Abstract
Objective
Factors associated with mineralization and osteophyte formation in osteoarthritis (OA) are incompletely understood. Genetic polymorphisms of matrix Gla protein (MGP), a mineralization inhibitor, have been associated clinically with conditions of abnormal calcification. We therefore evaluated the relationship of MGP concentrations and polymorphisms at the MGP locus to hand OA.
Methods
Ours was an ancillary study to a 3-year randomized trial assessing the effect of vitamin K supplementation on vascular calcification and bone loss among community-dwelling elders. We studied participants who had serum MGP concentration measured and DNA genotyped for 3 MGP genetic polymorphisms (rs1800802, rs1800801, and rs4236), and who had hand radiographs. We evaluated the cross-sectional associations of serum MGP and the 3 MGP genetic polymorphisms, respectively, with radiographic hand OA using logistic regression with generalized estimating equations, adjusting for potential confounders.
Results
Radiographic hand OA in ≥ 1 joint was present in 71% of the 376 participants (mean age 74 years, mean body mass index 28 kg/m2, 59% women). No significant association between serum MGP concentrations and radiographic hand OA was found [adjusted OR 1.0 (ref), 1.40, 1.21, and 1.21 for quartiles 1–4, respectively]. Homozygosity of the rs1800802 minor allele was associated with 0.56 times lower prevalence of hand OA compared with having ≥ 1 major allele at this locus (95% CI 0.32–0.99, p = 0.046).
Conclusion
There may be an association between hand OA and genetic polymorphism at the MGP locus that is not reflected by total MGP serum concentrations. Further studies are warranted to replicate and elucidate potential mechanisms underlying these observed associations.
Key Indexing Terms: MATRIX GLA PROTEIN, POLYMORPHISM, OSTEOARTHRITIS, VITAMIN K
Matrix Gla protein (MGP) is present in bone, cartilage, and vascular smooth muscle1. It requires vitamin K for conversion through γ-carboxylation of certain glutamic acid residues to confer function. In the carboxylated form, MGP acts as an inhibitor of calcification1. In the absence of functional MGP, changes that occur in animal models are similar to processes that occur in osteoarthritis (OA). For example, abnormal growth plate mineralization, which can lead to endochondral ossification, is the same process by which osteophytes form2. Chondrocytic abnormalities including aberrant chondrocyte proliferation3, apoptosis3, abnormal chondrocyte maturation, and lack of organized chondrocyte columns4 are akin to chondrocyte abnormalities in OA. Further, OA chondrocytes have recently been demonstrated to produce primarily undercar-boxylated MGP, while normal chondrocytes produce functional carboxylated MGP5.
It is thus plausible that insufficient functional MGP associated with inadequate concentration of vitamin K potentially plays a role in OA. We have reported that low circulating vitamin K concentrations are associated with radiographic OA6. A postulated mechanism for this association may be through inadequate functional MGP. In addition, genetic polymorphisms at the MGP locus have been associated with other disorders of abnormal mineralization such as vascular calcification7, nephrolithiasis8, and low bone mineral density9.
Because of the possible relation of lack of functional MGP to OA, and because MGP genetic polymorphisms have been associated with other conditions of abnormal mineralization, we evaluated the association of serum MGP concentration and MGP genetic polymorphisms, respectively, with radiographic hand OA. We focus on OA of the hand because knee OA is largely driven by biomechanical factors; any effects of a systemic factor may be more readily identified in the hand.
