Abstract
It is dentists’ dream to achieve bone repair with predictability, but without donor site morbidity as well as reconstruction of injured or pathologically damaged complex dental structures, however, this will no longer be a dream as these are being made into a reality using stem cell science. Stem cell science is clearly an intriguing and promising area of science. Stem cells have been isolated from a variety of embryonic and adult tissues. Dental stem cells are multipotent mesenchymal stem cells (MSCs) brought new enthusiasm among the researchers because of their easy accessibility, high quality and they don’t pose the same ethical concerns and controversy in comparison with embryonic stem cells. This review article provides brief insights about stem cell basics, the state of art in human dental stem cell research and its possible impact on future dentistry. Even though most of these modalities are still in infancy, it is evident that the 21st century dentist is going to play a critical role in the field of medicine. The aim of this article is to bring awareness among the dentists about the huge potential associated with the use of stem cells in a clinical setting, as well as proper understanding of related problems.
Keywords: Mesenchymal stem cells, stem cells, tooth engineering
INTRODUCTION
The immediate challenge for dentists is not only to be better able to address the questions that their patients have concerning stem cell-based therapy, but also to familiarize themselves with the spectrum of tools they may have in the near future to restore form and function of the tooth and -tooth-supporting structures effectively. Stem cells are not science fiction, but something that one day will become a part of each dentist's clinical practice. This article reviews what one should know about the about classification of stem cells, how they differ in their biological activity – dental stem cells osteogenic potential and results of clinical pilot studies using dental stem cells for bone repair-tissue engineering using dental stem cells-future challenges, future trends, and importance of dental surgeons role.
STEM CELLS
Stem cells are often called ‘master cells’ because they are a class of undifferentiated cells that are able to differentiate into specialized cell type. A stem cell “self-renews”-that is, when a stem cell is called into action, it undergoes cell division. One daughter cell remains a stem cell, while the other becomes more committed to forming a particular cell type (a “committed progenitor”) by a process called “asymmetric cell division”.[1] There are different types of stem cell along with different expectations of their biological activity.[2]
To be classified as a stem cell, a cell must satisfy three main criteria:[3]
It is not terminally differentiated: The cell is not committed to differentiate into a single cell type
It can divide without limit
It can renew the stem cell pool: During cell division each daughter cell can choose whether to commit to differentiation into single cell type o remain as a stem cell
Potency
Stem cells are categorized by their potential to differentiate into other types of cells.[1] Embryonic stem cells are the most potent since they must become every type of cell in the body.
Totipotent - the ability to differentiate into all possible cell types. Examples are the fertilized egg, capable of independently giving rise to all embryonic and extra-embryonic tissues
Pluripotent - the ability to differentiate into almost all cell types. Examples include the inner cell mass of blastocyst in the developing zygote and embryonic stem cells in culture, capable of giving rise to all embryonic cells and tissues
Multipotent - the ability to differentiate into a closely related family of cells. Examples include cells that are derived from the three embryonic germ layers (ectoderm, mesoderm and endoderm) that become more and more committed to generating particular cells as organs and tissues are formed
Oligopotent - the ability to differentiate into a few cells. Examples include (adult) lymphoid or myeloid stem cells
Unipotent - the ability to only produce cells of their own type, but have the property of self-renewal required to be labelled a stem cell. Examples include (adult) muscle stem cells
Embryonic stem cells are considered pluripotent instead of totipotent because they do not have the ability to become part of the extra-embryonic membranes or the placenta.
Plasticity
Plasticicity is the ability of a stem cell isolated from one tissue to “convert” to cells found in a different tissue, and sometimes even into cell types that originated from a completely different embryonic germ layer.[1] “Plasticity” have generated a great deal of enthusiasm as adult stem cell plasticity would fulfill the tissue engineer's dream of isolating stem cells from easily accessible sources like teeth, fat for regeneration of many different tissues by reprogramming the genes, a phenomenon called “Transdifferentiation” (e.g.,iPSC ).
