autophagosome formation (He and Levine, 2010), we next determined the effects of 3-MA, a class-III PI3 kinase inhibitor, on OA-induced GFP-LC3 puncta formation. We found that OA-induced GFP-LC3 puncta were also suppressed by 3-MA (Fig. 4, A and C). Taken together, these data suggest that OA-induced autophagy requires increased oxidative stress and the Beclin 1-Vps34 PI3 kinase complex.
Fig. 4.
NAC and 3-MA suppress OA-induced autophagy in HepG2 cells. A, HepG2 cells were first infected with Ad-GFP-LC3 (100 viral particles/cell) overnight and then treated with vehicle control (5% BSA), OA (500 μM), PA (500 μM), OA plus NAC (5 mM), PA plus NAC (5 mM), NAC (5 mM), OA plus 3-MA (10 mM), PA plus 3-MA (10 mM), or 3-MA (10 mM) alone for 6 h followed by fluorescence microscopy. B and C, the number of GFP-LC3 dots per cell (mean ± S.E.) was quantified from three independent experiments and more than 20 cells were counted in each individual experiment. #, p < 0.01 (one-way ANOVA with the Scheffé post hoc test). D, HepG2 cells were treated with OA or PA (500 μM) for 6, 12, and 24 h, and the expression levels of phosphorylated (p) 4EBP1/total 4EBP1 and p-p70S6k/total p70S6k were determined by immunoblot analysis from at least three independent experiments. E, HepG2 cells were treated with vehicle control (5% BSA) or various concentrations (125, 250, and 500 μM) of OA and PA for 6 h. The expression levels of p-4EBP1/total 4EBP1 and p-p70S6k/Total p70S6k were determined by immunoblot analysis from at least three independent experiments. Densitometry analysis for the expression levels of p-4EBP1 and p-p70S6K was performed using ImageJ software, which was further normalized with its loading control (β-actin).
We next determined whether mTOR, one of the key molecular signaling pathways regulating autophagy, would also be involved in fatty acid-induced autophagy. We found that neither PA nor OA treatment suppressed mTOR activity as determined by the level of phosphorylated 4EBP1 and p70S6K at different time points (Fig. 4D) and various concentrations (Fig. 4E). It seemed that OA treatment even increased the phosphorylated level of p70S6K after 12 and 24 h of treatment although it slightly decreased the phosphorylated 4EBP1 levels (Fig. 4D). The reasons for the different changes for p70S6K and 4EBP1 are not clear although they both are mTOR downstream substrates. In contrast, a high dose of PA (500 μM) reduced both total and phosphorylated 4EBP1, which is probably mediated by its cytotoxicity (see below). Taken together, these data suggest that OA-induced autophagy is mediated by ROS but not by mTOR suppression in HepG2 cells.
PA but Not OA Induces Apoptosis in HepG2 Cells.
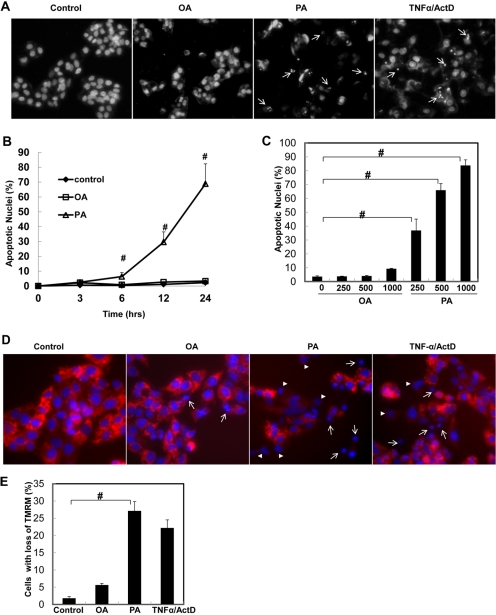
Because we found that OA and PA differentially regulated autophagy, we next determined whether OA and PA would also differentially regulate apoptosis in HepG2 cells. We found that PA significantly increased the number of apoptotic cells in a time- and dose-dependent manner, whereas cells treated with OA were barely affected (Fig. 5, A and B). The apoptotic nuclear changes in PA-treated cells were evident by the typical fragmented and condensed nuclear morphology (Fig. 5A, arrows), similar to apoptosis induced by TNF-α plus actinomycin D (ActD), a widely used model to trigger death receptor activation-induced apoptosis (Ding and Yin, 2004; Ding et al., 2004). Meanwhile, PA but not OA treatment also increased the number of cells with depolarized mitochondria (Fig. 5, D and E), suggesting that PA-induced apoptosis is mediated by the mitochondrial apoptotic pathway.
