Abstract
Otitis media and other middle ear diseases are extremely common among all children worldwide. Chronic otitis media is strongly associated with the presence of a bacterial middle ear biofilm, which if left untreated, may result in hearing loss or delays in the development of speech and language. Many animal models and methods used to study the progression of various middle ear diseases exist. However, there are no reported approaches to biofilm induction in which this infectious process can be investigated. Here we report a unique, non-invasive method of biofilm induction in the rat through repeated bacterial inoculations and pressure changes within the ear.
Keywords: Biofilm, Otitis Media, Middle ear, Rat model, Pressure changes
1. Introduction
Acute otitis media (OM) is one of the most common infant and childhood diseases, affecting approximately 50-85% of children by the age of 3 years [1-6]. Acute OM is characterized by acute inflammation and associated with a concurrent or subsequent suppurative process in the middle ear [7-8], such as biofilm formation. Chronic OM includes both OM with effusion and recurring OM. OM with effusion can result in hearing loss, which is linked to delays in the development of speech and language [9]. Recently, a critical link has been established between the presence of chronic OM and middle ear biofilms in the pediatric population [10].
A biofilm is a highly structured polymicrobial community characterized by bacterial cells embedded in a matrix of extracellular polymeric substances and attached to a surface [11]. More than 60% of bacterial infections involve biofilms [12], with some bacterial colonies exhibiting increased tolerance to antibiotic therapies. The dynamics of biofilm formation constitute a strategy of microbial survival by facilitating the transmission of pathogens, providing a stable protective environment, and acting as a reservoir for the dissemination of numerous microorganisms to new surfaces [13]. Currently, there is little research in the area of middle-ear biofilm formation, dynamics, and responses to antibiotics. Biofilms are believed to be responsible for chronic antibiotic-resistant OM in children. Our ability to identify and characterize biofilms in the middle ear will provide critical diagnostic information that will likely alter physician protocols for prescribing antibiotic therapies and the management of these common infectious diseases. In fact, this model was developed to evaluate the use of low-coherence interferometry and optical coherence tomography as possible non-invasive diagnostic techniques to detect and quantify biofilm growth behind the tympanic membrane (TM) in a clinical office-based setting [14].
The most common causative agents of OM and their frequencies of involvement are Streptococcus pneumonia (35-60%), Haemophilus influenza (12-23%), and Moraxella catarrhalis (5-15%) [7, 15-17]. Streptococcus pneumonia is naturally able to colonize in the human nasopharynx, causing infection in the middle ear and other remote tissues [18]. S. pneumonia is a major gram positive human pathogen and the leading cause of pneumonia, meningitis, and bloodstream infections in elderly and young patients, and in patients with immunosuppressive illnesses and chronic diseases. Because of its prevalence in middle ear effusions, S. pneumonia is the main causative agent of middle-ear infections in children, and has been linked to the formation of middle ear biofilms [12].
In most animal models, OM is induced by direct inoculation of bacteria into the middle ear via the TM or bulla, which is acceptable if the primary objective is to evaluate the effectiveness of antibiotics in treating OM. However, in order to focus on the pathogenesis or the prevention of OM, particularly chronic OM, animal models that closely resemble the natural course of disease in humans are needed, which is infection of the middle ear cavity via the Eustachian tube [19]. Here we present a novel, non-invasive method for inducing OM and a bacterial biofilm within the middle ear of the rat.
2. Materials and Methods
All animal studies were conducted under protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign. All animals were individually housed and provided standard rat chow and tap water ad libitum. Ibuprophen (15 mg/kg) was added to the drinking water to reduce general infection side-effects without compromising local bacterial growth in the animals.
