Abstract
Background:
Various mouth rinses have been used in the treatment of halitosis, but most of the evidence for the efficacy of mouth rinses is anecdotal. In the present study, 0.2% chlorhexidine rinse and an essential oil mouth rinse are compared for their efficacy in reducing the breath mercaptan levels.
Materials and Methods:
Fifteen patients with the chief complaint of oral malodor were randomly divided into 3 groups and were provided with the respective mouth rinses. Pre rinsing measurements were performed on the Day 1 and no other periodontal treatment was instituted. Post rinsing estimation of mercaptan levels was performed after 7 days.
Results:
When comparing chlorhexidine with the essential oil mouth rinse, the reduction in VSCs was highly significant in the chlorhexidine group (P<0.01). However, the reduction in the organoleptic scores was not significant among the two groups. Organoleptic scores showed very highly significant correlations with the VSC concentrations measured by the spectrophotometric method.
Conclusion:
The spectrophotometric technique employed in this study appears to be a promising new method for evaluation of oral malodor. Chlorhexidine still appears to be the agent of choice as a short term regimen in cases of oral malodor.
Keywords: Chlorhexidine, halitosis, organoleptic, spectrophotometry, volatile sulfur compounds (VSCs)
INTRODUCTION
Oral malodor, a common complaint, though having been recognized since ancient times, has only recently come to the fore, as it can be a significant social handicap in this increasingly sophisticated world. Bad breath has been recorded in the literature for thousands of years. The problem is discussed at length in the Jewish Talmud, as well as by the Greek and Roman writers. The prophet Mohammed is said to have thrown a congregant from the mosque for having the smell of garlic in his breath. Modern literature on bad breath dates back to a monograph published by Howe in the 19th century.
Though the true prevalence is unclear, since objective assessment is difficult, a study in Japan by Miyazaki et al. found that up to 25% of the population have volatile sulfur compounds (VSCs) in the breath in amounts higher than what was regarded as socially acceptable limit.[1] Another study by Loesche et al. in the USA, involving persons of 60 years of age and older, found 24% to have been told that they had oral malodor.[2] A similar study analyzing objective findings on halitosis from China reported a prevalence of 20-34%.[3]
There is some disagreement in the literature on the relative importance of oral contribution on the incidence of halitosis. Prinz suggested that more than 90% of all cases of objectionable breath originate from sources within the oral cavity. Rosenberg[4] and Tonzetich,[5] in separate studies, have reported that in as many as 85% of patients with bad breath, the odor originates in the mouth. In contrast, other investigators deemed oral malodor of importance in a smaller percentage of halitosis cases.
Oral malodor is caused by the presence of VSCs, especially methyl mercaptans (CH3SH) and hydrogen sulfide (H2S), and also short-chain fatty acids such as butyric, propionic and valeric acid, and polyamines, such as putrescine and cadaverine, in the exhaled air.[6–8]
These compounds result from the proteolytic degradation by oral bacteria of sulfur containing peptides and amino acids in saliva, gingival crevicular fluid, blood and desquamated epithelial cells.[9–10]
It appears from in vitro research that the gram-negative anaerobic microflora is responsible for odor formation. Fusobacterium nucleatum, Treponema denticola, Prevotella intermedia, Porphyromonas gingivalis, Bacteroides forsythus, Eubacterium and other sub-gingival species can produce large amounts of CH3SH and H2S from methionine, cysteine or serum proteins.[1,11–13]
The most simple and commonly used approach to sample and measure oral malodor is direct nasal sniffing of expelled air, also referred to as organoleptic or hedonic assessment. Recently, Kim's method of organoleptic testing using a gastight syringe and a paper cup connected to a plastic straw has been described.[14] Instrumental methods of odor analysis include gas chromatography and the use of an industrial sulfide monitor. Quantitative analysis of VSCs by a gas chromatography equipped with a flame photometric detector is considered one of the most reliable measurements for diagnosing halitosis.[15] A newly developed portable gas chromatograph (OralChroma™, Abimedical, Abilit Corp., Osaka, Japan) has now been described, which does not use a special carrier gas (uses air instead) and is highly sensitive, yet relatively of low cost, compared with a standard gas chromatograph.[16] Portable VSC detectors, such as a sulfide monitor, are widely used for the quantitative measurement of oral malodor. They have sufficient sensitivity to detect H2S, but also detect other volatiles existing in human oral air, even though they are not malodorous.[17] Cry-osmoscopy, semi-conductor gas sensors[18] and ion trap transportable monitors are some other methods to objectively measure halitosis.
