Abstract
Purpose
Biodegradable elastomers, which can possess favorable mechanical properties and degradation rates for soft tissue engineering applications, are more recently being explored as depots for biomolecule delivery. The objective of this study was to synthesize and process biodegradable, elastomeric poly(ester urethane)urea (PEUU) scaffolds and to characterize their ability to incorporate and release bioactive insulin-like growth factor–1 (IGF-1) and hepatocyte growth factor (HGF).
Methods
Porous PEUU scaffolds made from either 5 or 8 wt% PEUU were prepared with direct growth factor incorporation. Long-term in vitro IGF-1 release kinetics were investigated in saline or saline with 100 units/ml lipase to simulate in vivo degradation. Cellular assays were used to confirm released IGF-1 and HGF bioactivity.
Results
IGF-1 release into saline occurred in a complex multi-phasic manner for up to 440 days. Scaffolds generated from 5 wt% PEUU delivered protein faster than 8 wt% scaffolds. Lipase-accelerated scaffold degradation lead to delivery of >90% protein over 9 weeks for both polymer concentrations. IGF-1 and HGF bioactivity in the first 3 weeks was confirmed.
Conclusions
The capacity of a biodegradable elastomeric scaffold to provide long-term growth factor delivery was demonstrated. Such a system might provide functional benefit in cardiovascular and other soft tissue engineering applications.
Keywords: Growth factor delivery, elastomer, scaffold, polyurethane, tissue engineering
Introduction
The influence of substrate mechanical properties on cell proliferation, differentiation, and extracellular matrix production is increasingly appreciated.(1, 2) As such, more attention is being paid to the mechanical properties of candidate scaffolds for tissue engineering and regenerative medicine applications in an effort to improve functional outcomes. Historically, synthetic polymers used for cardiovascular replacement and repair have included non-degradable polymers such as poly(ethylene-terephthalate) and poly(tetrafluoroethylene) and tissue engineering approaches have frequently employed common polyesters such as poly(lactide), poly(glycolide), and their copolymers.(3) However, these polymers are relatively stiff and not mechanically similar to native cardiovascular tissue. Polyurethanes as a class of polymers are attractive for soft tissue applications because of their ability to exhibit elastic behavior generally similar to many soft tissues and to be able to do so through physical crosslinking. To this end, biodegradable, elastomeric polyurethanes have been developed using a variety of techniques to introduce lability (4–7), and these materials have been investigated in several locations in vivo, including both vascular and myocardial applications.(8, 9)
In order to enhance cellular infiltration into and survival within scaffolds and to direct the differentiation of endogenous and exogenous stem cell populations in situ, agents such as growth factors and adhesion molecules have been incorporated into polyurethane scaffolds for controlled release.(10–15) The kinetics describing drug release from biomaterial scaffolds can be complex and varied. For example, many systems demonstrate simple diffusion-controlled delivery characterized by a burst release of near-surface drug, a subsequent fast diffusion period, and ending with lower release rates as the agent is exhausted.(16) However, in the case of biodegradable materials a two-stage release profile can also be observed, wherein a significant amount of drug remains trapped in the material bulk until adequate degradation of the material occurs, at which point the remaining drug diffuses out quickly.(17) Other drug release profiles including constant, zero-order release, or even triphasic release can be achieved depending on factors such as material composition, material processing, drug physical properties, and drug loading.(17, 18) In the case of biomolecule delivery from polyurethane scaffolds, studies generally do not extend beyond a few weeks, meaning that the complexities of the release kinetics may be left unobserved. Additionally, whereas enzymes have been routinely used to investigate polyurethane degradation, reports of the use of enzymes in the context of controlled release are scarce.(19, 20)
Of particular interest for controlled release applications in myocardial tissue engineering is insulin-like growth factor-1 (IGF-1), which has been implicated in many physiological processes including tissue growth, protection and repair. Specifically, IGF-1 has been linked to several pathways in skeletal muscle formation and regeneration (21) and is expressed during the early and middle stages of muscle repair.(22–24). Following myocardial infarction, IGF-1 has been shown to stimulate hypertrophy and prevent cardiomyocyte death, thereby attenuating ventricular dilation.(25) Another growth factor that plays both a protective and reparative role in cardiac muscle is hepatocyte growth factor (HGF).(26–28) However, the therapeutic use of these growth factors is limited by their short half lives in vivo.(29, 30) Thus, the sustained delivery of IGF-1 or HGF coupled with the attractive mechanical properties of a biodegradable, elastomeric polyurethane scaffold may prove more advantageous for myocardial or skeletal muscle repair and regeneration than the administration of growth factor or polymer matrix alone.
The objective of the current study was to synthesize and process a biodegradable, elastomeric poly(ester urethane)urea (PEUU) with the ability to incorporate and release bioactive IGF-1 and HGF. Porous PEUU scaffolds were fabricated using a thermally induced phase separation technique, and growth factors were directly incorporated into scaffolds during preparation. The long-term in vitro release kinetics of IGF-1 from scaffolds were investigated in phosphate buffered saline (PBS) and in the presence of the enzyme lipase to simulate in vivo degradation. The bioactivity of released IGF-1 was confirmed with in vitro cell assays. HGF was also loaded into PEUU scaffolds, and while controlled release kinetics were not evaluated, it was also shown to maintain bioactivity upon release.
Materials and Methods
Biodegradable poly(ester urethane)urea (PEUU) synthesis
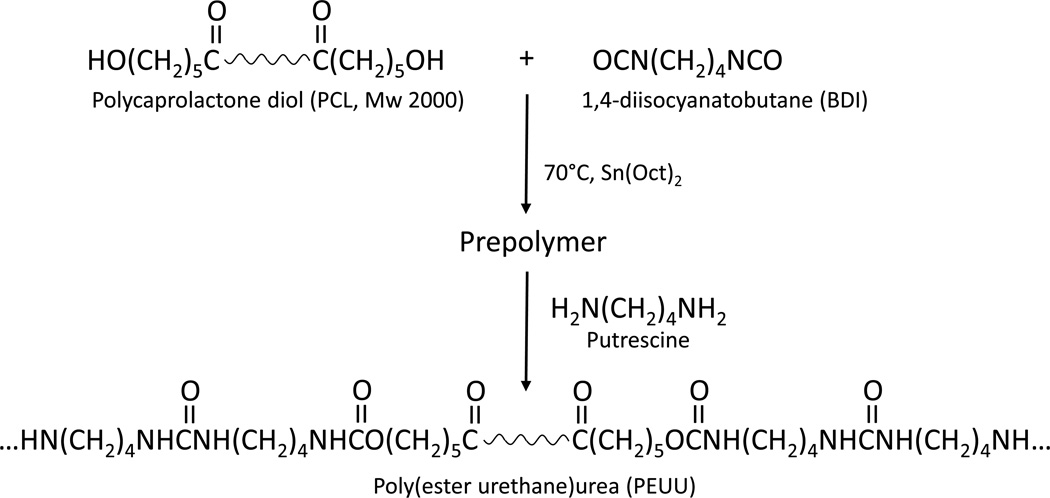
PEUU based on polycaprolactone diol (PCL, MW=2000, Aldrich), 1,4-diisocyanatobutane (BDI, Fluka), and putrescine (Aldrich) was synthesized in a two-step solution polymerization as previously reported (Figure 1).(4) In brief, a 15 wt% solution of PCL in dimethyl sulfoxide (DMSO) was stirred with a 5 wt% solution of BDI in DMSO under argon gas. Stannous octoate, the catalyst, was added, and the reaction allowed to continue at 80°C for three hours with constant stirring. After this time, the prepolymer solution was removed from heat and allowed to cool to room temperature. Putrescine in DMSO was then added dropwise, with constant stirring, to the prepolymer solution, and the reaction allowed to continue at room temperature for 12–18 hours. The stoichiometry of the reaction was 2:1:1 BDI:PCL:putrescine. The PEUU solution was precipitated in distilled water and immersed in isopropanol to remove unreacted monomers. Finally, the polymer was dried under vacuum at 50°C.
Figure 1.
Synthesis of PEUU. Poly(caprolactone) diol reacts with 1,4 diisocyanatobutane to form the prepolymer. The final product is formed by chain extension of prepolymer with putrescine.
