Abstract
The LIM homeodomain transcription factor Islet1 (Isl1) is expressed in multiple organs and plays essential roles during embryogenesis. Isl1 is required for the survival and specification of spinal cord motor neurons. Due to early embryonic lethality and loss of motor neurons, the role of Isl1 in other aspects of motor neuron development remains unclear. In this study, we generated Isl1 mutant mouse lines expressing graded doses of Isl1. Our study has revealed essential roles of Isl1 in multiple aspects of motor neuron development, including motor neuron cell body localization, motor column formation and axon growth. In addition, Isl1 is required for survival of cranial ganglia neurons.
Keywords: Motor neuron, V2 interneuron, Cranial ganglia, Cell death, Isl1, Axon growth
Introduction
Functional motor circuits are dependent on generation of diverse types of neurons and establishment of precise connections of these neurons with their respective targets. Distinct subclasses of neurons in spinal cord are identified by their soma position, stereotypical axon trajectories and combinatory gene expression (Tsuchida et al., 1994; Appel et al., 1995; Tanabe and Jessell, 1996; Sharma et al., 1998). Combinatorial expression of LIM homeodomain (LIM-HD) transcription factors (LIM code) are required for specification and maintenance of distinct neuronal identities and control coordinated cell migration and axon guidance (Jessell, 2000; Shirasaki and Pfaff, 2002). In ventral spinal cord, motor neurons (MN) and V2 interneurons (IN) are derived from adjacent progenitors that share several components of their genetic programs, such as highly related LIM-HD factors Lhx3 and Lhx4. Lhx3 and Lhx4 are expressed in progenitor cells that give rise to both MNs and V2 INs and play a role in specifying the ventral MN identity (Sharma et al., 1998). Overexpression of Lhx3 alone in chick neural tube promotes the generation of V2 INs, whereas in combination with Isl1, it promotes MN generation (Tanabe et al., 1998; Thaler et al., 2002). LIM-HD factor Isl1 and Homeodomain protein HB9 are among the first MN genes expressed in postmitotic MNs in spinal cord. Hb9 is critical for the consolidation of MN identity by actively suppressing the V2 IN genetic program (Arber et al., 1999; Thaler et al., 1999). Mice deficient in HB9 display aberrant expression of the V2 interneuron marker Chx10, disorganized motor columns and axon pathfinding defects (Arber et al., 1999; Thaler et al., 1999). Recent biochemical and genetic studies have provided further insights into molecular mechanisms regulating fate specification of MN and V2 IN (Thaler et al., 2002; Lee and Pfaff, 2003; Nakano et al., 2005). A MN hexamer composed of 2NLI:2Isl1:2Lhx3 binds to and directly activates the Hb9 enhancer, whereas a V2 IN tetrameric protein complex without Isl1 (2Lhx3;2NLI) drives V2 IN genesis (Thaler et al., 2002). MNs express two V2 IN repressors: LIM only protein LMO4 and Hb9. LMO4 can block V2-tetramer assembly while Hb9 binds directly V2-tetramer response elements and suppresses their activation. Similarly, in V2 INs, V2-tetramer induces Chx10, a repressor that binds MN-hexamer response elements and blocks their activation. Thus, these cross regulatory feedback loops ensure precise assignment of MN and V2 IN fates (Lee et al., 2008).
Isl1 is expressed in all postmitotic MNs and is required for various aspects of MN development (Thor et al., 1991; Ericson et al., 1992; Lundgren et al., 1995; Pfaff et al., 1996; Thor and Thomas, 1997; Segawa et al., 2001). In Drosophila, Islet is required for motor axon pathfinding and neurotransmitter expression (Thor and Thomas, 1997). In zebrafish, knockdown of Isl2 leads to abnormal spinal MN soma localization and defects in motor axon projection and neurotransmitter expression (Segawa et al., 2001). In mice, ablation of Isl1 results in complete elimination of spinal MNs immediately after cell cycle exit (Pfaff et al., 1996). Reduced levels of total Islet proteins lead to an increase in V2a IN generation at the expense of MN formation (Song et al., 2009). Due to early embryonic lethality and early loss of MNs in Isl1 null mice, the role of Isl1 in later MN development remains unclear.
Isl1 is expressed in forebrain striatum and ablation of Isl1 in brain leads to a loss of cholinergic interneurons in the striatum and loss of cholinergic projection neurons in the nucleus basalis (Wang and Liu, 2001; Elshatory and Gan, 2008). Isl1 is expressed in sensory neurons of dorsal root ganglia and retina and is required for survival and differentiation of these cells (Elshatory et al., 2007; Pan et al., 2008; Sun et al., 2008). In addition, Isl1 is expressed in neurons of cranial ganglia and nucleus, but the role of Isl1 in these cell types has not been investigated (Thor et al., 1991; Inoue et al., 1994).
In the present study, we generated several mouse lines with graded reduction in Isl1 expression. We have shown that Isl1 is required, in a dose dependent manner, for specification and maintenance of spinal MN identity, proper cell soma settling and appropriate axonal trajectories of MNs. We have also shown that reduced Isl1 expression in Isl1 compound mutants leads to the conversion of prospective MNs to V2 INs. In Isl1 hypomorphic embryos, despite proper expression of HB9, MNs fail to form proper motor columns and motor axons which innervate axial muscles and diaphragm muscle are missing or truncated. In addition, we found that Isl1 is required for survival of cranial ganglia neurons.
Results
Generation of Isl1 hypomorphic and compound mutant Mice
Previously we generated an Isl1nLacZ knock-in mouse line in which a nuclear LacZ (nLacZ) gene was introduced into the endogenous mouse Isl1 locus immediately prior to the translation initiation site (ATG) (Sun et al., 2007). Heterozygous Isl1nLacZ/+ are fertile and viable, thus utilized as control mice in this study. Homozygous Isl1nLacZ/nLacZ mutant embryos die around E9.5 with phenotypes identical to that described previously for conventional Isl1 null mutation, and no Isl1 immunoreactivity was detected in Isl1 mutant spinal cord (not shown), thus demonstrating that the Isl1nLacZ allele is a null allele. In this study, we generated a floxed Isl1 mouse line. Mice heterozygous for floxed Isl1 (Isl1f/+) with or without the neo cassette, and mice homozygous for floxed Isl1 without the neo cassette (Isl1f/f) are fertile and viable. However, mice homozygous for floxed Isl1 with the neo cassette (Isl1f;neo /f;neo) died soon after birth with significantly reduced Isl1 expression in spinal MNs (Song et al., 2009), suggesting that the neo cassette interferes with Isl1 expression (hypomorphic allele). Although no general morphological defects were found, Isl1 hypomorphic mutant mice exhibited several motor dysfunctions, including an inability to move and breathe. Lungs of Isl1 hypomorphic mice were not inflated, or were barely filled with air (not shown).
To further compromise Isl1 expression, we crossed Isl1f:neo/+ mice to Isl1nLacZ/+ mice to generate an Isl1 compound mutant with one Isl1 null allele and one hypomorphic allele (Isl1nLacZ/f;Neo). Compound mutant mice die embryonically before E12.5, due to cardiac defects (YS, SE, unpublished observation). To better visualize neuronal migration and axon projections in Isl1 hypomorphic mice, we crossed Isl1 hypomorphic mice to Hb9-GFP mice, in which GFP expression is under the control of the mouse Hb9 promoter (Wichterle et al., 2002).
