Abstract
Chronic stress evokes profound structural and molecular changes in the hippocampus, which may underlie spatial memory deficits. Corticotropin-releasing hormone (CRH) and CRH receptor 1 (CRHR1) mediate some of the rapid effects of stress on dendritic spine morphology and modulate learning and memory, thus providing a potential molecular basis for impaired synaptic plasticity and spatial memory by repeated stress exposure. Using adult male mice with CRHR1 conditionally inactivated in the forebrain regions, we investigated the role of CRH-CRHR1 signaling in the effects of chronic social defeat stress on spatial memory, the dendritic morphology of hippocampal CA3 pyramidal neurons, and the hippocampal expression of nectin-3, a synaptic cell adhesion molecule important in synaptic remodeling. In chronically stressed wild-type mice, spatial memory was disrupted, and the complexity of apical dendrites of CA3 neurons reduced. In contrast, stressed mice with forebrain CRHR1 deficiency exhibited normal dendritic morphology of CA3 neurons and mild impairments in spatial memory. Additionally, we showed that the expression of nectin-3 in the CA3 area was regulated by chronic stress in a CRHR1-dependent fashion and associated with spatial memory and dendritic complexity. Moreover, forebrain CRHR1 deficiency prevented the down-regulation of hippocampal glucocorticoid receptor expression by chronic stress but induced increased body weight gain during persistent stress exposure. These findings underscore the important role of forebrain CRH-CRHR1 signaling in modulating chronic stress-induced cognitive, structural and molecular adaptations, with implications for stress-related psychiatric disorders.
Keywords: Chronic stress, Cognition, CRHR1, Dendrite, Hippocampus, Nectin-3
Introduction
Chronic psychosocial stress in adulthood modulates brain structure and function, resulting in cognitive deficits and an increased risk for psychiatric disorders (de Kloet et al., 2005; Lupien et al., 2009). The hippocampus, a limbic brain region critically involved in neuroendocrine regulation of stress hormones (Kim and Diamond, 2002) and spatial memory processing (Squire et al., 2007), is particularly vulnerable to uncontrollable stress (Chen et al., 2010; Joëls and Baram, 2009; Kim and Diamond, 2002). Various forms of chronic stress lead to reversible but long-lasting spatial memory impairments in adult male rodents (Bisaz et al., 2011; Conrad et al., 1996; Wright and Conrad, 2005). These effects are associated with atrophy of apical dendrites of CA3 pyramidal neurons (Kole et al., 2004; Magariños and McEwen, 1995; McLaughlin et al., 2007; Watanabe et al., 1992), suppression of hippocampal synaptic plasticity (Joëls et al., 2004; Kole et al., 2004; Pavlides et al., 2002), and altered expression of synaptic cell adhesion molecules in the hippocampus (Bisaz et al., 2011; Sandi, 2004).
The mechanisms of chronic stress-induced spatial memory impairments remain to be understood. Chronic exposure to elevated glucocorticoids has been shown to induce apical dendritic retraction of CA3 pyramidal neurons (Conrad et al., 2007; Woolley et al., 1990) and impair spatial memory (Coburn-Litvak et al., 2003), similar to the effects caused by chronic stress. Moreover, in some studies, inhibition of glucocorticoid elevations prevents spatial memory retrieval deficits due to CA3 damage (Roozendaal et al., 2001) and impairments of spatial recognition memory by chronic stress (Wright et al., 2006). It is therefore proposed that chronic stress reduces dendritic complexity of CA3 neurons, which in turn disrupts hypothalamic-pituitary-adrenal (HPA) axis function and elevates glucocorticoid levels, leading to spatial memory deficits (Conrad, 2006). However, discrepant findings have also been reported. Repeated exposure to high levels of glucocorticoids and stress produced CA3 dendritic atrophy, but failed to impair spatial memory function (Conrad et al., 2007) and even facilitated spatial memory in ovariectomized female rats (McLaughlin et al., 2005) and hippocampus-involved contextual fear conditioning (Conrad et al., 1999). Hence, factors other than elevated glucocorticoids and CA3 dendritic retraction must be responsible for chronic stress-induced spatial memory impairments.
Corticotropin-releasing hormone (CRH) and its receptors are not only key mediators of neuroendocrine and behavioral responses to stress, but are also involved in learning and memory (Arzt and Holsboer, 2006; Heinrichs and Koob, 2004; Joëls and Baram, 2009). Transient increase in CRH facilitates hippocampus-dependent learning and memory (Lee et al., 1993; Radulovic et al., 1999; Row and Dohanich, 2008), whereas prolonged exposure to elevated CRH impairs spatial memory performance (Heinrichs et al., 1996). In the hippocampus, CRH is expressed in inhibitory interneurons (Chen et al., 2001) and interacts primarily with CRH receptor 1 (CRHR1), which is enriched in dendritic spines of pyramidal neurons (Chen et al., 2004b; Van Pett et al., 2000). Acute psychological stress induces release of hippocampal CRH (Chen et al., 2004b), which activates hippocampal neurons through CRHR1 (Chen et al., 2006; Refojo et al., 2005), and leads to rapid but reversible reduction in dendritic spines of CA3 neurons (Chen et al., 2008). Additionally, chronic exposure to CRH in vitro results in atrophy of dendrites of hippocampal neurons (Chen et al., 2004a). These findings point to the possibility that a sustained elevation of endogenous CRH during chronic stress contributes to dendritic remodeling.
Chronic stress paradigms in rodents typically involve intermittent restraint for 6 h per day (Hains et al., 2009; Watanabe et al., 1992; Wright and Conrad, 2005), a monotonous procedure that may lead to habituation (Haile et al., 2001; Willner, 2005). Chronic social defeat provides a naturalistic and complex chronic stress in male mice that is relevant to human social interactions, and has a high validity for psychiatric disorders (Krishnan et al., 2007; Tsankova et al., 2006). However, the effects of such stress on dendritic arborization, specifically the role of CRH and CRHR1 in dendritic integrity and spatial memory during recurrent social defeat, have remained unclear.
To address these issues, we subjected conditional forebrain CRHR1-deficient mice and wild-type controls to chronic social defeat stress. The effects of chronic stress were evaluated, with a focus on cognitive function and its structural and molecular substrates. We hypothesized that chronic social defeat stress would impair spatial memory in a CRHR1-dependent manner, so that forebrain CRHR1 deficiency would protect from the memory impairments provoked by chronic stress.
Materials and methods
Animals
Transgenic mice with postnatal inactivation of the Crhr1 gene in forebrain neurons (CRHR1Camk2aCre mice) were generated as described previously (Müller et al., 2003) (see Supplementary Materials and Methods). All animals were housed under a 12-hour light/dark cycle (lights on at 6 AM) and constant temperature (22±1°C) with free access to both food and water. The experiments were carried out in accordance with European Communities Council Directive 86/609/EEC. The protocols were approved by the committee for the Care and Use of Laboratory Animals of the Government of Upper Bavaria, Germany.
