Abstract
Background:
The use of different bioactive materials as coating on dental implant to restore tooth function is a growing trend in modern Dentistry. In the present study, hydroxyapatite and the bioactive glass-coated implants were evaluated for their behavior in osseous tissue following implantation in 14 patients.
Materials and Methods:
Bioactive glass and hydroxyapatite formulated and prepared for coating on Ti-6Al-4V alloy. Hydroxyapatite coating was applied on the implant surface by air plasma spray technique and bioactive glass coating was applied by vitreous enameling technique. Their outcome was assessed after 6 months in vivo study in human.
Results:
Hydroxyapatite and bioactive glass coating materials were nontoxic and biocompatible. Uneventful healing was observed with both types of implants.
Conclusion:
The results showed bioactive glass is a good alternative coating material for dental implant.
Keywords: Bioactive glass, bioactive materials, biocompatible, hydroxyapatite, osseous tissue
INTRODUCTION
With the recent advancement in the field of biomaterials, a renewed interest has been focused on the research on dental implantology, more specifically in the area of implant-tissue interface. As bio-inert materials often become encapsulated in fibrous tissue, it is important to develop new biomaterials that will ensure extended lifetime of implant performances.[1] A bioactive material (i.e., Calcium phosphate ceramics, glass-ceramics) has a behavior to create a bond between the living tissue and the prosthesis as well as their chemical composition can be adjusted to obtain the desired bioactive properties.[2] Synthetic hydroxyapatite (HA) is a totally biocompatible, osteoconductive material,[3] and has a close structural and chemical resemblance to bone mineral, but not identical.[4] Thus, HA have a capacity to deceive the living bone tissues behaving as a natural bone.[5] Bioactive glass ceramics (BG) have osteoproductive and osteostimulatory properties[6] and when exposed to tissue fluid, promote proliferation and differentiation of osteoblast cells to form an extracellular matrix and mineralization.[7] It has been established that BG have good mechanical property and higher bioactivity in comparison to HA.[8,9] Consequently, the material has become a better choice to be used as a coating on metallic implants. The present study has been undertaken to develop a suitable bioactive glass composition for coating on single tooth dental implant, fabricated from titanium alloy (Ti6Al4V) and to evaluate the behavior of the BG-coated and HA-coated titanium dental implants in human jaw bone.
MATERIALS AND METHODS
The study was carried out in the Department of Periodontics, Dr. R. Ahmed Dental College and Hospital, Kolkata, in association with Bioceramic and Coating Division of Central Glass and Ceramic Research Institute, Kolkata, from January to December 2008. It was approved by Institutional Ethical Committee of Dr. R. Ahmed Dental College and Hospital, Kolkata.
Fabrication of Ti-6Al-4V alloy dental implant
Titanium alloy (Ti6Al4V) material (5 mm × 5 mm bar) was procured.¶ The internal-hex, cylindrical, threaded, two-staged dental implant system (4 mm diameter × 13 mm length) was designed and fabricated [Figure 1] in Central Glass and Ceramic Research Institute, Kolkata. The indigenous implant system consists of implant itself, healing/cover screw, abutment screw and metallic gingival former. The implant had crestal module height - 1.5 mm, pitch - 1 mm, and thread depth - 0.25 mm. The abutment screw (length - 7 mm) was 5° taper coronally ([Figure 1], inset).
Figure 1.

Implant body, (inset) healing cover screw and abutment screw
Preparation of hydroxyapatite
In this study, calcium nitrate tetrahydrate£ [Ca(NO3)2.4H2O], and di-ammonium hydrogen ortho-phosphate£ [DAP, (NH4)2HPO4] were used as raw materials. Glycine§ (C2H5NO2) was used as fuel. Aqueous stock solutions of calcium nitrate tetrahydrate (2.72 M) and DAP (2.09 M) were mixed in the ratio of 1.67, which are required for formation of hydroxyapatite. A few drops of concentrated nitric acid† were added to dissolve the resulting white precipitate to make a clear homogeneous solution. The detailed method of synthesis was reported elsewhere.[10] The as-synthesized products were typically voluminous, fluffy foam-like mass that occupied a large volume. The resulting soft-agglomerated powder was readily ground manually in an agate mortar/pestle into fine powder and was thoroughly characterized.
¶ Mishra Dhatu Nigam, Hyderabad, India.
£ S.D. Fine-Chem. Ltd., India.
§ Glaxo, Qualigens, India.
† Merck, India.