Sources of stem cells
Commonly, stem cells come from two main sources:
Embryos formed during the blastocyst phase of embryological development (embryonic stem cells); and
Adult tissue (adult stem cells).
Embryonic stem cells
Embryonic stem cells are derived from a four- or five-day-old human embryo that is in the blastocyst phase of development. The blastocyst consists of an inner cell mass (embryoblast) and an outer cell mass (trophoblast). This latter mass is the source of embryonic stem cells - totipotent cells (cells with total potential to develop into any cell in the body). Embryonic stem cells are able to differentiate into more cell types than adult stem cells, over 220 types of cells in the human adult body. Embryonic stem cells can be grown relatively easily in culture than adult stem cells [Figure 1]. Embryonic stem cell research has triggered enormous debate due to the destruction of an embryo following cell extraction.
Figure 1.
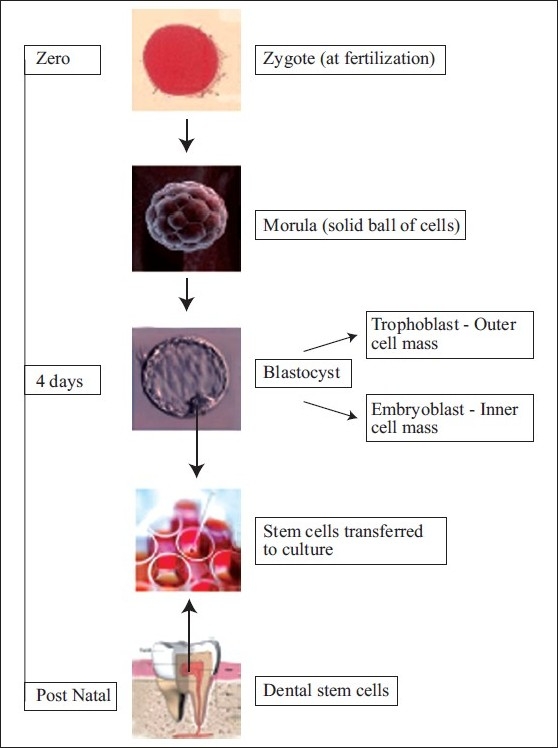
Stem cell culture
Umbilical cord blood stem cells are stem cells collected from the umbilical cord at birth that can produce all of the blood cells in the body (hematopoietic). Cord blood is currently used to treat patients who have undergone chemotherapy to destroy their bone marrow due to cancer or other blood-related.
Adult stem cells
Adult or somatic stem cells exist throughout the body after embryonic development and are found inside of different types of tissue. These stem cells have been found in tissues such as the brain, bone marrow, blood, blood vessels, skeletal muscles, teeth, heart, gut, skin, liver, ovarian epithelium, and testes. They remain in a quiescent or non-dividing state for years until activated by disease or tissue injury.
Unfortunately, adult stem cells are present in miniscule quantities and this can present difficulty for identifying and isolating them in numbers great enough to use therapeutically. Because adult stem cells aren′t as ‘young’ as embryonic stem cells, they contain more DNA abnormalities acquired with age. These can be caused by the environment, toxins or errors in DNA replication.
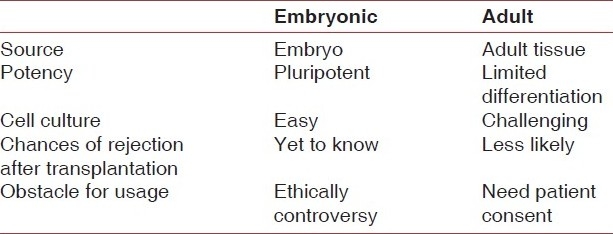
[Table 1] summarizes the differences between embryonic and adult stem cells.
Table 1.
