Abstract
Background: Recently, the analysis of gastric and colorectal tumor specimens determined that 78-kiloDalton glucose-regulated protein (GRP78), an endoplasmic reticulum chaperone, up-regulation serves as an efficient mechanism protecting cells against apoptosis and can confer drug resistance. We tested whether functional polymorphisms within the GRP78 gene are related to clinical outcome in gastric and colorectal cancer (CRC) patients.
Patients and methods: Blood samples of 234 stage II/III CRC patients at the University of Southern California (USC) and formalin-fixed paraffin-embedded tissues of 137 patients with localized gastric adenocarcinoma (GA) at USC and Memorial Sloan-Kettering Cancer Centers were obtained. GRP78 polymorphisms analyzed on germline DNA were correlated with clinical outcome using univariate and multivariate analyses.
Results: GA patients with the combined GRP78 rs391957 C/T and T/T genotype were at higher risk for tumor recurrence and death [hazard ratio (HR) 2.61; P < 0.001 and HR 3.17; P < 0.001, respectively], than those with C/C. These findings were subsequently tested in a CRC cohort where patients with the homozygous T/T genotype were at highest risk for tumor recurrence (HR 2.61; P = 0.015). The results remained significant after adjusting for clinicopathologic determinants.
Conclusion: These data provide the first evidence that the GRP78 rs391957 polymorphism can predict clinical outcome in localized GA and locally advanced CRC patients.
Keywords: colorectal cancer, gastric cancer, GRP78, outcome, polymorphism
introduction
In 2010, an estimated 142 570 new cases of colorectal cancer (CRC) and 21 100 new cases of gastric adenocarcinoma (GA) will be diagnosed in the United States [1]. Globally, CRC and GA are responsible for an estimated 529 000 and 700 000 deaths annually, yielding to a case–fatality ratio (CFR) of 0.75 and 0.52, respectively, which is much higher than in other common malignancies like breast cancer (CFR 0.36) and prostate cancer (CFR 0.33) [2]. Pathological tumor staging (T stage, N stage) remains the main prognostic determinant for CRC and GA [3]. Patients in early stages who are fortunate enough to undergo surgery, are considered candidates for cure. However, ∼30%–40% of CRC patients and ∼40%–60% of GA patients who underwent surgery followed by adjuvant (radio)chemotherapy will develop recurrence [4, 5]. Consequently, the development of molecular prognostic markers as an adjunct to the conventional clinicopathologic staging is essential in selecting patients at high risk of tumor recurrence, thereby rationalizing treatment strategies and improving outcomes.
GRP78 (78-kiloDalton glucose-regulated protein (), also referred to as BiP (immunglobulin heavy-chain binding protein), belongs to the heat-shock protein 70 family that resides primarily in the endoplasmic reticulum (ER) [6]. As a major ER chaperone, it facilitates correct protein folding and assembly, controls the activation of transmembrane ER stress sensors, binds Ca2+, and targets misfolded proteins to the proteasome for degradation [7, 8]. Other physiological functions of GRP78 include acting as a regulator of the unfolded protein response thereby attenuating ER stress and protecting cells against cell death [9, 10]. It has also been reported that GRP78 mediates the presentation of antigenic peptides to major histocompatibility complex class I (MHC1) molecules [11]. GRP78 is expressed at low basal level in major adult organs such as brain, heart and lung but is strongly up-regulated in tumors including GA [12, 13] and CRC [14]. Cancer cells are subject to ER stress as a result of hypoxia, acidosis, glucose deprivation and cytotoxic insult. These factors lead to an induction of GRP78 expression in tumor cells, thatwhich has been shown to protect them against apoptosis, immune attack [15, 16] and confer drug resistance [17–20]. Subsequently, tumor cells that are subject to such stresses remain viable [15]. Recent studies have indicated that overexpression of GRP78 is associated with tumor progression and poor prognosis in CRC and GA [12–14]. In addition, knockdown of GRP78 expression inhibited gastric cancer cell invasion in vitro and growth and metastasis in vivo [12].
There are several mechanisms that may lead to aberrant GRP78 expression. Previous reports have shown that polymorphisms within the GRP78 gene, alone or in combination, alter promoter activity [21, 22]. Subsequently, these findings provide evidence that functional GRP78 polymorphisms may contribute to interindividual variability in the ER stress response. Based on these data, we hypothesized that functional GRP78 polymorphisms could potentially predict clinical outcome in patients with localized GA and locally advanced CRC.
patients and methods
patients
This study includes a total of 234 patients with locally advanced (stage II and III) CRC and a total of 137 patients with localized (stage Ib–IV) GA. CRC patients who were treated with 5-fluorouracil (5-FU)-based adjuvant chemotherapy [bolus 5-FU: n = 56 (24%); infusional 5-FU: n = 178 (76%)], either at the University of Southern California/Norris Comprehensive Cancer Center (USC/NCCC) or the Los Angeles County/USC Medical Center, from 1987 to 2007, were included in this study. Patients with localized GA were treated with surgery alone or surgery and adjuvant (radio)chemotherapy, at USC/NCCC, the Los Angeles County/USC Medical Center, or the Memorial Sloan-Kettering Cancer Center/Cornell University from 1992 to 2008. Patient data were collected retrospectively through chart review and recurrence rate and overall survival (OS) data were updated most recently in 2008. Study approval was obtained by the Institutional Review Boards of the USC and Cornell University for Medical Sciences. All participants signed informed consent for the analysis of molecular correlates.