Fig. 5.
Differential effects of OA and PA on apoptosis in HepG2 cells. A, HepG2 cells were treated with vehicle control (5% BSA), OA (500 μM), PA (500 μM), or TNF-α (10 ng/ml) plus ActD (0.2 μg/ml) for 24 h. Apoptotic nuclei were analyzed by nuclear staining with Hoechst 33342 (1 μg/ml) for fragmented or condensed nuclei (arrows). B, HepG2 cells were treated with OA or PA (500 μM) for 6, 12, or 24 h or various concentrations (125, 250, and 500 μM) of OA or PA (C), and the number of apoptotic nuclei was quantified (mean ± S.E., n = 3). #, p < 0.01 (one-way ANOVA with the Scheffé post hoc test). D, HepG2 cells were treated with vehicle control (5% BSA), OA (500 μM), PA (500 μM), or TNF-α (10 ng/ml) plus ActD (0.2 μg/ml) for 6 h. The cells were loaded with TMRM (50 nM) followed by fluorescence microscopy (arrowheads, cells with partially lost mitochondrial membrane potential; arrows, cells with completely lost mitochondrial membrane potential). E, the number of cells with loss (both partial and complete) of TMRM staining was quantified (mean ± S.E.M.) from at least three independent experiments. #, p < 0.01 (one-way ANOVA with the Scheffé post hoc test).
Caspase-Mediated Beclin 1 Cleavage Is Associated with the Suppression of Autophagy in PA-Treated Cells.
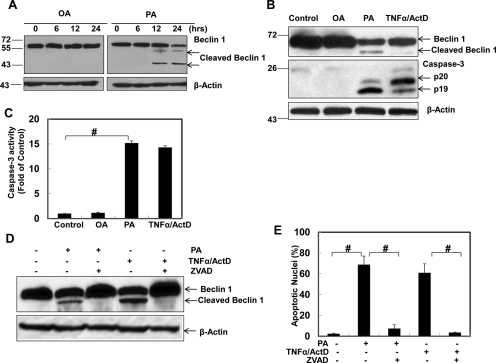
Increasing evidence suggests that apoptosis may suppress autophagy by caspase-mediated cleavage of essential autophagy proteins (Luo and Rubinsztein, 2010; Li et al., 2011a). Because we observed that OA and PA differentially regulate autophagy and apoptosis, we next determined whether PA-induced caspase activation would cleave Beclin 1, an essential autophagy protein serving as a key component in the Beclin 1-Vps34 PI3 kinase complex and in turn suppress autophagy in PA-treated cells. Indeed, we found that PA but not OA induced Beclin 1 cleavage in a time-dependent manner (Fig. 6A). We consistently observed a 50-kDa cleaved Beclin 1 in PA or TNF-α/ActD-treated cells as reported previously (Li et al., 2011a). Occasionally, an additional cleaved Beclin 1 band at approximately 45 kDa was also detected (Fig. 6A). However, this cleaved band could be less stable because it was not always detectable in our experiments. We further found that PA as well as TNF-α/ActD but not OA also induced caspase-3 cleavage (Fig. 6B) and increased caspase-3 activity (Fig. 6C). More importantly, PA and TNF-α/ActD-induced Beclin 1 cleavage and apoptosis were inhibited by a general caspase inhibitor, Z-VAD-fmk, further supporting the notion that PA-induced Beclin 1 cleavage and apoptosis are caspase-dependent (Fig. 6, D and E). Furthermore, in the presence of Z-VAD-fmk, the PA-induced number of GFP-LC3 puncta was significantly increased (Supplemental Fig. 5). These findings suggest that the cleavage of Beclin 1 by PA-induced caspase activation may suppress autophagy in PA-treated HepG2 cells as we observed in Fig. 2.
Fig. 6.