The differences between embryonic and adult stem cells
Mesenchymal stem cells
The history of research on adult stem cells began about 60 years ago when researchers discovered that the bone marrow contains at least two kinds of stem cells. One population, called hematopoietic stem cells, forms all the types of blood cells in the body. A second population, non-hematopoietic stem cells make up a small proportion of the stromal cell population in the bone marrow, and can generate bone, cartilage, fat, cells that support the formation of blood, and fibrous connective tissue (also called, or skeletal stem cells by some) were discovered a few years later. Owen and Friedenstein[4] called these cells “bone marrow stromal stem cells” (BMSCs) later Caplan[5] coined the term “mesenchymal stem cells” and some others[6,7] have chosen the term “skeletal stem cells” (SSCs) based on their ability to recreate all cell types associated with skeletal tissue.
Investigators have reported that mesenchymal stem cells (SSCs) like other adult stem cells, exhibit plasticity and are able to differentiate into cell types in addition to skeletal cells, such as muscle cells, nerve cells, cardiomyocytes.[8,9] Studies have shown that MSCs can differentiate into cells normally associated with the ectoderm and endoderm, such as neurons and muscle cells, respectively[10][Figure 2].
Figure 2.
Tissue engineering in dentistry
Dental stem cells
There are five recognized dental stem cell populations. Because of their ability to differentiate into bone and other periodontal tissues, dental stem cells have received much attention from both researchers and clinicians. Mesenchymal stem cells have isolated and characterized from dental pulp stem cells (DPSCs), periodontal ligament stem cells (PDLSCs), the remnant pulp of deciduous teeth stem cells from human exfoliated deciduous teeth (SHED), dental follicle stem cells (DFSCs), and stem cells from apical papilla (SCAP). The dental stem cells are usually obtained from deciduous teeth, wisdom teeth, and therapeutically extracted teeth.
Dental stem cells and their osteogenic potential
All five dental stem cell populations demonstrate the ability to undergo osteogenic differentiation in vitro and in vivo.[10] To initiate bone formation in vitro, dental stem cells are stimulated with chemicals such as ascorbic acid, dexamethasone and β-glycerophosphate which promote osteogenic differentiation and mineralization.[11] A widely used model which assesses bone formation in vivo is the immunocompromised mouse.[12] Immunocompromised mice lack the ability to raise an immune response to foreign transplanted cells allowing the cells to differentiate unchallenged.[12] Isolated stem cells are ectopically transplanted into immunocompromised mice and differentiate into mineralized tissue over time.[13]
DPSCs express genes associate with bone formation in vitro such as alkaline phosphatase (ALP), osteocalcin (OC), osteonectin (ON), and bone sialoprotein (SBP), in addition to producing mineral matrix (as judged by positive staining for Alizarin Red).[14] Histological analysis of DPSCs transplanted into immunocompromised mice revealed the formation of lamellar bone tissue and cells which stained positive for ALP, eight weeks following transplantation.[15]
Much like DPSCs, PDLSCs can be induced to express an osteoblast-like phenotype in vitro, as assessed by calcium accumulation, appearance of mineralized nodules and increased transcription of genes for ALP, BSP, and OC.[16] In vivo transplanted PDSSCs have been shown to generate tissue with striking similarity to both cementum and periodontal ligament.[16] Histological analysis of transplanted PDLSCs demonstrated the presence of cementum interspersed with collagen fibers, reminiscent of sharpey's fibers.[16]
SHED cells undergo osteogenic differentiation in vitro, as judged by expression of bone markers such a core-binding factor α-1 (CBFA1), ALP and BSP, and positive staining for Alizarin Red.[17] Following transplantation of SHEd cells into an immunocompromised mouse, muira et al. observed that SHED cells induced surrounding recipient tissue to generate bone. Interestingly, the authors noted that the transplanted SHED cells did not undergo osteogenesis directly.[17]
In vitro, SCAP cells can be stimulated to form mineralized tissue, however, induced SCAP cells also express dentin sialoprotein (DSP): A protein which is involved in dentine synthesis.[18] Similarly, in vivo transplantation of human SCAP into immunocompromised mice resulted in the generation of odontoblasts capable of depositing new dentine.[18,19] These results suggest that although SCAP can display certain osteogenic characteristics, they preferentially differentiate into dentine producing cells, reminiscent of odontoblast like cells.