MATERIALS AND METHODS
Study participants
The participants were part of a randomized controlled trial funded by the US National Institutes of Health assessing the effect of vitamin K supplementation on bone health and vascular calcification among community-dwelling elders, ages 60–80 years, recruited through direct mailings, newspaper advertisements, and notices in community centers. Of the 599 people initially screened, subjects were excluded if they had renal/hepatic disease, terminal illness, kidney stones within the past 5 years, a history of heart disease or osteoporosis, or were women < 5 years postmenopausal. In addition, subjects were excluded if they were taking bisphosphonates, calcitonin, hormone replacement, fluoride rinse, supplementation > 1500 mg/d of calcium, > 1500 IU of vitamin D, or warfarin within 6 months. The remaining subjects were randomized to oral phylloquinone (500 μg) plus multivitamin (n = 238) vs multivitamin only (n = 236) and followed for 3 years. Details of the study have been published elsewhere10. Posteroanterior hand radiographs were obtained at the last followup visit as part of an ancillary study. All participants received calcium and vitamin D supplementation. We included 376 participants, out of 452 initially enrolled, who had serum MGP concentrations, DNA genotyped for 3 MGP single nucleotide polymorphism (SNP), and hand radiographs available at the end of the 3-year trial. We asked participants whether they had any rheumatic disease, including specifically asking about rheumatoid arthritis or gout. We also used the Connective Tissue Disease Screening questionnaire to screen for potential rheumatic conditions11. None of our participants had identified themselves as having a rheumatic condition or screened positive on the screening questionnaire. The institutional review boards at Boston University Medical Center and Tufts Medical Center approved this study.
Serum MGP concentration
Total serum MGP concentrations (ng/ml) were measured at baseline and at every visit from blood samples collected after at least a 10-hour fast in a standardized manner using radioimmunoassay as described7,12. For our analysis, serum MGP concentrations from the last followup visit were used, which was concurrent with the hand radiographs obtained. The assay available at the time of our study did not distinguish between the functional and nonfunctional levels of MGP, and thus was a measure of total serum MGP concentrations.
MGP polymorphisms
Participants’ DNA was genotyped for 3 MGP SNP: rs1800802 (T-138C, promoter region), rs1800801 (G-7A, 5′UTR), and rs4236 (nonsynonymous polymorphism located on exon 4, with an alanine to threo-nine base change). The genotypes were in Hardy-Weinberg equilibrium7. Details of genotyping are described elsewhere7.
Hand radiographs
At the final 3-year followup visit, participants were invited to join a separately funded ancillary study in which bilateral posteroanterior hand radiographs were obtained using a standard technique. The radiographs were read by a board-certified musculoskeletal radiologist in a blinded fashion after calibration and adjudication. Each hand joint (4 distal interphalangeal, 4 proximal interphalangeal, 5 metacarpophalangeal, 1 interphalangeal, and 1 carpometacarpal) was scored for Kellgren-Lawrence (KL) grade (0–4), joint space narrowing (grade 0–3), and largest osteophyte (OST, grade 0–3), using the Osteoarthritis Research Society International atlas13. The musculoskeletal radiologists also read the radiographs for any evidence of other rheumatic conditions, such as rheumatoid arthritis, psoriatic arthritis, gout, or chondrocalcinosis.
Potential confounders
Information regarding age, sex, medical history, physical activity (by the Physical Activity Scale for the Elderly14), and smoking status (yes/no, current vs nonsmoker) was obtained at baseline and each followup visit. Height (nearest inch) by stadiometer and weight (pounds) by digital scale were measured in standing position. Body mass index (BMI) was calculated as weight (kg)/height2 (m2) at baseline and at each followup visit. Assays for measurement of plasma phylloquinone (nm/l) and serum 25-(OH) vitamin D (ng/ml) concentrations as described10 were drawn at baseline and at each followup visit. Information regarding highest grade completed (education) was obtained only at baseline.
