Abstract
Context:
Deficits in bone acquisition during growth may increase fracture risk. Assessment of bone health during childhood requires appropriate reference values relative to age, sex, and population ancestry to identify bone deficits.
Objective:
The objective of this study was to provide revised and extended reference curves for bone mineral content (BMC) and areal bone mineral density (aBMD) in children.
Design:
The Bone Mineral Density in Childhood Study was a multicenter longitudinal study with annual assessments for up to 7 yr.
Setting:
The study was conducted at five clinical centers in the United States.
Participants:
Two thousand fourteen healthy children (992 males, 22% African-Americans) aged 5–23 yr participated in the study.
Intervention:
There were no interventions.
Main Outcome Measures:
Reference percentiles for BMC and aBMD of the total body, lumbar spine, hip, and forearm were obtained using dual-energy x-ray absorptiometry for Black and non-Black children. Adjustment factors for height status were also calculated.
Results:
Extended reference curves for BMC and aBMD of the total body, total body less head, lumbar spine, total hip, femoral neck, and forearm for ages 5–20 yr were constructed relative to sex and age for Black and non-Black children. Curves are similar to those previously published for 7–17 year olds. BMC and aBMD values were greater for Black vs. non-Black children at all measurement sites.
Conclusions:
We provide here dual-energy x-ray absorptiometry reference data on a well-characterized cohort of 2012 children and adolescents. These reference curves provide the most robust reference values for the assessment and monitoring of bone health in children and adolescents in the literature to date.
Bone tissue is responsive to metabolic, genetic, and behavioral factors. A variety of chronic health conditions are known to affect bone mineral accretion in children and may also result in poor growth and delayed maturation (1). Assessment of bone health in pediatric patients is important to identify children who may be at risk of poor mineral accretion or future risk of osteoporosis due to low bone mineral density (BMD).
Dual-energy x-ray absorptiometry (DXA) is the most widely used method for assessing BMD and is a good surrogate measure of bone health. It is ideal for pediatric use because of its wide availability, rapid scan times, and low radiation exposure. In healthy, normally growing children, DXA measures of bone mineral content (BMC) and areal BMD (aBMD) increase as a function of age and sexual maturation. Therefore, accurate assessment of bone health in children depends on robust reference data to determine whether an individual child's BMC or aBMD is comparable with same-age and -sex peers.
Total body and lumbar spine scans are recommended for clinical assessment of bone health in children (2). Total body less head (TBLH) BMC or aBMD is preferred due to the changes in relative contribution of the head to total BMC and aBMD during growth and the importance of the postcranial skeleton in fracture risk assessment. However, there may be special circumstances for which the forearm or proximal femur may be the preferred scan site. DXA outcomes are influenced by bone size, so adjustment for body size is recommended for children, particularly those whose growth in height is at the extremes of the normal growth continuum (2, 3).
Previously we reported reference curves for BMC and aBMD of the total body, lumbar spine, forearm, and proximal femur for children aged 7–17 yr from the Bone Mineral Density in Childhood Study (BMDCS), a large national cohort of children for whom standardized DXA measurements were obtained (4). We also reported an adjustment procedure to account for tall or short stature relative to age (5). These previous reports were based on initial study data. We now present the complete data collected during four additional annual evaluations with additional recruitment of younger and older participants to produce enhanced reference curves for children aged 5–20 yr, and corresponding height adjustment equations.
Subjects and Methods
Study population
Children were recruited from five clinical centers in the United States: Children's Hospital of Los Angeles (Los Angeles, CA), Cincinnati Children's Hospital Medical Center (Cincinnati, OH), Creighton University (Omaha, NE), Children's Hospital of Philadelphia (Philadelphia, PA), and Columbia University (New York, NY). Initial recruitment occurred from July 2002 to November 2003 for girls aged 6–15 yr and boys aged 6–16 yr, with annual visits for 6 yr (up to seven visits). A second recruitment wave occurred between 2006 and 2007 to increase the number of younger (5 yr) and older (19 yr) participants to extend the reference percentiles from ages 5 to 20 yr. These subjects were evaluated annually for 2 yr (up to three visits).
As described previously (4), the criteria for study entry were designed to acquire a multiethnic sample of healthy, normally developing children. These criteria included anticipated residence in the United States for 3 yr or longer; school placement within 1 yr of expected for age; term birth (≥37 wk gestation or longer); birth weight greater than 2.3 kg; and no evidence of precocious or delayed puberty. For females, normal puberty was defined as onset of breast development at 8–13 yr, onset of menses between 10 and 16 yr, and onset of pubic hair present at 7 y or older in African-American and Hispanic girls and 8 yr or older in non-Hispanic white or other ethnicities. For males, the criteria were testes size 4 ml or greater between 9 and 14 yr and pubic hair development at 9 yr or older. Children were excluded for two or more fractures if age 10 yr or younger, or three or more fractures if age older than 10 yr; current or previous medication use or medical condition known to affect bone health; extended bed rest; height, weight, or body mass index (BMI) outside the range of the third to the 97th percentile; indwelling hardware; or scoliosis.
