Abstract
Type 1 Diabetes (T1D3) results from the immune-mediated destruction of the insulin producing β-islet cells in the pancreas. The genetic and environmental mechanisms promoting the development of this disease remain poorly understood. We have explored the cellular requirements for T1D development in DO11.10xRIP-OVA (DORmO) mice, which carry a T cell receptor transgene specific for an MHC class II-restricted epitope from ovalbumin (OVA) and express membrane-bound OVA in the pancreas under the control of the rat insulin promoter. We found that DORmO.RAG2−/− mice do not develop insulitis and are completely protected from diabetes, demonstrating that endogenous lymphocyte receptor rearrangement is required for disease development. Diabetes in DORmO mice is preceded by the development of OVA-specific autoantibodies, and is delayed in B cell-deficient DORmO.JHD−/− mice, demonstrating that B cells contribute to disease progression. In addition, transfer of CD8+ T cells from diabetic animals into DORmO.RAG2−/− mice promoted insulitis by OVA-specific CD4+ T cells. Finally, although diabetes develops in DORmO mice in the presence of a significant population of Foxp3+ OVA-specific regulatory T cells, boosting regulatory T cell numbers by injecting IL-2 immune complexes dampens autoantibody production and prevents development of insulitis and overt diabetes. These results help define the events leading to diabetes in DORmO mice, and provide new insights into the cellular interactions required for disease development in an antigen-specific model of T1D.
Keywords: Diabetes, Inflammation, Rodent, T cells, Tolerance
Introduction
Type 1 diabetes (T1D) is a polygenic autoimmune disease characterized by a smoldering inflammatory response directed against the insulin-producing β-islet cells of the pancreas . In Type 1 diabetics, the immune-mediated destruction of the pancreas is thought to begin years or even decades before metabolic dysregulation facilitates diagnosis (1). Therefore, the underlying environmental and genetic factors contributing to the initiation of T1D have been difficult to define. Indeed, at the time of T1D diagnosis, up to 80% of islet cell function has been lost due to immune destruction of the pancreas (2).
T cell-mediated destruction of the islets is clearly involved in T1D pathogenesis; in fact, in both animal models and human patients, the disease is strongly linked to polymorphisms in MHC class II (3,4). However, despite the use of autoantibodies as both diagnostic and prognostic markers of T1D (5–7), the direct involvement of autoreactive B cells in disease pathogenesis remains controversial (8). Moreover, the mechanisms by which diabetogenic T cells escape control by Foxp3+ regulatory T cells (Treg cells) have not been defined, but have important implications for the development of Treg cell-based therapies aimed at preventing or modulating disease (9).
RIPmOva mice express membrane-bound ovalbumin (OVA) under the control of the rat insulin promoter in the thymus and pancreas, and this system has been used extensively to model islet autoantigens (10). When crossed with mice expressing an OVA-specific MHC II-restricted T cell receptor transgene (DO11.10), the double transgenic DO11.10xRIPmOVA (DORmO) mice generate large numbers of OVA-specific effector and regulatory CD4+ T cells (11). Surprisingly, despite containing a high frequency of functional islet antigen-specific Treg cells, DORmO mice are spontaneously diabetic, with 100% of animals becoming hyperglycemic by 20 weeks of age (12). Thus, the DORmO mice provide a tractable, relatively synchronous antigen-specific model of T1D in which disease initiation and progression can be monitored and readily manipulated.
We used DORmO mice to examine the cellular requirements for disease development in a defined antigen-specific T1D model. Our data demonstrate that loss of immune tolerance, as indicated by insulitis and the production of islet antigen-specific autoantibodies, occurs weeks before changes in blood glucose are seen in this model of T1D. Surprisingly, DORmO.RAG2−/−mice do not develop insulitis and are completely protected from T1D development, indicating that additional, non-transgenic lymphocytes are needed to overcome tolerance in this model of T1D. Furthermore, T1D is significantly delayed in the B cell-deficient DORmO.JhD−/− mice, and this delay is associated with reduced activation and functional differentiation of the OVA-specific CD4+ T cells. Moreover, transfer of CD8+ T cells from pre-diabetic DORmO mice, into DORmO.RAG-2−/− promotes insulitis, suggesting that CD8+ T cells play a role in the breakdown of tolerance in DORmO mice. Finally, we show that augmenting Treg cell activity in pre-diabetic mice ameliorates autoantibody production and prevents disease, indicating that this may be an effective therapeutic strategy for preventing T1D in at-risk individuals.
Materials & Methods
Mice
All animals were bred and maintained under specific pathogen-free conditions, with free access to food and water, in the animal facility at the Benaroya Research Institute. DO11.10 transgenic mice were purchased from The Jackson Laboratory and bred with Balb/c mice expressing RIPmOva (kindly provided by Dr. Abul K. Abbas, University of California, San Francisco) to generate the DO11.10xRIPmOva (DORmO) double transgenic. Balb/c.JhD−/− mice and Balb/c.RAG2−/− mice were purchased from Taconic Laboratories and bred with the DORmO mice. All experiments and animal use procedures were approved by the Institutional Animal Use and Care committee at the Benaroya Research Institute.
Diabetes Monitoring
Beginning at four weeks of age, DORmO mice were bled weekly via the saphenous vein for the determination of their blood glucose level (BGL) using an Ascensia CONTOUR blood glucose meter and blood glucose monitoring strips (Bayer Healthcare LLC, Tarrytown, NY USA). When two consecutive blood glucose readings of > 200 mg/dL were recorded, animals were considered diabetic. When two consecutive blood glucose readings of > 300 mg/dL were recorded, animals were euthanized.
Lymphocyte Isolation
The spleen and pancreatic lymph nodes collected from each mouse. The tissues were pressed through a 100μm filter and washed with HBSS supplemented with 2% FBS and prepared for flow cytometric and functional analyses.