Reduced Isl1 expression in Isl1 compound mutant leads to a reduction in the number of spinal motor neurons and neurons in cranial ganglia
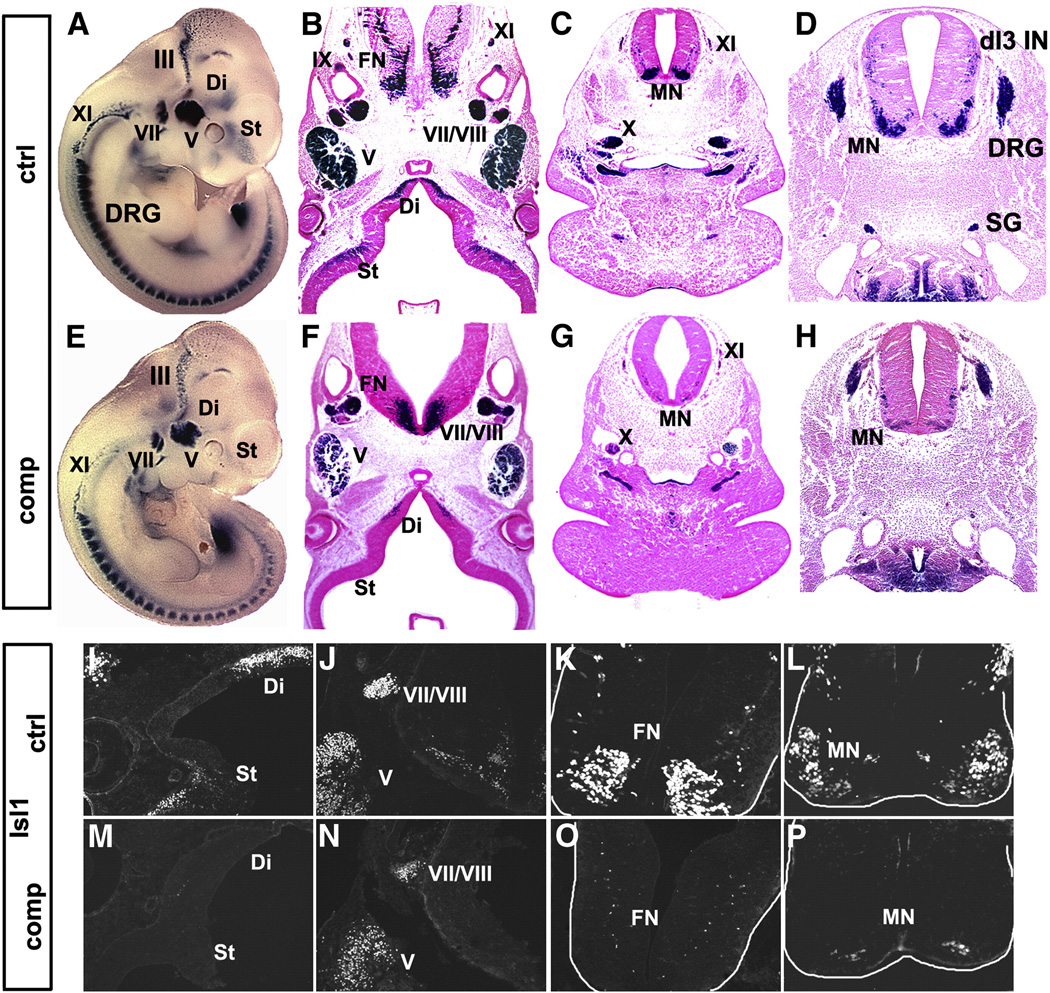
During development, Isl1 is expressed in multiple cell types and tissues and plays essential roles in these cells (Pfaff et al., 1996; Ahlgren et al., 1997; Cai et al., 2003; Laugwitz et al., 2005; Elshatory et al., 2007; Elshatory and Gan, 2008; Pan et al., 2008; Sun et al., 2008). We first examined how Isl1 expression is affected in Isl1 compound mutant embryos by analyzing Isl1 and β-gal stainings. Wholemount β-gal staining showed that Isl1-nLacZ was expressed in a pattern similar to the Isl1 mRNA expression pattern published previously (Fig. 1A) (Pfaff et al., 1996; Cai et al., 2003). In Isl1nLacZ/+ control embryos at E11.5, Isl1-nlacZ was expressed in various regions of the central nervous system, including the outer layer of the forebrain striatum, diencephalon, the ventral hindbrain MNs (Figs. 1A–C), the spinal MNs and a subpopulation of dorsal spinal interneurons (dI3 INs) (Figs.1A, D). Isl1-nlacZ was expressed in neurons of the peripheral nervous system, including neurons in the dorsal root ganglia (DRG) (Figs. 1A, D) and the sympathetic ganglia (SG) (Fig. 1D). Isl1-nlacZ was also expressed in most of the cranial ganglia with neurons derived from neural crest and/or placodes (Begbie and Graham, 2001) (Figs. 1A–C, shown are Oculomotor (III), Trigeminal (V), Facial/vestibulocochlear (VII/VIII), glossopharyngeal (IX), Vagal (X), Accessory (XI) ganglia/nerves). In compound mutant embryos, expression of β-gal and the number of β-gal expressing cells in most of these regions in central nervous system was significantly reduced (Figs. 1E–H). This result was confirmed by Isl1 antibody immunostaining (Figs. 1I–P). Compared to controls (Figs. 1I–L), there was a near absence of Isl1 immunoactivity in forebrain striatum and hindbrain MNs (Figs. 1M, O). Isl1 immunoreactivity in the V and VII/VIII cranial ganglia and the spinal MNs was significantly reduced (Figs. 1N, P).
Fig. 1.
Reduced Isl1 expression in regions of the central nervous system in Isl1 compound mutant. Isl1 is expressed in various regions of the central nervous system, including the outer layer of the striatum (St) and diencephalon (Di) (A, B, I), the motor neurons (MNs) of the ventral hindbrain (A, B, C, K), cranial ganglia/nucleus (shown are V, VII–XII) (A, B, J) and the spinal MNs (A, D, L) and dI3 interneurons (dI3 IN) (D). In Isl1 compound mutants (comp), the level of Isl1 expression and the number of Isl1 expressing cells are significantly reduced as revealed by Xgal staining (E–H) and Isl1 antibody staining (M–P, comp).
