Abstract
Therapies currently used for hemophilia involve injection of protein concentrates that are expensive, invasive and associated with side effects such as development of neutralizing antibodies (inhibitors) that diminish therapeutic efficacy. Gene transfer is an attractive alternative to circumvent these issues. However, until now, clinical trials using gene therapy to treat hemophilia have failed to demonstrate sustained efficacy, although a vector based on a self-complementary adeno-associated virus has recently shown promise. This article will briefly outline a novel gene-transfer approach using self-complementary adeno-associated viral vectors using hemophilia B as a target disorder. This approach is currently being evaluated in the clinic. We will provide an overview of the development of self-complementary adeno-associated virus vectors as well as preclinical and clinical data with this vector system.
Keywords: factor IX, hemophilia B, inhibitors, inverted terminal repeats, neutralizing antibodies, recombinant adeno-associated virus, self-complementary adeno-associated virus, single-stranded adeno-associated virus, terminal resolution sites
The term ‘hemophilia’ is used to refer to a group of disorders that are characterized by impairment of blood coagulation. Each of these disorders is caused by mutations in a single gene, resulting in deficiency of a single clotting factor in the coagulation cascade. The most common manifestation of these disorders is spontaneous hemorrhage in the absence of injury, predominantly in weight-bearing joints, resulting in painful chronic arthropathy. Spontaneous bleeding into a closed space such as the skull can result in death.
Hemophilia B is an X-linked recessive condition caused by deficiency of factor IX (FIX), and is the second most common of the hemophilias, affecting approximately one in 25,000 males. A number of mutations can occur in the gene encoding FIX, each compromising function of the clotting factor to a different degree. This disorder therefore occurs along a spectrum of severity that varies inversely with the levels of functional FIX in the plasma. Generally, individuals with <1% function of clotting factor are classified as severe hemophiliacs, those with 1–5% function as moderate hemophiliacs and those with >5% function as mild hemophiliacs [1].
Current therapy of hemophilia B involves administration of clotting factor concentrates delivered as injections, which have the disadvantages of being invasive and inconvenient to the patient, besides being prohibitively expensive for many patients around the world. Another concern with this mode of treatment is the development of circulating antibodies, or ‘inhibitors’ that neutralize the clotting factor administered as concentrate, thus rendering these ineffective. Acquisition of blood-borne diseases such as HIV/AIDS or hepatitis B through contaminated blood products was a large problem in the past but is less of a problem now, due to the availability of recombinant factors and more effective screening techniques on blood products [2].
The hemophilias are ideal candidates for gene therapy as they are caused by diminished function of a single protein, which is in turn caused by alteration (by mutation or deletion) of a single gene; restoring a functional copy of the affected gene could thereby completely ameliorate the clinical manifestations of the disease. It has also been shown that restoration of circulating clotting factor to 1–2% of its physiological levels greatly improves the bleeding diathesis, and so the therapeutic goals are modest. Clinical approaches for gene and cell therapy of the related disorder hemophilia A (summarized in Table 1) have so far not yielded sustained correction of the bleeding diathesis [3,4], but have on one occasion been associated with an inflammatory response triggered by the vector [5]. These studies have revealed a need for a vector that is safer and more efficacious than the vectors that have been used thus far.
Table 1.
Clinical studies for gene and cell therapy of hemophilia A and B.
| Disease | Vector | Route of administration |
Results | Ref. |
|---|---|---|---|---|
| Hemophilia A | Transient transfection of hFVIII into autologous dermal fibroblasts |
Transplantation of fibroblasts into omentum |
Transient elevation of FVIII levels in three out of six patients |
[3] |
| Hemophilia A | Retroviral vector expressing B-domain deleted hFVIII |
Intravenous: peripheral vein |
Transient elevation of FVIII levels in nine out of 13 patients | [4] |
| Hemophilia A | Gutless adenoviral vector expressing full-length hFVIII |
Intravenous | Toxic reaction in one patient, resulting in discontinuation of treatment |
[5] |
| Hemophilia B | rAAV2 vector expressing hFIX | Intramuscular: skeletal muscle |
Effective gene transfer into muscle tissue. Therapeutic levels of FIX were not seen |
[29–31] |
| Hemophilia B | rAAV2 vector expressing hFIX | Right hepatic artery | Therapeutic levels of FIX attained for 2–4 weeks at the highest dose of administered vector. This dose was also associated with an immune response against transduced hepatocytes, abrogating clinical benefits |
[34] |
FIX: Factor IX; FVIII: Factor VIII; hFIX: Human factor IX; hFVIII: Human factor VIII; rAAV: Recombinant adeno-associated viral.
Adeno-associated viral vectors for gene therapy of hemophilia B
Adeno-associated viruses are single-stranded DNA viruses that infect human cells but are naturally replication deficient, and are not associated with human disease. Recombinant vectors derived from these viruses consist of two 145 nucleotide inverted terminal repeats flanking an expression cassette encoding a therapeutic transgene, with deletion of all the viral open reading frames (Figure 1) [6,7]. Recombinant adeno-associated viral (rAAV) vectors are less immunogenic than several other viral vectors [8], but an immune response may still occur against the vector capsid proteins as well as the transgene-encoded protein. rAAV vectors have demonstrated promise due to their efficacy as a vector for gene transfer in nondividing tissues in vivo, such as neurons [9,10], photoreceptor cells [11,12], hepatocytes [13,14] and muscle cells [15,16]. In clinical settings, rAAV vectors have been shown to be efficacious in persistent transgene expression upon transduction of skeletal muscle [17,18] or retinal pigment epithelium [19,20].
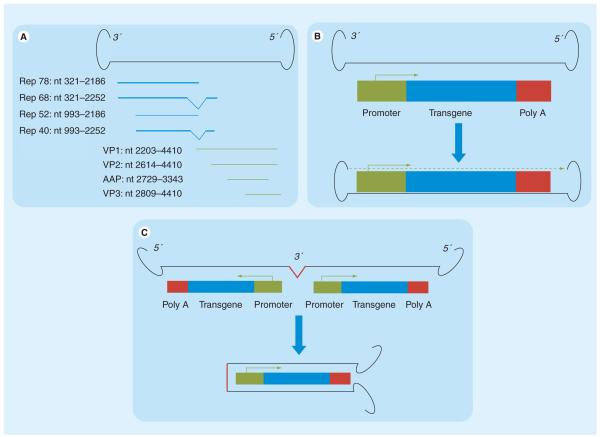
Figure 1. Adeno-associated virus variant genomes.
(A) Wild-type adeno-associated virus (AAV) genome, which consists of two inverted terminal repeats flanking coding sequences for four Rep proteins (Rep 78, Rep 68, Rep 52 and Rep 40) involved in genome replication, and four capsid proteins (VP1, VP2, AAP and VP3). (B) Recombinant AAV genome, where the coding sequences of the AAV genome are substituted with the transgenic cassette, which is flanked by the 3′ and 5′ inverted terminal repeats. Upon entry into the transduced cell (blue arrow), the single-stranded genome is converted by host factors into a transcriptionally active double-stranded form (dashed green arrow). (C) scAAV genome, in which the transgenic cassette is expressed as an inverted repeat with a deleted/mutated 3′ terminal repeats in the middle, and flanked by two intact 5′ terminal repeats. Upon entry into the transduced cell, the inverted repeats pair along their length, creating a double-stranded transcriptionally active genome, bypassing the need for second strand synthesis by host cell factors. Deletion/mutation of the 3′ terminal repeats prevents the generation of monomeric forms by the Rep endonucleases, thereby stabilizing the self-complementary AAV genome in its dimeric form.