PA but not OA induces caspase-mediated Beclin 1 cleavage. A, HepG2 cells were treated with OA or PA (500 μM) for 6, 12, or 24 h. Total cell lysates were subjected to immunoblot analysis using an anti-Beclin 1 antibody. B, HepG2 cells were treated with vehicle control (5% BSA), OA (500 μM), PA (500 μM), or TNF-α (10 ng/ml) plus ActD (0.2 μg/ml) for 24 h. Total cell lysates were subjected to immunoblot analysis for Beclin 1 and caspase 3. C, Total cell lysates (30 μg) were used for caspase-3 activity analysis (mean ± S.E., n = 3). #, p < 0.01 (one-way ANOVA with the Scheffé post hoc test). D, HepG2 cells were treated with vehicle control (5% BSA), PA (500 μM), PA (500 μM) plus Z-VAD-fmk (50 μM), TNF-α (10 ng/ml) plus ActD (0.2 μg/ml), or TNF-α (10 ng/ml) plus ActD (0.2 μg/ml) with Z-VAD-fmk (50 μM) for 24 h. Total cell lysates were subjected to immunoblot analysis for Beclin 1 and (E) apoptotic cell death was analyzed by nuclear staining with Hoechst 33342 (mean ± S.E., n = 3). #, p < 0.01 (one-way ANOVA with the Scheffé post hoc test).
Autophagy Attenuates Fatty Acid-Induced Apoptosis and Accumulation of Lipids.
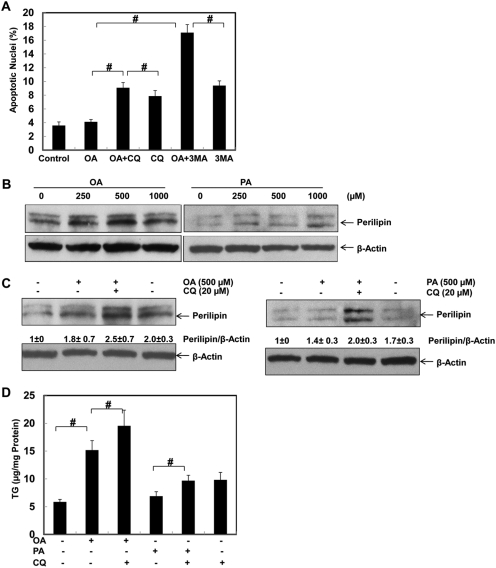
We next determined whether autophagy would play a protective role against fatty acid-induced apoptosis. We found that suppression of autophagy by using two pharmacological autophagy inhibitors, 3-MA, which suppresses the upstream PI3K (Beclin 1-Vps34 complex), and CQ, which inhibits the downstream lysosomal function, by increasing lysosomal pH, significantly increased OA-induced apoptosis (Fig. 7A). Interestingly, we further found that OA attenuated PA-induced apoptosis (Supplemental Fig. 6). This finding can be partially explained by OA-induced autophagy in HepG2 cells. Autophagy has been shown to remove excessive lipid droplets in hepatocytes and in alcohol-treated mouse liver (lipophagy) (Singh et al., 2009a; Ding et al., 2010a). Therefore, we next determined whether autophagy would also influence OA- and PA-induced accumulation of lipids in HepG2 cells. Perilipin is a lipid droplet-associated protein that is localized at the surface of the lipid droplet and whose protein levels may correlate with the number of lipid droplets (Ducharme and Bickel, 2008). We next determined the protein level of perilipin after OA and PA treatment in HepG2 cells. We found that both OA and PA increased the protein levels of perilipin in a dose-dependent manner (Fig. 7B). These results are generally in agreement with our earlier findings in Fig. 5 and previous reports (Listenberger et al., 2003; Ricchi et al., 2009). Of interest, we found that suppression of autophagy by CQ further increased both OA- and PA-induced expression of perilipin, supporting the current notion that autophagy may help to remove lipid droplets (Fig. 7C). In line with the findings for the perilipin changes, OA also significantly increased the total cellular TG level compared with that of control cells or PA-treated cells. Suppression of autophagy by CQ significantly increased TG levels in OA-treated cells (Fig. 7D). Furthermore, suppression of autophagy by 3-MA also increased OA-induced accumulation of LD (Supplemental Fig. 7A). In contrast, induction of autophagy by rapamycin tended to reduce TG levels in OA-treated cells (Supplemental Fig. 7B). Of interest, CQ alone also increased TG levels in HepG2 cells, suggesting that even inhibition of basal level autophagy could also increase the level of TG (Fig. 7D). The level of TG was higher in the PA and CQ treatment groups than that with PA treatment alone but was almost identical to that with CQ treatment alone. The lack of an additional increase of TG by CQ in CQ- and PA-treated cells is probably due to the already low autophagy activity induced by PA treatment in these cells (Fig. 7D). Taken together, these findings suggest that autophagy can attenuate fatty acid-induced apoptosis and the accumulation of lipids.
