Abstract
Biologic tumor necrosis factor (TNF)-α inhibitors do not cross the blood-brain barrier (BBB). A BBB-penetrating TNF-α inhibitor was engineered by fusion of the extracellular domain of the type II human TNF receptor (TNFR) to the carboxyl terminus of the heavy chain of a mouse/rat chimeric monoclonal antibody (MAb) against the mouse transferrin receptor (TfR), and this fusion protein is designated cTfRMAb-TNFR. The cTfRMAb-TNFR fusion protein and etanercept bound human TNF-α with high affinity and KD values of 374 ± 77 and 280 ± 80 pM, respectively. Neuroprotection in brain in vivo after intravenous administration of the fusion protein was examined in a mouse model of Parkinson's disease. Mice were also treated with saline or a non-BBB-penetrating TNF decoy receptor, etanercept. After intracerebral injection of the nigral-striatal toxin, 6-hydroxydopamine, mice were treated every other day for 3 weeks. Treatment with the cTfRMAb-TNFR fusion protein caused an 83% decrease in apomorphine-induced rotation, a 67% decrease in amphetamine-induced rotation, a 82% increase in vibrissae-elicited forelimb placing, and a 130% increase in striatal tyrosine hydroxylase (TH) enzyme activity. In contrast, chronic treatment with etanercept, which does not cross the BBB, had no effect on neurobehavior or striatal TH enzyme activity. A bridging enzyme-linked immunosorbent assay specific for the cTfRMAb-TNFR fusion protein showed that the immune response generated in the mice was low titer. In conclusion, a biologic TNF inhibitor is neuroprotective after intravenous administration in a mouse model of neurodegeneration, providing that the TNF decoy receptor is reengineered to cross the BBB.
Introduction
Tumor necrosis factor (TNF)-α is a proinflammatory cytokine in peripheral tissues, and the leading TNF-α-inhibitors (TNFIs) are biologic drugs, including etanercept, a TNF decoy receptor-Fc fusion protein, infliximab, a chimeric anti-TNF-α monoclonal antibody (MAb), and adalimumab, a human anti-TNF-α MAb (Tansey and Szymkowski, 2009). TNF-α also plays a pathologic role in brain disorders, including Parkinson's disease (PD) (McCoy et al., 2006), Alzheimer's disease (He et al., 2007), and depression (Himmerich et al., 2008). However, the biologic TNFIs cannot be developed for treatment of the brain, because these large molecule drugs do not cross the blood-brain barrier (BBB).
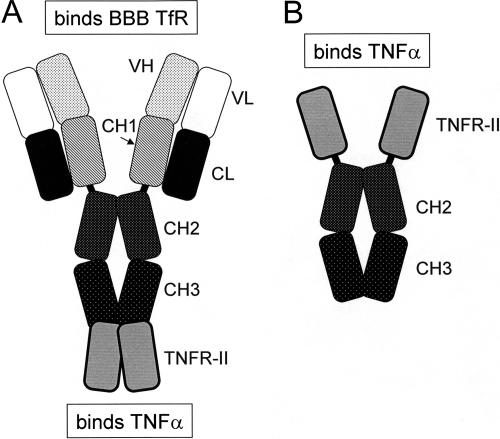
Biologic TNFIs can be reengineered for BBB penetration by engineering fusion proteins of the TNFI and a BBB molecular Trojan horse (MTH) (Pardridge, 2010). The latter is a peptide or peptidomimetic MAb that traverses the BBB via transport on an endogenous receptor-mediated transport system, such as the insulin receptor or the transferrin receptor (TfR). The most potent BBB MTH is a genetically engineered MAb against the human insulin receptor (HIR) (Boado et al., 2007). A fusion protein of the HIRMAb and the extracellular domain (ECD) of the type II TNF receptor (TNFR) has been engineered and is designated the HIRMAb-TNFR fusion protein (Hui et al., 2009). The brain uptake of the HIRMAb-TNFR fusion protein in the rhesus monkey is high, >3% of injected dose (ID)/brain, whereas etanercept does not cross the BBB in vivo (Boado et al., 2010). However, the HIRMAb only cross-reacts with the insulin receptor of the Old World primate, such as the rhesus monkey (Pardridge et al., 1995), and does not cross-react with the insulin receptor of New World primates or lower animals. There is no known MAb against the mouse insulin receptor that can be used as a BBB MTH in the mouse. Therefore, a surrogate MTH for the mouse has been engineered, which is a chimeric MAb against the mouse TfR, designated the cTfRMAb (Boado et al., 2009). A fusion protein of the cTfRMAb and the ECD of the TNFR-II has been engineered (Zhou et al., 2011a), and the structure of the cTfRMAb-TNFR fusion protein is shown in Fig. 1A. The decoy receptor is fused to the carboxyl terminus of the heavy chain of the IgG part of the fusion protein. In contrast, for the engineering of etanercept (Peppel et al., 1991), the TNFR decoy receptor is fused to the amino terminus of the heavy chain of the Fc fragment (Fig. 1B).
Fig. 1.
