Abstract
Context:
Nicotinic acid (NA), or niacin, lowers circulating levels of lipids, including triglycerides, very low-density lipoprotein-cholesterol, and low-density lipoprotein-cholesterol. The lipid-lowering effects have been attributed to its effect to inhibit lipolysis in adipocytes and thus lower plasma free fatty acid (FFA) level. However, evidence accumulates that the FFA-lowering effect may account for only a fraction of NA effects on plasma lipids, and other mechanisms may be involved. Recent studies have reported NA effects on gene expression in various tissues in vivo and in cultured cells in vitro.
Evidence Acquisition:
We reviewed articles reporting NA effects on gene expression, identified by searching PubMed, focusing on potential underlying mechanisms and implications for unexplained NA effects.
Conclusion:
The effects of NA on gene expression may be mediated directly via the NA receptor in the affected cells, indirectly via changes in circulating FFA or hormone levels induced by NA, or by activating the transcription factor FOXO1 in insulin-sensitive tissues. NA effects on gene expression provide new insights into previously unexplained NA effects, such as FFA-independent lipid-lowering effects, FFA rebound, and insulin resistance observed in clinics during NA treatment.
Nicotinic acid (NA), or niacin, is a B-group vitamin. In addition to its function as a vitamin, NA in high doses has been used as a lipid drug for five decades (1, 2). NA lowers circulating triglycerides (TG), very low-density lipoprotein (VLDL)-cholesterol, and low-density lipoprotein (LDL)-cholesterol levels while increasing high-density lipoprotein (HDL)-cholesterol (HDL-C) levels (3, 4). The lipid-lowering effects of NA have been attributed to its antilipolytic effect in adipocytes because this reduces plasma levels of free fatty acids (FFA), substrates for hepatic TG synthesis, and VLDL formation. However, circulating FFA levels rebound after long-term NA treatment, whereas its lipid-lowering effects persist (5). When this occurs, the hypolipidemic effects of NA may not be explained by reduced FFA delivery to the liver. In addition, the effect of NA to increase circulating HDL-C levels cannot be explained by the FFA-lowering effect of NA. Thus, despite a long history of clinical use, the precise mechanism by which NA modulates circulating lipids remains unclear. Also, insulin resistance often develops during NA treatment (6–8), but the underlying mechanism is unclear. We recently reported that NA has widespread effects on gene expression in major tissues of lipid metabolism, such as the liver, skeletal muscle, and adipose tissue (9), raising the possibility that the effects of NA on gene expression may explain some of the NA effects (wanted and unwanted) observed in clinical studies. In this review, we highlight literature data regarding NA effects on gene expression and discuss potential underlying mechanisms and implications for unexplained NA effects.
NA Action and Its Receptor
NA as a lipid drug
Dyslipidemias are major risk factors for cardiovascular disease, the number 1 cause of death in the United States. NA has been used as a drug for the treatment of dyslipidemias for five decades (1, 3). NA treatment results in a very desirable modification of atherogenic risk factors: decreases in VLDL-cholesterol, LDL-cholesterol, lipoprotein (a), and TG and increases in HDL-C (5, 10). Major clinical trials have demonstrated that NA treatment reduces the progression of atherosclerotic cardiovascular disease (3, 11). Despite these prominent beneficial effects and the fact that NA is both inexpensive and the most effective drug currently in use for increasing HDL-C (12), the use of NA has not been widespread because of its unwanted effects, such as the flush response (1, 3) and the development of insulin resistance. Clear understanding of the mechanisms underlying wanted and unwanted NA effects is critical to the development of a new strategy that maximizes the potentials of this class of drugs while eliminating unwanted side effects. Interest in NA has been rejuvenated by a recent discovery of its receptor (see NA receptor), which has enhanced our understanding of NA effects and has led to new strategies for treatment with NA or its derivatives. For example, partial agonists for the receptor are being developed as a drug that exerts the lipid-modifying effects of NA without causing the flush response (13, 14).
NA receptor
NA specifically binds to the plasma membrane of adipocytes via a specific G protein-coupled receptor (15, 16). In 2003, three independent groups discovered that the mouse orphan G protein-coupled receptor PUMA-G (protein up-regulated in macrophages by interferon γ) and its human ortholog HM74 are receptors for NA (17–19). These receptors (also known as GPR109) are expressed abundantly in adipose tissue but very little in other major tissues of lipid metabolism, such as the liver and skeletal muscle (18, 19). The NA receptor is coupled with the inhibitory G protein, and binding of NA to the receptor inhibits adenylate cyclase and thereby lowers cAMP levels (1). Decreased cAMP levels in turn reduce protein kinase A (PKA) activity, resulting in decreased phosphorylation and activity of hormone-sensitive lipase or other proteins involved in lipolysis, that is, hydrolysis of TG, in adipocytes.