Written informed consent was obtained from the study participants aged 18 yr or older. For participants younger than 18 yr of age, consent was obtained from the parent or guardian and assent was obtained from participants. The protocol was approved by the institutional review boards of each clinical center.
Bone densitometry
DXA scans were obtained using Hologic, Inc. (Bedford, MA) bone densitometers (QDR4500A, QDR4500W, Delphi A, and Apex models). One densitometer was used at each clinical center. The acquisition software versions varied slightly from version 11.1 to 12.7 (Apex 2.1).
Scans were obtained following manufacturer guidelines for patient positioning. Whole-body, posterioanterior lumbar spine (L1-L4, fast array), nondominant forearm, and left proximal femur (fast array) scans were acquired for each study participant. Cross-calibration of DXA devices and longitudinal calibration stability were monitored as previously described (4). All scans were analyzed centrally by the DXA Core Laboratory (University of California, San Francisco, San Francisco, CA) using Hologic software version Discovery 12.3 for baseline scans. Hologic's Apex 2.1 software was used for follow-up scan analysis using the compare feature. By design, there are no differences in the Discovery and APEX software for scan analysis in subjects younger than 20 yr old. However, the use of the compare feature during analysis forced APEX software to use the same analysis as used for the baseline regardless of age. An in-study validation determined that there were no systematic differences between the software versions for scan analysis over the course of the study when these procedures were followed (data not shown).
Descriptive measures
The study methods were identical to those previously described (4). Weight was measured on a digital scale, and height was measured using a stadiometer. Participants were dressed in examination gowns or light-weight clothing with shoes removed during the measurement. Height, weight, and BMI Z-scores were calculated using the 2000 growth charts from the Centers for Disease Control and Prevention (6).
Information on population of origin (African-American, Asian, etc.) and ethnicity (Hispanic/Latino vs. non-Hispanic/Latino) was elicited by questionnaire using the National Institutes of Health and U.S. Bureau of the Census classifications.
Sexual maturity stage was assessed by an experienced physician or nurse skilled in pediatric endocrinology. For this report, puberty stage was based on breast development in girls or testicular volume by orchidometer in boys as determined using standard clinical endocrine practice and according to the criteria of Tanner (7, 8).
Statistical analysis
aBMD and areal BMC reference curves were created as previously described (4). In brief, the power for the Box-Cox transformation, median, sd (LMS) approach (9) was used to generate BMC and aBMD curves relative to age using LMS Chartmaker version 1.16 (10). Sex-specific curves were constructed for Black and non-Black groups for each DXA measurement site. The LMS analysis generates age-specific values for the median (M), sd (S), and power for the Box-Cox transformation (L), which are used to construct centile curves using equation 1 as follows:
where Z is the Z-score that corresponds to a given percentile. For an individual DXA measurement (X), the Z-score can be calculated using the age-specific L, M, and S parameters and equation 2 as follows:
The fit of the centile curves was assessed by visual inspection as recommended in the software guidelines (10) on obtaining optimal L, M, and S parameter values and by comparison of the empirical centiles with the LMS-generated curves. The number of study participants over the age of 20 yr was sparse, so the age of all subjects with age 20 yr or older was recoded as 20 yr. Summary statistics were performed using Stata Version 10 software (StataCorp, College Station, TX) using the XTSUM procedure to account for the multiple observations per subject.
To confirm the need for separate curves for Black and non-Black groups, the non-Black reference curves were used to calculate Z-scores for subjects in the Black cohort. Mixed-effects regression analysis, accounting for multiple observations per subject, was used to examine these Z-scores. Age and sex trends were evaluated to determine whether separate curves for Black and non-Black groups were needed throughout childhood and adolescence.
We previously demonstrated an adjustment method for DXA outcome measures among children of short or tall stature using height Z-score (Ht-Z). Ht-Z, based on U.S. growth curves, can be calculated using the Epi Info Nutrition Calculator, a free software package available for download from the U.S. Centers for Disease Control and Prevention web site (http://www.cdc.gov/epiinfo/). The adjustment method requires three steps: 1) calculation of the BMC/aBMD for age Z-score (equation 2 above) and Ht-Z based on the U.S. growth curves; 2) calculation of a predicted BMC/aBMD Z-score based on age and Ht-Z (equation 3 below); and 3) calculation of the adjusted BMC/aBMD Z-score using the predicted BMC/aBMD Z-score and the BMC/aBMD for age Z-score (equation 4). Adjustment equations based on the expanded sample size and age range were calculated using generalized estimating equations with the population average option to determine relationships between Ht-Z and BMC/aBMD Z-scores. Separate equations were constructed for each bone outcome according to sex and Black vs. non-Black group. Equations with low explanatory value (R2 < 0.10) were excluded. The calculation of HZ × adjusted bone Z-score is accomplished with the following equations:
where the intercept and slope (β) are provided in the tables, and Ht-Z is calculated using the EpiInfo software; and
where the bone Z-score is the aBMD or BMC Z-score calculated using equation 2 and the L, M, and S values provided in the tables. For example, for a non-Black male aged 14.7 yr with a height of 152.1 cm and a spine BMD of 0.604, his rounded age would be 15 yr, and the L, M, and S values from Table 4 are 0.436, 0.873, and 0.121, respectively. To calculate the spine BMD Z-score, using equation 2, the spine BMD Z-score = [([0.604/0.873]0.436) − 1]/(0.436 × 0.121) = −2.81. His Ht-Z using the EpiInfo nutrition calculator is −1.95. We then use equation 3 to calculate his predicted spine BMD Z-score using the values given in Table 4 for 15 yr old non-Black males: [−0.071 + (−1.95 × 0.707)] −1.45. Using equation 4, we calculate his Ht-Z-adjusted spine BMD Z-score: [−2.81 − (−1.45)] = −1.36.