A, the cTfRMAb-TNFR fusion protein is formed by fusion of the ECD of the type II human TNFR to the carboxyl terminus of each heavy chain of the cTfRMAb. B, the etanercept fusion protein is formed by fusion of the same ECD of the type II human TNFR to the amino terminus of the Fc fragment of human IgG1 heavy chain.
The present investigation tests the neuroprotective actions of both the cTfRMAb-TNFR fusion protein and etanercept in the 6-hydroxydopamine mouse model of PD. The fusion proteins are administered every other day over a 3-week period by intravenous injection in the tail vein. Neuroprotection is assessed with three assays of neurobehavior, with a striatal tyrosine hydroxylase (TH) enzyme activity, and with TH immunocytochemistry. The level of immune response in mice caused by chronic administration of the cTfRMAb-TNFR fusion protein is assessed with a bridging ELISA.
Materials and Methods
Mouse Parkinson's Disease Model and Treatment.
All procedures were approved by the UCLA Animal Research Committee. Adult male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) weighing 25 to 32 g were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) intraperitoneally. Animals received a unilateral intracerebral injection of a total of 12 μg of 6-hydroxydopamine·HBr (Sigma-Aldrich, St. Louis, MO) dissolved in 0.02% ascorbic acid in 0.9% saline. The 6-hydroxydopamine (6 μg in 2 μl) was injected into the right striatum at two locations as described previously (Fu et al., 2010). The toxin was injected into the striatum at sites with the following stereotaxic coordinates: +1.0 mm relative to bregma, 2.1 mm relative to midline, and 2.9 mm below the skull surface (site 1); and +0.3 mm relative to bregma, 2.3 mm relative to midline, and 2.9 mm below the skull surface (site 2). Mice were treated intravenously with one of three treatment drugs: saline (n = 10 mice); etanercept, 1.0 mg/kg (n = 10 mice); or the cTfRMAb-TNFR fusion protein, 1.0 mg/kg (n = 10 mice), every 2 days over the following 3 weeks, with the first dose given 1 h after toxin injection into the brain. Treatment drugs were injected intravenously via the tail vein in a volume of 50 μl/mouse. The mice were euthanized at 3 weeks after toxin administration for measurement of striatal TH enzyme activity.
Etanercept (Enbrel) was obtained from the UCLA Pharmacy. The cTfRMAb-TNFR fusion protein was purified by protein G affinity chromatography of serum-free medium conditioned by stably transfected Chinese hamster ovary (CHO) cells as described previously (Zhou et al., 2011a). The 235-amino acid extracellular domain of the type II human TNFR, minus the signal peptide, was fused to the carboxyl terminus of the heavy chain of the cTfRMAb (Fig. 1A) as described previously (Zhou et al., 2011a). The fusion protein was formulated in 0.01 M sodium acetate-buffered saline (pH 6.5) and was stored either sterile-filtered at 4°C or at −70°C. The molecular mass of the cTfRMAb-TNFR fusion protein is 195,200 Da (Zhou et al., 2011a), whereas the molecular mass of etanercept is 51,200 Da. Therefore, at a systemic dose of each fusion protein of 1 mg/kg, a nearly 4-fold molar excess of etanercept was administered.
TNF-α Radioreceptor Assay.
The saturable binding of human TNF-α to either etanercept or to the cTfRMAb-TNFR fusion protein was determined with a radioreceptor assay as described previously (Hui et al., 2009). For TNF-α binding to either the cTfRMAb-TNFR fusion protein or to a mouse IgG1 negative control, a goat anti-mouse IgG1 Fc antibody (Bethyl Laboratories, Montgomery, TX) was plated in 96-well plates (0.2 μg/well). For TNF-α binding to either etanercept or to a human IgG1 negative control, a mouse anti-human IgG1 Fc antibody (Invitrogen, Carlsbad, CA) was plated in 96-well plates (0.2 μg/well). The fusion protein or negative control antibody was plated (100 ng/well), followed by a 1-h incubation at room temperature. The wells were then washed with phosphate-buffered saline (PBS), followed by the addition of 100 μl/well of a comixture of 125I-human TNF-α (specific activity = 91 μCi/μg; PerkinElmer Life and Analytical Sciences, Waltham, MA) at a concentration of 0.01 μCi/well (0.1 μCi/ml; 1.1 ng/ml; 60 pM) and various concentrations of unlabeled human TNF-α, followed by a 3-h incubation at room temperature. The wells were washed, and bound radioactivity was determined as described previously (Hui et al., 2009). The half-saturation constant, KD, of TNF-α binding to the cTfRMAb-TNFR or etanercept fusion protein was determined by nonlinear regression analysis using BMDP2007e software (Statistical Solutions, Cork, Ireland), after fitting of binding data to the following equation: bound = [(Bmax)(C)]/(KD + C), where Bmax is the maximal binding and C = the concentration of TNF-α.
Behavioral Testing.
Beginning 1 week after the toxin administration, mice were tested weekly for apomorphine- and amphetamine-induced rotation, which was performed on separate days, as described previously (Fu et al., 2010). A vibrissae-elicited forelimb-placing trial in the mice was performed at the end of the 3 weeks of treatment (Fu et al., 2010).
Tyrosine Hydroxylase Enzyme Activity.
