Abstract
Background and Objectives:
Conventional diagnostic indicators cannot distinguish between disease activity and inactivity but can detect the past tissue destruction. A proper and true periodontal diagnosis is essential in order to have a rational treatment and preventive strategy and to identify sites at risk. The present longitudinal study was designed with an aim to examine the relationship between gingival crevicular fluid (GCF) levels of aspartate aminotransferase (AST) and periodontal disease progression and to analyze the level of AST in GCF before and after the initial therapy in chronic periodontitis patients and determine the relationship between AST and conventional measures of periodontal status.
Materials and Methods:
A total of 20 patients with chronic periodontitis were randomly selected. Two diseased sites and one healthy site were selected in each patient. The periodontal status and GCF-AST levels were recorded at baseline and 3 months post-initial therapy and statistically analyzed.
Results:
There was a statistically significant difference in AST levels between diseased periodontal sites and healthy sites (P<0.05), and between baseline and post-initial therapy (P<0.05). Improvements in clinical status were noted following periodontal therapy and there was a corresponding decrease in AST levels.
Interpretation and Conclusion:
In conclusion, it is suggested that AST levels may be a useful adjunct in the clinical assessment of periodontal disease sites since AST level decreases when periodontal status improves.
Keywords: Aspartate aminotransferase, chronic periodontitis, disease progression, initial therapy, gingival crevicular fluid
INTRODUCTION
Periodontitis is an inflammatory disease characterized by the destruction of periodontal ligament and alveolar bone.[1,2] It is suggested that the nature of periodontitis has an episodicity, in which active and stable periods follow each other successively.[3–5] In the subjects with low frequency of disease active sites, it becomes challenging to diagnose and to assess the outcome of therapy in the absence of adequate diagnostic methods. Conventional diagnostic indicators such as bleeding on probing, probing pocket depth (PPD), attachment level and assessment of alveolar bone on radiographs cannot distinguish between disease activity and inactivity. These methods detect the past tissue destruction.[6,7]
Therefore, new diagnostic methods to detect disease-active or -inactive sites have been developed. A proper and true periodontal diagnosis is essential in order to have a rational treatment and preventive strategy and to identify sites at risk.
Host derived enzymes and their inhibitors, inflammatory and immune markers, tissue-breakdown products and enzymes of bacterial origin have been determined from gingival crevicular fluid (GCF) samples in numerous biomarker studies. This has led to a shift of focus toward GCF as a potential diagnostic fluid.
Aspartate aminotransferase (AST) previously termed as glutamic oxaloacetate transferase (GOT) has proven to be a strong diagnostic indicator of periodontal inflammatory lesions.[8,9] It is a soluble cytosolic enzyme which is confined to the cell cytoplasm but is released by dead or dying cells. Since cell death is an integral and essential component of periodontal tissue destruction. AST should be released during this process and should pass with the inflammatory exudate into GCF. GCF-AST levels help in determining the ongoing tissue destruction and hence aid in identifying sites undergoing active disease process. Significant AST levels have also been found in human gingival epithelial cells, gingival fibroblasts and periodontal ligament fibroblasts. AST level of 800 μIU has been suggested as the most suitable cut-off point to distinguish the sites at risk and sites unlikely to progress and AST level ≥1200 μIU has the best positive predictive power.
Persson et al.[10] demonstrated that AST levels can be used to assess the presence and extent of periodontal inflammation. Magnusson et al.[11] concluded that the outcome of the test is an effective objective measure distinguishing between diseased sites and non-diseased sites in patients and control subjects when evaluated both prior to and following application of therapy.
Shimada[9] suggested that AST levels may be a useful adjunct in the clinical assessment of periodontal disease sites, since AST level decreases when periodontal status improves. Mehta[12] found similar results as Shimada et al.[9]
Studies have indicated that with the improvement in the periodontal condition after therapy there is a significant reduction in the GCF-AST levels. Therefore, this study was planned with an aim to evaluate the potential of GCF-AST level as a diagnostic marker to assess periodontal disease activity and to determine its relationship after initial therapy.
MATERIALS AND METHODS
The present clinical study included 20 subjects between 30 and 60 years of age. They belonged to the group of patients referred to Department of Periodontics, K. M. Shah Dental College and Hospital, Baroda, for the treatment of chronic periodontitis. The study protocol was approved by the Research Cell of Sumandeep Vidyapeeth University, Piparia.
Inclusion criteria
Twenty patients were selected randomly, of age 30-60 years, exhibiting chronic periodontitis.
