Abstract
Objective
People with fibromyalgia (FM) report a number of physical, cognitive, and psychological symptoms. The purpose of the current study was to determine whether people with FM differed based on the type and severity of symptoms, and if so, whether sub-groups differ with respect to health care utilization, functional ability, and work status.
Methods
Symptom, health care utilization, work and physical data were available for 2,182 female responders to an internet survey. Factor analysis was conducted on the (1) physical and (2) cognitive/psychological symptoms, and resulting factor scores were utilized in a cluster analysis to identify sub-groups based on symptoms. Cluster groups were compared on a set of variables (e.g., healthcare utilization, coping).
Results
FAs resulted in 3 symptom factor scores: musculoskeletal (MS), non-musculoskeletal (NMS), and cognitive/psychological (CP) symptoms. The optimal cluster solution to the cluster analysis revealed 4 clusters. Group 1 was high on all 3 symptom domains, group 2 was moderate on the 2 physical symptom domains, high on the CP, group 3 was moderate on the 2 physical symptom domains, low on the CP, whereas group 4 was low on all symptom domains. The more symptomatic groups reported the greatest amount of healthcare utilization and difficulty in coping with symptoms.
Conclusion
FM population is heterogeneous with regards to symptom reporting. Additional research is needed to better understand differential symptom experience among people with FM. Clarification of these differences may increase understanding of the mechanisms involved in FM and provide guidance for treatment decisions.
The diagnosis of Fibromyalgia Syndrome (FM) is based on 2 criteria: 1) chronic widespread pain, defined as pain in at least 3 quadrants of the body and axial, of at least 3 months duration, and 2) report of pain on palpation of at least 11 of 18 specified locations -- “tender points”(1). Although pain and fatigue are the core symptoms of FM, a plethora of concomitant symptoms (e.g., muscle spasms, headaches, problems with memory and concentration, and depression) are frequently present (2). Conceptually, these symptoms may be divided into 2 broad groups: 1) physical and 2) cognitive/psychological. The average number of symptoms reported by FM patients varies (3–4), and the variability is partially associated with the method used (e.g., spontaneous report, response to formal questionnaires) and number of symptoms presented in the questionnaires. The diversity and severity of co-morbid symptoms associated with FM probably contributes to the lengthy and expensive process patients undergo in an attempt to obtain a diagnosis and treatment. The broader a set of symptoms are, the greater the number of procedures that tend to be performed to rule out objective diseases prior to a diagnosis of FM. In an internet survey of over 2,500 people reporting FM, over 75% reported that they had seen more than 3 healthcare providers prior to obtaining their diagnosis, and approximately 25% had seen more than 6 health care providers prior to diagnosis (2). In a study of 2,260 newly diagnosed FM patients, the average number of visits to healthcare professionals during the year prior to diagnosis was 25 (5).
At a theoretical level, the diversity of symptoms reported by FM patients is consistent with the view held by some experts that FM is a multisystem disorder, rather than a disorder that primarily involves musculoskeletal symptoms (6). Moreover, the observation of wide variability in symptom reporting suggests that FM patients may be a heterogeneous group (7, 8).
The primary purpose of the present study was to examine symptom reporting patterns in a large cohort of FM patients. More specifically, the first goal was to identify whether patients varied in either patterns and/or severity of symptom reporting. In other words, do all patients report both psychological and physical symptoms, or do some only report physical symptoms? In addition, do all patients report that symptoms are very troubling, or do some symptoms have a more minimal impact? Secondly, if groups of patients are identified based on patterns of symptom reporting, do these groups differ with respect to indices of health care utilization, functional ability, and work status.
Methods
Sample
In February, 2006, the National Fibromyalgia Association (NFA, a layperson support and advocacy organization) posted a comprehensive survey on their website. The survey was completed by a cohort of 2,583 people in a 3 day period. A total of 86 responders were male, and were not included in the current study. Of the 2,497 female responders, 315 failed to provide information about symptoms and thus were excluded from the analyses of symptoms, leaving a final sample of 2,182. Using random sample generator (SPSS for Windows, version 14), the sample was divided into 2 approximately equal sized groups. Sample 1 (n=1071) was used for the primary cluster analysis, and Sample 2 (n=1111) was used to validate the results from the primary analysis.
Questionnaire
The questionnaire was developed by a multidisciplinary Task Force of FM experts consisting of physicians, physical therapists, psychologists, nurse, and patient advocates who attended a 2-day meeting organized by the NFA. The initial questionnaire included items regarding socioeconomic, functional, treatment, and health history, along with physical, cognitive, and emotional symptoms (2). The initial version was circulated among the panel attending the meeting and several FM experts who had been invited to the meeting but who had been unable to attend. A final version of the questionnaire was created based on feedback on the initial version. The specific items utilized in the current analysis were selected from the final NFA Questionnaire and are described in detail below.