Homogenates of mouse brain striatum (left and right side) and frontal cortex were prepared with a Polytron homogenizer in 5 mM KPO4-0.1% Triton X-100 (pH 6.3) followed by centrifugation. After an aliquot was removed for measurement of protein with the bicinchoninic acid assay, dithiothreitol was added to the supernatant to 1 mM, and the supernatant was stored at −70°C until assay. The TH enzyme activity in the supernatant was measured with [3,5-3H]l-tyrosine (PerkinElmer Life and Analytical Sciences) as substrate. The purity of the [3,5-3H]l-tyrosine was assessed by thin-layer chromatography. TH enzyme activity converts [3,5-3H]l-tyrosine to l-DOPA and [3H]water. The [3H]water product was separated from the [3H]tyrosine substrate with a charcoal separation technique, as described previously (Fu et al., 2010). Any residual [3H]water present in the [3,5-3H]l-tyrosine was accounted for with determinations of assay blanks in each assay. The assay was validated with [3H]water (PerkinElmer Life and Analytical Sciences), which showed that the [3H]water was 100% recovered in the supernatant after removal of amino acid by the charcoal. TH enzyme activity was measured at 37°C for 30 min and is expressed as picomoles per hour per milligram of protein.
Tyrosine Hydroxylase Immunocytochemistry.
The brain was removed and coronal blocks were frozen in powdered dry ice, followed by embedding in Tissue Tek OCT medium, and refrozen, and blocks were stored at −70°C. Frozen sections (20-μm thickness) were prepared at −20°C on a Micron Instruments (San Marcos, CA) cryostat. Sections were fixed in ice-cold acetone-methanol (1:1) at −20°C for 20 min. Immune staining was performed with an affinity-purified rabbit antibody against rat TH, which cross-reacts with all forms of mammalian TH (Pel-Freez, Rogers, AR), which was diluted 1:1000 in PBS with 0.3% Triton X-100 and 3% horse serum. The secondary antibody was 2 μg/ml biotinylated horse anti-rabbit IgG (Vector Laboratories, Burlingame, CA). Immune detection was performed with the ImmPACT DAB kit (Vector Laboratories) using diaminobenzidine. The sections were not counterstained and were scanned with a UMAX PowerLook III scanner with transparency adapter. Striatal immunostaining on the lesioned and nonlesioned side was quantitated by determination of optical density using Image software (version 1.62; National Institutes of Health, Bethesda, MD). The entire striatum on both the lesioned and nonlesioned side was outlined for measurement of density of staining. In addition, the cortex was outlined as a measure of the background, and the cortical density was subtracted from the striatal density to yield the background-corrected density of striatal immunostaining.
Immunity ELISA.
The presence of anti-cTfRMAb-TNFR fusion protein antibodies in mouse plasma was measured with a two-site sandwich ELISA described previously (Zhou et al., 2011b). The cTfRMAb-TNFR fusion protein is used as the capture reagent, and biotinylated cTfRMAb-TNFR fusion protein is used as the detector reagent. The mouse plasma was diluted 1:50 in PBS. The capture reagent was plated overnight at 4°C in 96-well plates at 100 μl (250 ng)/well in 0.05 M NaHCO3/pH 8.3. The wells were blocked with PBS containing 1% bovine serum albumin (PBSB), followed by the addition of 100 μl/well of the 1:50 dilution of mouse plasma. After a 60-min incubation at 37°C, the wells were washed with PBSB, and the wells were incubated with biotinylated cTfRMAb-TNFR fusion protein (25 ng/well) for 60 min. The wells were washed with PBSB, followed by incubation with 100 μl (500 ng/well) of a streptavidin-peroxidase conjugate (Vector Laboratories) for 30 min at room temperature. The wells were washed with PBSB, and 100 μl/well o-phenylenediamine-H2O2 developing solution (Sigma-Aldrich) was added for a 15-min incubation in the dark at room temperature. The reaction was stopped by the addition of 100 μl/well 1 M HCl, followed by the measurement of absorbance at 492 and 650 nm. The A650 was subtracted from the A492. The (A492 − A650) for the PBSB blank was then subtracted from the (A492 − A650) for the sample.
The cTfRMAb-TNFR fusion protein was biotinylated with sulfo-biotin-LC-LC-N-hydroxysuccinimide, where LC = long-chain (Pierce Chemical). The biotinylation of the cTfRMAb-TNFR fusion protein was confirmed by SDS-polyacrylamide gel electrophoresis and Western blotting, for which the blot was probed with avidin and biotinylated peroxidase. The nonbiotinylated cTfRMAb-TNFR fusion protein was tested as a negative control.
Statistical Analysis.
Statistical differences between saline-, etanercept-, and fusion protein-treated mice were determined with analysis of variance (ANOVA) with Bonferroni correction. Statistical differences between striatal immunostaining on the lesioned and nonlesioned side were determined with Student's t test.
