Abstract
Introduction: Black men have a higher incidence of advanced stage at diagnosis and mortality from prostate cancer than do men in other racial groups. Given that androgen-deprivation therapy (ADT) is one of the mainstays of treatment for advanced prostate cancer, we investigated the development of biochemical failure, or recurrence of elevated prostate-specific antigen (PSA) levels, among different races in men receiving ADT.
Methods: Patients with prostate cancer who received ADT in the Kaiser Permanente Southern California Cancer Registry between January 2003 and December 2006 were eligible for inclusion in our study. Patients who had prior treatment for their cancer with surgery or radiation were excluded. Treatment failure was defined as an increase in PSA of >2 ng/mL from PSA nadir, with no subsequent decrease in PSA. We compared the biochemical failure rate in white patients to those in black, Hispanic, and Asian/other patients. The Cox proportional hazards regression model was used to estimate hazards ratios.
Results: Our study population consisted of 681 patients: 416 (61%) were white; 107 (16%) were black; 107 (16%) were Hispanic; and 51 (7%) were Asian or another race. After we controlled for all demographic variables and for variables related to prostate cancer, blacks were the only group with a lower risk of treatment failure compared with whites. The hazard ratios for treatment failure were as follows: black versus white, 0.66 (p = 0.03); Hispanic versus white, 1.00 (p = 0.8); Asian/other race versus white, 1.5 (p = 0.1). In this multivariate analysis, pretreatment PSA level and cancer stage were the only other variables associated with a higher risk of treatment failure.
Conclusion: Among patients receiving ADT as primary monotherapy for prostate cancer, blacks may have a lower rate of biochemical failure compared with whites. Although the etiology of this finding is unclear, it suggests the possibility that prostate cancer in black men may be more androgen sensitive than it is in white men.
Introduction
Prostate cancer is one of the most common cancers among men and the second most common cause of cancer death in the US. However, its incidence is markedly different among various races and countries. Prostate cancer is diagnosed in black men at a higher frequency,1 at a higher grade of disease,2–4 and at a younger age than in white men.5 Black men are 2.5 times more likely to die of prostate cancer than white men are.6 Epidemiologic studies have attributed this higher mortality to the advanced stage at presentation and higher grade at diagnosis. Several factors, such as low income, lower literacy rate, and poor health insurance, in black men have been implicated in the increased proportion of advanced stage at presentation and subsequent greater mortality.7,8
Androgen-deprivation therapy (ADT) using luteinizing hormone–releasing hormone (LHRH) agonist is one of the mainstays of prostate cancer treatment. It is based on the castration of a hormonally sensitive prostate cancer.9 Although differences in prostate cancer incidence and mortality between black and white men are widely accepted, the existence of racial differences in treatment outcomes remains controversial.10 We conducted a study to determine the impact of race on the development of biochemical failure, or recurrence of elevated prostate-specific antigen (PSA) levels, after ADT therapy. Because Kaiser Permanente Southern California (KPSC) has a racially diverse patient population and all of the patients it serves have equal access to health care, our patient population was an ideal setting in which to evaluate the effectiveness of ADT among different racial groups. KPSC is a large multispecialty, managed-care organization that covers the area from Bakersfield to San Diego, CA. In 2006, KPSC had more than 3.2 million members from diverse racial groups.
Methods
After obtaining approval from our institutional review board, we performed a retrospective cohort study of patients with prostate cancer who received ADT and were part of the KPSC Cancer Registry; 1617 patients were considered for study participation. Inclusion criteria included receiving an initial diagnosis of prostate cancer between January 2003 and December 2006, receiving primary ADT monotherapy, and undergoing a minimum of two PSA tests after starting leuprolide. Exclusion criteria were: having an unknown treatment type, having an unknown baseline PSA level, and having an unknown baseline cancer stage. Patients who had undergone previous radical prostatectomy and/or radiation therapy for prostate cancer were also excluded. The remaining 681 patients who received primary ADT comprised our study participants and were grouped according to their respective race; white, black, Hispanic, and Asian/other.
