Abstract
Background:
The preservation or reduction of alveolar ridge resorption following tooth extraction is important in patients especially for those intended for implants at a later stage. One way to achieve this is by using membranes, graft materials, and biodegradable space fillers to prevent alveolar bone resorption and promote regeneration. A major attraction for using biodegradable and biocompatible polymers as space fillers for ridge preservation is their safety profile in comparison to xenograft materials like lyophilized bone and collagen.
Materials and Methods:
Biocompatible polylactide space fillers were fabricated by fusing porous polylactide particles. The sponges were loaded with drugs by placing them in the respective solutions. Pseudomonas aeruginosa was isolated from a chronic periodontitis patient and in vitro anti-microbial evaluation was done with the drug loaded sponges.
Results:
Chlorhexidine loaded space filler showed significant anti microbial effect against multiple drug resistant Pseudomonas aeruginosa isolated from a patient with chronic periodontitis.
Conclusion:
The results of this study indicate that biodegradable drug releasing polylactide space fillers has the potential to be used for ridge preservation following tooth extraction. Release of drugs in the socket may prove useful in preventing development of alveolar osteitis post extraction which can interfere with normal healing of the socket. Synthetic biodegradable polymers also exhibit a controlled degradation rate to achieve complete resorption within the intended time.
Keywords: Alveolar osteitis, chlorhexidine, ridge preservation, polylactide
INTRODUCTION
Alveolar ridge resorption following tooth extraction is a frequently observed phenomenon that may decrease the possibility of placing dental implants or impair its retention. Outcome of implant therapy is no longer measured by implant survival alone but by long term aesthetic and functional success.[1] Therefore, it is essential to maintain the original dimension of the alveolar process for successful long term outcome of the treatment.
Various ridge preservation techniques have been proposed and used for preserving the alveolar process. An usual technique is to use graft materials to fill the extraction socket and use membranes to cover up the extraction site. Freeze dried bone allografts and xenografts are commonly used for this purpose but there are certain associated disadvantages. These graft materials show variable degradation rates and presence of these materials in the socket even after six months have been reported.[2] Another concern is the danger of transmissible infections like Bovine spongiform encephalopathy (BSE) from graft materials of animal origin. A suitable alternative is the use of synthetic biodegradable and biocompatible polymers. Polylactic acid is one such polymer with a long record of safe human use for a wide range of applications.[3] Studies using polylactide scaffolds have shown that they can decrease bone resorption during the healing phase in the sockets following tooth extraction.[2] Incorporation of chlorhexidine into sponges may offer an added advantage of preventing the development of alveolar osteitis following extraction which may interfere with normal healing of the bony socket. The aims of the present study were:
Design and fabrication of polylactide space fillers suitable for ridge preservation
Evaluation of the surface suitability of polylactide filler for cell attachment and growth
Evaluation of the anti microbial efficacy of chlorhexidine loaded polylactide space fillers.
MATERIALS AND METHODS
Materials
Clinical grade Poly-DL-Lactic acid (PDLLA) was purchased from Durect Corporation Pelham, USA. The surfactants, cetyltrimethyl ammonium bromide (CTAB), and polyvinylalcohol (PVA) were procured from Amresco Chemicals, USA. All the culture media employed in the present study were procured from Hi Media, Mumbai, India.
Design and fabrication of polylactide space fillers suitable for ridge preservation
Polylactide space fillers were fabricated in a two step process. Initially, polylactide porous particles were formulated and they were later fused to form scaffolds in appropriate moulds suitable to be used for ridge preservation. Polylactide particles were prepared by double emulsion solvent evaporation method.[4] Particle size distribution was measured using ‘Malvern mastersizer 2000’ particle size analyzer (Malvern, UK). Fabrication of polylactide sponges were carried out by part solubilization of the particles in presence of ethanol to fuse them into stable polylactide sponges. The porous polylactide particles were filled in a suitable plastic mould (plastic eppendorf tube) and then wetted with ethanol for 10 minutes to effect part solubilization and fusion of particles into stable three dimensional sponges. The polylactide sponge was removed from the mould and repeatedly washed with sterile water to remove any residual ethanol.
