Abstract
Context:
Acupuncture may represent a nonpharmaceutical treatment for women with polycystic ovary syndrome (PCOS), based on four studies.
Objective:
The objective of the study was to determine whether true, as compared with sham, acupuncture normalizes pituitary gonadotropin hormones and increases ovulatory frequency in women with PCOS.
Design:
This was a randomized, double-blind, sham-controlled clinical trial (5 month protocol).
Setting:
The study was conducted in central Virginia.
Participants:
Eighty-four reproductive-aged women completed the intervention. Eligibility required a PCOS diagnosis and no hormonal intervention 60 d before enrollment.
Interventions:
Intervention included 12 sessions of true or sham acupuncture (Park sham device) for 8 wk.
Main Outcome Measures:
Serum LH and FSH at baseline, after intervention, and 3 months later were measured. Ovulation was measured with weekly urine or blood samples.
Results:
Both arms demonstrated a similar mean ovulation rate over the 5 months (0.37/month among n = 40 true acupuncture and 0.40/month among n = 44 sham participants, P = 0.6), similar LH to FSH ratio improvement (−0.5 and −0.8 true and sham, respectively, P < 0.04 after intervention vs. baseline) and a similar decline in LH over the 5-month protocol (P < 0.05). Neither arm experienced a change in FSH. There were seven pregnancies (no difference by intervention, P = 0.7). Lower fasting insulin and free testosterone were highly correlated with a higher ovulation rate within the true acupuncture group only (P = 0.03), controlling for prestudy menstrual frequency and body mass index.
Conclusion:
We were unable to discern a difference between the true and sham acupuncture protocols for these women with PCOS, and both groups had a similar improvement in their LH/FSH ratio.
Approximately 6.5% of women of reproductive age have polycystic ovary syndrome (PCOS) (1, 2). PCOS is characterized by irregular or absent menstrual periods, hyperandrogenic manifestations such as acne and hirsutism (3), an increased ratio of LH to FSH (4), and insulin resistance (5).
Of women seen in a reproductive endocrinology/fertility clinic, 22% had tried acupuncture therapy within 18 months of their initial clinic visit in the United States (6) [12.5% within 6 months in Australia (7) and 8% use in the United Kingdom (8)]. The four publications [sample sizes ranged from 24 to 45 women (9–12)] on acupuncture for women with ovulatory disorders reported acupuncture to be effective for restoring regular menses, regular ovulation, and/or achieving pregnancy. One of these prior studies was a randomized clinical trial (RCT), and the comparison intervention was physical exercise (12). All four reports have limitations that have been addressed by this study, most notably a lack of a comparison population (9, 11), collection of posttreatment blood samples midcycle (11) or a nonstandardized time in the cycle (9, 12), and inclusion of a variety of menstrual disorders in the eligibility criteria (10).
This study was a RCT of acupuncture in oligoovulatory and anovulatory untreated, adult female patients with PCOS. The goals of this study were to assess whether an acupuncture standardized protocol would increase the ovulatory frequency and normalize the ratio of LH to FSH relative to a sham acupuncture intervention.
Subjects and Methods
Trial design
This study was a randomized, double-blind, sham-controlled clinical trial of acupuncture in women diagnosed with PCOS. The 5-month protocol involved baseline questionnaires and biological sampling, two intervention months, postintervention repeat questionnaires and biological sampling, 3 months of follow-up without intervention, and post-follow-up questionnaires and biological sampling. Women provided urine or blood samples weekly throughout the entire 5 months for objective assessment of ovulation. Menses were self-reported. This trial was approved by the University of Virginia's Internal Review Board (no. 12045), and this report follows the Standards for Reporting Interventions in Controlled Trials of Acupuncture guidelines (13).
Geographic area and enrollment criteria
All study participants were residents of Virginia, with one exception of a woman living in the Washington, DC, metropolitan area. Recruitment was initially targeted to Charlottesville, VA, and the surrounding counties (population of 155,000). In yr 2, marketing efforts were expanded to the Richmond, VA, metropolitan area (state capital) with a population of 850,000 and to the counties between Charlottesville and Harrisonburg, VA (metropolitan population 70,000). For marketing and recruitment details, see Pastore and Dalal (14).
Women were eligible if they had PCOS, as confirmed through symptoms and blood tests for this study or through their health care provider if the confirmatory laboratory results were collected in the prior year. Inclusion criteria were: 1) a diagnosis of PCOS, as confirmed by the presence of both oligomenorrhea and hyperandrogenism (15), 2) aged 18–43 yr, 3) at least one menses in the past 6 months but no more than eight periods in the most recent 12 months without hormonal intervention, and 4) agreement to not take hormonal contraceptives, metformin, or fertility medication for the 5 months of study participation. The requirement of one menses in the past 6 months was implemented to ensure that all women, after the 5-month trial, would have at least one menses in the prior 12 months to minimize her risk of endometrial cancer. Hyperandrogenism was defined by self-reported hirsutism and/or acne and/or elevated free testosterone [>6.8 pg/ml (16)]. Free testosterone was calculated from testosterone and SHBG levels (17, 18).