Fabrication of three-dimensional, growth factor-loaded PEUU scaffolds
Three-dimensional, porous PEUU scaffolds were fabricated by a thermally induced phase separation (TIPS) method.(31) Following synthesis, PEUU was dissolved in DMSO at 80°C to make 5 or 8 wt% polymer solutions. Insoluble, cross-linked polymer was removed by centrifugation at 250×g, and PEUU solution was once again heated to 80°C. Hot polymer solution was injected into a cylindrical glass mold (inner diameter 10 mm) capped with rubber stoppers. The mold was immediately placed at −80°C for three hours. The cylinder end caps were then removed, and the mold was transferred to 200-proof ethanol for 3–7 days at 4°C for solvent extraction. Solvent extraction was considered complete once the polymer scaffold detached from the edges of the glass mold. Following DMSO removal, scaffolds were placed in water overnight to remove ethanol and reconstitute pore structure. Finally, the scaffolds were vacuum-dried for 24 hours. Electron microscopy was used to verify porous scaffold morphology.
To fabricate growth factor-loaded scaffolds, IGF-1 and HGF (R&D Systems) were first reconstituted in PBS in an excess of bovine serum albumin (BSA) (1:100, wt:wt) to stabilize the protein. Solutions containing growth factor and BSA were then snap-frozen in liquid nitrogen and vacuum-dried for 48 hours. Dried proteins with salts were mixed with DMSO to make IGF-1 and HGF stock solutions at a concentration 25 µg/ml. Growth factor in DMSO was added to the polymer solution at 80°C under rapid stirring for 15 s to make protein homogeneous in solution, after which the solution was injected into a cylindrical glass mold and immediately transferred to −80°C. 125I-IGF-1 was synthesized following previously published methods.(32) IGF and 125IIGF-1-loaded scaffolds contained 2.5 mg of BSA and a final IGF-1 concentration of 500 ng/ml in PEUU solution. HGF-loaded scaffolds contained 2.5 mg of BSA and a final HGF concentration of 250 ng/ml of PEUU solution. Scaffolds were also fabricated without growth factor, but with BSA.
Mechanical testing of PEUU scaffolds
Scaffolds with and without growth factor were snap frozen in liquid nitrogen and cut into 500 µm-thick discs. In order to demonstrate similarity to scaffolds previously reported (15), tensile properties were measured on an MTS Tytron™ 250 MicroForce Testing Workstation (10 mm/min crosshead speed) according to ASTM D638. Five samples were tested for each scaffold.
Degradation of PEUU scaffolds
BSA-loaded scaffold disks were weighed and then immersed in PBS with or without 2 µL lipase enzyme solution (Sigma Aldich, 100 units/ml final concentration) at 37°C. At specified time points, scaffolds (n=3) were collected, dried, and weighed. After collecting the data for each time point the studied scaffolds were discarded. The mass of dry scaffolds at each time point was compared to the starting dry mass of the sample. Release fluid was changed at regular intervals in order to maintain a constant pH and to keep an active concentration of enzyme present. Differential scanning calorimetry (DSC) of scaffold disks (n=3) at different stages of enzymatic degradation over 1 wk, or without enzyme over 2 wk, was completed using a Thermal Analyst 2000 (TA Instruments) DSC 2910 differential scanning calorimeter. Scaffold disks were heated from −100°C to 80°C at a heating rate of 10°C/min.
Quantification of growth factor release from PEUU scaffolds
Release kinetics for PEUU scaffolds containing 125I-IGF-1 were determined in vitro. Scaffold disks (750 µm thickness, 10 mm diameter) were placed in test tubes and incubated in release media consisting of either 2 ml PBS or 2 ml PBS + 100 U/ml lipase enzyme per well at 37°C. Releasate was collected at pre-determined time points and replaced with 2 ml of fresh release media. The calculation of protein release at every time point was adjusted to account for the radioactive decay of I125 which is 59.6 days. These studies extended for up to a 440 day period. Growth factor release was determined by quantifying the radioactivity of the release fluid using a gamma counter (Auto Gamma II, Perkin Elmer).
Verification of bioactivity of released growth factor
The bioactivity of IGF-1 released from scaffolds without lipase enzyme was measured by a cell mitogenicity assay, employing Balb/3T3 cells (a mouse embryonic fibroblast cell line, R&D Systems) and MG-63 cells (a human osteosarcoma cell line, R&D Systems). These cell types were selected due to their documented dose-dependent proliferation in response to IGF-1 treatment.(33, 34) Preliminary studies involving direct addition of IGF-1 to these cells over a range from 0–200 ng/ml showed a maximum growth response for both cell types at 150 ng/mL IGF-1. Thus, this concentration was used as the standard for comparison in bioactivity assays. The bioactivity of released HGF was measured by a cell motogenic assay, and human umbilical vein endothelial cells (HUVECs) were selected due to their documented motogenic response to HGF.(35) Scaffold disks (500 µm thickness, 10 mm diameter) with and without growth factor loading were placed into wells of 24 well tissue culture plates in cell basal media (Minimum Essential Medium (MEM) for MG-63 cells and Dulbecco's Modified Eagle Medium (DMEM) for Balb/3T3 cells, both without fetal bovine serum). Basal medium from wells containing scaffolds was collected at pre-determined time points over three weeks, sterile-filtered, and kept frozen at −20°C until transfer to cells.
Balb/3T3 and MG-63 cells were plated at 1 × 104 cells/cm2 in each well of 24 well TCPS plates in cell growth medium (MEM or DMEM supplemented with 10% fetal bovine serum and penicillin and streptomyocin). Prior to growth media exchange with polymer releasate (basal medium), cells were treated overnight with 1 µl/ml colcemid (Sigma Aldrich) to synchronize cell cycle. Cells were then washed with PBS to remove traces of colcemid and fed with the appropriate polymer releasate. Four days following releasate treatment, cell numbers were indirectly quantified using a colorimetric assay for mitochondrial activity (MTT assay).(34) MTT results were qualitatively confirmed by visual cell inspection. Cell numbers for cells cultured in releasate were normalized to cells maintained in growth media.
HUVECs were plated at 1 × 104 cells/cm2 in each well of 6 well TCPS plates in growth medium (Endothelial basal medium, Lonza Inc). An in vitro wound healing assay was used to confirm the motogenic effect of HGF on cells.(35) Briefly, once cells had grown to confluence, a cell scraper was used to create a wound down the center of each well of the 6 well plate. Cells were then washed with PBS to remove cell debris from the wound and treated with polymer releasate. A live-cell imaging system from Automated Cell Technology (ACT) was used to image cells at 10 min intervals over a four day period. Cell motility was quantified in terms of cell velocity (using ACT software) and cell migration into the wound (using NIH ImageJ software).
Statistical analyses
Data are reported as mean ± standard deviation. All statistical analyses were performed using SPSS software. For cell proliferation, mass loss, and protein release studies t-tests were used for evaluation of differences between 2 sets of data. For cell motility studies, mechanical properties, DSC data, and protein release data involving 3 or more data sets, data were analyzed by ANOVA with Tukey post-hoc testing for specific differences between various degradation solutions. Significance was defined as p < 0.05.
Results
Mechanical properties of PEUU scaffolds
TIPS scaffolds fabricated without the addition of any biomolecules (BSA or growth factors) had tensile strengths of 0.61±0.15 MPa. Scaffolds containing BSA alone had tensile strengths of 0.43±0.04 MPa, and scaffolds containing BSA and growth factor (IGF-1 or HGF) had tensile strengths of 0.32±0.18 MPa (p>0.05).