Cytocompatibility and drug loading of the polylactide space fillers
The surface of the polylactide sponges composed of the fused particles were visualized by scanning electron microscope (SEM)-model EVO 50 (Zeiss, Germany). The cytocompatibility of the polylactide sponges was assessed using cell lines (B-16 melanoma cells). Polylactide sponges were transferred to six well culture plates and incubated with RPMI complete media containing high concentration of B-16 cells for 50 minutes for initial cell attachment to the polylactide sponges to take place. The scaffolds were then cultured in two ml of RPMI complete medium in a CO2 incubator and culture medium was changed on alternate days. Optical microscope (Magnus) was used to observe the growth of cells on the polylactide sponges over days.
Drug loading of the polylactide sponges were carried out by simple diffusion of the drug molecules into the porous polylactide particles of the sponge. The polylactide sponges were kept in the drug solutions of chlorhexidine and other selected drug solutions for 20-30 minutes for the particles to be saturated with the drug molecules. Afterwards, they were removed from the drug solutions and air dried and stored till needed for the anti microbial tests.
Evaluation of antibacterial activity of the drug loaded polylactide sponges
Isolate was obtained from a patient suffering from chronic periodontitis. Sample collection was carried out in the morning, before breakfast, and the daily oral cleansing procedure. The sample was then placed in transport medium and within one hour of collection, specimen was spread on blood, McConkey and cetrimide agar plates were incubated at 37°C for 18 h. We were able to isolate a multi drug resistant pseudomonas strain from the culture. Identification was done based on colony characteristics, pyocyanin production (bluish green pigment), gram staining, oxidase test, indole test, Voges Proskauer and citrate utilization tests and growth on Hugh Leifson medium, cetrimide agar and β hemolysis on sheep blood agar. Sensitivity to antibiotics was determined using agar diffusion method (NCCLS 2000) and the isolate was confirmed to be multiple drugs resistant. Commercially availabe chlorhexidine gluconate oral rinse 0.12%, piperacillin + tazobactam, and doxycycline were purchased. The polylactide sponge was dipped in 1 ml of the chlorhexidine and antibiotic solutions and kept for half an hour after which it was allowed to dry at room temperature. Three wells of standard size (8 mm) were incised at specified distances in Mueller Hinton agar and 18 hr old nutrient broth cultures of the P. aeruginosa isolate was swabbed on the Mueller Hinton agar plate using a sterile cotton swab. A total of 0.1 ml of the three drug solutions were added into separate wells. The drug loaded polylactide sponges as well as untreated sponge were placed on the media surface at equidistance using a sterile forceps and pressed gently. After incubation at 37°C for 24 hrs, diameter of zone of inhibition was measured and consequently antibacterial activity was assessed.
RESULTS
Formulation and characterization of polylactide particles
Polylactide particles were prepared using double emulsion solvent evaporation method using clinical grade polylactic acid. These polymers have a well characterized biodegradability rate of four to six months. The polylactide macro particles were porous and had an average size of ~300 µ as measured by the Malvern particle size analyzer [Figure 1]. The polylactide macro particles were made porous by incorporating sucrose in the internal aqueous phase, as it was seen that addition of sucrose increases the porosity of the polymer particles [Figure 2]. Increasing the porosity of particles would be beneficial during drug loading of the sponge by diffusion of drug molecules.
Figure 1.
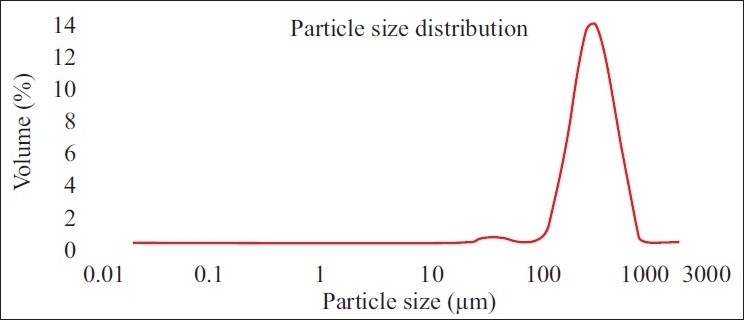
Size analysis of polylactide particles. The mean size of the particles is about 300 micrometers
Figure 2.