Flow Cytometric Analysis
Prior to incubating cells with the specified fluorochrome-conjugated antibodies, all cells were incubated in HBSS with 2% FBS and rat IgG (Sigma) to block non-specific binding. The following antibodies used for flow cytometry analyses were purchased from eBioscience: FITC-conjugated anti-CD62L, anti-CD8α, anti-IL-17 and anti-KJ1-26; Alex Fluor 488-conjugated anti-Foxp3; PE-conjugated anti-B220; APC-eFluor 780-conjugated anti-CD25; allophycocyanin (APC)-conjugated anti-CD25 and anti-CD62L; and, eFluor 450-conjugated Foxp3. Additional antibodies were purchased from Biolegend: PE-conjugated Foxp3; PerCP-Cy5.5-conjugated anti-CD4; PECy7-conjugated anti-CD8α and anti-B220; Alexa Fluor 700-conjugated anti-CD4 and anti-CD44; and, Pacific blue-conjugated anti-CD4 and anti-IFN-γ. Also, the following antibodies were purchased from Caltag: Alexa Fluor 488-conjugated anti-CD4; and, PE- and Alexa Fluor 647-conjugated anti-KJ1-26. PE- and PeCy7–conjugated anti-CD45RB and PeCy7-conjugated anti-B220 were also purchased from BD Bioscience. Data were acquired on a BD Biosciences LSR II flow cytometer and analyzed using the FlowJo software (TreeStar). For cell-sorting experiments, samples were first enriched for CD8+ cells, or depleted of CD4+ cells, using magnetic beads specific for CD8 or CD4, respectively, and magnetic columns (Miltenyi), and subsequently stained for the desired cell surface markers and isolated using a BD Biosciences FACSVantage cell sorter.
In vitro stimulation
Splenocytes were plated in 96-well, flat-bottom culture plates at a concentration of 5 × 105 to 1 × 106/200 mL/well in DMEM supplemented with 5% FBS. PMA (50 ng/mL) and ionomycin (1 mg/mL) were added to the culture and incubated for one hour at 37 °C in 5% CO2. Monensin (10 mg/mL, eBioscience) was then added and cells were cultured at 37 °C for an additional five hours. Following stimulation, the cells were stained with PerCP-Cy5.5–conjugated anti-CD4, PeCy7-conjugate anti-CD8, APC-eFluor 780–conjugated anti-CD25 and PE- or Alexa Fluor 647-conjgated anti-KJ1-26 antibodies in staining buffer (2% FBS-HBSS), prior to being fixed and permeabilized in eBioscience fixation/permeabilization buffer for one hour at 4 °C. Intracellular staining was performed using 0.5% saponin buffer supplemented with 2% FBS, containing labeled Pacific Blue-conjugated anti-IFN-γ, APC- or PE-conjugated anti-Foxp3 and FITC-conjugated anti-IL-17 for 30 min at 4 °C.
Adoptive Transfer of CD8+ T cells
Six to eight week old DORmO.RAG2−/−, DO11.10.RAG2−/− or RIPmOVA.RAG2−/− hosts were injected with 5 × 105 to 1 × 106 purified CD8+ cells via retro-orbital injection. Animals were monitored weekly thereafter for changes in BGL. All mice were sacrificed at 15 weeks post-transfer for analysis of the pancreatic inflammation and to examine lymphocytes in the pancreatic lymph nodes and spleen by FACS. Half of each pancreas was place in optimal cutting temperature (O.C.T.) media and snap frozen for immunofluorescence and half was placed in neutral-buffered formalin for immunohistochemistry. The lymph nodes and spleen were processed as described above and analyzed using flow cytometry.
IL-2/anti-IL-2 immune complexes
Purified recombinant IL-2 and anti-IL-2 monoclonal antibody (clone JES6-1A12) were purchased from eBioscience. IL-2 immune complexes (IL2C) contained 50 μg/mouse anti-IL-2 (clone JES6-1A12 ) and 1.5 μg/mouse purified IL-2 or 25 μg/mouse and 0.75 μg/mouse. The antibody was incubated with the purified IL-2 for 30 min at RT or O/N at 4 °C. Prior to injection, sterile PBS was added to a final injection volume of 100 μL per mouse. All injections were given intraperitoneally. Control mice received 25 μg or 50μg rat IgG in sterile PBS. Animals were injected weekly, beginning at two weeks of age, until the control animals became diabetic. Prior to, throughout and after treatment, all animals were monitored weekly for changes in their blood glucose levels.
ELISA
Serum OVA-specific IgG1 was determined by ELISA. Serum samples were diluted 1:1000 for IgG1 ELISA using ELISA buffer (1x PBS plus 2% normal goat serum and 0.05% Tween-20). High-binding, 96-well plates were coated with 100 μL/well of 2 mg/mL OVA in distilled water and incubated overnight at 4 °C. Plates were washed using the Skanwasher 300 version B (Molecular Devices Corp., Sunnyvale, CA USA) and PBS-Tween 20. To block non-specific binding, 200 μL/well of ELISA buffer was added to each well and the plate was incubated at 37°C for one hour in 5% CO2. Samples were plated in duplicate in the specified dilution and incubated overnight at 4 °C. The IgG1 standard was purchased from eBioscience. After washing the plate with PBS-Tween 20, alkaline phosphatase-conjugated anti-IgG1 (1:2500, Jackson Immunoresearch Laboratories, Inc., West Grove, PA USA) was added to each well. Plates were incubated at room temperature (RT) for 2 h, then washed with PBS-Tween 20. For the alkaline phosphatase substrate, para-nitrophenyl phosphate (p-NPP, Sigma) tablets were dissolved in a buffer containing 0.05 M Na2CO3 and 0.5 mM MgCl2, and 50 μL/well p-NPP-buffer was added to each plate. The chromagen was allowed to develop for up to 15 min at RT before the plate was analyzed using the the SoftMax Pro 5 software VersaMax tunable microplate reader at λ = 405 nm (Molecular Devices Corp., Sunnyvale, CA USA).