ADT is achieved by using an LHRH agonist, leuprolide acetate (Lupron Depot). Patients were treated with the following dosing regimens: calendar-based, testosterone-based, and intermittent. Calendar-based therapy has long been the standard and is defined as redosing patients with an LHRH agonist according to a preset schedule determined by the manufacturer. In our study, doses were administered every three months. Testosterone-based therapy is a newer method of administering an LHRH agonist that we adopted in 2006 and is based on previously published results.11 Testosterone-based therapy is defined as redosing patients with an LHRH agonist whenever serum testosterone levels increase to >50 ng/dL. (The castrate level of testosterone, 50 ng/mL, in our study was based on the phase III study12 conducted to obtain approval from the US Food and Drug Administration for the 22.5-mg formulation of Lupron.) Intermittent dosing was defined as redosing patients with LHRH on the basis of their PSA level, without regard to testosterone levels or elapsed time.
Because no definition of biochemical failure currently exists, we sought a definition that could readily apply to any method of administering LHRH-agonist therapy. Therefore, we chose to define failure as an increase in PSA of >2 ng/mL from PSA nadir, with no subsequent decrease in PSA. Cox proportional hazards regression was used to perform a multivariate analysis and to calculate the hazard ratios for treatment failure for all groups.
… prostate cancer presents the highest disparity in mortality between blacks and whites of any cancer in the US.10
Results
Baseline characteristics were similar among groups with respect to pretreatment PSA level, Gleason score, pretreatment testosterone levels, and cancer stage. The racial composition of our population was as follows: white, 416 (61%); black, 107 (16%); Hispanic, 107 (16%); and Asian/other, 51 (7%). Prostate cancer was diagnosed at a younger age in black and Hispanic patients than in white patients (Table 1).
Table 1.
Clinical and pathology characteristics in men with prostate cancer (adenocarcinoma only) diagnosed between 2003 and 2006 a
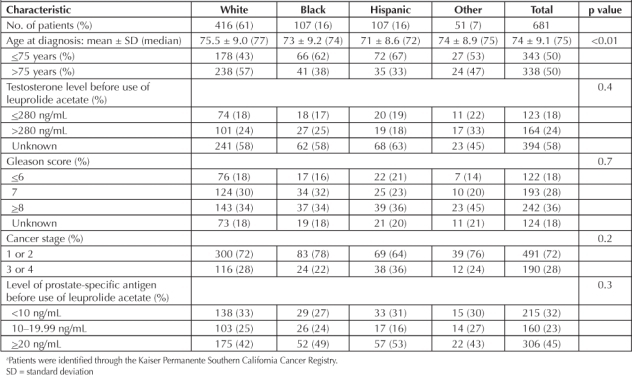
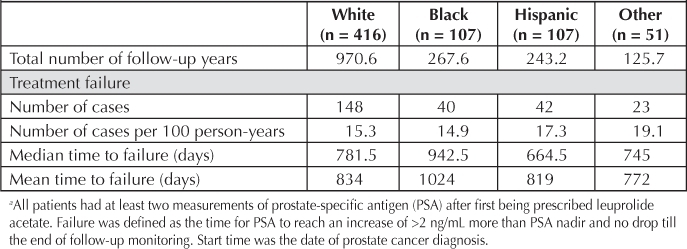
Cox proportional hazards regression was performed, controlling for all demographic variables and variables related to prostate cancer, to determine the hazard ratios for developing biochemical failure in each ethnic group compared with whites (reference group; Table 2): blacks versus whites, 0.66 (p = 0.03); Hispanics versus whites, 1.0 (p = 0.8); Asian/other versus whites, 1.50 (p = 0.1). The median number of days to PSA biochemical failure for each group was as follows (start time was the date of prostate cancer diagnosis): blacks, 942.5; whites, 781; Asians/other, 745; and Hispanics, 664.5 (Table 3).
Table 2.