Effects of NA on Gene Expression
In vivo studies
Recent studies have reported the effect of NA to alter gene expression in vivo in major tissues of lipid metabolism, such as the liver, adipose tissue, and skeletal muscle. NA treatment of obese dogs for 1 month was shown to decrease hepatic diacylglycerol O-acyltransferase (DGAT) 2 expression by approximately 60% (20). DGAT2 is a rate-limiting enzyme for hepatic TG synthesis (21), and decreased DGAT2 expression may explain NA-induced decreases in hepatic VLDL secretion and plasma lipoprotein levels in the absence of changes in plasma FFA levels. In addition, acute and chronic NA treatments in mice reduced hepatic expression of proliferator-activated receptor γ (PPARγ) coactivator (PGC)-1β and apolipoprotein C3 (APOC3) (22). In this study, PGC-1β was demonstrated to regulate plasma TG metabolism by increasing hepatic APOC3 expression and circulating APOC3 levels, suggesting that NA may lower plasma lipids by decreasing hepatic PGC-1β expression. NA also affects gene expression in vivo in adipocytes where the NA receptor is abundantly expressed. A 6-month treatment of patients with impaired glucose tolerance with extended-release (ER) NA led to a significant improvement of their atherogenic lipid profile, accompanied by changes in gene expression in adipose tissue, including increased adiponectin, C/EBPα, C/EBPδ, peroxisome PPARγ and decreased CPT2, hormone-sensitive lipase, NA receptor, and fatty-acid synthase mRNA expression, suggesting that some of the beneficial effects on lipid profiles may be due in part to altered gene expression (23). NA was shown to decrease circulating leptin concentrations in hypercholesterolemic rabbits, which was associated with a significant decrease in leptin mRNA expression in adipose tissue (24). The effect of NA to lower leptin mRNA expression was also demonstrated in cultured primary adipocytes where NA inhibited leptin expression and secretion and increased PPARγ expression in a dose-dependent manner (24). However, clinical studies also reported effects of acipimox, a long-acting NA analog, to increase circulating leptin levels (25, 26). Adiponectin, another adipokine, is known to exert atheroprotective effects and improve insulin action. Treatment of subjects with the metabolic syndrome with ER NA increased circulating adiponectin concentrations (26), primarily due to an increase in the active high-molecular weight complex (27, 28). Some of these effects may be due to increased adiponectin expression in adipose tissue, which increased by 35% after 6 months of ER NA treatment (23). NA was shown to acutely increase circulating IL-6 levels during exercise in humans, which was associated with increased IL-6 mRNA expression in adipose tissue (29). In skeletal muscle, NA infusion in humans acutely increased PPARα, PPARδ, and PGC-1α mRNA expression, and the effect on PGC-1α expression was suggested to be due to elevated epinephrine levels during NA infusion (30). Thus, numerous studies have demonstrated that NA treatment alters gene expression in vivo in various tissues. However, these in vivo studies are limited in testing whether the observed effects on gene expression represent direct effects of NA in the affected tissues or indirect effects mediated by changes in lipid or hormone levels induced by NA treatment.
In vitro studies
Direct effects of NA on gene expression have been studied in isolated cells. NA was shown to exert direct effects on adipokine expression in 3T3L1 adipocytes; NA inhibited TNF-α induction of proinflammatory chemokines, such as fractalkine, monocyte chemoattractant protein-1 and increased the expression of the atheroprotective adipokine, adiponectin, suggesting that some of the beneficial effects of NA on atherosclerosis and cardiovascular events may be mediated by these effects to regulate cytokine expression in adipocytes (31). NA was also shown to regulate monocyte adhesion to endothelial cells by altering the expression of cell-adhesion molecules in endothelial cells, such as ICAM-1 and PECAM-1, suggesting another potential mechanism underlying the beneficial effects of NA on atherogenesis (32, 33). Also, as discussed above, NA exerts direct effects to decrease leptin expression in primary adipocytes (24). ATP-binding cassette transporter A1 (ABCA1) plays a major role in the formation of HDL by transporting cellular cholesterol to lipid-poor apolipoprotein A-I (ApoA-I), a major apolipoprotein in HDL. NA was shown to promote ApoA-I-induced cholesterol efflux in adipocytes by increasing PPARγ, LXRα, and ABCA1 expression, revealing a potential mechanism by which NA increases circulating HDL-C levels (34). NA was also shown to increase PPARγ and ABCA1 expression in HepG2 cells and macrophages (35–37). In contrast, overexpression of the NA receptor GPR109A in mouse liver reduced ABCA1 expression, which was associated with decreased cholesterol efflux determined in primary hepatocytes (38).
Mechanisms of NA Effects on Gene Expression
NA receptor-mediated effects
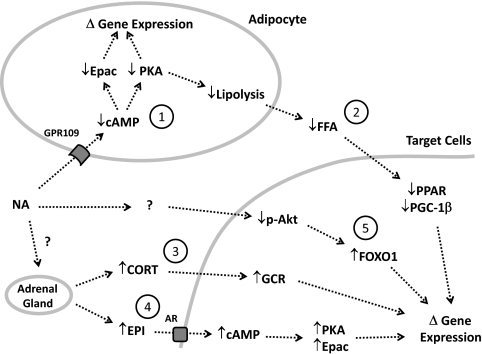
Many of the in vitro studies on the effects of NA to alter gene expression have demonstrated that activation of NA receptors was required for the effects. As discussed above (see NA receptor), activation of the NA receptor in adipocytes gives rise to an inhibitory G protein signal that inhibits adenylate cyclase and lowers cAMP levels. Decreased cAMP levels then alter the activities of the two intracellular cAMP targets, PKA and exchange protein activated by cAMP (Epac) (39). PKA and Epac are known to be involved in the regulation of expression for numerous genes, and therefore PKA- or Epac-dependent pathways may be involved in the NA effects on gene expression observed in vitro. In our recent study with microarray analysis (9), we found 121 genes whose expression was altered more than 4-fold by NA in vivo in rats. Interestingly, all of these changes in gene expression occurred in fat tissue, and none in the liver, heart, or skeletal muscle. This striking confinement of the 121 independent gene-expression changes to adipose tissue suggests the existence of NA-activated pathways for gene regulation exclusively in adipocytes (Fig. 1), which may be explained by the NA receptor that is expressed abundantly in adipose tissue but not in the other tissues (18, 19).
Fig. 1.
Potential mechanisms by which NA may alter gene expression in adipocytes and other target cells in vivo. Numbers represent individual pathways of NA effects. See the text for details. CORT, Cortisol or corticosterone; EPI, epinephrine; GCR, glucocorticoid receptor; AR, adrenergic receptor.