Exclusion criteria were: 1) diagnosed with Cushing's syndrome, uncontrolled thyroid disease, hyperprolactinemia, congenital adrenal hyperplasia, or diabetes mellitus; 2) use of metformin or hormonal contraceptives in the 60 d before enrollment; 3) use of any other hormonal drug in the 30 d before entry into study, including fertility medications, and over-the-counter hormonal supplements or herbs (i.e. black cohosh, clover, soy, dong quai/Chinese angelica root, fructus rubi, white peony root); 4) currently pregnant or breast-feeding during the prior 30 d; 5) any acupuncture treatment for ovulatory disorders in the prior 30 d; 6) weight more than 250 lb because these women were reported to be the most resistant to medical intervention at the time of our study design development (19); 7) currently taking anticoagulation medication other than low dose (≤81 mg) aspirin; 8) immune deficiency; and 9) history of any bleeding disorder.
Information regarding the PCOS symptoms (menstrual pattern, hirsutism, acne) was obtained using a phone screening questionnaire. Androgens, TSH, 17-hydroxyprogesterone, fasting glucose, hemoglobin A1C, and prolactin were assayed on fasting blood samples for diagnostic and eligibility confirmation purposes.
Eligibility of all potential enrollees was confirmed by a reproductive endocrinologist (C.D.W.). Signed informed consent was obtained before the diagnostic blood draw.
Interventions
Subjects in both groups had 12 acupuncture/sham session: twice each week for the first 4 wk followed by once per week for an additional 4 wk. There were four study acupuncturists, each of whom implemented both protocols. For the true acupuncture treatment, the following bilateral points were stimulated with electroacupuncture: bladder 23, bladder 28, spleen 6, and spleen 9. The following points were manually stimulated: pericardium 6, triple energizer 5, and governor vessel 20. The sham acupuncture was performed with the validated Park sham device (20, 21). The sham device was placed on the skin at standardized points on all four extremities (Achilles tendon and lateral head of the triceps) chosen to avoid standard acupuncture meridians and acupuncture points (22). For further details, see the Supplemental Data, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.
Longitudinal biological parameters and study clinic protocol
All participants had fasting 2.5-h study visits at a single study center at three time points: before the first intervention, within 2 wk after the last intervention, and 3 months after the last intervention. During each visit, a 75-g oral 2-h glucose tolerance test was performed. Blood samples were drawn for insulin, glucose, FSH, LH, dehydroepiandrosterone sulfate, progesterone, prolactin, testosterone, and SHBG assays. Height and weight were measured [for calculating body mass index (BMI)] by trained nurses. See the Supplemental Data for lab assay details.
Ovulation assessment
The participants provided weekly blood samples for serum progesterone measurement or collected first-void urine samples at home (stored in their home freezer) for pregnanediol glucuronide (PDG) measurement for the entire 5-month protocol. Ovulation was defined as progesterone of 3 ng/ml or greater or a ratio of the peak urinary PDG to the basal PDG level in the follicular phase of 4.0 or greater. See the Supplemental Data for further details.
Power analysis
Based on prior acupuncture/PCOS research, it was anticipated that 38% of the true acupuncture and 9% of the sham acupuncture group would have two or more ovulations during the time period encompassing the intervention and 3 months of follow-up (9). The power calculation presumed minimal placebo effect as the outcomes were hard biological end points, and the 9% ovulation rate in the sham arm was based on the preenrollment ovulatory frequency from Stener-Victorin et al. (9). With these rates of ovulation, the study required 78 total participants (1:1 ratio of acupuncture to sham) to see a relative odds of ovulation of 4.4 with 80% power and a two-sided type I error rate of 0.05 or less.
Randomization
Women were randomized to an intervention arm using a random number generator program. In blocks of four participants, stratified by obese (BMI ≥30 kg/m2) vs. nonobese (BMI <30 kg/m2) (23), consecutive study identifications were assigned to a treatment arm by a graduate student independent of the study team under the direction of a biostatistician. The weight strata ensured that the BMI distribution would be equally distributed between the acupuncture and sham treatment groups. These assignments were placed in individual sealed, opaque envelopes labeled with the study identifications. The lead acupuncturist (J.J.) drew the next consecutive envelope within the appropriate BMI strata, recorded who was assigned that envelope, communicated the intervention assignment directly and only to the acupuncturist who was selected for that participant, and stored all the envelopes until the final enrollee completed her final study visit. No participants switched their study intervention arm. The participants, principal investigator, clinical research coordinator, and biostatistician (J.T.P.) were all blind to the intervention arm. The lead acupuncturist and the acupuncturist who was treating the participant were the only individuals who knew the treatment arm for a given participant.