Statistical analysis
Each hand joint was defined as having osteoarthritis if the KL grade was ≥ 2. We also performed analyses with joint space narrowing (defined as ≥ 2 on a scale of 0–3) and large osteophytes (OST ≥ 2, on a scale of 0–3) as outcomes. Serum MGP concentrations were categorized into quartiles. Analyses were conducted on a per-joint basis. We evaluated the association of serum MGP concentrations and each of the 3 MGP polymorphisms with radiographic hand OA (ROA) using logistic regression with generalized estimating equations15 to adjust for correlations among hand joints within an individual. The polymorphisms of the MGP locus were first evaluated using an additive mode of inheritance. Where no trend was observed with that mode of inheritance, we tested in dominant modes of inheritance (e.g., GG vs AG + AA for rs1800802, AA vs AG + GG for rs1800801, and CC vs TC + TT for rs4236), and in recessive modes of inheritance (e.g., AA vs AG + GG for rs1800802, GG vs AG + AA for rs1800801, and TT vs TC + CC for rs4236). The analyses for association of MGP concentrations with ROA were adjusted for age, sex, BMI, education, physical activity, smoking status, serum 25-(OH) vitamin D, and plasma phylloquinone concentrations, all from the same clinic visit as when the hand radiographs were obtained. Since such factors cannot influence genetic polymorphisms, the analyses of MGP polymorphisms with ROA were adjusted for age, sex, and BMI only. Separate analyses were performed, additionally adjusting for treatment with vitamin K. Analyses were conducted in SAS 9.1 (SAS Institute, Cary, North Carolina, USA). P values < 0.05 were considered statistically significant.
RESULTS
The baseline characteristics of participants in the current study are presented in Table 1. Among the 376 participants enrolled, (59% women), the mean age was 71 years (SD 5.5), and the mean BMI was 28 kg/m2 (SD 5.1) at the same clinic visit where the radiographs were obtained. Seventy-one percent of the participants had ROA in ≥ 1 hand joint.
Table 1.
Participant characteristics.
| Characteristics (n = 376) | |
|---|---|
| Age, yrs, mean (SD) | 71 (5.5) |
| Women (%) | 59 |
| Mean BMI, kg/m2 (SD) | 28 (5.1) |
| Education (% attained any college) | 55.8 |
| Physical activity, mean PASE score (SD) | 133 (61.1) |
| Smoking (% ever smoked) | 52.6 |
| Mean plasma phylloquinone, nmol/l (SD) | 2.2 (2.4) |
| Mean serum 25-(OH) vitamin D, ng/ml (SD) | 25 (7.2) |
BMI: body mass index; PASE: Physical Activity Scale for the Elderly.
As shown in Table 2, we found no association between total serum MGP concentrations and hand ROA. Compared with the lowest quartile of serum MGP, the adjusted OR (95% CI) for having hand OA were 1.40 (0.94–2.06), 1.21 (0.80–1.81), and 1.21 (0.79–1.85) for the second through fourth quartiles, respectively (p for linear trend = 0.8).
Table 2.
Association of serum matrix Gla protein (MGP) concentration with radiographic hand osteoarthritis (OA).
| Serum MGP Quartiles (* Adjusted Mean Serum MGP, ng/ml ± SE) | Crude Prevalence† of Radiographic Hand OA (%) | Adjusted OR*† (95% CI) |
|---|---|---|
| Lowest quartile | ||
| 129.8 ± 20.5 | 10.68 | 1.0 (ref) |
| Second quartile | ||
| 174.2 ± 11.8 | 15.66 | 1.40 (0.94–2.06) |
| Third quartile | ||
| 214.9 ± 10.0 | 13.60 | 1.21 (0.80–1.81) |
| Highest quartile | ||
| 281.0 ± 45.8 | 17.10 | 1.21 (0.79–1.85) |
Adjusted for age, sex, body mass index, education, smoking status, physical activity, plasma phylloquinone, and 25-(OH) vitamin D.
The crude and adjusted OR for hand OA across quartiles of serum MGP concentration, among older men and women.
The minor allele frequencies for the 3 SNP rs1800802, rs1800801, and rs4236 were 0.25, 0.39, and 0.41, respectively. The genotype frequency distributions were evaluated and determined to be in Hardy-Weinberg equilibrium as described elsewhere7. Serum MGP concentrations significantly differed among the 3 genotypes for rs1800802, while for SNP rs1800801 and rs4236, only 1 genotype was significantly different (Table 3).
Table 3.
Association of matrix Gla protein (MGP) polymorphism with radiographic hand OA.