Results
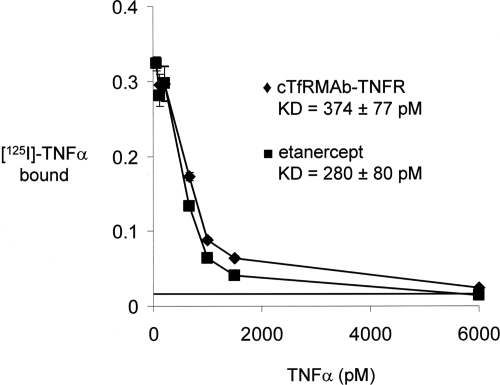
TNFα bound to both the cTfRMAb-TNFR fusion protein and to etanercept with high affinity and KD values of 374 ± 77 and 280 ± 80 pM, respectively (Fig. 2).
Fig. 2.
Radioreceptor assay shows saturation of binding of human TNF-α to either the cTfRMAb-TNFR fusion protein or to etanercept. The binding dissociation constant, KD, was determined by nonlinear regression analysis. The horizontal line at 1.5% binding represents the nonspecific binding observed when either human IgG1 or mouse IgG1 was plated in lieu of the fusion protein.
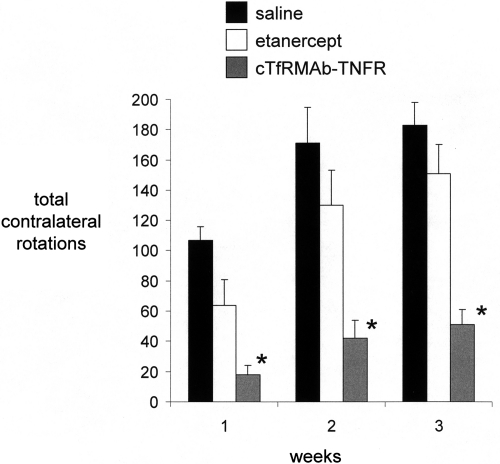
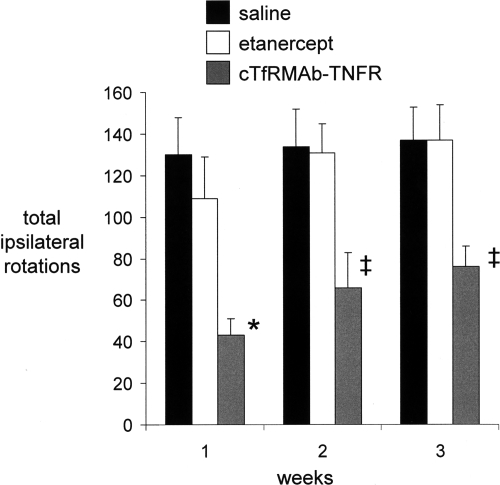
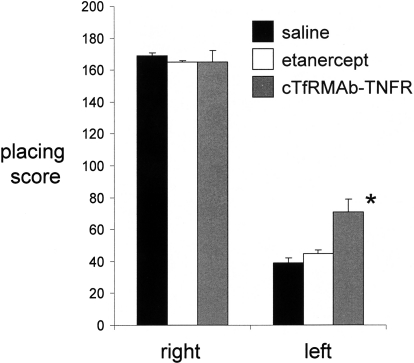
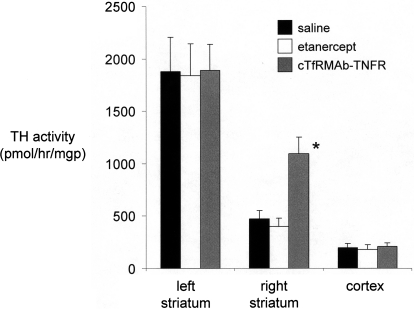
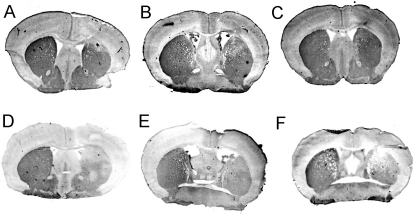
Mice tolerated the chronic treatment with either etanercept or the cTfRMAb-TNFR fusion protein, no mice exhibited signs of immune reaction to the study drugs, and no weight loss was observed in any of the treatment groups. Treatment of PD mice with chronic intravenous cTfRMAb-TNFR fusion protein resulted in a 75 to 83% reduction in apomorphine-induced rotation compared with that in saline-treated mice, whereas etanercept had no significant effect on drug-induced rotation (Fig. 3). Treatment of PD mice with chronic intravenous cTfRMAb-TNFR fusion protein caused a 45 to 67% reduction in amphetamine-induced rotation compared with that in saline-treated mice, whereas etanercept had no significant effect on drug-induced rotation (Fig. 4). Treatment of PD mice with chronic intravenous cTfRMAb-TNFR fusion protein caused a 82% increase in the vibrissae-elicited forelimb placing score compared with that for the saline-treated mice, whereas etanercept had no significant effect on forelimb placing (Fig. 5). Treatment of PD mice with chronic cTfRMAb-TNFR fusion protein caused a 130% increase in striatal TH enzyme activity on the lesioned (right) side, relative to that in saline-treated mice, whereas etanercept had no significant effect on striatal TH enzyme activity (Fig. 6). Chronic treatment with the cTfRMAb-TNFR fusion protein had no effect on TH enzyme activity in either the frontal cortex or the striatum on the nonlesioned (left) side (Fig. 6). The TH enzyme activity results were corroborated with TH immunocytochemistry, which shows the TH immunoreactivity in the striatum on the lesioned and nonlesioned side for three mice treated with the cTfRMAb-TNFR fusion protein (Fig. 7, A–C) and for three mice treated with saline (Fig. 7, D–F). The density of the striatal TH immunostaining on the lesioned side was increased 101% in the mice treated with the cTfRMAb-TNFR fusion protein compared with the saline-treated mice (Table 1).