Statistical analyses
Potential differences between the interventions and between the dropouts and completers were assessed with Kruskal Wallis and Wilcoxon rank sum tests (continuous variables) and Pearson χ2 tests (categorical variables). The monthly rates of ovulation by intervention were analyzed with a Poisson generalized linear model with the offset variable representing the logarithmic number of months of study participation. The gonadotropin results were analyzed with linear mixed models using the Welch's form of the Student t test, a test that is more robust to unequal group variances. Post hoc, the LH and FSH analyses were repeated excluding the women whose postintervention (n = 10 women, 12% of the cohort) or post-follow-up (n = 17, 20% of the cohort) samples were collected in the late follicular or luteal phase of the menstrual cycle phase or for whom missing samples prevented determination of cycle phase. For the primary aims, statistical significance was judged by a two-sided α = 0.05.
Secondary aims were to determine predictors of treatment success with acupuncture using Spearman correlations and Spearman partial correlations. Potential predictors are described in the Supplemental Data. An α = 0.10 was used for this analysis because the original study was not powered for this investigation and the intention was exploratory. Lastly, post hoc, the frequency of menses was compared during the trial with the hormone-free preenrollment time frame using the Wilcoxon paired sign test.
All statistical analyses were conducted with Spotfire S+ version 8.0 (TIBCO, Palo Alto, CA), SAS version 9.1.3 (SAS Institute Inc., Cary, NC), and EPI Info 3.5.1 (public domain) software.
Results
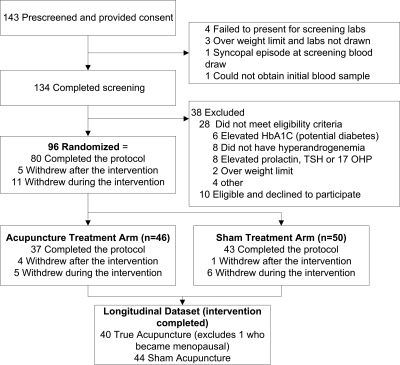
One hundred thirty-four women consented and were screened for this study, of which 38 (28%) were not eligible and 10 (7%) were eligible but declined to participate (Fig. 1). The remaining 96 eligible women were randomized between January 2006 and October 2009. Eleven women dropped out during the intervention phase; There was no age, education, BMI, or race difference between the dropouts and the remainder of the cohort (P > 0.06). One participant who was eligible at enrollment and entered the menopausal transition during the trial was excluded, as evidenced by increasing FSH levels (5.2 mIU/ml at enrollment, 73.0 mIU/ml after treatment, 40.7 mIU/ml after follow-up). Thus, the analytic cohort was restricted to 84 women who completed the intervention (n = 40 acupuncture, n = 44 sham).
Fig. 1.
Flow chart of study participation. HbA1C, Hemoglobin A1C; 17OHP, 17-hydroxyprogesterone.
Most of the women had some college education, and approximately half the cohort was obese (Table 1). There were no differences by intervention in age, education, BMI, race, or Hispanic ethnicity (P ≥ 0.20). Selected eligibility data and endocrine results are displayed (Table 2). On average, the participants had five menses in the most recent 12 months without hormonal intervention before enrollment. Nearly all the participants (94%) were acupuncture naïve.
Table 1.
Participant demographics/reproductive history by intervention arm
| Factor | True acupuncture (n = 40) | Sham acupuncture (n = 44) | P value |
|---|---|---|---|
| Age, yr, mean (sd) | 28.0 (6.3) | 26.5 (5.8) | 0.20 |
| Education, n (%) | |||
| High school or less | 4 (10.0) | 3 (6.8) | |
| Some college | 18 (45.0) | 18 (40.9) | 0.78 |
| College degree | 8 (20.0) | 13 (29.5) | |
| More than college | 10 (25.0) | 10 (22.7) | |
| BMI, mean (sd) | 30.1 (7.0) | 30.0 (6.8) | 0.99 |
| Race, n (%) | |||
| Caucasian | 30 (75.0) | 37 (84.1) | |
| African-American | 5 (12.5) | 4 (9.1) | 0.62 |
| Other | 5 (12.5) | 3 (6.8) | |
| Hispanic, n (%) | 1 (2.5) | 3 (6.8) | 0.62 |
Table 2.