FFA-mediated effects
Many of the effects of NA on gene expression observed in vivo may be indirectly mediated by humoral changes induced by NA. NA has the potent effect of lowering plasma FFA level, and some of the NA effects on gene expression may be mediated by altered plasma FFA (22). FFA are endogenous ligands for PPAR transcription factors, which play a major role in the regulation of genes involved in fuel metabolism and cellular signaling (40, 41). FFA are also known to be major regulators of PGC-1β, which regulates genes involved in hepatic lipid metabolism (42). Because insulin also lowers plasma FFA level (by inhibiting lipolysis in adipocytes), some effects on gene expression may be shared by NA and insulin. In fact, our study with microarray analysis (9) identified over 100 genes whose expression was altered similarly by NA and insulin. These genes may represent those regulated by plasma FFA. This idea is supported by the finding that this group included leptin and uncoupling protein 3, which are known to be regulated by plasma FFA (43, 44). Interestingly, we found that lipoprotein lipase (LPL) was up-regulated in skeletal muscle when plasma FFA levels were reduced by insulin or NA. Increased LPL expression in this major tissue increases the rate of VLDL removal and thereby lowers circulating lipid levels (10, 45).
Hormone-mediated effects
Our study with microarray analysis (9) showed that a 7-h NA infusion in rats had profound effects on mRNA expression for numerous genes, and the number of genes whose mRNA expression was altered by NA greatly exceeded the number of genes affected by insulin in skeletal muscle, fat, and liver tissues. Insulin is known for its profound effects to regulate gene expression in various tissues (46). Therefore, it was rather unexpected that the number of genes affected by NA exceeded that of genes affected by insulin in all of the insulin-sensitive tissues. However, many of the NA effects on gene expression may not represent direct effects of NA in the affected tissues. As discussed above (see NA receptor), the NA receptor is expressed in adipose tissue, but not in other insulin-sensitive tissues (18, 19). In addition, NA infusion or treatment increases plasma levels of GH (47), epinephrine (30, 48), corticosterone (49), and adiponectin (26, 27), which are known to regulate gene expression and cellular signaling in various tissues. Therefore, it is conceivable that many of the NA effects on gene expression may be mediated by changes in these hormones during NA infusion. In the same context, some unwanted NA effects may arise from these hormonal changes during NA treatment (see FFA rebound and Insulin resistance).
FOXO1-mediated effects
Our study (9) also showed that NA infusion in rats had profound effects to decrease FOXO1 phosphorylation (via Akt) and increase its transcriptional activity in insulin-sensitive tissues, including the liver, skeletal muscle, heart, and adipose tissue. These effects were opposite to the well-established effects of insulin to increase FOXO1 phosphorylation and inhibit its activity (50). In the same study, random, systematic analysis of microarray data revealed a few dozen genes whose expression was altered in opposing manners by insulin and NA, in correlation with FOXO1 or Akt phosphorylation. Interestingly, most of these genes encode proteins involved in energy metabolism and cellular signaling, consistent with the roles of Akt or FOXO1. Many of these genes may be regulated by FOXO1 and/or Akt. To support this idea, the gene list included PDK4, glucose 6-phosphatase, and IGF binding protein, known to be regulated by FOXO1 (51); and fatty acid synthase, ATP citrate lyase, 3-hydroxy-3-methylglutaryl-coenzyme A, and cAMP-responsive element modulator, reported to be regulated by Akt (52). The mechanism by which NA alters Akt and FOXO1 phosphorylation in insulin-sensitive tissues is unknown. These effects may not represent direct effects of NA in the affected tissues because NA had no significant effects on Akt and FOXO1 phosphorylation in vitro in adipose tissue explants or primary hepatocytes (9). These findings suggest that an in vivo (humoral) factor might have been activated during NA infusion and mediated the effects on Akt and FOXO1 phosphorylation.
Implications of NA Effects on Gene Expression for Unexplained NA Effects
FFA-independent lipid-lowering effects
Fasting plasma FFA levels were lowered during the first week of oral NA treatment in humans but rebounded to pretreatment levels after 2 wk of the treatment (6). After 1-month treatment with NA in female subjects, plasma TG and total cholesterol levels were significantly lowered despite FFA rebound or increased fasting FFA levels (53), suggesting FFA-independent lipid-lowering effects. DGAT catalyzes the final and rate-limiting step of hepatic TG synthesis. This is an important process because TG is used for VLDL formation and regulates cholesterol synthesis (54, 55). There are two isoforms of DGAT: DGAT1 and DGAT2. DGAT1 is involved in the formation of cytosolic lipid droplets and DGAT2 in TG synthesis for VLDL formation in the endoplasmic reticulum (55). Knockdown of DGAT2 in the liver using small interfering RNA led to hypolipidemia, indicating that DGAT2 plays a crucial role in hepatic synthesis and secretion of TG and VLDL (56, 57). In HepG2 cells, NA was shown to directly and noncompetitively inhibit DGAT2 but not DGAT1 enzyme activity, without altering DGAT2 expression (58), suggesting that this effect may account for FFA-independent effects of NA to decrease hepatic TG synthesis and VLDL secretion (4). In addition, as discussed above, NA treatment was shown to reduce hepatic DGAT2 expression by approximately 60% in dogs (20), providing another potential mechanism for FFA-independent lipid-lowering effects of NA. Our recent study showed that NA infusion (7–12 h) in rats significantly decreased hepatic DGAT2 mRNA and protein expression without altering DGAT1 expression (Oh, Y. and J. Youn, unpublished data). Taken together, these data suggest that NA may decrease plasma lipids not only by lowering substrates (i.e. FFA) but also by reducing the activity and/or the expression of the key enzyme of hepatic TG synthesis. NA was also shown to accelerate intracellular apolipoprotein B degradation by inhibiting TG synthesis in HepG2 cells (59). On the other hand, FFA-independent lipid-lowering effects of NA may be explained in part by the effect of NA to increase LPL expression and/or activity; increased LPL activity would increase the rate of VLDL removal and thereby lower circulating lipid levels (10, 45). However, there are very little data demonstrating NA effects on LPL expression or activity.