The rAAV genome is maintained in a predominantly episomal state within transduced cells [21], although a small degree of genomic integration has been observed in some studies [22]. Administration of rAAV vector in a rodent model of mucopolysaccharoidosis VII resulted in genomic vector integration and hepatocellular carcinoma development, raising concerns that rAAV vectors may not be completely free of genotoxic effects [23]. Nevertheless, other studies have failed to demonstrate genotoxic effects following rAAV vector transfer [24–26]. These studies raise hopes that rAAV vectors may be safer than vector systems such as oncoretroviruses that have been associated with genotoxicity and tumorigenesis in clinical trials [27,28].
Two clinical trials have attempted to use rAAV vectors in hemophilia B patients (summarized in Table 1). In the first trial, patients were administered rAAV to express human factor IX (hFIX) by intramuscular injection. Although all patients showed effective gene transfer at the site of injection, the clinical response was modest, with only one patient (out of eight) showing an increase in hFIX levels above 1% [29–31]. The second trial involved liver-targeted delivery of rAAV by direct infusion of vector into the hepatic artery, for several reasons: the liver is the natural site of hFIX synthesis; liver-directed expression (by intraportal administration of vector) leads to 1.5–4-fold higher levels of hFIX in the circulation when compared with intramuscular or tail vein administration in mice [32]; and expression from the liver has been shown to mediate immune tolerance, thereby reducing the risk of developing neutralizing antibodies [32,33]. Therapeutic levels of hFIX were obtained in two patients treated at the highest dose (2 × 1012 vector genomes [vg]/kg), although this effect was transient, lasting only 2–4 weeks after vector administration. In one of these patients, the decline in hFIX levels was associated with a transient rise in liver transaminases, which may have been caused by a cellular immune response to the adeno-associated virus (AAV)2 capsid that destroyed the transduced hepatocytes, thus abrogating clinical benefits. Patients administered low or intermediate doses of vector (8 × 1010 vg/kg or 4 × 1011 vg/kg) did not develop therapeutic levels of hFIX, although one patient treated at the intermediate dose developed an immune response targeting transduced hepatocytes [34].
High vector doses necessary for a therapeutic response may therefore be associated with liver toxicity due to an immune response to the vector capsid, which appears to be dose dependent, occurring at higher vector doses than are required for a clinical response, and abrogating the possibility of stable expression of the transgene product at therapeutic levels. High vector doses are shown to be associated with nonspecific biodistribution of vector in animal models [32,35]. Production of clinical-grade vector at 1014 vg per patient can also be cost-prohibitive [36]. It may be possible to abrogate the immune response to vector by using immunosuppressive therapy [37], but this has not yet been demonstrated in human subjects. An attractive alternative would therefore be the development of more potent vectors that can stably express the transgene at a lower vector dose without triggering an immune response.
Self-complementary AAV vectors
The AAV genome is single stranded, but upon transduction of target cells the viral genome must first be converted into a transcriptionally active double-stranded form, which is a crucial rate-limiting step in transgene expression from an rAAV vector [38]. This step may be facilitated by coadministration of adenovirus [39], expression of the adenoviral genes E1 and E4 [40], or coadministration of DNA-damaging agents [41]. However, these methods are associated with toxicity to the target cells that are to be transduced.
A different strategy towards overcoming this limiting step was the development of self-complementary AAV (scAAV) vectors (Figure 1). Here, the vector genome is designed as a single-stranded inverted repeat, which folds back upon itself to form a double-stranded genome when entering into infected cells. A genome <2.5 kilobases (kb; approximately half the size of the wild-type AAV genome) can therefore be packaged as a dimer, with the two inverted repeats pairing along their length, closed covalently by a hairpin at the terminal repeat. There is therefore no need for complementary strand synthesis, and this rate-limiting step is bypassed [42]. Dimerization of the genome in this manner can be stabilized by mutation or deletion of one of the two terminal resolution sites (trs; these are Rep-binding sites contained within each inverted terminal repeat [43]), which prevents cleavage by AAV Rep proteins to form monomers [44]. The replication fork initiates at the wild-type trs and proceeds through the genome and through the mutant trs, which is unable to facilitate resolution, causing the replication fork to proceed back across the genome where it terminates at the wild-type trs. The resultant self-complementary molecule is thus flanked by wild-type trs and has a mutant trs in the middle, and dimerizes along its length when packaged into the AAV [45].
Upon transduction of target cells, the scAAV genome exists either as circular genomes or concatemers, the former being much more effective in transgene expression [46]. scAAV vector genomes are more stable and more prone to circularization upon transduction of in vivo tissues than single-stranded AAV (ssAAV) vector [44]. The ends of the scAAV vector resemble a double-stranded DNA break, and circularization of the scAAV genome depends on the action of a number of double-stranded break repair proteins, such as Mre11, Nbs1, BLM, WRN, DNA-PK(CS) and ATM, the latter being required for circularizing of the scAAV genome in nondividing cells in vivo. This suggests that DNA repair via both homologous recombination and nonhomologous end repair pathways plays a role in scAAV genome circularization in target cells [47,48].
Unlike ssAAV, transduction by scAAV is not affected by coinfection with adenovirus or treatment with hydroxyurea or proteosome inhibitors, as the limiting step of second-strand synthesis is circumvented [49,50]. When used to transduce cells in culture, scAAV vectors were shown to be 5–140-fold more effective than the corresponding ssAAV [49]. A number of cell types have been effectively transduced by scAAV vectors in a therapeutically relevant context. Mesenchymal stem cells transduced with scAAV showed gene expression for up to 3 months when transplanted into rat brain [51]. scAAV vectors have been used to deliver CD40 ligand to lung carcinoma cells, and intratumoral administration of this vector can inhibit tumor growth by CD40 ligand-mediated activation of the immune response [52]. A therapeutic transgene can be targeted to hepatoblastoma cells and hepatocellular carcinoma cells by scAAV vectors, effecting apoptosis of the target cells in vitro [53]. Immature human dendritic cells can be transduced by scAAV without detectable change to their surface marker profiles, their functional properties or their ability to differentiate into mature dendritic cells [54].
The versatility of scAAV vectors is also demonstrated by their ability to transduce several different kinds of tissues in an in vivo or ex vivo setting. scAAV and ssAAV vectors were used to express erythropoietin upon injection into mouse muscle in vivo; the scAAV vectors expressed higher levels of erythropoietin, also leading to an increased hematocrit level due to expansion of red blood cell numbers by the transgenically expressed erythropoietin. An scAAV vector expressing green fluorescent protein of a liver-specific promoter transduced hepatocytes stably for 3 months in vivo. Transduction of cells in the CNS was also more widespread using scAAV as compared with ssAAV [45]. Dispersion of scAAV vector across various cell types in the CNS is accomplished by either intravenous or intracisternal administration [55]. scAAV vectors have been used to successfully transduce human and murine pancreatic islets without affecting their function [56]. scAAV vectors transduced the trabecular meshwork of the eye in rats and monkeys effectively with stable transgene expression lasting for months (in rats) to years (in monkeys) [57]. Transduction of an intact trabecular meshwork in the human eye [58] or the retinal pigment epithelium in the mouse [59,60] is more effective with scAAV than ssAAV vectors. scAAV vectors effectively transduce retinal ganglion cells of the primate retina [61], and can be used to deliver a therapeutic transgene into mouse retinal ganglion cells in vivo [62]. Transduction of the mouse myocardium is accomplished effectively with either vector, but earlier expression of transgene is observed with scAAV compared with ssAAV [63]. Percutaneous transendocardial delivery of scAAV vector results in effective transduction of approximately 60% of the canine myocardium [64,65]. Transduction of spinal cord motor neurons in rats was accomplished by retrograde axonal transport of scAAV vector following either intramuscular or intranerve injection [66]. scAAV vectors also enabled successful transduction of the thymus in murine and primate models [67], and the joint spaces of rabbit models of arthritis [68], as well as chondrocytes and synoviocytes from equine joint tissue [69].