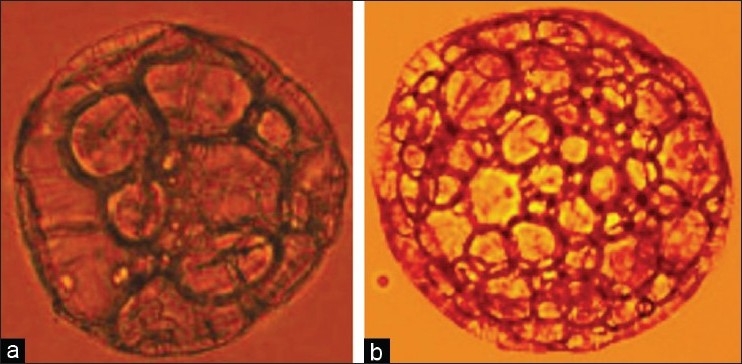
Microscopy picture of polylactide particles (a) Polylactide particle prepared without sucrose as an excipient (b) Polylactide particle prepared with sucrose in the internal aqueous phase showing increased porosity
Fabrication of polylactide sponges and their evaluation for cytocompatibilty
Fusion of polylactide macro particles into sponges and membranes were carried out at room temperature in suitable plastic moulds in presence of ethanol [Figures 3a and b]. Polylactic acid is sparingly soluble in ethanol with the result that the surface of the particles are partly solubilized resulting in fusion at the points of their contact in presence of ethanol, while still retaining the structural integrity of the particles. The polymeric sponge after its formation with ethanol is fragile in nature but with repeated washes with sterile water it stabilizes into a stable structure. The fusion regions between the particles of the sponge which are responsible for the stable structure of the sponge were observed using scanning electron microscope [Figure 4a]. Closer examination of the polylactide particles using scanning electron microscope revealed numerous pores on its surface [Figure 4b]. The polylactide sponges could fit into replicas of typical extraction socket sites made in alginate moulds. The sponge can be trimmed and shaped if necessary to fit into the extraction sockets and once it has been placed, it should be compacted without using excessive force so as to conform to the configuration of the defect. Also, polylactide membranes were fabricated suitable for placing as a barrier membrane after placement of the polylactide sponge in the extraction socket to afford further protection against in growth of epithelial cells [Figure 3b].
Figure 3.
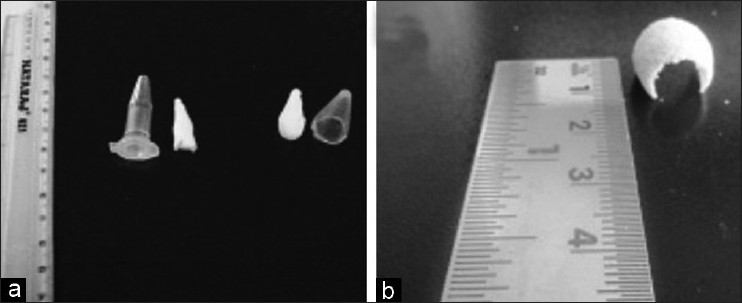
Fabrication of polylactide sponges and membranes (a) Particles fused into a sponge using eppendorf tube as a mould (b) Polylactide membrane fabricated to be used as barrier membrane
Figure 4.