Table 4.
Age- and sex-specific reference percentiles for lumbar spine aBMD for non-Black children
| Lumbar spine aBMD | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | Non-Black females |
Non-Black males |
||||||||||||||
| L | S | M |
HZ prediction equation | L | S | M |
HZ prediction equation | |||||||||
| 3rd | 10th | 50th | 90th | 97th | 3rd | 10th | 50th | 90th | 97th | |||||||
| 5 | −0.206 | 0.115 | 0.405 | 0.433 | 0.501 | 0.582 | 0.625 | −0.385 + (HZ × 0.430) | 0.436 | 0.121 | 0.380 | 0.412 | 0.483 | 0.562 | 0.601 | −0.129 + (HZ × 0.396) |
| 6 | −0.178 | 0.117 | 0.417 | 0.447 | 0.518 | 0.604 | 0.649 | −0.156 + (HZ × 0.427) | 0.436 | 0.121 | 0.399 | 0.432 | 0.507 | 0.589 | 0.630 | −0.077 + (HZ × 0.411) |
| 7 | −0.150 | 0.120 | 0.429 | 0.461 | 0.536 | 0.626 | 0.674 | −0.007 + (HZ × 0.473) | 0.436 | 0.121 | 0.417 | 0.451 | 0.530 | 0.616 | 0.658 | −0.057 + (HZ × 0.455) |
| 8 | −0.120 | 0.122 | 0.442 | 0.475 | 0.555 | 0.650 | 0.701 | 0.041 + (HZ × 0.522) | 0.436 | 0.121 | 0.434 | 0.470 | 0.552 | 0.641 | 0.685 | −0.037 + (HZ × 0.469) |
| 9 | −0.080 | 0.126 | 0.457 | 0.493 | 0.578 | 0.680 | 0.734 | 0.034 + (HZ × 0.501) | 0.436 | 0.121 | 0.450 | 0.488 | 0.572 | 0.665 | 0.711 | −0.005 + (HZ × 0.510) |
| 10 | −0.022 | 0.131 | 0.479 | 0.518 | 0.612 | 0.725 | 0.785 | −0.035 + (HZ × 0.485) | 0.436 | 0.121 | 0.467 | 0.506 | 0.594 | 0.690 | 0.737 | −0.005 + (HZ × 0.507) |
| 11 | 0.061 | 0.139 | 0.510 | 0.555 | 0.664 | 0.792 | 0.861 | −0.104 + (HZ × 0.542) | 0.436 | 0.121 | 0.487 | 0.527 | 0.619 | 0.719 | 0.769 | −0.039 + (HZ × 0.524) |
| 12 | 0.169 | 0.145 | 0.560 | 0.613 | 0.740 | 0.888 | 0.966 | −0.053 + (HZ × 0.593) | 0.436 | 0.121 | 0.516 | 0.558 | 0.655 | 0.761 | 0.814 | −0.100 + (HZ × 0.590) |
| 13 | 0.286 | 0.140 | 0.631 | 0.690 | 0.829 | 0.988 | 1.069 | −0.011 + (HZ × 0.592) | 0.436 | 0.121 | 0.559 | 0.605 | 0.710 | 0.825 | 0.882 | −0.151 + (HZ × 0.690) |
| 14 | 0.392 | 0.128 | 0.703 | 0.764 | 0.904 | 1.059 | 1.137 | −0.027 + (HZ × 0.539) | 0.436 | 0.121 | 0.619 | 0.670 | 0.787 | 0.914 | 0.978 | −0.099 + (HZ × 0.747) |
| 15 | 0.473 | 0.116 | 0.757 | 0.817 | 0.954 | 1.101 | 1.174 | −0.036 + (HZ × 0.553) | 0.436 | 0.121 | 0.687 | 0.744 | 0.873 | 1.014 | 1.084 | −0.071 + (HZ × 0.707) |
| 16 | 0.527 | 0.108 | 0.794 | 0.853 | 0.984 | 1.125 | 1.195 | −0.038 + (HZ × 0.595) | 0.436 | 0.121 | 0.743 | 0.804 | 0.944 | 1.096 | 1.172 | −0.032 + (HZ × 0.598) |
| 17 | 0.560 | 0.103 | 0.817 | 0.874 | 1.003 | 1.140 | 1.206 | −0.080 + (HZ × 0.589) | 0.436 | 0.121 | 0.781 | 0.846 | 0.993 | 1.153 | 1.233 | 0.022 + (HZ × 0.565) |
| 18 | 0.579 | 0.100 | 0.830 | 0.887 | 1.014 | 1.148 | 1.213 | −0.080 + (HZ × 0.574) | 0.436 | 0.121 | 0.805 | 0.872 | 1.023 | 1.189 | 1.271 | −0.011 + (HZ × 0.601) |
| 19 | 0.590 | 0.099 | 0.838 | 0.895 | 1.020 | 1.152 | 1.216 | −0.083 + (HZ × 0.515) | 0.436 | 0.121 | 0.821 | 0.889 | 1.043 | 1.212 | 1.296 | −0.044 + (HZ × 0.617) |
| 20 | 0.598 | 0.097 | 0.844 | 0.900 | 1.024 | 1.155 | 1.219 | −0.091 + (HZ × 0.451) | 0.436 | 0.121 | 0.833 | 0.902 | 1.059 | 1.230 | 1.316 | −0.071 + (HZ × 0.587) |
L, M, and S values to calculate Z-scores and HZ prediction equations to calculate height adjusted Z-scores are also shown. HZ, Ht-Z.