NA is the most effective drug currently available for raising HDL-C, but the underlying mechanism was unclear until recently when this effect could be demonstrated in animal models (60, 61). Cholesteryl ester transfer protein (CETP) is a protein, mainly produced by the liver, that transfers lipids (i.e. TG and cholesterol esters) between LDL and HDL particles. The importance of this protein in HDL-C metabolism was recognized by the discovery of genetic CETP deficiency in Japanese families with increased HDL-C and ApoA-I levels (62). Since then, CETP inhibition has been a major therapeutic strategy for raising HDL-C. Rodents naturally lack CETP, resulting in lipid profiles that are different from those of humans and are not altered by treatments with lipid drugs as in humans (60). When human CETP was introduced into mice, their plasma lipid profiles were changed to be closer to those of humans, and NA was able to increase HDL-C, demonstrating a critical role of this protein in mediating the effect of NA to raise HDL-C (60). In mice expressing human CETP under control of its natural flanking regions, NA was shown to increase HDL-C by decreasing hepatic expression and plasma levels of CETP (61). Taken together, these data suggest that the effect of NA to decrease hepatic expression and secretion of CETP may be a major, if not the major, mechanism by which NA raises HDL-C in humans. In addition, NA may increase plasma HDL-C by increasing ABCA1 expression, thereby increasing cholesterol efflux from various cells, including adipocytes and hepatocytes, as demonstrated in vitro (34–37). NA was also shown to inhibit surface expression of ATP synthase β chain, a receptor for HDL endocytosis, in HepG2 cells, associated with decreased HDL uptake (63), which may contribute to NA action to raise HDL. However, this effect occurred without changes in mRNA expression.
FFA rebound
Acute NA administration in humans (53) or rodents (49) results in a rapid decrease in the plasma FFA level, followed by a rebound and an overshoot above preinfusion levels. The rebound or overshoot of the plasma FFA level is probably due to the combination of waning antilipolytic effects of ingested or injected NA and stimulation of lipolysis by lipolytic hormones, such as epinephrine and cortisol, plasma levels of which increase after NA administration (30, 48, 49). Chronic treatment with NA is associated with increased basal (or fasting) FFA levels (8, 64). This may occur as a result of an overshoot of plasma FFA after NA administration the previous evening (65). However, when type 2 diabetic patients were treated extensively (i.e. with high and frequent dosages) for 3 d with acipimox, a long-acting NA analog, plasma FFA levels remained suppressed during the treatment period, but mean FFA levels gradually increased from d 1 to d 3 (65). These data suggest that slowly developing changes occur in adipocytes that increase lipolysis during repetitive or continuous exposure to acipimox (or NA). Consistent with this idea, fasting plasma FFA levels decreased in healthy subjects during the first week of NA treatment, but rebounded to control levels after 2 wk (6), and increased fasting FFA levels have been observed after 2 wk or more of NA treatment (8, 64).
The mechanisms underlying FFA rebound during chronic NA treatment have been unclear, in part due to a lack of appropriate animal models. Our recent studies showed that when NA was constantly infused in rats, the ability of NA to lower plasma FFA was maintained for at least 7 h (9), but when this infusion was extended to 24 h, plasma FFA levels rebounded to control levels (66). This was not due to a down-regulation of NA action because when the NA infusion was stopped, plasma FFA levels rapidly increased by more than 2-fold, indicating that basal lipolysis was increased. In the same study, microarray analysis revealed that the FFA rebound after 24-h NA infusion was associated with many changes in gene expression in adipocytes. Among these changes were 40–60% decreases in the expression of key enzymes of TG synthesis, including 1-acylglycerol-3-phosphate O-acyltransferase, DGAT1 and -2, GPD1, and PCK1. Decreased TG synthesis would help increase the net rate of lipolysis, that is, TG degradation into FFA and glycerol. In addition, perilipin expression decreased by approximately 40%. Perilipin coats lipid droplets in adipocytes and suppresses lipolysis by reducing lipase accessibility to the surface of lipid droplets (67). Therefore, decreased perilipin expression would contribute to the increase in lipolysis. Furthermore, phosphodiesterase (PDE) 3B gene expression decreased by approximately 60%. This change was specific because genes for other PDE isoforms were not affected. PDE3B degrades cAMP, a major regulator of lipolysis in adipocytes, and decreased PDE3B would help increase the cAMP level and thus lipolysis. Thus, these (and possibly other) changes in gene expression in adipocytes are likely to explain the increase in basal lipolysis in adipocytes (FFA rebound) after 24-h NA infusion in rats. Although a 24-h NA infusion in rats may be an excellent animal model in which to study FFA rebound, it may be crucial to test whether this acute model well represents similar phenomena observed during chronic oral NA treatments in humans. Because fat tissue sampling is feasible in humans, gene-expression analysis in fat tissue with or without NA treatment would provide answers to this critical question.
