Abstract
Doxorubicin (DOX) is a drug commonly used for the treatment of cancer. The development of resistance to DOX is common, and high cumulative doses cause potentially lethal cardiac side effects. HO-3867 (3,5-bis(4-fluorobenzylidene)-1-[(2,2,5,5-tetramethyl-2,5-dihydro-1-hydroxy-pyrrol-3-yl)methyl]piperidin-4-one), a synthetic curcumin analog, has been shown to exhibit both anticancer and cardioprotective effects. However, its cardioprotection in the setting of a conventional cancer therapy has not been established. This work investigated the use of HO-3867 and DOX to achieve a complementary outcome, i.e., increased toxicity toward cancer cells, and reduced cardiac toxicity. Combination treatment was investigated using DOX-resistant MCF-7 breast cancer cells [MCF-7 multidrug-resistant (MDR)] and BALB/c mice. Lower doses of HO-3867 and DOX (5 and 2.5 μM, respectively) reduced viability of MCF-7 MDR cells to an extent significantly greater than that when either drug was used alone, an effect equivalent to that induced by exposure to 50 μM DOX. In normal cardiac cells, the loss of viability from combination treatment was significantly lower than that induced by 50 μM DOX. Increases in apoptotic markers, e.g., cleaved caspase-3, and decreases in fatty acid synthase and pAkt expressions were observed by Western blotting. Mice treated with both HO-3867 and DOX showed significant improvement in cardiac functional parameters compared with mice treated with DOX alone. Reduced expression of Bcl-2 and pAkt was observed in mice treated with DOX alone, whereas mice given combination treatment showed levels similar to control. The study indicates that combination treatment of HO-3867 and DOX is a viable option for treatment of cancer with reduced cardiotoxic side effects.
Introduction
Doxorubicin (DOX) is commonly used in the treatment of several malignancies, including breast cancer. Although effective in the initial stages, repeated administration of DOX may lead to the development of drug resistance, which necessitates the use of increased doses to achieve sufficient therapeutic effect. Because increasing the dose increases the rate at which high cumulative doses are reached, DOX resistance is a serious issue to overcome (Szakács et al., 2006). In addition, at high cumulative doses of DOX, cardiotoxic side effects, such as congestive heart failure, dilated cardiomyopathy, and death, become increasingly more probable (Unverferth et al., 1982; Dunn, 1994; Jain, 2000; Tokarska-Schlattner et al., 2006; Takemura and Fujiwara, 2007; Ewer and Ewer, 2010). The pathogenesis of DOX-induced cardiotoxicity and heart failure is complex and may involve various signaling mechanisms, including oxidative stress, mitochondrial dysfunction/damage, and activation of mitogen-activated protein kinases (MAPK) (Kalyanaraman et al., 2002; Jin et al., 2003; Small et al., 2007; Takemura and Fujiwara, 2007; Vibet et al., 2008). There is increasing identification that activation of MAPK, p38, and c-Jun N-terminal kinase contributes to DOX-induced cell death and cardiotoxicity (Kim and Freeman, 2003; Timolati et al., 2006). Although the exact mechanism of DOX cardiotoxicity is not currently known, DOX-mediated generation of reactive oxygen species (ROS) has been implicated as being at least partially responsible (Zhou et al., 2001; Kalyanaraman et al., 2002; Kim et al., 2006). To ameliorate ROS-induced toxicity, there is a need to use detoxicants such as antioxidants that can protect cardiac cells by scavenging the oxidants (Cao and Li, 2004; Khan et al., 2006; Danz et al., 2009; Li et al., 2010).
HO-3867 (3,5-bis(4-fluorobenzylidene)-1-[(2,2,5,5-tetramethyl-2,5-dihydro-1-hydroxy-pyrrol-3-yl)methyl]piperidin-4-one) is a synthetic curcumin analog that we previously have shown to have antioxidant as well as anticancer properties (Selvendiran et al., 2010a,b,c). The compound targets STAT3 pathways in various cancer cell lines and inhibits xenograft ovarian tumor growth, without causing any significant side effects to healthy cells (Selvendiran et al., 2010a,b,c). In addition, we showed that HO-3867 inhibits Akt and MAPK expression by activation of phosphatase and tensin homolog on chromosome 10 in serum-stimulated (proliferating) human smooth muscle cells (Selvendiran et al., 2009). Furthermore, HO-3867 has been shown to have protective effects on healthy cells, at least partially because of its antioxidant properties (Selvendiran et al., 2010a). As such, it is an obvious complement to DOX for cancer therapeutics, because both of these drugs exhibit antitumor properties, and HO-3867 is additionally effective in scavenging ROS.
In this work, we examined the effects of DOX and HO-3867 combination treatment in vitro on breast cancer cells, including a drug-resistant strain. Normal cardiac and aortic endothelial cells were used as controls. We then examined the effect of combination treatment on the development of cardiotoxic side effects in vivo using a mouse model of breast tumor. The results showed that combination treatment allowed for equivalent loss of cell viability in cancerous cells compared with a high dose of DOX while reducing the toxic effects in healthy cells. Furthermore, it was observed that combination treatment eliminated the cardiotoxic side-effects of DOX.
Materials and Methods
Materials.