2.1 Bacterial Growth
Streptococcus pneumonia, serotype 19F (strain ST556), was chosen for these studies because it is the most common in OM [20]. The bacteria, generously provided by Dr. Jing-Ren Zhang (Albany Medical College, Albany, NY) [21], were routinely grown in 10 ml of Todd-Hewitt broth. The bacteria were centrifuged (3500 × g for 10 min), streaked in serial dilutions onto blood agar plates, and grown overnight at 37 °C to determine the concentration of vital bacteria. The bacteria were then suspended in a solution of saline/1% methylcellulose solution at a concentration of 1 × 1010 CFU/ml prior to use. The methylcellulose was added to enhance the bacterial adhesion to the nasopharyngeal epithelium [19].
2.2 Biofilm Formation
Healthy female Sprague-Dawley rats (Harlan, Indianapolis, IN), approximately 5-6 weeks of age, were used for these experiments. Animals were examined by otoscopy prior to bacterial inoculation, and none demonstrated visible signs of middle ear infections. Eight rats were given S. pneumonia inoculations, and one rat was given saline inoculations and used as a control. Prior to inoculations, rats were anesthetized by i.p. injection with a mixture of ketamine (100 mg/kg) / xylazine (10 mg/kg). The analgesic carprofen (5 mg/kg) was also provided to the animals prior to the bacterial inoculations to reduce any associated discomfort from the nasal canula.
The bacteria were inoculated nasally as previously described [19]. Briefly, 30 μl of bacterial solution was introduced through a Teflon canula (Howard Electric Instruments, El Dorado, KS) inserted approximately 2 cm into the left nostrils. After inoculation, the rats were immediately transferred to a custom-modified pressure chamber (Fig. 1, Sharpe Manufacturing Company, Minneapolis, MN) in which the chamber pressure was adjusted stepwise. The animals were orientated in a dorsal position to place the bacterial suspension closer to the nasopharyngeal entrance of the Eustachian tube and the pressure was slowly increased by 5 kPa every 30 seconds up to a maximum pressure of 50 kPa. After 2 min, the pressure was returned stepwise back to ambient atmospheric pressure, and the animals were returned to their cages in a dorsal position until they regained consciousness.
Figure 1. Prototype of Rat Pressure Chamber.
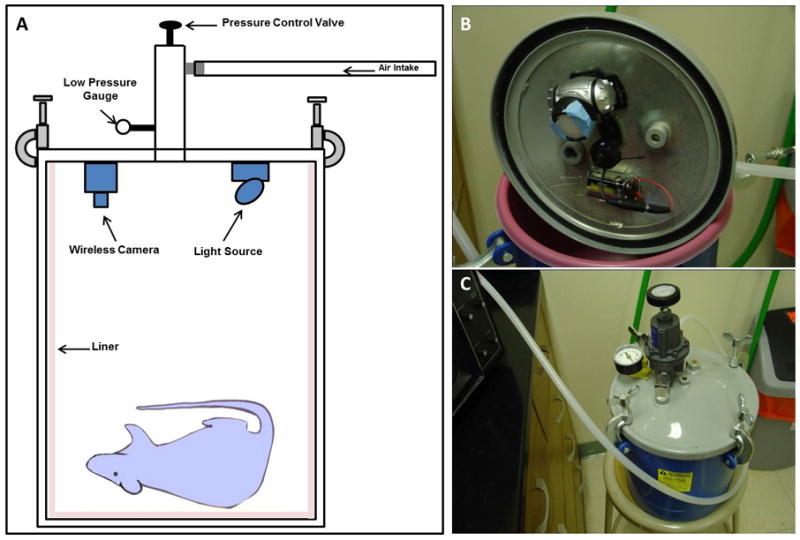
(A) Schematic diagram of custom-modified pressure chamber. (B) Inside view of chamber showing the attachment of the wireless camera and light source to the lid. (C) Full view of the completed chamber.