Insulin resistance
Insulin resistance often develops to deteriorate glucose tolerance during NA treatment, raising the concern of whether NA treatment is safe in glucose intolerant or diabetic patients despite its beneficial effects on lipid profile (thus reducing cardiovascular risk) (68, 69). The mechanism by which NA treatment results in insulin resistance remains unclear. As discussed above, changes in plasma adiponectin (26–28) levels cannot explain the development of insulin resistance during NA treatment. The possibility remains to be tested whether NA-induced increases in insulin antagonizing hormones, such as cortisol (49), epinephrine (30, 48), and GH (47), may cause insulin resistance. Previous studies have debated whether insulin resistance develops during NA treatment as a result of FFA rebound (7, 8). It is established that the plasma FFA level is a major determinant of insulin sensitivity (70); acute or chronic elevation of plasma FFA decreases insulin's action (71, 72). Lowering plasma FFA using NA or its analog acipimox for a short term (hours or days) has been shown to increase insulin sensitivity (73–75). In contrast, long-term treatments with NA have resulted in insulin resistance, often associated with increased basal FFA levels (8, 64), suggesting that FFA rebound and increased basal plasma FFA levels may be responsible for the development of insulin resistance. However, insulin resistance was observed during NA treatment even without increased basal FFA levels (6, 7), suggesting that other mechanisms may be involved. Our recent study showed that a continuous 24-h NA infusion in rats increased basal lipolysis in adipose tissue and basal plasma FFA levels by altering the expression of genes regulating lipolysis in adipocytes (66). If FFA rebound is responsible for insulin resistance during NA treatment, altered gene expression in adipocytes may drive the increases in adipocyte lipolysis and plasma FFA levels, resulting in insulin resistance. On the other hand, if insulin resistance develops independently of plasma FFA, there is the possibility that NA-induced changes in gene expression in other insulin-sensitive tissues, demonstrated in our recent study (9), may be responsible for the development of insulin resistance. Regarding this, the effect of NA to activate FOXO1 in insulin-sensitive tissues (9) may deserve attention from investigators because some FOXO1 target genes (e.g. PDK4, glucose 6-phosphatase, phosphoenolpyruvate carboxykinase, PGC-1, etc.) have been implicated in the regulation of blood-glucose control and/or insulin sensitivity (76–78).
Conclusion and Perspectives
The literature data reviewed here indicate that NA does more than merely decrease plasma FFA levels or suppress lipolysis in adipocytes. There is ample evidence that NA acutely or chronically alters gene expression and cellular signaling in various tissues and cells. The underlying mechanism may involve signaling through the NA receptor, but there is substantial evidence that many in vivo effects on gene expression may be mediated indirectly by changes in circulating lipid or hormone levels induced by NA treatment. Future studies are warranted to elucidate these mechanisms, which would contribute not only to the full understanding of NA effects (wanted and unwanted), but also to the discovery of novel pathways for gene regulation by lipids or hormones. Finally, NA has also been used as a tool for lowering plasma FFA (assuming that this is the only effect of NA) in studies on the role of plasma FFA. Data from such studies should be carefully interpreted because there are many ways NA could affect the target system under study.
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (MEST; no. 2010-0016115; to I.K.) and National Institutes of Health Grant DK090749 (to J.H.Y.).
Disclosure Summary: We have no conflicts of interest to disclose in connection with the manuscript.
Footnotes
- ABCA1
- ATP-binding cassette transporter A1
- ApoA-I
- apolipoprotein A-I
- APOC3
- apolipoprotein C3
- CETP
- cholesteryl ester transfer protein
- DGAT
- diacylglycerol O-acyltransferase
- Epac
- exchange protein activated by cAMP
- ER
- extended-release
- FFA
- free fatty acid
- HDL
- high-density lipoprotein
- HDL-C
- HDL-cholesterol
- LDL
- low-density lipoprotein
- LPL
- lipoprotein lipase
- NA
- nicotinic acid
- PDE
- phosphodiesterase
- PGC
- PPAR γ coactivator
- PKA
- protein kinase A
- PPAR
- peroxisome proliferator-activated receptor
- TG
- triglyceride
- VLDL
- very low-density lipoprotein.
References
- 1. Bodor ET, Offermanns S. 2008. Nicotinic acid: an old drug with a promising future. Br J Pharmacol 153(Suppl 1):S68–S75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karpe F, Frayn KN. 2004. The nicotinic acid receptor—a new mechanism for an old drug. Lancet 363:1892–1894 [DOI] [PubMed] [Google Scholar]
- 3. Carlson LA. 2005. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med 258:94–114 [DOI] [PubMed] [Google Scholar]
- 4. Kamanna VS, Kashyap ML. 2008. Mechanism of action of niacin. Am J Cardiol 101:20B–26B [DOI] [PubMed] [Google Scholar]
- 5. Walldius G, Wahlberg G. 1985. Effects of nicotinic acid and its derivatives on lipid metabolism and other metabolic factors related to atherosclerosis. Adv Exp Med Biol 183:281–293 [DOI] [PubMed] [Google Scholar]
