Abstract
Epoxyeicosatrienoic acids (EETs) and the cytochrome P450 epoxygenase CYP2J2 promote tumorogenesis in vivo and in vitro via direct stimulation of tumor cell growth and inhibition of tumor cell apoptosis. Herein, we describe a novel mechanism of inhibition of tumor cell apoptosis by EETs. In Tca-8113 cancer cells, the antileukemia drug arsenic trioxide (ATO) led to the generation of reactive oxygen species (ROS), impaired mitochondrial function, and induced apoptosis. 11,12-EET pretreatment increased expression of the antioxidant enzymes superoxide dismutase and catalase and inhibited ATO-induced apoptosis. 11,12-EET also prevented the ATO-induced activation of p38 mitogen-activated protein kinase, c-Jun NH2-terminal kinase, caspase-3, and caspase-9. Therefore, 11,12-EET-pretreatment attenuated the ROS generation, loss of mitochondrial function, and caspase activation observed after ATO treatment. Moreover, the CYP2J2-specific inhibitor compound 26 enhanced arsenic cytotoxicity to a clinically relevant concentration of ATO (1–2 μM). Both the thiol-containing antioxidant, N-acetyl-cysteine, and 11,12-EET reversed the synergistic effect of the two agents. Taken together, these data indicate that 11,12-EET inhibits apoptosis induced by ATO through a mechanism that involves induction of antioxidant proteins and attenuation of ROS-mediated mitochondrial dysfunction.
Introduction
Cytochrome P450 epoxygenases metabolize arachidonic acid to four regioisomeric cis-epoxyeicosatrienoic acids (EETs): 5,6-, 8,9-, 11,12-, and 14,15-EET (Zeldin, 2001). EETs possess multiple biological effects in the cardiovascular and renal systems, including anti-inflammatory (Node et al., 1999) and angiogenic (Michaelis et al., 2003; Wang et al., 2005) effects on endothelial cells, inhibition of vascular smooth muscle cell migration (Sun et al., 2002), and inhibition of apoptosis (Chen et al., 2001; Yang et al., 2007). We have shown that CYP2J2, a human cytochrome P450 epoxygenase that generates all four EETs, is highly expressed in several human organ tumors such as cancers of the esophagus, liver, breast, lung, and colon (Jiang et al., 2005). In addition, CYP2J2-derived EETs stimulate tumor cell proliferation, protect against apoptosis, and promote tumor invasion and metastasis in vitro and in vivo (Jiang et al., 2007).
The term reactive oxygen species (ROS) refers to highly reactive species that are oxygen metabolites or derivates. These include superoxide anions (O2˙̄), hydrogen peroxide (H2O2), hydroxyl radicals (·OH), singlet oxygen (1O2), and nitric oxide (NO). Several enzymes and the mitochondrial electron transport chain naturally produce ROS, and ROS production correlates with normal cell proliferation and signal transduction (D'Autréaux and Toledano, 2007; Valko et al., 2007). ROS have been implicated in the etiology of a wide range of human diseases including cancers (Valko et al., 2007). Extensive evidence indicates that ROS plays a critical role in tumor cell apoptosis whether induced by ischemia, drugs, or receptor-mediated factors (Pelicano et al., 2004; Engel and Evens, 2006; Orrenius, 2007; Ryter et al., 2007).
Arsenic trioxide (As2O3; ATO) was first used clinically to induce differentiation and apoptosis of all-trans-retinoic acid-resistant acute promyelocytic leukemia (Chen et al., 1997; Soignet et al., 1998). With accumulating experience and enhanced knowledge of the molecular actions of ATO and ROS, treatment of patients with ATO has been extended to solid tumors (Gazitt and Akay, 2005). ATO exerts its effect mainly by elevating intracellular ROS, disrupting cellular redox equilibrium, and inducing mitochondrial dysfunction (Perkins et al., 2000; Mahieux et al., 2001).
Our previous studies suggest that EETs inhibit tumor necrosis factor-α-induced apoptosis through up-regulation of the antiapoptotic proteins Bcl-2 and Bcl-xl and down-regulation of the proapoptotic protein Bax. However, it remains unknown how EETs modulate mitochondrial pathways that lead to apoptosis. In the present study, we extend our previous findings by elucidating the relationship between EETs and mitochondrial dysfunction induced by ATO.
Materials and Methods
Experimental Reagents.
Cell culture medium (Dulbecco's modified Eagle's medium) and fetal bovine serum were from Invitrogen (Carlsbad, CA). 2,7-Dichlorodihydrofluorescein diacetate (DCFH-DA), sulforhodamine B (SRB), JNK inhibitor [anthra[1–9-cd]pyrazol-6(2H)-one (SP600125)] and ERK inhibitor [2′-amino-3′-methoxyflavone (PD98059)] were from Sigma-Aldrich (St. Louis, MO). 8,9-EET, 11,12-EET, and 14,15-EET were from Cayman Chemical (Ann Arbor, MI). p38 inhibitor [4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine (SB203580)] was from Promega (Madison, WI). Antibodies against CYP2J2 and CypD were purchased from Abcam Inc. (Cambridge, MA). Antibodies against cytochrome c, superoxide dismutases (SODs), catalase, and β-actin were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Antibodies against ERK, p-ERK, P38, p-P38, JNK, and p-JNK were purchased from Cell Signaling Technology (Danvers, MA). The CYP2J2-specific inhibitor compound 26 (C26) was synthesized as described previously (Chen et al., 2009). siRNAs were synthesized by Ribobio Co. (Guangzhou, China). All other reagents were purchased from standard commercial suppliers unless otherwise indicated.
Cell Culture.
Tca-8113 cells and HepG2 cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained as recommended. Cells were cultured in Dulbecco's modified Eagle's medium, adjusted to contain 4 mM l-glutamine, 1.5 g/l sodium bicarbonate, 4.5 g/l glucose, 10% fetal bovine serum, 100 units/ml penicillin, and 65 units/ml streptomycin. All cell cultures were maintained at 37°C in constant humidified incubator containing 95% air/5% CO2 atmosphere.
Transfection.
Transfection was performed with Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions with cells plated in six-well plates at a density of approximately 106 cells/well. The cells were harvested 24 h after transfection with siRNAs.
Cell Cytotoxicity Assay.