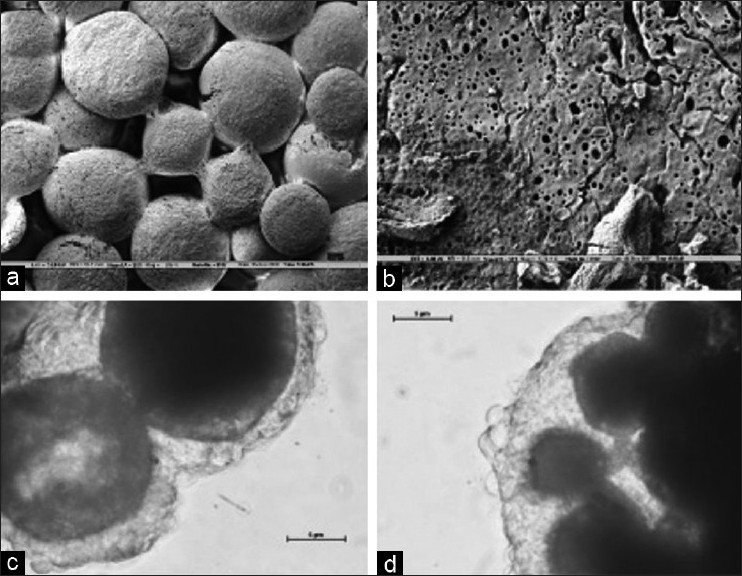
SEM of polylactide sponges after fusion of particles in ethanol (a) Prominent fusion regions between the particles can be seen in the Figure after the process of fusion (b) SEM of the surface of the particles reveals numerous pores (c and d) Growth of B16 melanoma cells on PLA sponge. The images shows the three dimensional growth of cells by day 6
Sterility checks were done as prescribed by ISO 10993 for biomedical devices for all the batches of polylactide sponges used for the experiments and were shown to be sterile. Evaluation of the polylactide sponges with B16 melanoma cell lines showed that they were non toxic to the cells and they readily adhered to the particles of the sponge and proliferated over days to form organized cellular masses on the sponges [Figures 4c and d]. Cells were disassociated from the sponge using cell disassociation buffer at different time points and viability of the cell population checked. It was seen that the cell population could maintain 90% viability even at two weeks of culture on the sponges. The polylactide sponge thus has the requisite characteristics necessary for promoting cell attachment and growth.
Evaluation of antibacterial activity of drug loaded polylactide sponges
The result of the disc diffusion method for antibiotic sensitivity of the clinical isolate showed that the bacteria are resistant to all antibiotics under study except piperacillin + tazobactam combination. The result of the disc diffusion method is shown in [Table 1]. Antibacterial activity of the individual drugs and drug loaded polylactide sponges is shown in [Table 2] which showed that piperacillin + tazobactam and polylactide sponges loaded with piperacillin + tazobactam showed maximum efficacy as the diameter of the zone of inhibition obtained was maximum in both the cases. [Figure 5] shows the antibacterial efficacy of drug loaded polylactide sponges to P. aeruginosa. It was also noted that the plain polylactide sponge without any drugs did not show the presence of inhibition zone. Significant diameter of zone of inhibition was noted around both the chlorhexidine loaded well and with the polylactide sponge loaded with chlorhexidine. Moderate zone of inhibition was observed around the well loaded with doxycycline and the sponge loaded with doxycycline. The antibacterial efficacy extended by chlorhexidine gluconate loaded sponges is noteworthy as the bacteria are prominent multi drug resistant species and chlorhexidine is widely used in dental applications. The drug loading process is a simple procedure that is achieved by placing the polylactide sponges in the drug solutions for half an hour prior to its evaluation.
Table 1.
Result of the sensitivity extended by P. aeruginosa isolate to antibiotics
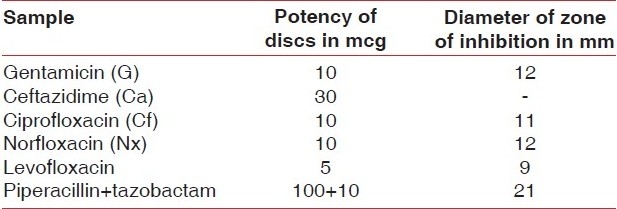
Table 2.