Chronic social defeat paradigm
The chronic social defeat paradigm was carried out as described previously (Krishnan et al., 2007; Tsankova et al., 2006) with minor modifications. Adult male CD1 mice with a body weight over 40 g were used as aggressive residents. Adult male wild-type and CRHR1Camk2aCre mice (2.5–4 months old) were single-housed 2 weeks before the start of the experiment, when the resident mice were housed in the defeat cages and their dominant behavior was trained in 3 sessions (5–10 min each) with young C57BL/6 males. CD1 males with attack latencies of more than 5 min in the last training session were not included in the experiment. The chronic social defeat stress procedure was carried out between 12 noon and 4 PM (6–10 h after lights on). Over a total of 21 days, wild-type or CRHR1Camk2aCre mice were introduced into the home cage of a different dominant CD1 mouse each day. All CD1 residents rapidly recognized and attacked the intruders within 2 min. To avoid serious injuries, the subordinate mouse was exposed to the CD1 aggressor for a maximum of 30 s after being defeated. After the aggressive encounter, the mice were separated by a holed metal partition, allowing the animals to keep continuous sensory, but not physical contact for the next 24 h. Control animals were single-housed. During the experiment, the controls were allowed to explore the defeat cages for 30 s in the absence of dominant mice and then returned to their home cages.
Experimental design
The body weight of the first batch of adult male mice (3– 4.5 months old; control wild-type, n = 13; control CRHR1Camk2aCre, n = 12; stressed wild-type, n = 13; stressed CRHR1Camk2aCre, n = 10) was monitored before and during the chronic stress procedure. Mice were tested in the object recognition task and the Y-maze task during the last week of the chronic stress paradigm. At 20 h after the last aggressive encounter, mice were rapidly anesthetized by isoflurane and decapitated. Trunk blood was collected for plasma corticosterone determination by radioimmunoassay as described previously (Schmidt et al., 2007). Hippocampi were quickly dissected from the right brain halves and immediately frozen on dry ice for Western blot analysis. The left brain halves were snap-frozen and stored at −80 °C for in situ hybridization. Adrenals were dissected and weighed.
To further assess the effects of chronic stress on spatial memory, a second batch of mice (control wild-type, n=8; control CRHR1Camk2aCre, n=8; stressed wild-type, n=7; stressed CRHR1Camk2aCre, n=7) were tested in the Y-maze task during the last week of the chronic stress paradigm. Peripheral blood was collected before and at 5 min, 30 min and 90 min after a 5-min restraint stress as described previously (Müller et al., 2003) on day 19 of the chronic social defeat paradigm to evaluate the endocrine response to acute stress. Plasma corticosterone levels were measured by radioimmunoassay (Schmidt et al.,2007). Mice were anesthetized and decapitated at 20 h after the last aggressive encounter. The left brain halves were stored at −80 °C until use, and the right halves were processed for Golgi staining (Gibb and Kolb, 1998). Another set of mice (control wild-type, n=3; control CRHR1Camk2aCre,n=3; stressed wild-type, n = 3; stressed CRHR1Camk2aCre, n = 2) were anesthetized with sodium pentobarbital (200 mg/kg of body weight, intraperitoneally) and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. Brains were postfixed in the same fixative, cryoprotected and stored at − 80 °C for double-fluorescence immunohistochemistry.
Behavioral and cognitive testing
All tests were performed between 8:00 AM and 12:00 noon and manually scored using the ANY-maze software (ANY-maze 4.50, Stoelting, Wood Dale, IL, USA).
Object recognition
The one-trial object recognition test was performed as described previously (Dere et al., 2007; Sterlemann et al., 2010) in an open fieldapparatus (50 × 50 × 50 cm3) under low illumination (30 lux). The floor of the apparatus was covered with sawdust bedding which was mixed between trials. During the first trial (acquisition phase; 10 min), the mice were presented a glass object placed in the center of the arena and allowed to explore the object freely. After a 30 min intertrial interval (ITI), the mice were presented the relocated familiar object alongside a novel one similar in size (retrieval phase; 5 min). The exploration of the objects was considered when the mice touched the objects with the nose, forepaws or vibrissae. Object preference was calculated as a ratio of time spent with the novel compared with the familiar object (Rice et al., 2008). The percentage of the time exploring and the number of contacts with the novel and the known objects was also calculated, with a higher preference for the novel object being rated as intact object recognition memory.
Y-maze
The Y-maze test was run using two batches of animals under the same conditions and results were pooled. The Y-maze apparatus was made of grey polyvinyl chloride with three symmetrical arms (30 × 10 × 15 cm3) marked by triangle-, bar- and plus-signs, respectively, as intra-maze spatial cues, and was evenly illuminated (30 lux). During the first trial (acquisition phase; 10 min), the mice were allowed to explore two of the three arms with the third arm blocked. After a 30 min ITI, the mice were placed in the center of the Y-maze and allowed to explore all arms freely (retrieval phase; 5 min). An arm entry was counted when all four limbs of the mouse were within an arm. Discrimination index was expressed as the percentage of time exploring (novel − familiar)/(novel + familiar) (Burgin et al., 2010). The percentage of time spent in and number of visits to the novel arm and the two familiar arms was also calculated, with a higher preference for the novel arm being rated as intact spatial recognition memory.
Golgi impregnation and the analysis of dendritic branches
After impregnation in Golgi–Cox solution for 21 days at room temperature, the brain tissues were kept in 30% sucrose for 2–7 days at 4 °C in the dark. Transverse hippocampal sections (200 μm) were collected and processed in 10% ammonium hydroxide (NH4OH) for 30 min, followed by 30 min of fixation in Kodak Fix (Eastman Kodak, Rochester, NY, USA). Golgi-impregnated pyramidal neurons in the CA3 area were reconstructed “blindly” using camera lucida. Neurons for analysis were chosen using unbiased, systematic sampling (8–12 neurons per animal) (Chen et al., 2004a) and included equal representation of long- and short-shaft neuronal populations. Dendritic branching was evaluated using Sholl analysis (Sholl, 1953), measuring total dendritic length and the number of intersections at concentric circles of increasing distance from the soma.
In situ hybridization
Brain halves were sectioned coronally at 16 μm through the hippocampus (Bregma −1.46 to −2.18) (Paxinos and Watson, 2001) at −20 °C in a cryotome. The sections were thaw-mounted on superfrost slides, dried, and kept at −80 °C. In situ hybridization was performed as previously described (Schmidt et al., 2007) (see Supplementary Materials and Methods).
Western blot
Western blot was performed as previously described (Sterlemann et al., 2010) (see Supplementary Materials and Methods). Because samples from the wild-type groups and the knockout groups were examined in two experiments, respective data were analyzed separately.
Double-fluorescence immunohistochemistry
Serial coronal sections were cut through the hippocampus (Bregma −1.46 to −2.18) at 30 μm thickness and 210 μm intervals in a cryotome. Double-labeling immunofluorescence was performed on free-floating sections (n = 3 per mouse) as described previously (Chen et al., 2004b). In short, after incubation with mouse anti-microtubule-associated protein 2 (MAP2; 1:1000, Abcam, Cambridge, UK) and rabbit anti-nectin-3 (1:500, Abcam) antibodies overnight at 4 °C, sections were rinsed and labeled with Alexa Fluor 488- and 647- conjugated donkey secondary antibodies (1:500, Invitrogen, Karlsruhe, Germany) for 2 h at room temperature. After rinsing, sections were transferred onto slides and coverslipped with Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (Vector laboratories, Burlingame, CA, USA).