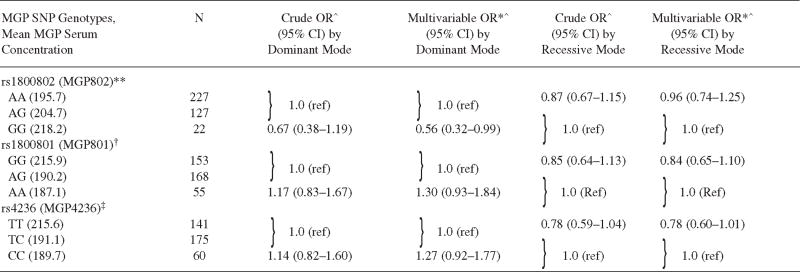
|
Crude and adjusted OR for radiographic hand OA with MGP polymorphism in dominant and recessive modes of inheritance.
Adjusted for age, sex, and BMI.
Adjusted mean serum MGP concentration is significantly different in the 3 genotypes.
Adjusted mean serum MGP concentration significantly different in GG compared with AG/AA.
Adjusted mean serum MGP concentration significantly different in TT compared with TC/CC. SNP: single-nucleotide polymorphism.
For the association of genetic polymorphisms of MGP with hand OA, no dose-response relationship was noted with the additive mode of inheritance. Using the dominant mode of inheritance, homozygosity for the minor allele of rs1800802 (GG) was associated with 44% lower odds of hand OA compared with having ≥ 1 major allele (AG + AA; adjusted OR 0.56, 95% CI 0.32–0.99, p = 0.05; Table 3). Adjustment for treatment with vitamin K did not change the result. Using the recessive mode of inheritance, a borderline association of rs4236 with odds of radiographic hand OA was also found. Homozygosity for the major allele (TT) was found to have 22% lower odds of radiographic OA compared with genotypes with ≥ 1 minor allele (TC + CC). The adjusted OR was 0.78 (95% CI 0.60–1.01) for the association. No significant association was found between the genetic polymorphism rs1800801 and radiographic hand OA (Table 3).
Similarly, using the dominant mode of inheritance, homozygosity for the minor allele of rs1800802 (GG) was associated with 75% lower odds of joint space narrowing (adjusted OR 0.25, 95% CI 0.11–0.58; Table 4) and 69% lower odds of osteophytosis (adjusted OR 0.31, 95% CI 0.17–0.58; Table 4) compared with having ≥ 1 major allele (AG + AA). Homozygosity for the major allele (TT) of rs4236 was found to have 48% lower odds of joint space narrowing compared with genotypes with at least 1 minor allele (TC + CC; adjusted OR 0.52, 95% CI 0.35–0.78; Table 4), while no such significant association with osteophytes could be found.
Table 4.
Association of matrix Gla protein (MGP) polymorphism with joint space narrowing and osteophytosis.
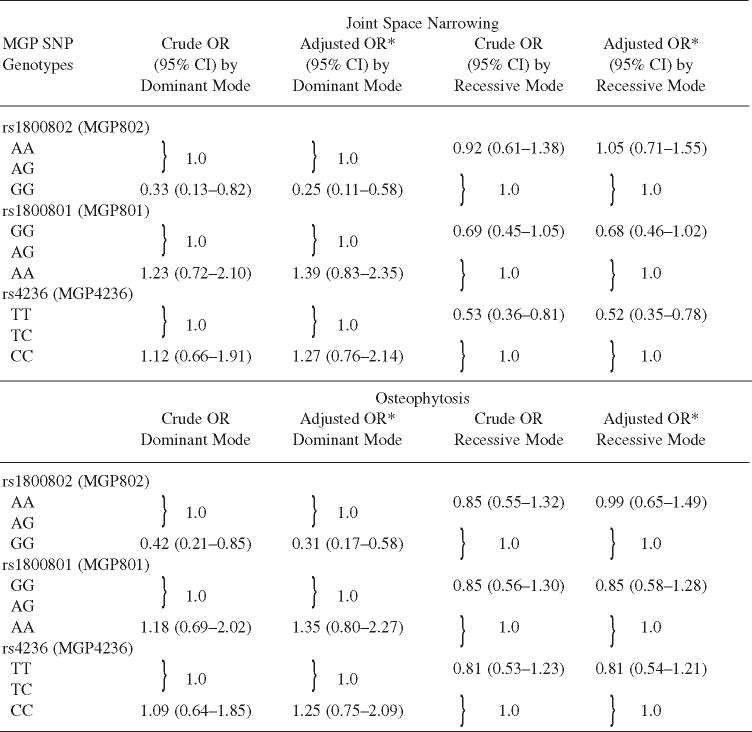
|
The crude and adjusted OR of joint space narrowing and osteophytosis with polymorphisms of MGP in dominant and recessive modes of inheritance, among older men and women.