Reduced Isl1 expression leads to malformation of cranial ganglia with increased cell death
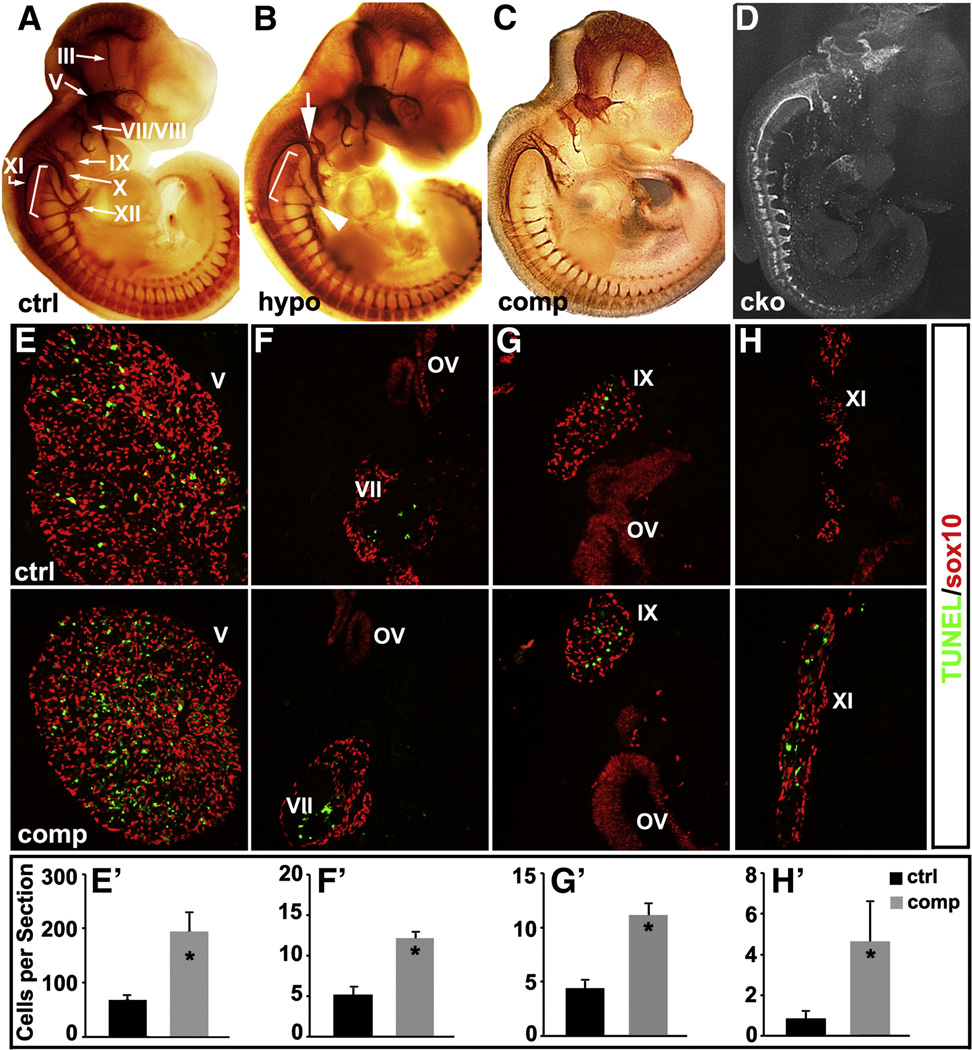
To assess the dose dependent effects of Isl1 on cranial ganglia development, we performed wholemount neurofilament antibody staining on E11.5 control and Isl1 mutant embryos with graded reductions in Isl1 expression (Figs. 2A–D). In Isl1 hypomorphic mutant embryos, the size and morphology of cranial ganglia/nerves III, V, VII/VIII, IX, X and XI appeared relatively normal (Fig. 2B). However, axons of cranial nerve XII were significantly thinner and blunted (Fig. 2B, bracket and arrowhead). An ectopic axonal projection was observed that extended from glossopharyngeal ganglion (IX) and connected to vagal (X) and accessory ganglia (XI) (Fig. 2B, white arrow). Further reduction in Isl1 expression in Isl1 compound mutants led to a significant reduction in the size of cranial ganglia V, VII/VIII, IX and X, and axonal projections of these ganglia were significantly thinner (VII, IX, X and IX), blunted (V, VII) or nearly absent (V, XII) (Fig. 2C). Spinal motor axons in Isl1 compound mutant were also significantly thinner or blunted (Fig. 2C). In addition, conditional knockout of Isl1 using Nestin-Cre, which is expressed in neural progenitors (Lendahl et al., 1990; Song et al., 2009), led to a severe loss of cranial ganglia. Cranial nerves and spinal motor axons were blunted or lost (Fig. 2D).
Fig. 2.
Reduced Isl1 expression in Isl1 compound mutant leads to increased apoptosis and abnormal development of cranial ganglia. Wholemount neurofilament staining of control (ctrl) (A), Isl1 hypomorphic (hypo) (B), Isl1 compound mutant (comp) (C) and Nestin-Cre; Isl1 conditional knockout (cko) (D) embryos at E11.5. Compared to control embryos, Isl1 hypomorphic mutant embryos displayed a relative normal size of cranial ganglia III, V, VII/VIII, X and XI (B). However, ectopic axonal projections from the cranial ganglion IX were observed (B, white arrow). Axonal projections of XI and XII ganglia were significantly reduced and blunted (B, bracket and arrowhead). In Isl1 compound mutant (C), further reduction in Isl1 expression led to reductions in the size of the cranial ganglia (V, VII/VIII, IX and X). Reductions in axon projections were observed in ganglia V (ophthalmic, maxillary and mandibular branches), VII, IX, X, XI and XII (C). Nestin-Cre; Isl1 conditional knockout embryos displayed an overall developmental defect with severe reduction in the size of cranial ganglia and their axon projections (D). TUNEL staining at E11.5 revealed significantly increased apoptosis in the cranial ganglia V, VII, IX and XI of Isl1 compound mutant compared to control embryo (E–H and E′–H′).
Reduced size of cranial ganglia might be attributed to decreased neurogenesis or increased neuronal apoptosis. To assess apoptosis in cranial ganglia of Isl1 compound mutant embryos, we performed TUNEL staining and Sox10 immunostaining. Sox10 is an HMG-box transcription factor expressed in neural crest and placodal progenitors that give rise to neurons and glial cells in cranial ganglia. In Isl1 compound mutant embryos at E11.5, Sox10 expression, which outlines cranial ganglia, was similar to that of control embryos, suggesting that migration and proliferation of the neural progenitors are not affected in Isl1 compound mutant embryos (Figs. 3E–H). However, significantly increased apoptosis in cranial ganglia V, VII, IX and XI was observed in Isl1 compound mutant embryos (Figs. 3E–H and E′–H′), indicating that Isl1 is required for survival of cranial ganglia neurons. We did not observe significantly increased apoptosis in MNs of the hindbrain and spinal cord of Isl1 compound mutant embryos, despite significantly reduced Isl1 expression in these regions (data not shown).
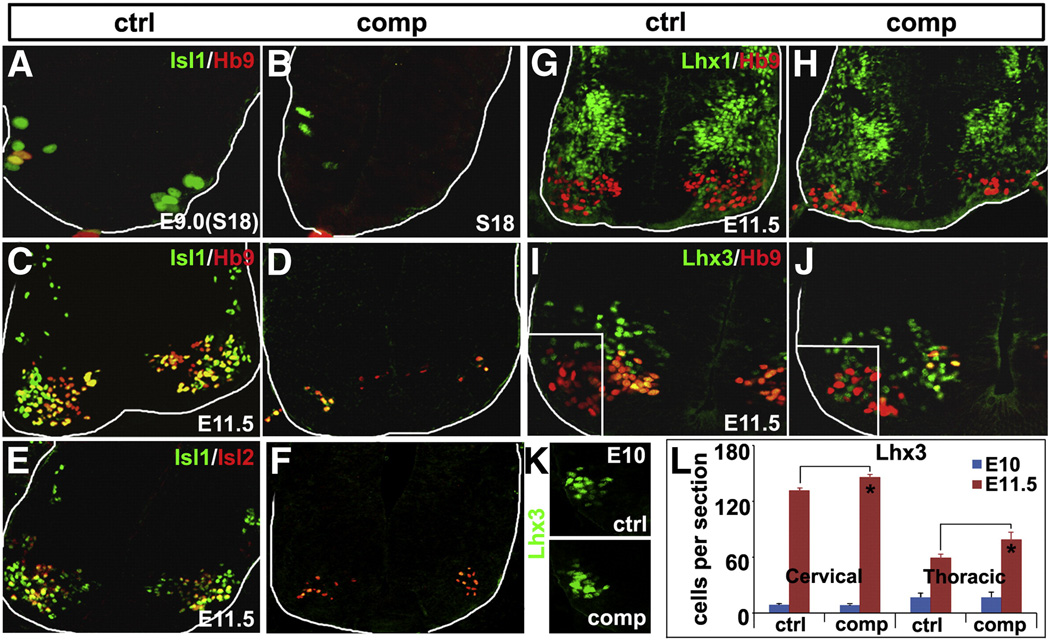
Fig. 3.