The fluorescent images (1600 × 1600 pixels) of area CA3 were obtained with an Olympus IX81 confocal microscope (Olympus, Tokyo, Japan) at 40× magnification using the Kalman filter and sequential scanning mode under identical settings for laser power, photomultiplier gain and offset. Images were imported into the NIH ImageJ software, converted to 8-bit grayscale and thresholded uniformly. MAP2 and nectin-3 colocalized puncta were revealed using the Colocalization Highlighter plugin of the ImageJ program. A square cursor (500 × 500 pixels) was placed on the stratum radiatum, stratum lucidum, stratum pyramidale, or stratum oriens of area CA3 to define the region of interest (ROI). The number of nectin-3-positive puncta and the number of MAP2 and nectin-3 colocalized puncta were determined using the Analyze Particle module of ImageJ within each ROI. Data were normalized by taking the value of the control wild-type group as 100%.
Statistical analysis
In the retrieval phase of the cognitive tasks, mice that failed to explore either of the objects (control wild-type, n = 3; stressed wild-type, n = 5; stressed CRHR1Camk2aCre, n = 4) or any of the three arms (control CRHR1Camk2aCre, n = 1; stressed wild-type, n = 4; stressed CRHR1Camk2aCre, n = 5) were excluded from analysis.
Data were analyzed by two-way analysis of variance (ANOVA) (condition × genotype) followed by Bonferroni post hoc test as necessary. Three-way ANOVA with condition and genotype as between-subjects factors and day as a within-subject factor was performed on body weight followed by post hoc testing when appropriate. Student t test was used to compare pairs of means. The level of statistical significance was set at p<0.05. Data are expressed as mean±SEM.
Results
Forebrain CRHR1 deficiency attenuates object recognition and spatial memory impairments induced by chronic social defeat stress
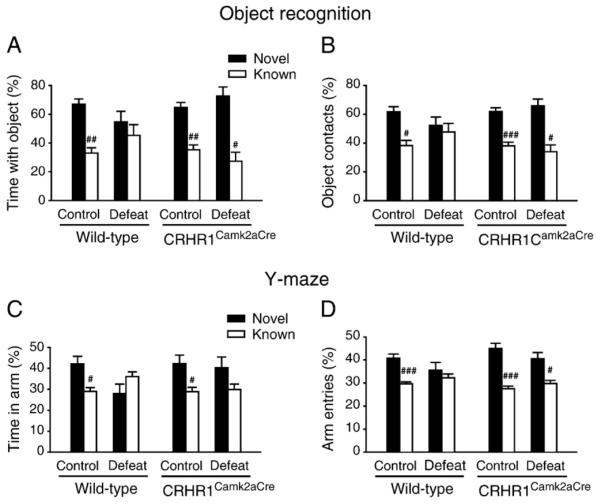
We evaluated the cognitive performance of the animals using the object recognition test, which involves medial temporal lobe structures including the hippocampus (Dere et al., 2007; Squire et al., 2007). A significant condition×genotype interaction effect [F(1,32)= 4.253, p<0.05] on object preference was observed. Control wild-type and control and stressed CRHR1Camk2aCre mice displayed intact memory as shown by significantly more exploration of the novel object than the familiar one in the retrieval phase [control wild-type: t(9)=4.623, p<0.01; control CRHR1Camk2aCre: t(11) = 4.302, p<0.01; stressed CRHR1Camk2aCre: t(5)=3.624, p<0.05; paired t test], while stressed wild-type mice failed to discriminate between the novel and familiar objects [t(7)=0.626, p=0.551; Fig. 1A]. Additionally, control mice and stressed CRHR1Camk2aCre mice made more visits to the novel object than the familiar one [control wild-type: t(9)=3.248, p<0.05; controlCRHR1Camk2aCre: t(11)=4.556, p<0.001; stressed CRHR1Camk2aCre: t(5)= 3.428, p<0.05]. In contrast, stressed wild-type mice visited both objects similarly [t(7)=0.379, p=0.716; Fig. 1B].
Fig. 1.
Object recognition and spatial recognition performance of wild-type and CRHR1Camk2aCre mice on day 15 and day 16 of the chronic social defeat paradigm, respectively. (A) Stressed wild-type mice spent comparable time with the novel and known objects, suggesting impaired object recognition memory. (B) Stressed wild-type mice explored the novel and known objects similarly. (C) Both stressed wild-type and CRHR1Camk2aCre mice spent comparable time in the novel and known arms. (D) Stressed wild-type mice visited the novel and known arms similarly, indicating impaired spatial recognition memory. #, p<0.05; ##, p<0.01; ###, p<0.001 versus respective novel object or arm. Mice: object recognition test, n = 6–12 per group; Y-maze test, n = 12–21 per group.
Hippocampus-dependent spatial recognition memory was characterized by the Y-maze test. In the retrieval phase, a significant main effect of stress [F(1,63) = 4.025, p<0.05] on discrimination index was revealed. Control wild-type and control CRHR1Camk2aCre mice significantly distinguished the novel arm from the known ones [control wild-type: t(20) = 2.394, p<0.05; control CRHR1Camk2aCre: t(18) = 2.215, p<0.05], while both stressed groups spent similar time in the novel and known arms [stressed wild-type: t(14) = −1.197, p = 0.251; stressed CRHR1Camk2aCre: t(11) = 1.341, p = 0.207; Fig. 1C]. However, control wild-type, control and defeated CRHR1Camk2aCre mice visited the novel arm more frequently than the known arms as shown by percentage of arm entries [control wild-type: t(20) = 4.252, p<0.001; control CRHR1Camk2aCre: t(18) = 5.074, p<0.001; stressed CRHR1Camk2aCre: t(11) = 2.672, p<0.05], whereas defeated wild-type mice entered the novel and familiar arms similarly [t(15) = 0.660, p = 0.519; Fig. 1D]. Poor performance of stressed wild-type mice suggests impaired hippocampus-dependent learning and memory by chronic stress, and this impairment was attenuated by CRHR1 deficiency in forebrain regions.