Symptom checklist
Participants were asked to rate on an 11-point scale the degree to which each of a set of 18 physical, psychological, and cognitive symptoms was “a problem” (from 0, no problem, to 10, extreme problem), in the “past week”. The symptoms included pain, fatigue, musculoskeletal symptoms (e.g., morning stiffness, spasms, spasticity, swelling), other physical symptoms commonly reported by FM patients (headaches/migraine, restless legs, abdominal pain, bladder problems, postural instability, dizziness, rashes), emotional symptoms (i.e., anxiety, depression, anger), and cognitive problems (i.e., concentration problems, forgetfulness).
Impact on physical ability
Participants were asked to respond to a set of 12 questions regarding their ability to perform activities associated with daily living (e.g., bathing, climbing stairs, shopping). The scale ranged from “can do” (= 4), to “can not do at all” (= 0). Since some respondents did not provide answers about each activity, an average physical impact score was calculated by summing across all activities and dividing by the total number of items answered.
Data Analyses
All data analyses were conducted utilizing SPSS for Windows, version 14 (SPSS, Chicago, IL). Based on our interest in symptom patterns on physical and psychological symptoms, symptoms were first grouped into 1) physical or 2) cognitive/psychological. Principal components factor analyses (FA) were then performed separately on each of the 2 groups of variables – physical and psychological/cognitive. Oblique rotations were utilized to aid in interpretation, and the number of factors chosen was based on evaluation of the scree plot. Regression scores from the 2 FA were then used in the subsequent cluster analysis (CA) procedure. FA of all physical symptoms resulted in 2 distinct factors: 1) “musculoskeletal symptoms (MS)” (i.e., pain, stiffness, spasticity, spasms), and 2) “non-musculoskeletal symptoms (NMS)” (i.e., abdominal pain, bladder problems, rashes). Cross-loading was defined as <0.15 difference among factor loadings, and a variable was considered to load with >0.39 loading (9). A total of 5 symptoms had high cross-loading between the 2 factors (i.e., restless leg, swelling, dizziness, postural instability, fatigue) and migraine did not load significantly on either factor and thus these were eliminated. The total model accounted for 52.07% of the variance (MS = 37.24%, NMS = 14.83%). The FA was performed on the cognitive and psychological symptoms (i.e., depression, anger, and anxiety, concentration and forgetfulness) all of which loaded on a single factor (CP) that accounted for 57.51% of the total variance. Factor scores for each of the 3 factors (MS, NMS, and CP) were used in the subsequent clustering procedure.
CA was performed to identify symptom profiles among respondents (10). The k-means clustering procedure, which allocates data points into a specified number of clusters based on the centroids of each data point, was utilized to classify respondents into unique clusters. The number of clusters retained was determined based on 2 criteria: stability (i.e., reproducibility) and interpretability. A solution was considered stable if the centroids produced in Sample 2 were not significantly different from the centroids produced in Sample 1. The cluster groups must also be interpretable, which refers to the alignment of the clusters with clinical reports and experience of working with FM patients.
The CA was first performed on Sample 1 (n=1071), and then cross-validated on Sample 2 (n=1111). Following determination of cluster groups, results of the CA were validated on Sample 1 through significance tests that compared groups defined by the cluster solution on a set of relevant clinical variables (10). Chi-square tests of significance were utilized on categorical variables, and analysis of variance (ANOVA) on continuous variables.
Results
Demographic data for responders in the initial (Sample 1) and confirmation (Sample 2) samples are presented in Table 1. Sample 1 was predominantly Caucasian (90.9%), with an average age of 47 years (±10.81). Nearly the entire cohort (98%) reported they had been diagnosed with FM by a healthcare professional and more than 76% of the sample indicated that they had been diagnosed with FM for 3 or more years. Over two-thirds of the sample was married (67.4%), and the majority (83%) had at least some college education. Approximately 31% of responders reported filing a disability claim due to the impact of their FM symptoms. Approximately half of the sample (53.2%) reported believing they were able to work at an income producing job. Statistical analyses comparing Sample 1 and Sample 2 revealed no significant differences on any of the demographic or healthcare variables, suggesting the 2 samples were representative of the total cohort.
Table 1.
Demographic characteristics for female responders to the National Fibromyalgia Association questionnaire for Sample 1 and Sample 2.