Recently, the electric nose technique has been introduced, but it cannot determine volatile chemical precisely, and it is difficult to distinguish mouth air compounds from others present using this equipment. The mouth air sample is contaminated with a certain amount of respiratory air by this sampling procedure.[19] A compact and simple gas chromatograph (GC) equipped with a newly invented indium oxide (In2O3) semiconductor gas sensor has also been developed.[20]
Whole saliva has served as a model of oral malodor generation for more than 100 years. One of the main constituents of whole saliva essential for malodor generation is its mixed bacteria. The second main constituent is the mix of saliva from various salivary glands, which, together with the shed bacteria and epithelial cells, is generally referred to as whole saliva. Depending on the degree of gingival inflammation, fluids from gingival crevices and periodontal pockets may also be present. Incubation of whole saliva at body temperature for various periods of time results in the generation of a malodor similar to that arising and emitted from the human mouth as breath.
Treatment strategies to control oral malodor are primarily directed at reduction of total bacterial counts in the oral cavity. Mouth rinsing is a common oral hygiene practice dating back to ancient times. Although in recent years, the plaque and gingivitis reducing properties of various mouth rinses have been emphasized, one of the major concerns which lead to frequent usage of mouth rinses in halitosis. Much of the evidence for the efficacy of mouth rinse in reducing bad breath is anecdotal and there are very few publications in the scientific literature on this subject. Few scientific investigators have addressed the ability of mouth rinses to reduce oral malodor for periods longer than 3 hours.
In the present study, a new technique has been attempted to assess the reduction in mercaptan levels produced by the use of 0.2% chlorhexidine rinse and an essential oil mouth rinse, when compared to a placebo rinse.
MATERIALS AND METHODS
In the present study, the comparison of the effect of an antiseptic mouthwash containing 0.2% chlorhexidine gluconate in reducing oral malodor with an essential oil mouth rinse and a placebo rinse has been done. The essential mouth rinse used was a commercial agent containing a combination of phenol related essential oils, thymol and eucalyptol, mixed with menthol and methylsalicylate.
Fifteen patients (nine males and six females) with the chief complaint of oral malodor were randomly divided into three groups and were provided with one of the three rinses:
Group A: Chlorhexidine mouth rinse‡
Group B: Essential oil mouth rinse§
Group C: Placebo
‡ 0.2% hexidine mouthwash, icpa health products
§ listerine mouthrinse, pfizer
No subjects had a history of periodontal treatment or treatment with antibiotics for the previous 3 months. Smokers were also excluded from the study.
Prerinsing measurements were performed on Day 1 and no other periodontal treatment was instituted.
Organoleptic estimations were carried out by a single judge. The subjects were instructed to exhale briefly through the mouth at a distance of approximately 10 cm from the nose of the judge and the results were rated on a scale of 0-5.[21]
Five milliliters of the subjects’ unstimulated saliva was collected in a glass impinger which was then sealed and incubated at 37°C for 24 hours.
All mouth rinses were provided in standard brown glass bottles. The subjects rinsed for 30 second periods, twice daily, for 1 week. No other changes, regarding oral hygiene, which may otherwise have an influence on oral flora, were instructed.