Consistent with other dental stem cell types, DFPCs undergo osteogenic differentiation in vitro, when cultured under defined conditions.[20,21] Bovine DFPCs transplanted into immunocompromised mice resulted in the generation of cementum like fibrous tissue.[22] In contrast, human DFPCs transplanted by the same method demonstrated no apparent mineralization, although the expression of osteogenic genes such as BSP and OC was elevated.[23]
Bone repair using dental stem cells
Materials currently used for repair of bone defect following periodontal surgery, trauma or maxillofacial surgery include autogenous bone, deproteinized xenograft, alloplastic bone substitutes. Dental stem cells are currently under investigation as a strategy for repairing defects in mandibular bone. Both pre clinical and clinical studies have revealed that dental stem cells have the ability to generate new bone and restore periodontal tissue.[24–27] These studies in which autologous dental stem cells have been grafted into mandibular defects have produced encouraging results. The ability of stem cells to differentiate into a variety of tissue types, such as vasculature and nerve, may enhance the viability of stem-cell-containing bone grafts resulting in a stronger bone foundation.[28]
In a study by kim et al.[24] autologous bone marrow stem cells (MBSCs) and PDLSCs were compared for their ability to regenerate bone in a canine peri-implant defect model. Mandibular defects were created in beagle dogs and grafted with a hydroxyapatite/β-tricalcium phosphate (HA/TCP) carrier containing either MBSCs or PDLSCs. Histological measurement of the mandible at eight-week post-surgery demonstrated a significant increase in new bone formation in defects treated with BMsCs and PDLSCs compared to control. At 16-week post-surgery defects treated with BMSCs, but not PDLSCs showed a significant increase in new bone compared to control. Parameters such as defects height and defect area showed no significant difference between stem-cell treated and control defects at the indicated time-pointed. Porcine SHED cells have been assessed for their ability to regenerate a mandibular defect in swine.[25] An experimental defect was created in the parasymphaseal area of the mandible and grafted with autologous porcine deciduous stem cells seeded into a TCP carrier. Histological assessment of sections from engrafted mandibles at six months post surgery revealed that defects treated with porcine deciduous stem cells showed a significant increase in ossification compared with TCP carrier alone.[25]
In a clinical study, d’Aquino et al.,[26] investigated the use of autologous DPSCs for the treatment of an alveolar bone defect following wisdom tooth extraction. DPSCs were isolated form patients maxillary molars and expanded ex vivo before seeding into a collagen scaffold. Patients treated with autologous DPSCs showed consistently enhanced regeneration of the bone defect as judged by gain of vertical bone height compared with patients treated with the collagen scaffold alone.
A recent clinical case study by Feng et al.,[27] indicated that autologous human progenitor cells form periodontal ligament may enhance tissue regeneration in patients with periodontitis. Three male patients with periodontitis were treated with autologous periodontal ligament progenitor cells (PDLPs) seeded onto CalcititeR 4060-2 bone grafting material at the periodontal defect site. Patients’ assessment at 32 months following PDLP implantation and demonstrated decreased tooth movement and probing depth at the treatment site, in addition to improvement of attachment gain, compared with pre-treatment conditions.