Degradation of PEUU Scaffolds
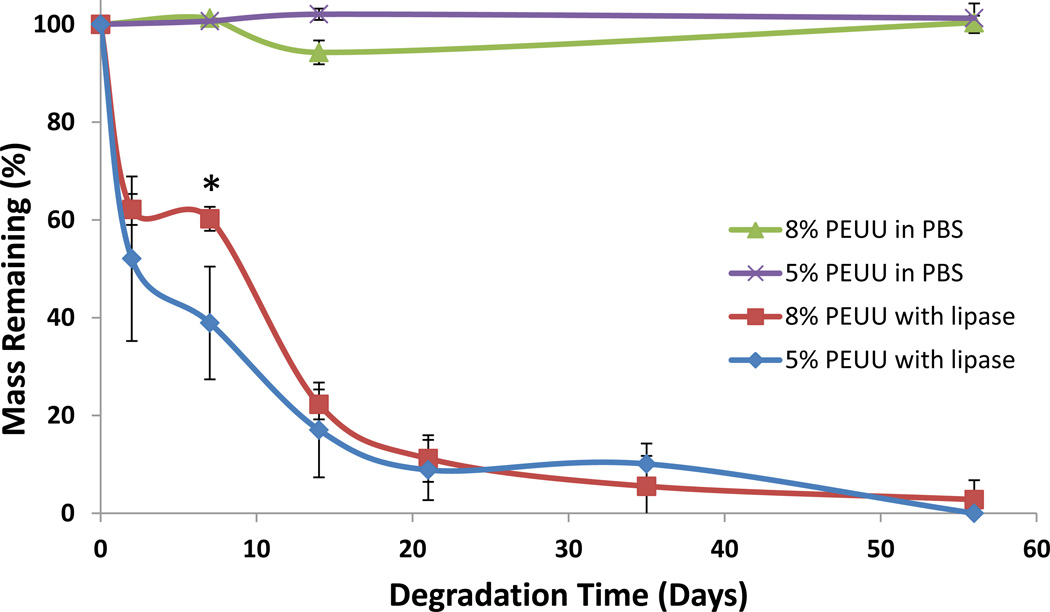
Scaffolds incubated in PBS did not demonstrate significant mass loss over the time period studied (Figure 2). In the presence of lipase scaffold mass loss was substantially accelerated, with both polymer concentrations losing nearly half their mass over the first two days. With enzyme present 5 wt% scaffolds lost more mass over the first week compared to 8 wt% scaffolds with 38.9 ± 11.5% and 60.2 ± 2.5% mass remaining, respectively (p<0.05). Polymer degradation was nearly complete after 8 weeks. Morphologically, scaffolds maintained an organized pore structure, and after one week of incubation with lipase the polymer surfaces were noticeably broken apart (Figure 3).
Figure 2.
The mass loss of PEUU scaffolds was determined over time for 5 and 8 wt% scaffolds soaked in PBS or with 100 units/ml lipase enzyme. * denotes p<0.05 between scaffold polymer concentrations
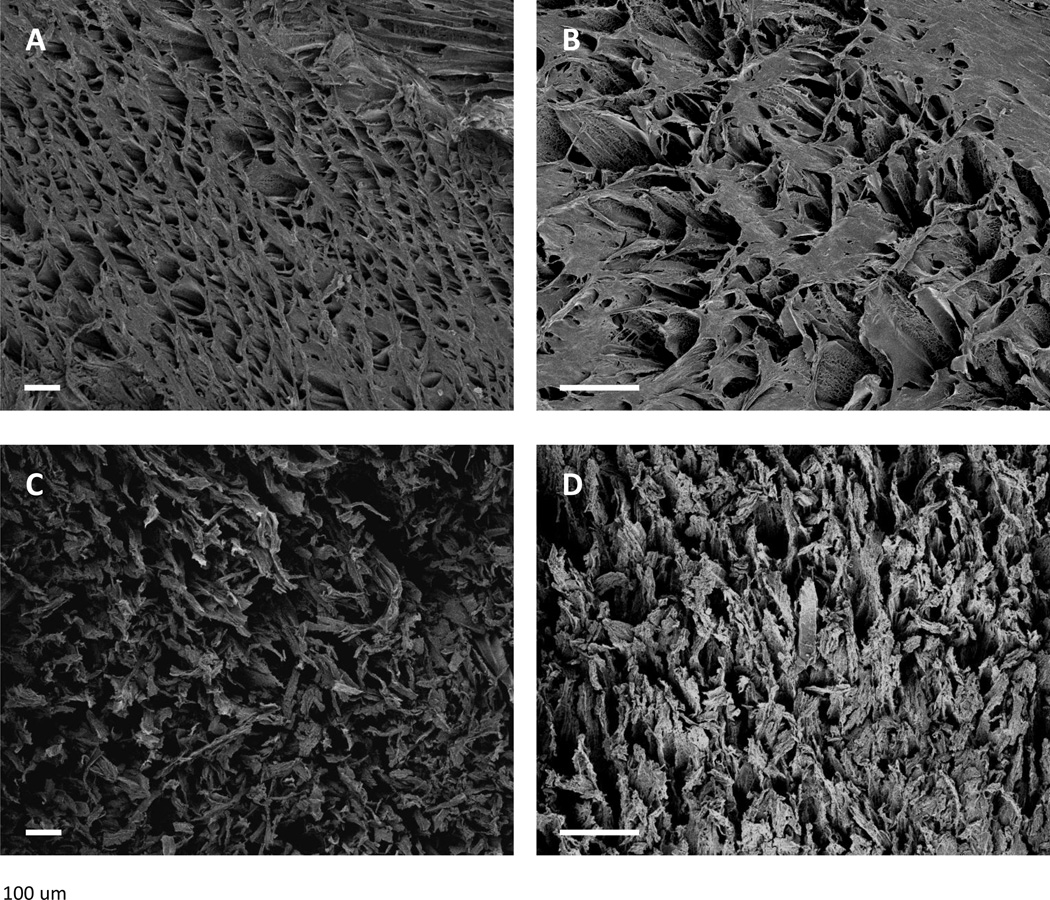
Figure 3.
Electron micrographs of TIPS scaffolds (5wt% A and C, 8 wt% B and D) incubated in PBS (top panels) or lipase solution (bottom panels) for 1 week. Scaffolds maintained an organized pore structure after one week in PBS (top panels). After one week of incubation with lipase, scaffold surfaces were noticeably broken apart. Scale bar = 100 µm.
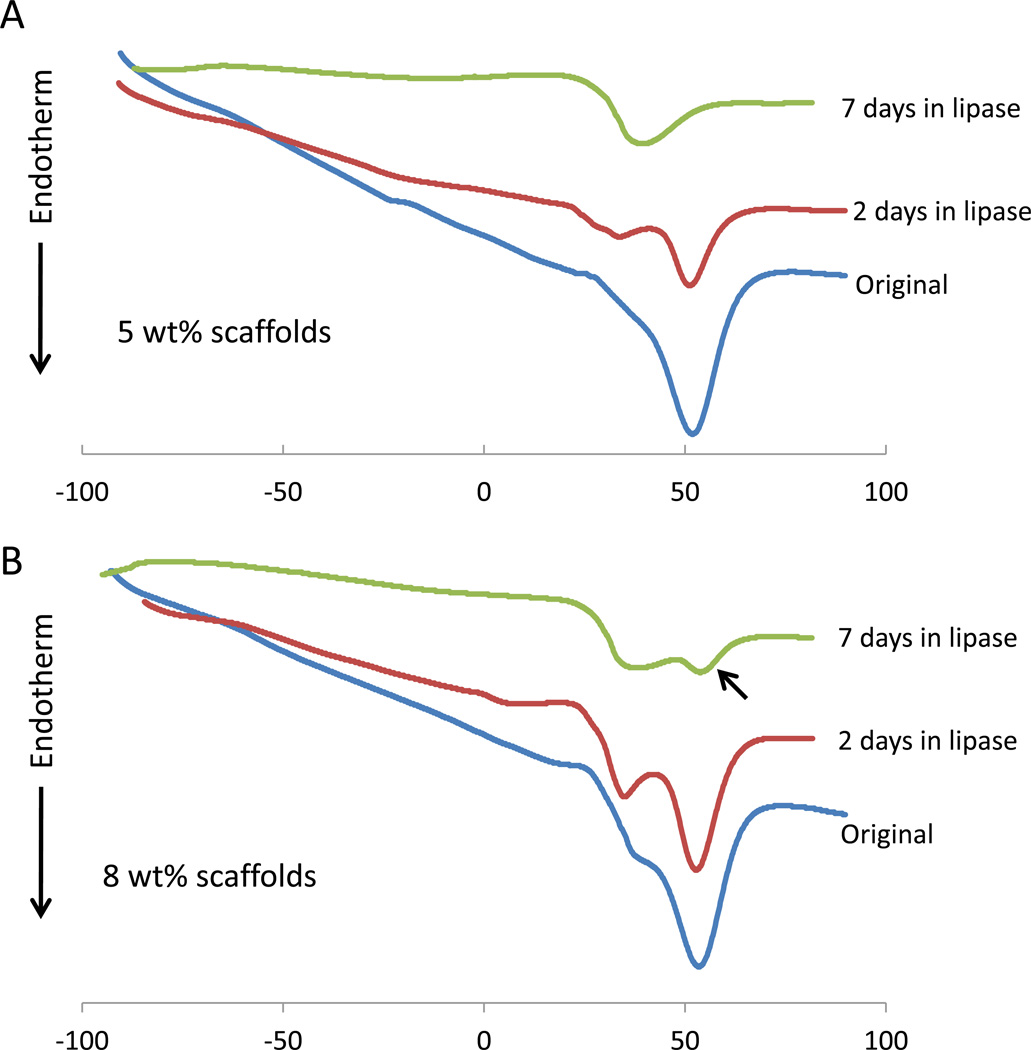
DSC confirmed the degradation of scaffolds in the presence of enzyme. The melting temperature (Tm) for original scaffolds was around 50°C for both 5 wt% and 8 wt% scaffolds – Table I and Figure 4. During degradation a significantly lower second melting peak grew near 30 °C as the primary peak weakened. By 7 days the original peak was gone in 5 wt% scaffolds though it continued to persist in 8 wt% scaffolds. Scaffolds in PBS did not show a significant change in Tm after 2 wk.