SRB was used to assess growth inhibition. This colorimetric assay estimates cell number indirectly by staining total cellular protein with SRB. In brief, cells were collected by trypsinization, counted, plated at a density of 5000 cells/well in 96-well, flat-bottomed microtiter plates (100 μl/well), and exposed as indicated. After exposure, the medium was removed, and cells were fixed with 20% (w/v) trichloroacetic acid at 4°C for 1 h and stained for 30 min with 0.4% (w/v) SRB dissolved in 1% acetic acid. Wells were washed five times with 1% acetic acid, and the protein-bound dye was solubilized with 10 mM Tris base, pH 10. The optical density of cells was determined at a wavelength of 540 nm using a colorimetric plate reader. Data are represented as a percentage of control cells.
Reactive Oxygen Species Detection.
H2O2 production was determined by using reagents and protocols provided in the Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Invitrogen). In brief, Amplex Red (50 μM) and horseradish peroxidase (0.1 U/ml) were added to the cellular samples. Fluorescence readings were made in a 96-well plate at excitation 530 nm and emission 590 nm. Background fluorescence, measured in the absence of cells, was subtracted from experimental values. To confirm hydrogen peroxide specificity, parallel samples were coincubated with 1000 U/ml catalase, and the specificity of the reaction was obtained by subtracting the amount of H2O2 produced in the presence of catalase from that in the absence of catalase. H2O2 concentration was calculated using a standard curve.
Alternatively, DCFH-DA was used as an ROS-capturing reagent using methods described previously (Zmijewski et al., 2005). DCFH-DA becomes deacetylated intracellularly by nonspecific esterases and is furthered oxidized by ROS to the fluorescent compound DCF. Cells were incubated with 10 μM DCFH-DA at 37°C for 20 min. Immediately after staining, DCF fluorescence was detected by inverted epifuorescence microscope or flow cytometry.
Mitochondrial Transmembrane Potential Measurement.
To determine the mitochondrial membrane potential (ΔΨm) of cells, a membrane potential-sensitive fluorescent probe, JC-1 (Agilent Technologies, Santa Clara, CA) was used. JC-1 is a cationic carbocyanine dye that presents itself as green fluorescent monomers at low concentration and yellow-red fluorescent J-aggregates when concentrated in functional mitochondria. In brief, cells were plated into 35-mm glass-bottom microwell dishes. After incubation, the cells were loaded with 10 μM JC-1 for 30 min at 37°C, washed, and viewed using an epifluorescence microscope with a blue excitation filter at 488 nm or a green excitation filter at 543 nm, to allow for the visualization of the green and yellow-red fluorescence, respectively. Both the yellow-red J-aggregates and the green monomer can be viewed with a “double-bandpass” filter designed to simultaneously detect fluorescein and rhodamine.
Caspase Activity Analysis.
Caspase-3 and caspase-9 activities were measured by colorimetric assays (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Cells were lysed in chilled lysis buffer after treatment with various agents for the indicated times. Cell lysates were centrifuged at 15,000g for 10 min, and the supernatants were added to 50 μl of 2× reaction buffer and 5 μl of 5 mM caspase-9 or 1 mM caspase-3 substrates. After incubation at 37°C for 1 h, absorbance was read at a wavelength of 405 nm.
Evaluation of Apoptosis.
According to previous publications (Han et al., 2009, 2010) and our pretest results, we treated cells with ATO with or without 11,12-EET for various times and doses as indicated. Flow cytometric assays with Annexin V and propidium iodide (PI) staining (BD Pharmingen, San Diego, CA) were done as described previously (Jiang et al., 2005).
Western Blotting.
Western blotting was performed as described previously (Wang et al., 2003). In brief, cell lysates were prepared by extracting proteins with lysis buffer (40 mM Tris-Cl, pH 8.0, 120 mM NaCl, and 0.1% NP-40) supplemented with protease and phosphatase inhibitors. Proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline and then incubated with primary antibodies overnight at 4°C. Blots were developed with peroxidase-conjugated secondary antibody, and proteins were visualized by enhanced chemiluminescence (Thermo Fisher Scientific, Waltham, MA).
SOD and Catalase Activity Assays.
Total SOD and catalase activity were measured with colorimetric kits (Jiancheng Bioengineering Institute, Nanjing, China). SOD activity was determined by hydroxylamine assay developed from xanthine oxidase assay. In brief, cells were sonicated and the lysates were reacted with reaction buffer and color developing reagent according to the manufacturer's instructions. The superoxide can oxidize hydroxylamine to form nitrite, which colors amaranth by the color developing agent, and it can be assayed by spectrophotometer. The SOD detected in the sample could specific inhibition on the formation of superoxide anion, and the quantity of produced nitrite is reduced. So the absorbance of test tube will be lower than that of control tube, we can calculate the activity of SOD in the sample with the formula. After incubation at 37°C for 40 min, absorbance was read at a wavelength of 550 nm, and optical density value was used for calculating SOD activity with the formula according to the manufacturer's instructions. The catalase activity was assayed likewise; cell lysates were reacted with reagents provided by the kit. The methodology assay catalase activity is based on the reaction of the enzyme in the presence of an optimal concentration of H2O2. The rate of dismutation of hydrogen peroxide to water and molecular oxygen is proportional to the concentration of catalase. Therefore, the sample containing catalase was incubated in the presence of a known concentration of hydrogen peroxide. After incubation for exactly 1 min, the reaction was quenched with ammonium molybdate. The amount of hydrogen peroxide remaining in the reaction mixture is then formation of its stable colored complex with ammonium molybdate and the complex was measured at 405 nm. Tone unit of catalase activity was defined as the amount enzyme that will decompose 1 μmol of hydrogen peroxide in 1 s per milligram of protein.
Statistical Analysis.
All data are presented as mean ± S.E.M. Significant differences between groups were determined using the unpaired Student's t test. A p value of less than 0.05 from two-tailed Student's t test analysis was used to indicate statistical significance. All figures are representative of at least three independent experiments.
Results
11,12-EET Decreases ATO-Induced Increase in ROS Level in Tumor Cells.
