Abstract
Context:
Soybean oil-based lipid emulsions are the only Food and Drug Administration-approved lipid formulation for clinical use in parenteral nutrition (PN). Recently concerns with its use have been raised due to the proinflammatory effects that may lead to increased complications because they are rich in ω-6 polyunsaturated fatty acids.
Methods:
This was a prospective, randomized, controlled, crossover study comparing the vascular, metabolic, immune, and inflammatory effects of 24-h infusion of PN containing soybean oil-based lipid emulsion (Intralipid), olive oil-based (ClinOleic), lipid free, and normal saline in 12 healthy subjects.
Results:
Soybean oil-PN increased systolic blood pressure compared with olive oil-PN (P < 0.05). Soybean oil PN reduced brachial artery flow-mediated dilatation from baseline (−23% at 4 h and −25% at 24 h, both P < 0.01); in contrast, olive oil PN, lipid free PN, and saline did not change either systolic blood pressure or flow-mediated dilatation. Compared with saline, soybean oil PN, olive oil PN, and lipid free PN similarly increased glucose and insulin concentrations during infusion (P < 0.05). There were no significant changes in plasma free fatty acids, lipid profile, inflammatory and oxidative stress markers, immune function parameters, or sympathetic activity between soybean oil- and olive oil-based lipid emulsions.
Conclusion:
The 24-h infusion of PN containing soybean oil-based lipid emulsion increased blood pressure and impaired endothelial function compared with PN containing olive oil-based lipid emulsion and lipid-free PN in healthy subjects. These vascular changes may have significant implications in worsening outcome in subjects receiving nutrition support. Randomized controlled trials with relevant clinical outcome measures are needed in patients receiving PN with olive oil-based and soybean oil-based lipid emulsions.
The beneficial effect of parenteral nutrition (PN) in improving the nutrition status of critically ill and other hospitalized patients is well established (1, 2). A number of clinical trials suggest that the administration of PN in severely malnourished patients lowers the rate of postoperative infections and other hospital complications (3–5). Recent randomized trials and metaanalyses, however, have raised questions about its safety and the increased rate of PN-associated hospital complications and mortality (1, 6). The increased risk of complications and mortality during PN therapy may be related, among other factors, to the development of hyperglycemia (7, 8) or to the lipid component (9, 10). For many decades, iv lipid emulsions used in PN have been based on soybean oil, which is rich in ω-6 polyunsaturated fatty acids (PUFA) (11). Due to its high content of linoleic acid, soybean-based lipid emulsions may promote the generation of arachidonic acid-derived eicosanoids and thus potentially exaggerate the inflammatory response during stress and trauma (12). Infusion of ω-6 PUFA can also exert immunosuppressive effects by suppressing a number of neutrophil, monocyte, and macrophage functions (9). We and others recently reported that a 48-h infusion of a commercial soybean oil lipid emulsion commonly used in PN (Intralipid; Fresenius Kabi, Uppsala, Sweden), resulted in a rapid and sustained increase in blood pressure (BP), endothelial dysfunction, inflammation, and abnormal autonomic nervous system activity (11, 13, 14).
Lipid emulsions with a lower PUFA content, partly by replacing soybean oil with olive oil (ClinOleic; Baxter, Chicago, IL), are an alternative lipid emulsion for PN (15, 16). This commercial product is an 80:20% mix of olive oil and soybean oil that has been approved by regulatory agencies for use in PN in European and other countries (but not in the United States, where only soybean oil-based lipid emulsions are available for clinical use). Several in vitro, animal, and human studies have shown that the impairment of immune function, oxidative stress, abnormal glucose metabolism, and inflammation that occur with soybean oil-based lipid emulsion may be mitigated by substitution with emulsions containing predominately olive oil (9, 14, 16). Recent clinical studies have suggested that use of the olive oil-based lipid emulsion in PN may be associated with improved immune function in critically ill patients compared with PN containing soybean oil-based emulsion (15). We hypothesized that compared with olive oil-based lipid emulsion, the use of the soybean-containing lipid emulsion would be associated with worsened endothelial function, inflammation, oxidative stress, and increased sympathetic activity in healthy subjects.
Participants and Methods
Participants
We studied 12 normotensive, healthy subjects. All participants had a BP less than 140/90 mm Hg at screening and had no prior history of prediabetes or diabetes mellitus, hypertension or use of antihypertensive medications, or use of lipid medications before the study. Diabetes mellitus was excluded with a 2-h glucose of less than 200 mg/dl during a 75-g oral glucose tolerance test and a fasting glucose of less than 126 mg/dl. Subjects with fasting triglyceride levels greater than 250 mg/dl, hepatic disease (serum glutamic-oxaloacetic transaminase/aspartate aminotransferase and serum glutamate pyruvate transaminase/aminotransferase, >3 times of ULN), renal insufficiency (creatinine >1.5 mg/dl), pregnancy, breast-feeding status, recent drug abuse (within 3 months), significant mental condition, or alcoholism or tobacco use were excluded. The Institutional Review Board at Emory University approved the research protocol, and all subjects gave written and signed consent before participation.
Study protocol
Participants were admitted to the Grady Memorial Hospital Clinical Interactions Network unit of the Atlanta Clinical and Translational Science Institute on four separate occasions to receive, in random order, a 24-h infusion of normal saline, lipid-free PN, PN with Intralipid 20% (Fresenius Kabi), and PN with ClinOleic 20% (Baxter). Subjects were admitted on the afternoon before the study. The following morning, after an overnight fast, subjects underwent research studies in the morning with the infusion starting between 1200 and 1300 h. During each of the four infusions, subjects were fed a standardized 400-calorie, low-fat chicken salad for lunch and dinner prepared by the research center's metabolic kitchen and after that they were allowed nothing by mouth until completion of infusion the following day. Subjects stayed in their room on the research unit and were not allowed to exercise during the study.