| Variable Mean (SD); n (%) |
Sample 1 (n=1071) |
Sample 2 (n=1111) |
p value |
|---|---|---|---|
| Average age (years) | 46.88 (10.81) |
46.78 (10.71) |
0.831 |
| Length of diagnosis (months) | |||
| Not officially diagnosed | 19 (1.8) | 19 (1.7) | |
| 0–6 months | 67 (6.3) | 80 (7.2) | |
| 7–12 months | 68 (6.4) | 46 (4.1) | 0.27 |
| 1–2 years | 93 (8.7) | 101 (9.1) | |
| 3–4 years | 215 (20.1) | 237 (21.4) | |
| >4 years | 608 (56.8) | 626 (56.4) | |
| Length of symptoms (months) |
|||
| 0–6 months | 2 (0.2) | 10 (0.9) | |
| 7–12 months | 19 (1.8) | 19 (1.7) | 0.169 |
| 1–2 years | 39 (3.7) | 51 (4.6) | |
| 3–4 years | 151 (14.2) | 153 (13.9) | |
| >4 years | 854 (80.2) | 869 (78.9) | |
| Race | |||
| Hispanic | 4 (0.4) | 6 (0.5) | 0.65 |
| White | 973 (90.9) | 1021 (91.9) | |
| Black | 18 (1.7) | 20 (1.8) | |
| Asian | 5 (0.5) | 4 (0.4) | |
| Native American | 46 (4.3) | 33 (3.0) | |
| Other | 24 (2.2) | 27 (2.4) | |
| Marriage | |||
| Never married | 129 (12.1) | 120 (10.8) | |
| Divorced/separated | 193 (18.1) | 205 (18.5) | 0.82 |
| Widowed | 26 (2.4) | 29 (2.6) | |
| Married | 721 (67.4) | 756 (68.1) | |
| Living Arrangement | |||
| Living with someone | 915 (86.5) | 939 (85.4) | 0.47 |
| Not living w/someone | 134 (12.7) | 146 (13.3) | |
| Education | |||
| Grade school | 2 (0.2) | 1 (0.1) | |
| High school | 178 (16.8) | 154 (14.0) | 0.34 |
| Some college | 397 (37.4) | 437 (39.8) | |
| College | 274 (25.8) | 299 (27.3) | |
| Graduate/Professional | 210 (19.8) | 206 (18.8) | |
| Income | 45–50k (4k) | 45–50k (4k) | 0.99 |
The frequency and average rating of symptoms for responders that provided symptom information (n = 2182) are reported in Table 2. The most frequently reported symptoms were fatigue (n = 2175, 99.7%), pain (n = 1990, 91.2%), and stiffness (n = 1954, 89.6%). Respondents reported their most problematic symptoms were stiffness (7.17, sd = ±2.49), fatigue (7.05, sd = ±2.07), and pain (6.45, sd = ±1.97). The average number of symptoms endorsed was 10.59 (sd = ±1.39) out of a possible 18.
Table 2.
Frequency and average rating of symptoms among female responders to the NFA questionnaire (n=2182).
| Variable n (%) |
Frequency (>3) N (%) |
Average rating Mean (SD) |
|---|---|---|
| Core Physical | ||
| Stiffness | 1954 (89.6) | 7.17 (2.49) |
| Pain | 1990 (91.2) | 6.45 (1.97) |
| Spasticity | 1522 (69.8) | 5.13 (2.98) |
| Muscle spasms | 1359 (62.3) | 4.82 (3.23) |
| Extraneous Physical | ||
| Abdominal pain | 1030 (47.2) | 3.60 (2.89) |
| Bladder problems | 666 (30.5) | 2.50 (2.98) |
| Rashes | 471 (21.6) | 1.89 (2.96) |
| Cognitive/Psychological | ||
| Forgetfulness | 1721 (78.9) | 5.88 (2.67) |
| Concentration | 1750 (80.2) | 5.87 (2.6) |
| Anxiety | 1274 (58.4) | 4.52 (3.07) |
| Anger | 1042 (47.8) | 3.87 (2.96) |
| Depression | 1229 (56.3) | 4.37 (3.14) |
| Other symptoms | ||
| Fatigue | 2175 (99.7) | 7.05 (2.07) |
| Migraine | 1230 (56.4) | 4.36 (3.09) |
| Balance | 981 (45.0) | 3.48 (2.87) |
| Dizziness | 800 (36.7) | 2.90 (2.82) |
| Swelling | 860 (39.4) | 3.20 (3.09) |
| Restless leg | 1001 (45.9) | 3.58 (3.44) |
Development of participant profiles
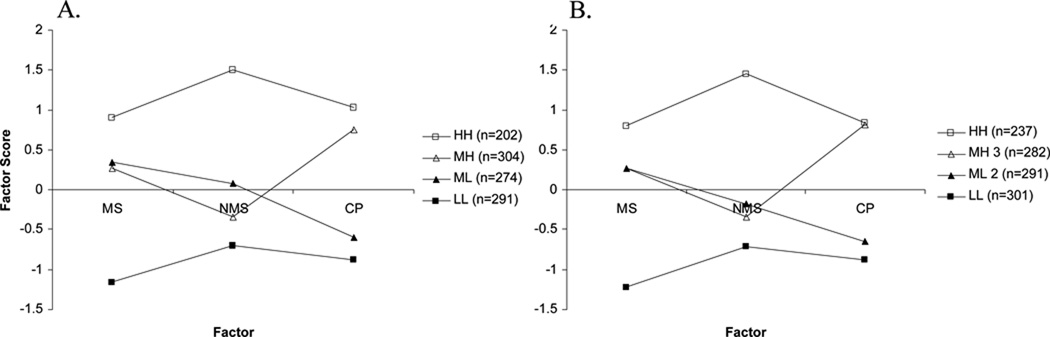
The k-means clustering procedure was conducted with 3 factor scores (MS, NMS, and CP) as the clustering variables, with iterations of 2, 3, 4, 5, and 6 cluster solutions. The CAs were repeated on Sample 2 for cross-validation, with successful replication with up to 4 groups (Figure 1). Statistical comparisons between centroids indicated the mean factor scores were not different for 11 out of the 12 comparisons between Sample 1 and 2. The scores on the NMS factor were different for the ML group (p<.001), however the total amount of variance accounted for by the relationship was only 2.8%, suggesting the difference was not meaningful. Additionally, differences were identified on external variables of interest with 4 groups (Table 3–4), and thus the 4 cluster solution was retained.