- 6. Alvarsson M, Grill V. 1996. Impact of nicotinic acid treatment on insulin secretion and insulin sensitivity in low and high insulin responders. Scand J Clin Lab Invest 56:563–570 [DOI] [PubMed] [Google Scholar]
- 7. Kelly JJ, Lawson JA, Campbell LV, Storlien LH, Jenkins AB, Whitworth JA, O'Sullivan AJ. 2000. Effects of nicotinic acid on insulin sensitivity and blood pressure in healthy subjects. J Hum Hypertens 14:567–572 [DOI] [PubMed] [Google Scholar]
- 8. Poynten AM, Gan SK, Kriketos AD, O'Sullivan A, Kelly JJ, Ellis BA, Chisholm DJ, Campbell LV. 2003. Nicotinic acid-induced insulin resistance is related to increased circulating fatty acids and fat oxidation but not muscle lipid content. Metabolism 52:699–704 [DOI] [PubMed] [Google Scholar]
- 9. Choi S, Yoon H, Oh KS, Oh YT, Kim YI, Kang I, Youn JH. 2011. Widespread effects of nicotinic acid on gene expression in insulin-sensitive tissues: implications for unwanted effects of nicotinic acid treatment. Metabolism 60:134–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drood JM, Zimetbaum PJ, Frishman WH. 1991. Nicotinic acid for the treatment of hyperlipoproteinemia. J Clin Pharmacol 31:641–650 [DOI] [PubMed] [Google Scholar]
- 11. Guyton JR, Capuzzi DM. 1998. Treatment of hyperlipidemia with combined niacin-statin regimens. Am J Cardiol 82:82U–84U; discussion 85U–86U [DOI] [PubMed] [Google Scholar]
- 12. Drexel H. 2009. Statins, fibrates, nicotinic acid, cholesterol absorption inhibitors, anion-exchange resins, omega-3 fatty acids: which drugs for which patients? Fundam Clin Pharmacol 23:687–692 [DOI] [PubMed] [Google Scholar]
- 13. Semple G, Skinner PJ, Gharbaoui T, Shin YJ, Jung JK, Cherrier MC, Webb PJ, Tamura SY, Boatman PD, Sage CR, Schrader TO, Chen R, Colletti SL, Tata JR, Waters MG, Cheng K, Taggart AK, Cai TQ, Carballo-Jane E, Behan DP, Connolly DT, Richman JG. 2008. 3-(1H-tetrazol-5-yl)-1,4,5,6-tetrahydro-cyclopentapyrazole (MK-0354): a partial agonist of the nicotinic acid receptor, G-protein coupled receptor 109a, with antilipolytic but no vasodilatory activity in mice. J Med Chem 51:5101–5108 [DOI] [PubMed] [Google Scholar]
- 14. Shen HC, Colletti SL. 2009. Novel patent publications on high-affinity nicotinic acid receptor agonists. Expert Opin Ther Pat 19:957–967 [DOI] [PubMed] [Google Scholar]
- 15. Aktories K, Schultz G, Jakobs KH. 1980. Regulation of adenylate cyclase activity in hamster adipocytes. Inhibition by prostaglandins, α-adrenergic agonists and nicotinic acid. Naunyn Schmiedebergs Arch Pharmacol 312:167–173 [DOI] [PubMed] [Google Scholar]
- 16. Lorenzen A, Stannek C, Lang H, Andrianov V, Kalvinsh I, Schwabe U. 2001. Characterization of a G protein-coupled receptor for nicotinic acid. Mol Pharmacol 59:349–357 [DOI] [PubMed] [Google Scholar]
- 17. Soga T, Kamohara M, Takasaki J, Matsumoto S, Saito T, Ohishi T, Hiyama H, Matsuo A, Matsushime H, Furuichi K. 2003. Molecular identification of nicotinic acid receptor. Biochem Biophys Res Commun 303:364–369 [DOI] [PubMed] [Google Scholar]
- 18. Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S. 2003. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med 9:352–355 [DOI] [PubMed] [Google Scholar]
- 19. Wise A, Foord SM, Fraser NJ, Barnes AA, Elshourbagy N, Eilert M, Ignar DM, Murdock PR, Steplewski K, Green A, Brown AJ, Dowell SJ, Szekeres PG, Hassall DG, Marshall FH, Wilson S, Pike NB. 2003. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem 278:9869–9874 [DOI] [PubMed] [Google Scholar]
- 20. Le Bloc'h J, Leray V, Chetiveaux M, Freuchet B, Magot T, Krempf M, Nguyen P, Ouguerram K. 2010. Nicotinic acid decreases apolipoprotein B100-containing lipoprotein levels by reducing hepatic very low density lipoprotein secretion through a possible diacylglycerol acyltransferase 2 inhibition in obese dogs. J Pharmacol Exp Ther 334:583–589 [DOI] [PubMed] [Google Scholar]
- 21. Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, Farese RV., Jr 2004. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem 279:11767–11776 [DOI] [PubMed] [Google Scholar]
- 22. Hernandez C, Molusky M, Li Y, Li S, Lin JD. 2010. Regulation of hepatic ApoC3 expression by PGC-1β mediates hypolipidemic effect of nicotinic acid. Cell Metab 12:411–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Linke A, Sonnabend M, Fasshauer M, Höllriegel R, Schuler G, Niebauer J, Stumvoll M, Blüher M. 2009. Effects of extended-release niacin on lipid profile and adipocyte biology in patients with impaired glucose tolerance. Atherosclerosis 205:207–213 [DOI] [PubMed] [Google Scholar]
- 24. Yang J, Zhao SP, Li J, Dong SZ. 2008. Effect of niacin on adipocyte leptin in hypercholesterolemic rabbits. Cardiovasc Pathol 17:219–225 [DOI] [PubMed] [Google Scholar]
- 25. Worm D, Vinten J, Vaag A, Henriksen JE, Beck-Nielsen H. 2000. The nicotinic acid analogue acipimox increases plasma leptin and decreases free fatty acids in type 2 diabetic patients. Eur J Endocrinol 143:389–395 [DOI] [PubMed] [Google Scholar]
- 26. Westphal S, Borucki K, Taneva E, Makarova R, Luley C. 2007. Extended-release niacin raises adiponectin and leptin. Atherosclerosis 193:361–365 [DOI] [PubMed] [Google Scholar]
- 27. Plaisance EP, Grandjean PW, Brunson BL, Judd RL. 2008. Increased total and high-molecular weight adiponectin after extended-release niacin. Metabolism 57:404–409 [DOI] [PubMed] [Google Scholar]
- 28. Westphal S, Luley C. 2008. Preferential increase in high-molecular weight adiponectin after niacin. Atherosclerosis 198:179–183 [DOI] [PubMed] [Google Scholar]