BP was measured with a manual cuff in triplicate on admission and every 4 h during infusion. At baseline while fasting and then every 4 h, blood samples were drawn for metabolic assessments, lipoprotein, neutrophil function, and inflammatory and oxidative stress analysis; insulin sensitivity (as outlined below) was measured before and after each infusion.
Administration of parenteral nutrition
PN was administered using uniform guidelines for PN support. Briefly, the nutritional goal was to provide total daily calorie (kilocalories) of 1.3 × basal energy expenditure (per the Harris-Benedict equation). The total amino acid/protein intake goal was 1.5 g/kg · d. One liter of the standard PN formula included 40 g/liter, nitrogen 6.4 g/liter, dextrose 150 g/liter, lipid 30 g/liter, with a total caloric density of 970 kcal/liter.
The Intralipid 20% solution is a long-chain triglyceride emulsion composed of 50% PUFA, 26% monounsaturated fatty acids, and 19% saturated fatty acids composed of linoleic acid, 50%; oleic acid, 26%; palmitic acid, 10%; stearic acid, 9%; egg yolk; and phospholipids, 3.5%. The fatty acid composition of ClinOleic 20% formulation contains 56% oleic acid, 12.9% palmitic, 17.2% linoleic, 3.5% stearic, 2.3% α-linolenic, and less than 1% of palmitoleic, arachidonic, and docosafexaenoic acids (11).
Flow-mediated dilatation (FMD)
Endothelial function.
Endothelium-dependent brachial artery dilatation was assessed at baseline and after 4 and 24 h of infusion using established methodology (13). Briefly, ultrasound images of the brachial artery were obtained at baseline under standardized conditions and 60 sec after induction of reactive hyperemia by 5-min cuff occlusion of the forearm. Image landmarks as well as surface markers were used to ensure anatomical consistency between serial imaging studies. All images were digitized online, and arterial diameters were measured with customized software (Medical Imaging Applications, Inc., Coralville, IA) by individuals blinded to the clinical and laboratory status of the subjects. FMD was expressed as the percentage increase in diameter from baseline. In our laboratory, the mean difference in FMD between two consecutive assessments at least 1 wk apart is 1.26 ± 0.76% (r = 0.75); the mean difference in the FMD between two readings of the same subjects is 0.82 ± 0.48% (r = 0.97).
Heart rate variability
Power spectrum analysis of R-R interval variability was used to assess the state of the autonomic nervous system at baseline and after 4 and 24 h of infusion (17). Based on a generated digital rhythm strip (SphygmaCor; AtCor Medical, West Ryde, Australia), we analyzed the absolute number of consecutive R-R intervals differing by more than 50 msec (NN50) and heart rate and (18).
Laboratory assays
Plasma glucose and lipids were measured on the CX7 chemistry analyzer (Beckman Diagnostics, Fullerton, CA) using reagents and calibrators from Beckman Diagnostics. Levels of low-density cholesterol (LDL) and high-density cholesterol (HDL) cholesterol were determined using homogeneous enzymatic kits (Genzyme Diagnostics, Elkton, PA). Free fatty acid (FFA) levels were determined by a colorimetric method (Genzyme Diagnostics). Levels of IL-6, TNF-α, C-reactive protein (CRP), insulin, and C-peptide were measured in plasma using solid-phase, two-site sequential chemiluminescent immunometric assays on the DPC Immulite analyzer (Diagnostic Products Corp., Los Angeles, CA). Plasma glutathione and related redox parameters were measured as indicators of oxidative stress (19). Briefly, samples were collected in a preservation solution and stored at −80 C under conditions known to result in negligible oxidation. Samples showing visual evidence of hemolysis were discarded. Samples were treated to form dansyl derivatives, analyzed by HPLC with fluorescence detection and quantified relative to γ-glutamyl-glutamate as an internal standard. Redox potential values were calculated using the Nernst equation with Eo −264 mV for the glutathione/glutathione disulfide couple and −250 mV for the cysteine/cystine couple at pH 7.4 (19). Coefficients of variation for concentration measurements were 5–6% for all parameters except cysteine and glutathione disulfide, which were 9–10%.
Immune function
The phagocytic and oxidative burst activity of monocytes and granulocytes in heparinized whole blood was assessed according to manufacturer's instructions using specific reagent kits for this purpose at baseline and again at 24 h after the four study infusions in each subject. Briefly, granulocyte and monocyte phagocytic activity was quantitated by incubation of whole blood with fluorescein isothiocyanate-labeled, opsonized Escherichia coli bacteria at 37 C (Phagotest; Orpegen Pharma, Heidelberg, Germany) with detection of fluorescence of internalized particles as a percentage of positive cells by flow cytometry (FACSort; Becton Dickinson Biosciences, Franklin Lakes, NJ), analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Oxidative burst activity of monocytes and granulocytes was quantitated in whole blood using a kit containing unlabeled opsonized bacteria (E. coli), phorbol-12-myristate-13 acetate and the chemotactic peptide N formyl-Met-Leu-Phe as stimulants, and dihydrorhodamine-123 as a fluorogenic substrate to determine the percentage of phagocytic cells which produce reactive oxidants (Phagoburst; Orpegen Pharma). A sample without stimulants served as a negative background control for each experiment. The percentage of positive cells was determined by flow cytometry and analyzed using FlowJo software (Tree Star).
Statistical analysis
The primary end point of interest was endothelial function, with secondary end points including inflammatory marker, oxidative stress, immune function, autonomic nerve system, insulin sensitivity, and carbohydrate metabolism. We first used one-sample t tests to assess 4- and 24-h changes of endothelial function from baseline and changes in 24-h immune function parameters from baseline. Repeated-measures ANOVA models were also fit for each occasion to evaluate the overall time effect on the endothelial function during the 24-h infusion. In addition, we used nonparametric Wilcoxon tests to compare 4- and 24-h endothelial function changes among the four infusion occasions and between any two distinct occasions that subjects received infusions of saline and lipid-free PN, soybean oil-based PN, and olive oil-based PN. We further used repeated-measures ANOVA to examine the difference among the four infusion occasions and the time effect simultaneously. Similar analyses were applied to secondary endpoints of interests. All proposed methods were implemented by SAS (version 9.2; SAS Institute, Cary, NC).