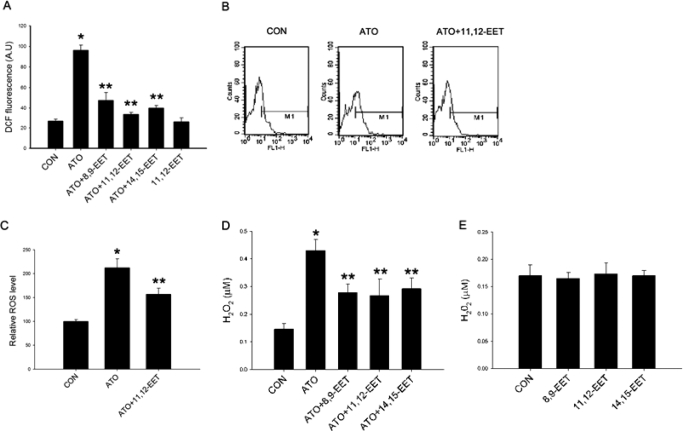
Treatment of Tca-8113 cells with 10 μM ATO for 2 h led to a significant increase in ROS production. The effects were dose-dependent in the range of 1 to 10 μM (Supplemental Fig. 1). To test whether EETs increases the capacity of tumor cells to scavenge ROS, Tca-8113 cells were incubated for 24 h with 100 nM 8,9-EET, 11,12-EET, or 14,15-EET before treatment with ATO. Preincubation with EETs significantly lowered ATO-induced increase in ROS level in these cells, but EET alone did not show the effect (Fig. 1A). We further confirmed this result in additional experiments, and results showed that 11,12-EET reduced ATO-induced increase in ROS level measured by flow cytometry (Fig. 1, B and C), and addition of the CYP2J2-specific inhibitor C26 significantly enhanced the effect of ATO (Supplemental Fig. 2). Because DCFH is limited by its lack of specificity and tendency to be spontaneously photo-oxidized, we performed more rigorous experiments to measure the intracellular hydrogen peroxide production via extracellular leakage of H2O2 by using the Amplex Red method. Likewise, EETs reduced ATO-induced ROS level measured by the Amplex Red method (Fig. 1D). None of the isoforms of EETs treated alone altered the ROS level significantly as shown in Fig. 1E. In the subsequent investigations, we used 11,12-EET, which is a potent and abundant EET in the human body.
Fig. 1.
Effect of EETs on ROS production in ATO-treated Tca-8113 cells. Cells were preincubated with 100 nM EETs or vehicle for 24 h, then exposed to 10 μM ATO for 2 h. DCFH-DA was then incubated with cells at 37°C for 15 min to detect ROS. A, quantification of mean ROS levels in Tca-8113 cells after ATO and EETs treatment. B, representative flow cytometric histogram of DCF fluorescent levels. C, quantification of mean ROS levels in Tca-8113 cells after ATO and 11,12-EET treatment (n = 3). D and E, production of hydrogen peroxide measured by Amplex Red (n = 6). Results shown are mean ± S.E.M. *, p < 0.05 versus control; **, p < 0.05 versus ATO treatment alone.
11,12-EET Up-Regulates the Expression and Activity of Antioxidant Enzymes.
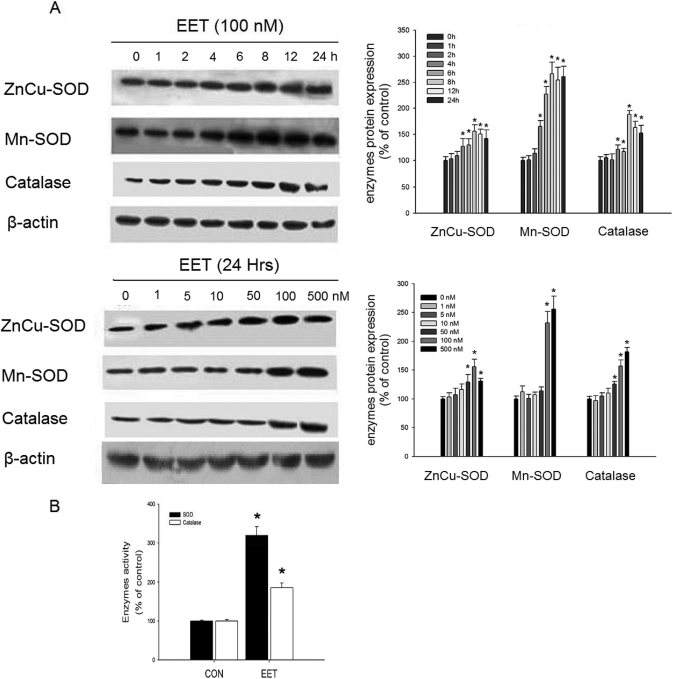
EETs have been shown to protect against injury after ischemia/reperfusion in endothelial cells (Wang et al., 2005), where ROS-induced lipid peroxidation causes significant cytotoxicity (Valko et al., 2007). Therefore, we hypothesized that antioxidant enzymes may account for the beneficial actions of EETs. We investigated the regulation of antioxidant enzyme expression by 11,12-EET in tumor cells. As shown in Fig. 2A, 11,12-EET increased ZnCu-SOD, Mn-SOD, and catalase protein levels in a time- and dose-dependent manner. Moreover, incubation of Tca-8113 cells with 100 nM EET for 12 h increased both total SOD and catalase activity (Fig. 2B).
Fig. 2.
Effect of 11,12-EET on antioxidant enzyme expression and activity. A, antioxidant enzyme expression after 11,12-EET treatment for the indicated times and doses. B, total SOD and catalase activity. Tca-8113 cells were preincubated with 100 nM 11,12-EET for 12 h, and enzymatic activities were then measured. Results are shown as percentage of control ± S.E.M. *, p < 0.05 versus control.
11,12-EET Attenuates Mitochondrial Transmembrane Potential Collapse and Caspase Activation after Treatment of Tumor Cells with ATO.
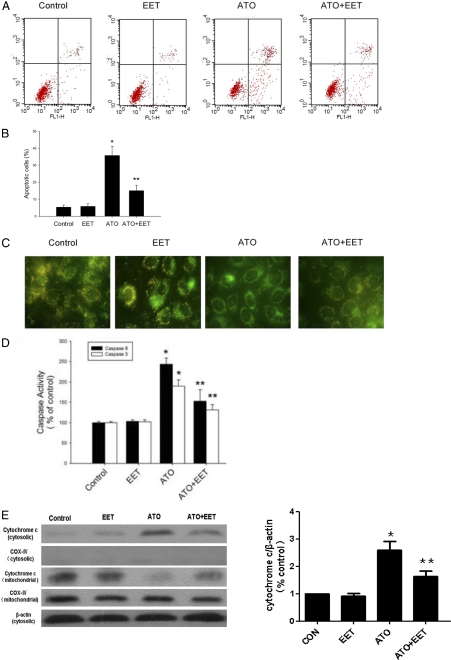
Oxidative damage plays an important role in the anticancer effects of ATO (Chen et al., 1998). Therefore, we examined whether EET-induced changes in SOD and catalase expression/activity could significantly affect ROS levels and apoptotic induction by ATO in tumor cells. Apoptotic cells were identified by Annexin V staining after 24-h treatment with ATO with/without 11,12-EET. As shown in Fig. 3, A and B, 11,12-EET significantly attenuated ATO-induced apoptosis.
Fig. 3.