Preparation of bioactive glass
The suitable bioactive glass was prepared using a conventional glass melting technique. The appropriate amounts of reagents/raw materials silica (SiO2), calcium carbonate (CaCO3), dry soda ash (Na2CO3), decahydrated borax (Na2B4O7.10H 2O), TiO2, DAP were mixed together in water or acetone. The mixture was thoroughly dried at 120°C to remove the liquid completely, and then heated in air at temperature 1000°C for 1 h for carbonate decomposition and finally melted at 1400°C for 1 h in a platinum crucible. The melt was quenched in distilled water to obtain flaky glass particles, dried and stored for subsequent use; alternatively the melt was poured in a preheated steel mould and annealed at 500°C for 30 min. The frit was subsequently milled to fine powder in aqueous medium using alumina balls in a mill for 4-8 h. The batches used to prepare bioactive glass (BG) consist of SiO2=43-44 wt. %, Na2B4O7.10H2O=6-7 wt. %, Na2CO3=11-12 wt. %, CaCO3=29-30 wt. %, (NH4)2HPO4=8-9 wt. %, TiO2=1-2 wt. %] and the resultant frit was characterized by different in vitro techniques.[9]
Cytotoxicity test
In vitro cytotoxicity test was performed at Tissue Culture Laboratory, Sree Chitra Thirunal Institute for Medical Sciences and Technology, Tiruvananthapuram, India, for both coating materials.
Powder morphology and particle size
The particle morphology and the agglomerate structure of HA and BG were investigated using a scanning electron microscope (SEM) before coating on dental implant.
Fabrication of coating on Ti-6Al-4V implants
The surface to be coated was mechanically cleaned to the required roughness by sand blasting technique and ultrasonically cleaned to remove any chemical impurity using different cleaning agent (acetone). HA coating was applied on the implant surface by air plasma spray technique (i.e., argon gas as vehicle and heat source) using a table top micro-plasma spray machine # [Figure 2a and b]. BG coating was applied on the implant surface by conventional vitreous enameling technique at 800–820°C in ambient atmosphere [Figure 3a and b]. The thickness of the coating was within the range of 70–100 μm. Coating material was not applied on coronal 2 mm and apical 3 mm of the implant body [Figure 4].
Figure 2.
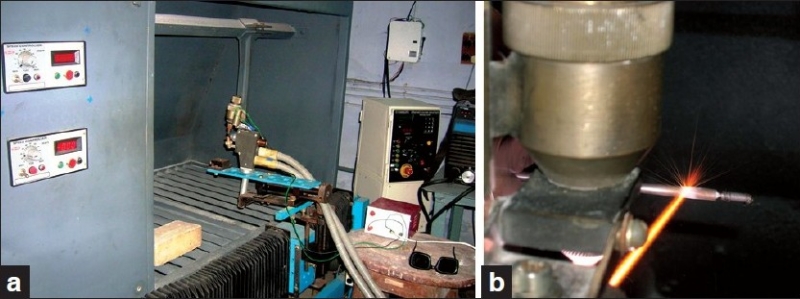
Micro-plasma spray technique for HA coating on implant (right) using a- top plasma machine (left)
Figure 3.
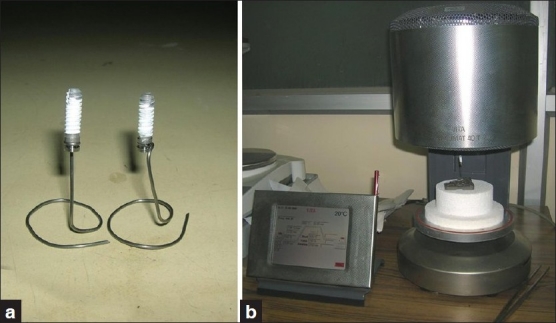
Conventional vitreous enameling technique for BG coating (left) on implant using Vita Vacumat dental furnace (right)
Figure 4.

HA coated (left) and BG coated titanium implant (right)
Packing and sterilization of coated Ti-6Al-4V dental implants
Each component of Ti-6Al-4V dental implants was ultrasonically cleaned to remove any chemical impurity using cleaning agent (acetone), dried and packed in an air tight plastic pouch. All the implant systems were finally sterilized by gamma radiation (GAMMA CHAMBER 5000).The human study was carried out in the Department of Periodontics, Dr. R. Ahmed Dental College and Hospital, Kolkata. Fourteen systemically healthy patients (10 males and 4 females), aged 20-54 years (mean age - 36 years) were selected for this study. A total of 28 implants (14 HA-coated Ti, 14 BG-coated Ti implants) have been placed in 14 patients. Twelve implants were placed in anterior maxilla and 16 implants were placed in anterior mandible. A written consent for this study was obtained from all the patients after explaining the pros and cons of the study and before initiation of any sort of treatment.