Results
Patient characteristics
The clinical characteristics of study subjects are shown in Table 1. All were normotensive and nonsmokers, and none had impaired glucose tolerance or diabetes by oral glucose tolerance test or fulfilled American Heart Association diagnostic criteria for metabolic syndrome (20).
Table 1.
Clinical characteristics of study subjects
| Characteristic | Value |
|---|---|
| Age (yr) | 41 ± 7 |
| Gender (male/female) | 7/5 |
| Body weight (kg) | 90 ± 15 |
| Body mass index (kg/m2) | 32 ± 2 |
| SBP (mm Hg) | 113 ± 21 |
| Diastolic blood pressure (mm Hg) | 65 ± 12 |
| Fasting glucose (mg/dl) | 86 ± 9 |
| 2-h OGTT glucose (mg/dl) | 110 ± 26 |
| Plasma insulin (μU/ml) | 8 ± 5 |
| C-peptide (ng/ml) | 2.21 ± 1 |
| HOMA-IR | 2.0 ± 1 |
| Free fatty acids (mmol/liter) | 0.72 ± 0.3 |
| Triglycerides (mg/dl) | 102 ± 58 |
| Total cholesterol (mg/dl) | 178 ± 35 |
| LDL-cholesterol (mg/dl) | 109 ± 23 |
| HDL-cholesterol (mg/dl) | 45 ± 17 |
Values are mean ± sd. OGTT, Oral glucose tolerance test; HOMA-IR, homeostatic model assessment of insulin resistance.
Blood pressure
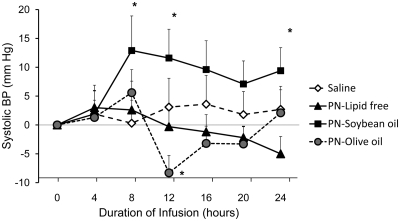
The infusion of saline and lipid free PN did not change systolic blood pressure (SBP) from baseline (Fig. 1). The administration of soybean-based PN raised SBP from baseline by 11.6 ± 16.5 mm Hg (P = 0.04) at 12 h and 9.4 ± 14.3 mm Hg (P = 0.054) at 24 h. In contrast, the infusion of olive oil-based PN resulted in a transient but significant decrease in SBP at 12 h of −8.3 ± 10.6 mm Hg from baseline (P = 0.02), but SBP levels were not significant different from baseline at the 24-h time point. Diastolic blood pressure did not change significantly during any of the study infusions (data not shown).
Fig. 1.
Changes in SBP during 24-h administration of PN containing soybean oil-based lipid emulsion, olive oil-based lipid emulsion, lipid free PN, and normal saline in healthy subjects. Values are mean ± sem. *, P < 0.05.
Endothelial function
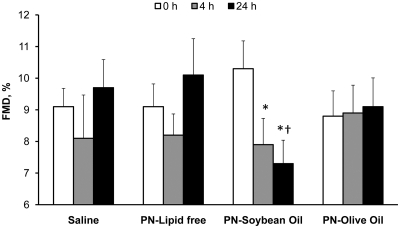
Brachial artery diameter was similar among study groups before cuff occlusion at 0, 4, and 24 h of study. The infusion of saline, lipid-free, and olive oil-based PN did not alter FMD from baseline at either 4 or 24 h of infusion (Fig. 2). In contrast, the infusion of soybean oil-based PN resulted in a significant decline in FMD from baseline at 4 and 24 h (−23 ± 17 and −26 ± 23%, both P < 0.05). Differences in FMD between groups were significantly different between subjects receiving soybean oil-based and olive oil-based PN after 24 h (P = 0.02), respectively.
Fig. 2.
FMD at 0, 4, and 24 h administration of PN containing soybean oil-based lipid emulsion, olive oil-based lipid emulsion, lipid free PN, and normal saline in healthy subjects. Values are mean ± sem. *, P < 0.05 vs. baseline; †, P = 0.02 vs. olive oil-based PN after 24 h of infusion.
Heart rate and autonomic nervous system
We observed a transient reduction in heart rate from baseline at 12 h in patients receiving saline and soybean oil- and olive oil-based PN (P < 0.05). However, there were no significant differences at 24 h, and differences in heart rate between groups were not significantly different at any time of the study. Similarly, there were no differences in Heart rate variability analysis in any of the groups from baseline or in between groups during the 24-h infusions.
Plasma FFA, glucose, insulin, and C-peptide concentrations
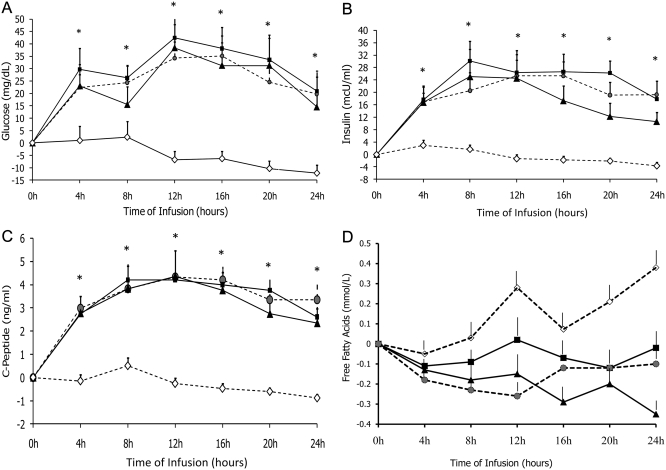
The infusion of saline resulted in no significant changes in glucose, insulin, or C-peptide concentrations (Fig. 4).