Result of the antibacterial efficacy of the drug loaded polylactide sponges to P. aeruginosa isolate
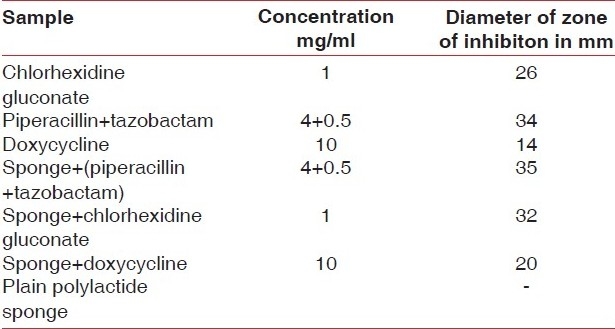
Figure 5.
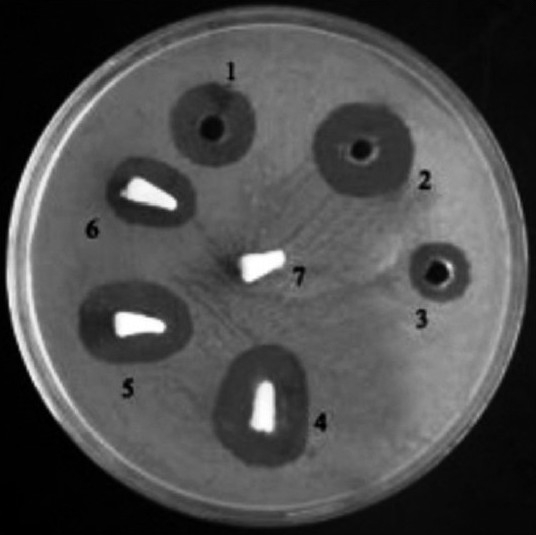
Antibacterial efficacy of the drug loaded polylactide sponges to P. aeruginosa. 1. Chlorhexidine gluconate, 2. Piperacillin+tazobactam, 3. Doxycycline, 4. Polylactide sponge+(piperacillin+tazobactam), 5. Polylactide sponge+Chlorhexidine gluconate, 6. Polylactide sponge+Doxycycline, 7. Plain polylactide sponge
DISCUSSION
The results of this study indicate that biodegradable drug releasing polylactide space fillers has the potential to be used for ridge preservation following tooth extraction. Release of drugs in the socket may prove useful in preventing development of alveolar osteitis post extraction which can interfere with normal healing of the socket. It has been shown that polylactide sponges at extraction sites decrease alveolar bone loss in comparison to natural healing by clot formation.[2] But in cases of underlying infections like alveolar osteitis, failure of the sponge or scaffold to integrate in the bony socket can take place. In such a scenario, a drug loaded implant which can manage the underlying infection would be beneficial in ensuring the success of the ridge preservation therapy. Drug loaded polylactide sponges fabricated in the present study showed significant activity against a multi drug resistant P. aeruginosa isolated from a patient suffering from chronic periodontitis. Of particular interest are chlorhexidine loaded polylactide sponges which has the potential to effect anti microbial activity against a wide range of bacteria. Another major advantage of chlorhexidine formulations is that microorganisms seldom develop resistance against it.
The volume as well as the shape of the alveolar process is determined by the form of the root, their axis of rotation, and eventual inclination.[5–7] Alveolar process being a tooth dependent tissue that develops in conjunction with the eruption of teeth will undergo atrophy subsequent to the removal of teeth.[8–10] The bundle bone at the site will lose its function.[11,12] Undisturbed extraction sockets heal uneventfully with bony tissue following extraction. This healing process usually occurs with substantial reduction of the original height and width of the alveolar bone. The greatest amount of bone loss is in horizontal dimension and occurs mainly on the facial aspect of the ridge, there is also loss of vertical ridge height which has been described to be most pronounced on buccal aspect.[12] This resorption results in narrower and shorter ridge and the effect of this resorptive pattern is the relocation of the ridge to a more palatal/lingual position.[13] The defect resulting from the loss of tooth may be complicated by previous bone loss due to periodontal disease, endodontic lesion, or a traumatic episode.