A new method was used to measure the VSCs from the headspace of suspended saliva samples. In this method, mercaptans and other sulfur containing compounds were collected by aspirating a measured volume of air through an aqueous solution of mercuric acetate-acetic acid. The collected mercaptans were subsequently determined by spectrophotometric measurement of the red complex produced by the reaction between mercaptans and a strongly acid solution of N,N-dimethyl-p-phenylenediamine and ferric chloride. The measurements were made using a UV spectrophotometer at 550 nm against the mercaptan free reference blank. The minimal detectable amount of methyl mercaptan by this technique is 0.04 μg/ml in a final solution of 25 ml. This technique is used for the estimation of mercaptans in the atmosphere.[22]
After the initial examination on the first visit, the subjects were instructed to use the assigned mouthwash for a period of 1 week. The patients were instructed not to rinse with the mouthwash on the morning of the final examination.
Statistical analysis
Statistical analysis was performed using the SPSS statistics 17.0 software. Correlation between the results of the organoleptic test and the spectrophotometric values was evaluated using the Spearman correlation coefficient.
Pre-rinsing and post-rinsing scores in each group were evaluated by the Student's t-test.
RESULTS
Comparison of the three groups based on the organoleptic scores and volatile sulfide measurements is shown in Tables 1–3.
Table 1.
Chlorhexidine mouth rinse group
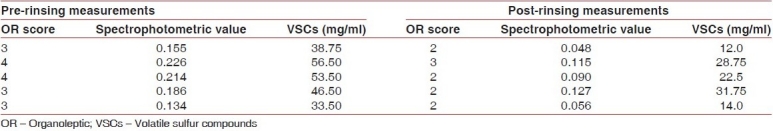
Table 3.
Placebo mouth rinse group
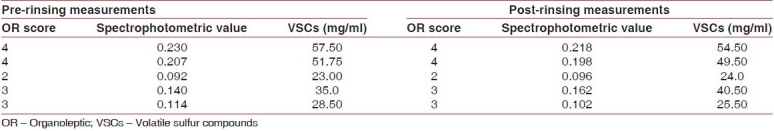
Table 2.
Essential oil mouth rinse group
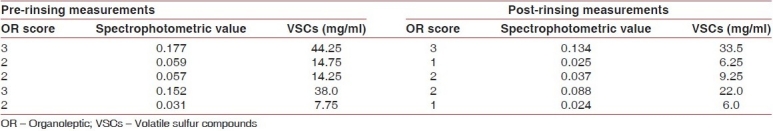
When compared to the placebo group, the chlorhexidine group showed highly significant reductions in VSCs (P<0.01) as shown in Table 4.
Table 4.
Volatile sulfide measurements
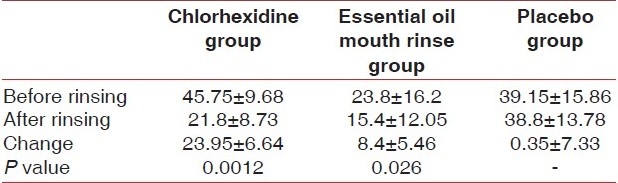
The reduction in VSCs in the essential oil mouth rinse group in comparison to the placebo was statistically significant (P<0.05) [Table 4].
The decrease in organoleptic scores was not significant in the essential oil mouth rinse group and was very significant in the chlorhexidine group (P<0.01) [Table 5].
Table 5.
Organoleptic scores
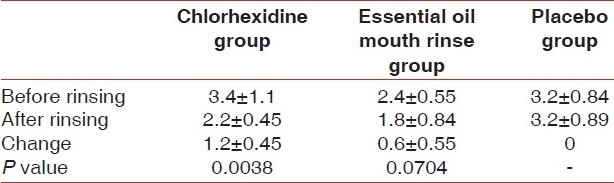
When comparing chlorhexidine with the essential oil mouth rinse, the reduction in VSCs was found to be statistically highly significant in the chlorhexidine group (P<0.01) [Table 4].
However, the reduction in the organoleptic scores was not significant among the two groups.
The Spearman correlation coefficients are shown in Table 6.
Table 6.