HO-3867 was synthesized as reported previously (Selvendiran et al., 2010a; Kaìlai et al., 2011). Stock solutions (20 mM) of the compound were freshly prepared in dimethyl sulfoxide. Cell culture medium (DMEM), fetal bovine serum, antibiotics, sodium pyruvate, trypsin, and phosphate-buffered saline were purchased from Invitrogen (Carlsbad, CA). Other cell culture media were purchased from the American Type Culture Collection (Manassas, VA) and Lonza (SmBM; Basel, Switzerland). Polyvinylidene fluoride membranes and molecular weight markers were obtained from Bio-Rad (Hercules, CA). Antibodies against pAkt, Akt, pERK1/2, ERK1/2, FAS, Bcl-2, Bcl-xL, caspases-3 and -7, PARP, and actin were purchased from Cell Signaling Technology (Danvers, MA). Antibodies specific for α-tubulin, cyclins D1 and D2, p53, p21, and p27 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Enhanced chemiluminescence reagents were obtained from (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). DOX and all other reagents of analytical grade or higher were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Lines and Culture.
MCF-7 human breast and MCF-7 MDR breast cancer cell lines were used. The MCF-7 MDR cells are partially resistant to anthracyclines, including DOX. Human aortic endothelial cells (HAEC) and cardiomyocytes (H9C2) were used as noncancerous (healthy) controls. The MCF-7 and MCF-7 MDR cells were grown in DMEM, H9C2 cells were grown in DMEM (American Type Culture Collection), and HAEC were grown in SmBM. All cell media were supplemented with 10% fetal bovine serum, 2% sodium pyruvate, 1% penicillin, and 1% streptomycin. Cells were grown in a 75-mm flask to 70% confluence at 37°C in an atmosphere of 5% CO2 and 95% air. Cells were routinely trypsinized (0.05% trypsin/EDTA) and counted using an automated counter (NucleoCounter; New Brunswick Scientific, Edison, NJ).
Cell Viability Assay.
Cell survival was assessed by MTT assay. Cells, grown to approximately 80% confluence in 75-mm flasks, were trypsinized, counted, seeded in 96-well plates with an average population of 7000 cells/well, incubated overnight, and then treated for 24 h with DOX alone (2.5, 25, or 50 μM), HO-3867 alone (5 μM), or in combination (2.5 μM DOX and 5 μM HO-3867). All experiments were done using six replicates and repeated at least three times. Untreated cells were used as controls.
Annexin V Assay.
Cells were treated with the drugs for a period of 24 h, trypsinized, washed in phosphate-buffered saline, and treated with annexin V and propidium iodide staining. Cells were sorted and analyzed by flow cytometry. Untreated cells were used as controls.
Immunoblot Analysis.
Cells in their respective media were treated with DOX alone (2.5 μM), HO-3867 alone (5 μM), or in combination using these concentrations for 24 h. Equal volumes of dimethyl sulfoxide (0.1% v/v) were present in each treatment. After treatment, the cell lysates were prepared in nondenaturing lysis buffer containing 10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 0.3 mM phenylmethylsulfonyl fluoride, 0.2 mM sodium orthovanadate, 0.5% Nonidet P40, 1 μg/ml aprotinin, and 1 μg/ml leupeptin. The lysates were centrifuged at 10,000g for 20 min at 4°C, and the supernatant was separated. The protein concentration in the lysates was determined using a Pierce detergent-compatible protein assay kit (Thermo Fisher Scientific, Waltham, MA). For Western blotting, 25 to 50 μg of protein lysate per sample was denatured in 2× SDS-polyacrylamide gel electrophoresis sample buffer and subjected to SDS-polyacrylamide gel electrophoresis on a 10% Tris-glycine gel. The separated proteins were transferred to a polyvinylidene fluoride membrane and blocked with 5% (w/v) nonfat milk powder in Tris-buffered saline with Tween 20 (10 mM Tris, 10 mM NaCl, 0.1% Tween 20) for 1 h at room temperature or overnight at 4°C. The membranes were then incubated with the primary antibodies. The bound antibodies were detected with horseradish peroxidase-labeled sheep antimouse IgG or horseradish peroxidase-labeled donkey anti-rabbit IgG using an enhanced chemiluminescence detection system (ECL Advanced kit; GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). Protein expression levels were quantified using Image Gauge version 3.45 (Fuji Film Corporation, Tokyo, Japan).
BALB/c Mouse Handling and Treatment.
Male BALB/c mice of approximately 20 g weight were split into four groups: control, DOX-treated, HO-3867-treated, or combination-treated. DOX was administered as an intraperitoneal injection of 2.5 mg/kg weekly for 4 weeks. HO-3867 was given continuously as 100 ppm in the feed. Combination-treated animals received both DOX injections and HO-3867 feed. After 5 weeks, the animals were euthanized. Tissue samples were collected at this time. Ventricular sections from euthanized mouse hearts were homogenized and treated using the same lysis buffer described above, after which the procedure for immunoblot analysis was the same as that described above for cells.
Echocardiography for Cardiac Functional Analysis.
Cardiac function was analyzed using echocardiography at baseline before the beginning of DOX treatment and at 4 weeks after DOX treatment. Mice were anesthetized using 1.5 to 2% isoflurane, and M-mode ultrasound images were acquired using a Vevo-2100 (VisualSonics, Toronto, ON, Canada) high-resolution ultrasound rodent imaging system.
Histopathology.
Slices of recovered heart tissues were fixed in 10% formalin for 48 h at room temperature, dehydrated by graded ethanol, and embedded in paraffin. Tissue sections (5-μm thick) were deparaffinized with xylene and stained with hematoxylin and eosin, and representative photomicrographs were taken using an inverted fluorescence microscope (Nikon TE 2000; Nikon, Tokyo, Japan).
Data Analysis.
The statistical significance of the results was evaluated using Student's t test or analysis of variance, as appropriate. A p value of less than 0.05 was considered significant.
Results
Combination Treatment with HO-3867 and DOX Reduces Cell Viability in MCF-7 and MCF-7 MDR Breast Cancer Cell Lines While Having Less Effect on Heart and Aortic Cells.