Alloplastic bone substitutes and bone derivatives of xenogenic origin have been used as osseoinductive, and osteoconductive materials. The use of grafting materials in fresh extraction sockets has been questioned because they seem to interfere with the normal healing process in the sockets in which oral implants have to be inserted.[14–17] Studies in humans using demineralized freeze-dried bone allograft (DFDBA)[18] and deproteinized natural bovine bone mineral (Bio-Osss)[19,20] or Bio active glass[21] have shown the presence of particles of the grafted material in the alveolar sockets 6-9 months following their insertion.[22,23] It is reported that use of resorbable membranes and sponges made of glycolide and lactide polymers in extraction sites partly prevent the occurrence of bone resorption.[2] Polylactide and polyglycolide acids are considered to be suitable matrices for bone and soft connective tissue.[24] Polylactic acid being a synthetic material does not carry the danger of being contaminated with infectious agents. They also undergo controlled degradation to be completely resorbed within 4-6 months.
A major advantage of polylactide sponges fabricated in this study is that they can be loaded with drugs to manage any potential infection at the extraction site. Chlorhexidine loaded polylactide sponges showed significant anti bacterial effect against P. aeruginosa in the study. Pseudomonas is described as a superinfectious microorganism that may contribute to periodontal break down.[25] Chlorhexidine is widely used for dental applications and pathogenic bacteria seldom develop resistance against it. It has been shown that chlorhexidine released from graft material may prevent growth of various bacteria and facilitate attachment of osteoblasts.[26] Verdugo et al. reported that exposure of human explants to 0.2% chlorhexidine did not have any adverse effect on osteoblast growth.[27] This was confirmed by SEM analysis showing absence of osteoblast phenotypic alterations after exposure. Lagares et al. showed that 0.2% of chlorhexidine gel seems to reduce the incidence of alveolar osteitis after removal of impacted third molars.[28] Hita-Iglesias et al. concluded that the topical application of bioadhesive chlorhexidine gel to surgical wound during the post operative week may decrease the incidence of alveolar osteitis after extraction of the mandibular third molars.[29] Thus, chlorhexidine loaded biocompatible and biodgradable polylactide sponges has the potential to be used for ridge preservation following tooth extraction especially in cases where there are chances of an underlying infection.
CONCLUSION
Porous polylactide sponges composed of fused particles were fabricated suitable to be used for ridge preservation following tooth extraction;
The sponges degrade in a controlled rate in 4-6 months allowing for bone formation in the sockets to take place;
The sponges can be loaded with drugs by diffusion and has the potential to prevent the development of alveolar osteitis which can lead to bone loss;
Chlorhexidine and piperacillin+tazobactam loaded polylactide sponges showed significant activity against a drug resistant P. aeruginosa isolated from a case of chronic periodontitis.