Adjusted for age, sex, and body mass index. SNP: single-nucleotide polymorphism.
DISCUSSION
In our hypothesis-generating study, we did not find an association of hand ROA with total MGP serum concentrations, but we did find associations with particular genetic polymorphisms at the MGP locus. Homozygosity for the minor allele (GG) of rs1800802 was associated with a reduced prevalence of hand ROA, joint space narrowing, and osteophytosis compared with genotypes with ≥ 1 major allele (AG + AA). In addition, a borderline association was found between rs4236 polymorphism and hand ROA. The association did not reach statistical significance, although a significant association was found with joint space narrowing. Homozygosity for the major allele (TT) of rs4236 was associated with reduced prevalence of ROA and joint space narrowing compared with genotypes with ≥ 1 minor allele (TC + CC). Although the potential mechanism for this observation is unknown, we speculate that it could be due to specific functional variants of MGP related to these genetic polymorphisms or one that is in linkage disequilibrium. These particular genetic polymorphisms were selected for genotyping based on evidence of altered promoter activity and some suggestion, although not consistent, with other conditions of abnormal mineralization7.
Studies have found an association between polymorphisms at the MGP locus and clinical syndromes of abnormal mineralization. The rs1800802 polymorphism has been associated with vascular calcification, and with homozygosity for the minor allele being associated with a lower risk of coronary artery calcification (CAC)7, mostly among white participants. This finding is consistent with the association of this SNP with a lower prevalence of hand OA in our study, also conducted primarily with white participants, suggesting a potential common set of pathways involved in mineralization. Crosier, et al7 also demonstrated an association of the other polymorphisms, rs1800801 and rs4236, with CAC. However, other studies have shown inconsistent results, with 1 study conducted among European participants (North Ireland and France)16 and the other among African American and non-Hispanic whites17. In terms of other clinical conditions of abnormal mineralization, the rs4236 polymorphism has been associated with nephrolithiasis8 among Japanese men and women. An association of an MGP genetic polymorphism with low bone mineral density among Japanese women has also been reported9. Thus, various MGP genetic polymorphisms have been associated with a variety of clinical conditions that exhibit abnormal mineralization, a finding that supports the importance of MGP in the process of mineralization.
For OA, MGP is likely important not only because of mineralization effects, but also for regulation of cartilage matrix, as implicated by chondrocyte abnormalities that can occur in the absence of functional MGP2,4. Biologically, the role of the functional form of MGP as a key regulator of mineralization is well known and has been demonstrated in vitro and in animal models. Paralleling the association of specific MGP polymorphisms, serum MGP concentrations have been associated with CAC18. Serum MGP concentration has also been associated with renal osteodystrophy19, a condition that has not been studied in the context of MGP genetic polymorphisms. In contrast to such studies, serum MGP concentrations were not associated with OA in our study. There are other instances in literature where MGP concentrations were not associated with a clinical syndrome of abnormal mineralization. For example, O’Donnell, et al20 were unable to detect a consistent association of serum MGP concentration with CAC, although they found an association with cardiovascular risk factors20. In the same study population they found an association of CAC with a polymorphism at the MGP locus. Thus it appears that serum total MGP concentration may not be a robust marker.