Decreased MN genesis in Isl1 compound mutant embryos. At E9 (18 Somites), a few Isl1 expressing MNs are generated in the anterior segments of the control spinal cord, a subpopulation of which coexpress Hb9 (A). However, in Isl1 compound mutant spinal cord, significantly fewer Isl1 expressing MNs are generated and none of which expresses Hb9 (B). At E11.5, compared to that of control mice (C, E), there is a dramatic reduction in the number of MNs marked by the expression of Isl1, Isl2 and Hb9 in Isl1 compound mutant (D, F). At E11.5, Lhx1 expression in the spinal cord of Isl1 compound mutant is comparable to that of control embryo (G, H). Lhx3 is expressed in V2 INs, and the MNs of the medial motor column where it is coexpressed with HB9 (I). However, the number of Lhx3 expressing cells in the spinal cord of Isl1 compound mutant embryos is significantly increased at E11.5 (J, L—red). Ectopic Lhx3 expressing cells were observed to invade into more ventrolateral region, a few of which coexpress HB9 (J). However, at E10.5 the number of Lhx3 expressing cells is comparable in mutant and control samples (K, L—blue).
Reduced Isl1 expression leads to decreased number of motor neuron
Consistent with previous studies, we found that reduced Isl1 expression in Isl1 compound mutant embryos led to reduced expression of two other MN markers Hb9 and Isl2 (Figs. 3A–F). We examined MN neurogenesis at the earliest time point during development. At E9 (18 Somite stage), a few Isl1 expressing MNs were generated in the anterior spinal cord of control embryos, of which a subpopulation co-expressed Hb9 (Fig. 3A). However, in Isl1 compound mutant embryos, significantly fewer Isl1 expressing cells were detected, none of which expressed Hb9 (Fig. 3B). At E11.5, there was a marked reduction in the number of MNs marked by the expression of Isl1, Hb9 and Isl2 in Isl1 compound mutant embryos (Figs. 3D, F). Reduced MN genesis was observed at all axial levels of the spinal cord, with more severe reductions seen at more anterior levels of Isl1 compound mutants. Lhx1 is expressed in the lateral part of the lateral motor column (LMC), and its expression pattern in Isl1 compound mutant embryos at E11.5 was similar to that of control embryos (Figs. 3G, H). Lhx3 is expressed in V2 INs and initially in all MNs, but is downregulated when MNs migrate laterally (Fig. 3I). At E10.5, the total number of Lhx3 expressing cells in Isl1 compound mutants embryos was comparable to that of controls (Figs. 3K, L). However, at E11.5, the number of Lhx3 expressing cells in Isl1 compound mutants was significantly increased, and a significant number of Lhx3 expressing cells were observed ectopically in more ventrolateral region, a few of which coexpressed Hb9 (Figs. 3J, L).
Reduced Isl1 expression leads to conversion of MNs to V2 INs
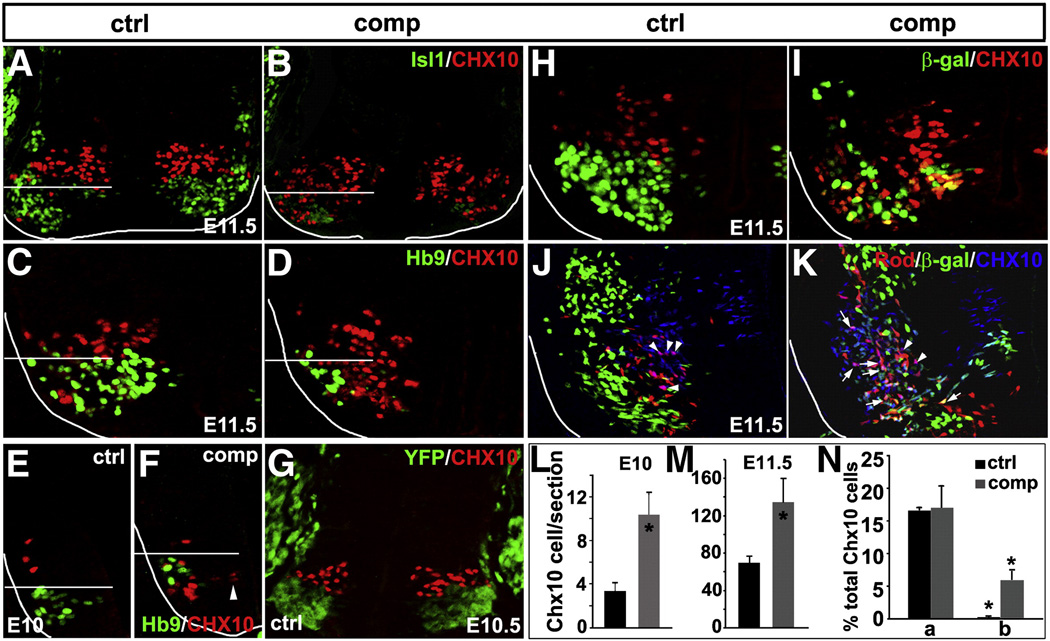
Isl1 plays an essential role in dictating the fate choice between MNs and V2 INs. We examined coexpression of MN markers Isl1 and Hb9 with the V2a IN marker Chx10 in Isl1 compound mutant embryos. At E11.5, the number of Isl1 and Hb9 expressing cells was significantly reduced with a concomitant increase in the number of Chx10+ V2a INs in Isl1 compound mutant embryos (Figs. 4A–D and M). A large number of Chx10 expressing cells in Isl1 compound mutant embryos were found ectopically in more ventral positions, mixed with remaining MNs (Figs. 4A–D, below the bar), suggesting a conversion of MNs to INs. However, we did not detect any cells that coexpressed MN markers and Chx10. We examined co-expression of Chx10 and Hb9 at E10, the earliest time of V2 IN genesis, to examine whether prospective MNs altered their identity immediately after birth. In control embryos, a few Chx10+ V2a INs have emerged in the spinal cord, dorsal to Hb9+ MNs (Fig. 4E). In Isl1 compound mutant embryos, there was a marked increase in the number of Chx10+ INs at this stage, which assumed a more ventral position relative to controls, and were mixed with Hb9 MNs (Fig. 4F, below the bar, and L). Some cells started to express Chx10 immediately after they leave the ventricular zone and start to differentiate (Fig. 4F, arrowhead). However, no coexpression of Chx10 and Hb9 was observed in either Isl1 compound mutants or control embryos at this early stage. We performed Isl1 lineage analysis with Isl1-Cre; Rosa-YFP and confirmed that Chx10 was never expressed in Isl1 lineages (YFP) in normal spinal cord (Fig. 4G), suggesting a complete segregation of these two cell types presumably at the progenitor level. These results suggested that the increase in V2a INs is due either to the conversion of MNs to INs immediately after their generation, or to increased genesis of V2a IN at the expense of MN genesis. Taking advantage of the fact that β-galactosidase is more stable and persists longer in cells (Thaler et al., 2004), we examined whether Isl1-β-gal was ever expressed in ectopic V2a INs. In control embryos, Isl1-β-gal was never coexpressed with Chx10 (Fig. 4H). However, in Isl1 compound mutant embryos, a substantial proportion of Chx10+ V2a INs coexpressed Isl1-β-gal (Fig. 4I), suggesting a conversion of prospective MNs to V2a INs in Isl1 mutants. To examine whether these aberrant Chx10+ V2a INs follow characteristic axonal trajectories of normal V2 INs, we performed intraspinal cord Rhodamine-dextran labeling. Chx10 V2a INs were similarly labeled by Rhodamine-dextran (Rhd) in both control (16.6 ± 0.5% of total Chx10+ cells) and Isl1 compound mutant spinal cords (17.2±3.44%) (Fig. 4J, K—arrowhead and N-a). A significant number of ectopic V2a INs in Isl1 compound mutant spinal cords, marked by the coexpression of Chx10 and Isl1-β-gal, were also labeled by Rhodamine-dextran (Fig. 4K—arrow and N-b), suggesting these ectopic INs extended axons intraspinally similarly to normal V2 INs. However, aberrant Chx10 V2 INs populated normal sites of MNs and intermingled with MNs, suggesting that these aberrant Chx10 V2 INs maintained a MN soma settling pattern.