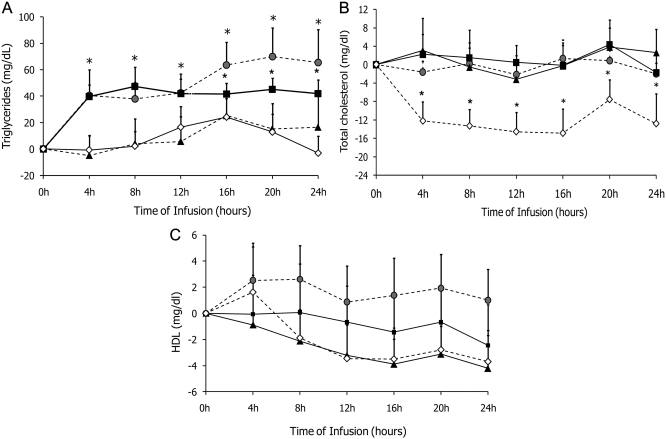
Fig. 4.
Changes in triglycerides (A), total cholesterol (B), and HDL cholesterol (C) concentration during 24 h administration of PN containing soybean oil-based lipid emulsion ( ), olive oil-based lipid emulsion (■), lipid free PN (
), olive oil-based lipid emulsion (■), lipid free PN ( ), and saline (♢) in healthy subjects. Values are mean ± sem. *, P < 0.05.
), and saline (♢) in healthy subjects. Values are mean ± sem. *, P < 0.05.
The infusion of lipid-free, soybean-oil based PN and olive oil-based PN resulted in significant increases in plasma glucose, insulin, and C-peptide concentrations from baseline, and values were significantly higher than saline infusion (P < 0.05). Glucose levels were significantly elevated during the 24-h infusion (P < 0.05); increases in glucose levels were between 20 and 30 mg/dl at 4 h, reached a peak level of 30 to 45 mg/dl at 12 h, and remained significantly elevated in until the end of the study period (Fig. 3A). Plasma insulin and C-peptide concentrations were significantly increased at 4 h, and levels remained similarly elevated in all three PN groups (Fig. 3, B and C). There were no differences in FFA levels between the three PN groups at baseline or at any point during the 24-h infusion period (Fig. 3A).
Fig. 3.
Changes in plasma glucose (A), insulin (B), and C-peptide (C) during 24 h administration of PN containing soybean oil-based lipid emulsion ( ), olive oil-based lipid emulsion (■), lipid-free PN (
), olive oil-based lipid emulsion (■), lipid-free PN ( ), and saline (♢) in healthy subjects. Values are mean ± sem. *, P < 0.05.
), and saline (♢) in healthy subjects. Values are mean ± sem. *, P < 0.05.
Plasma lipid profile
Triglyceride levels increased significantly from baseline after infusion of either soybean oil- or olive oil-based PN (each P < 0.05) compared with lipid free PN and saline infusion (Fig. 4A). Significant changes in triglycerides were observed after 4 h and levels remained likewise elevated during PN with either lipid emulsion. There were no differences in total, LDL, and HDL cholesterol concentrations during the 24-h infusion among the three PN groups (Fig. 4, B and C). Levels of total cholesterol were reduced in the saline infusion group, whereas levels remained quite constant in the three PN groups.
Plasma levels of inflammation and oxidative stress markers
Table 2 shows circulating levels of inflammatory markers (CRP IL-6, and TNF-α) and oxidative stress (cystine, cysteine, cysteine-glutathione disulfide, glutathione, glutathione disulfide, redox potential of glutathione/glutathione disulfide couple; redox potential of cysteine/cystine couple; sum of glutathione + 2*glutathione disulfide + cysteine-glutathione disulfide; total cysteine, sum of cysteine + 2*cystine + cysteine-glutathione disulfide) at baseline and during saline and the three PN infusions at any time point during the study (Table 2).
Table 2.
Plasma concentration of inflammatory and oxidative stress markers after 24 h administration of PN containing soybean oil-based lipid emulsion, olive oil-based lipid emulsion, lipid-free PN, and normal saline in healthy subjects
| Variable | 0 h | 12 h | 24 h | P valuea |
|---|---|---|---|---|
| CRP (mg/liter) | 0.198 | |||
| Saline | 3.88 ± 3.34 | 3.89 ± 2.63 | 4.41 ± 2.87 | |
| Lipid free | 5.91 ± 5.58 | 5.73 ± 4.17 | 4.59 ± 3.77 | |
| Soybean oil based | 3.94 ± 2.85 | 5.15 ± 4.18 | 4.35 ± 3.07 | |
| Olive oil based | 4.03 ± 2.73 | 5.64 ± 3.39 | 5.59 ± 3.64 | |
| TNF-α (pg/ml) | 0.422 | |||
| Saline | 10.50 ± 4.71 | 10.29 ± 4.24 | 10.99 ± 4.36 | |
| Lipid free | 12.25 ± 5.01 | 13.83 ± 5.99 | 11.83 ± 4.21 | |
| Soybean oil based | 11.5 ± 4.51 | 13.01 ± 5.63 | 12.06 ± 5.59 | |
| Olive oil based | 10.01 ± 3.67 | 10.16 ± 3.67 | 11.14 ± 4.72 | |
| IL-6 (pg/ml) | 0.795 | |||
