Abstract
Context: Population study data about relations of coffee drinking to arrhythmia are sparse.
Objective: To study relations of coffee drinking to risk of cardiac arrhythmia in 130,054 persons with previous data about coffee habits.
Design and Outcome Measure: We used Cox proportional hazards models with 8 covariates to study coffee-related risk in 3137 persons hospitalized for cardiac arrhythmia. We conducted a similar analysis of total caffeine-related risk in a subgroup with data about other caffeine intake (11,679 study participants; 198 hospitalized).
Results: With non-coffee-drinkers as the referent, the adjusted hazard ratio (HR) for any arrhythmia at the level of <1 cup of coffee per day was 1.0 (95% confidence interval [CI] = 0.9–1.1; p = 0.7); for 1–3 cups/day, it was 0.9 (CI, 0.8–1.0; p = 0.2), and for ≥4 cups/day, it was 0.8 (CI, 0.7–0.9; p = 0.002). With coffee intake as a continuous variable, the HR per cup per day was 0.97 (CI, 0.95–0.99; p = 0.001). Results were similar for several strata, including persons with history or symptoms of possible cardiore-spiratory disease and those without such history or symptoms. Coffee had similar relations to atrial fibrillation (48% of participants with arrhythmia) and most other specific arrhythmia diagnoses. Controlled for number of cups of coffee per day, total caffeine intake was inversely related to risk (HR highest quartile vs lowest = 0.6; p = 0.03).
Conclusion: The inverse relations of coffee and caffeine intake to hospitalization for arrhythmias make it unlikely that moderate caffeine intake increases arrhythmia risk.
Background
Cardiac rhythm disturbances are among the toxic effects from very large doses of caffeine administered in animal studies1–3 and taken in human suicide attempts.4 Patients frequently report palpitations after caffeine ingestion, and physicians often advise patients with arrhythmias to avoid caffeinated coffee. However, human experimental5–9 and prospective population study10–12 data about relations of commonly ingested amounts of coffee and caffeine to arrhythmia have yielded inconsistent results. Controlled experiments in humans have shown no relation to ventricular premature beats (VPBs), including data about caffeine restriction in persons with symptomatic VPBs,9 caffeine administration in patients with recent myocardial infarction,7 and inducibility of VPBs.8 A controlled experiment of ingestion of the caffeine equivalent of 4 to 5 cups of coffee showed no relationship to heart rate and number of atrial or VPBs on taped electrocardiograms.5 Two earlier population studies of coffee drinking and incident atrial fibrillation risk11,12 showed no association; another10 showed a weak positive association but with an inconsistent dose–response relationship.
In view of the need for more data about the association of arrhythmia risk with commonly ingested amounts of coffee, we examined and report here data about relations of reported coffee intake to subsequent risk of hospitalization for various arrhythmias in a large free-living population.
Methods
Study Participants and Data
The study protocols were approved by the institutional review board of the Kaiser Permanente Medical Care Program. Study participants were 130,054 members of a Northern California comprehensive health care plan who voluntarily underwent a health examination13 between 1978 and 1985. For many years, the examination was offered to adult members as a way to have a routine health checkup. Examination questionnaire items included ethnicity, other demographics, habits, and medical history. One query was “Do you drink coffee?” Options for answers to that question were “more than 6 cups/day, 4–6 cups/day, 1–3 cups/day, less than 1 cup/day, and never or seldom.” The questionnaire included a similar query about tea intake. Coffee and tea consumption were ascertained one time only. Measurements included body mass index (BMI), blood pressure, fasting blood glucose level, total blood cholesterol level, and leukocyte count. Total coffee and tea intake were ascertained for all 130,054 participants.
Examinees in 1984–1985 (n = 11,679, or 9.3% of all participants) answered queries about type of coffee, with answer options being “none, caffeinated only, decaffeinated only, both caffeinated and decaffeinated”. These persons also supplied data about caffeine in tea, soft drinks, and medications, enabling estimation of total caffeine intake. Caffeine intake was studied among these 11,679 participants.
