Abstract
Context:
The American Heart Association Nutrition Committee recommends women and men consume no more than 100 and 150 kcal of added sugar per day, respectively, whereas the Dietary Guidelines for Americans, 2010, suggests a maximal added sugar intake of 25% or less of total energy.
Objective:
To address this discrepancy, we compared the effects of consuming glucose, fructose, or high-fructose corn syrup (HFCS) at 25% of energy requirements (E) on risk factors for cardiovascular disease.
Participants, Design and Setting, and Intervention:
Forty-eight adults (aged 18–40 yr; body mass index 18–35 kg/m2) resided at the Clinical Research Center for 3.5 d of baseline testing while consuming energy-balanced diets containing 55% E complex carbohydrate. For 12 outpatient days, they consumed usual ad libitum diets along with three servings per day of glucose, fructose, or HFCS-sweetened beverages (n = 16/group), which provided 25% E requirements. Subjects then consumed energy-balanced diets containing 25% E sugar-sweetened beverages/30% E complex carbohydrate during 3.5 d of inpatient intervention testing.
Main Outcome Measures:
Twenty-four-hour triglyceride area under the curve, fasting plasma low-density lipoprotein (LDL), and apolipoprotein B (apoB) concentrations were measured.
Results:
Twenty-four-hour triglyceride area under the curve was increased compared with baseline during consumption of fructose (+4.7 ± 1.2 mmol/liter × 24 h, P = 0.0032) and HFCS (+1.8 ± 1.4 mmol/liter × 24 h, P = 0.035) but not glucose (−1.9 ± 0.9 mmol/liter × 24 h, P = 0.14). Fasting LDL and apoB concentrations were increased during consumption of fructose (LDL: +0.29 ± 0.082 mmol/liter, P = 0.0023; apoB: +0.093 ± 0.022 g/liter, P = 0.0005) and HFCS (LDL: +0.42 ± 0.11 mmol/liter, P < 0.0001; apoB: +0.12 ± 0.031 g/liter, P < 0.0001) but not glucose (LDL: +0.012 ± 0.071 mmol/liter, P = 0.86; apoB: +0.0097 ± 0.019 g/liter, P = 0.90).
Conclusions:
Consumption of HFCS-sweetened beverages for 2 wk at 25% E increased risk factors for cardiovascular disease comparably with fructose and more than glucose in young adults.
In epidemiological studies, consumption of sugar and/or sugar-sweetened beverages has been linked to the presence of unfavorable lipid levels (1–5), insulin resistance (6, 7), fatty liver (8, 9), type 2 diabetes (10–12), cardiovascular disease (13), and metabolic syndrome (14). We have recently reported that consumption of fructose-sweetened beverages at 25% of energy requirements (E) increased visceral adipose deposition and de novo lipogenesis, produced dyslipidemia, and decreased glucose tolerance/insulin sensitivity in older, overweight/obese men and women, whereas consumption of glucose-sweetened beverages did not (15). Because the commonly consumed sugars, sucrose and high-fructose corn syrup (HFCS), are composed of 50–55% fructose, these results provide a potential mechanistic explanation for the associations between sugar consumption and metabolic disease. However, the adverse metabolic effects of fructose consumption observed in the older, overweight/obese population (15) may not occur in a younger, leaner population.
Authors of three recent reviews have concluded that long-term sugar intakes as high as 25–50% E have no adverse effects with respect to components of metabolic syndrome (16) and that fructose consumption up to 140 g/d does not result in biologically relevant increases of fasting or postprandial triglycerides (TG) in healthy, normal-weight (17), or overweight or obese (18) humans. These reviews (16, 17) are cited in the Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans 2010, released June of 2010, in which a maximal intake level of 25% or less of total energy from added sugars is suggested (19). However, in August of 2009, the American Heart Association Nutrition Committee recommended that women consume no more than 100 kcal/d and men consume no more than 150 kcal/d of added sugar (20). This equates to differences between the two guidelines of 400 kcal/d for women consuming 2000 kcal/d and 525 kcal/d for men consuming 2500 kcal/d. To address this discrepancy, we compared the effects of consuming 25%E as glucose, fructose or HFCS for 2 weeks on risk factors for cardiovascular disease in young adults.
Materials and Methods
The subjects who participated in this study are a subgroup of participants from an ongoing 5-yr National Institutes of Health-funded investigation in which a total of eight experimental groups (n = 25/group) will be studied. The objectives include comparing the metabolic effects of fructose, glucose, and HFCS consumption at 25% E and to compare the metabolic effects of fructose and HFCS consumption at 0, 10, 17.5, and 25% E. The results reported in this paper are from the first 48 subjects to complete the study protocol in the experimental groups consuming 25% E as glucose, fructose, or HFCS (n = 16/group). Participants were recruited through an internet listing (Craigslist.com) and underwent telephone and in-person interviews with medical history, complete blood count, and serum biochemistry panel to assess eligibility. Inclusion criteria included age 18–40 yr and body mass index (BMI) 18–35 kg/m2 with a self-report of stable body weight during the prior 6 months. Exclusion criteria included diabetes (fasting glucose >125 mg/dl), evidence of renal or hepatic disease, fasting plasma TG greater than 400 mg/dl, hypertension (>140/90 mm Hg), or surgery for weight loss. Individuals who smoked, habitually ingested more than two alcoholic beverages per day, exercised more than 3.5 h/wk at a level more vigorous than walking, or used thyroid, lipid-lowering, glucose-lowering, antihypertensive, antidepressant, or weight loss medications were also excluded. The University of California, Davis, Institutional Review Board approved the experimental protocol for this study, and subjects provided written informed consent to participate.
For the 5 wk before study, subjects were asked to limit daily consumption of sugar-containing beverages to one 8-oz serving of fruit juice. Fifty-five subjects were enrolled in the experimental groups consuming 25% E as glucose, fructose, or HFCS. Four subjects withdrew due to unwillingness to comply with the study protocol (two in the HFCS group, two before group assignment), and two were withdrawn due to medical conditions not apparent during screening (HFCS and glucose group). The samples from one subject (HFCS group) who completed the study protocol were not analyzed because of illness during the 24-h serial blood collection. The experimental groups were matched for gender (nine men, seven women/group), BMI, fasting TG, cholesterol, high-density lipoprotein (HDL) and insulin concentrations. The subjects and University of California, Davis, Clinical Research Center (CCRC) and technical personnel were blinded to the sugar assignments.