11,12-EET inhibition of ATO-induced mitochondrial impairment. A, flow cytometric analysis of Tca-8113 cells treated with ATO (10 μM) and 11,12-EET (100 nM) for 24 h by using Annexin V-FITC and PI staining. The lower left quadrants represent nonapoptotic cells; the upper left quadrants represent cells that have lost their cell membranes and are dead, either end-stage apoptotic or necrotic (Annexin V-negative, PI-positive); the lower right quadrants represent early apoptotic cells (Annexin V-positive, PI-negative); and the upper right quadrants represent late apoptotic or necrotic cells (Annexin V- and PI-positive). B, graph represents the mean percentage of Annexin V-positive Tca-8113 cells expressed as the proportion of positive cells in each group (n = 3). C, detection of the mitochondrial transmembrane potential collapse by JC-1 staining. In cells with normal mitochondrial function, membrane potential-driven accumulation of these dyes results in the formation of red fluorescent J-aggregates as shown in the control panel. In cells treated with ATO, the green mitochondria stained with JC-1 dye indicate depressed ΔΨm. D, caspase-3 and caspase-9 activities in Tca-8113 cells. Cells were treated with 10 μM ATO and 100 nM of 11,12-EET for 24 h, lysed, and analyzed spectrophotometrically for caspase activity. E, cytochrome c release in Tca-8113 cells. Cells were treated with 10 μM ATO and 100 nM of 11,12-EET for 24 h and lysed, and cytosolic protein were analyzed by Western blot. Data are reported as mean absorption relative to control ± S.E.M. (n = 5). *, p < 0.05 versus control; **, p < 0.05 versus ATO treatment alone.
ATO may also cause a collapse of the ΔΨm before induction of apoptosis (Mahieux et al., 2001). ΔΨm was determined by JC-1 staining as shown in Fig. 3C. In cells with normal mitochondrial function, membrane potential-driven accumulation of these dyes results in the formation of yellow-red fluorescent J-aggregates as shown in Fig. 3C, control. In cells treated with ATO, the green mitochondria stained with JC-1 dye indicate depressed ΔΨm. It is noteworthy that 11,12-EET cotreatment prevented the ΔΨm collapse caused by ATO. Arsenic-induced apoptosis involves the mitochondrial pathway and activation of caspase-3 and caspase-9. Coincident with changes in ΔΨm, treatment of cells with ATO caused caspase activation, an effect that was partially inhibited by 11,12-EET (Fig. 3D). Cytochrome c release also participated in mitochondrial dysfunction. Consistent with the effects on caspase activities, ATO-induced cytochrome c release was partially reversed by 11,12-EET (Fig. 3E). EET alone, however, did not have effects on apoptosis, ΔΨm, and cytochrome c release.
11,12-EET Reverses ATO-Induced Activation of Stress Response Kinases.
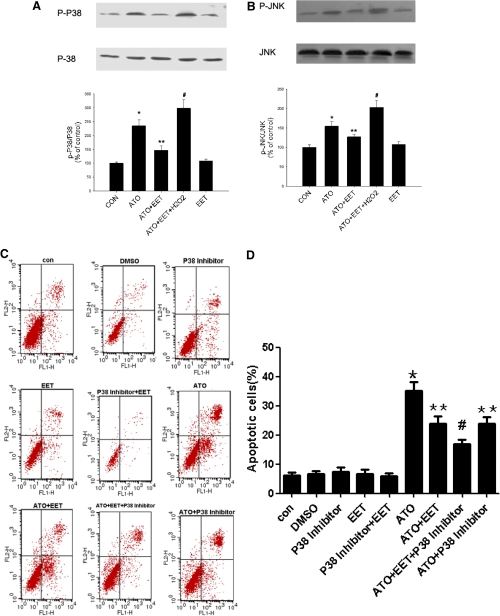
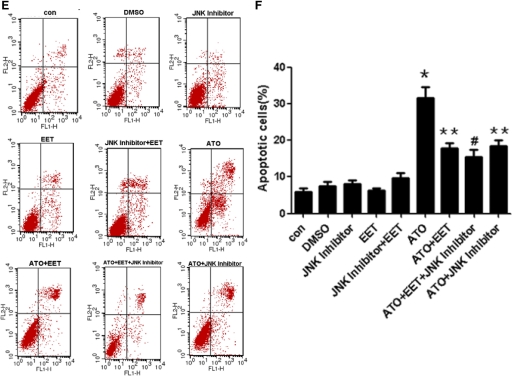
Among the three MAP kinases (ERK, JNK, and p38), JNK and p38 are important factors in apoptosis signaling and are sensitive to ROS levels (Kang and Lee, 2008b). Therefore, we investigated the effects of ATO and 11,12-EET on p38, JNK, and ERK phosphorylation. Preincubation with 11,12-EET blocked ATO-induced phosphorylation and hence activation of p38 and JNK. However, the effect of 11,12-EET was reversed by up-regulation of ROS through hydrogen peroxide administration (Fig. 4, A and B). Furthermore, to figure out whether MAPKs induced by ATO and inhibited by 11,12-EET are partially responsible for caspase activation and apoptosis, we investigated the effects of ATO and 11,12-EET on apoptosis by using specific inhibitors, and results showed that EET and inhibitors of JNK and p38 inhibited ATO induced apoptosis (Fig. 4, C-F). Moreover, the effects of EET on p38, JNK phosphorylation, and ROS production were determined with specific inhibitors, and the results showed that EET partially decreased the phosphorylation and ROS generation induced by ATO, but MAPK inhibitors did not influence ROS generation (Supplemental Fig. 3). We also measured the ERK pathways in the same way and found that ERK inhibitor blocked ERK phosphorylation induced by EETs. However, 10 μM ATO had no significant effect on ERK phosphorylation, and ERK inhibitor had no significant effect on ATO- and ATO+EET-induced apoptosis (Supplemental Fig. 4). These data suggested that EET played an important role in cell apoptosis via p38 and JNK induced by ROS.
Fig. 4.
11,12-EET suppression of ATO-induced p38 and JNK activation. Cells were starved overnight, preincubated with 11,12-EET or p38/JNK inhibitor for 12 h, and incubated with ATO or H2O2 for 15 min. Data are representative of three independent experiments. Results shown are mean ± S.E.M. (n = 3). A and B, p38 and JNK activation in Tca-8113 cells treated with ATO (10 μM), 11,12-EET (100 nM), and H2O2 (200 μM) as indicated. C and D, flow cytometric analysis of Tca-8113 cells treated with p38 inhibitor (20 μM) as indicated for 24 h using Annexin V-FITC and PI staining. E and F, flow cytometric analysis of Tca-8113 cells treated with JNK inhibitor (50 μM) as indicated for 24 h using Annexin V-FITC and PI staining. *, p < 0.05 versus control; **, p < 0.05 versus ATO; #, p < 0.05 versus ATO+EET or ATO+p38/JNK Inhibitor.