Fig. 7.
Suppression of autophagy enhances fatty acid-induced cell death and lipid accumulation. A, HepG2 cells were treated with vehicle control (5% BSA), OA (500 μM), OA plus CQ (20 μM), CQ alone, OA plus 3-MA (10 mM), or 3-MA alone for 24 h, and apoptotic cell death was analyzed by nuclear staining with Hoechst 33342 (mean ± S.E., n = 3). #, p < 0.01 (one-way ANOVA with the Scheffé post hoc test). B and C, HepG2 cells were treated with vehicle control (5% BSA) or various concentrations (125, 250, or 500) of OA or PA for 6 h (B) or with OA (500 μM) or PA (500 μM) in the presence or absence of CQ (20 μM) for 6 h (C). Total cell lysates were subjected to immunoblot analysis for perilipin A. Densitometry analysis for the expression level of perilipin was performed using ImageJ software, which was further normalized with its loading control (β-actin). D, HepG2 cells were treated as in C, and cellular TG levels (mean ± S.E., n = 3) were quantified as described under Materials and Methods. #, p < 0.01 (one-way ANOVA with the Scheffé post hoc test).
Induction of Steatosis and Autophagy in High-Fat Diet Mouse Liver.
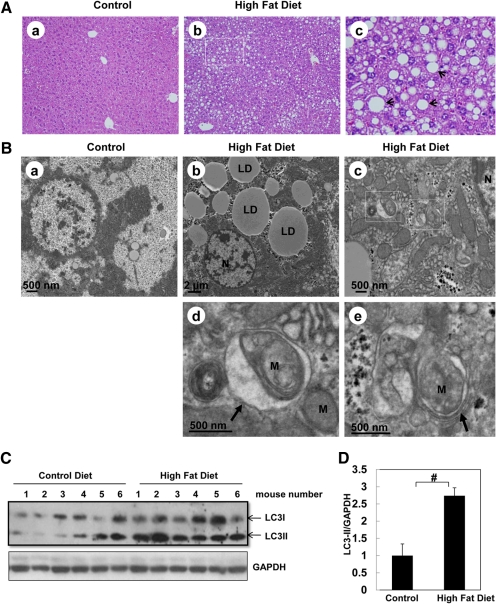
To determine the effects of a high-fat diet on steatosis and autophagy in the mouse liver, C57BL/6 mice were fed a high-fat diet or a control diet for 12 weeks. Macro- and microvesicular steatosis was evident in livers of mice fed with the high-fat diet (Fig. 8A). EM studies further confirmed that both the number and size of the hepatic lipid droplets were markedly increased in livers of mice fed a high-fat diet (Fig. 8B, b). Moreover, we also often found double-membrane autophagosomes that enwrapped damaged mitochondria in livers from mice fed a high-fat diet (Fig. 8B, c–e). Results from Western blot analysis indicate that there was an increased level of LC3-II in livers from mice fed a high-fat diet (Fig. 8, C and D), suggesting a possible increased number of autophagosomes or autolysosomes in high-fat diet-fed mouse livers. Taken together, these data suggest that a high-fat diet increases steatosis and may induce autophagy in mouse liver.
Fig. 8.
A high-fat diet induces steatosis and autophagy in mouse liver. Male C57BL/6J mice were fed either a control diet or a Western diet for 3 months. All the mice were starved for 16 h before they were sacrificed. A, representative photomicrograph of hematoxylin and eosin-stained liver section from a mouse fed a control diet (a) and from a mouse fed the high-fat diet (b). c, enlarged photomicrograph from b showing typical macrovesicular hepatic steatosis (arrows). B, liver samples were processed for EM. a, control diet. b and c, high-fat diet. d and e, enlarged photomicrographs from the boxed areas in c. Arrows, double membrane autophagosomes; N, nuclei, LD, lipid droplets; M, mitochondria. C, total liver lysates were subjected to Western blot analysis using an anti-LC3 antibody. The same membrane was blotted for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the loading control. D, densitometry analysis for the expression level of LC3-II was performed using ImageJ software, which was further normalized with its loading control (GAPDH). Data are presented as the fold of the control diet mouse livers (mean ± S.E., n = 6). #, p < 0.01, Student's t test.