Reduced apical dendritic complexity of hippocampal CA3 neurons by chronic stress in wild-type but not CRHR1Camk2aCre mice
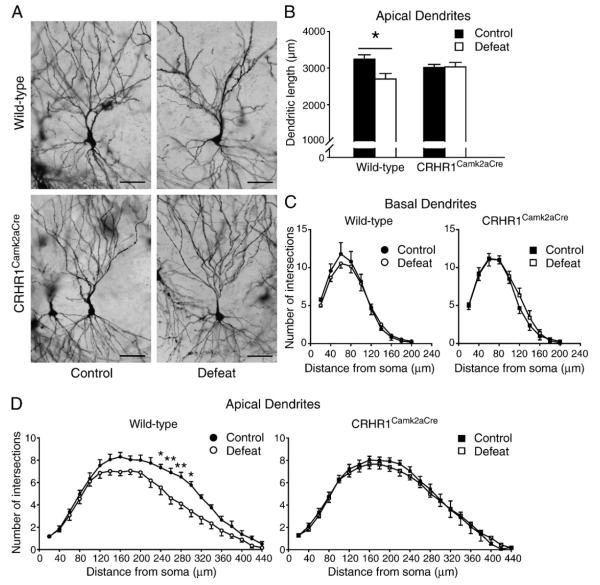
Apical dendrites of CA3 pyramidal neurons are highly vulnerable to stress-induced dendritic regression (McEwen, 1999). We therefore measured the dendritic length and the number of dendritic branching points from soma in both short- and long-shaft CA3 pyramidal neurons (Figs. 2A–D). A significant effect of stress [F(1,18) = 6.793, p<0.05] on apical dendritic length of short-shaft neurons (Figure S1A in the supplement) and a significant effect of stress [F(1,18) = 4.445, p<0.05] and condition × genotype interaction [F(1,18) = 4.931, p<0.05] on average dendritic length of both short- and long-shaft neurons (Fig. 2B) were found. Post hoc analyses suggested that chronic stress induced atrophy of apical dendrites in short-shaft CA3 pyramidal neurons and all CA3 pyramidal neurons on average (both p<0.05, Bonferroni’s test) in wild-type but not CRHR1Camk2aCre mice. Moreover, the number of branch intersections at 220–300 μm from the soma was significantly influenced by condition and/or condition × genotype interaction (Fig. 2D). Compared to the controls, defeated wild-type mice had significantly fewer branching points at 240–300 μm from the soma. However, there was no difference between groups in dendritic length (Figure S1C and S1D) or the number of branch intersections (Fig. 2C) of basal dendrites. These findings suggest that chronic stress reduces apical dendritic complexity of CA3 pyramidal neurons in a cell type-specific and dendritic segment-dependent manner, but could be abolished by hippocampal CRHR1 inactivation.
Fig. 2.
Effects of chronic social defeat stress on dendritic morphology of CA3 pyramidal neurons in wild-type and CRHR1Camk2aCre mice. (A) Representative Golgi-impregnated CA3 pyramidal neurons illustrate the reduction in apical dendritic branching in neurons from stressed wild-type mice (upper right panel), and the normalization of dendritic arborization of neurons from stressed CRHR1Camk2aCre mice (lower right panel). Scale bars = 70 μm. (B) Stressed wild-type mice showed reduced apical dendritic length in all CA3 pyramidal neurons on average compared to wild-type controls. (C) Basal dendritic complexity was not affected by chronic stress in wild-type and CRHR1Camk2aCre mice. (D) Compared to the controls, stressed wild-type mice had significantly less apical dendrite branch points at 240–300 μm from the soma of CA3 pyramidal neurons. *, p<0.05; **, p<0.01 versus control wild-type group. Mice: control wild-type, n = 4; the other three groups, n = 6.
Dysregulated nectin-3 expression in hippocampal CA3 neurons in stressed wild-type but not CRHR1Camk2aCre mice
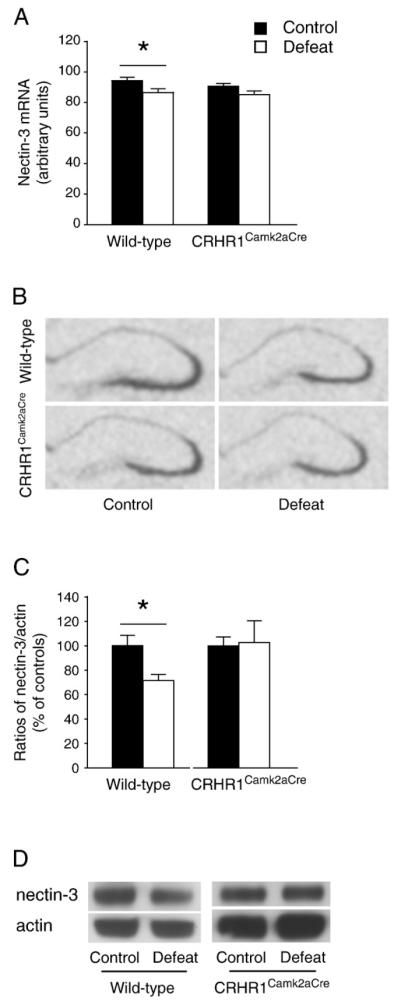
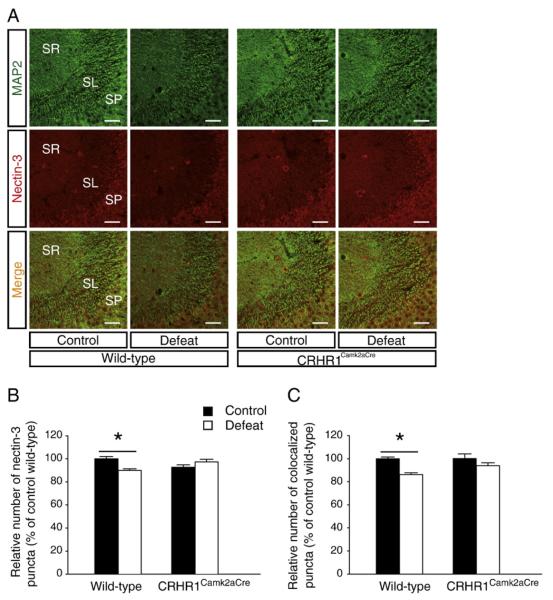
Nectin-3 is highly enriched in hippocampal CA3 neurons and implicated in synaptic remodeling (Honda et al., 2006; Majima et al., 2009; Mizoguchi et al., 2002). Because the spatial distribution pattern of nectin-3 overlapped the sites of dendritic impoverishment observed here, we examined nectin-3 mRNA and protein expression in the hippocampus (Figs. 3 and 4). A significant effect of stress [F(1,33) = 8.010, p<0.01] on nectin-3 mRNA expression was found (Figs. 3A and B). Nectin-3 mRNA levels in stressed wild-type mice were significantly decreased in area CA3 (p<0.05 versus wild-type controls, Bonferroni’s test). The mRNA results were supported by reduced nectin-3 protein expression level in hippocampal lysates of stressed wild-type mice [t(10) = 2.893, p<0.05, unpaired t test;Figs. 3C and D]. Detailed analyses revealed that the number of nectin-3-positive puncta in area CA3 was significantly influenced bycondition × genotype interaction [F(1,7) = 13.370, p<0.01] in the stratum radiatum and by stress [F(1,7) = 6.539, p<0.05] in the stratum pyramidale (Fig. 4A). In stressed wild-type mice, decreased nectin-3 level was prominent in the stratum radiatum (p<0.05, Bonferroni’s test; Fig. 4B). Moreover, an effect of stress [F(1,7) = 12.800, p<0.01] was noticed on the number of MAP2 and nectin-3 colocalized puncta in the stratum radiatum, which was reduced in stressed wild-type mice (p<0.05 versus the controls, Bonferroni’s test; Fig. 4C). No difference in either gene or protein expression of hippocampal nectin-3 between control and stressed CRHR1Camk2aCre mice was found.
Fig. 3.