It is not entirely clear why some studies have found a relation of serum MGP concentration and/or MGP genetic polymorphisms to phenotypes of abnormal mineralization while others have not. It is likely a combination of factors, including differences in ethnic groups, different ages at which phenotypes manifest, the ages at which the participants were studied, the rate of progression of diseases studied, and the prevalence of other pertinent risk factors that are likely important and contribute to these discordant findings. For serum concentration studies, the study design and number of measurements are also important methodologic considerations. As well, it is possible that total serum concentration is a sufficient correlate for some diseases, while functional MGP levels or even tissue levels of functional MGP are of greater relevance for OA, as is thought to be the case with other potential bio-markers of OA. Indeed, while both normal and OA-derived chondrocytes are capable of producing MGP, OA chondrocytes have been demonstrated to produce predominantly undercar-boxylated MGP while non-OA chondrocytes produce the functional carboxylated MGP5. Although functional levels of circulating MGP can be measured now, at the time of our study, the assays available were able to measure only total serum MGP concentration and not functional MGP concentration21.
Our study has limitations. First, our sample size may be too small to have sufficient power to precisely estimate effects of MGP concentrations with OA, and may compromise our ability to conduct more robust genetic association analyses. We also did not have available to us a validation cohort to replicate these findings. Second, there is a possibility that OA itself can lead to changes in serum MGP concentrations, leading to reverse causation in the cross-sectional analyses. Nonetheless, we did not find any association of serum MGP concentrations with OA, positive or negative, making reverse causation less likely. Further, reverse causation is not an issue for genetic polymorphism analyses because the polymorphisms are present prior to any OA occurrence. Third, the assay available at the time of our study reliably provides only total serum MGP concentration. Therefore, we are unable to comment on the association of serum functional MGP concentration (i.e., the degree of γ-carboxylation), which is likely more clinically relevant with OA. Fourth, we cannot be certain about the absence of secondary OA, but our screening methods did not identify any participants with rheumatic conditions other than a few who had chondrocalcinosis. We cannot exclude the possibility that any hand OA identified in those few with chondrocalcinosis was secondary to the chondrocalcinosis. Due to very few joints with chondrocalcinosis on hand radiographs in this sample (4 left hand, 4 right hand), we were unable to more directly evaluate this particular phenotype of abnormal mineralization in the joints. Fifth, our participant population was mostly white, a circumstance that aids the genetic analyses but limits the generalizability of our findings to other ethnic groups. Nonetheless, we consider this work exploratory and hypothesis-generating.
In our study, we tested our hypothesis that MGP concentration or polymorphism may be associated with OA. Although we were unable to find an association of MGP concentration, specific MGP genetic polymorphisms were in fact found to be associated with hand OA. Further studies are needed to replicate our results and explore mechanisms underlying these potential associations.
Acknowledgments
Support from an Arthritis Foundation Innovative Research Grant, and based upon work supported by the US Department of Agriculture, Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707, and the National Institutes of Health (AR055127, AG14759, HL696272, and AR47785).
We acknowledge Dr. Paul Price, University of California-San Diego, who measured participants’ serum MGP concentrations, and Dr. Robert Sarno, Tufts Medical Center, who read the radiographs for our study.