Results
Study sample
The sample consisted of 2,014 study participants (1022 girls) who completed 10,671 study visits. After study enrollment, 615 exclusion criteria were identified on 214 study participants as follows: medication use such as chronic steroid (number of observations was 260), anticonvulsants, oral isotrentinoin, psychiatric drugs; stimulants such as Ritalin, depoprovera/norplants (number of observations was 313); pregnancy (number of observations was 34); or serious illness (number of observations was eight) that might adversely affect bone mineral accrual. The final number of visits used for the creation of the reference curves was 10,066. Forty-one percent of subjects contributed seven observations to the final data set; 65% contributed at least four observations and only 7% had only one observation. The mean age was 13.5 ± 4.2 yr. The race and ethnic distribution of the sample was 48% Caucasian, 24% African-American, 17% Hispanic, and 11% other. In keeping with the previously published reference curves from this cohort, all reference curves were based on the categorization of either Black or non-Black race based on the parent's report of the child's race.
The distribution of observations across puberty stages were: 27% stage 1, 9% stage 2, 8% stage 3, 11% stage 4, and 45% in stage 5. Mean height, weight, and BMI Z-scores were significantly greater than zero (0.2 ± 0.9, 0.4 ± 0.8, 0.3 ± 0.9, respectively). The percent of observations involving children with a BMI in the range of at risk of overweight (85th to 95th BMI percentile) was 16%, and for the overweight range (BMI > 95th percentile) was 6%, indicating that few were in the obese range according to the Centers for Disease Control and Prevention guidelines (11). Height, weight, and BMI Z-scores were significantly greater (all P < 0.0001) for Black compared with non-Black children. Black girls were significantly (P < 0.001) younger than non-Black girls in prepuberty (7.7 vs. 8.1 yr, respectively) and at puberty stages 2–5 by 0.6–0.8 yr, signaling earlier timing of sexual maturation. Black and non-Black boys significantly differed (P = 0.001) in maturational timing only in puberty stage 4 (14.5 vs. 14.0 yr, respectively).
DXA reference curves
Reference percentiles for TBLH BMC and lumbar spine aBMD are given in Tables 1–4 for Black and non-Black boys and girls ages 5–20 yr. Reference percentiles for BMC and aBMD of the total body, hip, femoral neck, and distal one third radius BMD, lumbar spine BMC, and TBLH aBMD are given in Supplemental Tables 1–10, published on The Endocrine Society's Journal Online web site at http://jcem.endojournals.org. Each table also shows the L, M, and S values needed for calculating Z-scores using equation 2. The age-specific values shown are based on rounded ages; for example, the values for 10 yr olds should be applied to children who are 9.5–10.4 yr of age.
Table 1.