Chronic social defeat stress reduced hippocampal CA3 nectin-3 gene (A) and protein (C) expression in wild-type but not CRHR1Camk2aCre mice. Representative in situ hybridization (B) and Western blot (D) images show nectin-3 mRNA and protein expression in the hippocampus, respectively. *, p<0.05 versus control wild-type group. Mice: in situ hybridization, n = 8–11 per group; Western blot, n = 6 per group.
Fig. 4.
Effects of chronic social defeat stress on nectin-3 protein expression in area CA3 in wild-type and CRHR1Camk2aCre mice. (A) Representative confocal images of area CA3 immunostained for MAP2 and nectin-3. SR, stratum radiatum; SL, stratum lucidum; SP, stratum pyramidale. Scale bars = 50 μm. Stressed wild-type mice had reduced number of nectin-3-positive puncta (B) and MAP2 and nectin-3 colocalized puncta (C) in the stratum radiatum of area CA3. *, p<0.05 versus control wild-type group. Mice: stressed CRHR1Camk2aCre, n = 2; the other three groups, n = 3.
Altered hippocampal GR, but not MR, gene expression in stressed wild-type mice
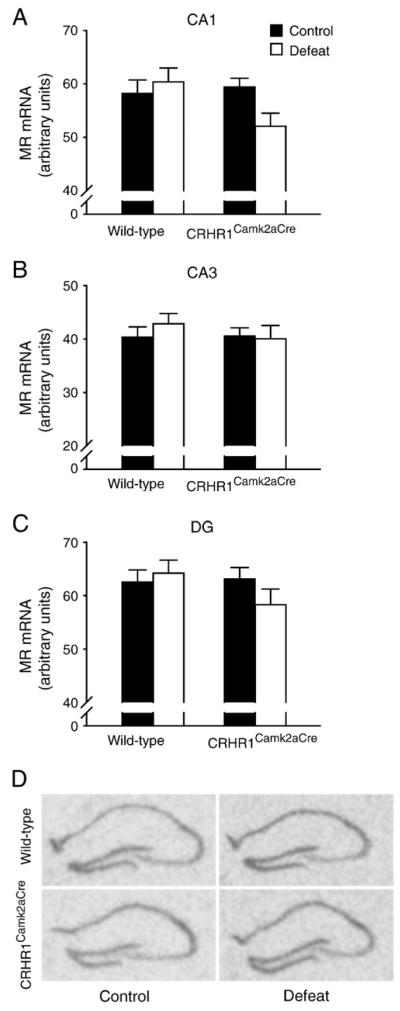
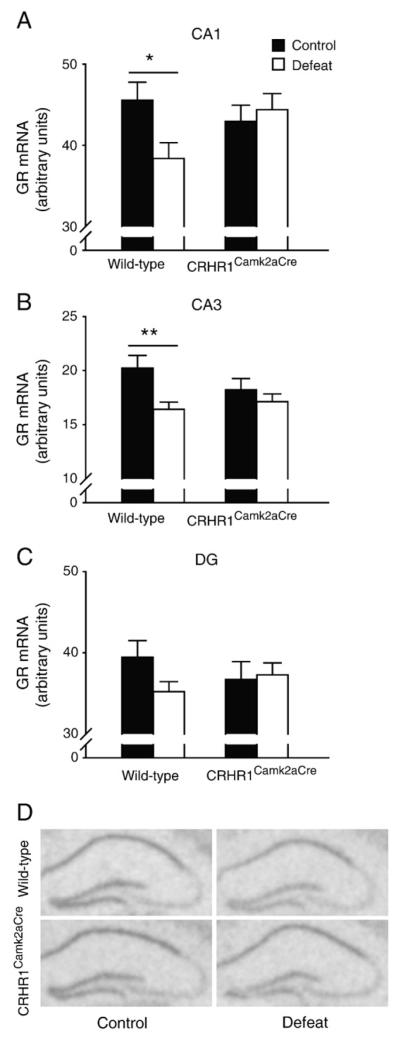
To investigate whether the hippocampal dendritic loss that accompanied the cognitive deficits in stressed wild-type mice was related to genes involved in HPA axis regulation and neuroendocrine response to stress, mRNA levels of mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) in the hippocampus were measured. MR gene expression in hippocampal CA1, CA3 and dentate gyrus regions was not altered by chronic stress in wild-type and CRHR1Camk2aCre mice (Figs. 5A–D). A significant interaction effect [F(1,33) = 4.380, p<0.05] on CA1 GR mRNA expression (Fig. 6A) and a significant effect of stress [F(1,33) = 7.314, p<0.05] on CA3 GR mRNA level (Fig. 6B) were noticed. Similar to previous findings (Kitraki et al., 1999; Wright et al., 2006), GR gene expression in CA1 and CA3 regions was significantly reduced by chronic stress in wild-type (p<0.05 and p<0.01 versus the controls, respectively, Bonferroni’s test) but not in CRHR1Camk2aCre mice. No difference in GR mRNA expression was observed in the dentate gyrus (Figs. 6C and D).
Fig. 5.
Effects of chronic social defeat stress on hippocampal MR gene expression in wild-type and CRHR1Camk2aCre mice. MR mRNA levels in CA1 (A), CA3 (B), and dentate gyrus (DG) (C) were not altered by chronic stress in wild-type and CRHR1Camk2aCre mice. (D) Representative in situ hybridization images showing MR mRNA expression in the hippocampus. Mice: n = 8–11 per group.
Fig. 6.
Effects of chronic social defeat stress on hippocampal GR gene expression in wild-type and CRHR1Camk2aCre mice. GR mRNA signals were significantly decreased in CA1 (A) and CA3 (B), but not DG (C), in stressed wild-type mice versus wild-type controls. (D) Representative in situ hybridization images showing GR mRNA expression in the hippocampus. *, p<0.05; **, p<0.01 versus control wild-type group. Mice: n = 8–11 per group.
Metabolic and endocrine consequences of chronic stress
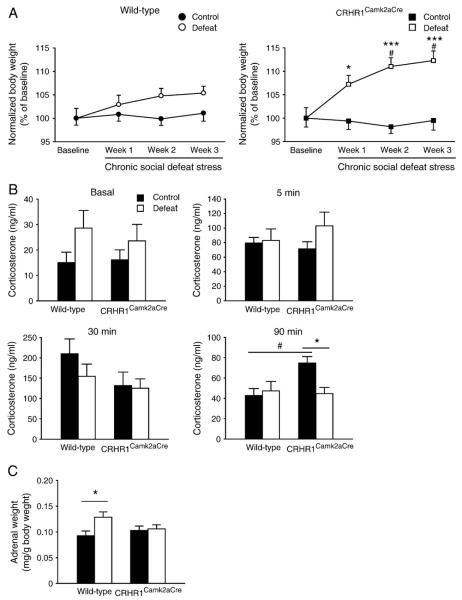
To assess the effects of chronic stress on metabolic function, body weight was monitored (Fig. 7A). All groups had similar baseline body weight (data not shown). A main effect of stress [F(1,44) = 10.746, p<0.01] on body weight change was detected. CRHR1Camk2aCre mice undergoing the stress paradigm showed a significantly higher body weight gain compared to unstressed controls (week 1, p<0.05; week 2, p<0.001; week 3, p<0.001; Bonferroni’s test). Moreover, during the last two weeks of the stress paradigm, CRHR1Camk2aCre mice gained more weight than wild-type mice under stressful situations (p<0.05 for both weeks, Bonferroni’s test). There was no significant effect of genotype or interaction on body weight.