Tissue engineering using dental stem cells
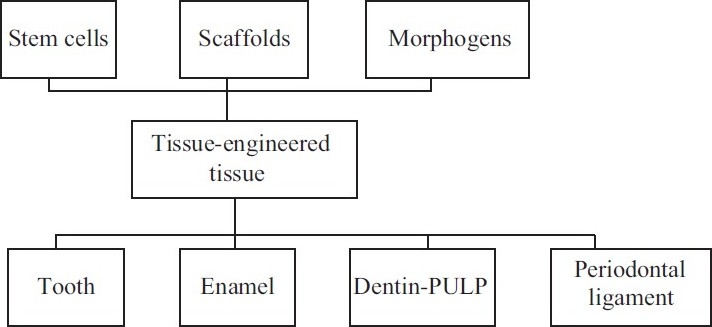
“Tissue Engineering” is the general term for a number of ways by which tissue lost as a result of trauma and disease might be restored.[29]
It requires the three key elements to combine together to form the basic framework for the formation of engineered tooth tissues [Figure 2].
Progenitor/stem cells;
Inductive morphogenetic signals[30,31] in an environment conductive to regeneration of a vital and functional tissue and/or organ; and
An extracellular matrix scaffold (which can be synthetic).
It has been tried to regenerate and/or engineer tooth tissues like enamel, dentine- pulp complex, and periodontal ligament by using different strategies of tissue engineering like guided tissue regeneration, stem cell therapy,[32] gene therepy,[33] injectable scaffolds, nano engineering either alone or in combination technologies. Dental tissue engineering using dental stem cells have recently shown promising results for novel approaches to treat diseases like periodontitis, dental caries or to improve dental pulp healing and the regeneration of teeth[34] and craniofacial bone by favoring alveolar ridge augmentation procedures[35] and methods to generate vascularized bone grafts.[36]
Future challenges, future trends and 21st century dentistry
The prime challenge for researchers is to solve the mystery about the precise mechanism responsible for cell growth and differentiation and to find ways to control stem cell differentiation. Once the modus operandi is established with control, scientists can more easily grow cells and tissues for specific functions.[37]
The complete predictable regeneration of periodontal tissue, the major goal of periodontal therapy, is still unobtainable. This can possibly be achieved by use of stem cells. In addition to these dental implants, interface with bone may be improved by the development of cementum on the implant surface along with re-establishment of a PDL between the newly formed cementum and the alveolar bone. This would mark a revolutionary achievement in the dental field. There is substantial evidence that dental ectomesenchymal stem cells are more promising for dentistry in future.[38]
Developments are taking place in such a way that they are beyond our expectation at present. Using stem cells and developing cell-based regenerative therapies for the repair of the tooth (enamel, dentin and cementum) and -tooth-supporting,- periodontal ligament is a new attractive approach. As a departure from the reliance of current clinical practice on durable materials such as amalgam, composites, and metallic alloys, biological therapies utilize mesenchymal stem cells, delivered or internally recruited to generate craniofacial structures in temporary scaffolding biomaterials. Craniofacial tissue engineering is likely to be realized in the foreseeable future and represents an opportunity that dentistry cannot afford to miss.[39,40]
Field of dentistry and the role of dentist in the field of medicine are going to rise to new heights. Dental pulp tissue is thought to be derived from migrating neural crest cells during development. Dental ectomesenchymal stem cells, DPSCs were found to be useful in cell based therapies to treat neurological diseases and injury.[41] The discovery that odontogenic tissues are a source of high quality adult stem cells has opened up a new role for dentists in the field of medicine [Figure 3]. In the future, a dentist's office could become a “stem cell bank” for patients who, in later life, may require new bone, teeth or other oral tissues. Dentists are positioned to become one of the key providers of stem cells, and as a result, their linkage with the medical field will become very intimate. Dentists can be involved in the extraction, collection, and storage of the stem cells from their patients’ teeth. In order for dentists to fully participate in this new role, they should become aware of the applications, clinical use, and banking of dental stem cells.[42]
Figure 3.