| Saline | 2.45 ± 0.62 | 9.11 ± 3.94 | 6.06 ± 5.58 | |
| Lipid free | 3.13 ± 1.70 | 8.2 ± 5.58 | 5.11 ± 2.25 | |
| Soybean oil based | 2.61 ± 1.12 | 8.52 ± 3.71 | 6.54 ± 4.44 | |
| Olive oil based | 2.76 ± 0.69 | 6.36 ± 2.67 | 5.59 ± 2.69 | |
| Cystine (μm) | 0.978 | |||
| Saline | 66.46 ± 17.50 | 66.69 ± 10.52 | 69.01 ± 14.23 | |
| Lipid free | 75.26 ± 23.71 | 81.31 ± 20.67 | 78.95 ± 15.06 | |
| Soybean oil based | 70.08 ± 22.62 | 77.99 ± 18.74 | 72.48 ± 15.16 | |
| Olive oil based | 76.60 ± 25.90 | 81.26 ± 19.79 | 80.97 ± 19.79 | |
| Cysteine (μm) | 0.101 | |||
| Saline | 8.99 ± 2.60 | 8.34 ± 2.66 | 8.29 ± 1.95 | |
| Lipid free | 9.42 ± 2.78 | 10.35 ± 3.35 | 9.81 ± 2.85 | |
| Soybean oil based | 7.89 ± 2.92 | 9.33 ± 3.57 | 8.28 ± 1.66 | |
| Olive oil based | 8.26 ± 2.86 | 10.18 ± 2.93 | 8.78 ± 2.85 | |
| Cysteine-glutathione disulfide (μm) | 0.338 | |||
| Saline | 1.77 ± 0.74 | 2.24 ± 1.36 | 1.82 ± 0.95 | |
| Lipid free | 2.10 ± 0.99 | 1.40 ± 0.66 | 1.75 ± 0.83 | |
| Soybean oil based | 1.96 ± 0.83 | 1.63 ± 0.62 | 1.38 ± 0.45 | |
| Olive oil based | 1.97 ± 0.97 | 1.64 ± 0.65 | 1.79 ± 0.89 | |
| Glutathione (μm) | 0.374 | |||
| Saline | 1.13 ± 0.83 | 1.10 ± 1.01 | 0.88 ± 0.42 | |
| Lipid free | 1.01 ± 0.80 | 0.62 ± 0.33 | 0.73 ± 0.38 | |
| Soybean oil based | 0.88 ± 0.64 | 0.73 ± 0.47 | 0.75 ± 0.29 | |
| Olive oil based | 0.74 ± 0.29 | 0.84 ± 0.71 | 0.78 ± 0.49 | |
| Glutathione disulfide (μm) | 0.387 | |||
| Saline | 0.04 ± 0.03 | 0.04 ± 0.05 | 0.03 ± 0.04 | |
| Lipid free | 0.06 ± 0.08 | 0.03 ± 0.02 | 0.05 ± 0.06 | |
| Soybean oil based | 0.04 ± 0.03 | 0.04 ± 0.05 | 0.04 ± 0.03 | |
| Olive oil based | 0.03 ± 0.01 | 0.13 ± 0.30 | 0.05 ± 0.04 | |
| Glutathione redox potential (μm) | 0.021 | |||
| Saline | −129.13 ± 20.42 | −125.78 ± 23.59 | −129.09 ± 12.51 | |
| Lipid free | −122.75 ± 13.58 | −121.30 ± 14.34 | −118.03 ± 15.08 | |
| Soybean oil based | −117.12 ± 36.97 | −114.56 ± 27.39 | −121.90 ± 16.22 | |
| Olive oil based | −120.23 ± 12.07 | −119.09 ± 20.32 | −116.03 ± 17.17 | |
| Cysteine redox potential (μm) | 0.051 | |||
| Saline | −72.08 ± 7.42 | −68.50 ± 13.79 | −68.73 ± 7.20 | |
| Lipid free | −71.20 ± 7.41 | −72.20 ± 8.79 | −71.73 ± 8.34 | |
| Soybean oil based | −67.00 ± 11.78 | −69.20 ± 14.41 | −69.06 ± 7.20 | |
| Olive oil based | −67.87 ± 8.91 | −72.54 ± 6.87 | −69.27 ± 7.45 | |
| Total glutathione, sum of glutathione + 2* glutathione disulfide + cysteine-glutathione disulfide (μm) | 0.565 | |||
| Saline | 2.97 ± 1.54 | 3.43 ± 2.41 | 2.76 ± 1.40 | |
| Lipid free | 3.24 ± 1.55 | 2.05 ± 0.89 | 2.63 ± 1.17 | |
| Soybean oil based | 2.88 ± 1.15 | 2.46 ± 1.08 | 2.17 ± 0.64 | |
| Olive oil based | 2.77 ± 1.13 | 2.55 ± 1.22 | 2.66 ± 1.25 | |
| Total cysteine, sum of cysteine + 2*cystine + cysteine-glutathione disulfide (μm) | 0.983 | |||
| Saline | 143.74 ± 36.23 | 143.93 ± 21.94 | 148.12 ± 29.15 | |
| Lipid free | 162.05 ± 50.21 | 170.62 ± 43.32 | 169.47 ± 3098 | |
| Soybean oil based | 150.03 ± 46.17 | 166.91 ± 38.34 | 154.58 ± 29.83 | |
| Olive oil based | 163.43 ± 52.90 | 174.34 ± 41.09 | 172.50 ± 40.95 |
P value for comparing the changing trend of each variable across time points (0, 4, 24 h) among the four infusion groups based on repeated-measures ANOVA models.
Immune function
In general, no significant changes were noted for granulocyte phagocytosis, granulocyte reactive oxygen species (ROS) generation, and monocyte reactive oxygen species generation from baseline and between groups during the 24 h of infusion in the four study groups (Table 3). Only monocyte phagocytosis was found to be significantly different among the four infusion groups (P = 0.023). Monocyte phagocytosis in the lipid-free infusion group and soybean-based infusion groups were significantly higher than in the normal saline infusion group (P = 0.032 and P = 0.009, respectively).
Table 3.