In most OM animal models, a bacterial infection usually has a self-limiting course that resolves in 8 – 10 days [1,7]. Therefore to induce biofilm formation, repeated inoculations every 4-7 days were administered, for up to 7 months. Over the course of the experiment, the TMs was observed for physiologic changes using a standard otoscope (Welch-Allyn, New York), and animals were sacrificed at various time points to access the progression of biofilm formation. Following biofilm formation, the animals were sacrificed and the TMs were harvested for histological examination.
2.3 Pressure Chamber Construction
A commercial 2.5 gallon pressure tank (product #7025, Sharpe Manufacturing Company, Minneapolis, MN) was modified to be used for our low pressure application (Fig. 1). The pressure gauge was replaced with a low-reading pressure gauge and a pressure control valve was installed in order to be able to control the intake and release of pressure. A light source and wireless camera were mounted on the underside of the lid, providing the ability to monitor the animal during the pressurization procedure. Finally, the desired pressure was achieved by attaching the chamber to a compressed air tank. As a safety precaution, the entire pressurization procedure took place inside a biosafety cabinet.
3. Results
To demonstrate the feasibility of the pressurization procedure, experiments were first performed using a solution of Indocyanine Green (ICG) dye instead of the bacterial solution. A 30 μl ICG solution (20 mg/ml, ~ 784 nm absorbance) was introduced into an anesthetized rat. The animal was pressurized, and following return to room pressure, was imaged in vivo using a darkbox (Maestro, CRI, Inc.) that permitted fluorescence excitation and imaging. In vivo imaging showed ICG fluorescence at the site of injection and in the ear canal (Fig. 2A). After imaging, the animal was sacrificed, and the TMs were removed and imaged ex vivo in the darkbox and compared to TMs from a non-dye-inoculated (control) animal (Fig. 2B). Fluorescence was only observed in inoculated and pressurized animals. No fluorescence was observed in animals that were inoculated, but not pressurized. Therefore, the sharp contrast between the fluorescence images of the inoculated rat and the control rat indicates that the dye was efficiently transferred from the nostril to the middle ears during the pressurization procedure, thus demonstrating the feasibility of passing a bacterial solution from the nostril into the middle ear in the rat model to induce biofilm formation.
Figure 2. Evaluation of Pressurization Protocol.
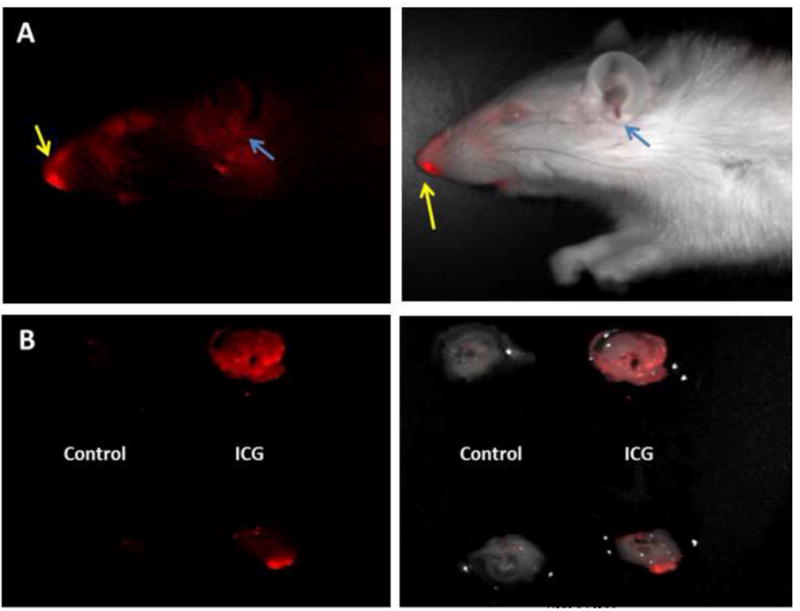
For bacteria and biofilm induction, a pressure chamber was constructed in which rats were placed during the pressurization procedure. For testing, ICG was inoculated intranasally in vivo prior to pressurization. (A) In vivo fluorescence darkbox imaging of an animal after ICG inoculation into the left nostril and pressurization. Yellow arrows indicate site of inoculation and blue arrows indicate presence of ICG in the ear canal. (B) Ex vivo fluorescence darkbox imaging of the TMs from inoculated (ICG) and control animals. In both figures, left images show fluorescence and right images show fluorescence signal overlay on brightfield images. In (B), the left and right TMs are shown at the top and bottom, respectively.