Each subject was required to have more than two diseased sites and at least one healthy site. The PPD of all teeth in each subject was measured at three sites per tooth: mesiobuccal, midbuccal and distobuccal. Only the site representing the deepest pocket of the tooth was used.
A diseased site was defined as
One with a minimum PPD ≥5 mm and ≤8 mm
Bleeding on probing
Bone loss as manifested by a distance of ≥3 mm from cementoenamel junction (CEJ) to the alveolar crest on bite-wing radiographs
A healthy site was defined as
PPD≤ 3 mm
No bleeding on probing
No bone loss
Exclusion criteria
Age less than 30 years
Use of antibiotics within the previous 3 months
Diseases known to affect oral tissue such as diabetes
Patients who were suffering from any liver, heart, kidney, muscle or joint diseases, which are known to affect AST levels
Periodontal therapy other than standard prophylaxis during the previous 6 months
An inadequate number of qualifying diseased and healthy sites
Smokers
A thorough medical and dental history was obtained, followed by a complete oral and clinical examination. All subjects were explained about the nature and duration of the study schedule and informed consents were obtained.
Clinical study design
At 0 day (at baseline)-Baseline clinical examination;
-recording of ancillary parameters;
-recording of soft tissue parameters;
-collection of GCF; -Phase I therapy (scaling and root planing)
At 1 month follow-up-Oral hygiene reinforcement; Follow-up done every 15 days-oral hygiene reinforcement
At 3 months follow-up-Post-initial therapy clinical examination;
-recording of ancillary parameters; -recording of soft tissue parameters; -collection of GCF from treated sites
-Biochemical estimation of AST in GCF was done at baseline (on 0 day) and post-initial therapy (on 90th day). Periodontal status was assessed by using PPD, clinical attachment level (CAL), bleeding on probing (Papillary Bleeding Index by Muhlemann, 1977) and gingival index (Loe and Silness, 1963) using UNC 15 probe. PPD and CAL at selected tooth sites were recorded with the help of pre-formed acrylic stents. A fixed point was marked on the stent and the CAL was measured as the distance from the fixed point to the base of the pocket.
The GCF was collected using micro-pipettes in this study. The collection was done separately from diseased and healthy sites. Prior to GCF collection, all the patients were instructed to gently massage the gingiva for about 10 minutes to mechanically stimulate an increase in flow rate of GCF. GCF was allowed to accumulate along the gingival crevice for about 10 minutes and was collected with the micro-pipettes. The collected fluid was transferred to collecting ampoules and subjected to analysis for AST content.
For the present study, dry biochemistry method was used to estimate the levels of AST in GCF. This method gives accurate results even with very minimal amount of the sample (~10 μl). The test is performed on VITROS DT 60 II System. A 10 μl sample of the specimen is deposited on the slide and evenly distributed by the Spreading Layer. The slide contains high concentration of pyridoxal-5-phosphate (P-5-P) which rapidly activates the apoenzyme, so that AST is fully active at the end of the Lag Phase without the need for a long pre-incubation. In this assay, the amino group of l-aspartate is transferred to α-ketoglutarate in the presence of P-5-P to produce glutamate and oxaloacetate. The oxaloacetate produced by the deamination of the l-aspartate is converted to malate by malate dehydrogenase (MDH) in the presence of NADH+ which in turn is oxidized to NAD+ . The rate of oxidation of NADH+ is monitored by Reflectance Spectrophotometry at a wavelength of 340 nm at 37°C. This rate of change is then used to calculate the enzyme activity.
All processes performed by the analyzer are controlled by a self-contained microcomputer which can be operated with a keyboard and the results can be read on its display screen and printed. The analysis is based on the following chemical reaction sequence:
AST
Aspartate + α-Ketoglutarate → Oxaloacetate + Glutamate
P-5-P
MDH
Oxaloacetate + NADH + H+ → Malate + NAD+
Statistical analysis
The information obtained was entered into Microsoft excel 2003 format. The data were analyzed using SPSS statistical package-16 [Statistical package for social science (SPSS) version16; SPSS Inc., Chicago, IL,USA].
One-way analysis of variance (ANOVA) test was conducted to observe whether there was a statistical significant difference between the clinical parameters and GCF-AST levels.
All analyses were made at 0.05 level of significance.
RESULTS
A statistically significant difference (P<0.0001) between mean PPD, CAL and AST levels at baseline and after 3 -months of initial therapy was obtained, suggestive of a significant decrease in mean PPD, CAL and AST levels after therapy as compared to that of baseline.
The sensitivity and specificity of AST test for the present study were 97% and 90%, respectively, with a positive predictive value of 95% and negative predictive value of 94%.