In addition to an increased level of transgene expression by scAAV vectors as compared with ssAAV, subtle changes in patterns of expression have also been noted. An equivalent dose of scAAV was shown to transduce up to 90% of hepatocytes in vivo, compared with 2% of hepatocytes transduced by ssAAV [44]. At a slightly lower dose of vector, scAAV transduced 25–50% of mouse hepatocytes, compared with less than 5% by ssAAV [45]. Expression of hFIX from an ssAAV vector led to intense expression of transgene from a small proportion (5%) of hepatocytes, while expression from an scAAV vector led to more homogenous but moderate expression from a large population of hepatocytes. Expression of the scAAV vector thus paralleled more closely the natural expression of hFIX, which is normally expressed in a homogenous manner throughout the liver [70].
Limitations of scAAV & attempts to optimize scAAV-mediated gene transfer
Several natural serotypes of AAV have been identified [71,72], primarily based on the surface capsid protein, which determines viral tropism and transduction efficiency, as well as immunogenicity in a human host. The most common serotype used thus far has been AAV2, which effectively transduces a variety of cell types. As humans are the natural host for AAV2, seropositivity to AAV2 is highly prevalent in the general population, which may diminish the utility of AAV2 as a therapeutic vector; results obtained in animal studies using AAV2 may not be entirely predictive of clinical responses in human subjects [73]. Other serotypes such as AAV8, which was first noted for its efficacy in tranducing liver cells [71], are lower in prevalence in the general population. The AAV8 capsid protein is less immunogenic than AAV2 [74] and is also uncoated earlier following transduction [75], further reducing the likelihood of development of a neutralizing immune response. In studies performed on nonhuman primates, the AAV8 vector was found to be more effective than AAV2 in liver cell transduction [76]. Pre-existing immunity to AAV8, though rare, does considerably diminish efficacy of gene transfer in these primate models, redirecting the vector to the spleen and away from the liver [77,78]. AAV8 also mediates effective gene transfer to the thymus [67]. Other AAV serotypes have been shown to mediate effective gene transfer to other tissues, such as AAV6 for cardiac cells [64,79], AAV1 for hematopoietic stem cells [26,80] and AAV3 for liver tumor cells [53]. Nevertheless, the AAV2 capsid is the most effective in transduction of the majority of cell types tested thus far [52,79,81,82]. Pre-existing immunity to specific AAV serotypes, as well as the need for these serotypes to transduce a target cell type, may therefore limit the potential for AAV-mediated gene transfer.
A significant limitation with the scAAV vector system is its small packaging capacity; as the AAV genome is 4.7 kb in size, the maximum packaging capacity of the scAAV vector is approximately half of this length, or about 2.3 kb. The packaging capacity can be extended to 3.3 kb genomes, but the proportion of single-stranded genomes increases linearly with genome length. Genomes larger than 3.5 kb are packaged almost solely as single-stranded forms. AAV Rep proteins encoded by helper plasmids during vector production have been shown to influence the generation of single-stranded genomes, and the use of helper plasmids that resulted in lower levels of Rep enabled the generation of scAAV vectors that were capable of packaging up to 6.6 kb genomes in their double-stranded forms [83]. The use of these modified helper plasmids may therefore enable packaging of larger therapeutic transgenes into scAAV vectors. Another study demonstrated that packaging capacity may be capsid dependent, with the AAV5 capsid enabling packaging of up to 8.9 kb of single-stranded genomes [84], although other studies find no such variation between serotypes [85].
The small size of the scAAV genome does not preclude delivery of siRNA, and therefore scAAV vectors may be ideal for this mode of therapy. The PDHA1 gene was successfully targeted by scAAV-delivered siRNAs in vitro, leading to a decline in enzyme activity levels [86,87]. PDHA1 siRNAs have also been delivered directly to rat striatum, resulting in decreased levels and activity of target protein in the striatum [88]. The MDR1 gene, which confers a drug resistant phenotype to tumor cells, has been targeted in vitro with scAAV-delivered siRNA [89]. scAAV vectors have also been used to deliver an anti-hepatitis C virus miRNA cluster that showed efficacy both in vitro and in vivo in liver cells [90].
A gender-specific imbalance in therapeutic efficacy following scAAV vector administration has been noted in some animal models, with benefits more pronounced in males than in females. Administration of an scAAV–LP1–apo3 vector in a mouse model of atherosclerosis showed retardation of aortic atherosclerotic lesions of up to 58% in male animals, but only 33% in female animals [91]. scAAV-mediated hFIX expression was also shown to be higher in male mice than in female mice [70]. Pretreatment of the mice with bortezomib before scAAV transfer or exposure of the mice to adenovirus 10–20 weeks after scAAV transfer were both successful in raising transgene expression in female animals [92]. The cause of this gender imbalance in these studies, both of which involved liver-mediated transgene expression, is unclear.
Transgene expression and transduction via AAV vectors has been shown to be inhibited by the cellular EGF receptor–protein tyrosine kinase signaling pathway [93]. The EGF receptor–protein tyrosine kinase pathway was shown to directly phosphorylate surface tyrosine residues on AAV2 capsid proteins, which inhibited transduction in a manner that was independent of cell entry and second-strand DNA synthesis [94]. The authors hypothesized that tyrosine phosphorylation of the AAV2 capsid proteins led to ubiquitination and proteosomal degradation of the AAV2 vector. They therefore substituted the surface tyrosine residues on the AAV2 capsid, and demonstrated decreased ubiquitination of the capsid, which was associated with greater transduction, more efficient intracellular trafficking of the vector and higher levels of transgenic protein [95]. These tyrosine-substituted AAV2 capsids were shown to effectively transduce mouse retina, skeletal muscle and liver in vivo [96–99].
The cellular factor FKBP52 has also been shown to phosphorylate AAV capsids and mark them for degradation. FKBP52 is itself dephosphorylated and inactivated by the cellular T-cell protein tyrosine phosphatase (TC-PTP). scAAV–TC-PTP vectors, when coadministered with ssAAV vectors, therefore act as helper viruses by preventing degradation of the ssAAV capsid [80,100]. FKBP52 can also be dephosphorylated and inactivated by protein phosphatase 5 (PP5). Coadministration of scAAV–PP5 and scAAV–TC-PTP with an ssAAV2 vector increased the transduction efficiency of the ssAAV2 vector by several fold following tail vein injection in mice, without having any adverse effects on transduced hepatocytes [101,102]. As ssAAV vectors have a higher packaging capacity than scAAV vectors, and as there are larger transgenes for which ssAAV cannot be substituted for scAAV, the aforementioned strategy is a novel use of scAAV vectors to deliver cellular factors that can increase transgene expression from the ssAAV vector.
Adeno-associated viral vectors have been shown to trigger the immune system by directly modulating cellular signaling pathways. AAV transduction was shown to activate both the canonical and noncanonical NF-κB pathways, leading to the expression of proinflammatory molecules. This effect could be blocked by the use of inhibitors to the NF-κB pathway [103]. In a comparative study, scAAV vectors were found to have a more profound stimulatory effect on local innate immune responses than ssAAV vectors. Several factors such as Toll-like receptor (TLR)-9, TLR-2, MyD88, TNF-α, IFN-α and IFN-β were upregulated. scAAV vectors also increased infiltration of neutrophils, macrophages and natural killer cells into the liver. These effects were found to be mediated by TLR-9 signaling that was stimulated by scAAV, and could be partially blocked by TLR-9 inhibition [104]. It will be important to identify the molecular mechanisms by which scAAV modulates the immune system and to design therapies that can circumvent these effects.