Analytic Methods
Participants were monitored until December 31, 2008, death or other cause for Health Plan termination, or first hospitalization in a program facility with a primary discharge diagnosis of cardiac dysrhythmia, code 427 in the International Classification of Diseases, 9th Revision. Incompleteness of comprehensive computerized data about outpatient arrhythmia diagnoses plus the impracticality of reviewing >130,000 paper records precluded study of arrhythmia events not resulting in hospitalization. The average duration of follow-up monitoring was 17.64 years, with a total of 2,224,214 person-years of observation. There were 3137 persons with an arrhythmia diagnosis. Table 1 shows selected unadjusted distributions.
Table 1.
Selected baseline traits of study population and participants with arrhythmia
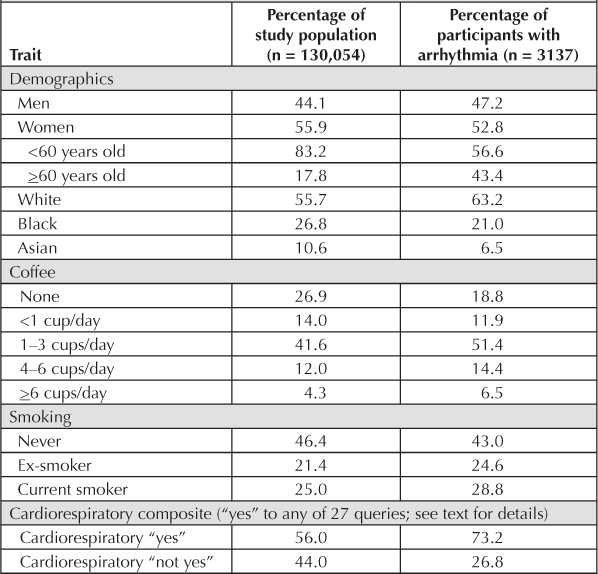
We used Cox proportional hazards models determined by the PHREG procedure described in version 8 of the user's guide for SAS Analytics (SAS Institute Inc, Cary, NC). Coffee was studied categorically, with never/seldom as referent and <1, 1–3, 4–6, ≥6 cups/day or <1, 1–3, ≥4 cups/day. With an assumption of linearity, coffee was also studied as a continuous per-cup-per-day variable with these assigned numbers: 0 for never/seldom, 0.5 for <1 cup/day, 2.0 for 1–3 cups, 5.0 for 4–6 cups, and 7 for ≥6 cups. Most multivariate models included age (continuous), sex, ethnicity (white as referent, with black, Asian, Hispanic, and other as additional groups), BMI (<25 kg/m2 as referent, with 25–29 kg/m2, ≥30 kg/m2 as additional groups), education (no college as referent, with some college and college graduate as additional groups), cigarette smoking (never as referent, with ex-smokers, <1 pack/day, and ≥1 pack/day as additional groups), alcohol intake (never as referent, with ex-drinkers and 4 current drinking categories as additional groups), and a cardiore-spiratory (CR) composite covariate.
The CR baseline risk covariate was considered positive if the participant answered yes to any of 27 queries involving current or past possible cardiovascular conditions (heart attack, angina, stroke, high blood pressure, diabetes, abnormal findings on electrocardiography, chest pain, palpitations, shortness of breath, blackouts, etc). The participants with a positive CR variable comprised 56.0% of the total study population and 73.2% of participants with arrhythmia.
The subset of 11,679 participants that supplied information about coffee types was also studied by comparing persons taking specific coffee types to non-coffee-drinkers as referent. In the same subset, total caffeine intake was studied as a continuous (per decile) variable. These were studied both with and without control for total amount of coffee intake.
For coffee intake, we studied risk of any arrhythmia and of specific diagnoses. The latter included supraventricular tachycardia (code 427.0; n = 257), paroxysmal ventricular tachycardia (code 427.1; n = 232), atrial fibrillation (code 427.31; n = 1512), atrial flutter (code 427.32; n = 273), ventricular fibrillation/flutter/cardiac arrest (code 427.4–5; n = 91), premature beats (code 427.6; n = 91), and other arrhythmia (code 427.8; n = 755). Analyses yielded estimates of hazard ratios (HRs), 95% confidence intervals (CIs), and p values. We performed similar analyses of the relation of tea to arrhythmia risk.