- 29. Holmes AG, Watt MJ, Febbraio MA. 2004. Suppressing lipolysis increases interleukin-6 at rest and during prolonged moderate-intensity exercise in humans. J Appl Physiol 97:689–696 [DOI] [PubMed] [Google Scholar]
- 30. Watt MJ, Holmes AG, Steinberg GR, Mesa JL, Kemp BE, Febbraio MA. 2004. Reduced plasma FFA availability increases net triacylglycerol degradation, but not GPAT or HSL activity, in human skeletal muscle. Am J Physiol Endocrinol Metab 287:E120–E127 [DOI] [PubMed] [Google Scholar]
- 31. Digby JE, McNeill E, Dyar OJ, Lam V, Greaves DR, Choudhury RP. 2010. Anti-inflammatory effects of nicotinic acid in adipocytes demonstrated by suppression of fractalkine, RANTES, and MCP-1 and upregulation of adiponectin. Atherosclerosis 209:89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tavintharan S, Lim SC, Sum CF. 2009. Effects of niacin on cell adhesion and early atherogenesis: biochemical and functional findings in endothelial cells. Basic Clin Pharmacol Toxicol 104:206–210 [DOI] [PubMed] [Google Scholar]
- 33. Tavintharan S, Woon K, Pek LT, Jauhar N, Dong X, Lim SC, Sum CF. 2011. Niacin results in reduced monocyte adhesion in patients with type 2 diabetes mellitus. Atherosclerosis 215:176–179 [DOI] [PubMed] [Google Scholar]
- 34. Wu ZH, Zhao SP. 2009. Niacin promotes cholesterol efflux through stimulation of the PPARγ-LXRα-ABCA1 pathway in 3T3–L1 adipocytes. Pharmacology 84:282–287 [DOI] [PubMed] [Google Scholar]
- 35. Siripurkpong P, Na-Bangchang K. 2009. Effects of niacin and chromium on the expression of ATP-binding cassette transporter A1 and apolipoprotein A-1 genes in HepG2 cells. J Nutr Biochem 20:261–268 [DOI] [PubMed] [Google Scholar]
- 36. Rubic T, Trottmann M, Lorenz RL. 2004. Stimulation of CD36 and the key effector of reverse cholesterol transport ATP-binding cassette A1 in monocytoid cells by niacin. Biochem Pharmacol 67:411–419 [DOI] [PubMed] [Google Scholar]
- 37. Knowles HJ, te Poele RH, Te Poole R, Workman P, Harris AL. 2006. Niacin induces PPARγ expression and transcriptional activation in macrophages via HM74 and HM74a-mediated induction of prostaglandin synthesis pathways. Biochem Pharmacol 71:646–656 [DOI] [PubMed] [Google Scholar]
- 38. Li X, Millar JS, Brownell N, Briand F, Rader DJ. 2010. Modulation of HDL metabolism by the niacin receptor GPR109A in mouse hepatocytes. Biochem Pharmacol 80:1450–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gloerich M, Bos JL. 2010. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 50:355–375 [DOI] [PubMed] [Google Scholar]
- 40. Grimaldi PA. 2007. Peroxisome proliferator-activated receptors as sensors of fatty acids and derivatives. Cell Mol Life Sci 64:2459–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nakamura MT, Cheon Y, Li Y, Nara TY. 2004. Mechanisms of regulation of gene expression by fatty acids. Lipids 39:1077–1083 [DOI] [PubMed] [Google Scholar]
- 42. Lin J, Tarr PT, Yang R, Rhee J, Puigserver P, Newgard CB, Spiegelman BM. 2003. PGC-1β in the regulation of hepatic glucose and energy metabolism. J Biol Chem 278:30843–30848 [DOI] [PubMed] [Google Scholar]
- 43. Nisoli E, Vettor R, Tonello C, Macor C, Federspil G, Carruba MO. 1999. Nutrient channelling-regulated peroxisome proliferator-activated receptor-γ-2 (PPARγ-2) and leptin gene expression in human subcutaneous fat. Diabetologia 42:495–497 [DOI] [PubMed] [Google Scholar]
- 44. Samec S, Seydoux J, Dulloo AG. 1999. Skeletal muscle UCP3 and UCP2 gene expression in response to inhibition of free fatty acid flux through mitochondrial β-oxidation. Pflugers Arch 438:452–457 [DOI] [PubMed] [Google Scholar]
- 45. Capurso A. 1991. Drugs affecting triglycerides. Cardiology 78:218–225 [DOI] [PubMed] [Google Scholar]
- 46. O'Brien RM, Streeper RS, Ayala JE, Stadelmaier BT, Hornbuckle LA. 2001. Insulin-regulated gene expression. Biochem Soc Trans 29:552–558 [DOI] [PubMed] [Google Scholar]
- 47. Quabbe HJ, Luyckx AS, L'Age M, Schwarz C. 1983. Growth hormone, cortisol, and glucagon concentrations during plasma free fatty acid depression: different effects of nicotinic acid and an adenosine derivative (BM 11.189). J Clin Endocrinol Metab 57:410–414 [DOI] [PubMed] [Google Scholar]
- 48. O'Neill M, Watt MJ, Heigenhauser GJ, Spriet LL. 2004. Effects of reduced free fatty acid availability on hormone-sensitive lipase activity in human skeletal muscle during aerobic exercise. J Appl Physiol 97:1938–1945 [DOI] [PubMed] [Google Scholar]
- 49. Pereira JN. 1967. The plasma free fatty acid rebound induced by nicotinic acid. J Lipid Res 8:239–244 [PubMed] [Google Scholar]
- 50. Tran H, Brunet A, Griffith EC, Greenberg ME. 2003. The many forks in FOXO's road. Sci STKE 2003:RE5. [DOI] [PubMed] [Google Scholar]
- 51. Lam EW, Francis RE, Petkovic M. 2006. FOXO transcription factors: key regulators of cell fate. Biochem Soc Trans 34:722–726 [DOI] [PubMed] [Google Scholar]
- 52. Porstmann T, Griffiths B, Chung YL, Delpuech O, Griffiths JR, Downward J, Schulze A. 2005. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene 24:6465–6481 [DOI] [PubMed] [Google Scholar]
- 53. Wang W, Basinger A, Neese RA, Christiansen M, Hellerstein MK. 2000. Effects of nicotinic acid on fatty acid kinetics, fuel selection, and pathways of glucose production in women. Am J Physiol Endocrinol Metab 279:E50–E59 [DOI] [PubMed] [Google Scholar]
- 54. Dixon JL, Ginsberg HN. 1993. Regulation of hepatic secretion of apolipoprotein B-containing lipoproteins: information obtained from cultured liver cells. J Lipid Res 34:167–179 [PubMed] [Google Scholar]