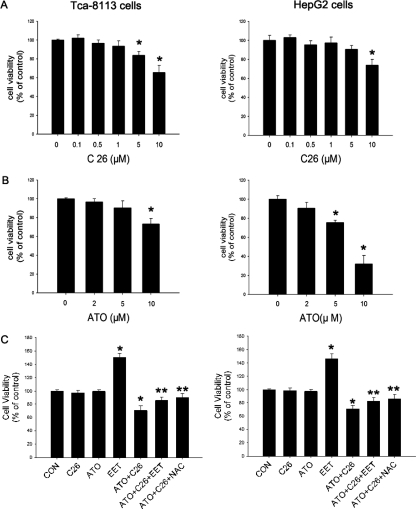
The CYP2J2-Specific Inhibitor C26 and ATO Synergistically Inhibit Cell Replication.
As reported previously, HepG2 cells expressed less CYP2J2 than Tca-8113 cells (Jiang et al., 2005).We tried to find out the effects of endogenous CYP2J2 on apoptosis by comparing the responses of two cell lines to apoptosis. In Tca-8113 cells, ATO (2–5 μM) and a CYP2J2-specific inhibitor, C26 (0.1–1 μM), caused a slight reduction in the number of viable cells when used in low doses (Fig. 5, A and B). More interestingly, HepG2 cells were more sensitive to ATO compared with Tca-8113 cells (Fig. 5B). Apoptosis assays also showed that C26 and ATO induced cell apoptosis in a dose-dependent manner (Supplemental Figs. 5 and 6). Moreover, ROS production in HepG2 cells was significantly more than in Tca-8113 cells with or without ATO treatment (Supplemental Fig. 7). Neither C26 treatment alone nor ATO treatment alone reduced the number of viable cells; however, cotreatment of 1 μM C26 and 2 μM ATO reduced tumor cell viability. Both 11,12-EET and the antioxidant N-acetyl-l-cysteine (NAC) prevented this effect, suggesting that, by inhibiting EET synthesis, C26 enhanced the cytotoxity of ATO through an ROS-dependent manner (Fig. 5C).
Fig. 5.
Effect of CYP2J2 inhibitor on cell proliferation. Cells were treated with the indicated drugs for 24 h, and cell number was measured by the SRB assay. A, effect of C26 on number of tumor cells. B, effect of ATO on number of tumor cells. C, effect of the combination of C26, ATO, 11,12-EET, and NAC on tumor cell number. Cells were incubated with 2 μM ATO, 1 μM C26, 100 of nM 11,12-EET, and/or 2 mM of NAC, as indicated for 24 h. Data are expressed as percentage of untreated controls ± S.E.M. (n = 5). *, p < 0.05 versus control; **, p < 0.05 versus ATO+C26.
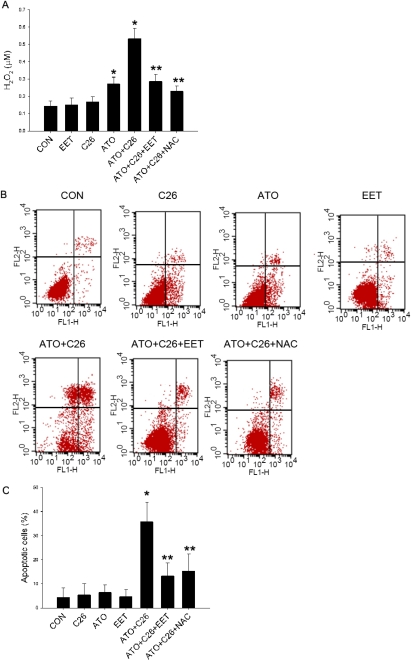
CYP2J2 Inhibition Sensitizes Tumor Cells to ATO-Induced ROS Elevation and Apoptosis.
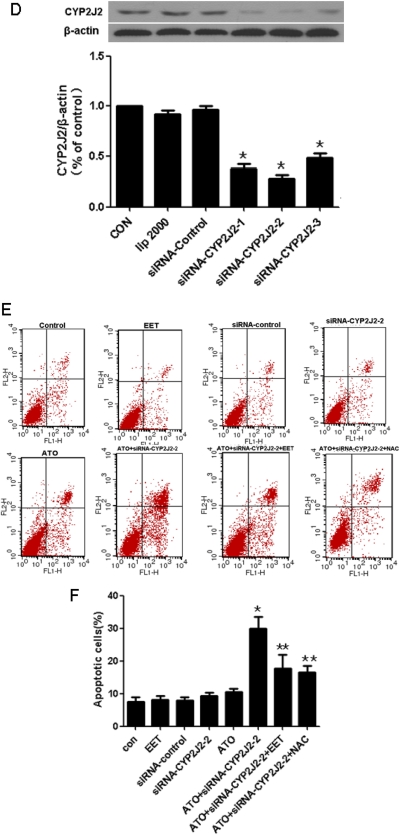
Exposure of Tca-8113 cells to C26 had minimal effect on cellular ROS levels. However, C26 had a synergistic effect with ATO on cellular ROS production. Pretreatment of 11,12-EET or 2 mM NAC for 24 h partially abolished the elevation of ROS caused by ATO and C26 (Fig. 6A). Likewise, cotreatment with C26 and ATO also increased cellular apoptosis compared with C26 or ATO alone, and this apoptosis was also attenuated by pretreatment with 11,12-EET or NAC (Fig. 6, B and C). To exclude the potential side effect of C26, we used CYP2J2-specific siRNA to knock down the expression of endogenous CYP2J2 (Fig. 6D). Confirming drug selectivity, the addition of CYP2J2-specific siRNAs (100 nM) led to a similar result as C26 treatment (Fig. 6, E and F).
Fig. 6.