In animals with repeated bacterial inoculations, early signs of infection were observed by otoscopy after approximately 6 weeks of inoculations. New, faint, small, blood vessels bisecting the TM on the inoculated side (left) were observed, whereas the non-inoculated side (right) appeared normal. Over time, these vessels appeared darker, more prominent, and branching, with the appearance of more dilated blood vessels across the TM. Also observed were faint, focal, opaque areas located behind the TM, suggesting early-stage ear infection with purulent effusion that eventually would lead to biofilm formation. Histologically, no biofilms were observed from two inoculated rats sacrificed at 3 and 5 weeks, although there was evidence of resident bacteria present behind the TM. Other early observations noted superficial blood vessels appearing larger and more dilated in the external ear canal of the inoculated animals, which also suggested early acute OM.
After continual bacterial inoculations, biofilm formation occurred. Reddish-cloudy areas could be seen just behind the TM. After approximately 7 months of inoculations, one animal developed severe OM (Fig. 3). As observed with video otoscopy, the right TM appeared cloudy, dark-red, and showed prominent hole-like areas. Histology confirmed an increased thickness of the TM and an abnormal film behind and on the TM, including hole-like structures as observed through the otoscope. At higher magnification, S. pneumonia colonies were present within the biofilm.
Figure 3. Video Otoscopy and Histological Observations.
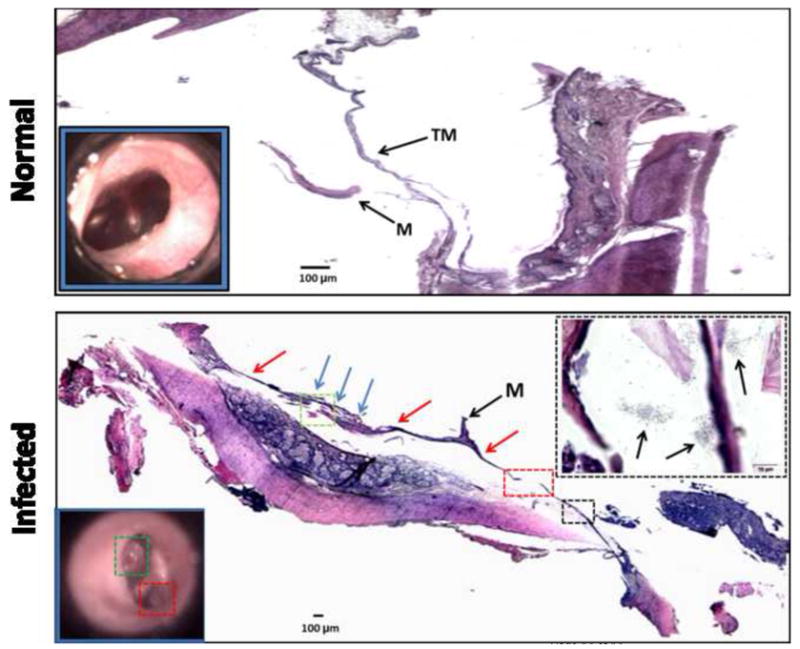
TOP: Histology (hematoxylin and eosin) of a TM from a normal (non-infected) animal showing a thin TM with no evidence of biofilm or bacterial colonies. Video otoscopy (inset lower left) shows a normal translucent TM. BOTTOM: Histology of a tympanic membrane from an infected animal. A biofilm (blue arrows) is present behind the TM (red arrows). The colored boxes on the histological section correspond to those in the video otoscope image (inset lower left). The histological section area in the black box is magnified and shown (inset upper right), revealing the presence of S. pneumonia colonies (arrows) located within and around the biofilm and TM. Abbreviations: M = malleus. TM = tympanic membrane.