Fig. 4.
Reduced Isl1 expression leads to conversion of prospective MNs to V2a INs. A significant increase in the number of Chx10 expressing V2a interneurons (INs) was observed in the spinal cord of Isl1 compound mutant embryos at E11.5 (A–D, M). These Chx10 cells found ectopically in more ventrolateral position, mixed with remaining MNs (B, D, below the bar). At E10, a few Chx10+ V2a INs start to emerge in the domain dorsal to that of Hb9+ MNs (E). A significant increase in the number of Chx10+ INs was readily detectable at this stage, which assumed a more ventral position and was mixed with Hb9 MNs (F, L). Some mutant cells started to express Chx10 immediately after they left the ventricular zone and started to differentiate (F, arrowhead). No coexpression of V2a IN marker Chx10 with MN marker Isl1 (A, B) and Hb9 (C–F) were observed in both Isl1 compound mutant and control embryos. Chx10 is never expressed in Isl1 lineage (YFP) in the normal spinal cord (G). Isl1-βgal is not coexpressed with Chx10 in control embryos (H). However, a significant number of ventral ectopic Chx10 V2a INs in Isl1 compound mutant embryos coexpress Isl1-βgal (45.4+/−23.4 per section) (I). Intraspinal cord labeling revealed that Chx10 V2a INs were similarly labeled by Rhodamine-dextran (Rhd) in both control (16.6 ± 0.5% of total Chx10+ cells) and Isl1 compound mutant (17.2 ± 3.44%) (J, K, arrowhead and N-a). A significant number of ectopic V2a INs in Isl1 compound mutant, marked by the coexpression of Chx10 and Isl1-βgal, were also labeled by Rhodamine-dextran (K-arrow and N-b). a: Rhd+/Chx10+; b: Rhd+/Chx10+/βgal+.
Isl1 is required for MN migration, motor column formation and axon growth
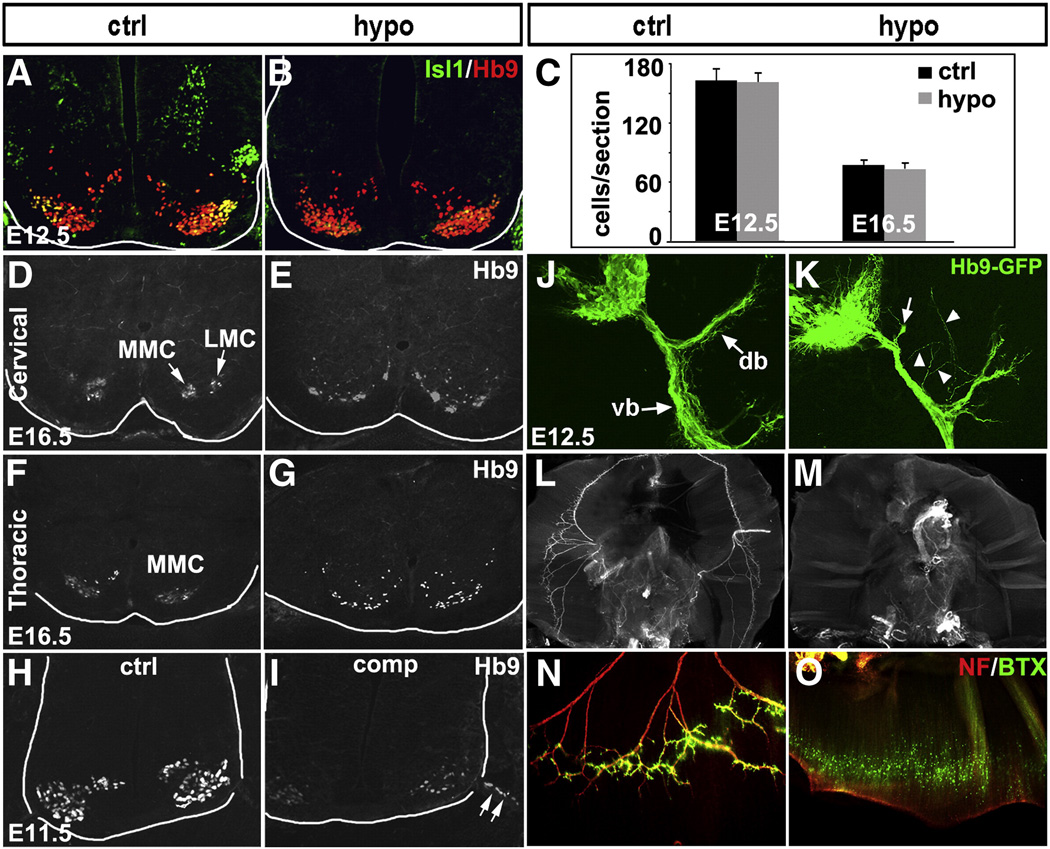
In Drosophila and zebrafish, Isl is required for correct soma settling and axon trajectory of the MNs (Thor et al., 1999; Segawa et al., 2001; Tanaka et al., 2011). We analyzed Isl1 hypomorphic mice, which survive perinatally. Despite a significant reduction in Isl1 expression in spinal MNs of Isl1 hypomorphic embryos, the level of Hb9 expression and the number of Hb9 expressing MNs did not significantly change during development (Figs. 5A, B and C). At E16.5, Hb9+ MNs in the spinal cord of control embryos were segregated into medial (MMC) and lateral (LMC) motor columns at the cervical level or MMC at the thoracic level (Figs. 5D, F). However, in Isl1 hypomorphic mice, Hb9+ MNs appeared to be scattered and ectopically positioned or abnormally clustered and failed to form distinct motor columns (Figs. 5E, G). In Isl1 compound mutant embryos, MNs were frequently observed to migrate along ventral roots from the neural tube (Fig. 5I arrow). These results demonstrated that Isl1 is required for MN soma settling or migration, and the formation of the motor column.
Fig. 5.