Immunohistochemistry & Immunofluorescence
For immunohistochemistry, tissues were fixed in 10% neutral buffered formalin, embedded in paraffin and 5-μm sections were stained with H&E; brightfield images of the H&E-stained tissue were acquired using a Leica DM2500 microscope equipped with an Insight 4-megapixel color CCD camera (Diagnostic Instruments, Sterling Heights, MI). For immunofluorescence, tissues were placed in O.C.T, snap frozen and stored at −80 °C until sectioned. For each tissue, 6-μm sections were cut and placed on Superfrost Plus charged slides (VWR International, LLC, West Chester, PA). Slides were fixed in cold acetone for 10 min and allowed to air-dry at room temperature for one hour. All slides were stored at −20 °C until stained for microscopy. Prior to incubating the slides with the specified fluorochrome-conjugated antibodies, slides were allowed to come to RT and, then, re-hydrated with 1x PBS. Non-specific binding was blocked by incubating the slides with PBS supplemented with 2% BSA and 2% normal goat serum for 30 min at RT. Then, sections were incubated with 1:200 guinea pig anti-insulin (Abcam, Cambridge, MA); slides were incubated for one hour at RT, washed with the staining buffer (1x PBS plus 2% BSA) three times for five minutes/wash. Sections were then incubated with Alexa Fluor 488- or Alexa Fluor 568–conjugated rabbit anti-guinea pig IgG (H+L) (Molecular Probes Invitrogen, Carlsbad, CA) and FITC-, Alexa Fluor-488-, Alexa Fluor 647- or APC–conjugated anti-KJ1-26, anti-CD4, anti-CD8 or anti-B220 for one hour at RT. After one hour, slides were washed as before. Images shown were visualized at a 20 × or 40 × magnification using Leica DMIRB inverted fluorescence microscope (Leica Microsystems, Bannockburn, IL) equipped with a Pursuit 4-megapixel cooled color/monochrome CCD camera (Diagnostic Instruments, Sterling Heights, MI) or Bio-Rad MRC-1024 UV laser scanning confocal microscope system (LSCM; Bio-Rad Laboratories, Hercules, CA) attached to a Nikon Diaphot 200 inverted microscope (Nikon Instruments, Inc., Melville, NY). Images were acquired using a Windows 2000 PC and the Spot Pursuit camera and Spot Advance Software (SPOT Imaging Solutions, Diagnostic Instruments, Inc., Sterling Heights, MI) for the Leica DM IRB microscope or the Laser Sharp 2000 acquisition software (Bio-Rad Laboratories, Hercules, CA) for the LSCM system.
Statistical Analysis
The statistical differences were assessed by Student’s t-test or one-way ANOVA (using Bonferroni post-tests for pairwise comparisons), where appropriate. Statistical significance was determined by a p-value < .05.
Results
Autoantibody generation coincides with the onset of insulitis in DORmO mice
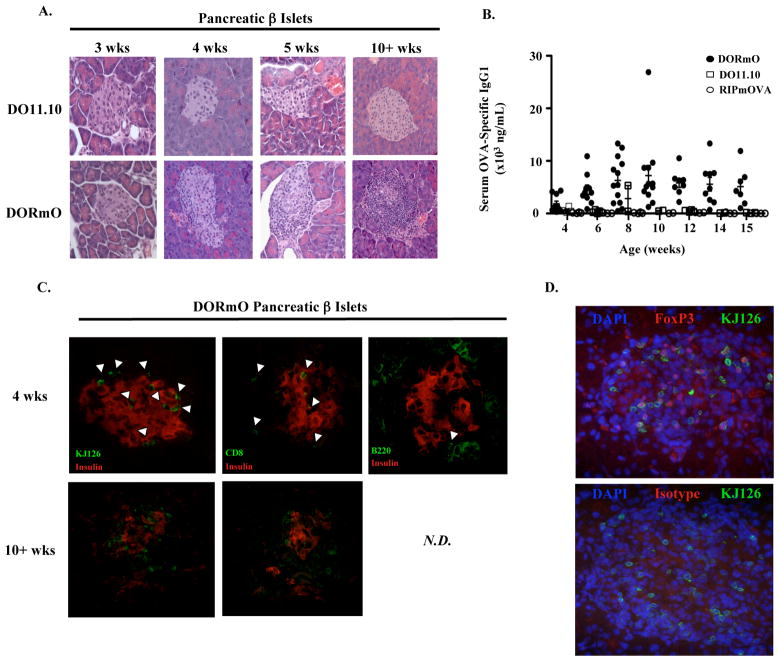
Consistent with a recently published report (12), we found that >90% of DORmO animals are diabetic by 15 weeks of age, and that all mice became diabetic by 20 weeks of age despite their high frequency of fully functional, islet antigen-specific Treg cells. In T1D, disease is thought to begin long before the loss of β-islet cells becomes severe enough to cause hyperglycemia. Therefore, to determine when the breakdown in tolerance occurs, we examined DORmO mice of various ages for development of insulitis and islet destruction. At three weeks of age, islets from DORmO mice are indistinguishable from control DO11.10 littermates (Fig. 1A). However, in the majority of DORmO mice, progressively destructive insulitis began by four weeks of age—at least 4 to 16 weeks prior to detectable alterations in glucose metabolism (Fig. 1A). Correlating with insulitis induction, OVA-specific autoantibodies were readily detected in the serum of DORmO mice beginning at 3–4 weeks of age, with titers peaking several weeks before the onset of diabetes (Fig. 1B and Fig. 2A). Immunofluorescence analysis demonstrated that, in addition to OVA-specific CD4+ T cells (identified by staining with the clonotypic antibody KJ1-26 (13)), the islet infiltrate also contained increasing numbers of B and CD8+ T cells (Fig. 1C). Notably, diabetes in DORmO mice occurs despite a large population of OVA-specific, KJ1-26+Foxp3+ Treg cells that co-exist with effector T cells in inflamed islets of diabetic mice (Fig. 1D). Thus, the DORmO model of T1D recapitulates several features of disease observed in other animal models and in human patients, including a protracted period of asymptomatic autoimmunity accompanied by the production of prognostic autoantibodies, and islet infiltration by effector and regulatory CD4+ T cells, CD8+ T cells and B cells.