DNA extraction, single-nucleotide polymorphisms selection and genotyping
DNA was extracted either from whole blood or from formalin-fixed paraffin-embedded tumor tissue using QIAamp kit (Qiagen, Valencia, CA). The following criteria were used to select candidate single-nucleotide polymorphisms (SNPs): (i) a minor allele frequency ≥10% in Caucasians according to the HapMap Project database (www.hapmap.org); (ii) functional polymorphisms located in the promoter region or 3′ untranslated region and were shown to be of biological significance according to the literature review; and (iii) were associated with disease risk (Table 2). GRP78 polymorphisms were tested either by using PCR–restriction fragment length polymorphism technique or by direct sequencing. Briefly, forward and reverse primers were used for PCR amplification, and PCR products were digested by restriction endonucleases (New England Biolabs, Ipswich, MA). Further, alleles were separated using a 4% NuSieve ethidium bromide-stained agarose gel. If no- restriction endonuclease could be found, samples were analyzed by direct sequencing. Genotyping results were validated by direct DNA sequencing in a random 5% of samples for additional quality control. Genotype concordance was ≥99%. The genes, reference SNPs' identification numbers, location, forward and reverse primer, restriction enzymes and annealing temperatures are summarized in Table 1.
Table 2.
Baseline demographic and clinical characteristics and clinical outcome in patients with localized GA and locally advanced CRC
| Localized GA |
Locally advanced CRC |
||||||||||
| Time to tumor recurrence |
OS |
Time to tumor recurrence |
|||||||||
| n | Median TTR, years (95% CI) | Hazard ratio (95% CI) | P valuea | Median OS, years (95% CI) | Hazard ratio (95% CI) | P valuea | n | Median TTR, years (95% CI) | Hazard ratio (95% CI) | P valuea | |
| Age, years | |||||||||||
| <60 | 80 | 2.2 (1.5–14.5b) | 1 | 0.42 | 4.7 (3.8–14.6b) | 1 | 0.65 | 132 | 9.4 (4.9–12.2b) | 1 | 0.60 |
| ≥60 | 57 | 3.7 (2.1–12.3b) | 0.81 (0.48–1.36) | 4.5 (3.3–7.3) | 1.14 (0.64–2.05) | 102 | 5.7 (3.5–16.8b) | 1.12 (0.74–1.69) | |||
| Sex | |||||||||||
| Male | 83 | 2.3 (1.8–7.0) | 1 | 0.85 | 4.1 (3.3–7.3) | 1 | 0.32 | 127 | 7.1 (3.9–11.1) | 1 | 0.70 |
| Female | 54 | 7.0 (1.5–8.3b) | 0.95 (0.56–1.63) | 7.3 (3.8–8.3b) | 0.72 (0.37–1.39) | 107 | 9.4 (4.9–16.8b) | 0.92 (0.61–1.40) | |||
| Race | |||||||||||
| White | 63 | 1.7 (1.2–4.4) | 1 | 0.085 | 3.8 (2.7–5.5) | 1 | 0.040 | 123 | 5.9 (4.8–11.1) | 1 | 0.58 |
| African-American | 1 | 0.5b | c | 0.5b | c | 15 | 2.6 (0.8–10.3b) | 1.42 (0.64–3.12) | |||
| Asian | 28 | 7.0 (2.3–14.5b) | 0.45 (0.23–0.91) | 7.3 (3.3–14.6b) | 0.45 (0.20–1.03) | 34 | 7.1 (2.4–10.6b) | 1.00 (0.54–1.84) | |||
| Hispanic | 45 | 3.7 (2.1–10.7b) | 0.63 (0.34–1.17) | 10.7b (3.6–10.7b) | 0.36 (0.15–0.85) | 62 | 10.4b (3.9–10.4b) | 0.77 (0.44–1.35) | |||
| Stage | |||||||||||
| I | 12 | 4.3b (2.2–4.3b) | 1 | 0.030 | 4.4b | c | 0.32 | 0.006 | |||
| II | 36 | 7.0 (2.9–10.7b) | 1.56 (0.35–6.98) | 5.4 (4.1–10.7b) | 1 | 105 | 10.7 (5.9–16.8b) | 1 | |||
| III | 71 | 1.8 (1.4–2.8) | 3.24 (0.78–13.5) | 3.8 (2.8–7.3) | 1.31 (0.69–2.50) | 129 | 5.2 (2.6–11.1) | 1.84 (1.18–2.85) | |||