Participant eligibility criteria and additional endocrine levels by intervention arm
| Factor | True acupuncture (n = 40) | Sham acupuncture (n = 44) | P value |
|---|---|---|---|
| Number of menses in most recent 12-month time period without hormonal medication, mean (sd) | 5.5 (2.3) | 4.8 (2.3) | 0.15 |
| Self-reported acne, n (%) | 20 (50.0) | 21 (47.7) | 1.00 |
| Self-reported hirsutism, n (%) | 27 (67.5) | 33 (75.0) | 0.48 |
| Fasting plasma glucose (mg/dl), mean (sd) | 92.7 (7.8) | 92.8 (7.0) | 0.96 |
| Fasting serum insulin (mIU/ml), mean (sd) | 12.3 (10.4) | 11.0 (9.7) | 0.59 |
| TSH (μIU/ml), mean (sd) | 1.52 (0.70) | 1.57 (0.75) | 0.81 |
| Prolactin (ng/ml), mean (sd) | 9.7 (5.1) | 9.7 (4.9) | 0.96 |
| 17 OHP (ng/dl), mean (sd) | 119.4 (39.7) | 118.0 (46.1) | 0.81 |
| HbA1C, mean (sd) | 5.3 (0.3) | 5.4 (0.3) | 0.60 |
| Testosterone (ng/dl), mean (sd) | 59.4 (26.0) | 66.3 (36.2) | 0.64 |
| DHEAS (μg/dl), mean (sd) | 192.6 (117.2) | 178.1 (79.5) | 0.84 |
| Free testosterone (pg/ml), mean (sd) | 12.9 (7.2) | 14.4 (9.9) | 0.83 |
| SHBG (nmol/liter), mean (sd) | 41.8 (27.8) | 41.8 (28.8) | 0.89 |
17 OHP, 17-Hydroxyprogesterone; HbA1C, hemoglobin A1C; DHEAS, dehydroepiandrosterone sulfate.
Eighty-seven percent (74 of 85) of the participants had all 12 scheduled sessions with the acupuncturist. Ten women (12%) had 11 sessions, and one woman had only 10 sessions. The final session with the acupuncturist was sometimes purposefully canceled by the principal investigator to time the posttreatment 2.5-hour study visit for the early follicular phase.
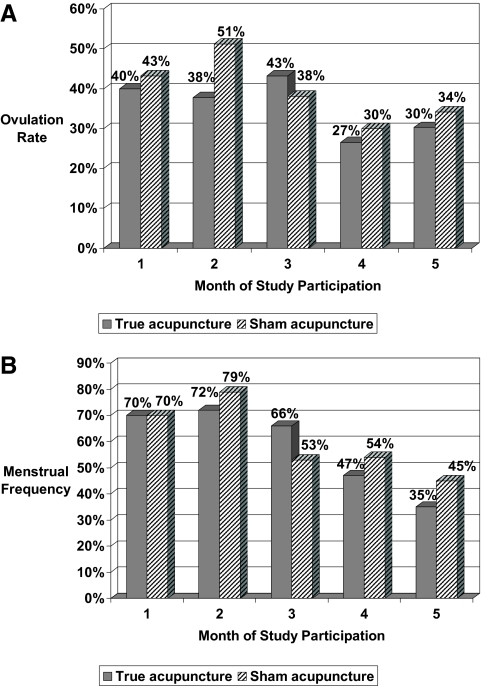
The monthly ovulation frequencies did not differ by intervention (Fig. 2A). The mean rate of ovulation over the 5-month protocol was 0.37 (95% confidence interval 0.29–0.46) in the true acupuncture group and 0.40 (95% confidence interval 0.32–0.49) in the sham acupuncture arm (P = 0.64). Both intervention arms experienced an improvement in the ratio of LH to FSH during the 8 wk of intervention (P < 0.04), and this persisted for the 3 months of follow-up in the acupuncture arm (P = 0.001, Table 3). There was no change in the FSH levels across time in either intervention arm (P > 0.11). LH declined during the intervention phase in both arms, although only significantly so in the sham arm (P = 0.04), and LH declined over the entire 5-month study time frame in both arms (P < 0.05). When the cohort was restricted to the women whose postintervention serum sampling could be documented to be in the early follicular menstrual phase (n = 38 true acupuncture, n = 43 sham acupuncture), there were two differences to the hormonal results reported above. First, the LH to FSH ratio reduction (from 1.8 to 1.4) in the true acupuncture arm during the intervention time frame did not quite reach statistical significance (P = 0.06, whereas P = 0.04 in the full cohort). Second, the decline in the LH level between the baseline and post-follow-up assessments (from 12.9 to 8.1 mIU/ml) was not significant in the sham arm (P = 0.10, whereas P = 0.048 in the full cohort).
Fig. 2.
A and B, Monthly ovulation (A) and menstrual (B) frequency by intervention arm and study month. Accup, Acupuncture.
Table 3.