Fig. 3.
Contralateral rotation (over 20 min) after the administration of apomorphine to PD mice treated with saline, etanercept, or the cTfRMAb-TNFR fusion protein at 1, 2, and 3 weeks after toxin injection. Data are means ± S.E. (n = 10 mice/group). Statistical differences from the saline-treated animals: *, p < 0.01 at weeks 1, 2, and 3, as determined by ANOVA with Bonferroni correction.
Fig. 4.
Ipsilateral rotation (over 20 min) after the administration of amphetamine to PD mice treated with saline, etanercept, or the cTfRMAb-TNFR fusion protein at 1, 2, and 3 weeks after toxin injection. Data are means ± S.E. (n = 10 mice/group). Statistical differences from the saline-treated animals: *, p < 0.01; ‡, p < 0.05, at weeks 1, 2, and 3, as determined by ANOVA with Bonferroni correction.
Fig. 5.
Vibrissae-elicited forelimb placing test scores for the right side, which is ipsilateral to the toxin lesion, and for the left side, which is contralateral to the toxin lesion, for the saline-, etanercept-, and the cTfRMAb-TNFR fusion protein-treated mice. All scores were measured at 3 weeks after toxin injection. Data are means ± S.E. (n = 10 mice/group). Statistical differences from the saline-treated animals: *, p < 0.01, as determined by ANOVA with Bonferroni correction.
Fig. 6.
TH enzyme activity on the lesioned side (right) and the nonlesioned side (left) in the striatum and in the frontal cortex of mice treated with saline, etanercept, or the cTfRMAb-TNFR fusion protein. Brain TH activity was measured at 3 weeks after toxin administration. Data are means ± S.E. (n = 10 mice/group). Statistical differences from the saline-treated animals in the right striatum: *, p < 0.01, as determined by ANOVA with Bonferroni correction.
Fig. 7.
TH immunocytochemistry for three mice treated with the cTfRMAb-TNFR fusion protein (A–C) and three mice treated with saline (D–F). The lesioned side of the brain corresponds to the right side of the figure.
TABLE 1.
Scanning densitometry of striatal TH immunoreactivity
| Treatment | Density of Striatal Staining |
|
|---|---|---|
| Lesioned | Nonlesioned | |
| cTfRMAb-TNFR | 0.167 ± 0.028* | 0.290 ± 0.062 |
| Saline | 0.083 ± 0.009 | 0.260 ± 0.006 |
P < 0.05, difference from saline treatment. Striatal density was corrected for background density over cortex.
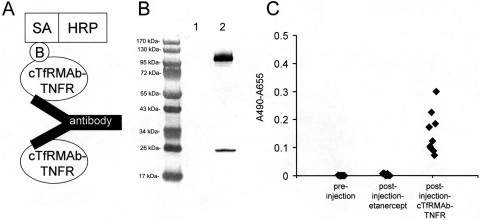
The design of the immunity ELISA is shown in Fig. 8A. The detector reagent is biotinylated cTfRMAb-TNFR fusion protein, and biotinylation of the fusion protein is verified by Western blot analysis (Fig. 8B). The preinjection mouse plasma and the terminal plasma from the etanercept-treated mice produced no immune reaction with cTfRMAb-TNFR fusion protein (Fig. 8C). There was a variable immune reaction observed at the end of the study in the mice treated with the cTfRMAb-TNFR fusion protein at 1:50 dilutions of plasma (Fig. 8C).
Fig. 8.
A, structure of the two-site ELISA for detection of antibodies against the cTfRMAb-TNFR fusion protein. The cTfRMAb-TNFR fusion protein is used as the capture reagent, and the biotinylated cTfRMAb-TNFR fusion protein is used as the detector reagent, along with a complex of streptavidin (SA) and horseradish peroxidase (HRP); the biotin moiety is designated, B. B, Western blot of nonbiotinylated cTfRMAb-TNFR fusion protein (lane 1) and biotinylated cTfRMAb-TNFR fusion protein (lane 2) with a conjugate of avidin and biotinylated peroxidase. C, absorbance at 1:50 dilutions of mouse plasma taken preinjection or postinjection after 3 weeks of either intravenous injections with either etanercept or the cTfRMAb-TNFR fusion protein. Data are shown for all 10 mice in each group.