This was a parallel-arm, diet intervention study with three phases: 1) a 3.5-d inpatient baseline period during which subjects resided at the CCRC; 2) a 12-d outpatient intervention period; and 3) a 3.5-d inpatient intervention period at the CCRC. During d 2 and 3 of the baseline and intervention inpatient periods, subjects consumed energy-balanced meals consisting of conventional foods. Daily energy requirements were calculated by the Mifflin equation (21) with adjustment of 1.3 for activity on the days of the 24-h serial blood collections, and adjustment of 1.5 for the other days. The baseline diet contained 55% E mainly as complex carbohydrate, 30% fat, and 15% protein. The intervention inpatient meals were as identical as possible to baseline meals, excepting the carbohydrate component consisted of 25% E as glucose-, fructose-, or HFCS-sweetened beverages and 30% E as complex carbohydrate. Sugar-sweetened beverages were provided to subjects as three daily servings consumed with meals and were flavored with an unsweetened drink mix (Kool-Aid; Kraft Foods, Northfield, IL). The timing of inpatient meal service and the energy distribution were: breakfast, 0900 h (25%); lunch, 1300 h (35%); dinner, 1800 h (40%).
During the 12-d outpatient phase of the study, the subjects were provided with and instructed to drink three servings of sugar-sweetened beverage per day (one per meal), to consume their usual diet, and to not consume other sugar-containing beverages, including fruit juice. To monitor compliance, the sugar-sweetened beverages contained a biomarker (riboflavin), which was measured fluorometrically in urine samples collected at the time of beverage pickup. These measurements indicated that the three groups of subjects were comparably compliant.
Twenty-four-hour serial blood collections were conducted on the third day of the baseline (0 wk) and intervention (2 wk) inpatient periods. Three fasting blood samples were collected at 0800, 0830, and 0900 h. Twenty-nine postprandial blood samples were collected at 30- to 60-min intervals from 0930 until 0800 h the next morning. Additional 6-ml samples were collected at the fasting time points, 0800, 0830, and 0900 h and also at 2200, 2300, and 2400 h, the period during which TG concentrations peaked during our previous study (15). The additional plasma from the three fasting samples was pooled, as was that from the three late-evening postprandial samples; multiple aliquots of each pooled sample were stored at −80 C.
Analyses
Primary outcomes include fasting TG, 24-h TG incremental area under the curve (AUC), late-evening postprandial TG concentrations, and fasting LDL, non-HDL-cholesterol (-C), apolipoprotein (apo)B concentrations, and the apoB to apoAI ratio. Secondary outcomes included body weight, fasting HDL, postprandial LDL, non-HDL-C, apoB, remnant lipoprotein-cholesterol (RLP)-C and RLP-TG, and fasting and postprandial small dense LDL-cholesterol (sdLDL-C). Fasting concentrations, 24-h AUC, and postmeal peaks for glucose and insulin, and homeostasis model assessment insulin resistance index (HOMA-IR) are presented in the online supplement, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org. Fasting measures were conducted on samples collected or pooled from the 0800, 0830, and 0900 h time points, and postprandial measures were conducted on samples collected or pooled from the 2200, 2300, and 2400 h time points. Lipid and lipoprotein concentrations (total cholesterol, HDL, TG, apoB, apoA1) were determined with a Polychem chemistry analyzer (PolyMedCo, Inc., Cortlandt Manor, NY). LDL concentrations were determined by direct homogenous assay using detergents (Denka Seiken, Tokyo, Japan) (22) and sdLDL-C concentrations were quantified using the sdLDL-C-EX“SEIKEN” homogeneous assay kit (Denka Seiken) (23). RLP concentrations were quantified with an immunoseparation assay (24). Glucose was measured with an automated glucose analyzer (YSI, Inc., Yellow Springs, OH), and insulin by RIA (Millipore, St. Charles, MO).
The incremental 24-h area AUC was calculated for TG, glucose, and insulin by the trapezoidal method. Glucose and insulin postmeal peaks were assessed as the mean amplitudes of the three postmeal peaks; specifically the peak postmeal value minus the premeal value was averaged for breakfast, lunch, and dinner for each subject. The absolute change (Δ from 2 wk when 25% E sugar/30% E complex carbohydrate was consumed compared with 0 wk when 55% E complex carbohydrate was consumed) for each outcome was analyzed with SAS 9.2 (SAS, Cary, NC) in a mixed procedures (PROC MIXED) model with sugar and gender as factors, and BMI, the change (2 to 0 wk) in body weight (ΔBW), and outcome concentration at baseline (outcomeB) as continuous covariables. ΔBW and outcomeB were removed if they did not improve the precision of the model. Significant differences (P < 0.05) among the three sugars were identified by the Tukey's multiple comparisons test. Outcomes that were significantly affected by 2 wk of glucose, fructose, or HFCS consumption were identified as least squares means (LS means) of the change significantly different than zero. Primary outcomes were also analyzed with BMI as a factor (BMI <25 m/kg2 vs. >25 m/kg2). Data are presented as mean ± sem.
Results
There were no significant differences among the three experimental groups in anthropomorphic (Table 1) or outcome measures at baseline (Tables 2 and 3 and Supplemental Table 1). Body weight (Table 3) and blood pressure (data not shown) were not affected by 2 wk consumption of glucose, fructose, or HFCS.
Table 1.
Subjects' baseline anthropomorphic and metabolic parameters
| Parameter | Glucose (n = 16) | Fructose (n = 16) | HFCS (n = 16) |
|---|---|---|---|
| Age (yr) | 27.0 ± 7.2 | 28.0 ± 6.8 | 27.8 ± 7.6 |
| Weight (kg) | 76.8 ± 14.1 | 76.8 ± 10.6 | 74.3 ± 14.9 |
| BMI (kg/m2) | 26.2 ± 3.6 | 25.4 ± 3.8 | 24.9 ± 4.8 |
| Waist circumference (cm) | 80.6 ± 10.4 | 77.8 ± 9.6 | 78.0 ± 10.8 |
| Body fat (%) | 28.0 ± 9.3 | 26.7 ± 11.8 | 25.0 ± 10.1 |
| TG (mmol/liter) | 1.2 ± 0.5 | 1.2 ± 0.4 | 1.3 ± 0.6 |
| Total cholesterol (mmol/liter) | 4.5 ± 0.8 | 3.9 ± 0.8 | 4.1 ± 0.8 |
| HDL-C (mmol/liter) | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.4 |
| Insulin (pmol/liter) | 97.9 ± 30.4 | 102.8 ± 86.4 | 89.1 ± 31.6 |
P > 0.05 for differences among groups at baseline for all parameters, PROC MIXED ANOVA. Mean ± sd.