Age- and sex-specific reference percentiles for total body less head bone mineral content for Black children
| TBLH BMC (g) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | Black females |
Black males |
||||||||||||||
| L | S | M |
HZ prediction equation | L | S | M |
HZ prediction equation | |||||||||
| 3rd | 10th | 50th | 90th | 97th | 3rd | 10th | 50th | 90th | 97th | |||||||
| 5 | −0.051 | 0.154 | 324 | 355 | 432 | 527 | 579 | −0.042 + (HZ × 0.911) | 1.209 | 0.155 | 309 | 353 | 443 | 529 | 569 | −0.188 + (HZ × 0.733) |
| 6 | −0.051 | 0.154 | 395 | 432 | 526 | 641 | 704 | 0.094 + (HZ × 0.524) | 1.032 | 0.154 | 380 | 430 | 536 | 642 | 691 | −0.165 + (HZ × 0.830) |
| 7 | −0.051 | 0.153 | 469 | 514 | 625 | 761 | 835 | 0.267 + (HZ × 0.511) | 0.852 | 0.153 | 458 | 514 | 637 | 763 | 824 | −0.122 + (HZ × 0.689) |
| 8 | −0.051 | 0.153 | 532 | 583 | 708 | 863 | 947 | 0.158 + (HZ × 0.525) | 0.686 | 0.153 | 534 | 597 | 736 | 884 | 956 | −0.050 + (HZ × 0.692) |
| 9 | −0.051 | 0.154 | 596 | 653 | 794 | 967 | 1062 | 0.053 + (HZ × 0.495) | 0.539 | 0.154 | 605 | 673 | 829 | 1000 | 1085 | −0.090 + (HZ × 0.607) |
| 10 | −0.051 | 0.155 | 678 | 743 | 905 | 1104 | 1213 | −0.229 + (HZ × 0.596) | 0.395 | 0.158 | 677 | 753 | 929 | 1128 | 1230 | −0.186 + (HZ × 0.727) |
| 11 | −0.051 | 0.156 | 796 | 873 | 1065 | 1302 | 1432 | −0.384 + (HZ × 0.832) | 0.241 | 0.165 | 757 | 842 | 1045 | 1283 | 1409 | −0.318 + (HZ × 0.729) |
| 12 | −0.051 | 0.155 | 942 | 1033 | 1260 | 1539 | 1691 | −0.356 + (HZ × 0.910) | 0.069 | 0.176 | 857 | 954 | 1197 | 1497 | 1660 | −0.411 + (HZ × 0.710) |
| 13 | −0.051 | 0.151 | 1085 | 1187 | 1439 | 1749 | 1917 | −0.192 + (HZ × 0.873) | −0.090 | 0.188 | 996 | 1112 | 1411 | 1800 | 2022 | −0.557 + (HZ × 0.877) |
| 14 | −0.051 | 0.146 | 1203 | 1313 | 1582 | 1910 | 2088 | −0.079 + (HZ × 0.794) | −0.177 | 0.191 | 1184 | 1320 | 1677 | 2152 | 2429 | −0.402 + (HZ × 0.742) |
| 15 | −0.051 | 0.143 | 1286 | 1400 | 1679 | 2016 | 2199 | −0.108 + (HZ × 0.797) | −0.192 | 0.180 | 1381 | 1531 | 1918 | 2429 | 2724 | −0.329 + (HZ × 0.786) |
| 16 | −0.051 | 0.140 | 1335 | 1451 | 1735 | 2077 | 2262 | −0.143 + (HZ × 0.817) | −0.177 | 0.164 | 1568 | 1723 | 2118 | 2625 | 2911 | −0.276 + (HZ × 0.730) |
| 17 | −0.051 | 0.139 | 1360 | 1477 | 1763 | 2108 | 2294 | −0.258 + (HZ × 0.939) | −0.158 | 0.150 | 1717 | 1873 | 2264 | 2752 | 3023 | −0.283 + (HZ × 0.754) |
| 18 | −0.051 | 0.138 | 1373 | 1491 | 1778 | 2124 | 2311 | −0.365 + (HZ × 0.794) | −0.145 | 0.141 | 1815 | 1971 | 2355 | 2827 | 3086 | −0.204 + (HZ × 0.796) |
| 19 | −0.051 | 0.138 | 1385 | 1503 | 1791 | 2139 | 2325 | −0.331 + (HZ × 0.827) | −0.140 | 0.137 | 1855 | 2010 | 2390 | 2856 | 3109 | −0.291 + (HZ × 0.946) |
| 20 | −0.051 | 0.137 | 1398 | 1517 | 1807 | 2155 | 2342 | −0.417 + (HZ × 0.938) | −0.139 | 0.136 | 1866 | 2020 | 2400 | 2863 | 3115 | −0.108 + (HZ × 0.881) |
L, M, and S values to calculate Z-scores and HZ prediction equations to calculate height adjusted Z-scores are also shown. This measure excludes the BMC of the head from the total body measurement. HZ, Ht-Z.
Table 2.