Dental clinic
CONCLUSION
Developments in dental stem cell research are taking place in such a way that they are beyond our expectation at present. However, it is a long journey, there are certain milestones to be passed and need to surmount obstacles encounter in the way before the use of stem cells in a clinical setting to transform dentistry as we know it, and even revolutionize contemporary dentistry. Along with technical aspects, various other issues like social, political, ethical, and religious viewpoints need to be addressed in the scientific and clinical use of stem cells. Dental precursor cells are attractive for novel approaches to treat diseases like periodontitis, dental caries or to improve dental pulp healing and the regeneration of craniofacial bone and teeth. Further, dental stem cells can be utilized to regenerate different tissues like nerve and bone. Even though most of these modalities are still in infancy, it is evident that the 21st century dentist is going to play a critical role in the field of medicine.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lakshmipathy U, Verfaillie C. Stem cell plasticity. Blood Rev. 2005;19:29–38. doi: 10.1016/j.blre.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Van Os R, Kamminga LM, de Haan G. Stem cell assays: Something old, something new, something borrowed. Stem Cells. 2004;22:1181–90. doi: 10.1634/stemcells.2004-0095. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: Implications in tumorigenesis and metastatis. J Cell physiol. 2010;222:268–77. doi: 10.1002/jcp.21940. [DOI] [PubMed] [Google Scholar]
- 4.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 5.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells. 2001;19:180–92. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 6.Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, Benayahu D, et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–47. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 7.Bianco P, Robey PG. Skeletal stem cells. In: Lanza RP, editor. Handbook of adult and fetal stem cells. San Diego: Academic Press; 2004. pp. 415–24. [Google Scholar]
- 8.Barry FP, Murphy JM. Mesenchymal stem cells: Clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Zipori D. Mesenchymal stem cells: Harnessing cell plasticity to tissue and organ repair. Blood Cells Mol Dis. 2004;33:211–5. doi: 10.1016/j.bcmd.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources; their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, ling J, Wei X, Wu L, Xiao Y. Stem cell regulatory gene expression in human adult dental pulp and periodontal ligament cells undergoing odontogenic/osteogenic differentiation. J Endod. 2009;35:1368–76. doi: 10.1016/j.joen.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Pryzborski SA. Differentiation of human embryonic stem cells after transplantation in immune-deficient mice. Stem Cells. 2005;23:1242–50. doi: 10.1634/stemcells.2005-0014. [DOI] [PubMed] [Google Scholar]
- 13.Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8:191–9. doi: 10.1111/j.1601-6343.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 14.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraft DC, Bindslev DA, Melsen B, Abdallah BM, Kassem M, Klein-Nulend J. Mechanosensitivity of dental pulp stem cells is related to their osteogenic maturity. Eur J Oral Sci. 2010;118:29–38. doi: 10.1111/j.1600-0722.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 16.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 17.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: Stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonoyama W, Liu y, Fang D, Yamaza T, Seo BM, Zhang C, et al. Mesenchymal stem cell-medicated functional tooth regeneration in swine. PloS One. 2006;1:1–8. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe S, Yamaguchi S, Watanabe A, Hamada K, Amagasa T. Hard tissue regeneration capacity of apical pulp derived cells (APDCs) from human tooth with immature apex. Biochem Biophys Res Commun. 2008;371:90–3. doi: 10.1016/j.bbrc.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Kemoun P, Laurencin-Dalicieux S, Rue J, Farges JC, Gennero I, Conte-Auriol F, et al. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res. 2007;329:283–94. doi: 10.1007/s00441-007-0397-3. [DOI] [PubMed] [Google Scholar]