Table 2.
Primary outcomes during consumption of complex carbohydrates at 0 wk and during consumption of glucose-, fructose-, or HFCS-sweetened beverages at 2 wk
| Primary outcomes | Glucose |
Fructose |
HFCS |
Effects | P value | |||
|---|---|---|---|---|---|---|---|---|
| 0 wk | 2 wk | 0 wk | 2 wk | 0 wk | 2 wk | |||
| 24-h TG AUC (mmol/liter per 24 h)a | 5.6 ± 1.1 | 3.6 ± 1.3b | 2.9 ± 1.5 | 7.6 ± 1.9*c | 3.8 ± 1.4 | 5.5 ± 1.7**c | Sugar | 0.0058 |
| Gender | 0.13 | |||||||
| BMI | 0.0033 | |||||||
| Late-evening TG (mmol/liter)d | 1.3 ± 0.2 | 1.5 ± 0.2b | 1.2 ± 0.1 | 1.8 ± 0.2***c | 1.3 ± 0.2 | 1.8 ± 0.2***,b,c | Sugar | 0.016 |
| Gender | 0.40 | |||||||
| BMI | 0.015 | |||||||
| Fasting TG (mmol/liter)e | 1.2 ± 0.1 | 1.4 ± 0.2* | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.2 | 1.4 ± 0.1 | Sugar | 0.54 |
| Gender | 0.035 | |||||||
| BMI | 0.94 | |||||||
| Fasting LDL-C (mmol/liter)a | 2.6 ± 0.2 | 2.6 ± 0.2b | 2.1 ± 0.2 | 2.4 ± 0.2*,b,c | 2.3 ± 0.2 | 2.7 ± 0.2***c | Sugar | 0.0098 |
| Gender | 0.057 | |||||||
| BMI | 0.40 | |||||||
| Fasting non-HDL-C (mmol/liter)a | 3.2 ± 0.2 | 3.3 ± 0.2b | 2.7 ± 0.2 | 3.0 ± 0.2*,b,c | 2.9 ± 0.2 | 3.4 ± 0.2***c | Sugar | 0.0077 |
| Gender | 0.017 | |||||||
| BMI | 0.48 | |||||||
| Fasting apoB (g/liter)a | 0.82 ± 0.06 | 0.83 ± 0.06b | 0.65 ± 0.04 | 0.74 ± 0.05****c | 0.73 ± 0.05 | 0.85 ± 0.06***c | Sugar | 0.0051 |
| Gender | 0.027 | |||||||
| BMI | 0.47 | |||||||
| apoB to apoAI ratioe | 0.70 ± 0.06 | 0.70 ± 0.05b | 0.54 ± 0.04 | 0.63 ± 0.05*c | 0.60 ± 0.06 | 0.71 ± 0.07***c | Sugar | 0.0031 |
| Gender | 0.34 | |||||||
| BMI | 0.61 | |||||||
P > 0.05 for differences among groups at baseline for all outcomes. Mean ± sem.
PROC MIXED two-factor (sugar, gender) analysis with adjustment for BMI, ΔBW (2 wk to 0 wk), and outcomeB on absolute Δ (2 wk vs. 0 wk).
Δ (2 wk vs. 0 wk) significantly different from c Δ (2 wk vs. 0 wk), Tukey's multiple comparison test.
PROC MIXED two-factor (sugar, gender) analysis with adjustment for BMI on absolute Δ (2 wk vs. 0 wk).
PROC MIXED two-factor (sugar, gender) analysis with adjustment for BMI and ▵BW (2 wk to 0 wk) on absolute ▵ (2 wk vs. 0 wk).
P < 0.01,
P < 0.05,
P < 0.0001,
P < 0.001, LS means of Δ different from zero.
Table 3.
Secondary outcomes during consumption of complex carbohydrates at 0 wk and during consumption of glucose-, fructose-, or HFCS-sweetened beverages at 2 wk
| Secondary outcomes | Glucose |
Fructose |
HFCS |
Effects | P value | |||
|---|---|---|---|---|---|---|---|---|
| 0 wk | 2 wk | 0 wk | 2 wk | 0 wk | 2 wk | |||
| Body weight (kg)a | 76.8 ± 3.5 | 77.2 ± 3.7 | 76.8 ± 2.6 | 76.7 ± 2.6 | 74.3 ± 3.7 | 74.7 ± 3.7 | Sugar | 0.32 |
| Gender | 0.62 | |||||||
| BMI | 0.50 | |||||||
| Fasting HDL (mmol/liter)b | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | Sugar | 0.92 |
| Gender | 0.37 | |||||||
| BMI | 0.22 | |||||||
| PP LDL (mmol/liter)b | 2.5 ± 0.2 | 2.6 ± 0.2c | 2.0 ± 0.2 | 2.3 ± 0.2*,c,d | 2.1 ± 0.2 | 2.7 ± 0.2**,d | Sugar | 0.0033 |
| Gender | 0.010 | |||||||
| BMI | 0.54 | |||||||
| PP non-HDL-C (mmol/liter)b | 3.0 ± 0.2 | 3.2 ± 0.2c | 2.5 ± 0.2 | 3.0 ± 0.2**,c,d | 2.6 ± 0.2 | 3.4 ± 0.2**,d | Sugar | 0.0012 |
| Gender | 0.017 | |||||||
| BMI | 0.27 | |||||||
| PP apoB (g/liter)b | 0.78 ± 0.05 | 0.83 ± 0.05c | 0.62 ± 0.04 | 0.73 ± 0.05***,c,b | 0.68 ± 0.05 | 0.84 ± 0.06**,d | Sugar | 0.031 |
| Gender | 0.10 | |||||||
| BMI | 0.56 | |||||||
| PP RLP-C (mmol/liter)a | 0.17 ± 0.02 | 0.19 ± 0.02c | 0.16 ± 0.02 | 0.23 ± 0.03**,b | 0.15 ± 0.02 | 0.21 ± 0.02***,c,b | Sugar | 0.035 |
| Gender | 0.37 | |||||||
| BMI | 0.034 | |||||||
| PP RLP-TG (mmol/liter)e | 0.34 ± 0.07 | 0.44 ± 0.06 | 0.35 ± 0.06 | 0.58 ± 0.08*** | 0.33 ± 0.06 | 0.54 ± 0.09*** | Sugar | 0.088 |
| Gender | 0.20 | |||||||
| BMI | 0.012 | |||||||
| Fasting sdLDL-C (mmol/liter)f | 0.65 ± 0.08 | 0.77 ± 0.10**** | 0.47 ± 0.04 | 0.59 ± 0.06*** | 0.61 ± 0.08 | 0.78 ± 0.09** | Sugar | 0.37 |
| Gender | 0.0019 | |||||||
| BMI | 0.11 | |||||||
| PP sdLDL-C (mmol/liter)f | 0.65 ± 0.08 | 0.79 ± 0.10*,c | 0.48 ± 0.04 | 0.64 ± 0.07**,c,d | 0.60 ± 0.08 | 0.86 ± 0.10**,d | Sugar | 0.019 |
| Gender | <0.0001 | |||||||
| BMI | 0.0125 | |||||||
P > 0.05 for differences among groups at baseline for all outcomes. Mean ± sem. PP, Postprandial.