Gonadotropin hormones across time by intervention arm (mean, sd)
| Measure | Preintervention baseline | After intervention | P value (vs. baseline) | Three-month follow-up | P value (vs. baseline) |
|---|---|---|---|---|---|
| True acupuncture | n = 38 | n = 38 | n = 30 | ||
| LH (mIU/ml) | 10.2 (10.7) | 7.0 (5.0) | 0.095 | 6.0 (3.6) | 0.004 |
| FSH (mIU/ml) | 5.2 (1.8) | 4.8 (1.6) | 0.197 | 4.7 (1.7) | 0.116 |
| LH to FSH ratio | 1.9 (1.2) | 1.4 (1.4) | 0.039 | 1.3 (0.7) | 0.001 |
| Sham acupuncture | n = 43 | n = 43 | n = 39 | ||
| LH | 12.9 (15.6) | 7.8 (6.1) | 0.037 | 8.1 (4.4) | 0.048 |
| FSH | 5.5 (1.5) | 5.8 (3.1) | 0.519 | 5.7 (2.9) | 0.772 |
| LH to FSH ratio | 2.2 (1.9) | 1.4 (1.1) | 0.019 | 1.5 (1.0) | 0.067 |
| P value (true vs. sham) | |||||
| LH | 0.364 | 0.604 | 0.040 | ||
| FSH | 0.394 | 0.077 | 0.094 | ||
| LH to FSH ratio | 0.360 | 0.876 | 0.276 | ||
Data were analyzed by way of linear mixed effects models for repeated measures. All comparisons were adjusted via analysis of covariance to a common baseline response.
In bivariate analyses (Table 4), a greater preenrollment annual menstrual frequency, lower free testosterone, and higher SHBG were suggestive of being predictive of the ovulation rate in both the acupuncture and sham arms (P < 0.10). Neither baseline BMI nor the percentage weight change during the 2 months of intervention was related to the ovulation rate in either arm (P > 0.25). Insulin was predictive of the mean ovulation within the true acupuncture group (P ≤ 0.05) but not the women in the sham arm. Partial correlations controlling for BMI and preenrollment menstrual frequency indicated that lower fasting serum insulin (P = 0.03) and lower free testosterone (P = 0.03) were highly correlated with a higher ovulation frequency within the acupuncture arm. Within the sham arm, corresponding partial correlations indicated a potential relationship with free testosterone and SHBG (P = 0.06 and P = 0.09, respectively, data not shown), and neither insulin measure was associated with the ovulation rate (P > 0.50).
Table 4.
Correlation between mean ovulation rate and potential predictors of response by intervention among women who completed the interventiona
| Factor | True acupuncture (n = 40), r (P value) | Sham acupuncture (n = 44), r (P value) | True acupuncture controlling for BMI and prestudy menstrual frequency (n = 38), r (P value) |
|---|---|---|---|
| Number of menses in most recent 12-month time period without hormonal medication | 0.28 (0.07) | 0.28 (0.07) | N/A |
| Free testosterone | −0.27 (0.09) | −0.37 (0.01) | −0.35 (0.03) |
| SHBG | 0.34 (0.03) | 0.32 (0.04) | 0.31 (0.07) |
| Fasting insulin (log transformed) | −0.37 (0.02) | NS | −0.35 (0.03) |
| AUC insulin (log transformed) | −0.31 (0.05) | NS | −0.31 (0.06) |
| Age | NS | 0.27 (0.08) | |
| DHEAS | NS | −0.35 (0.02) | |
| Testosterone | NS | −0.27 (0.07) |
Data were analyzed by way of the Spearman correlation and Spearman partial correlation. AUC, Area under the curve; DHEAS, dehydroepiandrosterone sulfate; NS, not significant.
Correlations of potential predictors of response with P < 0.10 are displayed.
The monthly rate of menses by the intervention arm is higher than the rate of ovulation (Fig. 2B, no statistical test was run), thus indicating that a proportion of the periods were anovulatory. Among those receiving true acupuncture, the overall frequency of periods during the 5-month study protocol (median 0.60/month, mean 0.56/month) was higher than the preenrollment menstrual frequency (median 0.50/month, mean 0.46/month), although this did not reach statistical significance (P = 0.066). Among those receiving sham acupuncture, the overall frequency of periods during the 5-month study protocol (median 0.60/month, mean 0.60/month) was higher than the preenrollment menstrual frequency (median 0.42/month, mean 0.40/month) and was statistically significant (P < 0.001).
Of the 45 participants who were eligible to become pregnant, defined as sexually active women who reported either no contraception or irregular use of contraception, seven women became pregnant during the 5-month trial. There was no difference by intervention (four of 20 = 20% in true acupuncture vs. three of 25 = 12% in the sham acupuncture, P = 0.68). An additional three women reported a pregnancy within 3 months after their study participation ended, all of whom were in the sham arm.