DISCUSSION
Detection of deteriorating areas helps to control periodontal diseases and provides a rationale for implementing treatment. At the present time, the only means of detecting deteriorating sites is longitudinal assessment of attachment level or of alveolar bone resorption on radiographs.
This lack of correlation between clinical measures of existing periodontal disease and current disease status or tendency for future disease progression has focused the attention toward the need for other means of assessing periodontal disease activity.
In a thorough evidence-based review, Cobb[13] calculated the mean probing depth reduction and gain of clinical attachment that can be achieved with root planing at sites that initially were 4 to 6 mm in depth and 7 mm or greater in depth. He reported mean pocket depth reductions of 1.29 mm and 2.16 mm, respectively, and mean gains of clinical attachment of 0.55 mm and 1.29 mm, respectively.
Similar results were found in the present study as shown in Tables 1 and 2.
Table 1.
Mean PPD at baseline and after 3 months of initial therapy
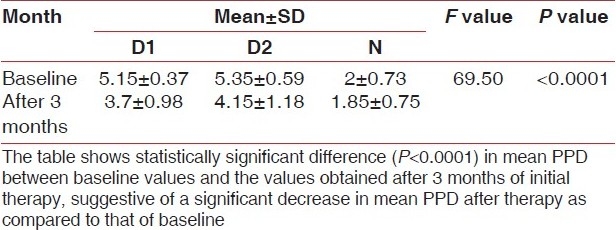
Table 2.
Mean CAL at baseline and after 3 months of initial therapy
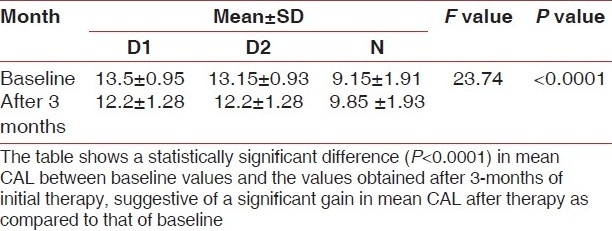
AST is one among the enzymes that are most commonly associated with tissue damage. AST is particularly important in the transport of reducing equivalents across the mitochondrial membrane via malate aspartate shuttle and is a sensitive indicator of necrosis in a number of tissues. The present study was based upon establishing the correlation between a biochemical cell death-marker, i.e. AST, in GCF and the status of periodontal disease activity.
The results in the present study demonstrate a degree of correlation existing between the GCF-AST and periodontal pocket depth wherein increased levels of AST were associated with increased pocket depth [Table 3]. After 3 months of therapy, with the reduction in pocket depth, the levels of AST decreased [Table 4]. The study by Chambers et al.[14] provides evidence that increased levels of AST in crevicular fluid may reflect active tissue destruction in the periodontium in ligature-induced periodontitis in the beagle dog.[14] A similar correlation has been reported between progressing periodontitis lesions and GCF-AST levels in humans.[12,14]
Table 3.
Correlation between mean PPD and AST levels after 3 months of initial therapy

Table 4.
Correlation between mean CAL and AST levels after 3 months of initial therapy

Attachment loss occurs when a periodontal pocket is active and the cells in contact with the pocket are destroyed by necrosis. Measurement of AST levels provides an indication of progression of periodontal destruction, and AST is therefore a potential marker for distinguishing between active and inactive disease sites.[9]
The AST activity in GCF has been shown to correlate with the severity of gingival inflammation and with active sites as detected by longitudinal assessment (3 months) of CAL.[10] In the present study there is a significant correlation between sites with high levels of AST and CAL as shown in Table 5. Also, with the gain in CAL the levels of AST decrease as shown in Table 6. The correlation between the Gingival Index and AST levels is significant suggesting that AST activity may be indicative of soft tissue changes occurring within the gingival tissues rather than deeper-seated changes in the periodontium as reported by Smith et al.[15]
Table 5.
Correlation between mean CAL and AST levels at baseline

Table 6.
Mean AST at baseline and after 3 months of initial therapy

In a longitudinal study by Shimada et al.[9] a statistically significant difference was reported between the reduction of probing depth at baseline and post-initial therapy. Similarly, in the present study, as shown in Table 3, there is statistically a highly significant correlation (P<0.0001) between mean PPD and the AST levels in GCF at baseline (0-month) suggesting that during the diseased state, as the mean PPD increases, the mean AST levels in GCF also increase. A highly significant correlation (P<0.0001) also exists between mean PPD and the AST levels in GCF even after 3-months of initial therapy as shown in Table 4 suggesting that even after initial therapy (i.e. in relatively healthy state), as the mean PPD decreases, the mean AST levels in GCF also decrease.