PROC MIXED two-factor (sugar, gender) analysis with adjustment for BMI on absolute Δ (2 wk vs. 0 wk).
PROC MIXED two-factor (sugar, gender) analysis with adjustment for BMI, ΔBW (2 wk to 0 wk), and outcomeB on absolute Δ (2 wk vs. 0 wk).
Δ (2 wk vs. 0 wk) significantly different from d Δ (2 wk vs. 0 wk), Tukey's multiple comparison test.
PROC MIXED two-factor (sugar, gender) analysis with adjustment for BMI and outcomeB on absolute Δ (2 wk vs. 0 wk).
PROC MIXED two-factor (sugar, gender) analysis with adjustment for BMI and ΔBW (2 wk to 0 wk) on absolute Δ (2 wk vs. 0 wk).
P < 0.01,
P < 0.0001,
P < 0.001,
P < 0.05, LS means of Δ different from zero.
Primary outcomes: comparing glucose, fructose, and HFCS with complex carbohydrate consumption
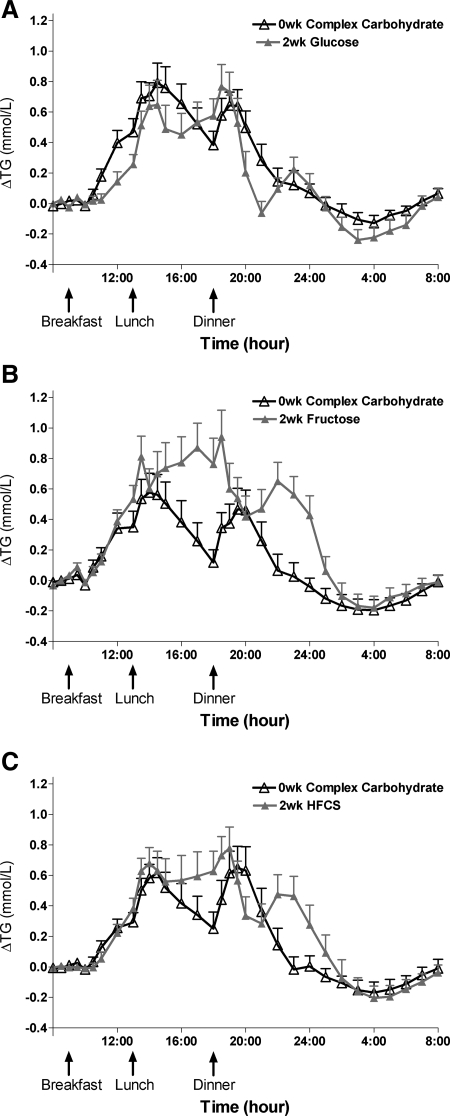
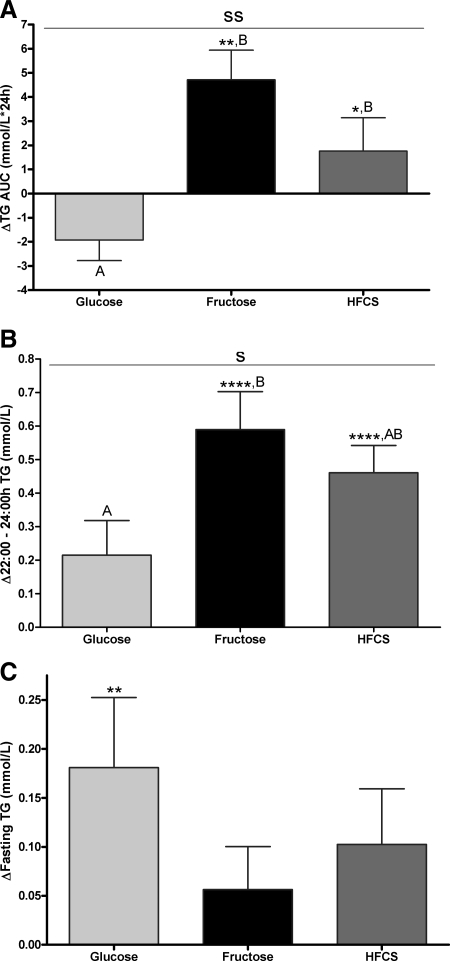
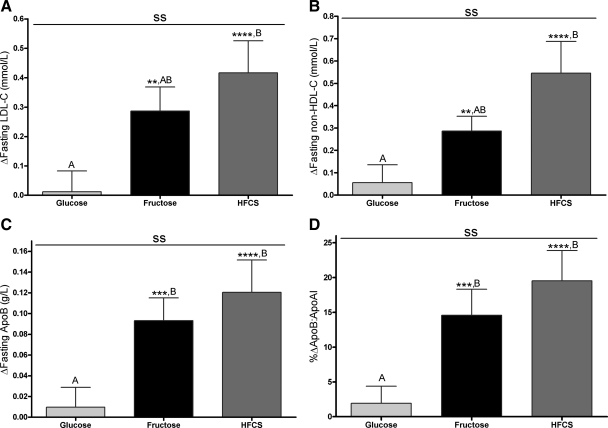
Table 2 presents the primary outcomes during consumption of complex carbohydrate at baseline (0 wk) and at the end of the 2-wk sugar interventions. The 24-h TG profiles during baseline and the end of the 2-wk intervention are shown in Fig. 1, A–C. The 24-h TG AUC (Fig. 2A) was significantly increased compared with baseline (LS means of Δ different from zero) in subjects consuming fructose (+4.7 ± 1.2 mmol/liter × 24 h, P = 0.0032) and HFCS (+1.8 ± 1.4 mmol/liter × 24 h, P = 0.035), whereas it tended to decrease during consumption of glucose (−1.9 ± 0.9 mmol/liter × 24 h, P = 0.14). The consumption of all three sugars resulted in a late-evening TG peak between 2200 and 2400 h that was not apparent when complex carbohydrate was consumed (Fig. 1, A–C). The late-evening peaks (Fig. 2B) were significantly increased compared with baseline during consumption of fructose (+0.59 ± 0.11 mmol/liter, P < 0.0001) and HFCS (+0.46 ± 0.082 mmol/liter, P < 0.0001) but not by glucose (+0.22 ± 0.10 mmol/liter, P = 0.077). All three sugars tended to increase fasting TG, but this was significant only in the group consuming glucose (Fig. 2C). Fasting LDL-C concentrations (Fig. 3A) were increased during consumption of fructose (+0.29 ± 0.082 mmol/liter, P = 0.0023) and HFCS (+0.42 ± 0.11 mmol/liter, P < 0.0001) but not glucose (+0.012 ± 0.071 mmol/liter, P = 0.86). Similarly, fasting non-HDL-C (Fig. 3B), apoB (Fig. 3C), and the apoB to apoAI ratio (Fig. 3D) were all significantly increased in subjects consuming fructose (non-HDL-C: +0.29 ± 0.066 mmol/liter, P = 0.0081; apoB: +0.093 ± 0.022 g/liter, P = 0.0005; apoB to apoAI ratio: +14.6 ± 3.8%, P = 0.0006) and HFCS (non-HDL-C: +0.55 ± 0.14 mmol/liter, P < 0.0001; apoB: +0.12 ± 0.031 g/liter, P < 0.0001; apoB to apoAI ratio: +19.5 ± 4.4%, P < 0.0001) compared with baseline but not in subjects consuming glucose (non-HDL-C: +0.055 ± 0.080 mmol/liter, P = 0.49; apoB: +0.0097 ± 0.019 g/liter, P = 0.90; apoB to apoAI ratio: +1.9 ± 2.5%, P = 0.81).
Fig. 1.
Twenty-four-hour TG profiles during consumption of complex carbohydrate and during consumption of sugar-sweetened beverages. The change of 24-h TG concentrations over fasting concentrations during consumption of energy-balanced baseline diet containing 55% E complex carbohydrate at 0 wk and during consumption of energy-balanced intervention diet containing 30% E complex carbohydrate and 25% E glucose (A), fructose (B), or HFCS (C) at 2 wk (n = 16/group). Data are mean ± sem.