Owing to the small packaging capacity of the AAV genome, it is important to determine the ideal regulatory elements that could optimally express a transgene from an scAAV vector. Among 14 different small promoters that were tested, the liver-specific transthyretin (TTR) enhancer promoter produced optimal hFIX expression upon vector administration directly into the portal vein in C57BL/6 mice. Addition of the minute virus of mice (MVM) small intron and the bovine growth hormone polyA (BGHpA) was also shown to facilitate transgene expression. Codon optimization of hFIX led to a fourfold increase in expression in vivo compared with endogenous hFIX, with the increase in expression shown to be independent of vector copy number. Interestingly, the scAAV vector was distributed evenly in liver cells, thereby mimicking endogenous hFIX synthesis, whereas an ssAAV vector administered in a parallel study was localized to a small subsection of liver cells [70]. These principles (using smaller promoters that may also be tissue-specific, gene-regulatory elements such as introns and codon-optimized coding sequences) can be established for a number of gene transfer conditions [105].
Manufacture of clinical-grade scAAV vector for therapy
Several protocols have been described for the generation of AAV vector on a large scale for gene therapy [106–108]. One such report describes the generation of good manufacturing practice-grade scAAV vector at a large scale for hemophilia B clinical trials [109]. A calcium phosphate-based method was used to transiently transfect two plasmids (one expressing the transgene of interest in the scAAV backbone along with adenoviral helper sequences and the other expressing AAV helper sequences) into 293T cells expanded into ten-stack culture chambers. After 40–48 h the cells were harvested, lysed, benzonase-treated, filtered and subjected to size-exclusion and ion-exchange chromatography. The material obtained was pooled, subjected to another round of size-exclusion chromatography and eluted in a lower volume. Eluates from several such preparations were pooled to obtain the final vialed product. A total of 432 ten-stack culture chambers yielded 2 × 1015 vector genomes.
Transfection efficiency was assessed by Western blotting, and viral titer assessed by quantitative PCR (qPCR) at each stage of the manufacturing process. Standard assays were used to test for sterility, presence of DNA contaminants, mycoplasma, adventitious viruses, retroviruses, bovine viruses and porcine viruses. sodium dodecyl sulfate polyacrylamide gel electrophoresis confirmed the presence of the three viral capsid proteins, normal and alkaline agarose gel electrophoresis confirmed integrity of the viral genome and transmission electron microscopy confirmed the presence of AAV-like particles. Binchoninic acid and UV were used to measure the total protein and the capsid protein, respectively, and qPCR and UV to measure viral titer. An replication-competent AAV assay was used to test for recombinants with replicative potential. The product was also tested for potency at various doses in mice, and transgenic protein (hFIX) levels assessed at various time points by ELISA. The final vialed product was tested for sterility, pH and general appearance, titer, general safety and seal integrity, and endotoxins.
Of importance, it was found that qPCR underestimates the viral titer by as much as tenfold as compared with UV due to the fact that re-annealing of genome templates interferes with primer binding, whereas dot-blot hybridization yields titers comparable to UV [109]. This is consistent with a report showing that restriction endonuclease treatment of the viral genome to cleave the hairpins and facilitate primer binding yields higher titers consistent with UV [110]. An underestimation of viral titer used in clinical trials may influence the interpretation of the efficacy of scAAV-based therapy. Also of importance is the fact that the final product contained a proportion of empty capsids along with viral vector; the A260 nm/A280 nm reading suggests 5.3 capsid equivalents per vg, while binchoninic acid suggests a twofold increase in capsid protein [109]. The clinical relevance of this is significant, as capsid proteins may trigger a T-cell-based immune response against the vector and host cells transduced by the vector [34,73].
scAAV vectors in preclinical & clinical studies for hemophilia B
A mini-hFIX cassette was developed to facilitate its packaging into scAAV vectors. The hFIX transgene was expressed off the synthetic liver promoter 1 (LP1), which consists of liver-specific elements from the human apolipoprotein E/C-I gene locus control region and the human α1-antitrypsin promoter. The LP1 promoter has the advantages of being compact and highly liver-specific. Also included in this construct are an SV40-modified small T antigen intron and an SV40 late polyA. The hFIX cDNA is a codon-optimized 1.6 kb fragment. This construct was inserted into a modified AAV2 back-bone with an intact 5′ trs and a deleted 3′ trs. Relatively low doses of vector could deliver therapeutic levels of hFIX in FIX-knockout mice, leading to correction of the bleeding diathesis. The scAAV vector successfully transduced up to 90% of hepatocytes in 6 weeks, and had a several fold increased expression of hFIX than the corresponding ssAAV vector. Analysis of the livers of these mice showed persistence of AAV genomes as concatamers or monomeric circles. Although scAAV genomes were detected in all tissues 6 weeks after administration, transgene expression was only detected from the liver. Upon administration to nonhuman primates, scAAV vectors were shown to express therapeutic levels of hFIX that were stably maintained when administered at the low dose of 4 × 1011 vg/kg [111].
Following these promising results, the scAAV–LP1–hFIX vector was further tested for therapeutic efficacy in nonhuman primates. Upon pseudotyping with the hepatotropic AAV8 capsid, administration of the vector via peripheral vein led to stable therapeutic levels of hFIX over a period of 9 months. These levels were similar to those obtained upon administration via portal vein; an important point as patients with a bleeding diathesis may not tolerate portal vein injection, and the efficacy of peripheral vein administration of AAV8-coated vector may significantly improve therapeutic ease and safety. hFIX protein expressed off the transgene was found to be post-transcriptionally processed appropriately and was biologically active in vivo. In animals with pre-existing immunity to AAV8, pseudotyping of the vector with AAV5 capsid was shown to lead to similar levels of hFIX, showing that pre-existing immunity to different strains of AAV can be circumvented by switching between different capsids [14].
A follow-up on these animals showed that even the highest dose of vector (2 × 1012 vg/kg) produced no abnormalities detected by clinical or biochemical tests, while leading to the highest levels of hFIX expression with nearly 100% of hepatocytes transduced. A lower dose of vector (2 × 1011 vg/kg) led to expression of hFIX at >10% of physiological levels, which was maintained for 5 years of observation, although some decline in transgene copy number and the proportion of transduced hepatocytes was observed, and all animals did develop antibodies to vector capsid proteins. Clinical, biochemical and histological assays as well as ultrasound analysis during this period revealed no toxicity, demonstrating the safety and efficacy of this vector system [35].
A clinical trial is underway to evaluate efficacy of scAAV2/8–LP1–hFIXco in hemophilia B patients, and preliminary results have been reported at the 2010 Annual Meeting of the American Society of Hematology [112] and the 2011 Annual Meeting of the American Society of Gene and Cell Therapy [113]. Encouragingly, patients treated with the lowest vector dose (2 × 1010 vg/kg) showed sustained low-level hFIX expression (1–2%) with no side effects or immune response against the vector. At a slightly higher dose of vector, hFIX expression was again detected (2–4%) but, in addition, an expansion of capsid-specific T cells was detected; however, this was not associated with elevation in liver enzymes or a decline in hFIX expression. Further data from this trial will shed more light on the efficacy of the scAAV vector system.