Effects of CYP2J2 inhibition on sensitization of tumor cells to ATO-induced ROS production and apoptosis. Tca-8113 cells were incubated with 2 μM ATO, 1 μM C26, 100 nM 11,12-EET, and/or 2 mM NAC. A, Tca-8113 cells were preincubated with C26 and NAC for 1 h, followed by 24-h incubation with 11,12-EET, and 2 h before detection, ATO was added to induce ROS. Production of hydrogen peroxide was measured by Amplex Red. Results shown are mean ± S.E.M. (n = 6). B, Tca-8113 cells were preincubated with C26 and NAC for 1 h, and then EET and ATO were added for 24 h. Density plots of Annexin V/PI staining were measured by flow cytometry. C, graph represents the mean number of Annexin V-positive Tca-8113 cells expressed as percentage of control untreated cells ± S.E.M. (n = 3). Each sample was run in duplicate, and the data are representative of three independent assays. D, Tca-8113 cells were treated with CYP2J2-specific siRNA (100 nM) for 24 h. CYP2J2 expression was determined by Western blot (n = 3). E, Tca-8113 cells were treated with CYP2J2-specific siRNA (100 nM), EET, and ATO for 24 h. Density plots of Annexin V/PI staining for apoptosis were measured by flow cytometry. F, graph represents the mean number of Annexin V-positive Tca-8113 cells expressed as percentage of control untreated cells ± S.E.M. Each sample was run in duplicate, and the data are representative of three independent assays. *, p < 0.05 versus control; **, p < 0.05 versus ATO+C26.
Discussion
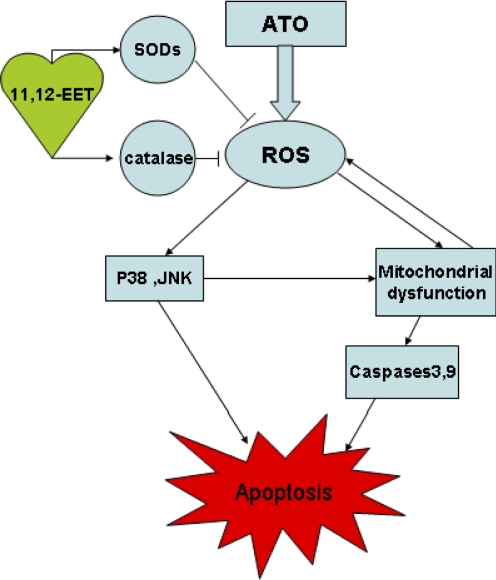
ATO disturbs the natural oxidation and reduction equilibrium, which in turn increases cellular ROS. In the present study, we show for the first time that EETs attenuate arsenic-induced ROS generation in candidate tumor cells. It is now well established that the proapoptotic effect of ATO is mediated by ROS (Dai et al., 1999; Jing et al., 1999; Kang et al., 2004). Moreover, growing evidence suggests that mitochondrial dysfunction plays a key role in oxidative stress (Brookes et al., 2004; Hail, 2005), and ROS generation further impairs mitochondrial electron transport and enhances more ROS production (Simon et al., 2000; Zorov et al., 2006). Consistent with these reports, our results indicate that EETs, through induction of SODs and catalase, reduce intracellular ROS level and attenuate several major apoptotic signaling events. These events include MAP kinase activation, the collapse of the mitochondrial transmembrane potential, and the activation of caspase-3 and caspase-9, which ultimately inhibit tumor cell apoptosis induced by ATO (Scheme 1). This work extends our knowledge of the antiapoptotic mechanism of EETs to the indirect suppression of ROS and mitochondrial damage in cells treated with ATO.
Scheme 1.
The hypothesis of the functions of EETs in tumor cell apoptosis induced by ATO.
MAP kinases are essential factors in apoptosis signaling and become activated in response to cellular redox state (Herr and Debatin, 2001). ATO was able to activate the p38 (Verma et al., 2002) and JNK/stress-activated protein kinase (Davison et al., 2004) in tumor cells, an effect known to be mediated by ROS (Kang and Lee, 2008a). In this study, the alleviation of ATO-induced p38, JNK activation by EET could be reversed by exogenous administration of H2O2, and p38 inhibitor, JNK inhibitor, and ERK inhibitor showed their interaction effects with EET treatment, indicating that EETs altered MAPK signaling in a ROS-dependent manner. The relationship between ROS generation, mitochondrial dysfunction, and p38 activation has been well examined. Herein, we demonstrated that EET altered the p38 and mitochondrial responses by reducing ROS levels. Reports show that in several leukemia cell lines the p38 MAPK pathway acts as a negative regulator of ATO-induced apoptosis and inhibits malignant cell growth (Giafis et al., 2006). However, inhibition of p38 activation failed to protect cells from ATO-induced apoptosis in solid tumor cell lines (Yi et al., 2004). In contrast, p38 activation does account for the mitochondrial translocation of Bax and the phosphorylation of Bcl-2 in response to ATO (Kang and Lee, 2008a,b). These data indicate that p38 activation by ATO plays different roles in different tumor cell types, and it may not be the major determinant in the course of apoptotic processes. For this reason, the significance of altered MAPK signaling pathways by EET in certain tumor cells remains to be investigated. We also found that ERK inhibition can block EET-induced ERK phosphorylation. However, ATO (10 μM) does not have significant effect on ERK phosphorylation, and ERK inhibitor does not have significant effect on ATO- and ATO+EET-induced apoptosis, suggesting ERK may not involve the ATO-related apoptosis.
CYP2J2 is a major enzyme found in extrahepatic tissue, with predominant expression in the cardiovascular system, including endothelial cells and cardiomyocytes (Node et al., 1999). We first demonstrated that CYP2J2 was overexpressed in various human solid cancers and human-derived cancer cell lines (Jiang et al., 2005). This study clearly illustrates that EETs up-regulate the expression and activity of two subtypes of SOD. This may be, at least in part, the underlying mechanism by which EETs increase ROS scavenging. However, there are also other possible mechanisms through which EETs may induce ROS scavenging. For example, EETs are reported to up-regulate heme oxygenase-1 in endothelial cells (Sacerdoti et al., 2007). Increased enzymatic activity of heme oxygenase results in decreased oxidative stress and a lower rate of apoptosis (Jozkowicz et al., 2007).
That Tca-8113 cells are apparently less sensitive to ATO than other tumor cells may be caused by a relatively high level of CYP2J2 expression compared with other tumor lines such as HepG2 (Jiang et al., 2005). EETs are involved in apoptosis, cell cycle, cell adhesion, chromosome stability, and DNA repair networks that are frequently affected in carcinogenesis (Croce, 2008). As shown in Fig. 5B, HepG2 cells generate fewer EETs than Tca-8113 cells, which may result in lower antioxidant effects. Consistent with this hypothesis, we detected significantly more ROS in HepG2 cells than Tca-8113 cells, and HepG2 cells were more susceptible to apoptosis. Low ATO concentrations showed similar effects in Tca-8113 and HepG2 cells, although differences were observed with increasing concentrations, which is consistent with our hypothesis (Fig. 5B). The susceptibility of tumor cells to ATO is associated with the inherent cellular ROS level (Yi et al., 2002), and some compounds enhance the arsenic cytotoxicity via increasing intracellular ROS production (Yi et al., 2004). We have demonstrated that CYP2J2-specific inhibitors can block tumor proliferation and metastasis in vivo and in vitro (Chen et al., 2009).