Fig. 2.
Effects of sugar-sweetened beverage consumption on TG concentrations. The change in 24-h TG AUC (A), late-night to late-evening TG (B), and fasting TG concentrations (C) compared with baseline after consuming 25% of energy requirements as glucose-, fructose-, or HFCS-sweetened beverages for 2 wk is shown. S, P < 0.05; SS, P < 0.01, effect of sugar; two-factor (sugar, gender) PROC MIXED analysis on Δ with adjustment for BMI (B), ΔBW (C), and outcome at baseline (A). *, P < 0.05, **, P < 0.01, ****, P < 0.0001, LS means different from zero. A, Δ different from B; Δ, Tukey's (n = 16/group). Data are mean ± sem.
Fig. 3.
Effects of sugar-sweetened beverage consumption on risk factors for cardiovascular disease. The change in fasting LDL (A), non-HDL-C (B), apoB concentrations (C), and apoB to apoA1 ratio (D) after consuming 25% of energy requirements as glucose-, fructose-, or HFCS-sweetened beverages for 2 wk. ss, P < 0.01, effect of sugar; two-factor (sugar, gender) PROC MIXED analysis on Δ with adjustment for BMI, ΔBW (D), and outcome at baseline (A–C). **, P < 0.01, ***, P < 0.001, ****, P < 0.0001, LS means different from zero. A, Δ different from B, Δ, Tukey's (n = 16/group). Data are mean ± sem.
Primary outcomes: comparing glucose, fructose, and HFCS consumption
The effects of the three sugars were significantly different (PROC MIXED two factor analysis with adjustment for BMI, ΔBW and outcomeB) for all primary outcomes except fasting TG (see effects of sugar P values in Table 2). The effects of HFCS compared with fructose consumption on all primary outcomes were not significantly different (P > 0.05, Tukey's). The increases in 24-h TG AUC (P = 0.0068), late evening TG peaks (P = 0.015), fasting apoB (P = 0.037), and the apoB to apoA1 ratio (P = 0.028) were larger after fructose consumption compared with glucose consumption. The increases in 24-h TG AUC (P = 0.034), fasting LDL (P = 0.0083), non-HDL-C (P = 0.0055), apoB (P = 0.0056), and apoB to apoAI ratio (P = 0.0034) were larger after HFCS consumption than glucose consumption.
With regard to BMI, although the statistical results presented in Table 1 and Figs. 2 and 3 include adjustment for BMI, Online Supplemental Fig. 1, A–F, presents the changes of the primary outcomes with subjects grouped as normal weight (BMI <25 kg/m2) or overweight/obese (BMI >25 kg/m2). The effect of BMI status was significant for the change of the 24-h TG AUC (P = 0.016) and the late-evening TG peaks (P = 0.019) but not for the fasting TG (P = 0.55, data not shown), LDL-C (P = 0.30), non-HDL-C (P = 0.93), apoB (P = 0.62), and apoB to apoAI ratio (P = 0.51). Normal weight and overweight/obese subjects consuming HFCS had comparable absolute (Supplemental Fig. 1) and percent increases of late-evening TG (BMI <25 kg/m2: +46 ± 11%; BMI >25 kg/m2: +31 ± 6%), fasting LDL-C (BMI <25 kg/m2: +22 ± 1%; BMI >25 kg/m2: +28 ± 1%), non-HDL-C (BMI <25 kg/m2: +36 ± 19%, BMI >25 kg/m2: +17 ± 7), apoB (BMI < 25 kg/m2: +17 ± 6%; BMI >25 kg/m2: +20 ± 8%), and the apoB to apoAI ratio (BMI <25 kg/m2: +22 ± 7%; BMI >25 kg/m2: +20 ± 9%).