Change in immune function after 24 h administration of PN containing soybean oil- and olive oil-based lipid emulsion, lipid-free PN, and normal saline in healthy subjects
| Normal saline | Lipid free | Soybean oil based | Olive oil based | P valuea | |
|---|---|---|---|---|---|
| Granulocyte phagocytosis (%) | 11.5 ± 55.8 | 1.5 ± 5.3 | 0.0 ± 8.3 | 1.6 ± 10.4 | 0.90 |
| Monocyte phagocytosis (%) | 04.1 ± 51.3 | −1.5 ± 14.1 | 2.7 ± 17.7 | −4.8 ± 16.1 | 0.22 |
| Granulocyte ROS generation (%) | −0.6 ± 30.8 | 9.1 ± 18.3 | −12.1 ± 32.9 | 9.1 ± 30.2 | 0.56 |
| Monocyte ROS generation (%) | −4.9 ± 32.0 | 6.5 ± 23.9 | −15.4 ± 34.9 | −3.6 ± 33.9 | 0.59 |
P value for testing whether the change in immune function is the same as among the four PN groups.
Discussion
This is the first prospective, randomized, controlled study to compare the vascular, metabolic, and inflammatory effects of lipid-free PN, soybean oil-based PN, and olive oil-based PN in healthy subjects. We found that the administration of soybean oil-based PN resulted in a rapid and sustained increase in BP and decreased endothelial function compared with olive oil-based PN and lipid-free PN. In contrast, olive oil-based PN had neutral effects on BP and endothelial function compared with lipid-free PN and saline infusion. Compared with saline, the 24 h infusion of PN with either soybean oil or olive oil resulted in similar increases in plasma glucose, insulin, C-peptide, and FFA, but there were no significant changes in inflammatory markers or heart rate variability between groups.
Malnutrition is common with a prevalence of 20–40% in intensive care unit (ICU) patients (2, 21) and has been shown to be an independent risk factor impacting on higher complications and increased mortality, length of hospital stay, and costs (22). PN improves nutritional status and provides essential substrates and micronutrients for cell and organ functions in hospitalized patients (11, 23–25). Despite improving nutrition status, the use of PN is associated with increased risk for hospital complications and mortality in some critically ill patients (1, 6, 26–28). The increased risk of complications and mortality during PN therapy may be related, among other factors, to excessive glucose and lipid loads that can lead to hyperglycemia, proinflammatory responses, altered leukocyte function, endothelial dysfunction, and increased oxidative stress in some patients (29–31). We previously reported that Intralipid infusion (Fresenius Kabi), the most commonly used soybean oil-based lipid emulsion used in PN formulations, results in rapid and sustained increases in blood pressure, endothelial dysfunction, inflammatory markers, and autonomic nervous system activity in patients with and without diabetes (13, 32). Due to its high content of PUFA, soybean-based lipid emulsions have the potential to exert immunosuppressive effects (9), endothelial dysfunction, nitric oxide production, proinflammatory response, and increased sympathetic activity (11, 14) as well as dose-dependent insulin resistance in diabetic and nondiabetic individuals (33–35). Short-term infusion of PUFA has also been shown to increase lipid peroxide formation and to decrease plasma glutathione, the most abundant extracellular antioxidant (36, 37). At the cellular level, fatty acids have been shown to activate both typical and atypical isoforms of protein kinase C (38), which are involved in the regulation of vascular tone and vascular smooth muscle cell growth and may contribute to endothelial dysfunction (38).
Newer lipid emulsions, such as the olive oil-based formula used in the current study, have been developed as a strategy to decrease potential deleterious effects observed with traditional soybean-based PN formulations (15, 16). The formulation we used (ClinOleic; Baxter) contains 80% olive oil and 20% soybean oil (11). The effects of this olive oil-based lipid emulsion have been examined in a number of settings including in vitro, animal, healthy human volunteer, and clinical studies (8, 13, 39). ClinOleic has been shown to have a comparable nutritional efficacy to Intralipid (9, 14, 39); however, experimental studies indicate that ClinOleic has less immunosuppressive and proinflammatory responses than soybean oil-based formulations (40, 41). Unblinded clinical studies in surgical patients have suggested that a shorter ICU stay and time on mechanical ventilation for patients treated with ClinOleic rather than with soybean oil formulas (42–44). Other studies, however, have reported no differences with regard to mortality, septicemia, infectious complications, multiple organ dysfunction, length of ICU, hospital stay, and mortality with soybean oil-based vs. olive-oil based lipid emulsion in PN (14, 45).
The underlying mechanisms for the increased in BP and endothelial dysfunction with soybean based lipid emulsion are not completely understood. We recently reported that high FFA levels after Intralipid (Fresenius Kabi) infusion for 48 h were associated with a rapid and sustained elevation in BP and endothelial dysfunction in obese diabetic individuals (13). The vascular abnormalities after chronic low-dose Intralipid infusion can also occur in nondiabetic individuals as well as was demonstrated in recent studies (46, 47). Some have hypothesized that increased FFA stimulate production of reactive oxygen species (36), increase lipid peroxidation (37), and regulate vascular tone contributing to endothelial dysfunction (38). Our results indicate that the observed changes in BP and endothelial function with soybean-based PN cannot be explained solely by acute changes in circulating FFA concentration because levels were similar to those observed after the infusion of olive oil-based and lipid free-PN. In addition, we found no difference in the rate of oxidative stress, inflammation, and carbohydrate tolerance between lipid emulsions.
In summary, our study provides novel information on human responses to PN containing traditional soybean-derived lipid emulsion and PN containing an olive oil-based lipid emulsion as new alternative formulation for PN. In agreement with previous reports, we observed that a 24-h administration of soybean-based lipid emulsion resulted in significant endothelial dysfunction and increased BP in healthy adults (13). In contrast, administration of olive oil-based and lipid-free PN had neutral effects on BP and endothelial function. These preliminary studies support the need for future randomized controlled trials to determine benefits in clinical outcome with olive oil-based vs. soybean-based lipid emulsions in hospitalized patients treated with PN. However, our studies were of short duration and involved healthy volunteers; thus, it is not clear whether the use of olive oil-based lipid emulsions may result in improved clinical outcomes over traditional lipid emulsions in hospitalized patients under the stress of medical and surgical illness.