There were two adverse events reported in this trial. One woman fainted with the initial blood draw (for determination of eligibility); she was not allowed to enroll due to the repeated blood draws required in this protocol. A second woman reported back spasms during a true acupuncture session; subsequent evaluation by a physician outside the study team determined that the spasm was unrelated to the treatment. Neither event was deemed serious.
Discussion
Our investigation revealed that this acupuncture protocol and this sham protocol resulted in similar rates of ovulation and similar reduction in the LH to FSH ratio in women with PCOS. After controlling for BMI and preenrollment menstrual frequency, these data suggest that this acupuncture protocol was more effective for women with less severe metabolic disturbance (lower fasting insulin) or less androgen production (lower free testosterone) because neither of those factors were related to the ovulation rate among the participants in the sham arm.
Commentary on the equivalent effect of the sham acupuncture is warranted. Other studies of different diseases, published after this grant was funded, also failed to show greater benefit of true compared with sham acupuncture. For example, no difference was detected in several large scale RCT of acupuncture for chronic pain patients (24–27). [For publications on acupuncturists' and acupuncture researchers' interpretation of the acupuncture placebo/control group results, the reader is referred elsewhere (28, 29).] Using neuroimaging techniques, differential effects on the brain were demonstrated in true vs. sham acupuncture treatment of women with fibromyalgia, which indicated different biological pathways between the interventions (30). In our data, although the ovulation rate overall and by month did not differ between the study arms, two biological factors (fasting insulin and free testosterone) demonstrated strong inverse correlations with the ovulatory frequency in the true acupuncture arm but not the sham arm. The key unanswered question in this study is whether there was a different biological pathway underlying the two distinct interventions, which resulted in similar ovulation and gonadotropin responses.
Comparison with relevant acupuncture literature
Of the four prior studies of acupuncture in women with various menstrual/infertility diagnoses, the most relevant to this RCT was a Stener-Victorin single-arm study of 24 women with PCOS all of whom had 2 months of acupuncture (same acupuncture points as in this RCT) and 3 months of posttreatment follow-up (9). They reported a similar decline in the LH to FSH ratio (from 1.7 to 1.47, P = 0.04) and no significant change in LH or FSH. The responders in the cohort of Stener-Victorin et al. (9) were more likely to have lower BMI, lower baseline fasting insulin, lower total testosterone, and higher baseline SHBG than the treated nonresponders(P ≤ 0.01). Our data support their lower fasting insulin and lower free testosterone association, after controlling for BMI and preenrollment menstrual frequency.
More recently Jedel et al. conducted a three-arm RCT (16 wk of acupuncture vs. 16 wk of physical exercise vs. an observation only arm) (12). They reported an increase in the monthly ovulation rate from 28% at baseline to 69% after 16 wk of acupuncture in 24 women with PCOS using a different acupuncture protocol. They reported no change in the LH to FSH ratio and notable declines (−30%) in the circulating testosterone level in the acupuncture arm; neither of these latter observations is supported by our study (our testosterone data were not displayed and are available from the first author upon request).
Strengths and limitations
Our results beg the question as to whether both arms or neither arm experienced an improvement in ovulatory function. We are unable to definitively answer this question with our data, although the improvement in the LH to FSH ratio in both arms and the increase in menstrual frequency during the trial compared with preenrollment suggest that both interventions may have been beneficial. The most important study limitation is the lack of preintervention ovulation data in our population. The findings are additionally limited by the fact that the acupuncture protocol did not allow for individuation; thus, the findings are not reflective of real-world acupuncture practice. Although the protocol was not too different from what the acupuncturists might have used on their own (personal communication with the study acupuncturists), deviations in acupuncture point selection or the addition of moxibustion, for example, were not allowed. Therefore, the interpretation of the results is limited to this particular acupuncture protocol. Of a lesser magnitude, the operational design of the clinical trial lacked objective measures of acne and hirsutism, which might or might not have been informative.
The strengths of this study are the RCT design, with its prospective data collection and blinding of the intervention. This trial attained the target sample size, thus there was adequate statistical power for the primary ovulation aim. This study has the largest sample size of any prospective acupuncture study yet published with PCOS women. The cohort represented a wide range of ages and BMI, which improves the generalizability of our findings. The majority of the gonadotropin hormone assessments occurred in the early follicular phase, which is the time when interindividual differences would be minimized, thus reducing measurement bias. This study included PCOS women from the general community (approximately 40% had not been diagnosed with PCOS before study enrollment) as opposed to a clinic population with potentially more bothersome symptoms.
External validity
These findings are applicable to women with PCOS as diagnosed with Eunice Kennedy Shriver National Institute of Child Health and Human Development criteria. The impact of acupuncture treatment for women diagnosed with ultrasonic ovarian cysts is unknown and cannot be extrapolated from these results. Because a desire for pregnancy was not a prerequisite, these findings are applicable to women whether or not they are seeking pregnancy. Because both professional non-MD acupuncturists and MD/acupuncturists were study practitioners and no differential difference was observed in the results by practitioner, the findings should be generalizable, regardless of an acupuncturist's training within the traditional Chinese medicine framework.