Age- and sex-specific reference percentiles for total body less head bone mineral content for non-Black children
| TBLH BMC (g) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | Non-Black females |
Non-Black males |
||||||||||||||
| L | S | M |
HZ prediction equation | L | S | M |
HZ prediction equation | |||||||||
| 3rd | 10th | 50th | 90th | 97th | 3rd | 10th | 50th | 90th | 97th | |||||||
| 5 | −0.019 | 0.186 | 258 | 288 | 365 | 463 | 518 | −0.051 + (HZ × 0.958) | −0.058 | 0.160 | 268 | 295 | 362 | 445 | 490 | 0.207 + (HZ × 0.941) |
| 6 | −0.019 | 0.166 | 344 | 380 | 470 | 581 | 643 | 0.134 + (HZ × 0.755) | −0.058 | 0.160 | 347 | 382 | 468 | 575 | 634 | 0.244 + (HZ × 0.546) |
| 7 | −0.019 | 0.152 | 422 | 463 | 562 | 683 | 749 | 0.181 + (HZ × 0.793) | −0.058 | 0.160 | 418 | 459 | 563 | 692 | 763 | 0.114 + (HZ × 0.716) |
| 8 | −0.019 | 0.146 | 483 | 527 | 635 | 766 | 837 | 0.182 + (HZ × 0.827) | −0.058 | 0.160 | 479 | 527 | 646 | 794 | 876 | 0.062 + (HZ × 0.775) |
| 9 | −0.019 | 0.146 | 537 | 586 | 706 | 851 | 929 | 0.127 + (HZ × 0.742) | −0.058 | 0.160 | 540 | 594 | 728 | 895 | 986 | 0.069 + (HZ × 0.756) |
| 10 | −0.019 | 0.152 | 598 | 656 | 797 | 968 | 1062 | −0.024 + (HZ × 0.769) | −0.058 | 0.160 | 596 | 656 | 804 | 988 | 1089 | −0.002 + (HZ × 0.823) |
| 11 | −0.019 | 0.169 | 674 | 745 | 926 | 1150 | 1274 | −0.125 + (HZ × 0.765) | −0.058 | 0.160 | 664 | 730 | 895 | 1100 | 1213 | −0.032 + (HZ × 0.885) |
| 12 | −0.019 | 0.185 | 777 | 868 | 1099 | 1393 | 1558 | −0.103 + (HZ × 0.757) | −0.058 | 0.160 | 762 | 838 | 1028 | 1263 | 1393 | −0.154 + (HZ × 0.961) |
| 13 | −0.019 | 0.182 | 914 | 1019 | 1286 | 1624 | 1812 | −0.090 + (HZ × 0.740) | −0.058 | 0.160 | 898 | 988 | 1211 | 1489 | 1642 | −0.220 + (HZ × 1.022) |
| 14 | −0.019 | 0.165 | 1049 | 1158 | 1431 | 1769 | 1954 | −0.125 + (HZ × 0.693) | −0.058 | 0.160 | 1079 | 1186 | 1454 | 1788 | 1971 | −0.219 + (HZ × 1.041) |
| 15 | −0.019 | 0.151 | 1149 | 1258 | 1526 | 1852 | 2028 | −0.165 + (HZ × 0.701) | −0.058 | 0.160 | 1276 | 1403 | 1720 | 2114 | 2331 | −0.201 + (HZ × 0.903) |
| 16 | −0.019 | 0.142 | 1212 | 1320 | 1582 | 1897 | 2067 | −0.201 + (HZ × 0.753) | −0.058 | 0.160 | 1427 | 1568 | 1923 | 2364 | 2606 | −0.185 + (HZ × 0.792) |
| 17 | −0.019 | 0.137 | 1247 | 1354 | 1612 | 1920 | 2085 | −0.235 + (HZ × 0.758) | −0.058 | 0.160 | 1524 | 1676 | 2055 | 2525 | 2785 | −0.164 + (HZ × 0.751) |
| 18 | −0.019 | 0.134 | 1263 | 1369 | 1625 | 1930 | 2093 | −0.226 + (HZ × 0.785) | −0.058 | 0.160 | 1577 | 1734 | 2127 | 2614 | 2882 | −0.195 + (HZ × 0.741) |
| 19 | −0.019 | 0.134 | 1267 | 1372 | 1628 | 1932 | 2094 | −0.235 + (HZ × 0.829) | −0.058 | 0.160 | 1605 | 1764 | 2163 | 2659 | 2932 | −0.190 + (HZ × 0.739) |
| 20 | −0.019 | 0.133 | 1268 | 1374 | 1629 | 1933 | 2095 | −0.218 + (HZ × 0.774) | −0.058 | 0.160 | 1624 | 1785 | 2189 | 2690 | 2967 | −0.195 + (HZ × 0.727) |
L, M, and S values to calculate Z-scores and HZ prediction equations to calculate height adjusted Z-scores are also shown. This measure excludes the BMC of the head from the total body measurement. HZ, Ht-Z.
Table 3.