Defects in MN migration, motor column formation and axon growth in Isl1 hypomorphic embryos. Despite significant reduction in Isl1 expression in spinal MNs of Isl1 hypomorphic mutant, the level of Hb9 expression and the number of Hb9 expressing MNs during development are not changed (A, B and C). At E16.5, Hb9+ MNs are segregated into medial (MMC) and lateral (LMC) motor columns at the cervical spinal cord, or MMC at the thoracic spinal cord of control mice (D, F). However, in Isl1 hypomorphic embryos, Hb9+ MNs are scattered and ectopically positioned or abnormally clustered and fail to form distinct motor columns (E, G). In Isl1 compound mutant spinal cord, MNs were frequently observed to migrate along ventral roots out of neural tube (H, I arrow). In Hb9-GFP transgenic mice, GFP expression marks the axons of the spinal MNs, which after exit from vertebral column, are divided into dorsal branch (db) and ventral branch (vb) (J). In Isl1 hypomorphic mice that have been crossed onto Hb9-GFP transgenic background, the dorsal branch of the spinal motor axons appeared to be blunt (K, arrow) and frequent ectopic motor projections were observed (K, arrowhead). Wholemount neurofilament immunostaining and Alexa 488-α-bungarotoxin (BTX) staining revealed motor innervation of diaphragm by phrenic nerve and the formation of neuromuscular junctions on the diaphragm of control mice (L, N). However, diaphragm of Isl1 hypomorphic mutant mice were deinnervated and no functional neuromuscular junctions were detected (M, O).
To examine MN axonal projections, we utilized Isl1 hypomorphic mice crossed into an Hb9-GFP transgenic mouse background that allowed us to visualize motor axon trajectories. In control embryos, spinal MNs extended motor axons that, after exiting from the vertebral column, divided into the dorsal branch (db) and ventral branch (vb) (Fig. 5J). In Isl1 hypomorphic mutants, dorsal branches of motor axons were lost or blunted (Fig. 5K, arrow) and frequent ectopic motor projections were observed (Fig. 5K, arrowhead).
Isl1 hypomorphic mice died soon after birth, presumably due to defects in respiratory motor activity. Therefore we examined phrenic nerve innervation of diaphragm muscle. Wholemount neurofilament immunostaining and rhodamine-α-bungarotoxin (BTX) staining revealed phrenic motor innervation and the formation of neuromuscular junctions in the diaphragm of control mice (Figs. 5L, N). However, the diaphragm of Isl1 hypomorphic mutant mice was deinnervated and no functional neuromuscular junctions were observed (Figs. 5M, O). Together, these results suggested that Isl1 is required for correct axon pathfinding and growth. It is likely that Isl1 hypomorphic mice died of respiratory defects and a lack of diaphragm innervation.
Discussion
Due to early lethality and loss of MNs in Isl1 conventional knockout mice, the role of Isl1 in MN development remains unclear. In this study, we have generated two Isl1 mutant mouse lines with graded reductions in Isl1 expression. Analysis of these mice has revealed essential roles of Isl1 in MN soma settling and axon projection, and provided further evidence that reduced Isl1 expression leads to conversion of prospective MNs to V2a INs. In addition, our study has demonstrated a requirement for Isl1 in survival of the cranial ganglia neurons.
Isl1 plays essential roles in MN survival and MN cell fate choice. It has been shown that reduced level of total Islet proteins leads to reduced number of MNs and conversion of MNs to V2a Ins (Song et al., 2009). Consistent with these studies, we found that reduced Isl1 expression in Isl1 compound mutant lead to a reduction in the number of MNs with a concomitant increase in the number of V2 INs. Loss of Hb9 expression and thus MNs occur at the very beginning of MN genesis (E9.0). However, at this stage no premature generation of Chx10 cells was detected. We observed a significantly increased number of Chx10 cells in Isl1 compound mutant spinal cord at E10 (30—somite), a time when V2 IN genesis is just beginning. A few mutant cells started to express Chx10 immediately after their emergence from the ventricular zone of the spinal cord. These results suggest an early conversion of MNs to V2 INs, or an increase in V2a IN genesis at the expense of MN genesis. We did not observe coexpression of MN marker Hb9 or Isl1 with V2 IN marker Chx10 in either control or Isl1 compound mutant embryos. Lineage analysis with Isl1-Cre; Rosa-YFP confirmed that Chx10 was never expressed in Isl1 lineages in normal spinal cord. However, by using an Isl1-LacZ mouse line, in which Isl1 nuclear LacZ expression recapitulates endogenous Isl1 expression, but nuclear LacZ persists longer than Isl1 protein in cells, we found that a substantial proportion of Isl1 mutant cells coexpressed Isl1-LacZ and Chx10, thus providing direct evidence that with reduced Isl1 expression, prospective MNs are converted to V2 INs, which are capable of projecting axon intraspinally.
The phenotype seen in the Isl1 mutant embryos is similar but distinct to that of Hb9 mutant mice. Hb9 is not required for initial MN development. Deletion of Hb9 leads to a gradual conversion of MNs to V2 INs over a period between E10.5 and E13.5, and this conversion is less complete, thus resulting in hybrid cells with mixed identities (Arber et al., 1999; Thaler et al., 1999). In contrast, the conversion of MNs to V2 INs in Isl1 compound mutant embryos occurs earlier, suggesting that Isl1 and Hb9 may not function in the same pathway, and other targets may exist downstream of Isl1 in specifying MN fate. Interestingly, in Drosophila, dHb9 and Isl1 do not regulate each other, but rather, function in parallel to specify MN fates (Broihier and Skeath, 2002). However, overexpression of Hb9, but not Isl1, is sufficient to induce MN differentiation, suggesting that Isl1 must function together with other factors. In this context, delayed downregulation of Isl1 in Hb9 mutants, but early loss of Hb9 expression in Isl1 compound mutants may contribute to phenotypic differences in these two mutant mice. In the future, it would be interesting to see to what extent Hb9 overexpression could rescue the MN phenotype caused by loss of Isl1.
Lhx3 is expressed transiently in all MNs, but downregulated when MNs migrate laterally. Overexpression of Lhx3 is sufficient to induce ectopic V2 IN differentiation (Tanabe et al., 1998; Thaler et al., 2002). We found that, in contrast to Hb9 mutants, initial expression of Lhx3 in Isl1 compound mutants was comparable to that of controls, but increased at E11.5, mainly due to conversion of MNs to V2a INs. Interestingly, a previous study has shown that deletion of Isl1 leads to ectopic Lhx3 expression in DRG sensory neurons, suggesting a role of Isl1 in repressing Lhx3 expression (Sun et al., 2008). Thus, increased Lhx3 expression in certain cellular contexts with reduced Isl1 and Hb9 promotes conversion of MNs to V2a INs.
Subclasses of spinal MNs are identified by their distinct soma position and stereotypical axonal trajectories. Studies in Drosophila and zebrafish have demonstrated that Isl1 is required for MN migration and axon pathfinding (Thor and Thomas, 1997; Segawa et al., 2001; Tanaka et al., 2011). However, due to the early loss of MNs in Isl1 null mice, the role of Isl1 in these later developmental events remains unclear. In this study, we have generated Isl1 hypomorphic mice, which survive until birth, allowing us to address these questions. During early development, the number and distribution of Hb9+ MNs in Isl1 hypomorphic mice was comparable to that of control mice. At later stages, control MNs are further sorted and consolidated into distinct motor columns. Although similar numbers of Hb9 MNs were observed in Isl1 hypomorphic mutant and control mice at this stage, Isl1 mutant MNs failed to form distinct motor columns and remained dispersed or formed small clusters. Some mutant MNs migrated ectopically and extraspinally, similar to behaviors observed in Hb9 and Isl2 null mice (Arber et al., 1999; Thaler et al., 2004). Our results demonstrated that Isl1 is required for MN soma settling. In addition, Isl1 hypomorphic mutant MNs projected axons along ventral pathway to peripheral targets. However, dorsal branches of Isl1 mutant motor axons and phrenic nerve were lost. So far the underlying mechanisms for this remain unclear. Although initially Isl1 is expressed in all spinal MNs, its expression level varies among motor columns (Tsuchida et al., 1994). Thus the dose-dependent requirement for Isl1 in each of the motor columns may vary significantly, but higher levels of Isl1 expression may be required for MNs of MMC to elaborate axons properly. Phrenic MNs that innervate diaphragm express Isl1, but not Isl2 (Ericson et al., 1997). Thus development of this population of MNs may also be critically dependent on high levels of Isl1 expression.