Proportional hazard model comparing biochemical failure rate among races a
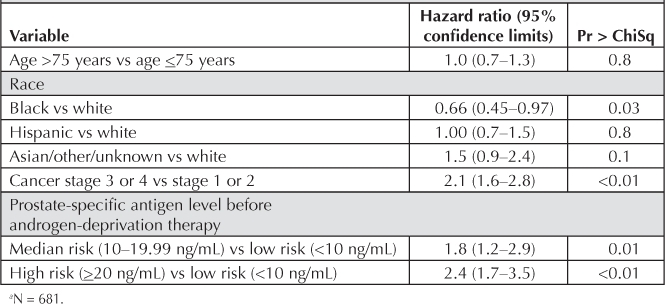
Table 3.
Biochemical failure a by race (number of cases per 100 person-years)
In this multivariate analysis, as expected, pretreatment PSA and stage were also associated with higher rates of treatment failure (Table 2).
Discussion
The incidence of prostate cancer differs among various races, being highest in blacks. In addition, age-adjusted mortality secondary to prostate cancer is higher for blacks than for whites.13 Considering absolute and relative mortality, prostate cancer presents the highest disparity in mortality between blacks and whites of any cancer in the US.10
Tewari et al14 suggested that the lower prostate cancer survival rate for blacks can be explained by differences in socioeconomic status among races and by lower surgical treatment rates for blacks. Other studies have suggested that higher prostate cancer mortality in black men may be secondary to higher tumor grades, younger age at diagnosis, and higher cancer stage. Our population is unique in that all patients, regardless of their ethnicity, have equal access to all components of health care screening and treatments. Therefore, many of the potential confounding factors that have been proposed for racial differences in prostate cancer outcomes were not present in our study. Our findings confirmed that age at prostate-cancer diagnosis is lower in blacks than in other racial groups. However, we found no intergroup differences in Gleason grade or cancer stage at diagnosis. This may have been because of the aggressive screening and easier access to medical care with KPSC.
We found that blacks with prostate cancer have a 34% lower risk of treatment failure when treated with ADT as primary therapy, compared with whites. One possible explanation for this result is different levels of serum testosterone and androgen-receptor expression in blacks compared with whites. Ross et al15 reported 15% higher mean serum testosterone levels in young black men compared with white men. Determining whether this difference in testosterone level leads to better response to hormonal therapy requires further investigation. Our study did not show a difference in pretreatment testosterone levels between blacks and whites. This could be secondary to small sample size, as data on pretreatment testosterone levels were available for only 42% of patients in our study.
Multiple studies have investigated the racial differences in prostate cancer outcome after hormonal therapy. However, we are aware of only 5 studies16–20 that specifically addressed outcomes among blacks and whites. Three of those studies measured overall survival, 1 measured disease-free survival, and the other measured biochemical failure. Four studies showed no association between race and treatment outcome. A study by Thompson et al16 showed lower overall survival for blacks compared with whites. All patients in the 5 studies just mentioned had metastatic prostate cancer. The majority of reports on the studies did not comment on whether variables such as socioeconomic factors and access to health care were controlled for in the study. These factors have frequently been used to explain racial differences in prostate cancer. All members of our managed-care population have equal access to health care. Thus, we believe that our results are more reflective of the true biologic disease progression in the two populations. In addition, our black and Hispanic populations are younger than those for the 5 studies, reflecting the American Cancer Society guideline that PSA levels in blacks and Hispanics should be checked starting when these men reach age 40 years.21 Further, we believe that using our strict inclusion and exclusion criteria, particularly the fact that we included only those patients receiving primary monotherapy, allowed for a cleaner analysis of biochemical failure in this population.
In addition, higher androgen-receptor expression in black men may play an important role. Gaston et al22 analyzed androgen-receptor protein expression in prostatic tissue from 25 black men and 25 white men who underwent radical prostatectomy for clinically localized prostate cancer. Androgen-receptor expression was 81% higher in prostate cancer in black men than in white men. The higher expression of androgen receptors in blacks could explain the better response to ADT.
Additionally, recent evidence has demonstrated that castrate-resistant prostate cancer is still very much a hormone-dependent cancer.23 Racial differences have also been found among variants of the genes of the enzymes involved in androgen biosynthesis and metabolism.24 It is possible that there is delayed and/or less upregulation of the paracrine/autocrine steroidogenesis enzymes in black men receiving ADT.