Figure 1. Final cluster centers for Sample 1 (a) and Sample 2 (b) set.
A. Final cluster centers (mean standardized scores) for symptom domains by cluster group for Sample 1 (n=1071). B. Final cluster centers for symptom domains by cluster group for Sample 2 (n=1111).
Table 3.
Demographic characteristics for responders by cluster group for Sample 1 (n=1071).
| Variable Mean (SD); n (%) |
HH (n=202) |
MH (n=304) |
ML (n=274) |
LL (n=291) |
P value |
|---|---|---|---|---|---|
| Average age, years |
46.80 (9.46) |
47.43 (10.29) |
47.96 (10.62) |
46.38 (12.27) |
0.20 |
| Length symptoms (months) |
|||||
| 0–6 months | 0 (0) | 0 (0) | 0 (0) | 2 (0.7) | |
| 0.18 | |||||
| 7–12 months | 4 (2.0) | 4 (1.3) | 6 (2.2) | 5 (1.7) | |
| 1–2 years | 3 (1.5) | 13 (4.3) | 8 (3.0) | 15 (5.2) | |
| 3–4 years | 34 (16.9) | 47 (15.5) | 28 (10.3) | 42 (14.5) | |
| >4 years | 160 (79.6) | 239 (78.9) | 229 (84.5) | 226 (77.9) | |
| Length diagnosis (months) |
|||||
| Not diagnosed | 4 (2.0) | 4 (1.3) | 7 (2.6) | 4 (1.4) | |
| 0–6 months | 14 (6.9) | 13 (4.3) | 15 (5.5) | 25 (8.6) | |
| 0.28 | |||||
| 7–12 months | 12 (5.9) | 23 (7.6) | 11 (4.0) | 22 (7.6) | |
| 1–2 years | 21 (10.4) | 26 (8.6) | 17 (6.2) | 29 (10.0) | |
| 3–4 years | 38 (18.8) | 67 (22.0) | 51 (18.6) | 59 (20.3) | |
| >4 years | 113 (55.9) | 171 (56.3) | 173 (63.1) | 151 (52.1) | |
| Race | 0.86 | ||||
| Hispanic/Latino | 1 (0.5) | 1 (0.3) | 1 (0.4) | 1 (0.3) | |
| White | 179 (88.6) | 282 (92.8) | 247 (90.1) | 265 (91.4) | |
| Black | 3 (1.5) | 3 (1.0) | 7 (2.6) | 5 (1.7) | |
| Asian | 1 (0.5) | 2 (0.7) | 0 (0) | 2 (0.7) | |
| Native American | 12 (5.9) | 12 (3.9) | 10 (3.6) | 12 (4.1) | |
| Other | 6 (3.0) | 4 (1.3) | 9 (3.3) | 5 (1.7) | |
| Marriage | |||||
| Never married | 14 (7.0) | 48 (15.8) | 31 (11.3) | 36 (12.4) | |
| Divor/Sep | 53 (26.4) | 54 (17.8) | 47 (17.2) | 39 (13.4) | 0.005 |
| Widowed | 3 (1.5) | 7 (2.3) | 10 (3.6) | 6 (2.1) | |
| Married | 131 (65.2) | 195 (64.1) | 186 (67.9) | 209 (72.1) | |
| Living Status | |||||
| Living someone | 179 (89.9) | 256 (85.3) | 233 (86.0) | 247 (85.8) | 0.41 |
| Living alone | 18 (13.4) | 41 (13.7) | 37 (13.7) | 38 (13.2) | |
| Education | |||||
| Grade school | 0 (0) | 1 (0.3) | 0 (0) | 1 (0.3) | |
| High school | 53 (26.2) | 54 (17.9) | 47 (17.3) | 24 (8.4) | |
| Some college | 91 (45.0) | 120 (39.7) | 100 (36.9) | 86 (30.1) | <.001 |
| College | 30 (14.9) | 87 (28.8) | 65 (24.0) | 92 (32.2) | |
| Graduate school | 28 (13.9) | 40 (13.2) | 59 (21.8) | 83 (29.0) | |
| Income | 39k (3k) | 45k (3k) | 52k (3k) | 59k (3k) | <.001 |
Table 4.