Patient evaluation
Patients enrolled in this study were partially edentulous and indicated for an implant supported fixed prosthesis (crown/bridge) in the anterior sextant of both maxilla and mandible. Patients’ extensive medical and dental history, clinical examination, oral hygiene habits, anatomic acceptability (i.e., alveolar ridge height and `width, vital structure), inclusion (i.e., both male and female with age limit of 18–60 years, motivated and willing to complete follow up, anterior edentulous areas that accommodate at least two implants of same diameter and length, minimum bone requirements were 6 mm of alveolar ridge width and 18 mm of ridge height, good general health, and oral hygiene habits, no history of previous implant failure or invasive perio-therapy for the past six months at the recipient site) and exclusion criteria (i.e., careless, non co-operative patients, pregnant or lactating women, patients having tobacco habit, history of radiation therapy, presence of systemic diseases, severe parafunctional habit) were thoroughly assessed before admitting a patient in the study.[11]
# Spraymet, Bangalore, India.
Operative procedures
Phase-I therapy was completed and oral hygiene instructions were given to the patients at least one month prior to surgery. Stage-1 surgery was performed for implant placement and exposure of head (Stage-2 surgery) after three months of Stage-1 surgery in aseptic conditions. After extraoral and intraoral disinfection and local anaesthesia application, crestal incision was given 1.5 mm short of gingival margin of the adjacent teeth that was modified at both the ends extending labially and lingually within the confines of attached gingiva. Full thickness flap was elevated both labially and lingually to expose the top of the alveolar ridge. A low speed, high torque (850–1250 rpm, 20–50 Ncm) drilling system (i.e., physio-dispenser) with copious normal saline irrigation were used to minimize excessive heat generation and trauma to the bone.[12] A surgical template or stent was placed on the occlusal table of same arch to direct accurate placement of implant and 2.5 mm diameter drill bit was used to penetrate at least 3–4 mm crestal bone for all the osteotomy sites. The stent was then removed and the facio-lingual and mesio-distal dimensions of the osteotomy sites were checked by inserting similar diameter guide pins. Keeping the guide pins inserted in the other sites, the same drill bit was used to establish the required depth (i.e., 13.5 mm) of osteotomy sites one by one to ensure accurate parallelism of two prepared sites. Holes were drilled 0.5 mm in addition to implant length to assure the level of implant at or just below the level of crestal bone as all two staged osseointegrated implant system confirm early post operative crestal bone loss as a result of surgery. According to implant size, same diameter drill bit (4 mm) was chosen as last widening drill for final osteotomy site preparation. All the drilling procedures were accomplished with steady hand, without wobbling to minimize funneling of the coronal portion of the osteotomy sites. No tap was used but countersinking was utilized in few cases. Implants were threaded into place without touching its surface, at the level of bone using hand wrench. Almost all the implants achieved primary stability at the time of placement except two. After placement, healing cover screw was threaded over the implants and tension free primary flap closure was obtained with a combination of horizontal mattress suture and interrupted sutures or continuous locking suture to ensure complete sealing of wound edges, preventing saliva contamination and to maintain a sterile environment around the implant.
Patients were given topical (0.2% chlorhexidine) and oral antimicrobials (500 mg amoxycillin thrice daily × 7 days), analgesic medications (400 mg ibuprofen thrice daily × 2 days with antacids) and routine post surgical instructions before release from clinic. Two weeks after Stage-1 surgery, the sutures were removed and the patients were instructed to gently brush the area with an ultra soft bristle toothbrush. Similar to Stage-1 surgery, routine post surgical instructions and medications were given to the patients after Stage-2 surgery. After two weeks of Stage-2 surgery, abutment screw was screwed into the implant body in place of gingival former and temporary prosthesis was fabricated. Patients were then recalled after one week to receive permanent restoration [Figure 5].
Figure 5.