- 55. Owen MR, Corstorphine CC, Zammit VA. 1997. Overt and latent activities of diacylglycerol acytransferase in rat liver microsomes: possible roles in very-low-density lipoprotein triacylglycerol secretion. Biochem J 323:17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yu XX, Murray SF, Pandey SK, Booten SL, Bao D, Song XZ, Kelly S, Chen S, McKay R, Monia BP, Bhanot S. 2005. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology 42:362–371 [DOI] [PubMed] [Google Scholar]
- 57. Liu Y, Millar JS, Cromley DA, Graham M, Crooke R, Billheimer JT, Rader DJ. 2008. Knockdown of acyl-CoA:diacylglycerol acyltransferase 2 with antisense oligonucleotide reduces VLDL TG and ApoB secretion in mice. Biochim Biophys Acta 1781:97–104 [DOI] [PubMed] [Google Scholar]
- 58. Ganji SH, Tavintharan S, Zhu D, Xing Y, Kamanna VS, Kashyap ML. 2004. Niacin noncompetitively inhibits DGAT2 but not DGAT1 activity in HepG2 cells. J Lipid Res 45:1835–1845 [DOI] [PubMed] [Google Scholar]
- 59. Jin FY, Kamanna VS, Kashyap ML. 1999. Niacin accelerates intracellular ApoB degradation by inhibiting triacylglycerol synthesis in human hepatoblastoma (HepG2) cells. Arterioscler Thromb Vasc Biol 19:1051–1059 [DOI] [PubMed] [Google Scholar]
- 60. Hernandez M, Wright SD, Cai TQ. 2007. Critical role of cholesterol ester transfer protein in nicotinic acid-mediated HDL elevation in mice. Biochem Biophys Res Commun 355:1075–1080 [DOI] [PubMed] [Google Scholar]
- 61. van der Hoorn JW, de Haan W, Berbée JF, Havekes LM, Jukema JW, Rensen PC, Princen HM. 2008. Niacin increases HDL by reducing hepatic expression and plasma levels of cholesteryl ester transfer protein in APOE*3Leiden.CETP mice. Arterioscler Thromb Vasc Biol 28:2016–2022 [DOI] [PubMed] [Google Scholar]
- 62. Inazu A, Brown ML, Hesler CB, Agellon LB, Koizumi J, Takata K, Maruhama Y, Mabuchi H, Tall AR. 1990. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N Engl J Med 323:1234–1238 [DOI] [PubMed] [Google Scholar]
- 63. Zhang LH, Kamanna VS, Zhang MC, Kashyap ML. 2008. Niacin inhibits surface expression of ATP synthase β chain in HepG2 cells: implications for raising HDL. J Lipid Res 49:1195–1201 [DOI] [PubMed] [Google Scholar]
- 64. Rasouli N, Hale T, Kahn SE, Spencer HJ, Elbein SC. 2005. Effects of short-term experimental insulin resistance and family history of diabetes on pancreatic β-cell function in nondiabetic individuals. J Clin Endocrinol Metab 90:5825–5833 [DOI] [PubMed] [Google Scholar]
- 65. Worm D, Henriksen JE, Vaag A, Thye-Rønn P, Melander A, Beck-Nielsen H. 1994. Pronounced blood glucose-lowering effect of the antilipolytic drug acipimox in noninsulin-dependent diabetes mellitus patients during a 3-day intensified treatment period. J Clin Endocrinol Metab 78:717–721 [DOI] [PubMed] [Google Scholar]
- 66. Oh YT, Oh KS, Choi YM, Jokiaho A, Donovan C, Choi S, Kang I, Youn JH. 2011. Continuous 24-h nicotinic acid infusion in rats causes FFA rebound and insulin resistance by altering gene expression and basal lipolysis in adipose tissue. Am J Physiol Endocrinol Metab 300:E1012–E1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR, Londos C. 2001. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA 98:6494–6499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ginsberg HN. 2006. Niacin in the metabolic syndrome: more risk than benefit? Nat Clin Pract Endocrinol Metab 2:300–301 [DOI] [PubMed] [Google Scholar]
- 69. Rosenson RS. 2007. Measure for measure—sugar or fats? Reconciling cardiovascular and diabetes risk with niacin therapy. Nat Clin Pract Endocrinol Metab 3:72–73 [DOI] [PubMed] [Google Scholar]
- 70. Bergman RN, Ader M. 2000. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab 11:351–356 [DOI] [PubMed] [Google Scholar]
- 71. Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. 1996. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97:2859–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim JK, Wi JK, Youn JH. 1996. Metabolic impairment precedes insulin resistance in skeletal muscle during high-fat feeding in rats. Diabetes 45:651–658 [DOI] [PubMed] [Google Scholar]
- 73. Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL. 1999. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 48:1836–1841 [DOI] [PubMed] [Google Scholar]
- 74. Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA. 2005. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes 54:3148–3153 [DOI] [PubMed] [Google Scholar]
- 75. Cusi K, Kashyap S, Gastaldelli A, Bajaj M, Cersosimo E. 2007. Effects on insulin secretion and insulin action of a 48-h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Endocrinol Metab 292:E1775–E1781 [DOI] [PubMed] [Google Scholar]
- 76. Majer M, Popov KM, Harris RA, Bogardus C, Prochazka M. 1998. Insulin downregulates pyruvate dehydrogenase kinase (PDK) mRNA: potential mechanism contributing to increased lipid oxidation in insulin-resistant subjects. Mol Genet Metab 65:181–186 [DOI] [PubMed] [Google Scholar]
- 77. Altomonte J, Richter A, Harbaran S, Suriawinata J, Nakae J, Thung SN, Meseck M, Accili D, Dong H. 2003. Inhibition of Foxo1 function is associated with improved fasting glycemia in diabetic mice. Am J Physiol Endocrinol Metab 285:E718–E728 [DOI] [PubMed] [Google Scholar]
- 78. Daitoku H, Yamagata K, Matsuzaki H, Hatta M, Fukamizu A. 2003. Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes 52:642–649 [DOI] [PubMed] [Google Scholar]