Health and disability variables for responders by cluster group for Sample 1 (n=1071).
| Variable mean (SD); n (%) |
HH (n=202) |
MH (n=304) |
ML (n=274) |
LL n=291) |
P value |
|---|---|---|---|---|---|
| Filed Workers Compensation Claim |
15 (7.6) | 18 (6.0) | 21 (7.9) | 14 (4.9) | 0.48 |
| Filed Disability Claim |
94 (48.0) | 113 (37.8) | 76 (28.3) | 42 (14.9) | <.001 |
| Times seen in ER in past year |
|||||
| 0 | 97 (48.87) | 186 (61.6) | 185 (68.5) | 224 (78.6) | |
| 1–4 | 86 (43.2) | 109 (36.1) | 79 (29.3) | 60 (21.1) | <.001 |
| 5–8 | 10 (5.0) | 5 (1.7) | 2 (0.7) | 1 (0.4) | |
| 9–12 | 3 (1.5) | 1 (0.3) | 4 (1.5) | 0 (0) | |
| >12 | 3 (1.5) | 1 (0.3) | 0 (0) | 0 (0) | |
| Times seen provider in past year |
|||||
| 0 | 4 (2.0) | 14 (4.6) | 7 (2.6) | 11 (3.8) | |
| 1–4 | 57 (28.2) | 113 (37.2) | 132 (48.2) | 175 (60.3) | <.001 |
| 5–8 | 48 (23.8) | 74 (24.3) | 65 (23.7) | 66 (22.8) | |
| 9–12 | 47 (23.3) | 57 (18.8) | 32 (11.7) | 19 (6.6) | |
| >12 | 46 (22.8) | 46 (15.1) | 38 (13.9) | 19 (6.6) | |
| Providers seen for diagnosis |
|||||
| none | 3 (1.5) | 6 (2.0) | 2 (0.7) | 5 (1.7) | |
| 1–2 | 31 (15.5) | 63 (20.9) | 74 (27.0) | 99 (34.1) | <.001 |
| 3–4 | 58 (29.0) | 105 (34.8) | 88 (32.1) | 102 (35.2) | |
| 5–6 | 35 (17.5) | 47 (15.6) | 40 (14.6) | 36 (12.4) | |
| >6 | 73 (36.5) | 81 (26.8) | 70 (25.5) | 48 (16.6) | |
| Believe able to work |
63 (31.7) | 131 (43.4) | 166 (61.3) | 204 (70.8) | <.001 |
| Believe provider treats FM legitimately |
|||||
| Not at all | 17 (8.6) | 28 (9.5) | 11 (4.1) | 7 (2.5) | |
| Somewhat | 60 (30.3) | 9 (23.3) | 66 (24.6) | 43 (15.4) | <.001 |
| Legitimate | 57 (28.8) | 88 (29.7) | 78 (29.1) | 92 (33.0) | |
| Very | 64 (32.3) | 111 (37.5) | 113 (42.2) | 137 (49.1) | |
| Lack of social support |
5.19 (3.22) | 4.62 (3.17) | 4.53 (3.23) | 3.82 (3.20) | <.001 |
| Ability to cope symptoms |
6.40 (2.24) | 5.68 (2.14) | 4.87 (2.32) | 3.79 (2.26) | <.001 |
| Sleep symptoms |
|||||
| Fall asleep | 6.8 (3.21) | 6.47 (3.0) | 5.7 (3.21) | 3.95 (3.1) | <.001 |
| Stay asleep | 7.05 (2.91) | 6.86 (2.62) | 6.49 (2.93) | 5.01 (2.99) | <.001 |
| Awake rested | 7.64 (.64) | 7.29 (2.64) | 6.63 (2.65) | 5.55 (2.54) | <.001 |
| Physical impact score |
25.28 (9.53) |
30.47 (9.51) |
31.60 (9.37) |
37.47 (7.94) |
<.001 |
scale items on a 0–10 scale, 0 = no problem
Cluster 1: High physical/high cognitive-psychological symptoms group (HH)
Final cluster centers (“centroids”, the mean scores of the clustering variables for each cluster) are presented in Figure 1. The scores are based on the z-distribution of the factor scores with a mean of 0 and SD of 1, thus the greater a cluster centroid deviates from 0, the greater the magnitude of difference on that variable with respect to the other clusters. Examination of Figure 1 suggests that Cluster 1, which included 202 respondents (18.86%), is highest on all 3 symptom domains. All scores were greater than a ½ SD from the mean of 0 (MS=.91, NMS=1.51, CP=1.04), suggesting they were different from the average symptom scores. With respect to all other clusters, the first cluster scored at least 50% higher on all symptom domains, and was thus labeled the high physical/high-cognitive-psychological group (HH).