Discussion
The results of this study are consistent with the following conclusions. First, the cTfRMAb-TNFR fusion protein and the TNFR-Fc fusion protein (etanercept) have comparable affinity of binding of TNFα, with a low a KD < 1 nM (Fig. 2). Second, chronic intravenous treatment of mice with experimental PD with the cTfRMAb-TNFR fusion protein results in neuroprotection, based on three assays of neurobehavior (Figs. 3–5), striatal TH enzyme activity (Fig. 6), and striatal TH immunocytochemistry (Fig. 7; Table 1). Third, chronic intravenous treatment with a comparable dose of the TNFR-Fc fusion protein (etanercept) has no therapeutic effect in experimental PD (Figs. 3–7). Fourth, chronic treatment of mice with the cTfRMAb-TNFR fusion protein causes a low-titer immune response against the cTfRMAb part of the fusion protein (Fig. 8).
IgG-decoy receptor fusion proteins are formed by fusion of the carboxyl terminus of the decoy receptor to the amino terminus of the IgG heavy chain (Scallon et al., 1995), as shown for etanercept in Fig. 1B. The engineering of the cTfRMAb-TNFR fusion protein is a departure from all prior IgG-decoy receptor fusion proteins in that the amino terminus of the TNFR-II ECD is fused to the carboxyl terminus of the antibody heavy chain (Fig. 1A). Despite this novel structure, the affinity of the cTfRMAb-TNFR fusion protein for TNF-α is high and is comparable to the binding affinity of etanercept (Fig. 2). The KD of cTfRMAb-TNFR fusion protein binding of human TNF-α, 374 ± 77 pM (Fig. 2), is comparable to the KD of TNF-α binding to the intact membrane receptor, which is 0.3 to 0.4 nM (Morita et al., 2001). The human TNFR-II receptor binds mouse TNF-α to the same extent as human TNF-α (Scallon et al., 2002). In addition to TNF-α, the cTfRMAb-TNFR fusion protein binds the murine BBB TfR with high affinity, and the brain uptake of the fusion protein in the mouse after intravenous injection is high, 2.8 ± 0.5% ID/g brain (Zhou et al., 2011a). In contrast, the brain uptake of an IgG that does not cross the BBB is 0.06% ID/g in the mouse (Lee et al., 2000). Given a brain uptake of 2.8% ID/g, the brain concentration of the cTfRMAb-TNFR fusion protein is 840 ng/g brain or approximately 6 nM, with the systemic injection dose of 1 mg/kg used in these studies. Because the cerebral concentration of TNF-α increases to approximately 0.5 nM in excitotoxic conditions (Shohami et al., 1994), the 1 mg/kg dose of fusion protein is sufficient to sequester the TNF-α produced in brain.
TNF-α induces apoptosis (Chen and Goeddel, 2002) and is elevated in the brains of patients with PD (Machado et al., 2011). The important role played by TNF-α in the pathogenesis of experimental PD was demonstrated in knockout mice. Deletion of the TNFR in mice produced resistance to PD-inducing neurotoxins such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (Sriram et al., 2002; Ferger et al., 2004). The intracerebral injection of a dominant-negative TNFα mutant protein is neuroprotective in experimental PD (McCoy et al., 2006). Intracerebral injection of the TNFI was necessary, because large-molecule TNFIs do not cross the BBB. The present study shows that a BBB-penetrating TNFI, the cTfRMAb-TNFR fusion protein, is neuroprotective in experimental PD in the mouse after intravenous administration. Chronic treatment of 6-hydroxydopamine-injected mice with intravenous cTfRMAb-TNFR fusion protein causes an improvement in three assays of neurobehavior (Figs. 3–5) and a 130% increase in TH enzyme activity in the striatum on the lesioned side (Fig. 6), which correlates with an increase in striatal immunoreactive TH as observed with immunocytochemistry (Fig. 7; Table 1).
Chronic treatment of PD mice with intravenous etanercept has no therapeutic effect on neurobehavior (Figs. 3–5) or striatal TH enzyme activity (Fig. 6). The lack of therapeutic effect of intravenous etanercept is consistent with prior work showing that etanercept does not cross the BBB (Boado et al., 2010). Owing to the lack of BBB penetration, it was necessary to administer etanercept by direct injection into the spinal cord in the rat model of spinal cord injury (Marchand et al., 2009). There is evidence for focal BBB disruption in toxin-induced PD, and mice with knockout of the TNFR have less BBB disruption after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration (Zhao et al., 2007). Therefore, treatment of PD mice with the cTfRMAb-TNFR fusion protein would be expected to reduce BBB disruption mediated by TNF-α. However, any disruption of the BBB that occurs in the 6-hydroxydopamine model in the mouse is insufficient to enable brain penetration of a therapeutic agent such as etanercept, because chronic dosing of PD mice with etanercept is not therapeutic (Figs. 3–6).