Similar analyses of total coffee intake were performed, including—one at a time—systolic blood pressure, diastolic blood pressure, total blood cholesterol, glucose, and leukocyte count.
Finally, we used similar models to examine risk of hospitalization for the three most numerous cardiovascular diagnostic groups. These were coronary artery disease ([CAD] codes 410–414; n = 7658), cerebrovascular disease (codes 430–438; n = 5108), and heart failure (code 428; n = 3418).
Results
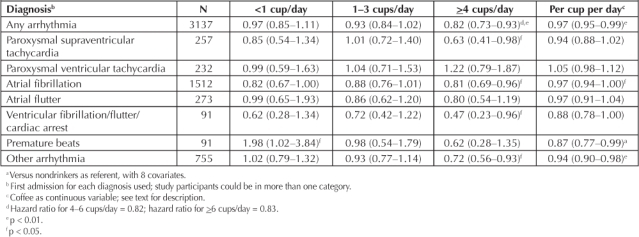
In adjusted models (Table 2), we observed an inverse relation of coffee to risk of hospitalization for arrhythmia, especially supraventricular arrhythmias, that was statistically significant for heavier coffee drinkers (≥4 cups/day) and for coffee as a continuous variable. This inverse relation was progressive in the largest intake categories: for example, at 4–6 cups/day, the HR for all arrhythmias was 0.84 (p = 0.05), and at >6 cups/day, it was 0.73 (p = 0.02). The results were similar for most of the specific supraventricular arrhythmia diagnoses. The HR for heavy coffee drinkers was >1.0 for paroxysmal ventricular tachycardia, but it was 0.5 for the composite of “ventricular fibrillation/flutter/cardiac arrest.”
Table 2.
Adjusted a hazard ratio (95% confidence interval) of arrhythmia diagnoses by coffee intake
The inverse relation of coffee drinking to arrhythmia was consistent in men, women, whites, blacks, and persons younger than or older than 60 years of age at baseline (Table 3). The association was nearly identical in persons with a yes for CR composite and those without a yes for the composite (Table 3). The inverse coffee relation was slightly stronger for risk within 10 years of examination (vs ≥10 years) and for risk of never-smokers (vs ex-smokers or current smokers). Tea drinking was less prevalent in our study population and was unrelated to any endpoint. For the 24,945 persons reporting intake of ≥1 cup/day of tea (vs none), the HR was 1.01; the HR per cup per day was 1.00.
Table 3.
Adjusted a hazard ratio (95% confidence interval) of arrhythmia in selected groups by coffee consumption
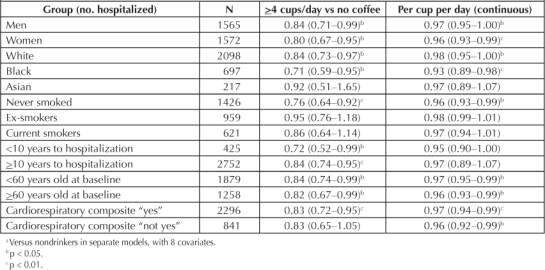
The inverse relation of coffee drinking to arrhythmia was consistent in men, women, whites, blacks, and persons younger than or older than 60 years of age …
We performed most stratified analyses separately for atrial fibrillation, the diagnosis for half of all participants. The results for atrial fibrillation were consistently similar to those for all arrhythmias; for example, the HR of those drinking ≥4 cups/day were 0.83 for men, 0.78 for women, 0.78 for white persons, 0.65 for black persons, 0.79 if <60 years old at baseline, 0.83 if ≥60 years old at baseline, 0.64 if <10 years to diagnosis, 0.84 if ≥10 years to diagnosis, 0.81 if CR “yes,” and 0.84 if CR “not yes.”