Correlations
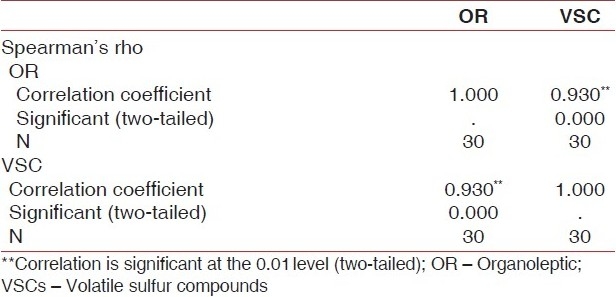
Organoleptic scores showed very highly significant correlations with the VSC concentrations measured by the spectrophotometric method for the 15 subjects.
DISCUSSION
In the present study, an attempt has been made to assess the reduction in malodor associated variables following rinsing with two active mouthwashes as opposed to a placebo rinse.
Fifteen volunteers, randomly divided into three groups, rinsed twice daily with one of the mouthwashes for a week. Measurements carried out 12-14 hours after the last rinse were compared with the baseline measurements. Measurements were carried out in a double-blind fashion using a organoleptic and a spectrophotometric technique.
A very highly significant correlation was demonstrated between the organoleptic scores and the spectrophotometric values for all the subjects (P<0.001). This technique appears to be of immense clinical importance and should be evaluated further for clinical applications.
Chlorhexidine reduced the organoleptic scores significantly, but this was not true for the essential oil mouth rinse. The results are in agreement with several studies in which 0.2% chlorhexidine mouth rinse produced significant reductions in volatile sulfur-containing compound levels and in organoleptic scores.[21,23,24]
Our results showed that the reduction in mercaptan values obtained with both the essential oil mouth rinse and chlorhexidine was statistically significant. Chlorhexidine when compared to the essential oil mouth rinse was significantly more efficacious in reducing the mercaptan levels (P<0.01). This is in agreement with the results of Carvalho et al. who showed that 0.2% chlorhexidine mouth rinse is more effective than an essential oil mouth rinse.[23] On the other hand, Rosenberg and coworkers have shown 0.2% chlorhexidine mouth rinse to be equally efficacious to a cetylpyridinium chloride (CPC)/essential oil mouth rinse.[21] The difference in the methodology applied and the use of a combination of CPC and essential oil mouth rinse in their study might explain the differences in the results.
In our study, the use of 0.2% chlorhexidine reduced the organoleptic scores by around 35% and the VSC measurements by around 52%. These results are similar to those reported by Pitts and coworkers which showed that mouthwashes containing chlorhexidine (0.2%) decrease the peak VSCs by 43% and the organoleptic score by 50%.[25]
Similarly, Rosenberg et al. reported 50% reduction in VSCs at 8-10 hours with chlorhexidine compared with placebo.[26]
Chemical reduction of micro-organisms by antimicrobial ingredients in oral healthcare products is only temporarily effective. Good short-term results were reported with chlorhexidine. Essential oils and CPC are only effective for short time periods of up to 2 or 3 hours.[27]
Listerine, which is an alcohol-based antiseptic mouth rinse containing essential oils, kills the odorogenic micro-organisms, and hence reduces the oral odor.[28]
While chlorhexidine appears to be clinically effective, it is not an agent that should be used routinely or for long periods of time. In spite of its undesirable effects, a short-term chlorhexidine rinsing regimen may be of potential diagnostic benefit in helping to determine whether the presenting malodor is of an oral etiology.
The essential oil mouth rinses are considered safer and are devoid of the long-term side effects of mouth rinses containing chlorhexidine.[29] However, some studies have reported on the erosive effects of long-term essential oil use on enamel.[30]
The essential oil mouth rinse, though effective in reducing oral malodor, does not have the efficacy of chlorhexidine.
CONCLUSION
The spectrophotometric technique employed in this study appears to be a promising new method for evaluation of oral malodor. Further investigations using larger sample sizes will help in establishing this technique as a method for halitosis evaluation.