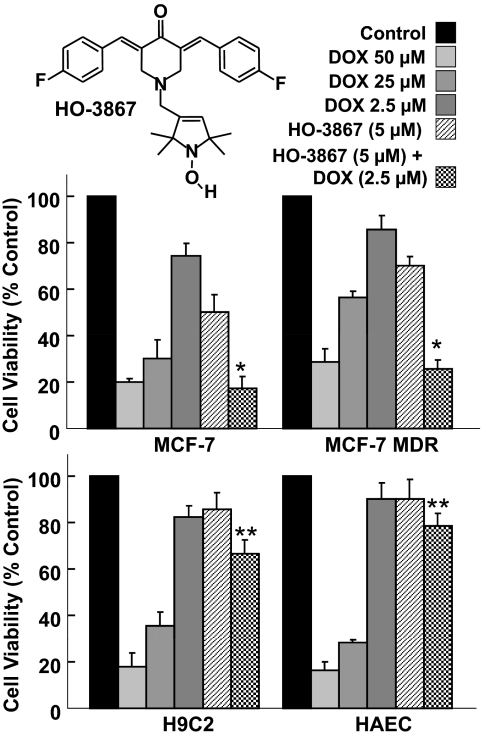
Using MTT assay, we examined the viability of several cell lines after treatment with low doses of DOX and HO-3867 alone (2.5 and 5 μM, respectively) or a combination of both of these concentrations. We compared these with effects observed using higher doses of DOX alone (25 and 50 μM). The results showed that combination treatment reduced the viability of MCF-7 and MCF-7 MDR breast cancer cells to an extent significantly greater than that seen by treatment with either drug alone (Fig. 1). Treatment with the higher doses of DOX had a similar effect on the viability of the cells. MCF-7 cells had slightly lower viability after treatment with DOX than MCF-7 MDR cells. In the noncancerous cell lines, H9C2 and HAEC, combination-treated cells had a significantly higher viability than those cells treated with higher doses of DOX. The results suggested that the combination treatment was more cytotoxic to cancer cells than DOX or HO-3867 alone at the doses used. Furthermore, the combination treatment induced much less cytotoxicity to normal cells.
Fig. 1.
DOX+HO-3867 combination treatment decreases viability in cancer cells, has less toxic effect upon normal cells. MTT assay of breast cancer cells (MCF-7, MCF-7 MDR) and normal cells (HAEC, H9C2) was performed using various concentrations of DOX and HO-3867 (structure shown) for 24 h. Combination treatment shows a greater loss of viability in cancer cells than treatment with either drug alone, at the same dose. Loss of viability is comparable to that seen in cells treated with a greater dose of DOX. In contrast, loss of viability in normal cells is significantly less than that in cells that were given a higher dose of DOX. *, p < 0.05 versus DOX (2.5 μM) and HO-3867 (5 μM), **, p < 0.05 versus DOX (50 μM). Values are mean ± S.D., n = 6.
Combination Treatment with Lowered Doses of HO-3867 and DOX Induces More Apoptosis in MCF-7 MDR Cells Than Treatment with Either Drug Alone.
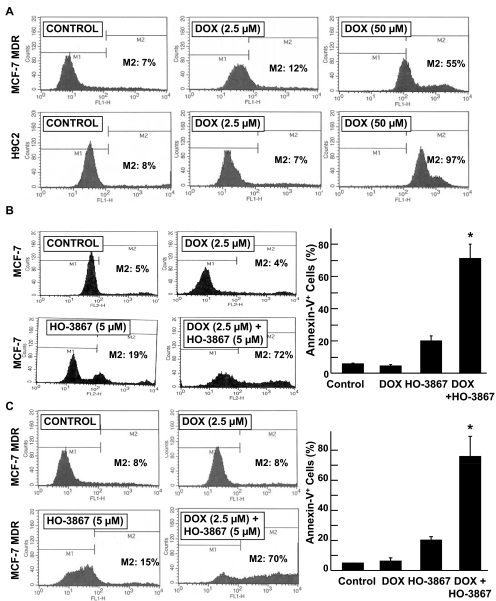
The inhibition of cell growth in tumor cells is usually associated with an increase in apoptosis. To analyze the effect of HO-3867 with DOX on apoptosis, we performed annexin V flow cytometry. In both MCF-7 MDR and HAEC cells, the higher dose of DOX induced a greater percentage of apoptotic cells (Fig. 2A). The apoptotic fraction was significantly increased in cells given combination treatment compared with those given DOX or HO-3867 alone in MCF-7 (Fig. 2B) and MCF-7 MDR (Fig. 2B) cells. The results indicated that the combination treatment induced substantially higher apoptosis in cancer cells than DOX or HO-3867 alone at the doses used.
Fig. 2.
DOX+HO-3867 combination treatment induces a greater percentage of annexin V-positive cancer cells than treatment with either drug alone. A, annexin V flow-cytometry data from MCF-7 MDR and H9C2 cells treated with either 5 or 50 μM DOX for 24 h. B and C, annexin V flow-cytometric data from MCF-7 (B) and MCF-7 MDR (C) cells treated with either DOX, HO-3867, or both (2.5 μM DOX, 5 μM HO-3867) for 24 h. Values in the bar graph are mean ± S.D., n = 3. *, p < 0.05 versus all other groups.
Combination Treatment of MCF-7 MDR Cells with HO-3867 and DOX Reduces Expression of Cell Cycle-Related Proteins and Increases Expression of Apoptotic Markers.