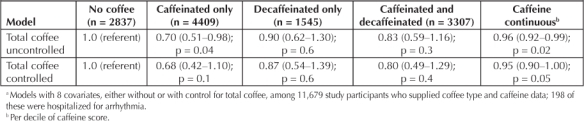
In the subset with caffeine data, total caffeine intake was related to lower arrhythmia risk, and the inverse relation to arrhythmia was stronger for persons reporting drinking only caffeinated coffee than for those reporting decaffeinated or both types (Table 4), associations little affected by controlling for total amount of coffee intake.
Table 4.
Adjusted a hazard ratio (confidence interval) of arrhythmia by coffee type and total caffeine
Among covariate relations, age, male sex, white ethnicity, adiposity (BMI ≥ 30kg/m2), and blood pressure were all related to increased risk (data not shown). Cigarette smoking was weakly related to risk. For example, compared with never smokers, the HR for <1 pack/day smokers was 1.08 (CI, 0.96–1.21), and for ≥1 pack/day smokers, the HR was 1.18 (CI, 1.02–1.36; p = 0.03). The CR composite was related to risk with a HR of 1.64 (CI, 1.51–1.78; p < 0.001). Inclusion of total blood cholesterol level, systolic or diastolic blood pressure, blood glucose level, or leukocyte count in models had a negligible effect on the HRs for arrhythmia. Although both systolic and diastolic blood pressures were related to risk, the HR for ≥4 cups of coffee was 0.82 (CI, 0.73–0.93) with or without inclusion of either systolic or diastolic blood pressure.
As detailed in the “Study Participants and Methods” section, there were three broad causes of cardiovascular hospitalizations with greater numbers than arrhythmias. The HRs associated with ≥4 cups/day for these were as follows: CAD, 1.13 (CI, 1.04–1.21; p = 0.002); cerebrovascular disease, 0.95 (CI, 0.87–1.04; p = 0.3); and heart failure, 1.04 (CI, 0.93–1.17; p = 0.5).
Discussion
It is unclear what, if any, rhythm disturbances are represented by anecdotal reports of “palpitations” after caffeine ingestion. Yet we are unaware of previous population studies that examine all arrhythmia in relation to coffee drinking.
The validity of the inverse relation of coffee drinking to risk of hospitalization for arrhythmias in these data is reinforced by consistency in most strata. Although the pathophysiologic mechanisms of various rhythm disturbances are not identical, it seems noteworthy that we found similar coffee associations with various supraventricular rhythm diagnoses. It is also important that other common cardiovascular causes of hospitalization, especially CAD and heart failure, did not show similar inverse associations with coffee intake. The relative specificity of the inverse coffee–arrhythmia association may provide further support for the results.
Although of borderline statistical significance, the coffee type and caffeine score data suggest that caffeine is involved. In this population, heavier coffee drinkers drink mostly the caffeinated type. Caffeine from coffee comprised 80% of all caffeine in the subset with caffeine data. Thus, caffeine intake may be a marker for total coffee consumption. Considerable detail about distributions and traits of persons in this population reporting caffeinated and decaffeinated coffee has been published.14 Tea drinking was unrelated to arrhythmia risk. On average, tea contains less caffeine than coffee,14–16 and tea drinkers in this population consume fewer cups per day than coffee drinkers do. Thus, the absence of a relation to tea does not rule out a role for caffeine.
A study of risk of hospitalization for atrial fibrillation in men reported by Wilhelmsen et al10 showed, in 754 cases, a borderline increase in risk at 1–4 cups/day (odds ratio = 1.24 [CI, 1.00–1.54]) but no significantly increased risk at ≥5 cups/day (odds ratio = 1.09 [CI, 0.87–1.38]).11 Frost and Vestergaard11 presented data from 555 cases of atrial fibrillation/flutter in 160,000 men and women, studying caffeine intake, mostly from coffee, by quintile. The HR of quintile 5 versus quintile 1 was 0.91 (CI, 0.70–1.19). A report by Conen et al12 of 945 incident atrial fibrillation events in relation to caffeine intake in 33,638 women showed an HR of quintile 5 versus quintile 1 of 0.89 (CI, 0.72–1.09). In view of overlapping CIs and differences in methodology, our results for atrial fibrillation are not incompatible with these reports.