Chlorhexidine still appears to be the agent of choice as a short-term regimen in cases of oral malodor.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Miyazaki H, Sakao S, Katoh Y, Takehara T. Correlation between volatile sulfur compounds and certain oral health measurements in general population. J Periodontol. 1995;66:679–84. doi: 10.1902/jop.1995.66.8.679. [DOI] [PubMed] [Google Scholar]
- 2.Loesche WJ, Grossman N, Dominguez L, Schork MA. Oral malodor in the elderly. In: Van St D, Rosenberg M, editors. Bad Breath. A multidisciplinary approach. Leuven Belgium: Leuven University Press; 1996. pp. 181–94. [Google Scholar]
- 3.Liu XN, Shinada K, Chen XC, Zhang GX, Yaegaki K, Kawaguchi Y. Oral malodor-related parameters in the Chinese general population. J Clin Periodontol. 2006;33:31–6. doi: 10.1111/j.1600-051X.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg M. Clinical assessment of bad breath: Current concepts. J Am Dent Assoc. 1996;127:475. doi: 10.14219/jada.archive.1996.0239. [DOI] [PubMed] [Google Scholar]
- 5.Tonzetich J. Production and origin of oral malodour: A review of mechanisms and methods of analysis. J Periodontol. 1977;48:1320. doi: 10.1902/jop.1977.48.1.13. [DOI] [PubMed] [Google Scholar]
- 6.Kleinberg I, Westbay G. Salivary and metabolic factors involved in oral malodor formation. J Periodontol. 1992;63:768–75. doi: 10.1902/jop.1992.63.9.768. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg S, Rosenberg M. Production of oral malodor in an in vitro system. In: Van St D, Rosenberg M, editors. Bad breath: A multidisciplinary approach. Leuven: Leuven University Press; 1996. pp. 143–50. [Google Scholar]
- 8.Tonzetich J, Kestenbaum RC. Odor production by human salivary fractions and plaque. Arch Oral Biol. 1969;14:815–27. doi: 10.1016/0003-9969(69)90172-1. [DOI] [PubMed] [Google Scholar]
- 9.Kleinberg I, Codipilly M. The biological basis of oral malodor formation. In: Rosenberg M, editor. Bad breath: research perspectives. Ramat Aviv: Ramot Publishing-Tel Aviv University; 1997. pp. 13–39. [Google Scholar]
- 10.Perrson S, Claesson R, Carlsson J. The capacity of subgingival species to produce volatile sulfur compounds in human serum. Oral Microbiol Immunol. 1989;4:169–72. doi: 10.1111/j.1399-302x.1989.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 11.Socransky SS, Haffajee AD, CuginiI MA, Smith C, Kent RL., JR Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 12.Loesche WJ, Lopatin DE, Stoll J, Van PN, Hujoel PP. Comparison of various detection methods for periodontopathic bacteria: Can culture be considered the primary reference standard? J Clin Microbiol. 1992;30:418–26. doi: 10.1128/jcm.30.2.418-426.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonzetich J, McBride BC. Characterization of volatile sulphur production by pathogenic and non-pathogenic strains of oral bacteroides. Arch Oral Biol. 1981;26:963–9. doi: 10.1016/0003-9969(81)90104-7. [DOI] [PubMed] [Google Scholar]
- 14.Kim DJ, Lee JY, Kho HS, Chung JW, Park HK, Kim YK. A new organoleptic testing method for evaluating halitosis.J. Periodontol. 2009;80:93–7. doi: 10.1902/jop.2009.080389. [DOI] [PubMed] [Google Scholar]
- 15.Murata T, Rahardjo A, Fujiyama Y, Yamaga T, Hanada M, Yaegaki K, et al. Development of a compact and simple gas chromatography for oral malodor measurement. J Periodontol. 2006;77:1142–7. doi: 10.1902/jop.2006.050388. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M, Anguri H, Nishida N, Ojima M, Nagata H, Shizukuishi S. Reliability of clinical parameters for predicting the outcome of oral malodor treatment. J Dent Res. 2003;82:518–22. doi: 10.1177/154405910308200706. [DOI] [PubMed] [Google Scholar]