Secondary outcomes: comparing glucose, fructose, and HFCS with complex carbohydrate consumption
Table 3 presents the secondary outcomes during consumption of complex carbohydrate at baseline and at the end of the 2-wk sugar interventions. Fasting HDL concentrations were unaffected by consumption of the three sugar-sweetened beverages. Similar to the responses in the fasting state, subjects consuming fructose and HFCS had increased postprandial concentrations of LDL-C, non-HDL-C, and apoB compared with baseline, whereas subjects consuming glucose did not. Fructose and HFCS consumption increased postprandial concentrations of RLP-C and RLP-TG compared with baseline, whereas consumption of glucose did not. Consumption of all three sugars increased fasting and postprandial sdLDL-C compared with baseline.
Secondary outcomes: comparing glucose, fructose, and HFCS consumption
The effects of the three sugars were significantly different (PROC MIXED two factor analysis with adjustment for BMI, ΔBW, and outcomeB) for postprandial LDL, non-HDL-C, apoB, RLP-C, and sdLDL-C (see effects of sugar P values in Table 3). The effects of HFCS compared with fructose consumption on all secondary outcomes were not significantly different (P > 0.05, Tukey's). The increases in postprandial RLP-C were larger during consumption of fructose compared with glucose (P = 0.044), and HFCS consumption caused larger increases in postprandial LDL (P = 0.0024), non-HDL-C (P = 0.0007), apoB (P = 0.025), and sdLDL-C (P = 0.014) (Tukey's) than glucose consumption.
For glucose, insulin, and HOMA-IR, the 24-h glucose and insulin profiles during baseline (0 wk) and at the end of the 2-wk intervention are presented in Online Supplemental Figs. 2, A–C, and 3, A–C, respectively. Compared with baseline, the 24-h glucose and insulin 24-h AUC and the postmeal insulin peaks were significantly increased in subjects consuming glucose, significantly decreased in subjects consuming fructose, and were unchanged in subjects consuming HFCS (Online Supplemental Table 1). Postmeal glucose peaks were increased in subjects consuming glucose and HFCS. Fasting glucose concentrations were significantly decreased in subjects consuming glucose, whereas fasting insulin concentrations and HOMA-IR did not change significantly in any group.
Gender
Although there were no significant sugar-gender interactions for any of the primary or secondary outcomes, men exhibited larger increases of fasting TG, non-HDL-C, apoB, and sdLDL-C concentrations and postprandial LDL, non-HDL-C, and sdLDL-C concentrations in response to sugar consumption than women (see effects of gender P values in Tables 2 and 3). However, postprandial TG responses, as assessed by the 24-h TG AUC, late-evening TG peaks, postprandial apoB, and RLP-TG concentrations, were not different between genders. The subjects consuming glucose exhibited the most divergent gender responses, particularly in sdLDL-C. Fasting and postprandial sdLDL-C levels were increased compared with baseline by +0.22 ± 0.07 mmol/liter (P = 0.0001) and +0.24 ± 0.05 mmol/liter (P < 0.0001), respectively, in men after glucose consumption but were unchanged in women (fasting sdLDL-C: −0.004 ± 0.02 mmol/liter, P = 0.61; postprandial sdLDL-C: +0.006 ± 0.019 mmol/liter, P = 0.69).
Discussion
The current study provides evidence that postprandial TG and fasting and postprandial concentrations of LDL, non-HDL-C, apoB, and the apoB to apoAI ratio, established risk factors for coronary heart disease (25), are significantly increased in response to 2 wk consumption of 25% of E as fructose and HFCS, but not glucose, in younger, normal-weight, and overweight subjects. In contrast and as was observed in older subjects (15), fasting TG concentrations were increased in subjects consuming glucose but not in those consuming fructose-containing sugars. The differential effects of fructose and glucose consumption on fasting and postprandial TG responses in subjects from both studies suggest that fasting TG concentrations are not a reliable indicator of the adverse changes in postprandial TG and other lipid/lipoprotein risk factors induced by fructose consumption. There is growing evidence linking increases of postprandial TG concentrations with proatherogenic conditions (26–28). It is important to note that for both the current and previous study (15), the differential effects of fructose and HFCS compared with complex carbohydrate on the 24-h TG profile were most marked in the late evening, approximately 4 and 6 h after dinner. Studies investigating the relationship between this late-evening peak and proatherogenic changes would be of interest, as would investigations into the sources of the TG that contributes to these peaks (de novo lipogenesis, diet, or fatty acids derived from adipose lipolysis).
To our knowledge this is the first study to directly compare the effects of sustained consumption of HFCS with 100% fructose and glucose-sweetened beverages. This comparison is important because it would seem likely that the effects of HFCS-sweetened beverages on circulating lipids and lipoproteins would be less than those of pure fructose-sweetened beverage because they contain 45% less fructose. And indeed, the postprandial TG and RLP responses exhibited the expected pattern based on the fructose content of the sugars, with increases being greatest in subjects who consumed 145 ± 4 g fructose per day from beverages, lowest in subjects who consumed 144 ± 5 g glucose per day and 0 g fructose per day from beverages and intermediate in subjects who consumed HFCS-sweetened beverages providing 64 ± 2 g glucose per day and 79 ± 3 g fructose per day. However, the changes of fasting and postprandial concentrations of LDL, non-HDL-C, apoB, and the apoB to apoAI ratio in subjects consuming HFCS were significantly larger compared with subjects consuming glucose and tended to be higher compared with subjects consuming pure fructose. More studies are needed to confirm this unexpected pattern and to determine whether it is a result of a synergistic effect of consuming fructose and glucose in combination. Additional studies are also needed to determine whether the substantial increases, seen after just 2 wk, are further aggravated with longer-term consumption of HFCS-sweetened beverages.
Compared with baseline, postmeal glucose and insulin responses (indexed as 24 h AUC and postmeal peaks) were mainly increased during glucose consumption, decreased during fructose consumption, and unchanged during HFCS consumption. This pattern is expected and further supports our data indicating that the adverse effects associated with chronic consumption of sugar-sweetened beverages result from the specific effects of fructose (29), rather than from increased circulating glucose and insulin excursions (i.e. glycemic index) (30–32). Although consumption of fructose increased fasting glucose and insulin concentrations in 2 wk and decreased insulin sensitivity by 17% in 10 wk (15), in the current study, HOMA-IR was unchanged after 2 wk consumption of fructose, HFCS, or glucose. This may be related to the subjects in the current study being younger and leaner (28 ± 7 yr; 25.5 ± 4.0 kg/m2) than the subjects in the previous study (54 ± 8 yr; 29.1 ± 2.9 kg/m2). In a study by Le et al. (33), inclusion of fructose with an energy-balanced diet for 4 wk in young, normal-weight men (24.7 ± 1.3 yr; ∼22 kg/m2) increased fasting glucose levels, but other indices of insulin sensitivity were unaffected. However, it was recently reported that consumption of fructose or glucose (150 g/d) for 4 wk lowered insulin sensitivity and increased HOMA-IR in subjects of similar age and BMI (31 ± 9 yr; 25.9 ± 2.2 kg/m2) (34).