Figure 1. DORmO mice develop insulitis and autoantibodies prior to overt diabetes.
A, Representative H & E staining of islets from either DO11.10 (top panels) or DORmO (bottom panels) mice at the indicated ages. B, ELISA analysis of serum OVA-specific IgG1 at the indicated ages in a cohort of DORmO, DO11.10 or RIPmOVA mice. Each point represents an individual animal and the mean value for each time point is indicated by a line. C, Representative immunofluorescence analysis of insulin, KJ1-26, CD8 and B220 staining as indicated in the pancreatic islets of DORmO mice at either 4 (top panels) or > 10 (bottom panels) weeks of age. N.D., not detectable. (D) Representative immunofluorescence analysis of KJ1-26 and FoxP3 (top) or isotype control (bottom) staining in the pancreatic islets of diabetic DORmO mice. DAPI staining (blue) was used to identify cell nuclei.
Figure 2. Diabetes in DORmO mice is RAG-dependent cells and delayed in the absence of B cells.
A, Comparison of diabetes induction in DORmO, DORmO.JHD−/− and DORmO.RAG2−/−mice. B, Representative H & E staining of pancreatic islets from 5–8 week old DORmO, DORmO.JHD−/− and DORmO.RAG2−/− mice. C, Quantitative comparison of insulitis in 5–8 week old DORmO, DORmO.JHD−/− and DORmO.RAG2−/− mice. Each point represents the percentage of insulitic islets from one animal (at least 20 islets/mouse were evaluated), and the mean percent of insulitic islets ± SEM for 5–6 animals/genotype is shown.
B cells accelerate T1D development in DORmO mice
It is evident that both DO11.10 Tg CD4+ T cells and islet-expressed ovalbumin are necessary for T1D development, as neither DO11.10 nor RIPmOva single transgenic mice develop insulitis or diabetes. However, rearrangement of endogenous T cell receptor and immunoglobulin loci results in the development of polyclonal populations of B cells and CD8+ T cells that may also contribute to disease development. To determine if these non-Tg lymphocyte populations were necessary for disease development in the DORmO model, DORmO animals were bred to RAG2−/− mice and monitored for disease development. Surprisingly, despite containing a full compartment of islet antigen-specific CD4+ T cells, DORmO.RAG2−/− mice failed to develop diabetes and were fully protected from insulitis (Fig. 2A, B).
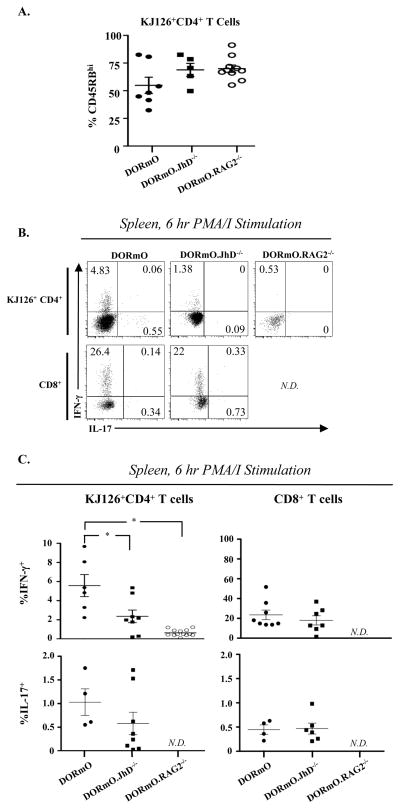
The lack of T1D in DORmO.RAG2−/− mice is not due to the absence of B cells, as DORmO.JhD−/− mice, which are B cell-deficient due to a targeted mutation in the immunoglobulin heavy chain gene (14), still developed disease, albeit with a significant delay (Fig 2A). Protection from diabetes in DORmO.RAG2−/− animals and the delay seen in the DORmO.JhD−/− mice were each associated with impaired effector differentiation of OVA-specific CD4+ T cells, evidenced by a decreased frequency of CD45RBloCD6L− cells among CD4+KJ1-26+Foxp3− T cells in the pancreatic lymph nodes compared to wild-type DORmO animals (Fig. 3A). Additionally, following ex vivo PMA/ionomycin stimulation, KJ1-26+CD4+ T cells from DORmO.RAG-2−/− mice did not produce either IFN-γ+ or IL-17+, and significantly fewer OVA-specific CD4+ T cells from DORmO.JhD−/− produced IFN-γ+ compared to wild-type DORmO controls (Fig. 3B, C). By contrast, the frequency of IFN-γ+ or IL-17+ CD8+ T cells did not differ significantly between the DORmO and DORmO.JhD−/− mice (Fig. 3B, C). As expected, CD8+ T cells were not observed in DORmO.RAG2−/− animals. The impaired differentiation of IFN-γ-producing effector cells in DORmO.JhD−/− mice was not due to reduced access to antigen, as roughly equal fractions of CD4+KJ1-26+Foxp3− T cells were expressed the early activation marker CD69 in the pancreatic lymph nodes of DORmO and DORmO.Jhd−/− animals. Collectively, these data indicate that, in addition to producing islet antigen-specific autoantibodies, B cells contribute to the activation and functional differentiation of islet antigen-specific T cells, thereby accelerating T1D development in DORmO mice.
Figure 3. Reduced activation OVA-specific CD4+ T cells in DORmO.JhD−/− mice.
A, Quantitative analysis of CD45RB expression by gated CD4+KJ1-26+ splenocytes from 6–8 week old DORmO, DORmO.JhD−/− and DORmO.RAG2−/− mice. Each point represents the percentage of CD45RBhi cells from one animal, and the mean ± SEM for 5–6 animals/genotype is shown. B, Representative flow cytometry analysis of IFN-γ and IL-17 production by gated CD4+KJ-126+ (top panels) and CD8+ (bottom panel) splenocytes from 6–8 week old DORmO, DORmO.JhD−/−and DORmO.RAG2−/− mice following in vitro stimulation with PMA/ionomycin. C, Quantitative analysis of IFN-γ and IL-17 production by CD4+KJ1-26+ (top panels) and CD8+ (bottom panel) splenocytes from 6–8 week old DORmO, DORmO.JhD−/− and DORmO.RAG2−/− mice following in vitro stimulation with PMA/ionomycin. Each point represents the percent of cytokine positive cells from one animal, and the mean ± SEM for 5–6 animals/genotype is shown. Data is representative of four independent experiments. N.D., not detectable.