Expert commentary
The development of scAAV vectors has greatly improved the prospects of successful gene transfer in the clinic. However, this approach is limited to a few diseases where the therapeutic transgene is approximately 1.5 kb, and thus small enough to be packaged into scAAV vectors. Possible target disorders include Gaucher’s disease [114], Krabbe’s disease, phenylketonurea [115] and ornithine transcarbamylase deficiency [116]. Promising results have been obtained for hemophilia B, a disease where liver-directed expression of hFIX to 1–2% of physiological levels can result in a marked improvement in clinical outcome.
Five-year view
Within the next few years, we will have mature data from the scAAV clinical trial for hemophilia B. This will inform us whether scAAV vectors are associated with improved clinical outcomes when compared with ssAAV vectors, as preliminary data from the first patients seem to suggest, although there are other novel aspects of the vector design used in the current clinical trial. This clinical trial will also shed light on whether higher vector doses are associated with significantly higher transgene expression, and whether this is associated with significant toxicity. The knowledge gained from these studies will directly impact therapy for other monogenic disorders.
Key issues.
Adeno-associated virus (AAV)-based vectors show promise as these viruses are nonpathogenic, efficacious in transduction of nondividing tissues in vivo and lead to long-term expression of transgenes.
AAV-based vectors have thus far shown the greatest promise for gene therapy of hemophilia B, but progress has been hampered by the need for high vector doses for efficient gene transfer and to compensate for a concomitant immune response to the vector capsid.
Self-complementary AAV (scAAV) vectors in particular are characterized by greater expression of transgene, more efficient transduction of target cells and more favorable patterns of expression.
These vectors can be encapsidated within capsid proteins from different serotypes in order to target them to particular tissues such as the liver, or to overcome pre-existing immunity to other serotypes.
A limitation of the AAV-based vector system is its small packaging capacity (which is further halved in scAAV vectors).
Manufacturing protocols have been described for production of large-scale quantities of good manufacturing practice-grade vectors for clinical use.
An scAAV2/8 vector expressing human factor IX from a liver-specific LP1 promoter has shown promise in animal models, and a clinical trial using this vector for hemophilia B is underway.
The ongoing clinical trial using scAAV vectors will be crucial to determine the direction of future clinical trials for hemophilia B, as well as other disorders.
Acknowledgments
The authors are supported by The Katharine Dormandy Trust and The National Blood Service in the UK, as well as The ASSISI Foundation of Memphis and The American Lebanese Syrian Associated Charities in the USA. Andrew M Davidoff is supported by National Heart, Lung and Blood Institute grant HL094396.
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.White GC, 2nd, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J. Factor VIII and Factor IX Subcommittee. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb. Haemost. 2001;85(3):560. [PubMed] [Google Scholar]
- 2.Lee CA. The best of times, the worst of times: a story of haemophilia. Clin Med. 2009;9(5):453–458. doi: 10.7861/clinmedicine.9-5-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth DA, Tawa NE, Jr, O’Brien JM, Treco DA, Selden RF. Nonviral transfer of the gene encoding coagulation factor VIII in patients with severe hemophilia A. N. Engl. J. Med. 2001;344(23):1735–1742. doi: 10.1056/NEJM200106073442301. • The first study to use gene therapy for hemophilia A, resulting in temporary amelioration of bleeding diathesis.
- 4.Powell JS, Ragni MV, White GC, 2nd, et al. Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion. Blood. 2003;102(6):2038–2045. doi: 10.1182/blood-2003-01-0167. [DOI] [PubMed] [Google Scholar]
- 5.Chuah MK, Collen D, Driessche T Vanden. Clinical gene transfer studies for hemophilia A. Semin. Thromb. Hemost. 2004;30(2):249–256. doi: 10.1055/s-2004-825638. [DOI] [PubMed] [Google Scholar]
- 6.Hermonat PL, Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc. Natl Acad. Sci. USA. 1984;81(20):6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tratschin JD, Miller IL, Smith MG, Carter BJ. Adeno-associated virus vector for high-frequency integration, expression, and rescue of genes in mammalian cells. Mol. Cell. Biol. 1985;5(11):3251–3260. doi: 10.1128/mcb.5.11.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr. Top. Microbiol. Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 9.Mandel RJ, Spratt SK, Snyder RO, Leff SE. Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson’s disease in rats. Proc. Natl Acad. Sci. USA. 1997;94(25):14083–14088. doi: 10.1073/pnas.94.25.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplitt MG, Leone P, Samulski RJ, et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat. Genet. 1994;8(2):148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 11.Ali RR, Sarra GM, Stephens C, et al. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat. Genet. 2000;25(3):306–310. doi: 10.1038/77068. [DOI] [PubMed] [Google Scholar]
- 12.Flannery JG, Zolotukhin S, Vaquero MI, LaVail MM, Muzyczka N, Hauswirth WW. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc. Natl Acad. Sci. USA. 1997;94(13):6916–6921. doi: 10.1073/pnas.94.13.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathwani AC, Davidoff AM, Hanawa H, et al. Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood. 2002;100(5):1662–1669. doi: 10.1182/blood-2002-02-0589. [DOI] [PubMed] [Google Scholar]
- 14.Nathwani AC, Gray JT, McIntosh J, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109(4):1414–1421. doi: 10.1182/blood-2006-03-010181. • Demonstration in a nonhuman primate model that delivery of adeno-associated virus (AAV)8 factor IX (FIX) either via a peripheral vein or the portal vein led to similar stable FIX expression and similar biodistribution. This is clinically relevant, as vector delivery via a peripheral vein in human hemophilia patients is easier and safer than vector delivery via an invasive procedure.
- 15.Fisher KJ, Jooss K, Alston J, et al. Recombinant adeno-associated virus for muscle directed gene therapy. Nat. Med. 1997;3(3):306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 16.Herzog RW, Hagstrom JN, Kung SH, et al. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc. Natl Acad. Sci. USA. 1997;94(11):5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brantly ML, Chulay JD, Wang L, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl Acad. Sci. USA. 2009;106(38):16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendell JR, Rodino-Klapac LR, Rosales XQ, et al. Sustained α-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann. Neurol. 2010;68(5):629–638. doi: 10.1002/ana.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonelli F, Maguire AM, Testa F, et al. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol. Ther. 2010;18(3):643–650. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a Phase 1 dose-escalation trial. Lancet. 2009;374(9701):1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakai H, Yant SR, Storm TA, Fuess S, Meuse L, Kay MA. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J. Virol. 2001;75(15):6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hargrove PW, Vanin EF, Kurtzman GJ, Nienhuis AW. High-level globin gene expression mediated by a recombinant adeno-associated virus genome that contains the 3′ γ globin gene regulatory element and integrates as tandem copies in erythroid cells. Blood. 1997;89(6):2167–2175. [PubMed] [Google Scholar]
- 23.Donsante A, Miller DG, Li Y, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317(5837):477. doi: 10.1126/science.1142658. • Shows that AAV vectors may integrate into the genome and drive tumorigenesis in certain circumstances.
- 24.Li H, Malani N, Hamilton SR, et al. Assessing the potential for AAV vector genotoxicity in a murine model. Blood. 2011;117(12):3311–3319. doi: 10.1182/blood-2010-08-302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han Z, Zhong L, Maina N, et al. Stable integration of recombinant adeno-associated virus vector genomes after transduction of murine hematopoietic stem cells. Hum. Gene Ther. 2008;19(3):267–278. doi: 10.1089/hum.2007.161. [DOI] [PubMed] [Google Scholar]
- 26.Maina N, Han Z, Li X, et al. Recombinant self-complementary adeno-associated virus serotype vector-mediated hematopoietic stem cell transduction and lineage-restricted, long-term transgene expression in a murine serial bone marrow transplantation model. Hum. Gene Ther. 2008;19(4):376–383. doi: 10.1089/hum.2007.143. [DOI] [PubMed] [Google Scholar]
- 27.Hacein-Bey-Abina S, von Kalle C, Schmidt M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003;348(3):255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 28.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302(5644):415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 29.Kay MA, Manno CS, Ragni MV, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat. Genet. 2000;24(3):257–261. doi: 10.1038/73464. • The first clinical trial to treat hemophilia B using AAV to deliver FIX; vector was administered intramuscularly. Safety was confirmed but systemic FIX expression was not.