Interestingly, while the animal received inoculations in the left nostril, these findings were observed in the right ear, with the left ear appearing moderately affected. This is not unexpected since the nasal cavity is an open system whereby fluid could drain to the right or left middle ear depending on the head angle during pressurization and normal activity. At early study time points (< 2 months), we observed signs of OM mainly in the left ears. By the end of the study (~ 6-7 months), we observed more chronic and persistent infections in both ears of the inoculated animals. Table 1 shows the progression of the biofilm formation in different animals over the course of this study. During the entire study, at no time did any of the inoculated animals appear sick or in pain or distress.
Table 1.
Progression of biofilm formation following S. pneumonia inoculations.
| Rat # | Timea | Otoscopic Observations |
Histologic Observations |
||
|---|---|---|---|---|---|
| Left Ear | Right Ear | Left Ear | Right Ear | ||
| 1 | 1 week | Normal appearance | Normal appearance | ||
| 3 weeks | Normal appearance | Normal appearance | Normal TM and middle ear | Normal TM and middle ear | |
| 2 | 3 weeks | Normal appearance | Normal appearance | ||
| 5 weeks | Normal appearance | Normal appearance | Slight thickening of TM | Normal TM and middle ear | |
| 3 | 1 month | TM red, inflamed | TM red, inflamed | ||
| 2 months | Small middle-ear effusion | Normal appearance | Bacteria present behind TM | Normal TM and middle ear | |
| 4 | 12 weeks | TM yellow, inflamed | TM yellow, inflamed | ||
| 16 weeks | TM clear | TM slightly red | |||
| 5 months | TM red, inflamed | TM dark red | Thickened TM, bacteria | Thickened TM, bacteria | |
| 5 | 6 weeks | TM with dilated blood vessels | Normal appearance | ||
| 9 weeks | TM slightly cloudy | TM cloudy | |||
| 15 weeks | TM very red | TM very red | |||
| 6 months | TM yellow-red | TM red, inflamed | Thickened TM, bacteria | Normal TM and middle ear | |
| 6 | 7 weeks | TM slightly cloudy | TM slightly red | ||
| 9 weeks | TM cloudy | TM cloudy | |||
| 26 weeks | Normal appearance | Normal appearance | |||
| 7 months | TM moderately red, cloudy | TM slightly red, cloudy | Moderately thickened TM | Normal TM and middle ear | |
| 7 | 6 weeks | TM cloudy | Normal appearance | ||
| 9 weeks | TM cloudy | TM cloudy | |||
| 31 weeks | TM red-yellow, cloudy | TM very red | |||
| 7 months | TM red, inflamed | TM red, inflamed | Moderate biofilm development | TM slightly inflamed | |
| 8 | 2 weeks | Normal appearance | TM slightly red | ||
| 6 weeks | Normal appearance | Normal appearance | |||
| 14 weeks | TM red, cloudy | TM dark red | |||
| 7 months | TM mildly red | TM very red, perforated | Thickened TM, bacteria | Thickened TM, bacteria, biofilm | |
| 9 | Non-injected control animal – normal otoscopic and histologic observations, bilaterally | ||||
Approximate experimental length of time between the first bacterial inoculation and otoscopic or histologic observations.
Abbreviation: TM, tympanic membrane
4. Discussion
There are many publications reporting a wide variety of experimental animal models, including dog, cat, ferret, monkey, gerbil, chinchilla, guinea pig, mouse, and rat, to study middle ear diseases. The choice of model species must be determined by the specific disease etiology in addition to other factors, including cost, ease of approach, animal physiology, human middle ear anatomy and disease similarities, and infection susceptibility. In fact, there is no single ideal non-human species that reflects the pathogenesis of all OM diseases and for all model applications [22]. We chose the rat model because it can be easily infected with human pathogens. The general anatomy of the rat middle ear and Eustachian tube is also similar to humans, where the middle ear mucosa layers of both rat and human have similar histological features of cell type and ciliary clearance tracts [23-26].