One shortcoming of our study is the use of different dosing regimens in our patient population (calendar-based, testosterone-based, and intermittent). However, these three methods of administering LHRH agonists have thus far not shown any differences in outcomes. Furthermore, all three methods are employed by urologists in everyday clinical practice. Thus, our patient population may reflect more of a real-world scenario than other studies' populations. It is important to note as well that the definition we used as evidence for early development of biochemical failure is applicable to any dosing regimen.
What remains unclear is whether the lower rate of PSA biochemical failure in our black population will lead to decreased morbidity and mortality. With longer follow-up periods, we will be able to assess whether early biochemical failure is prognostic factor for developing metastatic disease.
Conclusion
Of patients receiving ADT primary monotherapy for prostate cancer, black men may have a lower rate of biochemical failure than white men. Although the etiology of this finding is unclear, it suggests the possibility that prostate cancer in black men may be more androgen-sensitive than in white men.
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Acknowledgments
Katharine O'Moore-Klopf, ELS, of KOK Edit provided editorial assistance.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006 Mar–Apr;56(2):106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Cross CK, Shultz D, Malkowicz SB, et al. Impact of race on prostate-specific antigen outcome after radical prostatectomy for clinically localized adenocarcinoma of the prostate. J Clin Oncol. 2002 Jun 15;20(12):2863–8. doi: 10.1200/JCO.2002.11.054. [DOI] [PubMed] [Google Scholar]
- Moul JW, Wu H, Sun L, et al. Epidemiology of radical prostatectomy for localized prostate cancer in the era of prostate-specific antigen: an overview of the Department of Defense Center for Prostate Disease Research national database. Surgery. 2002 Aug;132(2):213–9. doi: 10.1067/msy.2002.125315. [DOI] [PubMed] [Google Scholar]
- Nielson ME, Han M, Mangold L, et al. Black race does not independently predict adverse outcome following radical retropubic prostatectomy at a tertiary referral center. J Urol. 2006 Aug;176(2):515–9. doi: 10.1016/j.juro.2006.03.100. [DOI] [PubMed] [Google Scholar]
- Eastham JA, Kattan MW. Disease recurrence in black and white men undergoing radical prostatectomy for clinical stage T1– T2 prostate cancer. J Urol. 2000 Jan;163(1):143–5. [PubMed] [Google Scholar]
- Powell IJ. Prostate cancer in the African American: is this a different disease? Semin Urol Oncol. 1998 Nov;16(4):221–6. [PubMed] [Google Scholar]
- Bennett CL, Ferreira MR, Davis TC, et al. Relation between literacy, race, and stage of presentation among low-income patients with prostate cancer. J Clin Oncol. 1998 Sep;16(9):3101–4. doi: 10.1200/JCO.1998.16.9.3101. [DOI] [PubMed] [Google Scholar]
- Conlisk EA, Lengerich EJ, Demark-Wahnefried W, Schildkraut JM, Aldrich TE. Prostate cancer: demographic and behavioral correlates of stage at diagnosis among blacks and whites in North Carolina. Urology. 1999 Jun;53(6):1194–9. doi: 10.1016/s0090-4295(99)00005-9. [DOI] [PubMed] [Google Scholar]
- Huggins C. Endocrine control of prostate cancer. Science. 1943 Jun 18;97(2529):541–4. doi: 10.1126/science.97.2529.541. [DOI] [PubMed] [Google Scholar]
- Peters N, Armstrong K. Racial differences in prostate cancer treatment outcomes: a systematic review. Cancer Nurs. 2005 Mar–Apr;28(2):108–18. doi: 10.1097/00002820-200503000-00004. [DOI] [PubMed] [Google Scholar]