- 30.Manno CS, Chew AJ, Hutchison S, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101(8):2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 31.Jiang H, Pierce GF, Ozelo MC, et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol. Ther. 2006;14(3):452–455. doi: 10.1016/j.ymthe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Nathwani AC, Davidoff A, Hanawa H, Zhou JF, Vanin EF, Nienhuis AW. Factors influencing in vivo transduction by recombinant adeno-associated viral vectors expressing the human factor IX cDNA. Blood. 2001;97(5):1258–1265. doi: 10.1182/blood.v97.5.1258. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Cao O, Swalm B, Dobrzynski E, Mingozzi F, Herzog RW. Major role of local immune responses in antibody formation to factor IX in AAV gene transfer. Gene Ther. 2005;12(19):1453–1464. doi: 10.1038/sj.gt.3302539. [DOI] [PubMed] [Google Scholar]
- 34.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV–factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12(3):342–347. doi: 10.1038/nm1358. •• The second clinical trial to treat hemophilia B using AAV to deliver FIX; the vector was administered via the hepatic artery. Clinical benefits were observed initially, but were abrogated by a cellular immune response to the vector.
- 35.Nathwani AC, Rosales C, McIntosh J, et al. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol. Ther. 2011;19(5):876–885. doi: 10.1038/mt.2010.274. • Confirms safety and efficacy of transgene expression upon administration of self-complementary AAV (scAAV)8–LP1–human FIX in a nonhuman primate model.
- 36.Hildinger M, Baldi L, Stettler M, Wurm FM. High-titer, serum-free production of adeno-associated virus vectors by polyethyleneimine-mediated plasmid transfection in mammalian suspension cells. Biotechnol. Lett. 2007;29(11):1713–1721. doi: 10.1007/s10529-007-9441-3. [DOI] [PubMed] [Google Scholar]
- 37.McIntosh JH, Cochrane M, Cobbold S, et al. Successful attenuation of humoral immunity to viral capsid and transgenic protein following AAV-mediated gene transfer with a non-depleting CD4 antibody and cyclosporine. Gene Ther. 2011 doi: 10.1038/gt.2011.64. DOI: 10.1038/gt.2011.64. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrari FK, Samulski T, Shenk T, Samulski RJ. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J. Virol. 1996;70(5):3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weitzman MD, Fisher KJ, Wilson JM. Recruitment of wild-type and recombinant adeno-associated virus into adenovirus replication centers. J. Virol. 1996;70(3):1845–1854. doi: 10.1128/jvi.70.3.1845-1854.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher KJ, Gao GP, Weitzman MD, DeMatteo R, Burda JF, Wilson JM. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J. Virol. 1996;70(1):520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexander IE, Russell DW, Miller AD. DNA-damaging agents greatly increase the transduction of nondividing cells by adeno-associated virus vectors. J. Virol. 1994;68(12):8282–8287. doi: 10.1128/jvi.68.12.8282-8287.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirata RK, Russell DW. Design and packaging of adeno-associated virus gene targeting vectors. J. Virol. 2000;74(10):4612–4620. doi: 10.1128/jvi.74.10.4612-4620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Im DS, Muzyczka N. The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell. 1990;61(3):447–457. doi: 10.1016/0092-8674(90)90526-k. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Ma HI, Li J, Sun L, Zhang J, Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10(26):2105–2111. doi: 10.1038/sj.gt.3302133. • One of two early studies showing that scAAV, generated by mutation of the 3′ inverted terminal repeat, is superior to conventional AAV vectors in transducing cell lines as well as liver and muscle in vivo.
- 45.McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10(26):2112–2118. doi: 10.1038/sj.gt.3302134. • The other of the two early studies independently showing that scAAV, generated by mutation of one of the terminal repeats, transduces liver, muscle and the CNS in vivo more efficiently than conventional AAV vectors.
- 46.Choi VW, Samulski RJ, McCarty DM. Effects of adeno-associated virus DNA hairpin structure on recombination. J. Virol. 2005;79(11):6801–6807. doi: 10.1128/JVI.79.11.6801-6807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi VW, McCarty DM, Samulski RJ. Host cell DNA repair pathways in adeno-associated viral genome processing. J. Virol. 2006;80(21):10346–10356. doi: 10.1128/JVI.00841-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cataldi MP, McCarty DM. Differential effects of DNA double-strand break repair pathways on single-strand and self-complementary adeno-associated virus vector genomes. J. Virol. 2010;84(17):8673–8682. doi: 10.1128/JVI.00641-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8(16):1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 50.Ding W, Yan Z, Zak R, Saavedra M, Rodman DM, Engelhardt JF. Second-strand genome conversion of adeno-associated virus type 2 (AAV-2) and AAV-5 is not rate limiting following apical infection of polarized human airway epithelia. J. Virol. 2003;77(13):7361–7366. doi: 10.1128/JVI.77.13.7361-7366.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SJ, Lee WI, Heo H, Shin O, Kwon YK, Lee H. Stable gene expression by self-complementary adeno-associated viruses in human MSCs. Biochem. Biophys. Res. Commun. 2007;360(3):573–579. doi: 10.1016/j.bbrc.2007.06.081. [DOI] [PubMed] [Google Scholar]
- 52.Wu JQ, Zhao WH, Li Y, Zhu B, Yin KS. Adeno-associated virus mediated gene transfer into lung cancer cells promoting CD40 ligand-based immunotherapy. Virology. 2007;368(2):309–316. doi: 10.1016/j.virol.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Glushakova LG, Lisankie MJ, Eruslanov EB, et al. AAV3-mediated transfer and expression of the pyruvate dehydrogenase E1 α subunit gene causes metabolic remodeling and apoptosis of human liver cancer cells. Mol. Genet. Metab. 2009;98(3):289–299. doi: 10.1016/j.ymgme.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin O, Kim SJ, Lee WI, Kim JY, Lee H. Effective transduction by self-complementary adeno-associated viruses of human dendritic cells with no alteration of their natural characteristics. J. Gene Med. 2008;10(7):762–769. doi: 10.1002/jgm.1204. [DOI] [PubMed] [Google Scholar]
- 55.Fu H, Muenzer J, Samulski RJ, et al. Self-complementary adeno-associated virus serotype 2 vector: global distribution and broad dispersion of AAV-mediated transgene expression in mouse brain. Mol. Ther. 2003;8(6):911–917. doi: 10.1016/j.ymthe.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 56.Rehman KK, Wang Z, Bottino R, et al. Efficient gene delivery to human and rodent islets with double-stranded (ds) AAV-based vectors. Gene Ther. 2005;12(17):1313–1323. doi: 10.1038/sj.gt.3302530. [DOI] [PubMed] [Google Scholar]
- 57.Buie LK, Rasmussen CA, Porterfield EC, et al. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Invest. Ophthalmol. Vis. Sci. 2010;51(1):236–248. doi: 10.1167/iovs.09-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borras T, Xue W, Choi VW, et al. Mechanisms of AAV transduction in glaucoma-associated human trabecular meshwork cells. J. Gene Med. 2006;8(5):589–602. doi: 10.1002/jgm.886. [DOI] [PubMed] [Google Scholar]
- 59.Yokoi K, Kachi S, Zhang HS, et al. Ocular gene transfer with self-complementary AAV vectors. Invest. Ophthalmol. Vis. Sci. 2007;48(7):3324–3328. doi: 10.1167/iovs.06-1306. [DOI] [PubMed] [Google Scholar]
- 60.Kong F, Li W, Li X, et al. Self-complementary AAV5 vector facilitates quicker transgene expression in photoreceptor and retinal pigment epithelial cells of normal mouse. Exp. Eye Res. 2010;90(5):546–554. doi: 10.1016/j.exer.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koilkonda RD, Hauswirth WW, Guy J. Efficient expression of self-complementary AAV in ganglion cells of the ex vivo primate retina. Mol. Vis. 2009;15:2796–2802. [PMC free article] [PubMed] [Google Scholar]