| IV | 18 | 1.6 (1.2–3.8b) | 4.00 (0.86–18.5) | 7.3b (1.4–7.3b) | 1.33 (0.43–4.09) | ||||||
| Tumor stage | |||||||||||
| T1d,e | 4 | 0.013 | 0.30 | 2 | 0.22 | ||||||
| T2d,e | 44 | 8.3b (2.9–8.3b) | 1 | 5.4 (4.1–8.3b) | 1 | 14 | |||||
| T3d,e | 79 | 1.7 (1.4–4.4) | 2.04 (1.14–3.67) | 4.5 (3.3–7.3) | 1.40 (0.73–2.68) | 187 | 9.4 (5.7–16.8b) | 1 | |||
| T4d,e | 10 | 27 | 3.2 (1.8–11.3b) | 1.42 (0.81–2.47) | |||||||
| Txe | 4 | ||||||||||
| N stage | |||||||||||
| Negative | 27 | 7.0 (1.8–10.7b) | 1 | 0.004 | 7.3 (3.4–10.7b) | 1 | 0.088 | 105 | 10.7 (5.9–16.8b) | 1 | 0.013 |
| N1 | 64 | 4.4 (2.2–14.5b) | 0.99 (0.47–2.11) | 5.5 (4.1–14.6b) | 1.07 (0.46–2.47) | 72 | 6.6 (2.7–11.3b) | 1.67 (1.01–2.74) | |||
| N2 | 31 | 1.3 (1.1–2.3) | 2.62 (1.15–5.94) | 3.3 (2.0–5.7b) | 2.27 (0.85–6.07) | 57 | 5.2 (1.8–12.4b) | 2.08 (1.24–3.49) | |||
| N3 | 15 | 1.6 (1.0–3.8b) | 1.96 (0.73–5.32) | 2.4 (1.1–3.8b) | 2.35 (0.66–8.41) | ||||||
| No. of resected lymph nodes | |||||||||||
| ≤12 | 70 | 6.6 (3.5–12.4b) | 1 | 0.71 | |||||||
| >12 | 145 | 9.4 (4.9–16.8b) | 0.92 (0.58–1.45) | ||||||||
| ECOG performance status | |||||||||||
| 0 | 62 | 2.5 (1.7–10.7b) | 1 | 0.77 | 4.5 (2.8–10.7b) | 1 | 0.25 | ||||
| 1 | 65 | 2.3 (1.6–14.5b) | 1.00 (0.60–1.68) | 5.7 (3.8–14.6b) | 0.68 (0.37–1.25) | ||||||
| 2 | 10 | 2.2b (1.7–2.2b) | 0.60 (0.14–2.53) | 2.2b (1.2–2.2b) | 1.67 (0.36–7.75) | ||||||
| Differentiation | |||||||||||
| Welle | 0.15 | 0.098 | 11 | 0.17 | |||||||
| Moderatee | 27 | 2.1 (1.1–3.8b) | 1 | 4.1 (2.7–4.7) | 1 | 151 | 10.7 (5.9–16.8b) | 1 | |||
| Poor/moderated | 10 | 3.7 (2.1–14.5b) | 0.65 (0.36–1.19) | 7.3 (3.8–14.6b) | 0.59 (0.31–1.13) | 54 | 5.4 (2.4–11.1b) | 1.40 (0.86–2.26) | |||
| Poord | 97 | ||||||||||
| Lauren | |||||||||||
| Diffuse | 40 | 3.7 (1.8–8.9b) | 1 | 0.87 | 7.3 (5.4–8.9b) | 1 | 0.74 | 7.3 (5.4–8.9b) | 1 | 0.74 | |
| Intestinal | 50 | 7.0 (2.1–14.5b) | 0.87 (0.45–1.67) | 5.7 (3.8–14.6b) | 1.10 (0.48–2.51) | 5.7 (3.8–14.6b) | 1.10 (0.48–2.51) | ||||
| Mixed | 21 | 12.3b (1.7–12.3b) | 1.04 (0.45–2.41) | 3.6 (1.9–12.3b) | 1.52 (0.51–4.58) | 3.6 (1.9–12.3b) | 1.52 (0.51–4.58) | ||||
| Type of chemotherapy | |||||||||||
| 5-FU | 0.0008 | 0.009 | 151 | 9.4 (5.4–16.8b) | 1 | 0.44 | |||||
| 5-FU/LV | 70 | 7.0 (2.8–10.6b) | 1 | 7.3 (3.8–10.6b) | 1 | ||||||
| 5-FU/LV/oxaliplatin | 19 | 1.6 (1.1–2.9) | 2.65 (1.22–5.75) | 2.4 (1.2–4.5b) | 4.07 (1.62–10.19) | 60 | 3.4 (2.1–5.2b) | 1.32 (0.76–2.28) | |||
| 5-FU/LV/CPT-11 | 23 | 7.1b (1.8–7.1b) | 1.35 (0.69–2.65) | ||||||||
| 5-FU, cis, CPT-11 | 23 | 14.5 (1.2–14.5) | 1.25 (0.58–2.69) | 4.1 (2.2–14.6b) | 1.32 (0.57–3.05) | ||||||
| None | 25 | 2.1 (0.8–2.5) | 2.99 (1.62–5.51) | 4.7 (2.8–5.7) | 1.95 (0.93–4.07) | ||||||
| Radiation | |||||||||||
| Yes | 88 | 2.5 (1.8–14.5b) | 1 | 0.92 | 4.5 (3.3–14.6b) | 1 | 0.68 | ||||
| No | 48 | 3.7 (1.7–12.3b) | 1.03 (0.60–1.76) | 5.4 (3.8–12.3b) | 0.89 (0.48–1.63) | ||||||
Based on log-rank test.
Estimates were not reached.
No events occurred and estimates were not obtained.
Grouped together for the estimates of hazard ratio in localized GA.
Grouped together for the estimates of hazard ratio in locally advanced CRC.