Fig. 7.
Effects of chronic social defeat stress on body weight, stress response and adrenal weight in wild-type and CRHR1Camk2aCre mice. (A) Body weight at 1 day before the chronic stress procedure was set as baseline. Mice were again weighed on days 7, 14 and 21 of stress. Stressed CRHR1Camk2aCre mice showed significantly increased body weight gain during the chronic stress paradigm compared to control CRHR1Camk2aCre and stressed wild-type mice (n = 10–13 per group). (B) Plasma corticosterone levels at basal conditions and 5 min and 30 min after restraint stress were comparable between groups during the chronic stress procedure. Control CRHR1Camk2aCre mice showed higher corticosterone levels than control wild-type and stressed CRHR1Camk2aCre mice at 90 min after the acute stress (n = 4–7 per group). (C) Stressed wild-type mice had significantly enlarged adrenals compared to wild-type controls (n = 8–11 per group). *, p<0.05; ***, p<0.001 versus respective control group. #, p<0.05 versus wild-type group under the same condition.
Basal plasma corticosterone levels were comparable between groups during (Fig. 7B) and immediately after (Table S1) chronic stress exposure. No difference in corticosterone levels was observed between groups at 5 min and 30 min after the restraint stress challenge. However, at 90 min after the acute stress, an interaction [F(1,19) = 5.282, p<0.05] effect on corticosterone concentrations was found, and control CRHR1Camk2aCre mice showed higher corticosterone levels than control wild-type and stressed CRHR1Camk2aCre mice (both p<0.05, Bonferroni’s test). In addition, a significant main effect of stress [F(1,34) = 4.248, p<0.05] on adrenal weight (Fig. 7C) was noticed. Post hoc analysis showed that chronic stress increased adrenal weight in wild-type animals (p<0.05 versus the controls, Bonferroni’s test), while stressed and stress-naïve CRHR1Camk2aCre mice had comparable adrenal weight. No other significant main or interaction effects on adrenal weight were detected.
Discussion
Chronic stress induces spatial memory impairments and dendritic remodeling. In the current study, we demonstrate that forebrain CRHR1 deficiency attenuates spatial memory deficits and prevents the dendritic regression of CA3 neurons and the loss of hippocampal nectin-3 expression induced by chronic social defeat stress. Moreover, the metabolic and neuroendocrine effects of chronic social defeat stress are also dependent on CRHR1 in the forebrain region. Together, our findings suggest that the forebrain CRH-CRHR1 system plays critical roles in the modulation of memory function under chronic stress.
Transient elevations of CRH in the hippocampus facilitate long-term potentiation under acute stress conditions (Blank et al., 2002), and enhance hippocampus-dependent learning and memory (Lee et al., 1993; Radulovic et al., 1999; Row and Dohanich, 2008). However, long-term consequences of hippocampal CRH and CRHR1 alterations and their involvement in chronic stress-induced cognitive deficits remain to be understood. Previous studies reported that both central CRH overexpressing (Heinrichs et al., 1996) and CRHR1 knockout mice (Contarino et al., 1999) showed impaired spatial memory, but the interpretation of the cognitive phenotype was limited by prominent physical and neuroendocrine dysfunctions in these mice. By using a mouse line in which CRHR1 is postnatally inactivated in forebrain neurons and basal HPA activity remains intact (Müller et al., 2003), we were able to dissect the involvement of hippocampal CRHR1 in chronic stress-modulated cognition. In the object recognition test, stressed wild-type mice, but not stressed CRHR1Camk2aCre mice, exhibited impaired memory performance, indicating that forebrain CRHR1 activation may mediate chronic stress-induced cognitive dysfunction. Though the role of the hippocampus in this test remains controversial (Dere et al., 2007), the involvement of dorsal hippocampus has been recently demonstrated (Broadbent et al., 2010). Additionally, in the context of stress, memory defects in this test strongly correlated with spine loss in apical dendrites of CA3 neurons, which was dependent on CRH-CRHR1 signaling (Chen et al., 2010). In the Y-maze task that specifically requires hippocampal network function, stressed wild-type mice exhibited profound spatial memory deficits whereas stressed CRHR1Camk2aCre mice displayed only mild cognitive impairments. Notably, cognition of unstressed CRHR1Camk2aCre mice was intact in the object recognition and Y-maze tests, suggesting that lack of hippocampal CRHR1 signaling does not interfere with cognitive function assessed by these tests under basal conditions, but could ameliorate the deleterious effects of chronic stress on spatial memory. Furthermore, based on our results and previous reports (Blank et al., 2002; Chen et al., 2010; Heinrichs et al., 1996; Hogan et al., 2005; Row and Dohanich, 2008), hippocampal CRH may modulate spatial memory through CRHR1 in a biphasic pattern: short-term exposure to moderate levels of CRH facilitates, whereas prolonged elevations of CRH negatively influence, the spatial memory performance.
Neuronal structural changes are thought to underlie synaptic plasticity and memory. Chronic exposure to stress reversibly alters the dendritic length and complexity of CA3 pyramidal neurons (Kole et al., 2004; Magariños and McEwen, 1995; McLaughlin et al., 2007; Watanabe et al., 1992), which is correlated with spatial memory deficits due to chronic stress (Conrad et al., 1996; Wright and Conrad, 2005). Repeated administration of glucocorticoids mimics the effectsof chronic stress on dendritic morphology (Conrad et al., 2007; Woolley et al., 1990), but fails to impair spatial memory consistently (Coburn-Litvak et al., 2003; Conrad et al., 2007). Recently, CRH has been shown to regulate stress-induced spine loss and dendritic remodeling of CA3 pyramidal neurons via CRHR1 (Chen et al., 2008). Therefore, CRH and CRHR1 are promising molecular substrates for chronic stress-induced structural and cognitive changes. Consistent with previous findings (Kole et al., 2004), chronic social defeat stress induced atrophy of apical dendrites in a subpopulation of CA3 neurons in a segment-dependent manner. The loss of dendritic branches and their respective spines provides a plausible basis for some of the cognitive defects found in stressed wild-type mice. In contrast, forebrain CRHR1 deficiency prevented the effects of chronic stress on CA3 dendritic length and complexity in CRHR1Camk2aCre mice. These results suggest that CRH-CRHR1 signaling plays a crucial role in chronic stress-evoked structural plasticity.