Our evidence suggests a synergistic effect of ATO and C26. The combination of C26 and ATO seemed to have more than an additive effect on tumor cell apoptosis than the individual treatments. In addition, the combined effect of C26 and ATO could be attenuated after EET or NAC pretreatment. Thus, the synergistic effect of C26 with ATO is through the inhibition of EET synthesis and subsequent reduction in cellular antioxidant capacity. This is the first finding that EETs play important roles in tumor cell proliferation and apoptosis via oxidant stress. This adds to the known antiapoptotic effects of EETs via enhancing Akt, MAPK1/2 signaling, and regulating expression of apoptosis-related proteins Bcl-2/Bax (Chen et al., 2001; Yang et al., 2007). These effects are distinct from ATO-mediated pathways, and therefore the combination of CYP2J2 inhibitor C26 with ATO may result in synergistic apoptotic effects. The present study suggests a novel strategy for treatment of human cancers. Enhanced apoptosis of arsenic-resistant tumor cells may be achieved by combined treatment with relatively low dosages of C26 and clinical acceptable dosages of ATO.
The results of the present study show for the first time that EETs increase antioxidant enzyme expression and activity in cancer cells. This effect enables tumor cells to resist the oxidative damage and the subsequent apoptosis induced by ATO. Moreover, by acting in synergy with ATO to cause tumor cell apoptosis, the CYP2J2-specific inhibitor C26 may be a promising molecule for clinical application. Further investigation of selective CYP2J2 inhibitors in anticancer therapies is warranted.
Supplementary Material
Acknowledgments
We thank Drs. Dongdong Yu and Leya He for help in analyzing the flow cytometry results.
This work was supported by the China Natural Science Foundation Committee [Grants 30700377, 30770882, 30430320]; the 973 Project [Grant 2007 CB512004]; and the Intramural Research Program of the National Institutes of Health National Institute of Environmental Health Sciences [Grant Z01-ES025034].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.180505.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- EET
- epoxyeicosatrienoic acid
- ERK
- extracellular signal-regulated kinase
- ATO
- arsenic trioxide
- ROS
- reactive oxygen species
- DCFH-DA
- 2,7-dichlorodihydrofluorescein diacetate
- SOD
- superoxide dismutase
- SRB
- sulforhodamine B
- MAPK
- mitogen-activated protein kinase
- JNK
- c-Jun NH2-terminal kinase
- C26
- compound 26
- PI
- propidium iodide
- NAC
- N-acetyl-l-cysteine
- FITC
- fluorescein isothiocyanate
- siRNA
- small interfering RNA
- ΔΨm
- mitochondrial transmembrane potential
- SP600125
- anthra[1–9-cd]pyrazol-6(2H)-one
- PD98059
- 2′-amino-3′-methoxyflavone
- SB203580
- 4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine.
Authorship Contributions
Participated in research design: Liu, Chen, and Wang.
Conducted experiments: Liu, Chen, Gong, and Li.
Performed data analysis: Liu, Chen, and Wang.
Wrote or contributed to the writing of the manuscript: Liu, Chen, Edin, Zeldin, and Wang.
References
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287:C817–C833 [DOI] [PubMed] [Google Scholar]
- Chen C, Li G, Liao W, Wu J, Liu L, Ma D, Zhou J, Elbekai RH, Edin ML, Zeldin DC, et al. (2009) Selective inhibitors of CYP2J2 related to terfenadine exhibit strong activity against human cancers in vitro and in vivo. J Pharmacol Exp Ther 329:908–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM, et al. (1997) Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood 89:3345–3353 [PubMed] [Google Scholar]
- Chen JK, Capdevila J, Harris RC. (2001) Cytochrome p450 epoxygenase metabolism of arachidonic acid inhibits apoptosis. Mol Cell Biol 21:6322–6331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Lin-Shiau SY, Lin JK. (1998) Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J Cell Physiol 177:324–333 [DOI] [PubMed] [Google Scholar]
- Croce CM. (2008) Oncogenes and cancer. N Engl J Med 358:502–511 [DOI] [PubMed] [Google Scholar]
- D'Autréaux B, Toledano MB. (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8:813–824 [DOI] [PubMed] [Google Scholar]
- Dai J, Weinberg RS, Waxman S, Jing Y. (1999) Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood 93:268–277 [PubMed] [Google Scholar]
- Davison K, Mann KK, Waxman S, Miller WH., Jr (2004) JNK activation is a mediator of arsenic trioxide-induced apoptosis in acute promyelocytic leukemia cells. Blood 103:3496–3502 [DOI] [PubMed] [Google Scholar]
- Engel RH, Evens AM. (2006) Oxidative stress and apoptosis: a new treatment paradigm in cancer. Front Biosci 11:300–312 [DOI] [PubMed] [Google Scholar]
- Gazitt Y, Akay C. (2005) Arsenic trioxide: an anti cancer missile with multiple warheads. Hematology 10:205–213 [DOI] [PubMed] [Google Scholar]
- Giafis N, Katsoulidis E, Sassano A, Tallman MS, Higgins LS, Nebreda AR, Davis RJ, Platanias LC. (2006) Role of the p38 mitogen-activated protein kinase pathway in the generation of arsenic trioxide-dependent cellular responses. Cancer Res 66:6763–6771 [DOI] [PubMed] [Google Scholar]
- Hail N., Jr (2005) Mitochondria: A novel target for the chemoprevention of cancer. Apoptosis 10:687–705 [DOI] [PubMed] [Google Scholar]
- Han YH, Moon HJ, You BR, Kim SZ, Kim SH, Park WH. (2009) The effect of MAPK inhibitors on arsenic trioxide-treated Calu-6 lung cells in relation to cell death, ROS and GSH levels. Anticancer Res 29:3837–3844 [PubMed] [Google Scholar]
- Han YH, Moon HJ, You BR, Kim SZ, Kim SH, Park WH. (2010) Effects of arsenic trioxide on cell death, reactive oxygen species and glutathione levels in different cell types. Int J Mol Med 25:121–128 [PubMed] [Google Scholar]
- Herr I, Debatin KM. (2001) Cellular stress response and apoptosis in cancer therapy. Blood 98:2603–2614 [DOI] [PubMed] [Google Scholar]