A statistically significant difference in AST levels in GCF between baseline and post-initial therapy was observed by Shimada et al.[9] Improvement in clinical status was noted following periodontal therapy and there was a corresponding decrease in AST levels. Similarly, in the present study, as shown in Table 7, a highly significant statistical difference (P<0.0001) exists between mean AST levels, after 3-months of initial therapy and at baseline, suggestive of a significant decrease in mean AST levels after therapy as compared to that of baseline.
Table 7.
Correlation between mean PPD and AST levels at baseline

Thus, levels of AST in GCF strongly correlate with periodontal disease activity. Overall, these findings suggest that the AST assay results correlate with the traditional clinical indices.
The presence of AST in GCF was first demonstrated by Chambers et al.,[15] who claimed that AST in GCF was 10-to 100-fold higher than in serum in active periodontal disease. AST activities of 800 units or higher appeared to be associated with active disease. The activity observed was site specific and was approximately 20 times greater than in the serum of normal periodontally healthy subjects and 5 times higher than the levels found in erythrocytes.[16] In spite of high degree of association between AST levels in GCF, inflammation, and attachment loss, a great deal of fluctuation between disease active and disease inactive sites was observed.[9] Fluctuation of values observed probably resulted from the known episodic nature of periodontal deterioration and inflammation, imprecision of the methods of measurement, and from the therapy rendered at each visit.
Assessment of GCF-AST levels harvested from periodontal pockets before and following successful therapy would permit a better estimate of validity of such measurements as a means of monitoring periodontal disease and periodontal health.
Paolantonio et al.[17] investigated the presence and activity levels of AST in peri-implant crevicular fluid (PCF) from diseased and healthy endosseous implants in order to assess if AST in PCF can be further studied as a possible objective diagnostic aid in oral implantology. PCF was collected by the insertion of a #40 standardized endodontic paper point to the base of the crevice or pocket for 30 seconds. The results suggested that PCF analysis may be further investigated in longitudinal studies as a suitable diagnostic strategy in the evaluation of dental implants.
Yucekal-Tuncer et al.,[18] performed a study to analyze the correlations between plaque index, gingival index, probing depth, CAL, AST, N-benzoyl-dl-arginine-2-naphthylamide (BANA) and sulfide ion activity (SIA) of diabetic patients with chronic periodontitis. Sixteen-subjects eight diabetic patients and eight systemically healthy patients were studied. The study indicated that the GCF metabolites had significant correlations with periodontally diseased sites in patients with chronic periodontitis, whether diabetic or systemically healthy and may help to confirm the clinical findings.
Perinetti et al.,[19] examined subgingival colonization of Actinobacillus actinomycetemcomitans (Aa) and alkaline phosphatase (ALP) and AST activities in GCF in order to assess whether these parameters have potential as biomarkers of tissue responses to orthodontic tooth movement in humans. Results suggest that A.a. subgingival colonization, and ALP and AST activities in GCF reflect the tissue responses that occur in the periodontium during orthodontic treatment.
Based on the recent studies, prominence has been given to AST activity in GCF as a diagnostic aid, and studies are still going on in order to know the extent to which AST levels can accurately distinguish between the disease-active and -inactive sites and to test whether the AST test might be used in a clinical setting.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Listgarten Pathogenesis of periodontitis. J Clin Periodontol. 1986;13:418–25. doi: 10.1111/j.1600-051x.1986.tb01485.x. [DOI] [PubMed] [Google Scholar]
- 2.Van Dyke TE, Lester MA, Shapira L. The role of the host response in periodontal disease progression: Implications for future treatment strategies. J Periodontol. 1993;64:792–806. doi: 10.1902/jop.1993.64.8s.792. [DOI] [PubMed] [Google Scholar]
- 3.Hirshfeld L, Wasserman A long-term survey of tooth loss in 600 treated periodontal patients. J Periodontol. 1978;49:225–37. doi: 10.1902/jop.1978.49.5.225. [DOI] [PubMed] [Google Scholar]