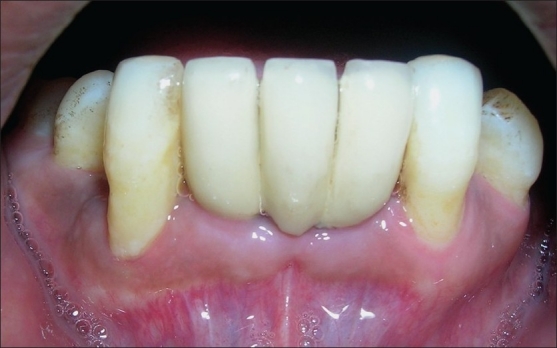
Intra-oral photograph after placement of permanent restoration
Implant-soft tissue interface was evaluated by plaque index (PI),[13] gingival index (GI),[14] probing pocket depth (PPD) distance from the gingival margin to the base of the probable crevice).[15] The clinical parameters were recorded with the help of a University of North Carolina (UNC-15) probeg for each implant site.
Implant-hard tissue interface was evaluated by:
Measurement of crestal bone loss in Intra-Oral Peri Apical (IOPA) X-ray: In determining actual bone loss, radiographic measurements of bone level were calibrated using divider and caliper with 1/10 mm gradation. The measurements from the top of the abutment to the point of bone-implant interface was calibrated using the known (i.e., L1=implant + abutment length) and radiographically measured distance from fixed reference point (i.e., top of the abutment) to the apical end of implant (L2). The actual average bone level of each implant measured multiplying the average bone level in radiograph (X’) by the ratio of the L1 to L2 [X (unknown)/X’ (known)=L1 (known)/L2 (known)]. Deduction of abutment length from actual average bone level(X) of baseline or X of subsequent follow-up represents the actual marginal bone loss of each implant at that visit because no marginal bone loss was considered at the time of implant placement (implant placed at the level of crestal bone). An implant was considered as failed if one or more of the following conditions were identified: Clinical mobility (following removal of prosthesis), unresolved chronic pain, implant loss, radiolucency around implant.[11]
γ Hu-Friedy Inc., Chicago
Statistical analysis
Statistical analysis was employed to compare crestal bone loss using a software program.§ To determine differences between the coated titanium implants, changes in crestal bone level were analyzed using ANOVA test. For each outcome measurement, at least 95% confidence limit was estimated. The present study was not a comparison between the two coating materials rather more concerned with their success and function in osseous tissue.
RESULTS AND OBSERVATIONS
Cytotoxicity test: Results showed no toxicity for both HA and bioactive glass using direct contact and extract method using L-929 mouse fibroblast cell [Figure 6].
Figure 6.
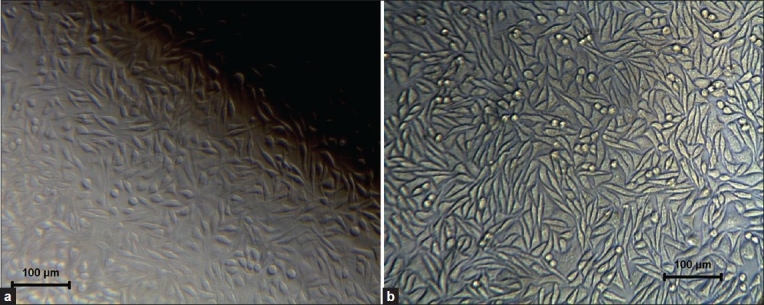
Cellular response after cytotoxicity test on HA (left) and BG (right) by direct contact method
Powder morphology and particle size: Powder used to fabricate the BG implants had the particle size in the range of 0.2-20 μm [Figure 7a]. The particle size of HA was roughly estimated to be of 0.1-10 from the SEM micrograph [Figure 7b].
Figure 7.
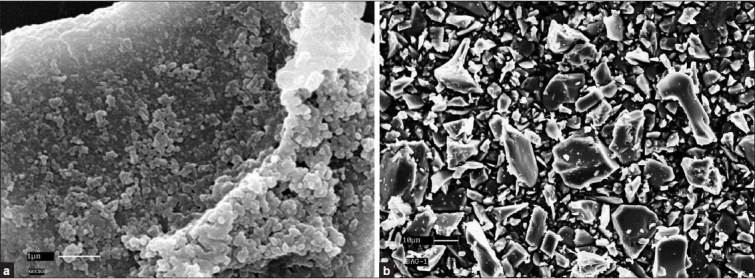
(a) SEM of HA powder and (b) BG powder
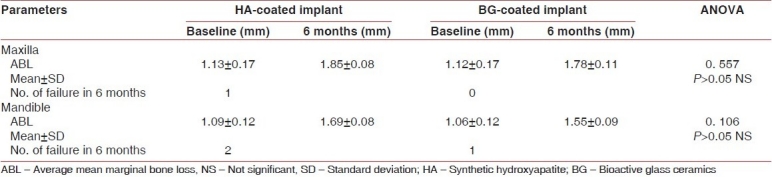
New indigenous implants were inserted in jaw bone without any difficulty. It was found clinically that healing was uneventful, in general. There was almost no sign of infection or untoward allergic and foreign-body type of reactions taking place postoperatively among the samples studied. In the six months post operative radiographic view both types of implant showed intimate bone apposition around them [Figure 8]. The range of plaque index, gingival index (GI) and mean probing pocket depth (PPD), scores were with in the range of 0-1, 0-1 and 2-3 mm respectively throughout the study period for both implant types in either arch. No analytical comparison was performed for PI, GI and PPD between the two coating materials because all scores were very near to normal limit. Mean average marginal bone loss with HA coated and BG coated implants upto six months of prosthetic loading in either arch were presented in [Table 1]. Evidence of suppuration, resorption of entire areas of HA coating and abnormal mobility with vertical pressure was observed with 1 HA-coated implant in lower jaw but no coating resorption and suppuration observed with any BG-coated implants after failure.