Age- and sex-specific reference percentiles for lumbar spine aBMD for Black children
| Lumbar spine aBMD | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | Black females |
Black males |
||||||||||||||
| L | S | M |
HZ prediction equation | L | S | M |
HZ prediction equation | |||||||||
| 3rd | 10th | 50th | 90th | 97th | 3rd | 10th | 50th | 90th | 97th | |||||||
| 5 | 0.827 | 0.131 | 0.410 | 0.451 | 0.541 | 0.632 | 0.676 | −0.459 + (HZ × 0.301) | 0.612 | 0.126 | 0.386 | 0.420 | 0.498 | 0.581 | 0.621 | −0.403 + (HZ × 0.082) |
| 6 | 0.802 | 0.130 | 0.424 | 0.465 | 0.557 | 0.651 | 0.696 | −0.319 + (HZ × 0.460) | 0.612 | 0.126 | 0.407 | 0.444 | 0.526 | 0.613 | 0.656 | −0.297 + (HZ × 0.214) |
| 7 | 0.773 | 0.130 | 0.438 | 0.481 | 0.575 | 0.672 | 0.719 | −0.096 + (HZ × 0.437) | 0.612 | 0.126 | 0.428 | 0.467 | 0.553 | 0.645 | 0.690 | −0.317 + (HZ × 0.267) |
| 8 | 0.741 | 0.129 | 0.454 | 0.498 | 0.594 | 0.694 | 0.742 | 0.018 + (HZ × 0.449) | 0.612 | 0.126 | 0.447 | 0.487 | 0.577 | 0.673 | 0.720 | −0.251 + (HZ × 0.291) |
| 9 | 0.696 | 0.128 | 0.477 | 0.521 | 0.620 | 0.725 | 0.775 | −0.044 + (HZ × 0.422) | 0.612 | 0.126 | 0.465 | 0.506 | 0.600 | 0.700 | 0.748 | −0.123 + (HZ × 0.337) |
| 10 | 0.625 | 0.127 | 0.514 | 0.561 | 0.665 | 0.776 | 0.830 | −0.184 + (HZ × 0.441) | 0.612 | 0.126 | 0.484 | 0.528 | 0.625 | 0.729 | 0.780 | −0.147 + (HZ × 0.334) |
| 11 | 0.526 | 0.124 | 0.573 | 0.623 | 0.736 | 0.858 | 0.918 | −0.398 + (HZ × 0.661) | 0.612 | 0.126 | 0.509 | 0.555 | 0.657 | 0.766 | 0.820 | −0.232 + (HZ × 0.338) |
| 12 | 0.409 | 0.122 | 0.648 | 0.702 | 0.825 | 0.960 | 1.027 | −0.413 + (HZ × 0.775) | 0.612 | 0.126 | 0.545 | 0.594 | 0.703 | 0.820 | 0.877 | −0.277 + (HZ × 0.397) |
| 13 | 0.295 | 0.119 | 0.718 | 0.775 | 0.906 | 1.052 | 1.126 | −0.202 + (HZ × 0.744) | 0.612 | 0.126 | 0.596 | 0.650 | 0.770 | 0.897 | 0.960 | −0.416 + (HZ × 0.598) |
| 14 | 0.198 | 0.117 | 0.775 | 0.834 | 0.971 | 1.126 | 1.205 | −0.088 + (HZ × 0.674) | 0.612 | 0.126 | 0.658 | 0.717 | 0.850 | 0.991 | 1.060 | −0.313 + (HZ × 0.604) |
| 15 | 0.124 | 0.116 | 0.817 | 0.877 | 1.019 | 1.179 | 1.262 | −0.040 + (HZ × 0.618) | 0.612 | 0.126 | 0.719 | 0.784 | 0.928 | 1.083 | 1.158 | −0.179 + (HZ × 0.588) |
| 16 | 0.072 | 0.115 | 0.847 | 0.908 | 1.052 | 1.218 | 1.303 | −0.086 + (HZ × 0.643) | 0.612 | 0.126 | 0.770 | 0.840 | 0.995 | 1.160 | 1.241 | −0.048 + (HZ × 0.570) |
| 17 | 0.037 | 0.114 | 0.867 | 0.929 | 1.075 | 1.243 | 1.331 | −0.169 + (HZ × 0.669) | 0.612 | 0.126 | 0.808 | 0.880 | 1.043 | 1.216 | 1.301 | 0.043 + (HZ × 0.562) |
| 18 | 0.016 | 0.113 | 0.879 | 0.942 | 1.089 | 1.258 | 1.347 | −0.149 + (HZ × 0.657) | 0.612 | 0.126 | 0.831 | 0.906 | 1.073 | 1.251 | 1.339 | 0.102 + (HZ × 0.564) |
| 19 | 0.005 | 0.113 | 0.886 | 0.948 | 1.096 | 1.267 | 1.356 | −0.114 + (HZ × 0.631) | 0.612 | 0.126 | 0.845 | 0.921 | 1.091 | 1.272 | 1.361 | 0.120 + (HZ × 0.584) |
| 20 | −0.003 | 0.113 | 0.891 | 0.953 | 1.101 | 1.273 | 1.362 | −0.100 + (HZ × 0.724) | 0.612 | 0.126 | 0.854 | 0.931 | 1.102 | 1.285 | 1.375 | 0.190 + (HZ × 0.604) |
L, M, and S values to calculate Z-scores and HZ prediction equations to calculate height adjusted Z-scores are also shown. HZ, Ht-Z.