Clinical significance and future directions
Women increasingly seek nonpharmaceutical options for fertility assistance. The demand for complementary/alternative medicine (CAM) remedies is not insignificant. In the United States, 29% of infertility patients had used a CAM modality for fertility treatment over a prospective 18-month time frame (6). In the United Kingdom, 23 and 40% of infertility patients in public and private clinics, respectively, had sought CAM for fertility purposes (8). The first line of treatment for PCOS women is clomiphene citrate and/or metformin if they are seeking pregnancy and oral contraceptives otherwise. For women who find these treatment options to be ineffective, insufficient, or unacceptable, nonpharmaceutical options would be well received if evidence showed efficacy. Perhaps other CAM modalities or other acupuncture protocols will offer potential solutions. This study suggests that this acupuncture regimen may be beneficial to women with lower fasting insulin and free testosterone levels. This is good news for women with PCOS who are seeking pregnancy because acupuncture may be a nonpharmaceutical option for them without any known fetal or multiple gestation risks. The safety of acupuncture is documented (31) and supported by few adverse events in this trial.
There is a clinical need for treatment options for women who are clomiphene resistant; thus, research on acupuncture treatment in this population would have high clinical value. It would also be informative to investigate the pathophysiology of both true and sham acupuncture. Not only is it confusing to the public to learn that a placebo is equivalent to an active intervention, but it also raises questions about the active intervention that may or may not be warranted.
Supplementary Material
Acknowledgments
We thank the clinical research coordinators (Parchayi Dalal and Virginia Hischman); the staff of the University of Virginia General Clinical Research Center; the study acupuncturists who are not a coauthor (Anne Smucker; David Groopman, MD; and Sallie Smithwick); the data safety monitor who also provided helpful suggestions on an earlier version of this article (Christopher McCartney, MD); and the women who volunteered to be a participant in this study.
This work was supported by Grant R21 AT002520 from the National Center for Complementary and Alternative Medicine at the National Institutes of Health and Grant M01RR000847 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, National Center for Complementary and Alternative Medicine, or the National Institutes of Health.
Disclosure Summary: No competing financial interests or conflicts of interest by the authors exist.
Footnotes
- BMI
- Body mass index
- CAM
- complementary/alternative medicine
- PCOS
- polycystic ovary syndrome
- PDG
- pregnanediol glucuronide
- RCT
- randomized clinical trial.
References
- 1. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. 2004. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749 [DOI] [PubMed] [Google Scholar]
- 2. Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. 2000. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab 85:2434–2438 [DOI] [PubMed] [Google Scholar]
- 3. Zawadzki J, Dunaif A.1992. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine F, Merriam GR. eds. The polycystic ovary syndrome. Cambridge, UK: Blackwell Scientific; 377–384 [Google Scholar]
- 4. Berga SL, Daniels TL. 1991. Use of the laboratory in disorders of reproductive neuroendocrinology. J Clin Immunoassay 14:23–28 [Google Scholar]
- 5. Legro R, Finegood D, Dunaif A. 1998. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinal Metab 83:2694–2698 [DOI] [PubMed] [Google Scholar]
- 6. Smith JF, Eisenberg ML, Millstein SG, Nachtigall RD, Shindel AW, Wing H, Cedars M, Pasch L, Katz PP. 2010. The use of complementary and alternative fertility treatment in couples seeking fertility care: data from a prospective cohort in the United States. Fertil Steril 93:2169–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stankiewicz M, Smith C, Alvino H, Norman R. 2007. The use of complementary medicine and therapies by patients attending a reproductive medicine unit in South Australia: a prospective survey. Aust N Z J Obstet Gynaecol 47:145–149 [DOI] [PubMed] [Google Scholar]
- 8. Coulson C, Jenkins J. 2005. Complementary and alternative medicine utilisation in NHS and private clinic settings: a United Kingdom survey of 400 infertility patients. J Exp Clin Assist Reprod 2:5–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stener-Victorin E, Waldenstrom U, Tagnfors U, Lundeberg T, Lindstedt G, Janson PO. 2000. Effects of electro-acupuncture on anovulation in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand 79:180–188 [PubMed] [Google Scholar]