As would be expected based on the evidence that both increasing age and postmenopausal status result in augmented postprandial lipid responses in women (35), more significant gender differences in lipid outcomes were observed in these younger subjects in the current study than in the older subjects previously studied (15). With the exception of postprandial TG, apoB, and RLP-C and RLP-TG, younger men exhibited larger lipoprotein responses after 2 wk of sugar consumption than younger women. The comparable responses in postprandial TG and apoB concentrations and the significantly different fasting TG and apoB responses between the genders suggest that rates of very low-density lipoprotein secretion may be similar between men and women, whereas rates of very low-density lipoprotein clearance are different. This is supported by kinetic studies, which demonstrate that women have higher TG-rich lipoprotein and LDL-apoB fractional catabolic rates than men, whereas production rates are comparable (36, 37).
The greater effect of glucose consumption on sdLDL-C levels in younger men compared with younger women represents the most marked difference between the current and our previous lipid results, which showed older men and women were comparably nonresponsive to consumption of glucose (15). The increase of fasting sdLDL-C concentrations compared with baseline in younger men consuming glucose was unexpected because they did not exhibit increases in fasting LDL and apoB concentrations.
The added sugar component of the typical U.S. diet consists of nearly equal amounts of HFCS and sucrose (38); therefore, it is a limitation of this study that we did not also investigate the effects of sucrose consumption. However, we expect that the effects of sucrose would be comparable with those of HFCS because its composition (50% glucose/50% fructose) is very similar to the composition of the HFCS used for this study (45% glucose/55% fructose). This is supported by results from a crossover study in which subjects consumed standardized diets containing 5, 18, or 33% of energy as sucrose, each for 6 wk. Compared with the 5% sucrose diet, LDL concentrations increased by 17% on the 18% sucrose diet and by 22% on the 33% sucrose diet (39).
Self-reported intake data suggest that 13% of the U.S. population consumes 25% or more of energy from added sugar (40). Importantly, the current results provide evidence that sugar consumption at this level increases risk factors for cardiovascular disease within 2 wk in young adults, thus providing direct experimental support for the epidemiological evidence linking sugar consumption with dyslipidemia (1–5) and cardiovascular disease (13). The results contradict the conclusions from recent reviews that sugar intakes as high as 25–50% of energy have no adverse long-term effects with respect to components of the metabolic syndrome (16) and that fructose consumption up to 140 g/d does not result in a biologically relevant increase of fasting or postprandial TG in healthy, normal-weight (17) or overweight or obese (18) humans. Additionally they provide evidence that the maximal upper limit of 25% of total energy requirements from added sugar, suggested by the Dietary Guidelines for Americans 2010 (19), may need to be reevaluated.
Supplementary Material
Acknowledgments
We thank James Graham and Marinelle Nunez for excellent technical support and the nursing staff at the University of California, Davis, Clinical Research Center for their dedicated nursing support. We also acknowledge and thank Janet Peerson for expert advice on the statistical analysis and Dr. Richard J. Havel for his expert advice and editing.
This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute Grant 1R01 HL09133. The project also received support from Grant UL1 RR024146 from the National Center for Research Resources, a component of the NIH, and NIH Roadmap for Medical Research.
Disclosure Summary: K.L.S., A.A.B., V.M., G.C., T.H.F., V.L., R.I.M., N.L.K, and P.J.H. have nothing to disclose. K.N. has consulted for Denka Seiken Co., Tokyo, Japan, and Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan. T.N. was previously employed by Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan. Y.I. is employed by Denka Seiken Co., Tokyo, Japan.
Footnotes
- apo
- Apolipoprotein
- AUC
- area under the curve
- BMI
- body mass index
- ΔBW
- change in body weight
- C
- cholesterol
- CCRC
- University of California, Davis, Clinical Research Center
- E
- energy requirements
- HDL
- high-density lipoprotein
- HFCS
- high-fructose corn syrup
- HOMA-IR
- homeostasis model assessment insulin resistance index
- LDL
- low-density lipoprotein
- LS means
- least squares means
- RLP
- remnant lipoprotein
- sdLDL-C
- small dense LDL-C
- TG
- triglyceride.