Our studies have demonstrated that Isl1 is required for MN soma settling and motor column formation and axonal growth, however, underlying mechanisms remain to be defined. Motor axon growth is regulated by a series of long-range and cell-contact chemoattractive or repulsive signals that involve several families of molecules and their respective receptors, including netrins/Unc, semaphorins/NRPs and several growth factor signaling pathways (Dickson, 2002; Klein, 2004). Mice deficit in each of these genes present defects in motor axon trajectories and/or motor neuron soma settling, which partially resemble defects observed here in Isl1 hypomorphic mice (Kawasaki et al., 1999; Chen et al., 2000; Giger et al., 2000; Haase et al., 2002; Takashima et al., 2002; Burgess et al., 2006). It has been shown that MMCm axons express FGFR1 and are chemoattracted by FGFs expressed in dermomyotome (Shirasaki et al., 2006)). In addition, differential expression of cell adhesion molecules such as cadherins and NCAM in subclasses of spinal motor neurons are involved in motor pool formation and axon guidance (Price et al., 2002). Future study is needed to examine whether alternations in expression of these genes are responsible for the phenotypes observed in Isl1 hypomorphic embryos.
Our study revealed a role of Isl1 in the survival and axon projection of cranial ganglia neurons, similar to that found in sensory neurons (Elshatory et al., 2007; Pan et al., 2008; Sun et al., 2008). We found that reduced Isl1 expression in Isl1 hypomorphic embryos resulted in misprojection of glossopharyngeal nerve (XI) and significant loss of hypoglossal nerve (XII). Further reduction or deletion of Isl1 lead to markedly increased cell death in neurons of multiple cranial ganglia and loss of axonal projections. However, loss of axons in these instances is probably secondary to the loss of neurons.
Experimental methods
Transgenic mice
Isl1nLacZ knock-in mouse line was generated by insertion of nuclear LacZ (nLacZ) gene into mouse Isl1 gene locus just before the Isl1 translation initiation site (ATG) (Sun et al., 2007). Floxed Isl1 mouse line was generated as described(Sun et al., 2008). Briefly, exon 4 of Isl1 gene was floxed by the insertion of a loxP site on one end and a loxP site and frt-flanked neomycin cassette (neo) on the other end that serves as an ES cell selection marker. By backcrossing mice heterozygous for floxed Isl1 with neo gene (Isl1f:Neo/+) to get mice homozygous for floxed Isl1with neo gene (Isl1f;neo/f;neo) (Isl1 hypomorphic mice). Isl1 compound mutant mice (Isl1nLacZ/f;Neo) were generated by crossing Isl1f;Neo/+ mice with Isl1nLacZ/+ mice. To better visualize the neuronal migration and axon projections in Isl1 hypomorphic mice, we crossed Isl1 hypomorphic mice with Hb9-GFP mice(Lee et al., 2004).
X-gal staining and histological analysis
Embryos were harvested at the desired time points during gestation and fixed for 1–2 h in 4% PFA. Embryos were stained several hours to overnight at 37 °C in X-gal substrate solution consisting of 5 mM K4Fe (CN)6, 5 mM K3Fe (CN)6, 2 mM MgCl2, 0.01% NP-40, 0.1% deoxycholate, and 0.1% X-gal in PBS. Wholemount samples were fixed with 4% paraformaldehyde, paraffin-embedded and sectioned, and counter stained with hematoxylin and eosin (H&E).
Immunostaining, TUNEL staining and retrograde labeling
Embryos were fixed for 1–3 h in 4% PFA, embedded in OCT, and sectioned at 10 µm. Immunofluorescence was performed with the following primary antibodies: mouse monoclonal anti-Islet1/2 (DSHB), rabbit anti-Islet1 (abcam), guinea pig anti-Isl2, guinea pig anti-Hb9, guinea pig anti-Sox10, mouse anti-Lhx1 and mouse anti-Lhx3, rabbit anti-Chx10, rabbit anti-caspase-3 (Cell Signal Tech. #9661), mouse anti-neurofilament (DSHB) and rabbit anti β-gal (Cappel, #55976). Sections were washed then incubated with the appropriate secondary antibodies fluorescently labeled with Alexa 488 or 594 (Invitrogen), and mounted in Vectashield DAPI medium (Vector Laboratories). TUNEL staining was performed as recommended (Roche). For the wholemount neurofilament immunostaining, E11.5 embryos were harvested and processed for immunostaining using rabbit anti-neurofilament 150 (DSHB and Chemicon) antibody. For the phrenic nerve labeling, the diaphragm muscles were fixed and processed for immunostaining using rabbit anti-neurofilament 150 antibody and Alexa 488-conjugated Bungarotoxin (Invitrogen) (Thaler et al., 1999).
Intraspinal labeling of the spinal interneurons was performed with rhodamine-dextran (3000 MW, Molecular Probes). Embryos were cultured in oxygenated Ringer's buffer overnight at room temperature and then fixed for immunocytochemistry.
Statistical analysis
Data are presented as mean ± SEM and student t-test was used for 2-group comparisons. Differences were considered statistically significant at a value of P < 0.05.
Acknowledgments
YFS was supported by a BGIA (0865143F) from the American Heart Association, a grant (NSFC 31071280) from the National Natural Science Foundation of China, the 973 Program (2011CB504006) from the Ministry of Science and Technology, China, and the Pujiang Program (10PJ1408600) from the Shanghai Science and Technology Commission; MS by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MEST) (NRF-2008-313-E00343) and SE by NIH5R01HL070867-04.