GA, gastric adenocarcinoma; CRC, colorectal cancer; OS, overall survival; TTR, time to tumor recurrence; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; 5-FU, 5-fluorouracil; LV, leucovorin; CPT-11, irinotecan; cis, cisplatin.
Table 1.
Location, primer sequences, restriction enzymes, and annealing temperature of the tested GRP78 polymorphisms
| Gene (rs number) | Location of polymorphisms | Forward primer (5′–3′) | Reverse primer (5′–3′) | Enzyme | Annealing |
| GRP78 rs391957 | Promoter region 57168556T>C | CTGACCCCGAGGCATTTC | GATGGAGGAAGGGAGAACAA | Mbo II | 60°C |
| GRP78 rs17840761 | Promoter region 57168511G>A | AGGCATTTCCGCTGGTAAC | AAAAGTTTCAGATCCCACAGC | Seq | 60°C |
| GRP78 rs12009 | 3′UTR 57161835G>A | GGCAGACCCTGAGCAGAATA | GCCCTAGTTGCTAACTACCATTT | Seq | 60°C |
GRP78, 78-kiloDalton glucose-regulated protein; UTR, untranslated region; Seq, direct sequencing.
statistical analysis
The primary end points of the associations between GRP78 polymorphisms and clinical outcome in two independent cohorts were time to tumor recurrence (TTR) and OS for patients with localized GA and TTR for patients with locally advanced CRC. The TTR was calculated from the date of diagnosis of the disease to the date of first observation of tumor recurrence or until last follow-up if the patient was recurrence free at that time. The OS was defined as the period from diagnosis to death from any cause or the last contact if the patient was alive. OS was not analyzed in the cohort of patients with locally advanced CRC, as the median OS had not been reached. All analyses were conducted separately in two cohorts.
The differences in baseline demographic, clinical and pathological characteristics by GRP78 polymorphisms were tested using Fisher's exact test. The association between each polymorphism and TTR and OS was analyzed using Kaplan–Meier curves and the log-rank test. While the genetic model of inheritance for GRP78 polymorphisms remained unknown, we considered the dominant, recessive, codominant, or additive model whenever appropriate.
Allelic distribution of GRP78 polymorphisms in each race/ethnic group was examined for deviation from Hardy–Weinberg equilibrium (HWE) using a one-degree-of-freedom chi-square test. Linkage disequilibrium among GRP78 polymorphisms was assessed using D′ and r2 values, and the haplotype frequencies were inferred using Haploview version 4.1 (www.broad.mit.edu/mpg/haploview).
The Cox proportional hazards regression model including tumor and lymph node stage as covariates and race and type of adjuvant chemotherapy as stratum variables was fitted to reevaluate the association between GRP78 polymorphisms and TTR and OS considering the imbalances in the distributions of baseline characteristics.
All statistical tests were two sided and carried out using the SAS statistical package version 9.2 (SAS Institute Inc., Cary, NC).
results
DNA was extracted for analysis from the blood samples of 234 locally advanced (stage II and III) CRC and tumor specimens of 137 localized (stage Ib–IV) GA patients. Of the 234 CRC patients, 90 (38.5%) had tumor recurrence and the probability of 3-year recurrence was 0.35 ± 0.034. The median TTR was 7.1 years [95% confidence interval (CI) 4.9 to 16.8+ years]. Forty-four (19%) of the 234 patients have died and the median OS for CRC cohort has not been reached. Patients with stage III disease were more likely to present with recurrence, compared with patients who were diagnosed with stage II disease (log-rank P < 0.001). However, there was no significant difference between GRP78 polymorphisms and tumor stage in the CRC cohort (P > 0.4).
Of the 137 patients with localized GA, 61 (45%) had tumor recurrence, with a probability of 3-year recurrence of 0.52 ± 0.05. Fifty-five of the 137 (40%) patients had recurrent disease within the first 3 years after surgery and the median time to recurrence (TTR) was 2.8 years (95% CI 2.1–7.0 years). Of the 137 patients, 45 (33%) have died and the median OS of the localized GA cohort is 4.7 years (95% CI 3.8–7.3 years). T- category (P = 0.013), N category (P = 0.004), and type of chemotherapy (P = 0.003) were significantly associated with TTR. There was no significant relationship between GRP78 polymorphisms tested and Laurens's classification (P > 0.6). Detailed clinicopathologic characteristics of both cohorts are summarized in Table 2. In both cohorts, GRP78 polymorphisms were not significantly associated with demographical (age, gender), clinical (type of chemotherapy) or pathological characteristics (tumor stage, grade or lymph node status) (P > 0.05). All genotype frequencies for GRP78 polymorphisms analyzed were within HWE (P > 0.05).