CD8+ T cells promote insulitis when transferred into the DORmO.RAG2−/− mice
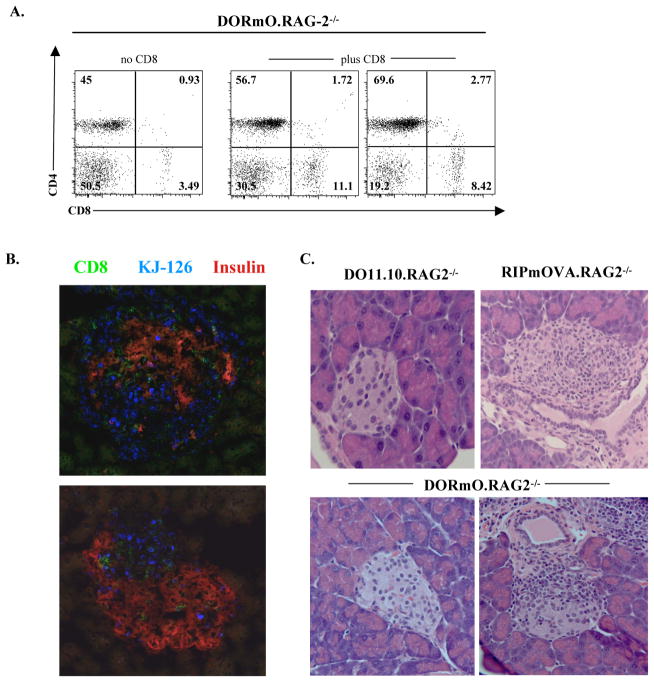
Although B cells accelerate T1D development in DORmO mice, their absence cannot account for the lack of diabetes in DORmO.RAG2−/− animals. In DORmO mice, CD8+ T cells are also found in the pancreatic infiltrate of diabetic DORmO animals (Fig. 1C). Loss of CD8+ T cells may therefore contribute to disease protection in DORmO.RAG2−/− mice. To test this, we transferred 5 × 105 to1 × 106 CD8+ T cells purified from pre-diabetic DORmO mice into 6–7 week old DORmO.RAG2−/−, RIPmOVA.RAG2−/− or DO11.10.RAG2−/− recipients (Fig. 4). These animals were monitored weekly for alterations in blood glucose levels and the presence of transferred cells in circulation for up to 15 weeks post-transfer (data not shown and Fig. 4A). All mice were sacrificed for analysis of pancreatic inflammation and T cell activation status in the associated lymph nodes and spleen (Fig. 4B, C and data not shown). Although one RIPmOVA.RAG2−/− recipient developed T1D during this period, we found that transfer of CD8+ T cells led to varying degrees of insulitis in all of the DORmO.RAG2−/− mice (Fig. 4C). Interestingly, no insulitis was observed in the DO11.10.RAG2−/− recipients, indicating that CD8+ T cells were not sufficient to cause insulitis in the absence of OVA expression in the pancreas. Rather, these data suggest that CD8+ T cells synergize with the OVA-specific KJ1-26+ CD4+ T cells to promote insulitis. Indeed, transfer of CD8+ T cells resulted in substantial islet infiltration by endogenous CD4+KJ1-26+ cells (Fig. 4B).
Figure 4. Transfer of CD8+ T cells from DORmO mice promotes insulitis but not diabetes in the DORmO.RAG2−/− mice.
A, Representative flow cytometry analysis of CD4 and CD8 expression by peripheral blood lymphocytes from either control (left) or CD8+ T cell-transferred (middle and right) DORmO.RAG2−/− mice. B, Representative immunofluorescence analysis of CD8, KJ1-26, and insulin staining in the pancreatic islets of CD8+ T cell-transferred DORmO.RAG2−/− mice. C, Representative H & E staining of pancreatic islets from CD8+ T cell-transferred DO11.10.RAG2−/−, RIPmOVA.RAG2−/−, and DORmO.RAG2−/− mice as indicated.