Acknowledgments
This investigator-initiated study was supported by a research grant from Baxter Pharmaceuticals (to G.E.U.) and National Institutes of Health Grant UL1 RR025008 (Atlanta Clinical and Translational Science Institute), American Diabetes Association Grant 7-03-CR-35 (to G.E.U.) and Grant K24 RR023356 (to T.R.Z.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BP
- Blood pressure
- CRP
- C-reactive protein
- FFA
- free fatty acid
- FMD
- flow-mediated dilatation
- HDL
- high-density cholesterol
- ICU
- intensive care unit
- LDL
- low-density cholesterol
- PN
- parenteral nutrition
- PUFA
- ω-6 polyunsaturated fatty acid
- ROS
- reactive oxygen species
- SBP
- systolic blood pressure.
References
- 1. Heyland DK, Montalvo M, MacDonald S, Keefe L, Su XY, Drover JW. 2001. Total parenteral nutrition in the surgical patient: a meta-analysis. Can J Surg 44:102–111 [PubMed] [Google Scholar]
- 2. Ziegler TR. 2009. Parenteral nutrition in the critically ill patient. N Engl J Med 361:1088–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muller JM, Brenner U, Dienst C, Pichlmaier H. 1982. Preoperative parenteral feeding in patients with gastrointestinal carcinoma. Lancet 1:68–71 [DOI] [PubMed] [Google Scholar]
- 4. 1991. Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med 325:525–532 [DOI] [PubMed] [Google Scholar]
- 5. Detsky AS, Baker JP, O'Rourke K, Goel V. 1987. Perioperative parenteral nutrition: a meta-analysis. Ann Intern Med 107:195–203 [DOI] [PubMed] [Google Scholar]
- 6. Anderson AD, Jain PK, MacFie J. 2003. Parenteral nutrition in the critically ill. Intensive Care Med 29:2103; author reply 29:2104. [DOI] [PubMed] [Google Scholar]
- 7. Cheung NW, Wong VW, McLean M. 2006. The Hyperglycemia: Intensive Insulin Infusion in Infarction (HI-5) study: a randomized controlled trial of insulin infusion therapy for myocardial infarction. Diabetes Care 29:765–770 [DOI] [PubMed] [Google Scholar]
- 8. Pasquel FJ, Spiegelman R, McCauley M, Smiley D, Umpierrez D, Johnson R, Rhee M, Gatcliffe C, Lin E, Umpierrez E, Peng L, Umpierrez GE. 2010. Hyperglycemia during total parenteral nutrition: an important marker of poor outcome and mortality in hospitalized patients. Diabetes Care 33:739–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buenestado A, Cortijo J, Sanz MJ, Naim-Abu-Nabah Y, Martinez-Losa M, Mata M, Issekutz AC, Martí-Bonmati E, Morcillo EJ. 2006. Olive oil-based lipid emulsion's neutral effects on neutrophil functions and leukocyte-endothelial cell interactions. JPEN J Parenter Enteral Nutr 30:286–296 [DOI] [PubMed] [Google Scholar]
- 10. Yaqoob P. 1998. Lipids and the immune response. Curr Opin Clin Nutr Metab Care 1:153–161 [DOI] [PubMed] [Google Scholar]
- 11. Waitzberg DL, Torrinhas RS, Jacintho TM. 2006. New parenteral lipid emulsions for clinical use. JPEN J Parenter Enteral Nutr 30:351–367 [DOI] [PubMed] [Google Scholar]
- 12. Yaqoob P. 2005. Monounsaturated fatty acids in parenteral nutrition; evaluation of risks and benefits. Br J Nutr 94:867–868 [DOI] [PubMed] [Google Scholar]
- 13. Umpierrez GE, Smiley D, Robalino G, Peng L, Kitabchi AE, Khan B, Le A, Quyyumi A, Brown V, Phillips LS. 2009. Intravenous intralipid-induced blood pressure elevation and endothelial dysfunction in obese African-Americans with type 2 diabetes. J Clin Endocrinol Metab 94:609–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sala-Vila A, Barbosa VM, Calder PC. 2007. Olive oil in parenteral nutrition. Curr Opin Clin Nutr Metab Care 10:165–174 [DOI] [PubMed] [Google Scholar]
- 15. García-de-Lorenzo A, Denia R, Atlan P, Martinez-Ratero S, Le Brun A, Evard D, Bereziat G. 2005. Parenteral nutrition providing a restricted amount of linoleic acid in severely burned patients: a randomised double-blind study of an olive oil-based lipid emulsion vs. medium/long-chain triacylglycerols. Br J Nutr 94:221–230 [DOI] [PubMed] [Google Scholar]
- 16. Yaqoob P. 1998. Monounsaturated fats and immune function. Proc Nutr Soc 57:511–520 [DOI] [PubMed] [Google Scholar]
- 17. 1996. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93:1043–1065 [PubMed] [Google Scholar]
- 18. O'Rourke MF, Gallagher DE. 1996. Pulse wave analysis. J Hypertens Suppl 14:S147–S157 [PubMed] [Google Scholar]
- 19. Jones DP, Liang Y. 2009. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med 47:1329–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. 2005. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 21. Giner M, Laviano A, Meguid MM, Gleason JR. 1996. In 1995 a correlation between malnutrition and poor outcome in critically ill patients still exists. Nutrition 12:23–29 [DOI] [PubMed] [Google Scholar]
- 22. Correia MI, Waitzberg DL. 2003. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr 22:235–239 [DOI] [PubMed] [Google Scholar]
- 23. Martindale RG, Cresci G. 2005. Preventing infectious complications with nutrition intervention. JPEN J Parenter Enteral Nutr 29:S53–S56 [DOI] [PubMed] [Google Scholar]
- 24. Strickland A, Brogan A, Krauss J, Martindale R, Cresci G. 2005. Is the use of specialized nutritional formulations a cost-effective strategy? A national database evaluation. JPEN J Parenter Enteral Nutr 29:S81–S91 [DOI] [PubMed] [Google Scholar]