univariate analysis for GRP78 rs391957 and TTR and OS in localized GA
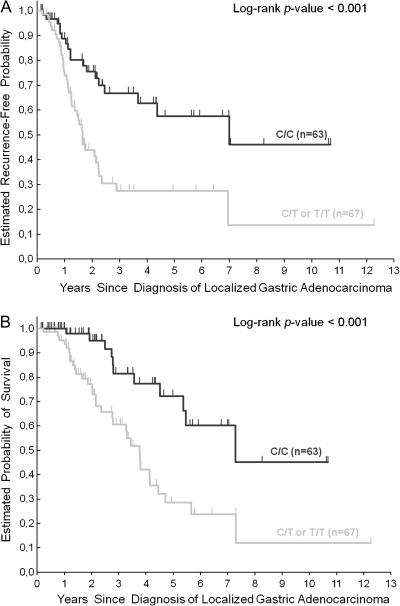
Genotyping of GRP78 rs391957 was successful in 130 (95%) of the 137 GA patients. In the remaining seven (5%) patients, genotyping was not successful because of limited quantity and quality of extracted genomic DNA. Patients harboring at least one T allele (C/T or T/T) of GRP78 rs391757 polymorphism had a significantly increased risk of tumor recurrence compared with those carrying C/C genotype [hazard ratio (HR) 2.61; 95% CI 1.48–4.59; P < 0.001; Figure 1]. In univariate analysis for OS, patients with at least one T allele (C/T or T/T) in the GRP78 rs391957 promoter region had a significantly increased risk of death compared with patients with C/C genotype (HR 3.17; 95% CI 1.60–6.29; P < 0.001, respectively; Figure 1; Table 3).
Figure 1.
Time to tumor recurrence (A) and overall survival (B) by GRP78 rs391957 polymorphism in localized gastric adenocarcinoma patients. Vertical hash marks indicate the time of last follow-up for those patients who have not recurred or died at the time of analysis of data. All censored patients and those who showed tumor recurrence are accounted for. GRP78, 78-kiloDalton glucose-regulated protein.
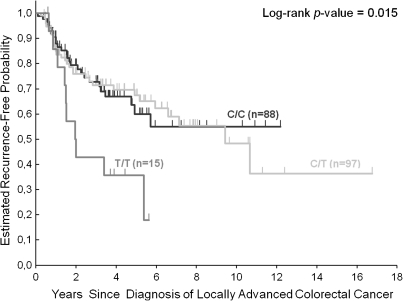
Figure 2.
Time to tumor recurrence by GRP78 rs391957 polymorphism in locally advanced (stage II and III) colorectal cancer patients. Vertical hash marks indicate the time of last follow-up for those patients who have not recurred or died at the time of analysis of data. All censored patients and those who showed tumor recurrence are accounted for. GRP78, 78-kiloDalton glucose-regulated protein.
Table 3.
Univariate analysis of GRP78 polymorphisms and clinical outcome in localized GA and locally advanced CRC
| Localized GA |
Locally advanced CRC |
|||||||
| Time to recurrence |
Overall survival |
Time to recurrence |
||||||
| n | Median, months (95% CI) | HR (95% CI) | Median, months (95% CI) | HR (95% CI) | n | Median, months (95% CI) | HR (95% CI) | |
| GRP78 rs391957 | ||||||||
| C/C | 63 | 7.0 (3.7–10.7a) | 1 | 7.3 (5.4–10.7a) | 1 | 88 | 12.2a (4.9–12.2a) | 1 |
| C/T | 56 | 1.6 (1.3–2.1) | 2.79 (1.56–4.97) | 3.8 (2.4–4.1) | 3.31 (1.65–6.62) | 97 | 9.4 (6.6–16.8a) | 1.01 (0.60–1.70) |
| T/T | 11 | 2.2 (0.8–4.9a) | 1.81 (0.67–4.91) | 4.9a (1.1–4.9a) | 2.25 (0.62–8.23) | 15 | 2.0 (1.4–5.4) | 2.61 (1.25–5.44) |
| P valueb | 0.001 | 0.002 | 0.015 | |||||
| C/T, T/Tc | 67 | 1.7 (1.3–2.2) | 2.61 (1.48–4.59) | 3.8 (2.8–4.5) | 3.17 (1.60–6.29) | |||
| P valueb | <0.001 | <0.001 | ||||||
| GRP78 rs17840761 | ||||||||
| A/A | 36 | 4.4 (2.5–10.7a) | 1 | 10.7a (4.5–10.7a) | 1 | 49 | 5.7 (3.5–12.2a) | 1 |
| A/G | 74 | 2.2 (1.7–12.3a) | 1.41 (0.73–2.72) | 4.1 (3.3–7.3) | 1.99 (0.87–4.55) | 126 | 10.7 (5.9–16.8a) | 0.87 (0.50–1.53) |
| G/G | 21 | 1.7 (0.8–7.0a) | 2.53 (1.12–5.69) | 3.8 (1.9–7.3a) | 3.24 (1.20–8.76) | 33 | 5.4 (2.0–12.4a) | 1.16 (0.58–2.31) |
| P valueb | 0.058 | 0.053 | 0.63 | |||||
| GRP78 rs12009 | ||||||||
| T/T | 55 | 3.2 (2.1–10.7a) | 1 | 5.4 (3.3–10.7a) | 1 | 62 | 5.7 (4.0–12.2a) | 1 |
| T/C | 58 | 2.1 (1.5–12.3a) | 1.26 (0.72–2.20) | 4.1 (2.8–12.3a) | 1.23 (0.64–2.38) | 116 | 10.7 (5.4–16.8a) | 0.88 (0.52–1.49) |
| C/C | 15 | 7.0 (1.1–7.0a) | 0.92 (0.37–2.26) | 4.7 (2.0–7.3a) | 1.19 (0.46–3.04) | 26 | 12.4a (1.5–12.4a) | 1.32 (0.65–2.68) |
| P valueb | 0.64 | 0.82 | 0.46 | |||||
Estimates were not reached.