Cluster 2: Moderate physical/high cognitive-psychological symptoms group (MH)
The second cluster was comprised of 304 respondents (28.38%), and scores on the physical factors were less than ½ a SD from the mean of 0 (MS= 0.26, NMS = −0.35), whereas the CP (.76) factor was more than ½ a SD from the mean of 0. Thus, the second cluster was considered moderate on the 2 physical factors, and high on the cognitive/psychological factor (MH).
Cluster 3: Moderate physical/low cognitive-psychological group (ML)
Approximately 25.58% (n = 274) of the sample were included in this third cluster group. Similar to the second cluster, the scores on both physical factors were less than ½ SD from the mean, and considered moderate (MS= 0.35, NMS = 0.07). However, the scores on the CP factor were greater than ½ SD from the mean, and considered low (−0.59), and the group was labeled moderate physical/low cognitive-psychological (ML).
Cluster 4: Low physical/low cognitive-psychological symptoms group (LL)
The remaining 291 respondents (27.17%) of Sample 1 comprised the fourth group. These respondents scored relatively lower (at least ½ SD from mean) on all 3 symptom factors. Cluster centroids were approximately twice as low as compared to the HH group in the MS (−1.16), NMS (−0.70), and CP factors (−0.87), thus the group was labeled low physical/low cognitive-psychological group (LL).
Validation of cluster solution
The cluster solution was validated by conducting identical k-means clustering procedures with iterations of 2, 3, and 4 cluster solutions on a second sample randomly selected from the total set, Sample 2. Inspection of Figure 1 reveals that the 4 cluster solution identified was virtually identical for both samples.
In addition to cross-validating the cluster solution on a separate sample, significance tests that compared the obtained clusters in Sample 1 on variables of clinical importance were conducted (11). Results are described below.
Demographic variables
Table 3 presents demographic characteristics for the 4 cluster profiles in Sample 1. Statistical comparisons indicated there were no significant differences among the 4 clusters in age (F3, 1049 = 1.05, P = 0.20), length of diagnosis (χ2 = 17.69, P = 0.28), race (χ2 = 9.31, P = 0.86), or living arrangement (χ2 = 2.91, P = 0.41). A significant difference among cluster profiles was detected in marital status (χ2 = 23.37, P = 0.005). The HH group was the most likely to have been divorced (26.4%), followed by the MH (17.8%), ML (17.2%), and LL (13.4%) groups, respectively. Analysis also revealed an overall effect for education (χ2 = 70.61, P < 0.001), with 29% of the LL group, 21.8 % of the ML, 13.9% of the HH and 13.2% of the MH group reporting attending graduate or professional school. A main effect for income suggested differences among profiles (F3, 1046 = 20.46, P <0.001), with pos hoc results indicating HH < MH < ML < LL (all P values < 0.009).
Healthcare, function, and work variables
Healthcare, functional status and disability/work beliefs for the cluster groups are presented in Table 4. Overall, the HH group was characterized by the greatest amount of healthcare utilization, reported worst physical function, and least favorable work characteristics. Conversely, the LL group was characterized by the least amount of healthcare utilization, and best physical function and work characteristics of all 4 groups.
Physical impact scores were significantly different among groups (F3, 946 = 66.03, P < 0.001), and discriminated between all but the 2 moderate physical groups, (HH < MH ≤ ML < LL, all significant P values < 0.001), with HH reporting the worst physical function (25.28, sd = ±9.53), and LL the best (37.47, sd = ±7.94). Lack of social support also varied among clusters (F3, 1063 = 7.59, P < 0.001), with post hoc tests indicating the HH group differed from all other groups (HH ≥ MH ≥ ML > LL all P values < 0.001), and the ML group differed from the HH group (ML < HH, P <0.03). A main effect for the amount of difficulty in managing symptoms indicated differences among the symptom profiles in coping (F3, 1056 = 6.13, P < 0.001), and all groups significantly differed from one another, with the greatest amount of difficulty reported by the HH group, (HH > MH > ML > LL, all P values < 0.001).
Symptom groups differed in their belief that they are able to work at an income producing job (χ2 = 91.82, P < 0.001), with LL the most likely to agree (70.8%), followed in descending order by ML (60.3%), MH (43.4%), and HH (31.7%). The number of group members filing a disability claim also varied among symptom clusters (χ2 = 67.48, P < 0.001), with 48% of the HH, 37.8% of the MH, 29.3% of the ML, and 14.9% of the LL reporting they had filed a claim.
The number of healthcare providers seen in the past year varied by symptom cluster (χ2 = 87.89, P < 0.001), as did the number of times they visited the emergency room (χ2 = 70.43, P < 0.001), and the number of healthcare providers they had seen before obtaining a diagnosis of FM (χ2 = 43.78, P < 0.001), with the greatest amount of healthcare utilization in the HH group, followed by the MH, ML, and LL groups, respectively.