- Pathak AS, Pacificar JS, Shapiro CE, Williams SG. Determining dosing intervals for luteinizing hormone releasing hormone agonists based on serum testosterone levels: a prospective study. J Urol. 2007 Jun;177(6):2132–5. doi: 10.1016/j.juro.2007.01.157. discussion 2135. [DOI] [PubMed] [Google Scholar]
- Sharifi R, Bruskewitz RC, Gittleman MC, Graham SD, Jr, Hudson PB, Stein B. Leuprolide acetate 22.5 mg 12-week depot formulation in the treatment of patients with advanced prostate cancer. Clin Ther. 2996 Jul–Aug;18(4):647–57. doi: 10.1016/s0149-2918(96)80215-3. [DOI] [PubMed] [Google Scholar]
- Stanford JL, Stephenson RA, Coyle L, et al. Prostate cancer trends 1973–1995, SEER Program, National Cancer Institute [monograph on the Internet] Bethesda, MD: National Institutes of Health; 1999. NIH Pub No. 99-4543. [cited 2011 Aug 1]. Available from: http://seer.cancer.gov/publications/prostate/ [Google Scholar]
- Tewari A, Horninger W, Pelzer AE, et al. Factors contributing to the racial differences in prostate cancer mortality. BJU Int. 2005 Dec;96(9):1247–52. doi: 10.1111/j.1464-410X.2005.05824.x. [DOI] [PubMed] [Google Scholar]
- Ross R, Bernstein L, Judd H, Hanisch R, Pike M, Henderson B. Serum testosterone levels in healthy young black and white men. J Natl Cancer Inst. 1986 Jan;76(1):45–8. [PubMed] [Google Scholar]
- Thompson I, Tangen C, Tolcher D, Crawford E, Eisenberger M, Moinpour C. Association of African-American ethnic background with survival in men with metastatic prostate cancer. J Natl Cancer Inst. 2001 Feb 7;93(3):219–25. doi: 10.1093/jnci/93.3.219. [DOI] [PubMed] [Google Scholar]
- Matzkin H, Rangel MC, Soloway MS. Relapse on endocrine treatment in patients with stage D2 prostate cancer. Does second-line hormonal therapy affect survival? Urology. 1993 Feb;41(2):144–8. doi: 10.1016/0090-4295(93)90167-9. [DOI] [PubMed] [Google Scholar]
- Bergan R, Walls R, Figg W, et al. Similar clinical outcomes in African-American and non-African-American males treated with suramin for metastatic prostate cancer. J Natl Med Assoc. 1997 Sep;89(9):622–8. [PMC free article] [PubMed] [Google Scholar]
- Fowler JE, Bigler SA, Renfroe DL, Dabagia MD. Prostate specific antigen in black and white men after hormonal therapy for prostate cancer. J Urol. 1997 Jul;158(1):150–4. doi: 10.1097/00005392-199707000-00047. [DOI] [PubMed] [Google Scholar]
- McLeod DG, Schellhammer PF, Vogelzang NJ, et al. Exploratory analysis on the effect of race on clinical outcome in patients with advanced prostate cancer receiving bicalutamide or flutamide, each in combination with LHRH analogues. The Casodex Combination Study Group. Prostate. 1999 Sep 1;40(4):218–24. doi: 10.1002/(sici)1097-0045(19990901)40:4<218::aid-pros2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- American Cancer Society Guidelines for the Early Detection of Cancer [monograph on the Internet] Atlanta, GA: American Cancer Society; revised 2011 Jun 23 [cited 2011 Aug 8]. Available from: www.cancer.org/Healthy/FindCancerEarly/Cancer-ScreeningGuidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. [Google Scholar]
- Gaston KE, Kim D, Singh S, Ford OH, 3rd, Mohler JL. Racial differences in androgen receptor protein expression in men with clinically localized prostate cancer. J Urol. 2003 Sep;170(3):990–3. doi: 10.1097/01.ju.0000079761.56154.e5. [DOI] [PubMed] [Google Scholar]
- Attard G, Reid AH, A'Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009 Aug 10;27(23):3742–8. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher D, Daniels G, Osman I, Lee P. Molecular mechanisms involving prostate cancer racial disparity. Am J Transl Res. 2009 Apr 20;1(3):235–48. [PMC free article] [PubMed] [Google Scholar]