Based on the log-rank test.
Dominant model.
GRP78, 78-kiloDalton glucose-regulated protein; GA, gastric adenocarcinoma; CRC, colorectal cancer; CI, confidence interval; HR, hazard ratio; CI, confidence interval.
univariate analysis for GRP78 rs391957 and TTR in CRC
Genotyping of GRP78 rs391957 was successful in 200 (86%) of the 234 CRC patients. In the remaining 34 (14%) patients, genotyping was not successful because of limited quantity and quality of extracted genomic DNA. Patients homozygous for GRP78 rs391757 T allele (T/T) had a significantly increased risk of tumor recurrence compared with those harboring C/C or C/T genotype (HR 2.61; 95% CI 1.25–5.44; P = 0.015; Figure 2; Table 3)
multivariable analysis of GRP78 rs391957 in localized GA and CRC
To adjust for potential confounding between GRP78 rs391957 polymorphism and both TTR and OS, a Cox proportional hazards model was constructed for both cohorts. The multivariable model for localized GA cohort was adjusted for T category and N category as covariates and stratified by race and type of chemotherapy. The CRC cohort included stage and type of adjuvant chemotherapy as covariates and stratified by race. GA patients carrying at least one T allele (T/T or C/T) of GRP78 rs391957 polymorphism remained significantly associated with decreased TTR and OS (adjusted P = 0.009 and adjusted P = 0.032, respectively). CRC patients harboring T/T genotype also remained significantly associated with TTR (adjusted P = 0.025; Table 4).
Table 4.
Multivariate analysis of GRP78 rs391957 and clinical outcome in localized GA and locally advanced CRC
| GRP78 rs391957 | Localized GA |
Locally advanced CRC |
|||
| n | TTR | OS | n | TTR | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| CC | 63 | 1 (reference) | 1 (reference) | 88 | 1 (reference) |
| CT | 56a | 2.497 (1.261–4.946) | 2.390 (1.076–5.306) | 97 | 0.980 (0.568–1.691) |
| TT | 11a | 15 | 2.632 (1.214–5.708) | ||
| P valueb | 0.009 | 0.032 | 0.025 | ||
Combined in the analysis for TTR and OS in gastric cancer cohort.
Wald test in Cox proportional hazards model including T category and N category as covariates and stratified by race and type of chemotherapy for the localized GA cohort; Cox models included stage and type of adjuvant therapy as covariates and stratified by race in the locally advanced CRC cohort.
GRP78, 78-kiloDalton glucose-regulated protein; GA, gastric adenocarcinoma; CRC, colorectal cancer; TTR, time to tumor recurrence; OS, overall survival; HR, hazard ratio; CI, confidence interval.
haplotype analysis
The three tested GRP78 polymorphisms (rs391957, rs17840761, and rs12009) were in linkage disequilibrium (data not shown). However, there were no significant relationships between the haplotypes and the clinical outcome parameters TTR and OS.
analysis of other tested GRP78 polymorphisms
We did not observe statistically significant relations between the two other tested GRP78 polymorphisms (rs17840761 and rs12009) and TTR and OS (Table 3).
discussion
The results of this translational study including patients with localized GA and locally advanced CRC led to the discovery that the functional GRP78 rs391957 promoter polymorphism may serve as a potential prognostic determinant for gastrointestinal cancers. These results remained significant even after adjusting for clinicopathologic characteristics. To our knowledge, this is the first report to indicate a significant association between a polymorphic variant of GRP78 (rs391957), a key molecule involved in host-stress response, and clinical outcome of patients from two independent GA and CRC cohorts.
Several studies have demonstrated that elevated- GRP78 level in cancer cell lines and solid tumors correlate with increased metastatic potential, drug resistance and poor patient survival [23]. Daneshmand et al. [24] have shown that prostate cancer patients with high GRP78 protein expression are at higher risk for tumor recurrence and death than patients with low GRP78 protein expression (P = 0.019 and P = 0.024, respectively). In GA and CRC, several independent studies have consistently confirmed that GRP78 protein overexpression is also a marker for aggressive disease and poor prognosis [12–14].
Polymorphisms in the promoter region of GRP78 are likely to influence clinical outcome if the different alleles alter transcriptional activity resulting in measurable and functional differences in gene expression. A functional study has demonstrated that GRP78 rs391957 polymorphism located in the promoter region of the GRP78 alters both the basal promoter activity and the promoter activity in response to ER stress [21]. More specifically, in non-stressed cells, the basal promoter activity of the variant T/T genotype was lower compared with C/C genotype. However, in response to ER stress, GRP78 messenger RNA and protein expression were significantly higher in cells harboring T/T genotype compared with C/C genotype [21]. These findings are consistent with the present study, which shows that localized GA patients carrying the GRP78 rs391957 C/C genotype had a significantly lower risk of tumor recurrence and death compared with patients possessing at least one T allele (C/T or T/T; P < 0.001 and P < 0.001, respectively; Table 3). These findings were subsequently tested in a cohort of patients with locally advanced CRC and showed consistently that patients carrying the GRP78 rs391957 C/C genotype had a significant lower risk for tumor recurrence compared with patients with C/T or T/T genotype (P = 0.015; Table 3). Moreover, it should be stressed that the prognostic significance of the GRP78 rs391957 polymorphism was independent of other established clinicopathologic determinants in both cohorts. Collectively, these data provide preliminary evidence suggesting GRP78 rs391957 promoter polymorphism as an independent prognostic marker for both localized GA and locally advanced CRC.