- Jiang JG, Chen CL, Card JW, Yang S, Chen JX, Fu XN, Ning YG, Xiao X, Zeldin DC, Wang DW. (2005) Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res 65:4707–4715 [DOI] [PubMed] [Google Scholar]
- Jiang JG, Ning YG, Chen C, Ma D, Liu ZJ, Yang S, Zhou J, Xiao X, Zhang XA, Edin ML, et al. (2007) Cytochrome p450 epoxygenase promotes human cancer metastasis. Cancer Res 67:6665–6674 [DOI] [PubMed] [Google Scholar]
- Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S. (1999) Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood 94:2102–2111 [PubMed] [Google Scholar]
- Jozkowicz A, Was H, Dulak J. (2007) Heme oxygenase-1 in tumors: is it a false friend? Antioxid Redox Signal 9:2099–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YH, Lee SJ. (2008a) The role of p38 MAPK and JNK in arsenic trioxide-induced mitochondrial cell death in human cervical cancer cells. J Cell Physiol 217:23–33 [DOI] [PubMed] [Google Scholar]
- Kang YH, Lee SJ. (2008b) Role of p38 MAPK and JNK in enhanced cervical cancer cell killing by the combination of arsenic trioxide and ionizing radiation. Oncol Rep 20:637–643 [PubMed] [Google Scholar]
- Kang YH, Yi MJ, Kim MJ, Park MT, Bae S, Kang CM, Cho CK, Park IC, Park MJ, Rhee CH, et al. (2004) Caspase-independent cell death by arsenic trioxide in human cervical cancer cells: reactive oxygen species-mediated poly(ADP-ribose) polymerase-1 activation signals apoptosis-inducing factor release from mitochondria. Cancer Res 64:8960–8967 [DOI] [PubMed] [Google Scholar]
- Mahieux R, Pise-Masison C, Gessain A, Brady JN, Olivier R, Perret E, Misteli T, Nicot C. (2001) Arsenic trioxide induces apoptosis in human T-cell leukemia virus type 1- and type 2-infected cells by a caspase-3-dependent mechanism involving Bcl-2 cleavage. Blood 98:3762–3769 [DOI] [PubMed] [Google Scholar]
- Michaelis UR, Fisslthaler B, Medhora M, Harder D, Fleming I, Busse R. (2003) Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR). FASEB J 17:770–772 [DOI] [PubMed] [Google Scholar]
- Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. (1999) Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285:1276–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S. (2007) Reactive oxygen species in mitochondria-mediated cell death. Drug Metab Rev 39:443–455 [DOI] [PubMed] [Google Scholar]
- Pelicano H, Carney D, Huang P. (2004) ROS stress in cancer cells and therapeutic implications. Drug Resist Updat 7:97–110 [DOI] [PubMed] [Google Scholar]
- Perkins C, Kim CN, Fang G, Bhalla KN. (2000) Arsenic induces apoptosis of multidrug-resistant human myeloid leukemia cells that express Bcr-Abl or overexpress MDR, MRP, Bcl-2, or Bcl-x(L). Blood 95:1014–1022 [PubMed] [Google Scholar]
- Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. (2007) Mechanisms of cell death in oxidative stress. Antioxid Redox Signal 9:49–89 [DOI] [PubMed] [Google Scholar]
- Sacerdoti D, Colombrita C, Di Pascoli M, Schwartzman ML, Bolognesi M, Falck JR, Gatta A, Abraham NG. (2007) 11,12-Epoxyeicosatrienoic acid stimulates heme-oxygenase-1 in endothelial cells. Prostaglandins Other Lipid Mediat 82:155–161 [DOI] [PubMed] [Google Scholar]
- Simon HU, Haj-Yehia A, Levi-Schaffer F. (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418 [DOI] [PubMed] [Google Scholar]
- Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, et al. (1998) Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med 339:1341–1348 [DOI] [PubMed] [Google Scholar]
- Sun J, Sui X, Bradbury JA, Zeldin DC, Conte MS, Liao JK. (2002) Inhibition of vascular smooth muscle cell migration by cytochrome p450 epoxygenase-derived eicosanoids. Circ Res 90:1020–1027 [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84 [DOI] [PubMed] [Google Scholar]
- Verma A, Mohindru M, Deb DK, Sassano A, Kambhampati S, Ravandi F, Minucci S, Kalvakolanu DV, Platanias LC. (2002) Activation of Rac1 and the p38 mitogen-activated protein kinase pathway in response to arsenic trioxide. J Biol Chem 277:44988–44995 [DOI] [PubMed] [Google Scholar]
- Wang H, Lin L, Jiang J, Wang Y, Lu ZY, Bradbury JA, Lih FB, Wang DW, Zeldin DC. (2003) Up-regulation of endothelial nitric-oxide synthase by endothelium-derived hyperpolarizing factor involves mitogen-activated protein kinase and protein kinase C signaling pathways. J Pharmacol Exp Ther 307:753–764 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wei X, Xiao X, Hui R, Card JW, Carey MA, Wang DW, Zeldin DC. (2005) Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J Pharmacol Exp Ther 314:522–532 [DOI] [PubMed] [Google Scholar]
- Yang S, Lin L, Chen JX, Lee CR, Seubert JM, Wang Y, Wang H, Chao ZR, Tao DD, Gong JP, et al. (2007) Cytochrome P-450 epoxygenases protect endothelial cells from apoptosis induced by tumor necrosis factor-α via MAPK and PI3K/Akt signaling pathways. Am J Physiol Heart Circ Physiol 293:H142–H151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J, Gao F, Shi G, Li H, Wang Z, Shi X, Tang X. (2002) The inherent cellular level of reactive oxygen species: one of the mechanisms determining apoptotic susceptibility of leukemic cells to arsenic trioxide. Apoptosis 7:209–215 [DOI] [PubMed] [Google Scholar]
- Yi J, Yang J, He R, Gao F, Sang H, Tang X, Ye RD. (2004) Emodin enhances arsenic trioxide-induced apoptosis via generation of reactive oxygen species and inhibition of survival signaling. Cancer Res 64:108–116 [DOI] [PubMed] [Google Scholar]
- Zeldin DC. (2001) Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem 276:36059–36062 [DOI] [PubMed] [Google Scholar]
- Zmijewski JW, Moellering DR, Le Goffe C, Landar A, Ramachandran A, Darley-Usmar VM. (2005) Oxidized LDL induces mitochondrially associated reactive oxygen/nitrogen species formation in endothelial cells. Am J Physiol Heart Circ Physiol 289:H852–H861 [DOI] [PubMed] [Google Scholar]
- Zorov DB, Juhaszova M, Sollott SJ. (2006) Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta 1757:509–517 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.