Chronic dosing of mice with the cTfRMAb-TNFR fusion protein causes an immune response against the fusion protein, whereas chronic administration of etanercept in mice causes no immune response (Fig. 8). This finding suggests that the immune response is directed against the cTfRMAb part of the antibody. However, the immune response against the cTfRMAb-TNFR fusion protein is low-titer and produces an average optical density reading of 0.2 with a 1:50 dilution of 100 μl of plasma (Fig. 8). The low-titer immune response in this study equates to 0.1 optical density/μl plasma. This titer is 100-fold lower than the immune response against biologic drugs that neutralize therapeutic effects of the drug (Dickson et al., 2008). Moreover, recent work has shown that the low-titer immune response produced with chronic treatment with cTfRMAb fusion proteins has no neutralizing effect on the TfR in vivo (Zhou et al., 2011b). The rate of brain uptake of fusion protein-treated mice and saline-treated mice is comparable at the end of 12 weeks of twice weekly treatment with 2 mg/kg doses of cTfRMAb fusion protein (Zhou et al., 2011b). The low-titer, non-neutralizing immune response to the fusion protein may be related to the presence of specific amino acid sequences within the constant region of the heavy chain that induce T-cell immune tolerance (De Groot et al., 2008). In addition, the fusion protein is produced in CHO cells, which secrete fucosylated glycoproteins, and fucosylated glycoproteins have minimal effects on antibody dependent cytotoxicity (Kanda et al., 2006). Etanercept produced in CHO cells is fucosylated and does not induce antibody-dependent cytotoxicity (Shoji-Hosaka et al., 2006).
In summary, neurodegenerative disease of brain has an inflammatory component triggered in part by TNF-α. Central nervous system disease should be treatable with biologic TNF inhibitors similar to the use of these agents for inflammation in peripheral conditions (Tansey and Szymkowski, 2009). However, the biologic TNF inhibitors, such as the TNFR decoy receptor, do not cross the BBB (Boado et al., 2010). Systemic etanercept administration has no therapeutic effect in the mouse model of PD, and this is attributed to the lack of etanercept transport across the BBB. However, the same TNF-α decoy receptor that forms etanercept is therapeutic after intravenous injection in a mouse model of PD, providing that the TNFα decoy receptor is reengineered to cross the BBB. Fusion of the TNF-α decoy receptor to the cTfRMAb, which acts as a BBB molecular Trojan horse, creates a new IgG-TNFR fusion protein. The Trojan horse-TNFR fusion protein both binds the BBB TfR, to enable brain penetration from blood, and binds TNF-α, to inhibit the action of this inflammatory cytokine in brain behind the BBB.
Acknowledgments
Winnie Tai and Phuong Tram provided expert technical assistance.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant R01-NS065917].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.185876.
- TNF
- tumor necrosis factor
- TNFI
- tumor necrosis inhibitor
- MAb
- monoclonal antibody
- PD
- Parkinson's disease
- BBB
- blood-brain barrier
- MTH
- molecular Trojan horse
- TfR
- transferrin receptor
- HIR
- human insulin receptor
- ECD
- extracellular domain
- TNFR
- tumor necrosis factor receptor
- ID
- injected dose
- cTfRMAb
- chimeric MAb against mouse TfR
- TH
- tyrosine hydroxylase
- ELISA
- enzyme-linked immunosorbent assay
- CHO
- Chinese hamster ovary
- ANOVA
- analysis of variance
- AD
- Alzheimer's disease
- PBS
- phosphate-buffered saline
- PBSB
- PBS containing 1% bovine serum albumin.
Authorship Contributions
Participated in research design: Zhou, Sumbria, Hui, Lu, Boado, and Pardridge.
Conducted experiments: Zhou, Sumbria, Hui, Lu, and Boado.
Performed data analysis: Zhou, Sumbria, Hui, Lu, Boado, and Pardridge.
Wrote or contributed to the writing of the manuscript: Zhou, Sumbria, Hui, Lu, Boado, and Pardridge.
References
- Boado RJ, Hui EK, Lu JZ, Pardridge WM. (2010) Drug targeting of erythropoietin across the primate blood-brain barrier with an IgG molecular Trojan horse. J Pharmacol Exp Ther 333:961–969 [DOI] [PubMed] [Google Scholar]
- Boado RJ, Zhang Y, Wang Y, Pardridge WM. (2009) Engineering and expression of a chimeric transferrin receptor monoclonal antibody for blood-brain barrier delivery in the mouse. Biotechnol Bioeng 102:1251–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Zhang Y, Zhang Y, Pardridge WM. (2007) Humanization of anti-human insulin receptor antibody for drug targeting across the human blood-brain barrier. Biotechnol Bioeng 96:381–391 [DOI] [PubMed] [Google Scholar]
- Chen G, Goeddel DV. (2002) TNF-R1 signaling: a beautiful pathway. Science 296:1634–1635 [DOI] [PubMed] [Google Scholar]
- De Groot AS, Moise L, McMurry JA, Wambre E, Van Overtvelt L, Moingeon P, Scott DW, Martin W. (2008) Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes.” Blood 112:3303–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson P, Peinovich M, McEntee M, Lester T, Le S, Krieger A, Manuel H, Jabagat C, Passage M, Kakkis ED. (2008) Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I. J Clin Invest 118:2868–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferger B, Leng A, Mura A, Hengerer B, Feldon J. (2004) Genetic ablation of tumor necrosis factor-α (TNF-α) and pharmacological inhibition of TNF-synthesis attenuates MPTP toxicity in mouse striatum. J Neurochem 89:822–833 [DOI] [PubMed] [Google Scholar]