- 62.Koilkonda RD, Chou TH, Porciatti V, Hauswirth WW, Guy J. Induction of rapid and highly efficient expression of the human ND4 complex I subunit in the mouse visual system by self-complementary adeno-associated virus. Arch. Ophthalmol. 2010;128(7):876–883. doi: 10.1001/archophthalmol.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andino LM, Conlon TJ, Porvasnik SL, Boye SL, Hauswirth WW, Lewin AS. Rapid, widespread transduction of the murine myocardium using self-complementary adeno-associated virus. Genet. Vaccines Ther. 2007;5:13. doi: 10.1186/1479-0556-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bish LT, Sleeper MM, Brainard B, et al. Percutaneous transendocardial delivery of self-complementary adeno-associated virus 6 achieves global cardiac gene transfer in canines. Mol. Ther. 2008;16(12):1953–1959. doi: 10.1038/mt.2008.202. [DOI] [PubMed] [Google Scholar]
- 65.Bish LT, Sleeper MM, Sweeney HL. Percutaneous transendocardial delivery of self-complementary adeno-associated virus 6 in the canine. Methods Mol. Biol. 2011;709:369–378. doi: 10.1007/978-1-61737-982-6_24. [DOI] [PubMed] [Google Scholar]
- 66.Hollis ER, 2nd, Kadoya K, Hirsch M, Samulski RJ, Tuszynski MH. Efficient retrograde neuronal transduction utilizing self-complementary AAV1. Mol. Ther. 2008;16(2):296–301. doi: 10.1038/sj.mt.6300367. [DOI] [PubMed] [Google Scholar]
- 67.Moreau A, Vicente R, Dubreil L, et al. Efficient intrathymic gene transfer following in situ administration of a rAAV serotype 8 vector in mice and nonhuman primates. Mol. Ther. 2009;17(3):472–479. doi: 10.1038/mt.2008.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kay JD, Gouze E, Oligino TJ, et al. Intra-articular gene delivery and expression of interleukin-1Ra mediated by self-complementary adeno-associated virus. J. Gene Med. 2009;11(7):605–614. doi: 10.1002/jgm.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goodrich LR, Choi VW, Carbone BA, McIlwraith CW, Samulski RJ. Ex vivo serotype-specific transduction of equine joint tissue by self-complementary adeno-associated viral vectors. Hum. Gene Ther. 2009;20(12):1697–1702. doi: 10.1089/hum.2009.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu Z, Sun J, Zhang T, et al. Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of hemophilia B at low vector dose. Mol. Ther. 2008;16(2):280–289. doi: 10.1038/sj.mt.6300355. [DOI] [PubMed] [Google Scholar]
- 71.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl Acad. Sci. USA. 2002;99(18):11854–11859. doi: 10.1073/pnas.182412299. • The first study that describes isolation of AAV8 and its efficacy in transducing liver cells in vivo.
- 72.Rabinowitz JE, Rolling F, Li C, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 2002;76(2):791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mingozzi F, Maus MV, Hui DJ, et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13(4):419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 74.Vandenberghe LH, Wang L, Somanathan S, et al. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat. Med. 2006;12(8):967–971. doi: 10.1038/nm1445. [DOI] [PubMed] [Google Scholar]
- 75.Thomas CE, Storm TA, Huang Z, Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 2004;78(6):3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sarkar R, Tetreault R, Gao G, et al. Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood. 2004;103(4):1253–1260. doi: 10.1182/blood-2003-08-2954. [DOI] [PubMed] [Google Scholar]
- 77.Wang L, Calcedo R, Wang H, et al. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol. Ther. 2010;18(1):126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang L, Calcedo R, Bell P, et al. Liver gene transfer with vectors based on adeno-associated virus 8 in non-human primates: impact of pre-existing immunity. Hum. Gene Ther. 2011 doi: 10.1089/hum.2011.031. DOI: 10.1089/hum.2011.031. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sipo I, Fechner H, Pinkert S, et al. Differential internalization and nuclear uncoating of self-complementary adeno-associated virus pseudotype vectors as determinants of cardiac cell transduction. Gene Ther. 2007;14(18):1319–1329. doi: 10.1038/sj.gt.3302987. [DOI] [PubMed] [Google Scholar]
- 80.Zhong L, Li W, Li Y, et al. Evaluation of primitive murine hematopoietic stem and progenitor cell transduction in vitro and in vivo by recombinant adeno-associated virus vector serotypes 1 through 5. Hum. Gene Ther. 2006;17(3):321–333. doi: 10.1089/hum.2006.17.321. [DOI] [PubMed] [Google Scholar]
- 81.Lee HS, Shin OK, Kim SJ, et al. Efficient gene expression by self-complementary adeno-associated virus serotype 2 and 5 in various human cancer cells. Oncol. Rep. 2007;18(3):611–616. [PubMed] [Google Scholar]
- 82.Natkunarajah M, Trittibach P, McIntosh J, et al. Assessment of ocular transduction using single-stranded and self-complementary recombinant adeno-associated virus serotype 2/8. Gene Ther. 2008;15(6):463–467. doi: 10.1038/sj.gt.3303074. [DOI] [PubMed] [Google Scholar]
- 83.Wu J, Zhao W, Zhong L, et al. Self-complementary recombinant adeno-associated viral vectors: packaging capacity and the role of rep proteins in vector purity. Hum. Gene Ther. 2007;18(2):171–182. doi: 10.1089/hum.2006.088. [DOI] [PubMed] [Google Scholar]
- 84.Allocca M, Doria M, Petrillo M, et al. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J. Clin. Invest. 2008;118(5):1955–1964. doi: 10.1172/JCI34316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu Z, Yang H, Colosi P. Effect of genome size on AAV vector packaging. Mol. Ther. 2010;18(1):80–86. doi: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Han Z, Gorbatyuk M, Thomas J, Jr, Lewin AS, Srivastava A, Stacpoole PW. Down-regulation of expression of rat pyruvate dehydrogenase E1α gene by self-complementary adeno-associated virus-mediated small interfering RNA delivery. Mitochondrion. 2007;7(4):253–259. doi: 10.1016/j.mito.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han Z, Berendzen K, Zhong L, et al. A combined therapeutic approach for pyruvate dehydrogenase deficiency using self-complementary adeno-associated virus serotype-specific vectors and dichloroacetate. Mol. Genet. Metab. 2008;93(4):381–387. doi: 10.1016/j.ymgme.2007.10.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ojano-Dirain C, Glushakova LG, Zhong L, et al. An animal model of PDH deficiency using AAV8-siRNA vector-mediated knockdown of pyruvate dehydrogenase E1α. Mol. Genet. Metab. 2010;101(2–3):183–191. doi: 10.1016/j.ymgme.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu D, McCarty D, Fernandes A, Fisher M, Samulski RJ, Juliano RL. Delivery of MDR1 small interfering RNA by self-complementary recombinant adeno-associated virus vector. Mol. Ther. 2005;11(4):523–530. doi: 10.1016/j.ymthe.2004.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang X, Haurigot V, Zhou S, Luo G, Couto LB. Inhibition of hepatitis C virus replication using adeno-associated virus vector delivery of an exogenous anti-hepatitis C virus microRNA cluster. Hepatology. 2010;52(6):1877–1887. doi: 10.1002/hep.23908. [DOI] [PubMed] [Google Scholar]
- 91.Osman E, Evans V, Graham IR, et al. Preliminary evaluation of a self-complementary AAV2/8 vector for hepatic gene transfer of human apoE3 to inhibit atherosclerotic lesion development in apoE-deficient mice. Atherosclerosis. 2009;204(1):121–126. doi: 10.1016/j.atherosclerosis.2008.08.043. [DOI] [PubMed] [Google Scholar]
- 92.Nathwani AC, Cochrane M, McIntosh J, et al. Enhancing transduction of the liver by adeno-associated viral vectors. Gene Ther. 2009;16(1):60–69. doi: 10.1038/gt.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhong L, Zhao W, Wu J, et al. A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol. Ther. 2007;15(7):1323–1330. doi: 10.1038/sj.mt.6300170. [DOI] [PubMed] [Google Scholar]
- 94.Zhong L, Li B, Jayandharan G, et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381(2):194–202. doi: 10.1016/j.virol.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhong L, Li B, Mah CS, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl Acad. Sci. USA. 2008;105(22):7827–7832. doi: 10.1073/pnas.0802866105. • Mutating the surface tyrosine residues on the AAV2 capsid prevented ubiquitination and proteosomal degradation of the vector, thereby allowing effective transgene expression at a lower vector dose.