Table I.
Melting Temperature of Polymer Scaffolds
| Scaffold wt% | Incubation Conditions |
Primary Peak (°C)* |
Secondary Peak (°C)* |
|---|---|---|---|
| 5 wt% | Original | 51.0 ± 1.3 | - |
| 2 Days in Lipase | 51.3 ± 0.2 | 36.2 ± 2.2† | |
| 7 Days in Lipase | 34.4 ± 4.6† | - | |
| 14 Days in PBS | 54.1 ± 0.8 | - | |
| 8 wt% | Original | 52.6 ± 1.3 | - |
| 2 Days in Lipase | 53.1 ± 0.7 | 36.3 ± 2.4† | |
| 7 Days in Lipase | 35.3 ± 3.1† | 53.8 ± 0.02 | |
| 14 Days in PBS | 54.8 ± 0.2 | - |
data presented as mean ± standard deviation
p<0.05 compared to original Tm of same wt% scaffolds
Figure 4.
DSC heating curves of 5 wt % (A) and 8 wt% (B) scaffolds during degradation with lipase enzyme. In all situations the initial primary melting temperature near 50°C is lost as a new substantial peak near 30°C is formed. Scaffolds with 8 wt% PEUU maintain the first peak for 7 days (black arrow) suggesting a slower degradation than 5 wt% scaffolds which have lost that peak by 7 days.
IGF-1 Release from PEUU scaffolds
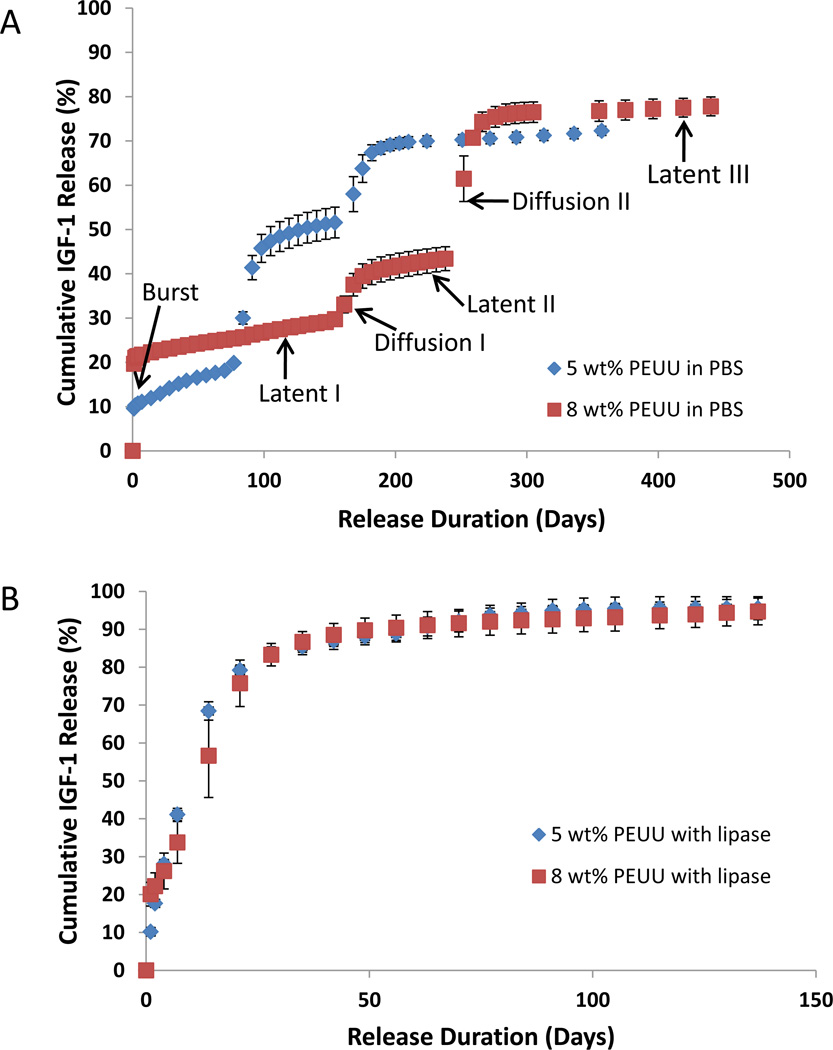
Before samples were utilized in the release kinetics study, a substantial amount of IGF-1 had already been lost from the cylindrical scaffolds during DMSO extraction in ethanol. In particular, 8 wt% scaffolds lost 15% of loaded protein during this step compared to 50% for 5 wt% scaffolds. Thus, the starting mass of IGF-1 in 8 wt% and 5 wt% scaffolds was 46.8 ± 7.5 and 22.5 ± 4.1 ng/scaffold, respectively. Once TIPS scaffold disks were immersed in PBS the initial burst release occurred over 48 hours, the extent of which was dependent on polymer concentration. Scaffolds with 8 wt% and 5 wt% polymer showed a burst release of 21.0 ± 1.0% and 10.3 ± 0.4 %, respectively (p<0.001) (Figure 5A, Table II). Following the burst release, there was an extended period of slow, steady protein release from scaffolds, termed latent phase I, and the release rate in this phase was faster in 5 wt% scaffolds (0.80%/week) than 8 wt% (0.39%/wk) scaffolds (p<0.001). Following latent phase I, a period of more rapid release occurred, labeled diffusion phase I, wherein 10–30% of protein was released. Observationally, the scaffolds showed distinct, moderate swelling at this point but maintained their shape. Upon handling, a qualitative reduction in modulus was noted. The initial burst, latent I, and diffusion I phases were followed by a second period of steady protein release (termed latent phase II) and then a second stage of accelerated protein release (termed diffusion phase II) from the scaffolds. Protein release after this point was very low for both polymer concentrations (latent phase III). Neither scaffold released all incorporated protein during the study, but radioactivity measurements confirmed that residual incorporated protein still resided within the polymer scaffolds, both types of which remained in a single piece throughout the study.
Figure 5.
A) Scaffolds in PBS demonstrated a tri-phasic release profile for IGF-1 over the time period studied. Alternating periods of slow, steady protein release (latent phases) and more rapid release (diffusion phases) followed an initial burst release. B) Both scaffold types incubated with 100 units/ml lipase enzyme released IGF-1 at a much faster rate than those without enzyme.
Table II.
IGF-1 Release Kinetics
| 5 wt% PEUU Scaffolds | |||||
|---|---|---|---|---|---|
| Release Phase |
Phase Begin (Day) |
Phase End (Day) |
Phase Duration (Day) |
Release Rate (%/week)* |
Total IGF-1 Release (%)* |
| Burst | 0 | 2 | 2 | -- | 10.3 ± 0.41 |
| Latent I | 2 | 70 | 68 | 0.80 ± 0.04 | 7.8 ± 0.38 |
| Diffusion I | 70 | 112 | 42 | -- | 30.3 ± 3.91 |
| Latent II | 112 | 154 | 42 | 0.53 ± 0.03 | 3.2 ± 0.16 |
| Diffusion II | 154 | 196 | 42 | -- | 17.5 ± 2.89 |
| Latent III | 196 | -- | -- | 0.14 ± 0.02 | -- |
| 8 wt% PEUU Scaffolds | |||||
| Phase Begin (Day) |
Phase End (Day) |
Phase Duration (Day) |
Release Rate (%/week)* |
Total IGF-1 Release (%)* |
|
| Burst | 0 | 2 | 2 | -- | 21.0 ± 1.0 |
| Latent I | 2 | 147 | 145 | 0.39 ± 0.01 | 8.1 ± 0.22 |
| Diffusion I | 147 | 189 | 42 | -- | 11.9 ± 2.10 |
| Latent II | 189 | 238 | 49 | 0.35 ± 0.02 | 2.4 ± 0.15 |
| Diffusion II | 238 | 284 | 46 | -- | 32.6 ± 4.71 |
| Latent III | 284 | -- | -- | 0.08 ± 0.01 | -- |
data presented as mean ± standard deviation
As is apparent in Figure 5A and Table II, the rate of protein release varied depending on the phase of the release profile and the initial polymer mass fraction. The rate of IGF-1 release decreased for each subsequent latency phase. For example, during latent phase I in 5 wt% scaffolds, IGF-1 released at 0.80%/wk compared to 0.53%/wk and 0.14%/wk for the second and third latency periods, respectively (p<.001 between groups). The release rates in 8 wt% scaffolds followed a similar pattern but were consistently lower than for the 5 wt% scaffolds during these same latent phases at 0.39, 0.35, and 0.08%/wk respectively (p<.001 between groups). The 8 wt% scaffolds consistently demonstrated longer latent periods. For example, the latent phase I lasted over 20 weeks compared to 10 weeks for 5 wt% scaffolds. Interestingly, diffusion I and diffusion II phases lasted similar amounts of time for both scaffold mass fractions. The diffusion I stage released more protein in 5 wt% scaffolds than 8 wt% scaffolds (30.3 ± 3.9% vs. 11.9 ± 2.1%, p<.001) but the diffusion II stage release was the opposite with less release from 5 wt% scaffolds (17.5 ± 2.9% vs. 32.6 ± 4.7%, p<.001) than the 8 wt% counterparts.