- 4.Goodson JM, Tanner AC, Haffajee AD, Sornberger GC, Socransky SS. Patterns of progression and regression of advanced destructive periodontal disease. J Clin Periodontol. 1982;9:472–81. doi: 10.1111/j.1600-051x.1982.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 5.Lindhe J, Haffajee AD, Socransky SS. Progression of periodontal disease in adult subjects in the absence of periodontal therapy. J Clin Periodontol. 1983;10:433–42. doi: 10.1111/j.1600-051x.1983.tb01292.x. [DOI] [PubMed] [Google Scholar]
- 6.Lindhe J, Okamoto H, Yoneyama T, Haffajee A, Socransky SS. Longitudinal changes in periodontal disease in untreated subjects. J Clin Periodontol. 1989;16:662–70. doi: 10.1111/j.1600-051x.1989.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 7.Lindhe J, Okamoto H, Yoneyama T, Haffajee A, Socransky SS. Periodontal loser sites in untreated adult periodontitis. J Clin Periodontol. 1989;16:671–8. doi: 10.1111/j.1600-051x.1989.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 8.Persson GR, Page RC. Diagnostic characteristics of crevicular fluid aspartate aminotransferase (AST) levels associated with periodontal disease activity. J Clin Periodontol. 1992;19:43–8. doi: 10.1111/j.1600-051x.1992.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 9.Shimada K, Mizuno T, Ohshio K, Kamaga M, Murai S, Ito K. Analysis of aspartate aminotransferase in gingival crevicular fluid assessed by using PocketWatch™ : A longitudinal study with initial therapy. J Clin Periodontol. 2000;27:819–23. doi: 10.1034/j.1600-051x.2000.027011819.x. [DOI] [PubMed] [Google Scholar]
- 10.Persson GR, DeRouen TA, Page RC. Relationship between aspartate aminotransferase in gingival crevicular fluid and gingival inflammation. J Periodontal Res. 1990;25:17–24. doi: 10.1111/j.1600-0765.1990.tb01203.x. [DOI] [PubMed] [Google Scholar]
- 11.Magnusson I, Persson RG, Page RC, DeRouen TA, Crawford JM, Cohen RL, et al. A multi-center clinical trial of a new chairside test in distinguishing between diseased and healthy periodontal sites. II. Association between site type and test outcome before and after therapy. J Periodontol. 1996;67:589–96. doi: 10.1902/jop.1996.67.6.589. [DOI] [PubMed] [Google Scholar]
- 12.Rajini, Mehta DS. Comparison of Aspartate Aminotransferase (AST) levels in Gingival Crevicular Fluid before and after periodontal phase I therapy, using PocketWatch™ (Periodontal tissue monitor system)- A rapid chairside test. J Indian Den Assoc. 2001;72:70–5. [Google Scholar]
- 13.Cobb CM. Non-surgical pocket therapy: mechanical. Ann Periodontol. 1996;1:443–90. doi: 10.1902/annals.1996.1.1.443. [DOI] [PubMed] [Google Scholar]
- 14.Chambers DA, Crawford JM, Mukherjee S, Cohen RL. Aspartate aminotransferase increases in crevicular fluid during experimental periodontitis in Beagle dogs. J Periodontol. 1984;55:526–30. doi: 10.1902/jop.1984.55.9.526. [DOI] [PubMed] [Google Scholar]
- 15.Chambers DA, Imrey PB, Cohen RL, Crawford JM, Alves ME, McSwiggin TA. A longitudinal study of aspartate aminotransferase in human gingival crevicular fluid. J Periodontal Res. 1990;26:65–74. doi: 10.1111/j.1600-0765.1991.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 16.Smith AJ, Alexander M, Mackenzie D, Lennon A, Riggio MP, MacFarlane TW. Microbial factors and gingival crevicular fluid aspartate aminotransferase levels.A cross sectional study. J Clin Periodontol. 1998;25:334–9. doi: 10.1111/j.1600-051x.1998.tb02450.x. [DOI] [PubMed] [Google Scholar]
- 17.Paolantonio M, Di Placido G, Tumini V, Di Stilio M, Contento A, Spoto G. Aspartate aminotransferase activity in crevicular fluid from dental implants. J Periodontol. 2000;71:1151–7. doi: 10.1902/jop.2000.71.7.1151. [DOI] [PubMed] [Google Scholar]
- 18.Yucekal-Tuncer B, Uygur C, Firatli E. Gingival crevicular fluid levels of aspartate aminotransferase, sulfide ions and N-benzoyl-DL-arginine-2-naphthylamide in diabetic patients with chronic periodontitis. J Clin Periodontol. 2003;30:1053–60. doi: 10.1046/j.0303-6979.2003.00426.x. [DOI] [PubMed] [Google Scholar]
- 19.Perinetti G, Paolantonio M, Serra E, D’Archivio D, D’Ercole S, Festa F, et al. Longitudinal monitoring of subgingival colonization by Actinobacillus actinomycetemcomitans, and crevicular alkaline phosphatase and aspartate aminotransferase activities around orthodontically treated teeth. J Clin Periodontol. 2004;31:60–7. doi: 10.1111/j.0303-6979.2004.00450.x. [DOI] [PubMed] [Google Scholar]