Discussion
Hepatic lipotoxicity is closely associated with the progression of fatty liver disease. However, the mechanisms by which excess fatty acids induce hepatotoxicity are not completely understood. Furthermore, the mechanisms by which hepatocytes tolerate lipotoxicity remain largely unknown. In the present study, we found that saturated and unsaturated fatty acids differentially regulate autophagy and apoptosis in HepG2 cells. Monounsaturated OA was readily converted to TG-enriched lipid droplets, induced autophagy, and was resistant to apoptosis in HepG2 cells. In contrast, saturated PA was only slightly converted to TG-enriched lipid droplets resulting in the induction of apoptosis without the activation of autophagy. We demonstrated that there is cross-talk between fatty acid-induced apoptosis and autophagy, in which saturated PA-induced apoptosis suppresses autophagy by caspase-mediated cleavage of Beclin 1. Conversely, autophagy also attenuated fatty acid-induced apoptosis and accumulation of lipids. We further found that a high-fat diet induced marked steatosis and autophagy in the mouse liver. Overall, the results reveal a novel mechanism underlying the differential role of saturated versus unsaturated fatty acids in hepatotoxicity and could suggest new therapeutic approaches for treating fatty liver diseases by modulating autophagy.
Induction of apoptosis by excessive free fatty acids is a key histological feature of nonalcoholic fatty liver disease and correlates with progressive inflammation and fibrosis. The accumulation of TG-enriched lipid droplets was once thought to be the underlying cause of liver injury and insulin resistance in tissues, but it has recently been suggested that the accumulation of lipid droplets is a parallel phenomenon and may even play a protective role against the lipotoxicity from free fatty acids and other fatty acid-derived mediators (Malhi and Gores, 2008; Garbarino and Sturley, 2009; Neuschwander-Tetri, 2010). In Chinese hamster ovary cells, OA is readily converted into TG and stored in lipid droplets, resulting in less apoptosis, whereas PA is poorly incorporated into triglyceride and increases apoptosis (Listenberger et al., 2003). In the present study, we also found that the number of lipid droplets and the levels of TG are significantly higher in OA-treated HepG2 cells than in PA-treated cells. As a result, OA fails to induce apoptosis and even protects against PA-induced apoptosis in HepG2 cells (Fig. 5C; Supplemental Fig. 6). It has been reported that overexpression of stearoyl-CoA desaturase 1, which increases the level of unsaturated fatty acids and TG formation, decreases PA-induced lipotoxicity (Listenberger et al., 2003). On the other hand, impairing the formation of TG by knockout of acyl CoA:diacylglycerol transferase 1 increases the lipotoxicity of OA (Listenberger et al., 2003). Taken together, our results along with other groups' findings suggest that the formation of TG may not be the cause of fatty acid-induced lipotoxicity.
It is interesting that the monounsaturated fatty acid OA but not the saturated fatty acid PA induces autophagy in HepG2 cells. This could be one additional important mechanistic basis for why OA is less toxic than PA in HepG2 cells. Autophagy is recognized as a critical cell survival mechanism induced by nutrient or growth factor deprivation, hypoxia, ROS, DNA damage, protein aggregates, damaged organelles, or intracellular pathogens (Kroemer et al., 2010; Ravikumar et al., 2010). Although the mechanisms by which autophagy protects against cell death are not fully understood, it is generally thought to involve multiple mechanisms including bulk protein degradation, recycling of misfolded and aggregate-prone proteins, relieving endoplasmic reticulum stress, and removing depolarized or permeabilized mitochondria (Ding et al., 2007a,b; Kroemer et al., 2010; Kim and Lemasters, 2011). Fatty acids have been shown to induce apoptosis through activation of the proapoptotic protein Bax and subsequent mitochondrial damage (Malhi et al., 2006). In the present study, we found that saturated fatty acids increased the number of cells with depolarized mitochondria (Fig. 5, D and E). Moreover, suppression of autophagy enhanced fatty acid-induced apoptosis. It is possible that the impaired autophagy in PA-treated cells may exacerbate mitochondrial damage and further increase apoptosis. Although our present study and other previous reports showed that saturated fatty acids (such as PA) are more toxic than unsaturated fatty acids (such as OA), it has also been reported that a diet enriched in saturated but not unsaturated fatty acids reversed alcohol-induced liver injury in a rat model (Nanji et al., 1995). This paradox could be due to cytochrome P450 2E1 activity being suppressed by saturated but not by unsaturated fatty acids and CYP2E1 being the key enzyme that promotes alcohol-induced liver injury (Nanji et al., 1995).