Mechanisms by which chronic stress promotes dendritic retraction are unclear. One possibility is via the dying-back of dendrites after loss of their synapses and spines. Synaptic cell adhesion molecules play essential roles in the growth, maintenance and retraction of synapse-carrying spines (Dalva et al., 2007), and have been implicated in chronic stress-induced structural changes and cognitive deficits (Bisaz et al., 2011; Sandi, 2004). Nectin-3, an immunoglobulin-like cell adhesion molecule highly enriched in CA3 neurons, primarily localizes at puncta adherentia junctions (PAJs) and participates in synaptogenesis and synaptic remodeling (Majima et al., 2009; Mizoguchi et al., 2002). Genetic deletion of nectin-3 destabilizes PAJs at the mossy fiber-CA3 synapses and leads to aberrant mossy fiber sprouting in the hippocampus, indicating its involvement in axodendritic adhesion and structural plasticity (Honda et al., 2006). Our results showed that the expression of hippocampal nectin-3, especially in the CA3 stratum radiatum where dendritic arborization was reduced by chronic stress, was down-regulated in stressed wild-type but not stressed CRHR1Camk2aCre mice. These findings are inconclusive about the behavioral significance of nectin-3 and the casual relationship between nectin-3 dysregulation and dendritic shrinkage. However, we provide evidence that hippocampal nectin-3 expression is regulated by chronic stress and modulated by CRH-CRHR1 signaling, which is associated with dendritic remodeling and cognitive function. Additionally, based on its specific spatial distribution pattern, nectin-3 is a potential molecular marker for compromised CA3 neurons in response to chronic stress.
Glucocorticoids bind to MR and GR, both abundantly expressed in the hippocampal neurons in rodents (Herman et al., 1989; van Steensel et al., 1996). Hippocampal MR and GR participate in the negative feedback inhibition of the HPA axis, and modulate learning and memory in a coordinated manner (de Kloet et al., 2005). Studies using several transgenic mouse lines have assessed the effects of long-term alterations of forebrain MR or GR on cognitive performance. Forebrain MR overexpression enhances spatial memory retention (Lai et al., 2007), whereas forebrain MR deficiency (Berger et al., 2006)or GR overexpression (Wei et al., 2007) leads to cognitive deficits. Interestingly, considerable evidence shows that GR and/or MR are reduced in the hippocampus under chronic stress and this down-regulation could last for days (Kitraki et al., 1999; Wright et al., 2006). However, though the imbalance in MR- and GR-mediated actions is hypothesized to contribute to the memory impairments by chronic stress (de Kloet et al., 1999), our findings that hippocampal GR, but not MR, is down-regulated in stressed wild-type mice could be interpreted as being adaptive to protect hippocampal neurons from neurotoxic or metabolic challenges (Conrad, 2008) rather than being a substrate of cognitive impairments. It should be noted that we only examined hippocampal GR mRNA levels using in situ hybridization. As shown by previous studies (Nishi et al., 2007; Usuku et al., 2005), GR immunoreactivity is very weak in mouse CA3 region. Therefore, it may be difficult to detect any subtle alterations of CA3 GR protein expression induced by stress. Furthermore, corticosterone levels did not differ between control and stressed wild-type mice basally or inresponse to stress. Hence, it is unlikely that dysregulated hippocampal GR and the potential alterations of glucocorticoids account for the behavioral, structural and molecular changes observed in stressed wild-type mice.
Chronic social defeat stress resulted in a mild HPA axis hyperactivity in stressed wild-type mice, as indicated by increased adrenal weights and, to a lesser extent, reduced hippocampal GR mRNA expression. In contrast, stressed CRHR1Camk2aCre mice had similar adrenal weights and hippocampal GR expression levels compared to the controls. Interestingly, the blunted HPA axis feedback to acute stress challenge in CRHR1Camk2aCre mice, as indicated by elevated plasma corticosterone levels at 90 min after restraint stress, was restored under recurrent stress exposure. These findings highlight the importance of hippocampal CRH-CRHR1 function and its interaction with chronic stress in regulating HPA axis activity.
Under chronic psychosocial stress situations, subordinate male mice show increased food intake and gain more weight, leading to a positive energy balance and increased vulnerability to hyperphagia-associated obesity, whereas individually housed mice show reduced body weight gain (Bartolomucci et al., 2009; Moles et al., 2006). In line with these findings, our results further suggest that forebrain CRHR1 inactivation increases body weight gain in chronically stressed mice,which may not be surprising since activation of central CRH receptors suppresses food intake (Bell et al., 1998; Grill et al., 2000), and the Crhr1 gene has been associated with obesity in humans (Challis et al., 2004).
In summary, the results demonstrate that forebrain CRHR1 inactivation ameliorates spatial memory deficits and prevents abnormal structural and molecular alterations in adult hippocampus that are induced by chronic stress. Though the involvement of CRHR1 in other forebrain regions in aforementioned changes needs to be delineated, hippocampal CRH-CRHR1 signaling may mediate the effects of chronic stress on cognition. These data highlight forebrain CRHR1 as an exciting molecular target for attenuating the cognitive effects of stress. However, our findings suggest that such inactivation may also increase the risk for metabolic disorders such as obesity.
Supplementary Material
Acknowledgments
We thank Stefanie Unkmeir and Robert Menz for their technical assistance. This work was supported by the European Community’s Seventh Framework Program (FP7, Project No. 201600), the Bundesministerium für Bildung und Forschung within the framework of the NGFN-Plus (FKZ: 01GS08151 and 01GS08155) and by the Initiative and Networking Fund of the Helmholtz Association in the framework of the Helmholtz Alliance for Mental Health in an Ageing Society (HA-215).
Footnotes
Appendix A. Supplementary data Supplementary data to this article can be found online at doi:10.1016/j.nbd.2011.01.020.