Potential Confounders
Persons at increased risk of rhythm disturbances because of symptoms or a diagnosis of heart disease might have quit drinking coffee before giving baseline data. Such a “sick quitter” phenomenon could raise the risk of the non-coffee-drinking referent group and cause a spurious inverse coffee association. The similar inverse relation in CR “yes” and “not yes” strata substantially refutes this explanation. We made the CR composite a broadly inclusive one, with the result that it included a majority of all participants. Some of the CR “yes” participants probably had no CR illness, but the “not yes” stratum is likely to be composed of healthy participants with few sick quitters. The specificity of the relation for arrhythmia also reduces the likelihood of this explanation. The weakening of the inverse relation after ten years could be interpreted as support for the sick-quitter phenomenon. However, it might also be because of reduction of coffee intake by some participants as they aged, thus supporting a true inverse coffee association.
Cigarette smoking is correlated with coffee drinking and is an important potential confounder of studies of coffee and health endpoints.15–17 Smoking was not strongly related to arrhythmias in our data, and the inverse coffee–arrhythmia relation was strongest in never-smokers, points against the likelihood of confounding by smoking. Both nicotine and caffeine are metabolized by the hepatic cytochrome P4501A1 enzyme.18–20 Benowitz et al20 called acceleration of hepatic metabolism of caffeine by cigarette smoking “a well-established observation.” We speculate that more prolonged presence of caffeine in the blood of never-smokers might be a factor in their stronger inverse coffee–arrhythmia relation.
Persons with rhythm problems are often not hospitalized. For the years of follow-up monitoring, we had no data about rhythm disturbances not resulting in hospitalization. Thus, it is likely that our participants disproportionately include persons with arrhythmias causing hemodynamic compromise or other reasons for concern about the rhythm disturbance. It is possible that the relation of coffee drinking to more benign rhythm problems might be different from the results reported here. We have no data that cast light on this possibility.
Among covariate relations, age, male sex, white ethnicity, adiposity (BMI ≥ 30kg/m2), and blood pressure were all related to increased risk …
We have no data about follow-up coffee use, but loss to follow-up monitoring is not systematically related to coffee habits in this population.15 Other limitations of this analysis include incomplete caffeine data; lack of validation of diagnoses by actual paper-chart review; and absence of data about circumstances leading to hospitalization, method of coffee preparation, size of cups, time of day coffee was imbibed, and dietary habits. Strengths include the large multiethnic study cohort, diversity of coffee habits, and presence of data about demographics and smoking.
Possible Mechanisms
Biologic mechanisms for a protective effect of coffee are speculative. Caffeine, the most prominent pharmacologically active ingredient in coffee, induces catechol release, but primarily in caffeine-naïve persons and not in regular ingesters.9 Probably the caffeine action of most hypothetical relevance is antagonism of adenosine by competitive binding to receptors. Pharmacologic doses of adenosine decrease atrioventricular nodal conduction, reduce atrial muscle contractility, decrease sinoatrial node activity, and cause coronary artery dilation.21 It is unclear how antagonism of these adenosine actions by caffeine might reduce risk of arrhythmias. Adenosine also shortens the refractory period of atrial tissue and can trigger atrial fibrillation and other supraventricular arrhythmias during pharmacologic coronary disease testing22–25; these aspects may more plausibly support a protective effect of adenosine antagonism by caffeine.
Noncaffeine ingredients abound in coffee. Relevant to cardiovascular disease is a diterpene compound, cafestol, in coffee prepared without paper filtering. Cafestol raises low-density-lipoprotein cholesterol levels,26 but if increase of this powerful CAD risk factor translated into more CAD in coffee drinkers, this would be more likely to increase, rather than decrease, arrhythmias. Coffee is a rich source of antioxidants,27 a possible source of health benefit, but not specifically for arrhythmias. We will not speculate further here.