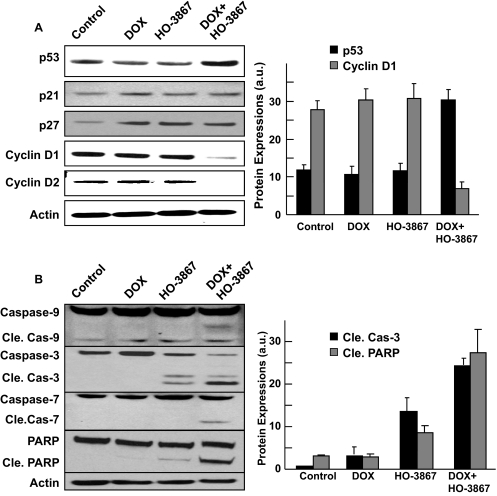
Western blotting was used to examine the effect of combination treatment on cell-cycle and apoptotic protein expressions in MCF-7 MDR cells. Cells treated with both DOX and HO-3867 had reduced levels of cyclins D1 and D2 compared with those treated with either drug alone or control (Fig. 3A). The expressions of caspases-3, -7, and -9, as well as cleaved PARP, were increased in combination-treatment cells compared with treatment with either of the drugs used alone or as control (Fig. 3B). The results revealed that the combination treatment caused greater inhibition of cell cycle-promoting proteins and promotion of apoptosis in cancer cells than DOX or HO-3867 alone at the doses used.
Fig. 3.
DOX+HO-3867 combination treatment decreases expression of cyclins D1 and D2 and induces expression of apoptotic markers. Western blotting was performed on MCF-7 MDR cells treated with DOX, HO-3867, or both (2.5 μM DOX, 5 μM HO-3867) for 24 h. A, the effect of treatment on cell cycle-related proteins and densitometric analysis of visualized bands for p53 and cyclin D1 expressions. B, the effect of treatment on apoptotic marker proteins and quantified the cleaved caspase-3 and PARP expressions (Cle. Cas, Cleaved caspase).
Combination Treatment of MCF-7 MDR Cells with HO-3867 and DOX Decreases Expression of FAS and pAkt (Ser473).
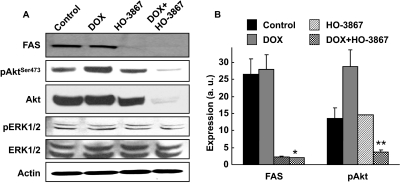
FAS is overexpressed in a variety of tumors and plays a key role in the migration and invasion of cancer cells. A number of reports have shown that anticancer agents induce apoptosis, in part, by blocking the activation of FAS and Akt pathways (Wrede et al., 2002; Coticchia et al., 2009; Zecchin et al., 2011). Akt prevents cells from undergoing apoptosis by inhibiting proapoptotic factors (Coticchia et al., 2009; Srinivasan et al., 2009). Recently, we have shown that HO-3867 suppressed the migration and invasion of the ovarian cancer cells by inhibiting the expression/activity of FAS (Selvendiran et al., 2010b). Hence, we determined the expression levels of FAS and Akt in cells treated with DOX and HO-3867 by Western blot analysis. The results showed that FAS, Akt, and pAkt (Ser473) were significantly decreased with combination treatment (Fig. 4).
Fig. 4.
DOX+HO-3867 combination treatment decreases expression of FAS and pAkt. Western blotting was performed on MCF-7 MDR cells treated with DOX, HO-3867, or both (2.5 μM DOX, 5 μM HO-3867) for 24 h. A, representative blots showing the effect of treatment on proteins in the FAS and Akt signaling pathways. B, quantitative representation of FAS and pAkT Ser473 (pAkTSer473). Values are mean ± S.D., n = 3. *, p < 0.05 versus control and DOX treatment. **, p < 0.05 versus all other groups.
Combination Treatment of BALB/c Mice with HO-3867 and DOX Decreases the Incidence of Cardiotoxic Side Effects Compared with DOX Treatment Alone.
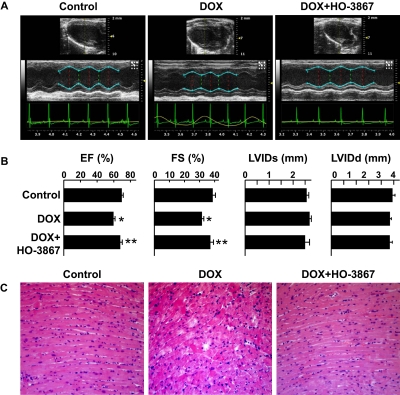
We examined the effect of combination treatment using an in vivo mice model of DOX-induced cardiotoxicity. Mice were treated with DOX alone or a combination of DOX and HO-3867 for 4 weeks and examined using M-mode echocardiography (Fig. 5A). Ejection fraction was significantly decreased in mice given DOX treatment alone, and mice given combination treatment had significantly greater ejection fraction by comparison (Fig. 5B). Likewise, fractional shortening was significantly less in the DOX-treated group but not in the combination-treated group compared with control. However, there were no significant differences in left ventricular dimensions at the systole and diastole between the groups. Histopathological analysis on animals treated with DOX alone showed characteristic cardiotoxic lesions, including mild to moderate multifocal cardiomyocyte degeneration, vacuolation, interstitial edema, and mild inflammatory cell infiltrates. Hearts from combination-treatment mice were devoid of such lesions and appeared histologically similar to that of untreated control animals (Fig. 5C). The results established that the combination treatment induced less cardiac dysfunction than DOX alone.
Fig. 5.