The administration of varying chamber pressures is necessary because the introduction of bacteria into the nasal cavity at ambient atmospheric pressure alone will not result in the formation of a middle ear biofilm. A normal functioning Eustachian tube prohibits bacteria from entering the middle ear cavity under normal atmospheric pressure. To achieve the desired results of chronic OM and biofilm formation, pressure must be increased under controlled conditions with consecutive steps to move the bacteria inoculum into the middle ear. In our procedure, small pressure changes were used to avoid pathological barotrauma in the TM, such as vascular dilation, hemorrhage, or perforation; none of which were observed after the rats were removed from the pressure chamber. It is also important to note that this procedure must occur while the animal is anesthetized, as it is then less likely that the bacteria suspension would be swallowed.
To date, most studies in this area have focused on effusions caused by acute bacterial infections, to which only a single inoculation of bacteria is necessary. However, a single inoculation of bacteria results in animals appearing clinically healthy for up to 28 days [1]. In fact, similar techniques have been reported in a chinchilla model, using nasal inoculations to transfer bacteria to the TM to form AOM [27]. This system appears to produce a higher percentage of infection in a quicker period of time. However, it does not utilize the advantage of continual bacterial inoculation and pressurization to form more severe OM conditions. Since our goal was to study bacterial biofilm formation and chronic ear infections, repeated bacterial inoculations over time was required to produce chronic-type infections that could not be cleared quickly, and thereby providing suitable conditions for biofilm formation.
5. Conclusions
In this study, we describe the development of the first physiological animal model for non-invasively inducing chronic OM and middle ear biofilms. We demonstrate that the application and pressurization of a small volume of pneumococcus administered at regular intervals over time results in biofilm formation in the middle ear. The development of this model is based on previous studies [28] demonstrating that positive nasopharyngeal pressures may result in infections secreted from the nasopharynx being transferred into the middle ear, provided that the ambient air pressure is higher than the middle ear pressure. In fact, a similar, single-inoculation method has been reported in a murine animal model for acute OM [29], but did not involve biofilm formation. Since our future goal is to optically image biofilms in vivo [30], the larger ear canal provided by the rat is necessary. Most research in middle ear diseases have investigated effusions caused by acute bacterial infections, for which only a single inoculation of bacteria was necessary. As such, no induction procedures performed repeated inoculations of bacteria to form biofilms in any animal model.
With an increasing appreciation of the role that biofilms play in human disease, and specifically in middle ear infections, the development of this model was motivated by the study of bacterial biofilm formation and chronic ear infections. This new method and animal model for middle ear biofilms will enable a wide range of future research to better understand the initiation, development, and resolution of middle ear biofilms and their role in chronic OM and other ear diseases. This scientific research has the potential to enable the development of new diagnostic technologies and new treatment strategies for the optimal management of OM.