Discussion
The concept of subgroups within FM was considered even prior to the development of the formal ACR diagnostic criteria (12–13). Since the publication of the ACR criteria in 1990, subgroups have been described based on psychosocial parameters (8), tender point thresholds, sensory-thresholds, and psychological variables (7). Our study is the first of which we are aware that identifies subgroups of people with FM based on patterns of reporting musculoskeletal symptoms (MS), other physical symptoms (NMS), cognitive and psychological (CP) symptoms. Although several symptoms commonly associated with FM (e.g., fatigue) were not included in the cluster analysis due to cross-loading, the intent of the sub-grouping was to describe patterns based on types of symptoms, and not for diagnostic purposes. The withdrawal of these symptoms does not suggest they do not play an important role in FM and should be considered in assessment. Four types of FM patients were identified based on their reporting of a set of these 3 symptom domains: 1) HH, 2) ML, 3) MH, and 4) LL. The analyses on external variables provided evidence that the 4 clusters differed significantly on several important variables, including healthcare utilization, degree of physical impairment, and ability to cope with symptoms. This, combined with the cross-validation on the test set, supports the validity of the methods used to identify patient clusters.
With regards to the physical symptom patterns, there was a tendency for FM patients within each of the 4 clusters to report similar degrees of musculoskeletal and non-musculoskeletal physical symptom severity, as evidenced by the relatively flat lines between MS and NMS factor scores in Figure 1. The comparable prevalence of MS and NMS within clusters suggests that FM may not be an exclusively musculoskeletal problem, consistent with Yunus’ research (6, 14–15).
Approximately 50% of the FM patients (both the HH and MH groups) reported major problems with psychological and cognitive symptoms. The HH and LL groups reported that their physical symptoms (MS and NMS) and their psychological symptoms were comparable in severity. In contrast, the MH group reported that psychological symptoms were more severe than physical symptoms, whereas the ML group showed the opposite pattern. The 2 groups that reported greater problems with the cognitive/psychological symptoms reported a relative inability to manage their symptoms as compared to the LL and ML groups. Based on their personal recognition of an inability to cope with their symptoms, these patients might benefit the most from psychological treatment aimed at improving coping skills (16–18).
There are a number of possible explanations to account for the differences in symptom reporting among FM patients. One possibility is that as FM progresses, symptom severity increases and it evolves into a multisystem syndrome. However, this hypothesis was not supported in the current study, since duration of symptoms and time elapsed since diagnosis was equivalent among all cluster groups. Another possibility is that patients with greater severity of symptoms have more extensive physical pathology, as a result, for example, of co-morbid medical conditions. Thirdly, it is possible that high symptom reporters experience more distressing symptoms because they have developed central nervous system sensitization, increased levels of inflammatory neurotransmitters such as Substance P, or hypothalamic-pituitary-adrenal axis dysfunction (for review of these mechanisms please see 6, 19). These 2 latter explanations warrant additional research to determine their relative contributions to patterns of symptom reporting.
One notable difference among the sub-groups was in education level, with LL > ML > MH > HH, reporting lower levels of education respectively. Thus, there was an inverse relation between participants’ education levels and the number and/severity of the symptoms they reported. This relation raises the possibility that responses of participants were influenced, at least to some extent, by an “education-related response set” such that what is really being measured is the degree to which a person will agree or disagree with an item, regardless of content (20). It has been suggested that the acquiescence is associated with item ambiguity (21), so that when respondents are unsure of an item, they will tend to answer in the affirmative. A study that alters the scale presentation, and either balances the number of positive and negative symptom items, or presents a negative scale to a sub-set and positive symptom scale to a sub-set, may provide insight into the role cognitive bias and response set may play in response to self-report questionnaires in general and, in particular, symptom reporting.
This study has several limitations. All of the data were derived from self-reports of a sample of participants who responded to an online survey conducted by the NFA. We did not have independent information about whether they actually met the diagnostic criteria for FM, or about any other medical data that they provided. Moreover, they were not a clinical sample and reported high levels of education. Thus, the results may not generalize to other samples people with FM or clinical populations. The percentage included in the high symptom reporting (HH) group is likely an underestimate compared to what may be observed in clinical settings since the sample may not have been specifically seeking treatment at the time of the survey.
Additionally, multiple analyses were performed to identify associations between the 4 clusters identified in this study and variables such as perceived work capacity and utilization of health resources. As a result, it is possible that some of the associations described above may be spurious. Since this was an exploratory study, we decided not to make any adjustment for the performance of multiple analyses. However, observation of the results reported does indicate that levels of statistical significance usually exceeded P < 0.001 and those may be reasonably valid.
The diagnosis of FM remains controversial, in part due to the failure to identify clear pathological mechanisms to aid in diagnosis. The current recommended classification criteria, positive tender point evaluation and chronic widespread pain of at least 3 month’s duration, are broad and non-specific. Application of these criteria has resulted in a diverse group of people being diagnosed with FM. Results from the current study confirm there are several subgroups within the FM population. The patterns of symptom reported by FM patients may reflect differences in the mechanisms underlying their conditions. For example, it is possible that musculoskeletal symptoms dominate the experiences of some patients with FM, whereas cognitive/psychological symptoms dominate the experiences of others. Research is needed to explore the associations among the sub-groups identified in previous research (7–8, 12–13) and the sub-groups identified in the current study. Understanding the differences within FM patients may guide future revisions of the diagnostic criteria for FM, serve as a basis for better understanding the mechanisms involved in FM, and inform treatment decision-making.