References
- Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- Appel B, Korzh V, Glasgow E, Thor S, Edlund T, Dawid IB, Eisen JS. Motoneuron fate specification revealed by patterned LIM homeobox gene expression in embryonic zebrafish. Development. 1995;121:4117–4125. doi: 10.1242/dev.121.12.4117. [DOI] [PubMed] [Google Scholar]
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- Begbie J, Graham A. Integration between the epibranchial placodes and the hindbrain. Science. 2001;294:595–598. doi: 10.1126/science.1062028. [DOI] [PubMed] [Google Scholar]
- Broihier HT, Skeath JB. Drosophila homeodomain protein dHb9 directs neuronal fate via crossrepressive and cell-nonautonomous mechanisms. Neuron. 2002;35:39–50. doi: 10.1016/s0896-6273(02)00743-2. [DOI] [PubMed] [Google Scholar]
- Burgess RW, Jucius TJ, Ackerman SL. Motor axon guidance of the mammalian trochlear and phrenic nerves: dependence on the netrin receptor Unc5c and modifier loci. J. Neurosci. 2006;26:5756–5766. doi: 10.1523/JNEUROSCI.0736-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Bagri A, Zupicich JA, Zou Y, Stoeckli E, Pleasure SJ, Lowenstein DH, Skarnes WC, Chedotal A, Tessier-Lavigne M. Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron. 2000;25:43–56. doi: 10.1016/s0896-6273(00)80870-3. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Elshatory Y, Gan L. The LIM-homeobox gene Islet-1 is required for the development of restricted forebrain cholinergic neurons. J. Neurosci. 2008;28:3291–3297. doi: 10.1523/JNEUROSCI.5730-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshatory Y, Everhart D, Deng M, Xie X, Barlow RB, Gan L. Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J. Neurosci. 2007;27:12707–12720. doi: 10.1523/JNEUROSCI.3951-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J, Thor S, Edlund T, Jessell TM, Yamada T. Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science. 1992;256:1555–1560. doi: 10.1126/science.1350865. [DOI] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell TM, Briscoe J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Giger RJ, Cloutier JF, Sahay A, Prinjha RK, Levengood DV, Moore SE, Pickering S, Simmons D, Rastan S, Walsh FS, Kolodkin AL, Ginty DD, Geppert M. Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron. 2000;25:29–41. doi: 10.1016/s0896-6273(00)80869-7. [DOI] [PubMed] [Google Scholar]
- Haase G, Dessaud E, Garces A, de Bovis B, Birling M, Filippi P, Schmalbruch H, Arber S, deLapeyriere O. GDNF acts through PEA3 to regulate cell body positioning and muscle innervation of specific motor neuron pools. Neuron. 2002;35:893–905. doi: 10.1016/s0896-6273(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Inoue A, Takahashi M, Hatta K, Hotta Y, Okamoto H. Developmental regulation of islet-1 mRNA expression during neuronal differentiation in embryonic zebrafish. Dev. Dyn. 1994;199:1–11. doi: 10.1002/aja.1001990102. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat. Rev. Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr. Opin. Cell Biol. 2004;16:580–589. doi: 10.1016/j.ceb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1 + cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Pfaff SL. Synchronization of neurogenesis and motor neuron specification by direct coupling of bHLH and homeodomain transcription factors. Neuron. 2003;38:731–745. doi: 10.1016/s0896-6273(03)00296-4. [DOI] [PubMed] [Google Scholar]
- Lee SK, Jurata LW, Funahashi J, Ruiz EC, Pfaff SL. Analysis of embryonic motoneuron gene regulation: derepression of general activators function in concert with enhancer factors. Development. 2004;131:3295–3306. doi: 10.1242/dev.01179. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee B, Joshi K, Pfaff SL, Lee JW, Lee SK. A regulatory network to segregate the identity of neuronal subtypes. Dev. Cell. 2008;14:877–889. doi: 10.1016/j.devcel.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Lundgren SE, Callahan CA, Thor S, Thomas JB. Control of neuronal pathway selection by the Drosophila LIM homeodomain gene apterous. Development. 1995;121:1769–1773. doi: 10.1242/dev.121.6.1769. [DOI] [PubMed] [Google Scholar]
- Nakano T, Windrem M, Zappavigna V, Goldman SA. Identification of a conserved 125 base-pair Hb9 enhancer that specifies gene expression to spinal motor neurons. Dev. Biol. 2005;283:474–485. doi: 10.1016/j.ydbio.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Pan L, Deng M, Xie X, Gan L. ISL1 and BRN3B co-regulate the differentiation of murine retinal ganglion cells. Development. 2008;135:1981–1990. doi: 10.1242/dev.010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84:309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- Price SR, De Marco Garcia NV, Ranscht B, Jessell TM. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell. 2002;109:205–216. doi: 10.1016/s0092-8674(02)00695-5. [DOI] [PubMed] [Google Scholar]
- Segawa H, Miyashita T, Hirate Y, Higashijima S, Chino N, Uyemura K, Kikuchi Y, Okamoto H. Functional repression of Islet-2 by disruption of complex with Ldb impairs peripheral axonal outgrowth in embryonic zebrafish. Neuron. 2001;30:423–436. doi: 10.1016/s0896-6273(01)00283-5. [DOI] [PubMed] [Google Scholar]
- Sharma K, Sheng HZ, Lettieri K, Li H, Karavanov A, Potter S, Westphal H, Pfaff SL. LIM homeodomain factors Lhx3 and Lhx4 assign subtype identities for motor neurons. Cell. 1998;95:817–828. doi: 10.1016/s0092-8674(00)81704-3. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu. Rev. Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Lewcock JW, Lettieri K, Pfaff SL. FGF as a target-derived chemoattractant for developing motor axons genetically programmed by the LIM code. Neuron. 2006;50:841–853. doi: 10.1016/j.neuron.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Song MR, Sun Y, Bryson A, Gill GN, Evans SM, Pfaff SL. Islet-to-LMO stoichiometries control the function of transcription complexes that specify motor neuron and V2a interneuron identity. Development. 2009;136:2923–2932. doi: 10.1242/dev.037986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Liang X, Najafi N, Cass M, Lin L, Cai CL, Chen J, Evans SM. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev. Biol. 2007;304:286–296. doi: 10.1016/j.ydbio.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Dykes IM, Liang X, Eng SR, Evans SM, Turner EE. A central role for Islet1 in sensory neuron development linking sensory and spinal gene regulatory programs. Nat. Neurosci. 2008;11:1283–1293. doi: 10.1038/nn.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki Ji J, Hirota S, Kitamura Y, Kitsukawa T, Fujisawa H, Klagsbrun M, Hori M. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2002;99:3657–3662. doi: 10.1073/pnas.022017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe Y, Jessell TM. Diversity and pattern in the developing spinal cord. Science. 1996;274:1115–1123. doi: 10.1126/science.274.5290.1115. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, William C, Jessell TM. Specification of motor neuron identity by the MNR2 homeodomain protein. Cell. 1998;95:67–80. doi: 10.1016/s0092-8674(00)81783-3. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Nojima Y, Shoji W, Sato M, Nakayama R, Ohshima T, Okamoto H. Islet1 selectively promotes peripheral axon outgrowth in Rohon–Beard primary sensory neurons. Dev. Dyn. 2011;240:9–22. doi: 10.1002/dvdy.22499. [DOI] [PubMed] [Google Scholar]
- Thaler J, Harrison K, Sharma K, Lettieri K, Kehrl J, Pfaff SL. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron. 1999;23:675–687. doi: 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Lee SK, Jurata LW, Gill GN, Pfaff SL. LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein–protein interactions. Cell. 2002;110:237–249. doi: 10.1016/s0092-8674(02)00823-1. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Koo SJ, Kania A, Lettieri K, Andrews S, Cox C, Jessell TM, Pfaff SL. A postmitotic role for Isl-class LIM homeodomain proteins in the assignment of visceral spinal motor neuron identity. Neuron. 2004;41:337–350. doi: 10.1016/s0896-6273(04)00011-x. [DOI] [PubMed] [Google Scholar]
- Thor S, Thomas JB. The Drosophila islet gene governs axon pathfinding and neurotransmitter identity. Neuron. 1997;18:397–409. doi: 10.1016/s0896-6273(00)81241-6. [DOI] [PubMed] [Google Scholar]
- Thor S, Ericson J, Brannstrom T, Edlund T. The homeodomain LIM protein Isl-1 is expressed in subsets of neurons and endocrine cells in the adult rat. Neuron. 1991;7:881–889. doi: 10.1016/0896-6273(91)90334-v. [DOI] [PubMed] [Google Scholar]
- Thor S, Andersson SG, Tomlinson A, Thomas JB. A LIM-homeodomain combinatorial code for motor-neuron pathway selection. Nature. 1999;397:76–80. doi: 10.1038/16275. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Wang HF, Liu FC. Developmental restriction of the LIM homeodomain transcription factor Islet-1 expression to cholinergic neurons in the rat striatum. Neuroscience. 2001;103:999–1016. doi: 10.1016/s0306-4522(00)00590-x. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]