DOX+HO-3867 combination treatment protects cardiac tissue in vivo. Mice were treated with DOX or DOX+HO-3867. Echocardiography was performed after 4 weeks, and tissues were harvested after 5 weeks. A, representative M-mode echocardiograms of control and DOX-treated and combination-treated groups showing wall motion. B, changes in ejection fraction (EF), fractional shortening (FS), left ventricular internal diameter at systole (LVIDs), and left ventricular internal diameter at diastole (LVIDd) in the three groups. *, p < 0.05 versus control, **, p < 0.05 versus DOX-treated. Values are mean ± S.D., n = 6. DOX+HO-3867 treatment ameliorated the significant decrease in the ejection fraction and fractional shortening induced by DOX. C, representative images (magnification, 200×) of hematoxylin and eosin staining performed on cross-sections harvested after 5 weeks of treatment with DOX or DOX+HO-3867. Cross-sections from animals treated with DOX alone exhibited lesions characteristic of DOX toxicity, such as cardiomyocyte degeneration and cytoplasmic vacuolation, whereas the cross-sections from the animals given combination treatment seemed normal comparable to control.
Combination Treatment of BALB/c Mice with HO-3867 and DOX Increases the Expression of Prosurvival Proteins in Heart Tissue Compared with Treatment with DOX Alone.
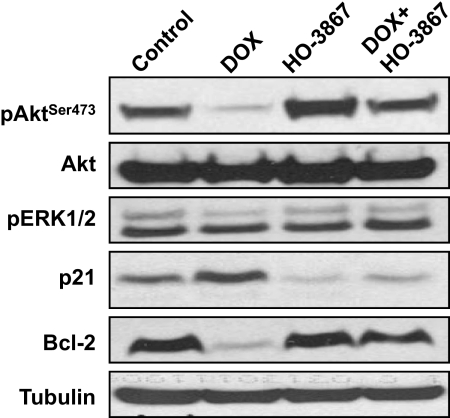
After 5 weeks of DOX treatment, the animals were euthanized, and the ventricular sections of the heart were harvested. Protein levels in these sections were analyzed by Western blotting. Levels of pAkt (Ser473) and Bcl-2 were decreased in DOX-treated mice but not in the combination-treated mice (Fig. 6). In contrast, p21 levels were increased in DOX-treated mice. This increase was not seen in combination-treated mice, in which p21 levels were similar to those seen in control mice. Our results indicate that HO-3867-induced up-regulation of Akt and Bcl-2 proteins may account, at least in part, for the cardioprotective effects observed in the combination-treated animals. The results established that the combination treatment up-regulated the levels of prosurvival proteins in the heart.
Fig. 6.
DOX+HO-3867 combination treatment induces protective proteins in heart tissue in vivo. Western blotting was performed on harvested ventricular tissue from mice after 5 weeks of treatment with DOX, HO-3867, or both. DOX significantly suppresses the pAkt and Bcl-2 proteins, whereas HO-3867 in combination with DOX reversed these proteins in cardiac tissues.
Discussion
Resistance to chemotherapy is a commonly encountered impediment in the treatment of breast cancer (Pivot et al., 2000; Rossi et al., 2005). Such resistance can prove to be a major limitation to the effectiveness of the treatment, necessitating the use of increased doses, which can lead to debilitating consequences that substantially reduce the quality of life of patients. In the case of DOX, increasing doses can lead to severe cardiac complications that may eventually promote an early death in patients (Unverferth et al., 1982; Jain, 2000; Takemura and Fujiwara, 2007; Ewer and Ewer, 2010). As such, development of strategies to counter drug resistance, while reducing both short-term and long-term impediments, is necessary. This study indicates that a low-dosage combination treatment using both HO-3867 and DOX can be more effective at inhibiting breast cancer cell growth than either drug working alone. Moreover, this combination therapy alleviates many of the side effects of high-dose DOX treatment.
This study shows that combination treatment of HO-3867 with a low dose of DOX has a similar effect on the viability of both wild-type and drug-resistant breast cancer cells compared with a much greater dose of DOX alone. The effect upon normal cardiomyocytes and aortic endothelial cells is markedly in contrast to that in cancer cells. The viability of the noncancerous (healthy) cells treated with the combination therapy is more than three times greater than that of cells treated with DOX. A similar trend is seen in the percentage of apoptotic cells in combination-treated cultures. It is clear that combination treatment with low-dose DOX and HO-3867 would reduce the amount of DOX exposure necessary to treat breast cancer. Because the side effects of DOX are known to be related to the total cumulative dose given, this is an important factor in assessing the clinical utility of such treatment.
The p53 tumor suppressor protein is to promote the induction of p21. The induction of p21 causes subsequent cell-cycle arrest by binding of the cyclin-cdk complex. In this study, we have shown that combination treatment of MDR cancer cells with HO-3867 and DOX resulted in the accumulation of p53, p21, and p27. The arrest of cell-cycle progression in tumor cells is usually associated with induction of apoptosis. Many chemotherapeutic agents induce apoptosis by activation of the p53-dependent pathway. The effect of the combination treatment is further seen in cell-cycle protein expression and expression of apoptotic markers. The expressions of cyclins D1 and D2 are reduced by combination treatment, whereas no effect is seen with treatment by either HO-3867 or DOX alone. Likewise, expression of cleaved caspases-3 and -7 is greatly increased by combination treatment, which is an indication of increased apoptosis-promoting signaling. These results indicate that the loss of viability seen in combination treatment may be caused by the complementary effects of both cell-cycle arrest and the induction of apoptosis.