- Fu A, Zhou QH, Hui EK, Lu JZ, Boado RJ, Pardridge WM. (2010) Intravenous treatment of experimental Parkinson's disease in the mouse with an IgG-GDNF fusion protein that penetrates the blood-brain barrier. Brain Res 1352:208–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Zhong Z, Lindholm K, Berning L, Lee W, Lemere C, Staufenbiel M, Li R, Shen Y. (2007) Deletion of tumor necrosis factor death receptor inhibits amyloid beta generation and prevents learning and memory deficits in Alzheimer's mice. J Cell Biol 178:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmerich H, Fulda S, Linseisen J, Seiler H, Wolfram G, Himmerich S, Gedrich K, Kloiber S, Lucae S, Ising M, et al. (2008) Depression, comorbidities and the TNF-α system. Eur Psychiatry 23:421–429 [DOI] [PubMed] [Google Scholar]
- Hui EK, Boado RJ, Pardridge WM. (2009) Tumor necrosis factor receptor-IgG fusion protein for targeted-drug delivery across the human blood-brain barrier. Mol Pharm 6:1536–1543 [DOI] [PubMed] [Google Scholar]
- Kanda Y, Yamane-Ohnuki N, Sakai N, Yamano K, Nakano R, Inoue M, Misaka H, Iida S, Wakitani M, Konno Y, et al. (2006) Comparison of cell lines for stable production of fucose-negative antibodies with enhanced ADCC. Biotechnol Bioeng 94:680–688 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Engelhardt B, Lesley J, Bickel U, Pardridge WM. (2000) Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse. J Pharmacol Exp Ther 292:1048–1052 [PubMed] [Google Scholar]
- Machado A, Herrera AJ, Venero JL, Santiago M, De Pablos RM, Villarán RF, Espinosa-Oliva AM, Argüelles S, Sarmiento M, Delgado-Cortés MJ, et al. (2011) Peripheral inflammation increases the damage in animal models of nigrostriatal dopaminergic neurodegeneration: possible implication in Parkinson's disease incidence. Parkinsons Dis 2011:393769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand F, Tsantoulas C, Singh D, Grist J, Clark AK, Bradbury EJ, McMahon SB. (2009) Effects of Etanercept and Minocycline in a rat model of spinal cord injury. Eur J Pain 13:673–681 [DOI] [PubMed] [Google Scholar]
- McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. (2006) Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease. J Neurosci 26:9365–9375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita C, Horiuchi T, Tsukamoto H, Hatta N, Kikuchi Y, Arinobu Y, Otsuka T, Sawabe T, Harashima S, Nagasawa K, et al. (2001) Association of tumor necrosis factor receptor type II polymorphism 196R with systemic lupus erythematosus in the Japanese: molecular and functional analysis. Arthritis Rheum 44:2819–2827 [DOI] [PubMed] [Google Scholar]
- Pardridge WM. (2010) Biopharmaceutical drug targeting to the brain. J Drug Target 18:157–167 [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Kang YS, Buciak JL, Yang J. (1995) Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. Pharm Res 12:807–816 [DOI] [PubMed] [Google Scholar]
- Peppel K, Crawford D, Beutler B. (1991) A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J Exp Med 174:1483–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallon B, Cai A, Solowski N, Rosenberg A, Song XY, Shealy D, Wagner C. (2002) Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther 301:418–426 [DOI] [PubMed] [Google Scholar]
- Scallon BJ, Trinh H, Nedelman M, Brennan FM, Feldmann M, Ghrayeb J. (1995) Functional comparisons of different tumour necrosis factor receptor/IgG fusion proteins. Cytokine 7:759–770 [DOI] [PubMed] [Google Scholar]
- Shohami E, Novikov M, Bass R, Yamin A, Gallily R. (1994) Closed head injury triggers early production of TNF α and IL-6 by brain tissue. J Cereb Blood Flow Metab 14:615–619 [DOI] [PubMed] [Google Scholar]
- Shoji-Hosaka E, Kobayashi Y, Wakitani M, Uchida K, Niwa R, Nakamura K, Shitara K. (2006) Enhanced Fc-dependent cellular cytotoxicity of Fc fusion proteins derived from TNF receptor II and LFA-3 by fucose removal from Asn-linked oligosaccharides. J Biochem 140:777–783 [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O'Callaghan JP. (2002) Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson's disease. FASEB J 16:1474–1476 [DOI] [PubMed] [Google Scholar]
- Tansey MG, Szymkowski DE. (2009) The TNF superfamily in 2009: new pathways, new indications, and new drugs. Drug Discov Today 14:1082–1088 [DOI] [PubMed] [Google Scholar]
- Zhao C, Ling Z, Newman MB, Bhatia A, Carvey PM. (2007) TNF-α knockout and minocycline treatment attenuates blood-brain barrier leakage in MPTP-treated mice. Neurobiol Dis 26:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QH, Boado RJ, Hui EK, Lu JZ, Pardridge WM. (2011a) Brain-penetrating tumor necrosis factor decoy receptor in the mouse. Drug Metab Dispos 39:71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QH, Boado RJ, Hui EK, Lu JZ, Pardridge WM. (2011b) Chronic dosing of mice with a transferrin receptor monoclonal antibody-glial-derived neurotrophic factor fusion protein. Drug Metab Dispos 39:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]