Scaffolds incubated with lipase enzyme released protein much faster than those without enzyme, with more than 90% of IGF-1 being released after 9 weeks for both polymer concentrations (Figure 5B). The distinct release phases observed with scaffolds in PBS were not apparent when enzyme was present. However, a noticeable attenuation of release rate was demonstrated in 8 wt% scaffolds compared to 5 wt% scaffolds during specific early time points. Specifically, IGF-1 release was slower in 8 versus 5 wt% scaffolds between days 1–2 (2.1 ± 0.8% vs. 7.5 ± 1.8% respectively, p<.05), days 2–4 (4.0 ± 1.6% vs. 10.4 ± 0.5%, p<.05), and days 4–7 (7.6 ± 1.4%, vs. 13.1 ± 2.1%, p<.05).
Bioactivity of released IGF-1 and HGF
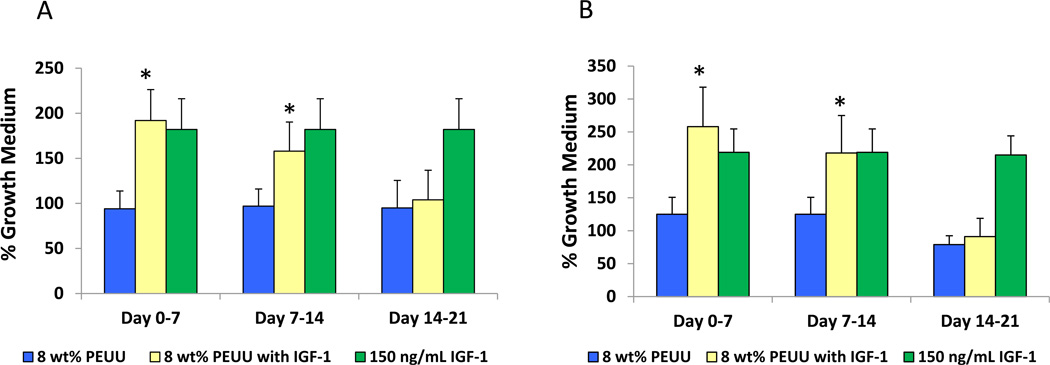
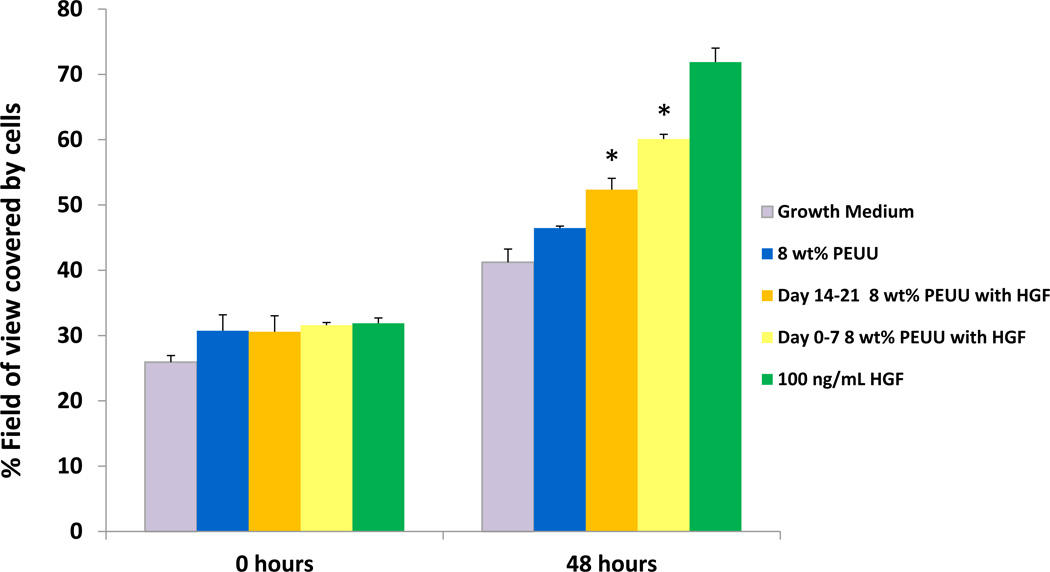
MG-63 and Balb/3T3 cells treated with releasate from IGF-1 containing scaffolds collected between days 0–7 and days 7–14 exhibited a significant increase in proliferation compared to those treated with degradation solutions from scaffolds without growth factor (p<0.05) (Figure 6) Cellular proliferation was comparable to that for cells treated with 150 ng/ml IGF-1. Specifically, cells treated with day 0–7 releasate from scaffolds containing IGF-1 exhibited a 2.0-fold (MG-63) and 2.1-fold (Balb/3T3) increase in cell number compared to cells treated with releasate from scaffolds without growth factor. Cells treated with day 7–14 releasate from scaffolds containing IGF-1 exhibited a 1.6-fold (MG-63) and 1.7-fold (Balb/3T3) increase over cells in releasate from scaffolds without growth factor. No significant difference in cell proliferation was seen between cells treated with releasate collected between days 14–21 from either set of scaffolds. For HGF, following treatment with the day 0–7 and day 14–21 polymer releasate from scaffolds containing HGF, endothelial cells grew into and repopulated the wound area more extensively than those maintained in growth medium or in releasate from scaffolds without growth factor (p<0.05) (Figure 7). Day 7–14 releasate was not collected to evaluate.
Figure 6.
MG-63 (A) and Balb/3T3 (B) cells cultured in releasate from polymer containing IGF-1 demonstrated significantly greater cell metabolic activity (as an index of cell number) compared to cells cultured in releasate from polymer without growth factor (*p<0.05). IGF-1 added at 150 ng/ml to releasate from polymer without growth factor served as a positive control.
Figure 7.
HUVECs maintained in releasate from scaffolds containing HGF grew into and repopulated the artificial wound area more extensively than those maintained in growth medium or in releasate from scaffolds without growth factor (*p<0.05). HGF added at 100 ng/ml to releasate from polymer without growth factor served as a positive control.
Discussion
The release profiles of drugs from biodegradable elastomers vary widely depending on material composition and processing. Biodegradable thermoset elastomers based on ε-caprolactone and D,L-lactide have been processed with solid drug particles to form an osmotically-driven drug delivery device that delivers drug in a zero-order fashion.(18, 36, 37) The release rates in this system can be altered by changing the molecular weight of the prepolymer or the amount of excipient included with the drug. In another report ascorbic acid was incorporated into the backbone of a thermoplastic polyurethane and released only after hydrolysis of ester bonds.(14) This led to a slow release rate initially which increased substantially over time in a way that corresponded with bulk polymer degradation. Many other biodegradable elastomers release molecules in a diffusion-controlled manner characterized by high initial release rates that quickly taper off.(11, 16, 38) One such group, a family of injectable biodegradable polyurethanes, has been of particular interest due to their ability to crosslink in situ to form porous polymer scaffolds in a way that does not substantially decrease the bioactivity of incorporated drugs. These systems generally show a high initial release that plateaus after a few days with 50–90% of drug being released depending on the drug loading mechanism and type of drug being delivered.(10, 39) Despite the fact that some members of this family are relatively slowly degrading (~30% mass loss over 36 weeks), release studies do not extend beyond a few weeks. This prevents understanding the mechanisms involved over time in a slow-degrading polyurethane and how the remaining 10–50% of protein is released from the system. In studying the long-term release kinetics of protein from PEUU, as has been done here, more insight may be gained toward developing extended release approaches for polyurethane systems.