Acknowledgments
We would like to thank Dr. Jing-Ren Zhang (Albany Medical College, Albany, NY) for his helpful and insightful conversations related to bacteria in OM, Dr. Daniel Marks (University of Illinois at Urbana-Champaign) for his assistance in the construction of the pressure chamber, and Dr. Charles N. Stewart (Blue Highway, LLC and Welch Allyn, Inc.) for the video otoscopy system.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raustyte G, Hermansson A. Development of mytingosclerosis during acute otitis media caused by Streptococcus pneumonia and non-typeable Haemophilus influenza: A clinical otomicroscopical study using the rat model. Medicina. 2005;41(8):661–7. [PubMed] [Google Scholar]
- 2.Alho OP, Koivu M, Sorri M, Rantakallio P. The occurrence of acute otitis media in infants. A life-table analysis. Int J Pediatr Otorhinolaryngol. 1991;21(1):7–14. doi: 10.1016/0165-5876(91)90054-f. [DOI] [PubMed] [Google Scholar]
- 3.Alho OP. How common is recurrent acute otitis media? Acta Otolaryngol Suppl. 1997;529:8–10. doi: 10.3109/00016489709124067. [DOI] [PubMed] [Google Scholar]
- 4.Paradise JL, Rockette HE, Colborn DK, Bernard BS, Smith CG, Kurs-Lasky M, Janosky JE. Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics. 1997;99(3):318–33. doi: 10.1542/peds.99.3.318. [DOI] [PubMed] [Google Scholar]
- 5.Daly KA, Brown JE, Lindgren BR, Meland MH, Le CT, Giebink GS. Epidemiology of otitis media onset by six months of age. Pediatrics. 1999;103(6 Pt 1):1158–66. doi: 10.1542/peds.103.6.1158. [DOI] [PubMed] [Google Scholar]
- 6.Daly KA, Rovers MM, Hoffman HJ, Uhari M, Casselbrant ML, Zielhuis G, Kvaerner KJ. Recent advances in otitis media. Epidemiology, natural history, and risk factors. Ann Otol Rhinol Laryngol Suppl. 2005;194:8–15. doi: 10.1177/00034894051140s104. [DOI] [PubMed] [Google Scholar]
- 7.Caye-Thomasen P, Tos M. Histopathologic differences due to bacterial species in acute otitis media. Int J Pediatr Otohinolarngol. 2002;63(2):99–110. doi: 10.1016/s0165-5876(01)00641-3. [DOI] [PubMed] [Google Scholar]
- 8.van der Ven L, van der Dobbelsteen GP, Nagarajah B, Van Dijken H, dortant PM, Vos JG, roholl PJ. A new rat model of otitis media caused by Streptococcus pneumoniae: conditions and application in immunization protocols. Infect Immun. 1999;67(11):6098–103. doi: 10.1128/iai.67.11.6098-6103.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett KE, Haggard MP, Silva PA, Stewart IA. Behaviour and developmental effects of otitis media with effusion into the teens. Arch Dis Child. 2001;85:91–95. doi: 10.1136/adc.85.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Hayes J, Forbes M, Greenberg DP, Dice B, Burrows A, Wackym PA, Stoodley P, Post JC, Ehrlich GD, Kerschner JE. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;12(296):202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–45. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 12.Moscoso M, Garcia E, Lopez R. Biofilm formation by Streptococcus pneumoniae: role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion. J Bacteriol. 2006;188(22):7785–95. doi: 10.1128/JB.00673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall-Stoodley L, Stoodley P. Biofilm formation and dispersal and the transmission of human pathogens. Trends Microbiol. 2005;13:7–10. doi: 10.1016/j.tim.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Xi C, Marks D, Schlachter S, Luo W, Boppart SA. High-resolution three-dimensional imaging of biofilm development using optical coherence tomography. J Biomed Opt. 2006;11(3):034001. doi: 10.1117/1.2209962. [DOI] [PubMed] [Google Scholar]
- 15.Lundgren K, Ingvarsson L. Microbiology in acute otitis media. In: Sade J, editor. Acute and Secretory Otitis Media. Kugler Publications; Amsterdam, The Netherlands: 1986. pp. 175–9. [Google Scholar]