Combination treatment significantly reduced the expression of FAS and both pAkt (Ser473) and total Akt. This implies that the mechanism of action for the combination treatment may involve one or both of these pathways. Our results indicate that FAS inhibition was solely caused by HO-3867 alone, because the combination treatment had no further inhibition (Fig. 4). We have recently shown that HO-3867 is capable of down-regulating FAS and focal adhesion kinase, thereby inhibiting the migration and invasion of human ovarian cancer cells (Selvendiran et al., 2010b). Because both FAS and Akt are often overexpressed in breast cancers (Esslimani-Sahla et al., 2007; Vazquez-Martin et al., 2008; Wu et al., 2008), this indicates that this combination may have clinical utility in these cases. It has previously been shown that the inhibition of FAS and Akt can lead to apoptosis in breast cancer, as well as other types of cancer (Coticchia et al., 2009; Zecchin et al., 2011). In contrast, cells overexpressing FAS and Akt exhibit resistance to apoptosis (Liu et al., 2008). By reducing the expression of FAS and Akt, the combination treatment may induce apoptosis through the mitochondrial pathway.
Side effects from DOX treatment commonly result in a loss of cardiac function. In the present study, the combination treatment permitted the use of reduced doses of DOX while maintaining the same level of cancer inhibition. Mice given combination treatment had significantly better cardiac function after 4 weeks than their counterparts that were given DOX alone. Both ejection fraction and fractional shortening were significantly reduced by DOX treatment, whereas mice given combination treatment experienced no such reduction. Furthermore, microscopic analysis of ventricular slices confirms that DOX-treated hearts are clearly physiologically different from control animals, whereas combination-treated animals have hearts that are physiologically similar to the control group. This similarity of the combination-treated animals to their control counterparts is also seen in the protein expression in the hearts. Both pAkt (Ser473) and Bcl-2 are significantly reduced in the DOX-treated animals; however, the expression of both proteins is recovered in the combination-treated group. This is especially interesting given the effect of combination treatment seen in cancer cells. In cancer cells, the combination treatment reduces the expression of pAkt (Ser473) compared with DOX treatment alone. In normal heart tissue, however, the effect of the combination is precisely the opposite; combination treatment results in a greater expression of pAkt (Ser473) than that seen in DOX-treated animals.
The design of HO-3867 was intended to provide both anticancer and antioxidant properties with cell-type-dependent cytotoxicity or protection (Kaìlai et al., 2011). Many chemotherapeutic agents such as DOX act by producing free radicals, which may increase oxidative stress in normal cells (Sinha and Mimnaugh, 1990; Minotti et al., 2004). HO-3867 has been shown to differentiate between healthy and cancerous cells and selectively protect healthy cells by scavenging free radicals (Selvendiran et al., 2010a). The protective (antioxidant) function stems from the N-hydroxypyrroline moiety of HO-3867, which is capable of scavenging oxygen radicals in cells that have normal oxygenation or redox status (Samuni et al., 2004). Most tumors are hypoxic in nature, and their cellular environment is more reducing (for example, thiol-rich) compared with healthy cells (Kuppusamy et al., 1998, 2002; Ilangovan et al., 2002). Under the reductive conditions in cancer cells, the antioxidant efficacy of HO-3867 is inhibited (Mitchell et al., 2001). We have previously shown that HO-3867 exhibited significant growth arrest and apoptosis in a number of human cancer cell lines, including breast, colon, head and neck, liver, lung, ovarian, and prostate cancer, with no apparent toxicity to noncancerous cells (Selvendiran et al., 2010a). We observed that the anticancer activity of HO-3867 was mediated by inhibition of STAT3 phosphorylation at Tyr705 and Ser727 residues and that induction of apoptotic markers cleaved caspase-3 and PARP (Selvendiran et al., 2010c). The protective activity of HO-3867 toward noncancerous cells was shown to be mediated by the ability of the compound to confer selective antioxidant protection to the healthy cells. We further demonstrated that HO-3867 significantly inhibited the growth of the ovarian xenograft tumors (A2780) in a dosage-dependent manner (Selvendiran et al., 2010c). Western blot analyses of the xenograft tumor tissues confirmed that HO-3867 inhibited pSTAT3 (Tyr705 and Ser727) and pJAK1 and increased apoptotic markers cleaved caspase-3 and PARP. In addition, it was observed that HO-3867 suppressed the migration and invasion of the ovarian cancer cells by inhibiting the expression/activity of FAS and focal adhesion kinase proteins (Selvendiran et al., 2010b). Whereas our previous studies suggested the potential of HO-3867 as a safe and effective anticancer agent for cancer therapy, for the first time, the present study provides confirmatory evidence that HO-3867 protects healthy tissue in an in vivo model of cardiotoxicity.
This study clearly demonstrated that combination treatment with DOX and HO-3867 can allow for greater toxicity to breast cancer cells while reducing cardiac side effects. Such reduction of side effects is seen in two ways: 1) by reducing the dose of DOX necessary to inhibit drug-resistant cancer cell growth, and 2) by directly reducing the cardiac side-effects of DOX even while maintaining the same dose. As such, combination treatment with both DOX and HO-3867 has clear potential as a therapy for the management of drug-resistant breast cancer.
Acknowledgments
We thank Brian Rivera for critical evaluation of the manuscript.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL095066]; and the National Institutes of Health National Institute of Biomedical Imaging and Bioengineering [Grant EB006153]. The development of HO-3867 was supported by the Hungarian Research Fund [Grant OTKA K81123].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.183681.
- DOX
- doxorubicin
- FAS
- fatty acid synthase
- ROS
- reactive oxygen species
- STAT3
- signal transducer and activator of transcription-3
- MDR
- multidrug-resistant
- MAPK
- mitogen-activated protein kinase
- DMEM
- Dulbecco's modified Eagle's medium
- ERK
- extracellular signal-regulated kinase
- PARP
- poly(ADP-ribose) polymerase
- HAEC
- human aortic endothelial cells
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
- HO-3867
- (3,5-bis(4-fluorobenzylidene)-1-[(2,2,5,5-tetramethyl-2,5-dihydro-1-hydroxy-pyrrol-3-yl)methyl]piperidin-4-one).