Figure 8.
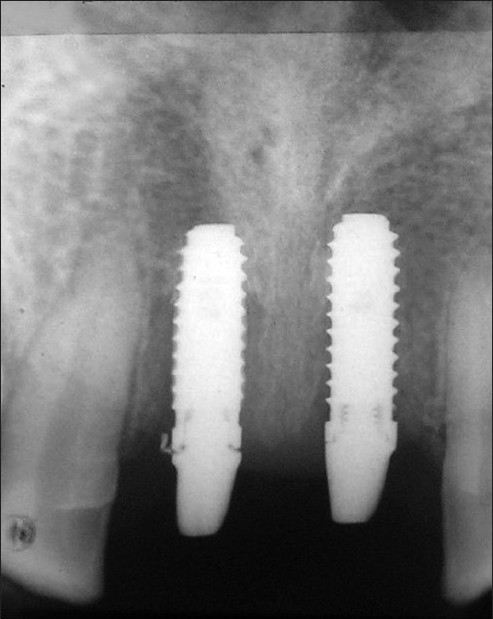
I.O.P.A X-ray of HA (left) and BG (right) coated implants 6 months after permanent prosthetic attachment in maxilla
Table 1.
Differences in mean marginal bone loss by ANOVA test and number of failures of HA coated and BG coated implant in maxilla and mandible after six months of prosthetic loading
§ SPSS version 11, SPSS Inc., Chicago
DISCUSSION
This study was a short term, prospective clinical study. The threaded implant design was chosen to provide increased surface area of coating in contact with bone tissue. The coating materials (HA and BG) were applied on titanium implant leaving 1–2 mm from crestal module to prevent early exposure of coating at oral environment during function because rough coating may invite more plaque accumulation. Apically 3 mm of area was also devoid of coating to facilitate cutting efficiency of the implants into bony tissue.
Plaque index (PI), mean gingival index (GI), and mean probing pocket depth (PPD) scores of the two coating materials were very near to normal limit throughout the study period in both the arches. This finding suggested that low level of plaque did not interfere with healing processes of any particular group. Low GI scores also indicate that all the coating materials were biocompatible, non-toxic, did not elicit any inflammatory and/or foreign body responses to the tissues and no apparent retardation of normal bone healing process around implants occurs, which is primary requisites for osseointegration.[16] Probing depth alone might have limited value to provide any comparable results because it might be changed with alteration in the position of gingival margin.[17] All the values of bone level changes observed in the present study were in the direction of bone loss for both coated types.[18]
Evidence of suppuration and entire resorption of HA coating indicated that local acidic environment was a reason for HA coating resorption in infected osteotomy sites, similar phenomenon was also observed by Jarcho.[5] The almost intact BG coating on failed implant demonstrated that BG produce an alkaline medium around the implant through dissolution of alkali ions, that might prevent coating resorption upon failure. No suppuration around the failed BG implant was observed, which suggested that antimicrobial property of BG might have some role in preventing infection.[19] Overall implant failure rate of HA coated implant was higher than that of BG coated implant [Table 1] might be due to lack of osteostimulatory property of HA or incapability to cope with surgical inaccuracies.
CONCLUSION
The BG and HA powder developed is suitable for coating on Ti-6Al-4V implants. The overall results showed bioactive glass coated implant is a good alternative coating material for dental implant at least under the present experimental condition.