- 25. Marik PE, Zaloga GP. 2004. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ 328:1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bellantone R, Doglietto G, Bossola M, Pacelli F, Negro F, Sofo L, Crucitti F. 1988. Preoperative parenteral nutrition of malnourished surgical patients. Acta Chir Scand 154:249–251 [PubMed] [Google Scholar]
- 27. Klein S, Kinney J, Jeejeebhoy K, Alpers D, Hellerstein M, Murray M, Twomey P. 1997. Nutrition support in clinical practice: review of published data and recommendations for future research directions. Summary of a conference sponsored by the National Institutes of Health, American Society for Parenteral and Enteral Nutrition, and American Society for Clinical Nutrition. Am J Clin Nutr 66:683–706 [DOI] [PubMed] [Google Scholar]
- 28. Marik PE. 2006. Death by total parenteral nutrition: part deaux. Crit Care Med 34:3062; author reply 34:3062–3063 [DOI] [PubMed] [Google Scholar]
- 29. Montori VM, Bistrian BR, McMahon MM. 2002. Hyperglycemia in acutely ill patients. JAMA 288:2167–2169 [DOI] [PubMed] [Google Scholar]
- 30. McCowen KC, Malhotra A, Bistrian BR. 2001. Stress-induced hyperglycemia. Crit Care Clin 17:107–124 [DOI] [PubMed] [Google Scholar]
- 31. Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, Hirsch IB, Hirsch IB. 2004. Management of diabetes and hyperglycemia in hospitals. Diabetes Care 27:553–591 [DOI] [PubMed] [Google Scholar]
- 32. Gosmanov AR, Smiley DD, Robalino G, Siquiera J, Khan B, Le NA, Patel RS, Quyyumi AA, Peng L, Kitabchi AE, Umpierrez GE. 2010. Effects of oral and intravenous fat load on blood pressure, endothelial function, sympathetic activity, and oxidative stress in obese healthy subjects. Am J Physiol Endocrinol Metab 299:E953–E958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boden G, Chen X, Rosner J, Barton M. 1995. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes 44:1239–1242 [DOI] [PubMed] [Google Scholar]
- 34. Boden G, Lebed B, Schatz M, Homko C, Lemieux S. 2001. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes 50:1612–1617 [DOI] [PubMed] [Google Scholar]
- 35. Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. 1996. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97:2859–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. 2000. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C–dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49:1939–1945 [DOI] [PubMed] [Google Scholar]
- 37. Paolisso G, Gambardella A, Tagliamonte MR, Saccomanno F, Salvatore T, Gualdiero P, D'Onofrio MV, Howard BV. 1996. Does free fatty acid infusion impair insulin action also through an increase in oxidative stress? J Clin Endocrinol Metab 81:4244–4248 [DOI] [PubMed] [Google Scholar]
- 38. Egan BM, Greene EL, Goodfriend TL. 2001. Insulin resistance and cardiovascular disease. Am J Hypertens 14:116S–125S [DOI] [PubMed] [Google Scholar]
- 39. Reimund JM, Rahmi G, Escalin G, Pinna G, Finck G, Muller CD, Duclos B, Baumann R. 2005. Efficacy and safety of an olive oil-based intravenous fat emulsion in adult patients on home parenteral nutrition. Aliment Pharmacol Ther 21:445–454 [DOI] [PubMed] [Google Scholar]
- 40. Granato D, Blum S, Rössle C, Le Boucher J, Malnoë A, Dutot G. 2000. Effects of parenteral lipid emulsions with different fatty acid composition on immune cell functions in vitro. JPEN J Parenter Enteral Nutr 24:113–118 [DOI] [PubMed] [Google Scholar]
- 41. Reimund JM, Scheer O, Muller CD, Pinna G, Duclos B, Baumann R. 2004. In vitro modulation of inflammatory cytokine production by three lipid emulsions with different fatty acid compositions. Clin Nutr 23:1324–1332 [DOI] [PubMed] [Google Scholar]
- 42. Huschak G, Zur Nieden K, Hoell T, Riemann D, Mast H, Stuttmann R. 2005. Olive oil based nutrition in multiple trauma patients: a pilot study. Intensive Care Med 31:1202–1208 [DOI] [PubMed] [Google Scholar]
- 43. Kohlhardt SR, Smith RC, Kee AJ. 1994. Metabolic response to a high-lipid, high-nitrogen peripheral intravenous nutrition solution after major upper-gastrointestinal surgery. Nutrition 10:317–326 [PubMed] [Google Scholar]
- 44. Tappy L, Schwarz JM, Schneiter P, Cayeux C, Revelly JP, Fagerquist CK, Jéquier E, Chioléro R. 1998. Effects of isoenergetic glucose-based or lipid-based parenteral nutrition on glucose metabolism, de novo lipogenesis, and respiratory gas exchanges in critically ill patients. Crit Care Med 26:860–867 [DOI] [PubMed] [Google Scholar]
- 45. Vahedi K, Atlan P, Joly F, Le Brun A, Evard D, Perennec V, Roux-Haguenau D, Bereziat G, Messing B. 2005. A 3-month double-blind randomised study comparing an olive oil- with a soyabean oil-based intravenous lipid emulsion in home parenteral nutrition patients. Br J Nutr 94:909–916 [DOI] [PubMed] [Google Scholar]
- 46. Kashyap SR, Belfort R, Cersosimo E, Lee S, Cusi K. 2008. Chronic low-dose lipid infusion in healthy patients induces markers of endothelial activation independent of its metabolic effects. J Cardiometab Syndr 3:141–146 [DOI] [PubMed] [Google Scholar]
- 47. Mathew M, Tay E, Cusi K. 2010. Elevated plasma free fatty acids increase cardiovascular risk by inducing plasma biomarkers of endothelial activation, myeloperoxidase and PAI-1 in healthy subjects. Cardiovasc Diabetol 9:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]