- 96.Petrs-Silva H, Dinculescu A, Li Q, et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol. Ther. 2009;17(3):463–471. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qiao C, Zhang W, Yuan Z, et al. Adeno-associated virus serotype 6 capsid tyrosine-to-phenylalanine mutations improve gene transfer to skeletal muscle. Hum. Gene Ther. 2010;21(10):1343–1348. doi: 10.1089/hum.2010.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Markusic DM, Herzog RW, Aslanidi GV, et al. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol. Ther. 2010;18(12):2048–2056. doi: 10.1038/mt.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ryals RC, Boye SL, Dinculescu A, Hauswirth WW, Boye SE. Quantifying transduction efficiencies of unmodified and tyrosine capsid mutant AAV vectors in vitro using two ocular cell lines. Mol. Vis. 2011;17:1090–1102. [PMC free article] [PubMed] [Google Scholar]
- 100.Zhong L, Chen L, Li Y, et al. Self-complementary adeno-associated virus 2 (AAV)-T cell protein tyrosine phosphatase vectors as helper viruses to improve transduction efficiency of conventional single-stranded AAV vectors in vitro and in vivo. Mol. Ther. 2004;10(5):950–957. doi: 10.1016/j.ymthe.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 101.Jayandharan GR, Zhong L, Li B, Kachniarz B, Srivastava A. Strategies for improving the transduction efficiency of single-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2008;15(18):1287–1293. doi: 10.1038/gt.2008.89. [DOI] [PubMed] [Google Scholar]
- 102.Jayandharan GR, Zhong L, Sack BK, et al. Optimized adeno-associated virus (AAV)-protein phosphatase-5 helper viruses for efficient liver transduction by single-stranded AAV vectors: therapeutic expression of factor IX at reduced vector doses. Hum. Gene Ther. 2010;21(3):271–283. doi: 10.1089/hum.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jayandharan GR, Aslanidi G, Martino AT, et al. Activation of the NF-κB pathway by adeno-associated virus (AAV) vectors and its implications in immune response and gene therapy. Proc. Natl Acad. Sci. USA. 2011;108(9):3743–3748. doi: 10.1073/pnas.1012753108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Martino AT, Suzuki M, Markusic DM, et al. The genome of self-complementary AAV vectors increases TLR9-dependent innate immune responses in the liver. Blood. 2011;117(24):6459–6468. doi: 10.1182/blood-2010-10-314518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maina N, Zhong L, Li X, et al. Optimization of recombinant adeno-associated viral vectors for human beta-globin gene transfer and transgene expression. Hum. Gene Ther. 2008;19(4):365–375. doi: 10.1089/hum.2007.173. [DOI] [PubMed] [Google Scholar]
- 106.Virag T, Cecchini S, Kotin RM. Producing recombinant adeno-associated virus in foster cells: overcoming production limitations using a baculovirus-insect cell expression strategy. Hum. Gene Ther. 2009;20(8):807–817. doi: 10.1089/hum.2009.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Clement N, Knop DR, Byrne BJ. Large-scale adeno-associated viral vector production using a herpesvirus-based system enables manufacturing for clinical studies. Hum. Gene Ther. 2009;20(8):796–806. doi: 10.1089/hum.2009.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yuan Z, Qiao C, Hu P, Li J, Xiao X. A versatile adeno-associated virus vector producer cell line method for scalable vector production of different serotypes. Hum. Gene Ther. 2011;22(5):613–624. doi: 10.1089/hum.2010.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Allay JA, Sleep S, Long S, et al. Good manufacturing practice production of self-complementary serotype 8 adeno-associated viral vector for a hemophilia B clinical trial. Hum. Gene Ther. 2011;22(5):595–604. doi: 10.1089/hum.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fagone P, Sleep S, Allay J, et al. Alternative vector titration methods for scAAV vectors eliminate systemic errors caused by template self-annealing during qPCR thermocycling. Presented at: 14th Annual Meeting of the American Society of Gene and Cell Therapy; Seattle, WA, USA. 18–21 May 2011; (Abstract 587) [Google Scholar]
- 111.Nathwani AC, Gray JT, Ng CY, et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107(7):2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nathwani A, Tuddenham E, Rosales C, et al. Early clinical trial results following administration of a low dose of a novel self complementary adeno-associated viral vector encoding human factor IX in two subjects with severe hemophilia B. Presented at: 52nd Annual Meeting of the American Society of Hematology; Orlando, FL, USA. 4–7 December 2010; (Abstract 248) [Google Scholar]
- 113.Basner-Tschakarjan E, Mingozzi F, Chen Y, et al. Dose-dependent activation of capsid-specific T cells after AAV serotype 8 vector administration in a clinical study for hemophilia B. Presented at: 14th Annual Meeting of the American Society of Gene and Cell Therapy; Seattle, WA, USA. 18–21 May 2011; (Abstract 602) [Google Scholar]
- 114.McEachern KA, Nietupski JB, Chuang WL, et al. AAV8-mediated expression of glucocerebrosidase ameliorates the storage pathology in the visceral organs of a mouse model of Gaucher disease. J. Gene Med. 2006;8(6):719–729. doi: 10.1002/jgm.901. [DOI] [PubMed] [Google Scholar]
- 115.Yagi H, Ogura T, Mizukami H, et al. Complete restoration of phenylalanine oxidation in phenylketonuria mouse by a self-complementary adeno-associated virus vector. J. Gene Med. 2011;13(2):114–122. doi: 10.1002/jgm.1543. [DOI] [PubMed] [Google Scholar]
- 116.Cunningham SC, Spinoulas A, Carpenter KH, Wilcken B, Kuchel PW, Alexander IE. AAV2/8-mediated correction of OTC deficiency is robust in adult but not neonatal Spf(ash) mice. Mol. Ther. 2009;17(8):1340–1346. doi: 10.1038/mt.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]