ACKNOWLEDGMENT
For this study, we thank our Professor and Head of department of periodontics, Dr. Thomas George, for his guidance and support which was very vital for the study. Also, we would like to thank Principal of our Dental College, Dr. Oommen Aju Jacob, for his guidance, support, and meticulous review of the manuscript. Our heartfelt thanks to Fr. Dr. Mathew Mazhavancheriyil, Director, Pushpagiri Research Center, for his guidance and kind permission to conduct the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Darby I, Chen ST, Buser D, Dent M. Ridge preservation techniques for implant therapy. Int J Oral Maxillofac Implants. 2009;24:260–71. [PubMed] [Google Scholar]
- 2.Serino G, Biancu S, Lezzi G, Piattelli A. Ridge preservation following tooth extraction using a polylactide and polyglycolide sponge as space filler: A clinical and histological study in humans. Clin Oral Implantol Res. 2003;14:651–8. doi: 10.1034/j.1600-0501.2003.00970.x. [DOI] [PubMed] [Google Scholar]
- 3.Barrows TH. Degradable implant materials: A review of synthetic absorbable polymers and their applications. Clin Mater. 1986;1:233–57. [Google Scholar]
- 4.Katare YK, Muthukumaran T, Panda AK. Influence of particle size, antigen load, dose and additional adjuvant on the immune response from antigen loaded PLA microparticles. Int J Pharm. 2005;301:149–60. doi: 10.1016/j.ijpharm.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 5.Nair R, Schug J. Observation on healing of human tooth extraction sockets implanted with bioabsorbable polylactic-polyglycolic acids copolymer root replicas: A clinical, radiographic, and histologic follow-up report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:559–69. doi: 10.1016/S1079210403006334. [DOI] [PubMed] [Google Scholar]
- 6.Van der WF, Dell’Acqua F, Slot DE. Alveolar bone dimensional changes ofpost-extraction sockets in humans: A systematic review. J Clin Periodontol. 2009;36:1048–58. doi: 10.1111/j.1600-051X.2009.01482.x. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder HE. The periodontium. In: Oksche A, Vollrath L, editors. Handbook of Microscopic Anatomy. Berlin: Springer; 1986. pp. 233–46. [Google Scholar]
- 8.Atwood DA. A cephalometric study of the clinical rest position of the mandible. Part II. The variability in the rate of bone loss following the removal of occlusal contacts. J Prosthet Dent. 1957;7:544–52. [Google Scholar]
- 9.Hedegard B. Some observations on tissue changes with immediate maxillary dentures. Dent Pract. 1962;13:70–8. [Google Scholar]
- 10.Tallgren A. The continuing reduction ofthe residual alveolar ridges in complete denture wearers: A mixed longitudinal studycovering 25 years. J Prosthet Dent. 1972;27:120–32. doi: 10.1016/0022-3913(72)90188-6. [DOI] [PubMed] [Google Scholar]
- 11.Botticelli D, Berglundh T, Lindhe J. Hard-tissue alterations following immediate implant placement in extraction sites. J Clin Periodontol. 2004;31:820–8. doi: 10.1111/j.1600-051X.2004.00565.x. [DOI] [PubMed] [Google Scholar]
- 12.Arau’jo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005;32:212–8. doi: 10.1111/j.1600-051X.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 13.Pinho MN, Roriz VL, Novaes AB, Jr, Taba M, Jr, Grisi MF, de Souza SL, et al. Titanium membranes inprevention of alveolar collapse after tooth extraction. Implant Dent. 2006;15:53–61. doi: 10.1097/01.id.0000202596.18254.e1. [DOI] [PubMed] [Google Scholar]
- 14.Pinholt EM, Bang G, Haanaes HR. Alveolar ridge augmentation in rats by Bio-Oss. Scand J Dent Res. 1991;99:154–61. doi: 10.1111/j.1600-0722.1991.tb01878.x. [DOI] [PubMed] [Google Scholar]
- 15.Becker W, Becker BE, Caffesse R. Acomparison of demineralized freeze-dried bone and autologous bone to induce bone formation inhuman extraction socket. J Periodontol. 1994;65:1128–33. doi: 10.1902/jop.1994.65.12.1128. [DOI] [PubMed] [Google Scholar]