Conclusion
In a large cohort, coffee drinking and caffeine intake are inversely related to risk of hospitalization for arrhythmias, especially atrial fibrillation and other supraventricular arrhythmias. These observational data do not establish causality, and thus a protective effect is not proven. It is highly unlikely that moderate caffeine intake increases arrhythmia risk.
Disclosure Statement
The research reported here was supported by the Robert Wood Johnson Foundation's Program of Research Integrating Substance Use in Mainstream Healthcare ([PRISM] proposal #51512 to Arthur L Klatsky, primary investigator) and by a grant from the Kaiser Foundation Research Institute. Data collection in 1978–1985 was supported by the Alcoholic Beverage Medical Research Foundation, Inc (Baltimore, MD). All authors have participated actively in the execution of the study and/or in preparation of the manuscript for this report, and all reviewed the final version. The authors have no conflicts of interest to disclose.
Acknowledgments
Katharine O'Moore-Klopf, ELS, of KOK Edit provided editorial assistance.
References
- Paspa P, Vassalle M. Mechanism of caffeine-induced arrhythmias in canine cardiac Purkinje fibers. Am J Cardiol. 1984 Jan 15;53(2):313–9. doi: 10.1016/0002-9149(84)90445-4. [DOI] [PubMed] [Google Scholar]
- Strubelt O, Diederich KW. Experimental treatment of the acute cardiovascular toxicity of caffeine. J Toxicol Clin Toxicol. 1999;37(1):29–33. doi: 10.1081/clt-100102405. [DOI] [PubMed] [Google Scholar]
- Mehta A, Jain AC, Mehta MC, Billie M. Caffeine and cardiac arrhythmias. An experimental study in dogs with review of literature. Acta Cardiol. 1997;52(3):273–83. [PubMed] [Google Scholar]
- Price KR, Fligner AJ. Treatment of caffeine toxicity with esmolol. Ann Emerg Med. 1990 Jan;19(1):44–6. doi: 10.1016/s0196-0644(05)82139-0. [DOI] [PubMed] [Google Scholar]
- Newcombe PF, Renton KW, Rautaharju CA, Spencer CA, Montague TJ. High-dose caffeine and cardiac rate and rhythm in normal subjects. Chest. 1988 Jul;94(1):90–4. doi: 10.1378/chest.94.1.90. [DOI] [PubMed] [Google Scholar]
- Myers MG. Caffeine and cardiac arrhythmias. Ann Intern Med. 1991 Jan 15;114(2):147–50. doi: 10.7326/0003-4819-114-2-147. [DOI] [PubMed] [Google Scholar]
- Myers MG, Harris L. High dose caffeine and ventricular arrhythmias. Can J Cardiol. 1990 Apr;6(3):95–8. [PubMed] [Google Scholar]
- Chelsky LB, Cutler JE, Griffith K, Kron J, McClelland JH, McAnulty JH. Caffeine and ventricular arrhythmias. An electrophysiological approach. JAMA. 1990 Nov 7;264(17):2236–40. [PubMed] [Google Scholar]
- Newby DE, Neilson JM, Jarvie DR, Boon NA. Caffeine restriction has no role in the management of patients with symptomatic idiopathic ventricular premature beats. Heart. 1996 Oct;76(4):355–7. doi: 10.1136/hrt.76.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen L, Rosengren A, Lappas G. Hospitalizations for atrial fibrillation in the general male population: morbidity and risk factors. J Intern Med. 2001 Nov;250(5):382–9. doi: 10.1046/j.1365-2796.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- Frost L, Vestergaard P. Caffeine and risk of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Clin Nutr. 2005 Mar;81(3):578–82. doi: 10.1093/ajcn/81.3.578. [DOI] [PubMed] [Google Scholar]