Expansion of Treg cells prevents autoantibody production and T1D development in DORmO mice
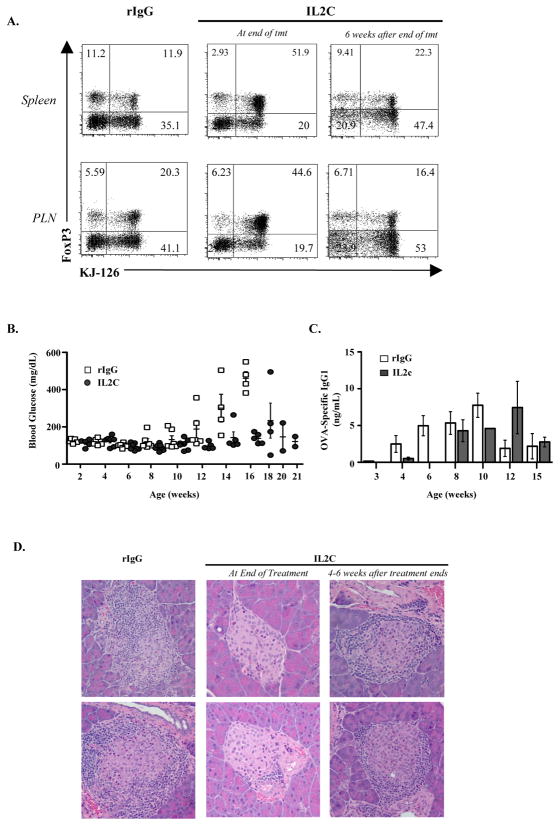
DORmO mice contain a high frequency of OVA-specific Foxp3+ Treg cells, and Treg cells can be found within the pancreatic islets in diabetic animals (Fig. 1D). Additionally, when DORmO mice are crossed with Foxp3-deficient scurfy mice, the absence of regulatory T cells leads to severe insulitis by four weeks of age (Supplemental Fig. 1). Clough et al. have demonstrated that Treg cells in DORmO mice are functional, but that their suppressive activity is overcome during the development of diabetes, perhaps by production of pro-inflammatory cytokines such as IL-21 (12). Modulating Treg cell activity has been proposed as a cellular therapy in T1D. Therefore, to determine if increasing the number of endogenous Treg cells can prevent the development of T1D, we treated DORmO mice with IL-2/anti-IL-2 immune complexes (IL2C) (Fig. 5). Using the JES6-1A12 mAb, IL2C induce the specific expansion of Treg cells in vivo with little impact on other IL-2 responsive immune cell populations (15). Indeed, weekly treatment with IL2C beginning between 1–2 weeks of age led to robust expansion of both OVA- (KJ1-26+) and non-OVA–specific (KJ1-26−) Treg cells and prevented diabetes development in DORmO mice (Fig. 5A,B). Additionally, IL2C-treated mice developed less severe insulitis and the majority of islet mass and function was preserved compared to control mice given rat IgG (Fig. 5D). Notably, protection from diabetes was also associated with a transient reduction in OVA-specific IgG1 autoantibodies, indicating that IL2C treatment also impaired the B cell response to autoantigen, most likely by limiting CD4+ T cell help (Fig. 5C). To determine if long-term IL2C treatment beginning before insulitis or autoantibodies are evident would lead to development of durable immune tolerance in the absence of continued Treg cell expansion, weekly IL2C treatment was stopped in one cohort of DORmO mice at around 15 weeks of age, a time at which all of the rat IgG-treated mice had already progressed to overt diabetes. However, within one to two months after treatment cessation, 80% (4/5) of the IL2C treated animals developed T1D that was indistinguishable from that observed in control-treated DORmO mice (Fig. 5D and Supplemental Fig. 2). Additionally, short-term (i.e., four weeks) treatment with IL2C, beginning at the onset of autoantibody production and insulitis, failed to prevent delay disease progression in any of the mice (Supplemental Fig. 2). Therefore, although IL2C treatment could prevent diabetes development, continued treatment was necessary for this protective effect.
Figure 5. In vivo expansion of Treg cells prevents diabetes in the DORmO mice.
A, Representative flow cytometry analysis of Foxp3and KJ1-26 expression by gated CD4+ T cells from the spleens (top panels) or PLNs (bottom panels) of rat IgG- (left panels) or IL2C-treated (middle and right panels) DORmO mice at the indicated treatment times. B, Blood glucose levels monitored weekly in rat IgG- or IL2C-treated DORmO mice. Each point represents a value from an individual animal. C, ELISA analysis of OVA-specific IgG1 in the serum of rat IgG- or IL2C-treated DORmO mice. The mean ± SEM is shown for 6–9 mice/treatment. D, Representative H & E staining of pancreatic islets from rat IgG- (left panels) or IL2C-treated (middle and right panels) DORmO mice at the indicated treatment times.
Discussion
The current report details the development of spontaneous diabetes in the DORmO double transgenic mouse model of T1D. The known antigen specificity of this model makes it an attractive tool for delineating the relative contributions of different lymphocyte populations to diabetes progression and for the examining the function of islet antigen-specific Treg cells during disease development. The novelty of this model is further highlighted by the requirement for a non-transgenic, RAG-dependent lymphocyte population for diabetes development. This contrasts with the most commonly used TCR transgenic model of T1D, the BDC2.5 mouse on the NOD genetic background. In these animals, T1D development is dramatically accelerated when they are rendered RAG-deficient. The enhanced disease likely stems from impaired development of various populations of regulatory T cells driven by endogenous T cell receptor rearrangement in BDC2.5 mice (16,17). By contrast, DORmO.RAG-2−/− animals develop a population of OVA-specific, Foxp3+ Treg cells due to expression of mOVA in the thymus, and these cells are capable of controlling the potentially diabetogenic KJ1-26+ Foxp3− CD4+ T cells (11,18). Indeed, the lack of disease development in the DORmO.RAG-2−/− is not due to an inability of Foxp3−KJ1-26+ CD4+ T cells to induce disease on their own, as insulitis rapidly develops when CD25−KJ1-26+CD4+ T cells from DO11.10 mice are transferred into RIPmOva.RAG2−/− hosts and TID can be induced in these hosts by immunization with OVA (19). These data suggest that in DORmO mice, endogenous lymphocyte populations help CD4+ T cells overcome Treg cell-mediated suppression and promote overt autoimmunity.
The significant delay in disease progression in the DORmO.JhD−/− mice demonstrates that B cells influence the initiation and/or progression of T1D in this model. The decreased activation of and reduced production of IFN-γ from OVA-specific T cells in the DORmO.JhD−/−animals suggest that B cells may have an important role as antigen presenting cells (APCs), facilitating the activation and functional differentiation of diabetogenic T cells. Indeed, in the context of diabetes, B cells have been shown to be important in T cell activation (20–23). Moreover, autoantibodies produced by OVA-specific B cells may directly cause islet damage, increasing the amount of islet antigen available for presentation to autoreactive T cells. Additionally, autoantibodies may directly induce the activation of dendritic cells and cross-presentation of islet antigens via Fc receptor binding, promoting the activation and recruitment of pancreatic Ag-specific T cells and promoting immune destruction of the islets (24,25). However, the fact that equal fractions of KJ1-26+Foxp3- express the early activation marker CD69 in the pancreatic lymph nodes of DORmO and DORmO.JhD−/− mice indicates that the influence of B cells as APCs in the activation of islet-specific CD4+ is more qualitative than quantitative.