Authorship Contributions
Participated in research design: Dayton, Selvendiran, and P. Kuppusamy.
Conducted experiments: Dayton, M. L. Kuppusamy, Meduru, Naidu, and Khan.
Contributed new reagents or analytic tools: Kaìlai and Hideg.
Performed data analysis: Dayton, Selvendiran, Meduru, and Khan.
Wrote or contributed to the writing of the manuscript: Dayton, Selvendiran, and P. Kuppusamy.
References
- Cao Z, Li Y. (2004) Potent induction of cellular antioxidants and phase 2 enzymes by resveratrol in cardiomyocytes: protection against oxidative and electrophilic injury. Eur J Pharmacol 489:39–48 [DOI] [PubMed] [Google Scholar]
- Coticchia CM, Revankar CM, Deb TB, Dickson RB, Johnson MD. (2009) Calmodulin modulates Akt activity in human breast cancer cell lines. Breast Cancer Res Treat 115:545–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danz ED, Skramsted J, Henry N, Bennett JA, Keller RS. (2009) Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic Biol Med 46:1589–1597 [DOI] [PubMed] [Google Scholar]
- Dunn J. (1994) Doxorubicin-induced cardiomyopathy. J Pediatr Oncol Nurs 11:152–160 [DOI] [PubMed] [Google Scholar]
- Esslimani-Sahla M, Thezenas S, Simony-Lafontaine J, Kramar A, Lavaill R, Chalbos D, Rochefort H. (2007) Increased expression of fatty acid synthase and progesterone receptor in early steps of human mammary carcinogenesis. Int J Cancer 120:224–229 [DOI] [PubMed] [Google Scholar]
- Ewer MS, Ewer SM. (2010) Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat Rev Cardiol 7:564–575 [DOI] [PubMed] [Google Scholar]
- Ilangovan G, Li H, Zweier JL, Krishna MC, Mitchell JB, Kuppusamy P. (2002) In vivo measurement of regional oxygenation and imaging of redox status in RIF-1 murine tumor: effect of carbogen-breathing. Magn Reson Med 48:723–730 [DOI] [PubMed] [Google Scholar]
- Jain D. (2000) Cardiotoxicity of doxorubicin and other anthracycline derivatives. J Nucl Cardiol 7:53–62 [DOI] [PubMed] [Google Scholar]
- Jin W, Wu L, Liang K, Liu B, Lu Y, Fan Z. (2003) Roles of the PI-3K and MEK pathways in Ras-mediated chemoresistance in breast cancer cells. Br J Cancer 89:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaìlai T, Kuppusamy ML, Balog M, Selvendiran K, Rivera BK, Kuppusamy P, Hideg K. (2011) Synthesis of N-substituted 3,5-bis(arylidene)-4-piperidones with high antitumor and antioxidant activity. J Med Chem 54:5414–5421 [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B, Joseph J, Kalivendi S, Wang S, Konorev E, Kotamraju S. (2002) Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol Cell Biochem 234–235:119–124 [PubMed] [Google Scholar]
- Khan M, Varadharaj S, Shobha JC, Naidu MU, Parinandi NL, Kutala VK, Kuppusamy P. (2006) C-phycocyanin ameliorates doxorubicin-induced oxidative stress and apoptosis in adult rat cardiomyocytes. J Cardiovasc Pharmacol 47:9–20 [DOI] [PubMed] [Google Scholar]
- Kim J, Freeman MR. (2003) JNK/SAPK mediates doxorubicin-induced differentiation and apoptosis in MCF-7 breast cancer cells. Breast Cancer Res Treat 79:321–328 [DOI] [PubMed] [Google Scholar]
- Kim SY, Kim SJ, Kim BJ, Rah SY, Chung SM, Im MJ, Kim UH. (2006) Doxorubicin-induced reactive oxygen species generation and intracellular Ca2+ increase are reciprocally modulated in rat cardiomyocytes. Exp Mol Med 38:535–545 [DOI] [PubMed] [Google Scholar]
- Kuppusamy P, Afeworki M, Shankar RA, Coffin D, Krishna MC, Hahn SM, Mitchell JB, Zweier JL. (1998) In vivo electron paramagnetic resonance imaging of tumor heterogeneity and oxygenation in a murine model. Cancer Res 58:1562–1568 [PubMed] [Google Scholar]
- Kuppusamy P, Li H, Ilangovan G, Cardounel AJ, Zweier JL, Yamada K, Krishna MC, Mitchell JB. (2002) Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res 62:307–312 [PubMed] [Google Scholar]
- Li J, Liu H, Ramachandran S, Waypa GB, Yin JJ, Li CQ, Han M, Huang HH, Sillard WW, Vanden Hoek TL, et al. (2010) Grape seed proanthocyanidins ameliorate Doxorubicin-induced cardiotoxicity. Am J Chin Med 38:569–584 [DOI] [PubMed] [Google Scholar]
- Liu H, Liu Y, Zhang JT. (2008) A new mechanism of drug resistance in breast cancer cells: fatty acid synthase overexpression-mediated palmitate overproduction. Mol Cancer Ther 7:263–270 [DOI] [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56:185–229 [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Krishna MC, Kuppusamy P, Cook JA, Russo A. (2001) Protection against oxidative stress by nitroxides. Exp Biol Med (Maywood) 226:620–621 [DOI] [PubMed] [Google Scholar]
- Pivot X, Asmar L, Buzdar AU, Valero V, Hortobagyi G. (2000) A unified definition of clinical anthracycline resistance breast cancer. Br J Cancer 82:529–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Colantuoni G, Maione P, Ferrara C, Airoma G, Barzelloni ML, Castaldo V, Gridelli C. (2005) Chemotherapy of breast cancer in the elderly. Curr Med Chem 12:297–310 [DOI] [PubMed] [Google Scholar]