References
- 1. Aeberli I, Zimmermann MB, Molinari L, Lehmann R, l'Allemand D, Spinas GA, Berneis K. 2007. Fructose intake is a predictor of LDL particle size in overweight schoolchildren. Am J Clin Nutr 86:1174–1178 [DOI] [PubMed] [Google Scholar]
- 2. Bortsov AV, Liese AD, Bell RA, Dabelea D, D'Agostino RB, Jr, Hamman RF, Klingensmith GJ, Lawrence JM, Maahs DM, McKeown R, Marcovina SM, Thomas J, Williams DE, Mayer-Davis EJ. 20 January 2011. Sugar-sweetened and diet beverage consumption is associated with cardiovascular risk factor profile in youth with type 1 diabetes. Acta Diabetol 10.1007/s00592-010-0246-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duffey KJ, Gordon-Larsen P, Steffen LM, Jacobs DR, Jr, Popkin BM. 2010. Drinking caloric beverages increases the risk of adverse cardiometabolic outcomes in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr 92:954–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Welsh JA, Sharma A, Abramson JL, Vaccarino V, Gillespie C, Vos MB. 2010. Caloric sweetener consumption and dyslipidemia among U.S. adults. JAMA 303:1490–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Welsh JA, Sharma A, Cunningham SA, Vos MB. 2011. Consumption of added sugars and indicators of cardiovascular disease risk among U.S. adolescents. Circulation 123:249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bremer AA, Auinger P, Byrd RS. 6 September 2009. Sugar-sweetened beverage intake trends in U.S. adolescents and their association with insulin resistance-related parameters. J Nutr Metab 10.1155/2010/196476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoshida M, McKeown NM, Rogers G, Meigs JB, Saltzman E, D'Agostino R, Jacques PF. 2007. Surrogate markers of insulin resistance are associated with consumption of sugar-sweetened drinks and fruit juice in middle and older-aged adults. J Nutr 137:2121–2127 [DOI] [PubMed] [Google Scholar]
- 8. Assy N, Nasser G, Kamayse I, Nseir W, Beniashvili Z, Djibre A, Grosovski M. 2008. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol 22:811–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. 2008. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol 48:993–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Montonen J, Järvinen R, Knekt P, Heliövaara M, Reunanen A. 2007. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr 137:1447–1454 [DOI] [PubMed] [Google Scholar]
- 11. Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. 2008. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med 168:1487–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. 2004. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 292:927–934 [DOI] [PubMed] [Google Scholar]
- 13. Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. 2009. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr 89:1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D'Agostino RB, Gaziano JM, Vasan RS. 2007. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 116:480–488 [DOI] [PubMed] [Google Scholar]
- 15. Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ. 2009. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119:1322–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruxton CH, Gardner EJ, McNulty HM. 2010. Is sugar consumption detrimental to health? A review of the evidence, 1995–2006. Crit Rev Food Sci Nutr 50:1–19 [DOI] [PubMed] [Google Scholar]
- 17. Dolan LC, Potter SM, Burdock GA. 2010. Evidence-based review on the effect of normal dietary consumption of fructose on development of hyperlipidemia and obesity in healthy, normal weight individuals. Crit Rev Food Sci Nutr 50:53–84 [DOI] [PubMed] [Google Scholar]
- 18. Dolan LC, Potter SM, Burdock GA. 2010. Evidence-based review on the effect of normal dietary consumption of fructose on blood lipids and body weight of overweight and obese individuals. Crit Rev Food Sci Nutr 50:889–918 [DOI] [PubMed] [Google Scholar]
- 19. DGAC 2010. Report of the Dietary Guidelines Advisory Committee (DGAC) on the dietary guidelines for Americans, 2010. http://www.cnpp.usda.gov/dgas2010-dgacreport.htm
- 20. Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J. 2009. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 120:1011–1020 [DOI] [PubMed] [Google Scholar]
- 21. Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. 1990. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 51:241–247 [DOI] [PubMed] [Google Scholar]
- 22. Sakaue T, Hirano T, Yoshino G, Sakai K, Takeuchi H, Adachi M. 2000. Reactions of direct LDL-cholesterol assays with pure LDL fraction and IDL: comparison of three homogeneous methods. Clin Chim Acta 295:97–106 [DOI] [PubMed] [Google Scholar]
- 23. Ito Y, Fujimura M, Ohta M, Hirano T. 2011. Development of a homogeneous assay for measurement of small dense LDL cholesterol. Clin Chem 57:57–65 [DOI] [PubMed] [Google Scholar]
- 24. Nakajima K, Saito T, Tamura A, Suzuki M, Nakano T, Adachi M, Tanaka A, Tada N, Nakamura H, Murase T. 1994. A new approach for the detection of type III hyperlipoproteinemia by RLP-cholesterol assay. J Atheroscler Thromb 1:30–36 [DOI] [PubMed] [Google Scholar]
- 25. Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. 2009. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302:1993–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. 2007. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 298:309–316 [DOI] [PubMed] [Google Scholar]
- 27. Karpe F. 1999. Postprandial lipoprotein metabolism and atherosclerosis. J Intern Med 246:341–355 [DOI] [PubMed] [Google Scholar]
- 28. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. 2007. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 298:299–308 [DOI] [PubMed] [Google Scholar]
- 29. Stanhope KL, Griffen SC, Bremer AA, Vink RG, Schaefer EJ, Nakajima K, Schwarz JM, Beysen C, Berglund L, Keim NL, Havel PJ. 2011. Metabolic responses to prolonged consumption of glucose- and fructose-sweetened beverages are not associated with postprandial or 24-h glucose and insulin excursions. Am J Clin Nutr 94:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ding EL, Malik VS. 2008. Convergence of obesity and high glycemic diet on compounding diabetes and cardiovascular risks in modernizing China: an emerging public health dilemma. Global Health 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu FB, Malik VS. 2010. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol Behav 100:47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schernhammer ES, Hu FB, Giovannucci E, Michaud DS, Colditz GA, Stampfer MJ, Fuchs CS. 2005. Sugar-sweetened soft drink consumption and risk of pancreatic cancer in two prospective cohorts. Cancer Epidemiol Biomarkers Prev 14:2098–2105 [DOI] [PubMed] [Google Scholar]
- 33. Lê KA, Faeh D, Stettler R, Ith M, Kreis R, Vermathen P, Boesch C, Ravussin E, Tappy L. 2006. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr 84:1374–1379 [DOI] [PubMed] [Google Scholar]
- 34. Silbernagel G, Machann J, Unmuth S, Schick F, Stefan N, Häring HU, Fritsche A. 2011. Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: an exploratory trial. Br J Nutr 106:79–86 [DOI] [PubMed] [Google Scholar]
- 35. Jackson KG, Abraham EC, Smith AM, Murray P, O'Malley B, Williams CM, Minihane AM. 2010. Impact of age and menopausal status on the postprandial triacylglycerol response in healthy women. Atherosclerosis 208:246–252 [DOI] [PubMed] [Google Scholar]
- 36. Matthan NR, Jalbert SM, Barrett PH, Dolnikowski GG, Schaefer EJ, Lichtenstein AH. 2008. Gender-specific differences in the kinetics of nonfasting TRL, IDL, and LDL apolipoprotein B-100 in men and premenopausal women. Arterioscler Thromb Vasc Biol 28:1838–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watts GF, Moroz P, Barrett PH. 2000. Kinetics of very-low-density lipoprotein apolipoprotein B-100 in normolipidemic subjects: pooled analysis of stable-isotope studies. Metabolism 49:1204–1210 [DOI] [PubMed] [Google Scholar]
- 38. Wells FW, Buzby JC. 2008. Dietary assessment of major trends in U.S. food consumption, 1970–2005. Economic Information Bulletin No. (EIB-33). Washington, DC: Department of Agriculture [Google Scholar]
- 39. Reiser S, Bickard MC, Hallfrisch J, Michaelis OE, 4th, Prather ES. 1981. Blood lipids and their distribution in lipoproteins in hyperinsulinemic subjects fed three different levels of sucrose. J Nutr 111:1045–1057 [DOI] [PubMed] [Google Scholar]
- 40. Marriott BP, Olsho L, Hadden L, Connor P. 2010. Intake of added sugars and selected nutrients in the United States, National Health and Nutrition Examination Survey (NHANES), 2003–2006. Crit Rev Food Sci Nutr 50:228–258 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.