ACKNOWLEDGMENTS
The authors wish to acknowledge the service of Dr. Prasanta Banerjee, Professor Department of Periodontics, Dr. R. Ahmed Dental College, Kolkata. The authors are grateful to Prof. I. Manna, Director, Central Glass and Ceramic Research Institute (CGCRI), Kolkata-32, India, for his kind permission to publish this work. Thanks are also due to Dr. Premananda Bharati and Dr. Suman Chakraborty, Indian Statistical Institute, Kolkata, for their kind help in statistical analysis of data.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hench LL. San Diego, CA: 1998. Bioactive materials. The potential for tissue regeneration. In 24th annual meeting of Society for Bio materials; pp. 22–6. [DOI] [PubMed] [Google Scholar]
- 2.Ravaglioli A, Krajewski A. London, New York, Tokyo, Melbourne: Chapman and Hall; 1992. Bioceramics: Materials, properties, applications. [Google Scholar]
- 3.Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Relate Res. 1981;157:259–78. [PubMed] [Google Scholar]
- 4.Mc Connell D. New York: Springer-Verlag; 1973. Apatite. [Google Scholar]
- 5.Jarcho M. Retrospective analysis of hydroxyapatite development for oral implant applications. Dent Clin North Am. 1992;36:19–26. [PubMed] [Google Scholar]
- 6.Hench LL. The story of Bioglass. J Mater Sci Mater Med. 2006;17:967–78. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 7.Xynox ID, Edgar AJ, Buttery LD, Hench LL, Polak JM. Ionic Products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem Biophys Res Comm. 2000;276:461–5. doi: 10.1006/bbrc.2000.3503. [DOI] [PubMed] [Google Scholar]
- 8.Mardare CC, Mardare AI, Fernandes JR, Pina SC, Joanni E, Fernandes JR, et al. Deposition of bioactive glass-ceramic thin-films by RF magnetron sputtering. J European Ceram Soc. 2003;23:1027–30. [Google Scholar]
- 9.Ghosh SK, Nandi SK, Kundu B, Datta S, De DK, Roy SK, et al. Interfacial response of hydroxyapatite and tri-calcium phosphate prepared by a novel aqueous combustion method: A comparison with bioglass in vivo implanted in goat. J Biomed Mater Res. 2008;86B:217–27. doi: 10.1002/jbm.b.31009. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh SK, Datta S, Basu D. Solution combustion synthesis of calcium hydroxyapatite nanoparticles. Trans Ind Ceram Soc. 2004;63:27–35. [Google Scholar]
- 11.Jeffcoat MK, McGlumphy EA, Reddy MS, Geurs NC, Proskin HM. A comparison of Hydroxyapatite (HA)-coated threaded, HA coated cylindric and Titanium threaded endosseous dental implants. Int J Oral Maxillofac Implants. 2003;18:406–10. [PubMed] [Google Scholar]
- 12.Eriksson RA, Adell R. Temperatures during drilling for the placement of implants using the osseointegration technique. J Oral Maxillofac Surg. 1986;44:4–7. doi: 10.1016/0278-2391(86)90006-6. [DOI] [PubMed] [Google Scholar]
- 13.Silness P, Loe H. Periodontal disease in pregnancy. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 14.Loe H, Silness J. Periodontal disease in pregnancy. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 15.Zlataric DK, Celebic A, Valentic-Peruzovic M. The effect of removable partial dentures on periodontal health of abutment and non-abutment teeth. J Periodontol. 2002;73:137–44. doi: 10.1902/jop.2002.73.2.137. [DOI] [PubMed] [Google Scholar]
- 16.Lamons J, Natiella J. Biomaterials, biocompatibility and periimplant consideration. Dent Clin North Am. 1986;30:3–23. [PubMed] [Google Scholar]
- 17.Klokkevold PR, Cochran DL. Clinical aspects and evaluation of the implant patients. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA, editors. Carranza's Clinical Periodontology. 10th ed. Louis: Saunders; 2006. pp. 1087–104. [Google Scholar]
- 18.Manz MC. Radiographic assessment of peri-implant vertical bone loss: DICRG interim report No. 9. J Oral Maxillofac Surg. 1997;55(12):62–71. doi: 10.1016/s0278-2391(16)31199-5. [DOI] [PubMed] [Google Scholar]
- 19.Allan I, Newman H, Wilson M. Antibacterial activity of particulate Bioglass® against supra- and subgingival bacteria. Biomaterials. 2001;22:1683–7. doi: 10.1016/s0142-9612(00)00330-6. [DOI] [PubMed] [Google Scholar]