Why would saturated and unsaturated fatty acids have different effects in autophagy induction? In mammalian cells, the mTOR pathway is the most studied pathway regulating autophagy. Many diverse signals such as growth factors and amino acids activate mTOR to suppress autophagy. In contrast, rapamycin suppresses mTOR and induces autophagy in various cell lines. Inhibition of mTOR leads to reduced phosphorylation of two of its downstream effectors, p70S6K and 4EBP1. How the suppression of mTOR leads to autophagy induction is not completely known, but data suggest that suppression of mTOR is probably coupled to the activation of the ULK1-ULK2 (mammalian orthologs of yeast Atg1) complex, which may recruit other autophagy proteins to the isolation membrane, the origin of the autophagosome membrane (Mizushima, 2010). In this study, we found that OA has little effect on mTOR suppression, suggesting that OA-induced autophagy could be mTOR-independent. Several stimuli have been shown to induce autophagy independent of mTOR such as lithium, carbamazepine, and valproic acid, all of which reduce intracellular inositol levels (Ravikumar et al., 2010). Although the effect of OA on intracellular inositol levels is not known, we found that OA-induced autophagy requires ROS formation and the classic PI3 kinase complex because an antioxidant (NAC) and a PI3 kinase inhibitor (3-MA) suppress OA-induced autophagy. Unlike OA, PA increased apoptosis and caspase-3 activation in HepG2 cells, whereas there is no or even decreased autophagy induction. Cleavage of Beclin 1 during apoptosis has been shown to block Beclin 1-dependent autophagy (Luo and Rubinsztein, 2010). Indeed we found that PA induced caspase-mediated cleavage of Beclin 1. Thus, our results support the emerging notion that there is cross-talk between apoptosis and autophagy, in which autophagy and apoptosis counteract each other. Therefore, the different capacity for apoptosis induction by PA and OA may determine their different effects on autophagy induction.
In addition to protecting against cell death, autophagy has recently been show to regulate lipid homeostasis by removing excess lipid droplets (Singh et al., 2009a). Liver-specific knockout of Atg7, an essential autophagy gene regulating conjugation of LC3 with PE, leads to steatosis in the mouse liver (Singh et al., 2009a). Our previous studies also demonstrated that activation of autophagy reduced alcohol-induced steatosis in an acute mouse model (Ding et al., 2010a, 2011). In the present study, we found that autophagy also regulates fatty acid-induced lipid accumulation. Suppression of autophagy by CQ increases the accumulation of lipids in hepatocytes, whereas induction of autophagy by rapamycin tends to decrease TG contents in OA-treated hepatocytes. These findings suggest that modulation of autophagy may provide a novel therapeutic approach for not only alcoholic liver disease but also general obesity-induced steatosis.
In conclusion, we found that unsaturated and saturated fatty acids differentially regulate apoptosis and autophagy in hepatocytes. Unsaturated OA promotes the formation of TG-enriched lipid droplets, induces autophagy, and has little effect on lipoapoptosis. Saturated PA is poorly converted into TG-enriched lipid droplets, induces lipoapoptosis, and decreases autophagy. Induction of autophagy protects against fatty acid-induced lipotoxicity. Our data also support the emerging concept that autophagy and apoptosis are two antagonistic events that tend to inhibit each other. The modulation of autophagy represents a novel approach that may have therapeutic benefits for obesity-induced steatosis and liver injury.
Supplementary Material
Acknowledgments
We thank Barbara Fegley (University of Kansas Medical Center Electron Microscopy Research Laboratory) for excellent assistance with the EM studies. We thank Drs. Hao Zhu and Ming Xu for technical support for the triglyceride measurement.