- 16.Luotonen J, Herva E, Karma P, Timonen M, Leinonen M, Makela PH. The bacteriology of acute otitis media in children with special reference to Streptococcus pneumoniae as studied by bacteriological and antigen detection methods. Scand J Infect Dis. 1981;13(3):177–83. doi: 10.3109/inf.1981.13.issue-3.04. [DOI] [PubMed] [Google Scholar]
- 17.Stephenson JS, Martin LM, Kardatzke D, Bluestone C. Prevalence of bacteria in middle ear effusions for the 1980s. In: Lim DJ, Bluestone C, Klein JO, et al., editors. Recent Advances in Otitis Media. BC Decker; Ontario, Canada: 1993. pp. 389–92. [Google Scholar]
- 18.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6:288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 19.Tonnaer EL, Sanders EA, Curfs JH. Bacterial otitis media: A non-invasive rat model. Vaccine. 2003;21(31):4539–44. doi: 10.1016/s0264-410x(03)00501-2. [DOI] [PubMed] [Google Scholar]
- 20.Kilpi T, Herva E, Kaijalainen T, Syrjanen R, Takal AK. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. J Infect Dis. 2001;20:654–62. doi: 10.1097/00006454-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Joloba ML, Windau A, Bajaksouzian S, Appelbaum PC, Hausdorf WP, Jacobs MR. Pneumococcal conjugate vaccine serotypes of Streptococcus pneumonia isolates and the antimicrobial susceptibility of such isolated in children with otitis media. Clin Infect Dis. 2001;33(9):1489–94. doi: 10.1086/323027. [DOI] [PubMed] [Google Scholar]
- 22.Piltcher OB, Swarts JD, Magnuson K, Alper CM, Doyle WJ, Hebda PA. A rat model of otitis media with effusion caused by eustacian tube obstruction with and without Streptococcus pneumoniae infection: methods and disease course. Otolaryngol Head Neck Surg. 2002;126(5):490–8. doi: 10.1067/mhn.2002.124935. [DOI] [PubMed] [Google Scholar]
- 23.Daniel HJ, 3rd, Fulghum RS, Brinn JE, Barrett KA. Comparative anatomy of eustachian tube and middle ear cavity in animal models for otitis media. Ann Otol Rhinol Laryngol. 1982;91(1 Pt 1):82–9. doi: 10.1177/000348948209100118. [DOI] [PubMed] [Google Scholar]
- 24.Hellstrom S, Salen B, Stenfors LE. Anatomy of the rat middle ear. A study under the dissection microscope. Acta Anat. 1982;112:346–52. doi: 10.1159/000145527. [DOI] [PubMed] [Google Scholar]
- 25.Albiin N, Hellstrom S, Salen B, Stenfors LE, Soderberg O. The anatomy of the eustachian tube in the rat: a macro- and microscopical study. Anat Rec. 1983;207(3):513–21. doi: 10.1002/ar.1092070313. [DOI] [PubMed] [Google Scholar]
- 26.Albiin N, Hellstrom S, Stenfors LE, Cerne A. Middle ear mucosa in rats and humans. Ann Otol Rhinol Laryngol Suppl. 1986;126:2–15. doi: 10.1177/00034894860950s501. [DOI] [PubMed] [Google Scholar]
- 27.Hoa M, Syamal M, Sachdeva L, Berk R, Coticchia J. Demonstration of nasopharyngeal and middle ear mucosal biofilms in an animal model of acute otitis media. Ann Otol Rhinol Laryngol. 2009;118:292–8. doi: 10.1177/000348940911800410. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfeld RM, Bluestone CD. Evidence-based otitis media. Hamilton: BC Decker Inc; 1999. [Google Scholar]
- 29.Stol K, van Selm S, vad den Berg S, Bootsma HJ, Blokx WA, Graamans K, Tonnaer EL, Hermans PW. Development of a non-invasive murine infection model for acute otitis media. Microbiology. 2009;155(Pt 12):4135–44. doi: 10.1099/mic.0.033175-0. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen C, Tu H, Chaney EJ, Stewart CN, Boppart SA. Non-invasive optical interferometry for the assessment of biofilm growth in the middle ear. Biomed Opt Express. 2010;1:1104–16. doi: 10.1364/BOE.1.001104. [DOI] [PMC free article] [PubMed] [Google Scholar]