IGF-1 release from this biodegradable porous scaffold system demonstrated a multi-phasic release profile. There are a number of drug delivery systems that follow a biphasic drug release profile characterized by two separate stages of quick drug release separated by a latency phase.(17, 40, 41) The first phase of quick drug delivery occurs as drug is released that is either close to the surface or can easily diffuse out of the bulk of the material. Following this, delivery is slow in the latency phase until material degradation becomes adequate to loosen the scaffold and allow trapped drug to quickly diffuse out. The current report demonstrates a release profile wherein there are three distinct occurrences of rapid, diffusion-controlled release. Importantly, because the quantitative studies of IGF-1 release used radiolabeled protein, the release kinetics observed may be different from what actually occurs when non-labeled IGF-1 is released, as in the case of the bioactivity studies. As the scaffolds for both studies were made with identical protocols and loading concentrations, the protein released at each stage would likely be similar. However, variation may arise due to potential differences between IGF-1 and 125I-IGF-1 and processing variability.
While the exact mechanisms of the complex release profile are not clear and warrant further investigation, it is likely that the phase segregated nature of PEUU plays a role. First, both hard and soft segments may act independently in relation to degradation and drug release. Additionally, PCL is known to contribute significant crystallinity to polyurethanes, the gradual breakdown of which can be delayed even in the presence of enzyme.(5, 42) It is possible that degradation follows a multi-step process where amorphous soft segment, crystalline soft segment, and hard-segment degradation occur separately with each stage influencing drug delivery. This hypothesis is supported by the DSC data which show a change in Tm during degradation. The transition from a Tm near 50°C to one near 30°C in the presence of lipase shows that the crystal structure of the polymer is being altered, particularly to a Tm below body temperature. In the case of the lower Tm, crystal structures would largely be melted above 37°C which could loosen the polymer network and allow more protein to be released. It may be that one of the diffusion phases seen for protein release from scaffolds in PBS is the result of a transition from the higher Tm to the lower one. Additionally, the delayed loss of the initial melting peak for 8 wt% scaffolds agrees well with the slower mass loss and slower protein release that was demonstrated during enzymatic degradation of 8 wt% scaffolds compared to 5 wt%.
The differences in protein release between 5 wt% and 8 wt% scaffolds illustrate the influence that polymer mass fraction had on drug release. First, 5 wt% scaffolds lost more protein in the solvent extraction step of scaffold processing, meaning these scaffolds had a lower loading efficiency. Second, the delivery rates of protein from 5 wt% scaffolds were consistently higher and occurred at earlier time points than for 8 wt% scaffolds. This phenomenon may be attributed to the smaller mass fraction of PEUU in the 5 wt% scaffold disks, which led to more free volume for molecular diffusion out of the scaffold and less material that required degradation. Both polymer mass fractions, however, exhibited slower release rates for IGF-1 than had been shown previously when basic fibroblast growth factor (bFGF) was released from similar scaffolds.(15) The reason for this difference is not clear but may be related to differences in the affinity of IGF-1 and bFGF to the polymer or the slower polymer degradation that was present here compared to the previous study. While no significant mass loss was seen in the scaffolds in PBS over the time period studied, in the presence of enzyme, scaffolds with 8 wt% polymer had attenuated mass loss, particularly in the first week compared to those with 5 wt% polymer. This difference in enzymatic degradation between 5 and 8 wt% scaffolds was noticeable in the release profiles where the rate of IGF-1 release was significantly lower between days 1 and 7 for the 8 wt% scaffolds.
The biodegradation of polyurethanes is a complex process that has gained significant attention in recent decades.(43) Hydrolytically labile bonds in the soft segment provide the most common mechanism of degradation. It has been shown that both oxidative and enzymatic processes encouraged by inflammatory cells may speed polymer breakdown in vivo.(44, 45) Two common esterase enzymes produced by macrophages that have been utilized in vitro to simulate in vivo soft-segment biodegradation mechanisms are cholesterol esterase and lipase.(20, 42, 44–46) The ability of cells and enzymes to degrade polyurethanes is dependent on many factors including hard and soft segment chemical composition, size, surface morphology and mechanical environment.(45, 47–49) These studies illustrate that numerous complex processes are involved in biomaterial degradation, which cannot be fully replicated in vitro.
The enzyme concentration in this report was chosen for its ability to provide a degradation rate that corresponded to scaffold break down over approximately 8 weeks, similar to what is histologically observed in vivo when a similar scaffold disk was implanted as a cardiac patch.(8) The release profile exhibited when scaffolds were incubated with lipase demonstrated that for in vivo environments where material degradation was accelerated, release rates were markedly different than in the PBS environments often used for in vitro controlled release studies. The rapid rate of polymer degradation in the presence of enzyme made for protein release kinetics that appeared more like the simple diffusion controlled systems discussed earlier, which was substantially different from the multiple phasic release profile seen in the PBS. Therefore, these data emphasize that researchers should consider the working environment when characterizing controlled release from biodegradable matrices, a practice not regularly seen in the literature.
Linear thermoplastic elastomers have an advantage over thermoset elastomers in that drugs can be readily incorporated during processing and in a way that does not risk drug inactivation during polymer crosslinking. In the current research, protein bioactivity was studied over the first three weeks of drug delivery. It is unclear if the loss of measurable cellular response to IGF-1 between day 14–21 was from a loss of bioactivity or simply due to the low protein release during that time period as seen in the release profile. The latter case would suggest that released protein was of insufficient concentration to influence cell behavior in the assay used, which might be corrected with a higher initial loading dose of protein. Additionally, it has been shown that growth factors incorporated into thermoset degradable elastomers can maintain bioactivity over at least 3 weeks (36), suggesting that longer-term maintenance of bioactivity is feasible.
One limitation of the current research deals with understanding the maintenance of protein bioactivity during scaffold processing and throughout the release duration. To determine changes in bioactivity the amount of protein released from a scaffold needs to be quantified and then the functionality of that protein would be tested. However, in this report two distinct experiments were employed to study IGF-1 release – quantitative release kinetics using radiolabeled protein and bioactivity of non-labeled released protein. Both were needed due to an inability to quantify non-labeled IGF-1 using enzyme-linked immunosorbent assays (ELISA) and other spectrophotometric techniques. The inability to detect both IGF-1 and HGF protein with these techniques may be related to the presence of polymer degradation products or conformational changes to the protein which would prevent binding of the monoclonal antibody in the ELISA sandwich assay. In particular, the TIPS processing involved mixing protein in organic solvent and brief exposure to high temperatures – both factors that could damage the loaded protein. In order to preserve bioactivity, BSA was added in excess to protect the protein of interest as has been done previously.(50) Importantly, in spite of any presumed destruction of protein or changes to protein binding in the ELISA, enough protein remained bioactive over the three wk studied to elicit the expected results on cell behavior in vitro.
One basis of controlled release formulations is that while proteins generally have a short half-life in vivo, their sequestration in a polymeric scaffold can act to protect them in vivo to prevent rapid degradation. An important finding in this research is that maintaining protein bioactivity for long durations as would be necessary for in vitro release studies may be irrelevant given the much faster release seen in conditions that mimic the in vivo environment. In the experiments using lipase, protein delivery was nearly complete after only 9 wk compared to nearly 15 months for studies without lipase.
The ideal duration of IGF-1 delivery that would be desired is dependent on the drug delivery application. For example, in the case of cardiac ischemia, rapid delivery of IGF-1 in the days following injury has been shown to rescue injured myocardium.(51) However, in the case of slowly-progressing, chronic degenerative diseases such as inherited cerebellar ataxia (52) or diabetes (53), therapeutic options to treat severely debilitating symptoms are currently limited by the short in vivo life-span of peptide molecules and the need to administer drugs to patients on a daily basis. In such cases, controlled-release formulations leading to sustained improvements in patient symptoms and/or disease conditions allows for more convenient dosage and increased compliance with treatment.
Scaffold processing was also noteworthy for its role in determining protein loading efficiency in PEUU scaffolds. The final step of scaffold synthesis using TIPS involved DMSO solvent extraction. This step required 3–7 days of soaking in ethanol during which time a substantial amount of protein was lost to the solvent. While this method was necessary to create the mechanically robust, porous scaffolds studied here, other techniques such as salt-leaching may be considered which do not have a lengthy liquid-phase solvent extraction step. The short duration required to remove salts from thin scaffold disks in water (<20 minutes) may lead to less protein loss in solution initially, even in spite of the higher solubility of protein in water compared to ethanol. Regardless of processing technique it is important to have a uniform distribution of protein throughout the scaffold. In this report disks cut from locations throughout the initial cylindrical scaffold were used in the release experiments. The narrow standard deviation in the protein release kinetics among all disks suggests a homogeneous distribution of protein throughout the cylindrical scaffolds.