- 17.Furne J, Majerus G, Lenton P, Springfield J, Levitt DG, Levitt MD. Comparison of volatile sulfur compound concentrations measured with a sulfide detector vs. gas chromatography. J Dent Res. 2002;81:140–3. [PubMed] [Google Scholar]
- 18.Shimura M, Yasuno Y, Iwakura M, Shimada Y, Sakai S, Suzuki K, et al. A new monitor with a Zinc Oxide thin film semiconductor sensor for the measurement of volatile sulfur compounds in mouth air. J Periodontol. 1996;67:396–402. doi: 10.1902/jop.1996.67.4.396. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka M, Anguri H, Nonaka A, Kataoka K, Nagata H, Kita J, et al. Clinical assessment of oral malodor by the electronic nose system. J Dent Res. 2004;83:317–21. doi: 10.1177/154405910408300409. [DOI] [PubMed] [Google Scholar]
- 20.Hanada M, Koda H, Onaga K, Tanaka K, Okabayashi T, Itoh T, et al. Portable oral malodor analyzer using highly sensitive In2O3 gas sensor combined with a simple gas chromatography system. Analytica Chimica Acta. 2003;47:27–35. [Google Scholar]
- 21.Rosenberg M, Kulkarni GV, Bosy A, McCulloch CA. Reproducibility and sensitivity of oral malodour measurements with a portable sulphide monitor. J Dent Res. 1991;70:1436–40. doi: 10.1177/00220345910700110801. [DOI] [PubMed] [Google Scholar]
- 22.Moore H, Helwig HL, Graul RJ. A spectrophotometric method for determination of mercaptans in air. Am Ind Hyg Assoc J. 1960;21:466–70. doi: 10.1080/00028896009344106. [DOI] [PubMed] [Google Scholar]
- 23.Carvalho MD, Tabchoury CM, Cury JA, Toledo S, Nogueira-Filho GR. Impact of mouthrinses on morning bad breath in healthy subjects. J Clin Periodontol. 2004;31:85–90. doi: 10.1111/j.0303-6979.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 24.van Steenberghe D, Avontroodt P, Peeters W, Pauwels M, Coucke W, Lijnen A, et al. Effect of different mouthrinses on morning breath. J Periodontol. 2001;72:1183–91. doi: 10.1902/jop.2000.72.9.1183. [DOI] [PubMed] [Google Scholar]
- 25.Pitts G, Brogdon C, Hu L, Masurat T, Pianotti R, Schumann P. Mechanism of action of an antiseptic, anti-odor mouthwash. J Dent Res. 1983;62:738–42. doi: 10.1177/00220345830620061001. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg M, Gelernter I, Barki M, Bar-Ness R. Day-long reduction of oral malodor by a two-phase oil: Water mouthrinse as compared to chlorhexidine and placebo rinses. J Periodontol. 1992;63:39–43. doi: 10.1902/jop.1992.63.1.39. [DOI] [PubMed] [Google Scholar]
- 27.van den Broek AM, Feenstra L, de Baat C. A review of the current literature on management of halitosis. Oral Dis. 2008;14:30–9. doi: 10.1111/j.1601-0825.2006.01350.x. [DOI] [PubMed] [Google Scholar]
- 28.Silwood CJ, Grootveld MC, Lynch E. A multifactorial investigation of the ability of oral health care products (OHCPs) to alleviate oral malodour. J Clin Periodontol. 2001;28:634–41. doi: 10.1034/j.1600-051x.2001.028007634.x. [DOI] [PubMed] [Google Scholar]
- 29.Stoeken JE, Paraskevas S, van der Weijden GA. The long-term effect of a mouthrinse containing essential oils on dental plaque and gingivitis: A systematic review. J Periodontol. 2007;78:1218–8. doi: 10.1902/jop.2007.060269. [DOI] [PubMed] [Google Scholar]
- 30.Pontefract H, Hughes J, Kemp K, Yates R, Newcombe RG, Addy M. The erosive effects of some mouthrinses on enamel.A study in situ. J Clin Periodontol. 2001;28:319–24. doi: 10.1034/j.1600-051x.2001.028004319.x. [DOI] [PubMed] [Google Scholar]