- 10. Gerhard I, Postneek F. 1992. Auricular acupuncture in the treatment of female infertility. Gynecol Endocrinol 6:171–181 [DOI] [PubMed] [Google Scholar]
- 11. Mo X, Li D, Pu Y, Xi G, Le X, Fu Z. 1993. Clinical studies on the mechanism for acupuncture stimulation of ovulation. J Tradit Chin Med 13:115–119 [PubMed] [Google Scholar]
- 12. Jedel E, Labrie F, Odén A, Holm G, Nilsson L, Janson PO, Lind AK, Ohlsson C, Stener-Victorin E. 2011. Impact of electroacupuncture and exercise on hyperandrogenism and oligo/amenorrhoea in women with polycystic ovary syndrome: a randomized controlled trial. Am J Physiol Endocrinol Metab 300:E37–E45 [DOI] [PubMed] [Google Scholar]
- 13. MacPherson H, Altman DG, Hammerschlag R, Youping L, Taixiang W, White A, Moher D. 2010. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT Statement. J Evid Based Med 3:140–155 [DOI] [PubMed] [Google Scholar]
- 14. Pastore LM, Dalal P. 2009. Recruitment strategies for an acupuncture randomized clinical trial of reproductive age women. Complement Ther Med 17:229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collins JA. 2003. Revised consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 80:19–2614568284 [Google Scholar]
- 16. ARUP Laboratories lab guide. In: http://www.aruplab.com/guides/clt/tests/clt__212b.htm Salt Lake City, UT: ARUP Laboratories [Google Scholar]
- 17. Vermeulen A, Verdonck L, Kaufman JM. 1999. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672 [DOI] [PubMed] [Google Scholar]
- 18. Södergård R, Bäckström T, Shanbhag V, Carstensen H. 1982. Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma proteins at body temperature. J Steroid Biochem 16:801–810 [DOI] [PubMed] [Google Scholar]
- 19. Shepard MK, Balmaceda JP, Leija CG. 1979. Relationship of weight to successful induction of ovulation with clomiphene citrate. Fertil Steril 32:641–645 [DOI] [PubMed] [Google Scholar]
- 20. Park J, White A, Lee H, Ernst E. 1999. Development of a new sham needle. Acupunct Med 17:110–112 [Google Scholar]
- 21. Park J, White A, Stevinson C, Ernst E, James M. 2002. Validating a new non-penetrating sham acupuncture device: two randomised controlled trials. Acupunct Med 20:168–174 [DOI] [PubMed] [Google Scholar]
- 22. Helms J. 1995. Acupuncture energetics: a clinical approach for physicians. Berkeley, CA: Medical Acupuncture Publishers [Google Scholar]
- 23. National Heart, Lung, and Blood Institute 1998. Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults: the evidence report. Obes Res 6:51S–209S [PubMed] [Google Scholar]
- 24. Linde K, Streng A, Jürgens S, Hoppe A, Brinkhaus B, Witt C, Wagenpfeil S, Pfaffenrath V, Hammes MG, Weidenhammer W, Willich SN, Melchart D. 2005. Acupuncture for patients with migraine: a randomized controlled trial. JAMA 293:2118–2125 [DOI] [PubMed] [Google Scholar]
- 25. Melchart D, Streng A, Hoppe A, Brinkhaus B, Witt C, Wagenpfeil S, Pfaffenrath V, Hammes M, Hummelsberger J, Irnich D, Weidenhammer W, Willich SN, Linde K. 2005. Acupuncture in patients with tension-type headache: randomised controlled trial. BMJ 331:376–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris RE, Tian X, Williams DA, Tian TX, Cupps TR, Petzke F, Groner KH, Biswas P, Gracely RH, Clauw DJ. 2005. Treatment of fibromyalgia with formula acupuncture: investigation of needle placement, needle stimulation, and treatment frequency. J Altern Complement Med 11:663–671 [DOI] [PubMed] [Google Scholar]
- 27. Brinkhaus B, Witt CM, Jena S, Linde K, Streng A, Wagenpfeil S, Irnich D, Walther HU, Melchart D, Willich SN. 2006. Acupuncture in patients with chronic low back pain: a randomized controlled trial. Arch Intern Med 166:450–457 [DOI] [PubMed] [Google Scholar]
- 28. Kaptchuk TJ, Chen KJ, Song J. 2010. Recent clinical trials of acupuncture in the West: responses from the practitioners. Chin J Integr Med 16:197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Langevin HM, Wayne PM, MacPherson H, Schnyer R, Milley RM, Napadow V, Lao L, Park J, Harris RE, Cohen M, Sherman KJ, Haramati A, Hammerschlag R. 2011. Paradoxes in acupuncture research: strategies for moving forward. Evid Based Complement Alternat Med 10.1155/2011/180805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harris RE, Zubieta JK, Scott DJ, Napadow V, Gracely RH, Clauw DJ. 2009. Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on μ-opioid receptors (MORs). Neuroimage 47:1077–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. National Center for Complementary and Alternative Medicine Acupuncture: NCCAM information and resources package. In: /www.medicalacupuncture.org/acu_info/articles/nccaminfo.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.