This work was supported in part by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant R21-AA017421]; the National Institutes of Health National Center for Research Resources [Grants P20-RR021940, P20-RR016475] (the first to W.-X.D. and J.P.L. and the second to W.-X.D); the National Institutes of Health National Institute of Environmental Health Sciences [Grant R01-ES017537] (to J.P.L.); and American Heart Association [Scientist Development Grant 0835121G] (to J.P.L.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.184341.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- PA
- palmitic acid
- OA
- oleic acid
- TG
- triglyceride
- LC3
- light chain 3
- PE
- phosphatidylethanolamine
- CQ
- chloroquine
- BAF
- bafilomycin A1
- Beclin 1
- Bcl-2-interacting protein 1
- PI3K
- phosphatidylinositol 3-kinase
- p70S6K
- 70-kDa ribosomal protein S6 kinase-1
- 4EBP1
- translational initiation factor 4E binding protein-1
- BSA
- bovine serum albumin
- 3-MA
- 3-methyladenine
- NAC
- N-acetylcysteine
- TNF-α
- tumor necrosis factor-α
- Z-VAD-fmk
- N-benzyloxycarbonyl-Val-Ala-Asp(O-Met) fluoromethyl ketone
- TMRM
- tetramethylrhodamine methyl ester
- PBS
- phosphate-buffered saline
- GFP
- green fluorescent protein
- ANOVA
- analysis of variance
- EM
- electron microscopy
- AV
- autophagosomes
- LD
- lipid droplets
- ROS
- reactive oxygen species
- mTOR
- mammalian target of rapamycin
- ActD
- actinomycin D.
Authorship Contributions
Participated in research design: Ding and Luyendyk.
Conducted experiments: Mei, Ni, Manley, Bockus, Kassel, Copple, and Ding.
Contributed new reagents or analytic tools: Luyendyk.
Performed data analysis: Mei, Ni, and Ding.
Wrote or contributed to the writing of the manuscript: Mei, Kassel, Luyendyk, and Ding.
References
- Baylin A, Kabagambe EK, Siles X, Campos H. (2002) Adipose tissue biomarkers of fatty acid intake. Am J Clin Nutr 76:750–757 [DOI] [PubMed] [Google Scholar]
- Chalasani N, Deeg MA, Crabb DW. (2004) Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 99:1497–1502 [DOI] [PubMed] [Google Scholar]
- Choi SE, Lee SM, Lee YJ, Li LJ, Lee SJ, Lee JH, Kim Y, Jun HS, Lee KW, Kang Y. (2009) Protective role of autophagy in palmitate-induced INS-1 beta-cell death. Endocrinology 150:126–134 [DOI] [PubMed] [Google Scholar]
- Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, Lu B, Stolz DB, Clemens DL, Yin XM. (2010a) Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology 139:1740–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Manley S, Ni HM. (2011) The emerging role of autophagy in alcoholic liver disease. Exp Biol Med (Maywood) 236:546–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Ni HM, DiFrancesca D, Stolz DB, Yin XM. (2004) Bid-dependent generation of oxygen radicals promotes death receptor activation-induced apoptosis in murine hepatocytes. Hepatology 40:403–413 [DOI] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Gao W, Chen X, Kang JH, Stolz DB, Liu J, Yin XM. (2009) Oncogenic transformation confers a selective susceptibility to the combined suppression of the proteasome and autophagy. Mol Cancer Ther 8:2036–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, Stolz DB, Shao ZM, Yin XM. (2007a) Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem 282:4702–4710 [DOI] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Gao W, Yoshimori T, Stolz DB, Ron D, Yin XM. (2007b) Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am J Pathol 171:513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Li M, Liao Y, Chen X, Stolz DB, Dorn GW, 2nd, Yin XM. (2010b) Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. J Biol Chem 285:27879–27890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Yin XM. (2004) Dissection of the multiple mechanisms of TNF-α-induced apoptosis in liver injury. J Cell Mol Med 8:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djavaheri-Mergny M, Maiuri MC, Kroemer G. (2010) Cross talk between apoptosis and autophagy by caspase-mediated cleavage of Beclin 1. Oncogene 29:1717–1719 [DOI] [PubMed] [Google Scholar]
- Ducharme NA, Bickel PE. (2008) Lipid droplets in lipogenesis and lipolysis. Endocrinology 149:942–949 [DOI] [PubMed] [Google Scholar]
- Fimia GM, Piacentini M. (2010) Regulation of autophagy in mammals and its interplay with apoptosis. Cell Mol Life Sci 67:1581–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarino J, Sturley SL. (2009) Saturated with fat: new perspectives on lipotoxicity. Curr Opin Clin Nutr Metab Care 12:110–116 [DOI] [PubMed] [Google Scholar]
- He C, Levine B. (2010) The Beclin 1 interactome. Curr Opin Cell Biol 22:140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa N, Hara Y, Mizushima N. (2006) Generation of cell lines with tetracycline-regulated autophagy and a role for autophagy in controlling cell size. FEBS Lett 580:2623–2629 [DOI] [PubMed] [Google Scholar]