- 21.Vollner F, Ernst W, Driemel O, Morsczeck C. A two-step strategy for neuronal differentiation in vitro of human dental follicle cells. Differentiation. 2009;77:433–41. doi: 10.1016/j.diff.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Handa K, Saito M, Yamauchi M, Kiyono T, Sato S, Teranaka T, et al. Gementum matrix formation in viov by cultured dental follicle cells. Bone. 2002;31:606–11. doi: 10.1016/s8756-3282(02)00868-2. [DOI] [PubMed] [Google Scholar]
- 23.Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–65. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Kim KH, Seo BM, Koo KT, Kim TI, Seol YJ, et al. Alveiolar bone regeneration by transplantation of periodontal ligament stem cells and bone marrow stem cells in a canine peri-implant defect model: A pilot study. J Periodontal. 2009;80:11815–23. doi: 10.1902/jop.2009.090249. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Y, Liu Y, Zhang CM, Zhang HY, Li WH, Shi S, et al. Stem cells from deciduous tooth repair mandibular defect in swine. J Dent Res. 2009;88:249–54. doi: 10.1177/0022034509333804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.d’Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, et al. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75–83. doi: 10.22203/ecm.v018a07. [DOI] [PubMed] [Google Scholar]
- 27.Feng F, Akiyama K, Liu Y, Yamaza T, Wang TM, Chen JH, et al. Utility of PDL progenitors for in vivo tissue regeneration: A report of 3 cases. Oral Dis. 2010;16:20–8. doi: 10.1111/j.1601-0825.2009.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakashima M, Iohara K, Sugiyama M. Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev. 2009;20:435–40. doi: 10.1016/j.cytogfr.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31:711–8. doi: 10.1097/01.don.0000164138.49923.e5. [DOI] [PubMed] [Google Scholar]
- 30.Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247–52. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 31.Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21:1025–32. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 32.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: A review of current status and a call for action. J Endod. 2007;33:377–90. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31:711–8. doi: 10.1097/01.don.0000164138.49923.e5. [DOI] [PubMed] [Google Scholar]
- 34.Honda MJ, Fong H, Iwatsuki S, Sumita Y, Sarikaya M. Tooth-forming potential in embryonic and postnatal tooth bud cells. Med Mol Morphol. 2008;41:183–92. doi: 10.1007/s00795-008-0416-9. [DOI] [PubMed] [Google Scholar]
- 35.De Kok IJ, Peter SJ, Archambault M, van den Bos C, Kadiyala S, Aukhil I, et al. Investigation of allogeneic mesenchymal stem cell-based alveolar bone formation: Preliminary findings. Clin Oral Implants Res. 2003;14:481–9. doi: 10.1034/j.1600-0501.2003.110770.x. [DOI] [PubMed] [Google Scholar]
- 36.Mankani MH, Krebsbach PH, Satomura K, Kuznetsov SA, Hoyt R, Robey PG. Pedicled bone flap formation using transplanted bone marrow stromal cells. Arch Surg. 2001;136:263–70. doi: 10.1001/archsurg.136.3.263. [DOI] [PubMed] [Google Scholar]
- 37.Murray PE, Garcia-Godoy F. Stem cell responses in tooth regeneration. Stem Cells Dev. 2004;13:255–62. doi: 10.1089/154732804323099181. [DOI] [PubMed] [Google Scholar]
- 38.Morsczeck C, Reichert TE, Völlner F, Gerlach T, Driemel O. The state of the art in human dental stem cell research. Mund Kiefer Gesichtschir. 2007;11:259–66. doi: 10.1007/s10006-007-0071-7. [DOI] [PubMed] [Google Scholar]
- 39.Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, et al. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966–79. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulmer FL, Winkel A, Kohorst P, Stiesch M. Stem cells - prospects in dentistry. Schweiz Monatsschr Zahnmed. 2010;120:860–72. [PubMed] [Google Scholar]
- 41.Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult Human Dental Pulp Stem Cells Differentiate Toward Functionally Active Neurons Under Appropriate Environmental Cues. Stem Cells. 2008;26:1787–95. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- 42.Krasner P, Verlander P. Stem cells in dentistry and medicine: The dentist's role. Dent Today. 2011;30(128):130–4. [PubMed] [Google Scholar]