Several hypotheses have been proposed to provide biologic mechanisms by which GRP78 can promote tumor progression and metastasis. In vitro studies have predicted that ER stress response can activate the mitogen-activated protein kinase kinase/extracellular pathway in gastric cancer cells and hence inhibit apoptotic signaling in the cells subjected to ER stress [25]. Although GRP78 is generally restricted to the lumen of the ER in normal tissues, it was recently reported that GRP78 can translocate from the ER to the cell surface of tumor cells [26–28]. It has been reported that cell surface GRP78 serves as a co-receptor for MHC1 antigen presentation and as a receptor for α2-macroglobulin (α2M) [29]. Activation of GRP78 by α2M is postulated to promote proliferation, survival and metastasis of prostate cancer cells [30]. In addition, the extracellular signaling protein Cripto is reported to form a complex with cell surface GRP78 and enhance tumor growth via inhibition of transforming growth factor-β [31]. Moreover, it has been demonstrated that high GRP78 protein levels are not only involved in tumor progression but can also modulate tumor sensitivity to chemotherapeutic agents. Lee et al. [20] revealed that GRP78- positivity is associated with shorter recurrence-free survival following doxorubicin-based treatment alone but may predict response to taxane-based adjuvant chemotherapy in breast cancer [19]. Moreover, knockdown of GRP78 expression resulted in increased sensitivity to temozolomide, 5-FU and CPT-11 (irinotecan) in glioma cell lines [32, 33] and sensitized- colon cancer cell lines to histone deacetylase inhibitors [34].
While the precise mechanism whereby GRP78 regulates tumor progression in GA and CRC awaits further investigation, the results of the present study might be explained by the fact that the T-variant of GRP78 promoter polymorphism rs391957 drives promoter activation, thereby increasing GRP78 expression, which yields to a cascade of downstream pathways that ultimately result in gastric cancer and CRC progression and may modulate drug sensitivity.
Importantly for clinical translation, GRP78 is expressed on the cell surface of tumors but not normal organs and therefore has become an attractive target for cancer treatment [23]. GRP78-targeting peptides linked with cytotoxic chemotherapeutics have been shown to induce melanoma cell death in vitro [35] and tumor cell death in vivo [36]. Recently, Arap et al. [26] showed that synthetic chimeric peptides composed of GRP78-binding motifs fused to a pro-apoptotic sequence suppressed tumor growth without affecting normal tissues in xenograft models. In addition, Fu et al. [37] revealed that Akt phosphorylation—the major antiapoptotic and pro-proliferative signaling mechanism—is inhibited by knockdown of GRP78 in prostate cancer [37]. These studies further support the notion of GRP78 as a new promising therapeutic target. Our results may aid in the selection of patients with an increased likelihood of response to these drugs.
Although these findings indicate for the first time that the GRP78 promoter polymorphism rs391957 is significantly associated with OS and/or TTR in two independent study cohorts, these observations should be considered hypothesis generating due to the retrospective design and relative numbers of patients involved. Once our data are validated by prospective biomarker-embedded clinical trials, this polymorphism in GRP78 may become a valuable biomarker aiding in the selection of patients at high risk for tumor recurrence in GA and CRC.
funding
National Institutes of Health (5 P30CA14089-27I); Dhont Family Foundation; Austrian Society of Hematology and Oncology and Kurt und Senta-Herrmann Foundation to T.W. Cancer & Solidarité Foundation, Genève, Switzerland to P.H.; Austrian Society of Hematology and Oncology and Bank Austria Visiting Scientists Program to A.G.
disclosure
The authors declare no conflict of interest.