- 16.Becker W, Urist M, Vincenzi G, De Georges D, Niederwanger M. Clinical and histological observation of sites implanted withintraoral autologous bone graft or allograft. Human case reports. J Periodontol. 1996;67:1025–33. doi: 10.1902/jop.1996.67.10.1025. [DOI] [PubMed] [Google Scholar]
- 17.Buser D, Hoffmann B, Bernard JP, Lussi A, Mettler D, Schenk RK. Evaluation offilling materials in membrane-protected defect. Clin Oral Implants Res. 1998;3:137–50. doi: 10.1034/j.1600-0501.1998.090301.x. [DOI] [PubMed] [Google Scholar]
- 18.Brugnami F, Then PR, Moroi H, Leone CW. Histologic evaluation of human extractionsocket treated with demineralized freeze-driedbone allograft (DFDBA) and cell occlusive membrane. J Periodontol. 1996;67:821–5. doi: 10.1902/jop.1996.67.8.821. [DOI] [PubMed] [Google Scholar]
- 19.Diès F, Etienne D, Abboud NB, Ouhayoun JP. Bone regeneration in extraction sites after immediate placement of an e-PTFE membrane with or without a biomaterial. A report of 12 consecutive cases. Clin Oral Implants Res. 1996;7:277–85. doi: 10.1034/j.1600-0501.1996.070310.x. [DOI] [PubMed] [Google Scholar]
- 20.Artzi Z, Tal H, Dayan D. Porous bovinebone mineral in healing of human extraction socket. Part 1. Histometric evaluation at months. J Periodontol. 2000;71:1015–23. doi: 10.1902/jop.2000.71.6.1015. [DOI] [PubMed] [Google Scholar]
- 21.Froum S, Cho SC, Rosenberg E, Rohrer M, Tarnow D. Histological comparison of healing extraction socket implanted with bioactiveglass or demineralized freeze-dried bone allograft: A pilot study. J Periodontol. 2002;73:94–102. doi: 10.1902/jop.2002.73.1.94. [DOI] [PubMed] [Google Scholar]
- 22.Lekovic V, Kenney EB, Weinlaender M, Han T, Klakkevold P, Nedic M, et al. Bone regenerative approach to alveolar ridgemaintenance following tooth extraction. Report of 10 cases. J Periodontol. 1997;68:563–70. doi: 10.1902/jop.1997.68.6.563. [DOI] [PubMed] [Google Scholar]
- 23.Lekovic V, Camargo PM, Klokkevold PR, Weinlaender M, Kenney EB, Nedic M, et al. Preservation of alveolar bone in extraction sockets using bioabsorbable membrane. J Periodontol. 1998;69:1044–9. doi: 10.1902/jop.1998.69.9.1044. [DOI] [PubMed] [Google Scholar]
- 24.Laurencin CT, Lane JM. Chicago: Quintessence Publishing Co; 1999. Poly-lactide acidand poly-glycolide acid: Orthopedic and surgery applications. In: Tissue Engineering: Application in maxillofacial surgery and periodontics; pp. 325–39. [Google Scholar]
- 25.Slots J, Feik D, Rams TE. Age and sex relationship of superinfecting microorganisms in periodontitis patients. Oral Microbiol Immunol. 1990;5:305–8. doi: 10.1111/j.1399-302x.1990.tb00430.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen YT, Hung SL, Lin LW, Ling LJ. Attachment of peridontal ligament cells to chlorhexidine loaded GTR membranes. J Periodontol. 2003;74:1652–9. doi: 10.1902/jop.2003.74.11.1652. [DOI] [PubMed] [Google Scholar]
- 27.Verdugo F, Sáez-Rosón A, Uribarri A, Martínez-Conde R, Cabezas-Olcoz J, Moragues MD, et al. Bone microbial decontamination agents in osseous grafting: An in vitro study with fresh human explants. J Periodontol. 2011;82:863–71. doi: 10.1902/jop.2010.100514. [DOI] [PubMed] [Google Scholar]
- 28.Torres-Lagares D, Gutierrez-Perez JL, Hita-Iglesias P, Magallanes-Abad N, Flores-Ruiz R, Basallote-Garcia M, et al. Randomized, double-blind study of effectiveness of intra-alveolar application of chlohexidine gel in reducing incidence of alveolar osteitis and bleeding complication in mandibular third molar surgery in patients with bleeding disorders. J Oral Maxillofac Surg. 2010;68:1322–6. doi: 10.1016/j.joms.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 29.Hita-Iglesias P, Torres-Lagares D, Flores-Ruiz R, Magallanes-Abad N, Basallote-Gonzalez M, Gutierrez-Perez JL. Effectiveness of chlorhexidine gel versus chlorhexidine rise in reducing alveolar osteitis in mandibular third molar surgery. J Oral Maxillofac Surg. 2008;66:441–5. doi: 10.1016/j.joms.2007.06.641. [DOI] [PubMed] [Google Scholar]