References
- Arzt E, Holsboer F. CRF signaling: molecular specificity for drug targeting in the CNS. Trends Pharmacol. Sci. 2006;27:531–538. doi: 10.1016/j.tips.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, et al. Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLoS ONE. 2009;4:e4331. doi: 10.1371/journal.pone.0004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SM, et al. Effects of third intracerebroventricular injections of corticotropin-releasing factor (CRF) on ethanol drinking and food intake. Psychopharmacol. Berl. 1998;139:128–135. doi: 10.1007/s002130050697. [DOI] [PubMed] [Google Scholar]
- Berger S, et al. Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc. Natl Acad. Sci. USA. 2006;103:195–200. doi: 10.1073/pnas.0503878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaz R, et al. Causal evidence for the involvement of the neural cell adhesion molecule, NCAM, in chronic stress-induced cognitive impairments. Hippocampus. 2011;21:56–71. doi: 10.1002/hipo.20723. [DOI] [PubMed] [Google Scholar]
- Blank T, et al. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J. Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, et al. Object recognition memory and the rodent hippocampus. Learn. Mem. 2010;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgin AB, et al. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat. Biotechnol. 2010;28:63–70. doi: 10.1038/nbt.1598. [DOI] [PubMed] [Google Scholar]
- Challis BG, et al. Genetic variation in the corticotrophin-releasing factor receptors: identification of single-nucleotide polymorphisms and association studies with obesity in UK Caucasians. Int. J. Obes. Relat. Metab. Disord. 2004;28:442–446. doi: 10.1038/sj.ijo.0802564. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J. Neurosci. 2001;21:7171–7181. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc. Natl Acad. Sci. USA. 2004a;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. Hippocampal corticotropin releasing hormone: pre- and postsynaptic location and release by stress. Neuroscience. 2004b;126:533–540. doi: 10.1016/j.neuroscience.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. Cellular and molecular mechanisms of hippocampal activation by acute stress are age-dependent. Mol. Psychiatry. 2006;11:992–1002. doi: 10.1038/sj.mp.4001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J. Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc. Natl Acad. Sci. USA. 2010;107:13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn-Litvak PS, et al. Chronic administration of corticosterone impairs spatial reference memory before spatial working memory in rats. Neurobiol. Learn. Mem. 2003;80:11–23. doi: 10.1016/s1074-7427(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav. Cogn. Neurosci. Rev. 2006;5:41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev. Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, et al. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav. Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, et al. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav. Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Conrad CD, et al. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. J. Neurosci. 2007;27:8278–8285. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contarino A, et al. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Res. 1999;835:1–9. doi: 10.1016/s0006-8993(98)01158-5. [DOI] [PubMed] [Google Scholar]
- Dalva MB, et al. Cell adhesion molecules: signalling functions at the synapse. Nat. Rev. Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, et al. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, et al. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Dere E, et al. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci. Biobehav. Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Gibb R, Kolb B. A method for vibratome sectioning of Golgi–Cox stained whole rat brain. J. Neurosci. Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Grill HJ, et al. Long-term effects on feeding and body weight after stimulation of forebrain or hindbrain CRH receptors with urocortin. Brain Res. 2000;867:19–28. doi: 10.1016/s0006-8993(00)02193-4. [DOI] [PubMed] [Google Scholar]
- Haile CN, et al. Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. Psychopharmacol. Berl. 2001;154:213–220. doi: 10.1007/s002130000650. [DOI] [PubMed] [Google Scholar]
- Hains AB, et al. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc. Natl Acad. Sci. USA. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J. Pharmacol. Exp. Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, et al. Learning impairment in transgenic mice with central overexpression of corticotropin-releasing factor. Neuroscience. 1996;74:303–311. doi: 10.1016/0306-4522(96)00140-6. [DOI] [PubMed] [Google Scholar]
- Herman JP, et al. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol. Endocrinol. 1989;3:1886–1894. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- Hogan JB, et al. Effects of CRF1 receptor antagonists and benzodiazepines in the Morris water maze and delayed non-matching to position tests. Psychopharmacol. Berl. 2005;178:410–419. doi: 10.1007/s00213-004-2028-y. [DOI] [PubMed] [Google Scholar]
- Honda T, et al. Involvement of nectins in the formation of puncta adherentia junctions and the mossy fiber trajectory in the mouse hippocampus. Mol. Cell. Neurosci. 2006;31:315–325. doi: 10.1016/j.mcn.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Joëls M, Baram TZ. The neuro-symphony of stress. Nat. Rev. Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, et al. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–231. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kitraki E, et al. Long-lasting effects of stress on glucocorticoid receptor gene expression in the rat brain. Neuroendocrinology. 1999;69:331–338. doi: 10.1159/000054435. [DOI] [PubMed] [Google Scholar]
- Kole MH, et al. Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. Neuroscience. 2004;125:337–347. doi: 10.1016/j.neuroscience.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lai M, et al. Forebrain mineralocorticoid receptor overexpression enhances memory, reduces anxiety and attenuates neuronal loss in cerebral ischaemia. Eur. J. Neurosci. 2007;25:1832–1842. doi: 10.1111/j.1460-9568.2007.05427.x. [DOI] [PubMed] [Google Scholar]
- Lee EH, et al. Hippocampal CRF, NE, and NMDA system interactions in memory processing in the rat. Synapse. 1993;14:144–153. doi: 10.1002/syn.890140207. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, et al. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Majima T, et al. Involvement of afadin in the formation and remodeling of synapses in the hippocampus. Biochem. Biophys. Res. Commun. 2009;385:539–544. doi: 10.1016/j.bbrc.2009.05.097. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, et al. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: possible involvement of CA1 neurons. Neuroscience. 2005;135:1045–1054. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, et al. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A, et al. Nectin: an adhesion molecule involved in formation of synapses. J. Cell Biol. 2002;156:555–565. doi: 10.1083/jcb.200103113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A, et al. Psychosocial stress affects energy balance in mice: modulation by social status. Psychoneuroendocrinology. 2006;31:623–633. doi: 10.1016/j.psyneuen.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Müller MB, et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat. Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Nishi M, et al. Direct visualization of glucocorticoid receptor positive cells in the hippocampal regions using green fluorescent protein transgenic mice. Neuroscience. 2007;146:1555–1560. doi: 10.1016/j.neuroscience.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Pavlides C, et al. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–257. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Mouse Brain in Stereotaxic Coordinates. Second Edition Academic Press; San Diego: 2001. [Google Scholar]
- Radulovic J, et al. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J. Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refojo D, et al. Corticotropin-releasing hormone activates ERK1/2 MAPK in specific brain areas. Proc. Natl Acad. Sci. USA. 2005;102:6183–6188. doi: 10.1073/pnas.0502070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CJ, et al. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, et al. Memory retrieval impairment induced by hippocampal CA3 lesions is blocked by adrenocortical suppression. Nat. Neurosci. 2001;4:1169–1171. doi: 10.1038/nn766. [DOI] [PubMed] [Google Scholar]
- Row BW, Dohanich GP. Post-training administration of corticotropin-releasing hormone (CRH) enhances retention of a spatial memory through a noradrenergic mechanism in male rats. Neurobiol. Learn. Mem. 2008;89:370–378. doi: 10.1016/j.nlm.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat. Rev. Neurosci. 2004;5:917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, et al. Persistent neuroendocrine and behavioral effects of a novel, etiologically relevant mouse paradigm for chronic social stress during adolescence. Psychoneuroendocrinology. 2007;32:417–429. doi: 10.1016/j.psyneuen.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Squire LR, et al. Recognition memory and the medial temporal lobe: a new perspective. Nat. Rev. Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlemann V, et al. Chronic social stress during adolescence induces cognitive impairment in aged mice. Hippocampus. 2010;20:540–549. doi: 10.1002/hipo.20655. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Usuku T, et al. Visualization of glucocorticoid receptor in the brain of green fluorescent protein-glucocorticoid receptor knockin mice. Neuroscience. 2005;135:1119–1128. doi: 10.1016/j.neuroscience.2005.06.071. [DOI] [PubMed] [Google Scholar]
- Van Pett K, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- van Steensel B, et al. Partial colocalization of glucocorticoid and mineralocorticoid receptors in discrete compartments in nuclei of rat hippocampus neurons. J. Cell Sci. 1996;109(Pt 4):787–792. doi: 10.1242/jcs.109.4.787. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, et al. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Wei Q, et al. Overexpressing the glucocorticoid receptor in forebrain causes an aging-like neuroendocrine phenotype and mild cognitive dysfunction. J. Neurosci. 2007;27:8836–8844. doi: 10.1523/JNEUROSCI.0910-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural– neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Woolley CS, et al. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8:151–154. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, et al. Attenuating corticosterone levels on the day of memory assessment prevents chronic stress-induced impairments in spatial memory. Eur. J. Neurosci. 2006;24:595–605. doi: 10.1111/j.1460-9568.2006.04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.