References
- 1.Zebboudj AF, Imura M, Bostrum K. Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J Biol Chem. 2002;277:4388–94. doi: 10.1074/jbc.M109683200. [DOI] [PubMed] [Google Scholar]
- 2.Yagami K, Suh J, Enomoto-Iwamoto M, Koyama E, Abrams W, Shapiro I, et al. Matrix GLA protein is a developmental regulator of chondrocyte mineralization and, when constitutively expressed, blocks endochondral and intramembranous ossification in the limb. J Cell Biol. 1999;147:1097–108. doi: 10.1083/jcb.147.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman B, Gigout L, Sudre L, Grant M, Wallis G. Coordinated expression of matrix Gla protein is required during endochondral ossification for chondrocyte survival. J Cell Biol. 2001;154:659–66. doi: 10.1083/jcb.200106040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo G, Ducy P, McKee M, Pinero G, Loyer E, Behringer R. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 5.Wallin R, Schurgers L, Loeser R. Biosynthesis of the vitamin K-dependent matrix Gla protein (MGP) in chondrocytes: A fetuin-MGP protein complex is assembled in vesicles shed from normal but not from osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2010;18:1096–103. doi: 10.1016/j.joca.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Neogi T, Booth S, Zhang Y, Jacques P, Terkeltaub R, Aliabadi P, et al. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum. 2006;54:1255–61. doi: 10.1002/art.21735. [DOI] [PubMed] [Google Scholar]
- 7.Crosier M, Booth S, Peter I, Ordovas J. Matrix Gla Protein polymorphisms are associated with coronary artery calcifications in men. J Nutr Sci Vitaminol (Tokyo) 2009;55:59–65. doi: 10.3177/jnsv.55.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao B, Yasui T, Itoh Y, Tozawa K, Hayashi Y, Kohri K. A polymorphism of matrix Gla protein gene is associated with kidney stones. J Urol. 2007;177:2361–5. doi: 10.1016/j.juro.2007.01.118. [DOI] [PubMed] [Google Scholar]
- 9.Tsukamoto K, Orimo H, Hosoi T, Miyao M, Yoshida H, Watanabe S, et al. Association of bone mineral density with polymorphism of the human matrix Gla protein locus in elderly women. J Bone Miner Metab. 2000;18:27–30. doi: 10.1007/s007740050006. [DOI] [PubMed] [Google Scholar]
- 10.Booth S, Dallal G, Shea M, Gundberg C, Peterson J, Dawson-Hughes B. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab. 2008;93:1217–23. doi: 10.1210/jc.2007-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlson E, Sanchez-Guerrero J, Wright E, Lew R, Daltroy L, Katz J, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5:297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 12.Otawara Y, Price P. Developmental appearance of matrix GLA protein during calcification in the rat. J Biol Chem. 1986;261:10828–32. [PubMed] [Google Scholar]
- 13.Felson D, Nevitt M, Yang M, Clancy M, Niu J, Torner J, et al. A new approach yields high rates of radiographic progression in knee osteoarthritis. J Rheumatol. 2008;35:2047–54. [PMC free article] [PubMed] [Google Scholar]
- 14.Washburn R, Ficker J. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness. 1999;39:336–40. [PubMed] [Google Scholar]
- 15.Zhang Y, Glynn RJ, Felson DT. Musculoskeletal disease research: should we analyze the joint or the person? J Rheumatol. 1996;23:1130–4. [PubMed] [Google Scholar]
- 16.Hermann S, Whatling C, Brand E, Nicaud V, Cambien F. Polymorphism of the human matrix gla protein (MGP) gene, vascular calcification, and myocardial infarction. Arterioscler Thromb Vasc Biol. 2000;20:2386–93. doi: 10.1161/01.atv.20.11.2386. [DOI] [PubMed] [Google Scholar]
- 17.Taylor B, Schreiner P, Doherty T. Matrix Gla protein and osteopontin genetic associations with coronary artery calcification and bone density: the CARDIA study. Hum Genet. 2005;116:525–8. doi: 10.1007/s00439-005-1258-3. [DOI] [PubMed] [Google Scholar]
- 18.Jono S, Ikari Y, Vermeer C, Saito S. Matrix Gla protein is associated with coronary artery calcification as assessed by electron-beam computed tomography. Thromb Haemost. 2004;91:790–4. doi: 10.1160/TH03-08-0572. [DOI] [PubMed] [Google Scholar]
- 19.Coen G, Ballanti P, Balducci A, Bonucci E. Renal osteodystrophy: alpha-Heremans Schmid glycoprotein/fetuin-A, matrix GLA protein serum levels, and bone histomorphometry. Am J Kidney Dis. 2006;48:106–13. doi: 10.1053/j.ajkd.2006.03.083. [DOI] [PubMed] [Google Scholar]
- 20.O’Donnell C, Shea M, Price P, Dawson-Hughes B, Booth S. Matrix Gla protein is associated with risk factors for atherosclerosis but not with coronary artery calcification. Arterioscler Thromb Vasc Biol. 2006;12:2769–74. doi: 10.1161/01.ATV.0000245793.83158.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker B, Schurgers L, Brandenburg V, Christenson R, Vermeer C, Ketteler M, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152:640–8. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]