Acknowledgements
The authors would like to thank Peter G. Waldo for his insightful comments regarding data analyses.
Support for this manuscript was provided by NIH (NIAMS) grant AR 44724 and an unrestricted grant provided to the National Fibromyalgia Association by Pfizer.
REFERENCES
- 1.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 2.Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord. 2007;8:27. doi: 10.1186/1471-2474-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson JP, Wilson H, Swanson KS, Turk DC. Symptom reporting in people with fibromyalgia- high and low endorsers. J Pain. 2008;9 suppl 2:p17 [164]. [Google Scholar]
- 4.Stuifbergen AK, Phillips L, Voelmack W, Browder R. Illness perceptions and related outcomes among women with fibromyalgia syndrome. Women’s Health Issues. 2006;16:353–360. doi: 10.1016/j.whi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Hughes G, Martinez C, Myon E, Taieb C, Wessely S. The impact of a diagnosis of fibromyalgia on health care resource use by primary care patients in the UK: an observational study based on clinical practice. Arthritis Rheum. 2006;54:177–183. doi: 10.1002/art.21545. [DOI] [PubMed] [Google Scholar]
- 6.Yunus MB. Role of central sensitization in symptoms beyond muscle pain, and the evaluation of a patient with widespread pain. Best Pract Res Clin Rheumatol. 2007;21:481–497. doi: 10.1016/j.berh.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Giesecke T, Williams DA, Harris RE, Cupps TR, Tian X, Tian TX, et al. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum. 2003;48:2916–2922. doi: 10.1002/art.11272. [DOI] [PubMed] [Google Scholar]
- 8.Turk DC, Okifuji A, Sinclair JD, Starz TW. Differential responses by psychosocial subgroups of fibromyalgia syndrome patients to an interdisciplinary treatment. Arthritis Care Res. 1998;11:397–404. doi: 10.1002/art.1790110511. [DOI] [PubMed] [Google Scholar]
- 9.Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory. Pain. 1985;23:343–356. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- 10.Milligan GW, Cooper MC. An examination of procedures for determining the number of clusters in a data set. Psychometrika. 1985;50:159–179. [Google Scholar]
- 11.Aldenderfer MS, Blashfield RK. In: Cluster Analysis. Lewis-Beck MS, editor. Newbury Park: Sage Publications; 1984. [Google Scholar]
- 12.Wolfe F. Fibromyalgia: the clinical syndrome. Rheum Dis Clin North Am. 1989;15:1–18. [PubMed] [Google Scholar]
- 13.Simms RW, Goldenberg DL, Felson DT, Mason JH. Tenderness in 75 anatomic sites; distinguishing fibromyalgia patients from controls. Arthritis Rheum. 1988;31:182–187. doi: 10.1002/art.1780310205. [DOI] [PubMed] [Google Scholar]
- 14.Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36:339–356. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Yunus MB. Central Sensitivity Syndromes: A New Paradigm and Group Nosology for Fibromyalgia and Overlapping Conditions, and the Related Issue of Disease versus Illness. Semin Arthritis Rheum. 2008;37:339–352. doi: 10.1016/j.semarthrit.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Turk DC. Fibromyalgia: a patient-oriented perspective. In: R.H. D, W.S. B, editors. Psychosocial and Psychiatric Aspects of Pain: a Handbook for Health Care Providers. Seattle: IASP Press; 2004. pp. 308–339. [Google Scholar]
- 17.Thieme K, Turk DC, Flor H. Responder criteria for operant and cognitive-behavioral treatment of fibromyalgia syndrome. Arthritis Rheum. 2007;57:830–836. doi: 10.1002/art.22778. [DOI] [PubMed] [Google Scholar]
- 18.Turk D, Robinson J, Swanson K, Wilson H. Effectiveness of psychological therapies in the rehabilitation of individuals with fibromyalgia: responder analysis. J Pain. 2008;9 suppl 2:17[166]. [Google Scholar]
- 19.Peterson EL. Fibromyalgia--management of a misunderstood disorder. J Am Acad Nurse Pract. 2007;19:341–348. doi: 10.1111/j.1745-7599.2007.00235.x. [DOI] [PubMed] [Google Scholar]
- 20.Jackman MR. Education and prejudice or education and response-set? American Sociological Review. 1973;38:327–339. [PubMed] [Google Scholar]
- 21.Bass BM. Authoritarianism or acquiescence? Journal of Abnormal and Social Psychology. 1955;51:616–623. doi: 10.1037/h0042890. [DOI] [PubMed] [Google Scholar]