Our data clearly demonstrate that B cells accelerate disease development in DORmO mice, however, they are not ultimately required for diabetes. Although islet antigen-specific CD4+ T cells are sufficient to cause disease in other TCR transgenic models of T1D, CD8+ T cells are thought to be important mediators of islet destruction in NOD mice and in T1D patients (26,27). Indeed, DORmO.RAG2−/− mice given purified CD8+ T cells from pre-diabetic DORmO donors developed insulitis by 15 weeks-post transfer, supporting a role these cells in disease pathogenesis. As with tissue damage caused by autoantibodies, islet destruction by CD8+ T cells may release large amounts of islet antigens, facilitating the activation and functional differentiation of autoreactive CD4+ T cells and epitope spreading. Additionally, CD8+ T cells could expedite lymphocytic infiltration of the islets by altering the extracellular matrix (ECM) surrounding the islets through secretion of matrix metalloproteinases, and such a mechanism for CD8+ T cell function has been proposed in NOD mice (28). Consistent with a promoting role for CD8+ T cells in islet inflammation, the islet infiltrate in DORmO.RAG2−/− mice given CD8+ cells is composed largely of endogenous KJ1-26+ T cells, with very few of the transferred CD8+ cells evident. Interestingly, in at least one instance, the transferred CD8+ T cells caused severe insulitis and T1D development in a RIPmOVA.RAG2−/− recipient, indicating that in the absence of OVA-specific CD4+ T cells, these CD8+ cells could themselves mediate disease. From these results, it is tempting to speculate that with only a small number of CD8+ T cells present, the OVA-specific Treg cells found in DORmO.RAG2−/− mice may limit CD8+ T cell-mediated islet destruction and act to prevent diabetes development in the 15-week window analyzed in these experiments. Moreover, transfer of these CD8+ T cells into DO11.10.RAG2−/− did not cause diabetes or insulitis, suggesting that recognition of OVA, perhaps through expression of the clonotypic KJ1-26 TCR, was required for these effects.
DORmO mice develop T1D despite containing a large population of OVA-specific Treg cells. These Treg cells are functional, as they can suppress T cell proliferation ex vivo (11). Their failure to prevent diabetes is not due to their inability to access the target tissue as we found islet antigen-specific Treg cells even with the inflamed islets of diabetic DORmO mice (Fig. 1D). Moreover, either genetic or antibody-mediated depletion of Treg cells in DORmO mice dramatically accelerates insulitis and T1D development, indicating that these Treg cells do function in vivo to delay T1D development in these animals ((12) and Supplemental Fig. 1). The processes underlying the failure of Treg cell suppression are not well understood. For instance, the cytokine IL-21 has been implicated in the inhibition of Treg cell function in DORmO mice (12). However, increased IL-21 production was first detected in the pancreatic lymph node at ~6 weeks of age, whereas immune tolerance is breached at least 2–3 weeks earlier, as evidenced by insulitis and production of OVA-specific autoantibodies (Fig. 1). Thus, although IL-21 may contribute to suppression of Treg cell function in the later stages of T1D development, its increased expression is unlikely to be the proximal cause of tolerance breakdown in DORmO mice. The pro-inflammatory cytokine IL-6 can both inhibit Treg cell function and prevent the de novo induction of Treg cells from Foxp3− precursors in the periphery (29,30), potentially contributing to the overall inhibition of Treg cell function during T1D development.
The protracted period of pre-clinical autoimmunity in T1D provides a unique opportunity for identification and therapeutic intervention in individuals before metabolic dysregulation begins. For instance, manipulating Treg cell activity in vivo during critical temporal windows during disease development may help restore immune homeostasis, leading to lasting immune tolerance (9). Indeed, expanding Treg cells with IL2C has proven effective in preventing experimental autoimmune encephalomyelitis and blocking allograft rejection in mice (31). By contrast, IL2C treatment of 10 week-old prediabetic NOD mice actually accelerated disease development, presumably through enhanced activation of CD25-expressing effector lymphocyte populations (32). We found that weekly injection of IL2C into DORmO mice beginning before any signs of detectable autoimmunity completely prevented insulitis and diabetes, and reduced autoantibody production. However, the expanded Treg cell population is not maintained once treatment is stopped, and all mice progressed to overt diabetes following cessation of treatment. Thus, despite greatly enhanced Treg cell activity during the temporal window in which loss of tolerance begins in DORmO mice, long-term IL2C treatment, on its own, did not result in the induction of durable, long-term tolerance. These results demonstrate that Treg-directed therapies have the potential to treat or manage diabetes and suggest that additional interventions, such as rapamycin treatment aimed at disarming effector T cells, may be necessary to induce full tolerance after Treg cell expansion (31).
Taken together, our results demonstrate that the disease process in the DORmO mouse model is a complex series of events that involves not only the transgenic OVA-specific CD4+ T cells but also multiple endogenous, non-Tg populations. Further examination of this model will be useful in delineating the mechanisms involved in the breakdown of T and B cell tolerance in a simplified, antigen-specific model of T1D. Additionally, these findings highlight the potential value of modulating endogenous Treg cell activity for the prevention, treatment or cure of autoimmune diseases.
Supplementary Material
Footnotes
This work was supported by grants from the National Institutes of Health to D.J.C. (DK072295, AI067750 and AR055695). J.D.W. was supported, in part, by a training grant from the University of Washington Department of Immunology (T32-AI07411).