- Kim I, Lemasters JJ. (2011) Mitophagy selectively degrades individual damaged mitochondria after photoirradiation. Antioxid Redox Signal 14:1919–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Cregg JM, Dunn WA, Jr, Emr SD, Sakai Y, Sandoval IV, Sibirny A, Subramani S, Thumm M, Veenhuis M, et al. (2003) A unified nomenclature for yeast autophagy-related genes. Dev Cell 5:539–545 [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC. (2008) Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy 4:849–950 [DOI] [PubMed] [Google Scholar]
- Komiya K, Uchida T, Ueno T, Koike M, Abe H, Hirose T, Kawamori R, Uchiyama Y, Kominami E, Fujitani Y, et al. (2010) Free fatty acids stimulate autophagy in pancreatic β-cells via JNK pathway. Biochem Biophys Res Commun 401:561–567 [DOI] [PubMed] [Google Scholar]
- Kroemer G, Mariño G, Levine B. (2010) Autophagy and the integrated stress response. Mol Cell 40:280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. (2004) The role of autophagy during the early neonatal starvation period. Nature 432:1032–1036 [DOI] [PubMed] [Google Scholar]
- Li H, Wang P, Sun Q, Ding WX, Yin XM, Sobol RW, Stolz DB, Yu J, Zhang L. (2011a) Following cytochrome c release, autophagy is inhibited during chemotherapy-induced apoptosis by caspase-8-mediated cleavage of Beclin-1. Cancer Res 71:3625–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Hou Y, Wang J, Chen X, Shao ZM, Yin XM. (2011b) Kinetics comparisons of mammalian Atg4 homologues indicate selective preferences toward diverse Atg8 substrates. J Biol Chem 286:7327–7338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. (2003) Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 100:3077–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Rubinsztein DC. (2010) Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ 17:268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyendyk JP, Sullivan BP, Guo GL, Wang R. (2010) Tissue factor-deficiency and protease activated receptor-1-deficiency reduce inflammation elicited by diet-induced steatohepatitis in mice. Am J Pathol 176:177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Bronk SF, Werneburg NW, Gores GJ. (2006) Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem 281:12093–12101 [DOI] [PubMed] [Google Scholar]
- Malhi H, Gores GJ. (2008) Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis 28:360–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N. (2010) The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol 22:132–139 [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. (2010) Methods in mammalian autophagy research. Cell 140:313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanji AA, Sadrzadeh SM, Yang EK, Fogt F, Meydani M, Dannenberg AJ. (1995) Dietary saturated fatty acids: a novel treatment for alcoholic liver disease. Gastroenterology 109:547–554 [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA. (2010) Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 52:774–788 [DOI] [PubMed] [Google Scholar]
- Ni HM, Bockus A, Wozniak AL, Jones K, Weinman S, Yin XM, Ding WX. (2011) Dissecting the dynamic turnover of GFP-LC3 in the autolysosome. Autophagy 7:188–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, et al. (2010) Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 90:1383–1435 [DOI] [PubMed] [Google Scholar]
- Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, Fantoni LI, Marra F, Bertolotti M, Banni S, et al. (2009) Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol 24:830–840 [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Cuervo AM, Ravikumar B, Sarkar S, Korolchuk V, Kaushik S, Klionsky DJ. (2009) In search of an “autophagomometer.” Autophagy 5:585–589 [DOI] [PubMed] [Google Scholar]
- Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. (2007) Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 26:1749–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S, Kitada T, Sakaguchi H. (2005) Clinicopathological significance of oxidative cellular damage in non-alcoholic fatty liver diseases. Hepatol Res 33:132–134 [DOI] [PubMed] [Google Scholar]
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. (2009a) Autophagy regulates lipid metabolism. Nature 458:1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ. (2009b) Autophagy regulates adipose mass and differentiation in mice. J Clin Invest 119:3329–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Ohsumi Y. (2010) Current knowledge of the pre-autophagosomal structure (PAS). FEBS Lett 584:1280–1286 [DOI] [PubMed] [Google Scholar]
- Wobser H, Dorn C, Weiss TS, Amann T, Bollheimer C, Büttner R, Schölmerich J, Hellerbrand C. (2009) Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res 19:996–1005 [DOI] [PubMed] [Google Scholar]
- Xu M, Wang W, Frontera JR, Neely MC, Lu J, Aires D, Hsu FF, Turk J, Swerdlow RH, Carlson SE, et al. (2011) Ncb5or deficiency increases fatty acid catabolism and oxidative stress. J Biol Chem 286:11141–11154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin S. (2009) Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci USA 106:19860–19865 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.