- Samuni Y, Gamson J, Samuni A, Yamada K, Russo A, Krishna MC, Mitchell JB. (2004) Factors influencing nitroxide reduction and cytotoxicity in vitro. Antioxid Redox Signal 6:587–595 [DOI] [PubMed] [Google Scholar]
- Selvendiran K, Ahmed S, Dayton A, Kuppusamy ML, Tazi M, Bratasz A, Tong L, Rivera BK, Kálai T, Hideg K, et al. (2010a) Safe and targeted anticancer efficacy of a novel class of antioxidant-conjugated difluorodiarylidenyl piperidones: differential cytotoxicity in healthy and cancer cells. Free Radic Biol Med 48:1228–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvendiran K, Ahmed S, Dayton A, Ravi Y, Kuppusamy ML, Bratasz A, Rivera BK, Kálai T, Hideg K, Kuppusamy P. (2010b) HO-3867, a synthetic compound, inhibits the migration and invasion of ovarian carcinoma cells through downregulation of fatty acid synthase and focal adhesion kinase. Mol Cancer Res 8:1188–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvendiran K, Kuppusamy ML, Bratasz A, Tong L, Rivera BK, Rink C, Sen CK, Kálai T, Hideg K, Kuppusamy P. (2009) Inhibition of vascular smooth-muscle cell proliferation and arterial restenosis by HO-3867, a novel synthetic curcuminoid, through up-regulation of PTEN expression. J Pharmacol Exp Ther 329:959–966 [DOI] [PubMed] [Google Scholar]
- Selvendiran K, Tong L, Bratasz A, Kuppusamy ML, Ahmed S, Ravi Y, Trigg NJ, Rivera BK, Kálai T, Hideg K, et al. (2010c) Anticancer efficacy of a difluorodiarylidenyl piperidone (HO-3867) in human ovarian cancer cells and tumor xenografts. Mol Cancer Ther 9:1169–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha BK, Mimnaugh EG. (1990) Free radicals and anticancer drug resistance: oxygen free radicals in the mechanisms of drug cytotoxicity and resistance by certain tumors. Free Radic Biol Med 8:567–581 [DOI] [PubMed] [Google Scholar]
- Small GW, Shi YY, Higgins LS, Orlowski RZ. (2007) Mitogen-activated protein kinase phosphatase-1 is a mediator of breast cancer chemoresistance. Cancer Res 67:4459–4466 [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Koduru S, Kumar R, Venguswamy G, Kyprianou N, Damodaran C. (2009) Diosgenin targets Akt-mediated prosurvival signaling in human breast cancer cells. Int J Cancer 125:961–967 [DOI] [PubMed] [Google Scholar]
- Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. (2006) Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5:219–234 [DOI] [PubMed] [Google Scholar]
- Takemura G, Fujiwara H. (2007) Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 49:330–352 [DOI] [PubMed] [Google Scholar]
- Timolati F, Ott D, Pentassuglia L, Giraud MN, Perriard JC, Suter TM, Zuppinger C. (2006) Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation-contraction coupling and reduces oxidative stress in adult rat cardiomyocytes. J Mol Cell Cardiol 41:845–854 [DOI] [PubMed] [Google Scholar]
- Tokarska-Schlattner M, Zaugg M, Zuppinger C, Wallimann T, Schlattner U. (2006) New insights into doxorubicin-induced cardiotoxicity: the critical role of cellular energetics. J Mol Cell Cardiol 41:389–405 [DOI] [PubMed] [Google Scholar]
- Unverferth DV, Magorien RD, Leier CV, Balcerzak SP. (1982) Doxorubicin cardiotoxicity. Cancer Treat Rev 9:149–164 [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin A, Colomer R, Brunet J, Lupu R, Menendez JA. (2008) Overexpression of fatty acid synthase gene activates HER1/HER2 tyrosine kinase receptors in human breast epithelial cells. Cell Prolif 41:59–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibet S, Goupille C, Bougnoux P, Steghens JP, Goré J, Mahéo K. (2008) Sensitization by docosahexaenoic acid (DHA) of breast cancer cells to anthracyclines through loss of glutathione peroxidase (GPx1) response. Free Radic Biol Med 44:1483–1491 [DOI] [PubMed] [Google Scholar]
- Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ. (2002) Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1). J Biol Chem 277:49676–49684 [DOI] [PubMed] [Google Scholar]
- Wu Y, Mohamed H, Chillar R, Ali I, Clayton S, Slamon D, Vadgama JV. (2008) Clinical significance of Akt and HER2/neu overexpression in African-American and Latina women with breast cancer. Breast Cancer Res 10:R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KI, Kuppusamy P, English S, Yoo J, Irie A, Subramanian S, Mitchell JB, Krishna MC. (2002) Feasibility and assessment of non-invasive in vivo redox status using electron paramagnetic resonance imaging. Acta Radiol 43:433–440 [DOI] [PubMed] [Google Scholar]
- Zecchin KG, Rossato FA, Raposo HF, Melo DR, Alberici LC, Oliveira HC, Castilho RF, Coletta RD, Vercesi AE, Graner E. (2011) Inhibition of fatty acid synthase in melanoma cells activates the intrinsic pathway of apoptosis. Lab Invest 91:232–240 [DOI] [PubMed] [Google Scholar]
- Zhou S, Palmeira CM, Wallace KB. (2001) Doxorubicin-induced persistent oxidative stress to cardiac myocytes. Toxicol Lett 121:151–157 [DOI] [PubMed] [Google Scholar]