Tissue engineering often seeks to combine appropriate scaffolding materials with important signaling molecules to encourage healthy tissue regeneration. It has been demonstrated that the controlled delivery of IGF-1 and HGF from biomaterials in the context of ischemic myocardium has been influential in cardiac repair.(51, 54) These studies, however, utilize biomaterials that provide minimal mechanical support in a setting where such support may be advantageous. A mechanically robust, elastomeric scaffold similar to the one studied here was able to prevent further cardiac deterioration after its application as a surface patch following myocardial infarction.(8) Combining the benefit of this temporary mechanical support system with growth factor delivery might act to abrogate disease progression and encourage functional tissue repair in injured myocardium more effectively than either system independently.
Conclusion
A biodegradable, elastomeric PEUU scaffold system was characterized for its ability to be loaded with and deliver growth factors (IGF-1 and HGF) of clinical relevance. Cellular assays confirmed that the bioactivity of IGF-1 and HGF was maintained during scaffold processing and for at least the early period of drug delivery. The kinetics of IGF-1 release over a period up to 440 days demonstrated a complex, triphasic profile. Much of this complexity was lost and replaced by a single phase release profile when enzyme was present to simulate in vivo scaffold degradation. The ease of processing associated with this thermoplastic biodegradable elastomer for both scaffold formation and drug loading make this material an attractive option for soft tissue applications where both drug delivery and appropriate mechanical support are desired.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) grant #HL069368. Dr. Baraniak and Mr. Nelson were supported by NIH training grants #T32-EB001026-01 and #T32-HL076124, respectively.
Abbreviations
- BDI
1,4-diisocyanatobutane
- bFGF
basic fibroblast growth factor
- BSA
bovine serum albumin
- DMEM
Dulbecco’s modified Eagle medium
- DMSO
dimethyl sulfoxide
- HGF
hepatocyte growth factor
- HUVECs
human umbilical vein endothelial cells
- IGF-1
insulin-like growth factor 1
- MEM
minimum essential medium
- PBS
phosphate buffered saline
- PCL
polycaprolactone
- PEUU
poly(ester urethane)urea
- TIPS
thermally induced phase separation
References
- 1.Rehfeldt F, Engler AJ, Eckhardt A, Ahmed F, Discher DE. Cell responses to the mechanochemical microenvironment--implications for regenerative medicine and drug delivery. Adv Drug Deliv Rev. 2007;59:1329–1339. doi: 10.1016/j.addr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 3.Ramaswami P, Wagner WR. Cardiovascular Tissue Engineering. In: Guelcher S, Hollinger JO, editors. An Introduction to Biomaterials. Boca Raton, FL: CRC Press; 2006. pp. 461–484. [Google Scholar]
- 4.Guan J, Sacks MS, Beckman EJ, Wagner WR. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J Biomed Mater Res. 2002;61:493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 5.Skarja GA, Woodhouse KA. In vitro degradation and erosion of degradable, segmented polyurethanes containing an amino acid-based chain extender. J Biomater Sci Polym Ed. 2001;12:851–873. doi: 10.1163/156856201753113060. [DOI] [PubMed] [Google Scholar]
- 6.Guan J, Wagner WR. Synthesis, characterization and cytocompatibility of polyurethaneurea elastomers with designed elastase sensitivity. Biomacromolecules. 2005;6:2833–2842. doi: 10.1021/bm0503322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gogolewski S, Galletti G. Degradable, microporous vascular prosthesis from segmented polyurethane. Colloid & Polymer Science. 1986;264:854–858. [Google Scholar]
- 8.Fujimoto KL, Tobita K, Merryman WD, Guan J, Momoi N, Stolz DB, Sacks MS, Keller BB, Wagner WR. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J Am Coll Cardiol. 2007;49:2292–2300. doi: 10.1016/j.jacc.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He W, Nieponice A, Soletti L, Hong Y, Gharaibeh B, Crisan M, Usas A, Peault B, Huard J, Wagner WR, Vorp DA. Pericyte-based human tissue engineered vascular grafts. Biomaterials. 2010;31:8235–8244. doi: 10.1016/j.biomaterials.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hafeman A, Li B, Yoshii T, Zienkiewicz K, Davidson J, Guelcher S. Injectable biodegradable polyurethane scaffolds with release of platelet-derived growth factor for tissue repair and regeneration. Pharmaceutical Research. 2008;25:2387–2399. doi: 10.1007/s11095-008-9618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hafeman AE, Zienkiewicz KJ, Carney E, Litzner B, Stratton C, Wenke JC, Guelcher SA. Local delivery of tobramycin from injectable biodegradable polyurethane scaffolds. J Biomater Sci Polym Ed. 2010;21:95–112. doi: 10.1163/156856209X410256. [DOI] [PubMed] [Google Scholar]
- 12.Sivak WN, Zhang J, Petoud S, Beckman EJ. Incorporation of ionic ligands accelerates drug release from LDI-glycerol polyurethanes. Acta Biomater. 2010;6:144–153. doi: 10.1016/j.actbio.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Stankus JJ, Freytes DO, Badylak SF, Wagner WR. Hybrid nanofibrous scaffolds from electrospinning of a synthetic biodegradable elastomer and urinary bladder matrix. J Biomater Sci Polym Ed. 2008;19:635–652. doi: 10.1163/156856208784089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Doll BA, Beckman EJ, Hollinger JO. A biodegradable polyurethane-ascorbic acid scaffold for bone tissue engineering. J Biomed Mater Res A. 2003;67A:389–400. doi: 10.1002/jbm.a.10015. [DOI] [PubMed] [Google Scholar]
- 15.Guan J, Stankus JJ, Wagner WR. Biodegradable elastomeric scaffolds with basic fibroblast growth factor release. J Control Release. 2007;120:70–78. doi: 10.1016/j.jconrel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li B, Brown KV, Wenke JC, Guelcher SA. Sustained release of vancomycin from polyurethane scaffolds inhibits infection of bone wounds in a rat femoral segmental defect model. J Control Release. 2010;145:221–230. doi: 10.1016/j.jconrel.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Lao LL, Venkatraman SS, Peppas NA. A novel model and experimental analysis of hydrophilic and hydrophobic agent release from biodegradable polymers. J Biomed Mater Res A. 2009;90:1054–1065. doi: 10.1002/jbm.a.32171. [DOI] [PubMed] [Google Scholar]
- 18.Amsden B. A model for osmotic pressure driven release from cylindrical rubbery polymer matrices. J Control Release. 2003;93:249–258. doi: 10.1016/j.jconrel.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Amsden BG. Biodegradable elastomers in drug delivery. Expert Opin Drug Deliv. 2008;5:175–187. doi: 10.1517/17425247.5.2.175. [DOI] [PubMed] [Google Scholar]
- 20.Woo GLY, Yang ML, Yin HQ, Jaffer F, Mittelman MW, Santerre JP. Biological characterization of a novel biodegradable antimicrobial polymer synthesized with fluoroquinolones. J Biomed Mater Res. 2002;59:35–45. doi: 10.1002/jbm.1214. [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal N, Musaro A. Gene therapy for cardiac cachexia? Int J Cardiol. 2002;85:185–191. doi: 10.1016/s0167-5273(02)00253-x. [DOI] [PubMed] [Google Scholar]
- 22.Schulze PC, Fang J, Kassik KA, Gannon J, Cupesi M, MacGillivray C, Lee RT, Rosenthal N. Transgenic overexpression of locally acting insulin-like growth factor-1 inhibits ubiquitin-mediated muscle atrophy in chronic left-ventricular dysfunction. Circ Res. 2005;97:418–426. doi: 10.1161/01.RES.0000179580.72375.c2. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi S, Aso H, Watanabe K, Nara H, Rose MT, Ohwada S, Yamaguchi T. Sequence of IGF-I, IGF-II, and HGF expression in regenerating skeletal muscle. Histochem Cell Biol. 2004;122:427–434. doi: 10.1007/s00418-004-0704-y. [DOI] [PubMed] [Google Scholar]