- Conen D, Chiuve SE, Everett BM, Zhang SM, Buring JE, Albert CM. Caffeine consumption and incident atrial fibrillation in women. Am J Clin Nutr. 2010 Sep;92(3):509–14. doi: 10.3945/ajcn.2010.29627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collen MF, Davis LF. The multitest laboratory in health care. J Occup Med. 1969 Jul;11(7):355–60. [PubMed] [Google Scholar]
- Kubo Shlonsky A, Klatsky AL, Armstrong MA. Traits of persons who drink decaffeinated coffee. Ann Epidemiol. 2003 Apr;13(4):273–9. doi: 10.1016/s1047-2797(02)00414-3. [DOI] [PubMed] [Google Scholar]
- Klatsky AL, Armstrong MA, Friedman GD. Coffee, tea, and mortality. Ann Epidemiol. 1993 Jul;3(4):375–81. doi: 10.1016/1047-2797(93)90064-b. [DOI] [PubMed] [Google Scholar]
- Klatsky AL, Koplik S, Kipp H, Friedman GD. The confounded relation of coffee drinking to coronary artery disease. Am J Cardiol. 2008 Mar 15;101(6):825–7. doi: 10.1016/j.amjcard.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Schreiber GB, Robins M, Maffeo CE, Masters MN, Bond AP, Morganstein D. Confounders contributing to the reported associations of coffee or caffeine with disease. Prev Med. 1988 May;17(3):295–309. doi: 10.1016/0091-7435(88)90005-9. [DOI] [PubMed] [Google Scholar]
- Joeres R, Klinker H, Heusler H, Epping J, Zilly W, Richter E. Influence of smoking on caffeine elimination in healthy volunteers and in patients with alcoholic liver cirrhosis. Hepatology. 1988 May–Jun;8(3):575–9. doi: 10.1002/hep.1840080323. [DOI] [PubMed] [Google Scholar]
- Brown CR, Benowitz NL. Caffeine and smoking: behavioral, cardiovascular, and metabolic interactions. Pharmacol Biochem Behav. 1989 Nov;34(3):565–70. doi: 10.1016/0091-3057(89)90559-5. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Peng M, Jacob P., 3rd. Effects of cigarette smoking and carbon monoxide on chlorzoxazone and caffeine metabolism. Clin Pharmacol Ther. 2003 Nov;74(5):468–74. doi: 10.1016/j.clpt.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Cabalag MS, Taylor DM, Knott JC, Buntine P, Smit D, Meyer A. Recent caffeine ingestion reduces adenosine efficacy in the treatment of paroxysmal supraventricular tachycardia. Acad Emerg Med. 2010 Jan;17(5):44–9. doi: 10.1111/j.1553-2712.2009.00616.x. [DOI] [PubMed] [Google Scholar]
- Turley AJ, Murray S, Thambyrajah J. Pre-excited atrial fibrillation triggered by intravenous adenosine: a commonly used drug with potentially life-threatening adverse effects. Emerg Med J. 2008 Jan;25(1):46–8. doi: 10.1136/emj.2007.051227. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Kobori A, Kuwahara T, Takahashi A. Adenosine triphosphate exposes dormant superior vena cava conduction responsible for recurrent atrial fibrillation. J Cardiovasc Electrophysiol. 2010 Apr;21(4):464–5. doi: 10.1111/j.1540-8167.2009.01647.x. [DOI] [PubMed] [Google Scholar]
- Atienza F, Jalife J. Reentry and atrial fibrillation. Heart Rhythm. 2007 Mar;4(3 Suppl):S13–6. doi: 10.1016/j.hrthm.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Kanei Y, Hanon S, Van-Tosh A, Schweitzer P. Adenosine-induced atrial fibrillation during pharmacologic stress testing: report of eight cases and review of the literature. Int J Cardiol. 2008 Sep 16;129(1):e15–7. doi: 10.1016/j.ijcard.2007.05.090. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Frei B. Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr. 2006;46(2):101–23. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- Halvorsen BL, Carlsen MH, Phillips KM, et al. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am J Clin Nutr. 2006 Jul;84(1):95–135. doi: 10.1093/ajcn/84.1.95. [DOI] [PubMed] [Google Scholar]