Acknowledgments
This work was previously presentationed of this work was at the Poster Discussion Session, Tumor Biology, ASCO 2010 (Abstract 10524). The work was carried out at the Sharon A. Carpenter Laboratory at the USC/NCCC in memory of David Donaldson and in honor of Dhont foundation.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Gunderson LL, Jessup JM, Sargent DJ, et al. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28:264–271. doi: 10.1200/JCO.2009.24.0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–816. doi: 10.1097/01.sla.0000143245.28656.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg RM, Fleming TR, Tangen CM, et al. Surgery for recurrent colon cancer: strategies for identifying resectable recurrence and success rates after resection. Eastern Cooperative Oncology Group, the North Central Cancer Treatment Group, and the Southwest Oncology Group. Ann Intern Med. 1998;129:27–35. doi: 10.7326/0003-4819-129-1-199807010-00007. [DOI] [PubMed] [Google Scholar]
- 6.Dong D, Ni M, Li J, et al. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68:498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- 7.Gething MJ. Role and regulation of the ER chaperone BiP. Semin Cell Dev Biol. 1999;10:465–472. doi: 10.1006/scdb.1999.0318. [DOI] [PubMed] [Google Scholar]
- 8.Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004;4:966–977. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Gronow M, Selim MA, Papalas J, Pizzo SV. GRP78: a multifunctional receptor on the cell surface. Antioxid Redox Signal. 2009;11:2299–2306. doi: 10.1089/ARS.2009.2568. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Jiang Y, Jia Z, et al. Association of elevated GRP78 expression with increased lymph node metastasis and poor prognosis in patients with gastric cancer. Clin Exp Metastasis. 2006;23:401–410. doi: 10.1007/s10585-006-9051-9. [DOI] [PubMed] [Google Scholar]
- 13.Zheng HC, Takahashi H, Li XH, et al. Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum Pathol. 2008;39:1042–1049. doi: 10.1016/j.humpath.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Xing X, Lai M, Wang Y, et al. Overexpression of glucose-regulated protein 78 in colon cancer. Clin Chim Acta. 2006;364:308–315. doi: 10.1016/j.cca.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Jamora C, Dennert G, Lee AS. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc Natl Acad Sci U S A. 1996;93:7690–7694. doi: 10.1073/pnas.93.15.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy RK, Mao C, Baumeister P, et al. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J Biol Chem. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- 17.Dong D, Ko B, Baumeister P, et al. Vascular targeting and antiangiogenesis agents induce drug resistance effector GRP78 within the tumor microenvironment. Cancer Res. 2005;65:5785–5791. doi: 10.1158/0008-5472.CAN-05-0754. [DOI] [PubMed] [Google Scholar]
- 18.Ermakova SP, Kang BS, Choi BY, et al. (-)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006;66:9260–9269. doi: 10.1158/0008-5472.CAN-06-1586. [DOI] [PubMed] [Google Scholar]
- 19.Lee E, Nichols P, Groshen S, et al. GRP78. as potential predictor for breast cancer response to adjuvant taxane therapy. Int J Cancer. 2010;28:726–731. doi: 10.1002/ijc.25370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee E, Nichols P, Spicer D, et al. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–7853. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 21.Hsu WC, Wang HK, Lee LC, et al. Promoter polymorphisms modulating HSPA5 expression may increase susceptibility to Taiwanese Alzheimer's disease. J Neural Transm. 2008;115:1537–1543. doi: 10.1007/s00702-008-0117-5. [DOI] [PubMed] [Google Scholar]
- 22.Kakiuchi C, Ishiwata M, Nanko S, et al. Functional polymorphisms of HSPA5: possible association with bipolar disorder. Biochem Biophys Res Commun. 2005;336:1136–1143. doi: 10.1016/j.bbrc.2005.08.248. [DOI] [PubMed] [Google Scholar]
- 23.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 24.Daneshmand S, Quek ML, Lin E, et al. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol. 2007;38:1547–1552. doi: 10.1016/j.humpath.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Zhang LJ, Chen S, Wu P, et al. Inhibition of MEK blocks GRP78 up-regulation and enhances apoptosis induced by ER stress in gastric cancer cells. Cancer Lett. 2009;274:40–46. doi: 10.1016/j.canlet.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 26.Arap MA, Lahdenranta J, Mintz PJ, et al. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6:275–284. doi: 10.1016/j.ccr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Wey S, Zhang Y, et al. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307–2316. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Liu R, Ni M, et al. Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J Biol Chem. 2010;285:15065–15075. doi: 10.1074/jbc.M109.087445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Misra UK, Sharma T, Pizzo SV. Ligation of cell surface-associated glucose-regulated protein 78 by receptor-recognized forms of alpha 2-macroglobulin: activation of p21-activated protein kinase-2-dependent signaling in murine peritoneal macrophages. J Immunol. 2005;175:2525–2533. doi: 10.4049/jimmunol.175.4.2525. [DOI] [PubMed] [Google Scholar]
- 30.Misra UK, Deedwania R, Pizzo SV. Activation and cross-talk between Akt, NF-kappaB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J Biol Chem. 2006;281:13694–13707. doi: 10.1074/jbc.M511694200. [DOI] [PubMed] [Google Scholar]
- 31.Kelber JA, Panopoulos AD, Shani G, et al. Blockade of Cripto binding to cell surface GRP78 inhibits oncogenic Cripto signaling via MAPK/PI3K and Smad2/3 pathways. Oncogene. 2009;28:2324–2336. doi: 10.1038/onc.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pyrko P, Schonthal AH, Hofman FM, et al. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–9816. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 33.Virrey JJ, Dong D, Stiles C, et al. Stress chaperone GRP78/BiP confers chemoresistance to tumor-associated endothelial cells. Mol Cancer Res. 2008;6:1268–1275. doi: 10.1158/1541-7786.MCR-08-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumeister P, Dong D, Fu Y, Lee AS. Transcriptional induction of GRP78/BiP by histone deacetylase inhibitors and resistance to histone deacetylase inhibitor-induced apoptosis. Mol Cancer Ther. 2009 May 5 doi: 10.1158/1535-7163.MCT-08-1166. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Steiniger SC, Kim Y, et al. Mechanistic studies of a peptidic GRP78 ligand for cancer cell-specific drug delivery. Mol Pharm. 2007;4:435–447. doi: 10.1021/mp060122j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Passarella RJ, Spratt DE, van der Ende AE, et al. Targeted nanoparticles that deliver a sustained, specific release of paclitaxel to irradiated tumors. Cancer Res. 2010;70:4550–4559. doi: 10.1158/0008-5472.CAN-10-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu Y, Wey S, Wang M, et al. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc Natl Acad Sci U S A. 2008;105:19444–19449. doi: 10.1073/pnas.0807691105. [DOI] [PMC free article] [PubMed] [Google Scholar]