- 24.Machida S, Booth FW. Insulin-like growth factor 1 and muscle growth: implication for satellite cell proliferation. Proc Nutr Soc. 2004;63:337–340. doi: 10.1079/PNS2004354. [DOI] [PubMed] [Google Scholar]
- 25.Padin-Iruegas ME, Misao Y, Davis ME, Segers VF, Esposito G, Tokunou T, Urbanek K, Hosoda T, Rota M, Anversa P, Leri A, Lee RT, Kajstura J. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120:876–887. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin H, Wyss JM, Yang R, Schwall R. The therapeutic potential of hepatocyte growth factor for myocardial infarction and heart failure. Curr Pharm Des. 2004;10:2525–2533. doi: 10.2174/1381612043383863. [DOI] [PubMed] [Google Scholar]
- 27.Duan HF, Wu CT, Wu DL, Lu Y, Liu HJ, Ha XQ, Zhang QW, Wang H, Jia XX, Wang LS. Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol Ther. 2003;8:467–474. doi: 10.1016/s1525-0016(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 28.Ueda H, Nakamura T, Matsumoto K, Sawa Y, Matsuda H. A potential cardioprotective role of hepatocyte growth factor in myocardial infarction in rats. Cardiovasc Res. 2001;51:41–50. doi: 10.1016/s0008-6363(01)00272-3. [DOI] [PubMed] [Google Scholar]
- 29.Pulavendran S, Rajam M, Rose C, Mandal AB. Hepatocyte growth factor incorporated chitosan nanoparticles differentiate murine bone marrow mesenchymal stem cell into hepatocytes in vitro. IET Nanobiotechnol. 2010;4:51. doi: 10.1049/iet-nbt.2009.0014. [DOI] [PubMed] [Google Scholar]
- 30.Kato N, Nakanishi K, Nemoto K. Efficacy of HGF gene transfer for various nervous injuries and disorders. Cent Nerv Syst Agents Med Chem. 2009;9:300–306. doi: 10.2174/187152409789630406. [DOI] [PubMed] [Google Scholar]
- 31.Guan J, Fujimoto KL, Sacks MS, Wagner WR. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials. 2005;26:3961–3971. doi: 10.1016/j.biomaterials.2004.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kricker JA, Towne CL, Firth SM, Herington AC, Upton Z. Structural and Functional Evidence for the Interaction of Insulin-Like Growth Factors (IGFs) and IGF Binding Proteins with Vitronectin. Endocrinology. 2003;144:2807–2815. doi: 10.1210/en.2002-221086. [DOI] [PubMed] [Google Scholar]
- 33.Liu XJ, Xie Q, Zhu YF, Chen C, Ling N. Identification of a nonpeptide ligand that releases bioactive insulin-like growth factor-I from its binding protein complex. J Biol Chem. 2001;276:32419–32422. doi: 10.1074/jbc.C100299200. [DOI] [PubMed] [Google Scholar]
- 34.Singh M, Shirley B, Bajwa K, Samara E, Hora M, O'Hagan D. Controlled release of recombinant insulin-like growth factor from a novel formulation of polylactide-co-glycolide microparticles. J Control Release. 2001;70:21–28. doi: 10.1016/s0168-3659(00)00313-8. [DOI] [PubMed] [Google Scholar]
- 35.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu F, Neufeld R, Amsden B. Sustained release of bioactive therapeutic proteins from a biodegradable elastomeric device. J Control Release. 2007;117:80–89. doi: 10.1016/j.jconrel.2006.09.077. [DOI] [PubMed] [Google Scholar]
- 37.Gu F, Neufeld R, Amsden B. Osmotic-driven release kinetics of bioactive therapeutic proteins from a biodegradable elastomer are linear, constant, similar, and adjustable. Pharm Res. 2006;23:782–789. doi: 10.1007/s11095-006-9750-6. [DOI] [PubMed] [Google Scholar]
- 38.Hirose K, Marui A, Arai Y, Nomura T, Inoue S, Kaneda K, Kamitani T, Fujita M, Mitsuyama M, Tabata Y, Komeda M. Sustained-release vancomycin sheet may help to prevent prosthetic graft methicillin-resistant Staphylococcus aureus infection. Journal of Vascular Surgery. 2006;44:377–382. doi: 10.1016/j.jvs.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 39.Li B, Davidson JM, Guelcher SA. The effect of the local delivery of platelet-derived growth factor from reactive two-component polyurethane scaffolds on the healing in rat skin excisional wounds. Biomaterials. 2009;30:3486–3494. doi: 10.1016/j.biomaterials.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui F, Cun D, Tao A, Yang M, Shi K, Zhao M, Guan Y. Preparation and characterization of melittin-loaded poly (dl-lactic acid) or poly (dl-lactic-co-glycolic acid) microspheres made by the double emulsion method. J Control Release. 2005;107:310–319. doi: 10.1016/j.jconrel.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Lin CC, Metters AT. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv Drug Deliv Rev. 2006;58:1379–1408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Cometa S, Bartolozzi I, Corti A, Chiellini F, De Giglio E, Chiellini E. Hydrolytic and microbial degradation of multi-block polyurethanes based on poly([var epsilon]-caprolactone)/poly(ethylene glycol) segments. Polymer Degradation and Stability. 2010;95:2013–2021. [Google Scholar]
- 43.Santerre JP, Woodhouse K, Laroche G, Labow RS. Understanding the biodegradation of polyurethanes: From classical implants to tissue engineering materials. Biomaterials. 2005;26:7457–7470. doi: 10.1016/j.biomaterials.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 44.Labow RS, Meek E, Santerre JP. Hydrolytic degradation of poly(carbonate)-urethanes by monocyte-derived macrophages. Biomaterials. 2001;22:3025–3033. doi: 10.1016/s0142-9612(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 45.Labow RS, Meek E, Santerre JP. Model systems to assess the destructive potential of human neutrophils and monocyte-derived macrophages during the acute and chronic phases of inflammation. J Biomed Mater Res. 2001;54:189–197. doi: 10.1002/1097-4636(200102)54:2<189::aid-jbm5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 46.Peng H, Ling J, Liu J, Zhu N, Ni X, Shen Z. Controlled enzymatic degradation of poly(caprolactone)-based copolymers in the presence of porcine pancreatic lipase. Polymer Degradation and Stability. 2010;95:643–650. [Google Scholar]
- 47.Labow RS, Erfle DJ, Santerre JP. Neutrophil-mediated degradation of segmented polyurethanes. Biomaterials. 1995;16:51–59. doi: 10.1016/0142-9612(95)91096-h. [DOI] [PubMed] [Google Scholar]
- 48.Labow RS, Sa D, Matheson LA, Dinnes DLM, Paul Santerre J. The human macrophage response during differentiation and biodegradation on polycarbonate-based polyurethanes: Dependence on hard segment chemistry. Biomaterials. 2005;26:7357–7366. doi: 10.1016/j.biomaterials.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 49.Tang YW, Labow RS, Revenko I, Santerre JP. Influence of surface morphology and chemistry on the enzyme catalyzed biodegradation of polycarbonate-urethanes. J Biomater Sci Polym Ed. 2002;13:463–483. doi: 10.1163/156856202320253965. [DOI] [PubMed] [Google Scholar]
- 50.Pérez C, Castellanos IJ, Costantino HR, Al-Azzam W, Griebenow K. Recent trends in stabilizing protein structure upon encapsulation and release from bioerodible polymers. Journal of Pharmacy and Pharmacology. 2002;54:301–313. doi: 10.1211/0022357021778448. [DOI] [PubMed] [Google Scholar]
- 51.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carrascosa C, Torres-Aleman I, Lopez-Lopez C, Carro E, Espejo L, Torrado S, Torrado JJ. Microspheres containing insulin-like growth factor I for treatment of chronic neurodegeneration. Biomaterials. 2004;25:707–714. doi: 10.1016/s0142-9612(03)00562-3. [DOI] [PubMed] [Google Scholar]
- 53.Lam XM, Duenas ET, Daugherty AL, Levin N, Cleland JL. Sustained release of recombinant human insulin-like growth factor-I for treatment of diabetes. Journal of Controlled Release. 2000;67:281–292. doi: 10.1016/s0168-3659(00)00224-8. [DOI] [PubMed] [Google Scholar]
- 54.Tambara K, Premaratne GU, Sakaguchi G, Kanemitsu N, Lin X, Nakajima H, Sakakibara Y, Kimura Y, Yamamoto M, Tabata Y, Ikeda T, Komeda M. Administration of control-released hepatocyte growth factor enhances the efficacy of skeletal myoblast transplantation in rat infarcted hearts by greatly increasing both quantity and quality of the graft. Circulation. 2005;112:I129–I134. doi: 10.1161/CIRCULATIONAHA.104.526293. [DOI] [PubMed] [Google Scholar]