Abbreviations: BGL, Blood glucose level; DORmO, DO11.10xRIPmOVA; IL2C, purified IL-2/anti-IL-2 mAb complex; NOD, Non-obese diabetic; RT, Room temperature; T1D, Type 1 Diabetes
References
- 1.Achenbach P, Bonifacio E, Koczwara K, Ziegler AG. Natural history of type 1 diabetes. Diabetes. 2005;54(Suppl 2):S25–S31. doi: 10.2337/diabetes.54.suppl_2.s25. [DOI] [PubMed] [Google Scholar]
- 2.Ludvigsson J, Heding LG. beta-cell function in children with diabetes. Diabetes. 1978;27(Suppl 1):230–234. doi: 10.2337/diab.27.1.s230. [DOI] [PubMed] [Google Scholar]
- 3.Nepom GT. Class II antigens and disease susceptibility. Annu Rev Med. 1995;46:17–25. doi: 10.1146/annurev.med.46.1.17. [DOI] [PubMed] [Google Scholar]
- 4.Suri A, Unanue ER. The murine diabetogenic class II histocompatibility molecule I-Ag7: structural and functional properties and specificity of peptide selection. Adv Immunol. 2005;88:235–265. doi: 10.1016/S0065-2776(05)88007-1. [DOI] [PubMed] [Google Scholar]
- 5.Kimpimaki T, Kulmala P, Savola K, Kupila A, Korhonen S, Simell T, Ilonen J, Simell O, Knip M. Natural history of beta-cell autoimmunity in young children with increased genetic susceptibility to type 1 diabetes recruited from the general population. J Clin Endocrinol Metab. 2002;87:4572–4579. doi: 10.1210/jc.2002-020018. [DOI] [PubMed] [Google Scholar]
- 6.Knip M, Karjalainen J, Akerblom HK. Islet cell antibodies are less predictive of IDDM among unaffected children in the general population than in sibs of children with diabetes. The Childhood Diabetes in Finland Study Group. Diabetes Care. 1998;21:1670–1673. doi: 10.2337/diacare.21.10.1670. [DOI] [PubMed] [Google Scholar]
- 7.LaGasse JM, Brantley MS, Leech NJ, Rowe RE, Monks S, Palmer JP, Nepom GT, McCulloch DK, Hagopian WA. Successful prospective prediction of type 1 diabetes in schoolchildren through multiple defined autoantibodies: an 8-year follow-up of the Washington State Diabetes Prediction Study. Diabetes Care. 2002;25:505–511. doi: 10.2337/diacare.25.3.505. [DOI] [PubMed] [Google Scholar]
- 8.Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–299. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 9.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 10.Kurts C, Heath WR, Kosaka H, Miller JF, Carbone FR. The peripheral deletion of autoreactive CD8+ T cells induced by cross-presentation of self-antigens involves signaling through CD95 (Fas, Apo-1) J Exp Med. 1998;188:415–420. doi: 10.1084/jem.188.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clough LE, Wang CJ, Schmidt EM, Booth G, Hou TZ, Ryan GA, Walker LS. Release from regulatory T cell-mediated suppression during the onset of tissue-specific autoimmunity is associated with elevated IL-21. J Immunol. 2008;180:5393–5401. doi: 10.4049/jimmunol.180.8.5393. [DOI] [PubMed] [Google Scholar]
- 13.Haskins K, Kubo R, White J, Pigeon M, Kappler J, Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J Exp Med. 1983;157:1149–1169. doi: 10.1084/jem.157.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Trounstine M, Alt FW, Young F, Kurahara C, Loring JF, Huszar D. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol. 1993;5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 15.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez A, Andre-Schmutz I, Carnaud C, Mathis D, Benoist C. Damage control, rather than unresponsiveness, effected by protective DX5+ T cells in autoimmune diabetes. Nat Immunol. 2001;2:1117–1125. doi: 10.1038/ni738. [DOI] [PubMed] [Google Scholar]
- 17.You S, Slehoffer G, Barriot S, Bach JF, Chatenoud L. Unique role of CD4+CD62L+ regulatory T cells in the control of autoimmune diabetes in T cell receptor transgenic mice. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14580–14585. doi: 10.1073/pnas.0404870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarween N, Chodos A, Raykundalia C, Khan M, Abbas AK, Walker LS. CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J Immunol. 2004;173:2942–2951. doi: 10.4049/jimmunol.173.5.2942. [DOI] [PubMed] [Google Scholar]
- 19.Eggena MP, Walker LS, Nagabhushanam V, Barron L, Chodos A, Abbas AK. Cooperative roles of CTLA-4 and regulatory T cells in tolerance to an islet cell antigen. J Exp Med. 2004;199:1725–1730. doi: 10.1084/jem.20040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouaziz JD, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC, Tedder TF. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci U S A. 2007;104:20878–20883. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1998;161:3912–3918. [PubMed] [Google Scholar]
- 22.Falcone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J Immunol. 1998;161:1163–1168. [PubMed] [Google Scholar]
- 23.Noorchashm H, Lieu YK, Noorchashm N, Rostami SY, Greeley SA, Schlachterman A, Song HK, Noto LE, Jevnikar AM, Barker CF, Naji A. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J Immunol. 1999;163:743–750. [PubMed] [Google Scholar]
- 24.Harbers SO, Crocker A, Catalano G, D’Agati V, Jung S, Desai DD, Clynes R. Antibody-enhanced cross-presentation of self antigen breaks T cell tolerance. J Clin Invest. 2007;117:1361–1369. doi: 10.1172/JCI29470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reijonen H, Daniels TL, Lernmark A, Nepom GT. GAD65-specific autoantibodies enhance the presentation of an immunodominant T-cell epitope from GAD65. Diabetes. 2000;49:1621–1626. doi: 10.2337/diabetes.49.10.1621. [DOI] [PubMed] [Google Scholar]
- 26.Bendelac A, Carnaud C, Boitard C, Bach JF. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987;166:823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faustman DL, Davis M. The primacy of CD8 T lymphocytes in type 1 diabetes and implications for therapies. J Mol Med. 2009;87:1173–1178. doi: 10.1007/s00109-009-0516-6. [DOI] [PubMed] [Google Scholar]
- 28.Savinov AY, Rozanov DV, Golubkov VS, Wong FS, Strongin AY. Inhibition of membrane type-1 matrix metalloproteinase by cancer drugs interferes with the homing of diabetogenic T cells into the pancreas. J Biol Chem. 2005;280:27755–27758. doi: 10.1074/jbc.M506016200. [DOI] [PubMed] [Google Scholar]
- 29.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 30.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 31.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.