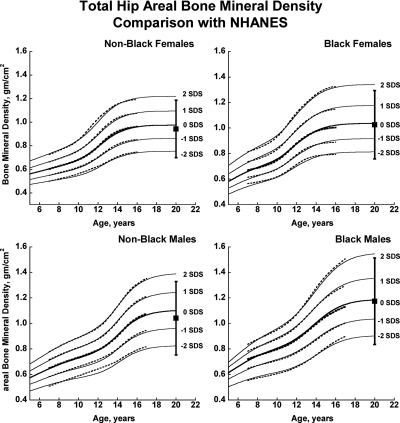
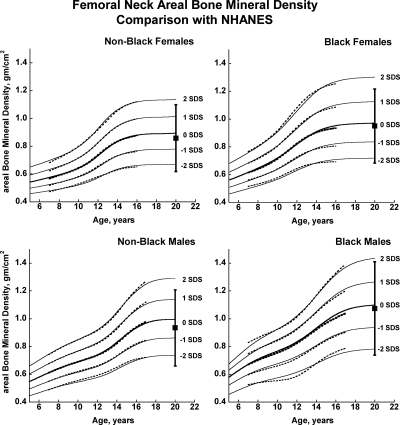
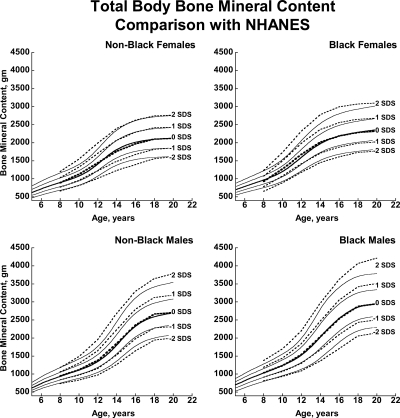
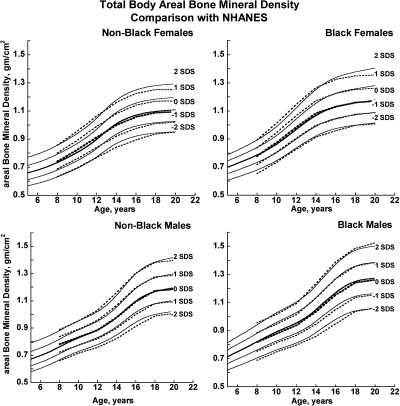
There was close agreement between the current curves and those previously published on a subset of observations and more limited age range (4) (see Supplemental Figures 1–5). Figures 1 and 2 also show previously published total hip and femoral neck reference ranges from the National Health and Nutrition Examination Surveys (NHANES) for young adults (ages 20–29 yr) based on data collected between 1988 and 1994 (12). The median for the NHANES data are generally similar to the BMDCS curves (Fig. 1) for total hip aBMD. However, the −2 sd levels are higher for the BMDCS curves compared with the NHANES data for both the total hip and the femoral neck sites. Total body BMC (TBBMC) and total body aBMD (TBaBMD) were compared with recently published NHANES results (13) (see Figs. 3 and 4). The BMDCS and NHANES distributions for TBaBMD were comparable. For TBBMC, the median curves were similar, but the upper and lower reference percentiles for the NHANES data were broader than those provided in the BMDCS curves.
Fig. 1.
Reference curves for total hip aBMD for healthy male and female, Black, and non-Black children aged 5–20 yr. Shown are the curves for −2, −1, 0, +1, and +2 sd (Z-scores). Corresponding percentile distributions and L, M, and S values are shown in Supplemental Table 6. The dotted line curves are the previously published BMDCS reference curves (4) for ages 7 to 17 yr based on a subset of observations used to generate the final curves. The error bar to the right of each curve shows the young adult (20–29 yr) reference range (mean ± 2 sd) from the NHANES (12).
Fig. 2.
Reference curves for femoral neck aBMD for healthy male and female, Black, and non-Black children aged 5–20 yr. Shown are the curves for −2, −1, 0, +1, and +2 sd (Z-scores). Corresponding percentile distributions and L, M, and S values are shown in Supplemental Table 8. The dotted line curves are the previously published BMDCS reference curves (4) for ages 7–17 yr based on a subset of observations used to generate the final curves. The error bar to the right of each curve shows the young adult (20–29 yr) reference range (mean ± 2 sd) from the NHANES (12).
Fig. 3.
BMDCS reference curves (solid lines) for TBBMC compared with published values from the NHANES (13) (dashed lines).
Fig. 4.
BMDCS reference curves (solid lines) for TBaBMD compared with published values from the NHANES (13) (dashed lines).
The previously noted pattern of greater BMC and aBMD for Blacks compared with non-Black subjects persisted. aBMD Z-scores and percentile ranks were computed for all participants using the reference values for non-Blacks. The median values for Black children were comparable to the 81st percentile for TBaBMD of the non-Black reference curves, the 70th percentile for spine, the 77th percentile for total hip, and the 75th percentile for radius aBMD of the non-Black reference curves. The difference between the Black and non-Black subsets was evident at all ages (data not shown), emphasizing the importance of separate reference curves for Black vs. non-Black children and adolescents.
Equations for calculating Ht-Z-adjusted bone Z-scores
Ht-Z was significantly associated with all bone Z-scores. The highest R2 were for TBBMC and TBLH BMC, ranging from 0.33 to 0.43. The equations for calculating Ht-Z-adjusted bone Z-scores are provided in each table.
Discussion
BMC and aBMD increase substantially during childhood and adolescence. Differences in body size and composition and maturational timing promote sex differences during this period. Consequently, BMC and aBMD must be evaluated as age- and sex-specific Z-scores to account for expected developmental changes in bone. Moreover, BMC and aBMD variability increases during adolescence. Therefore, a large healthy reference sample is essential to characterize the normal range of age-related changes in BMC and aBMD from childhood to adolescence. The results presented here describe BMC and aBMD from ages 5 to 20 yr based on about 10,000 observations of about 2,000 healthy participants from five centers in the United States. Standardized data collection methods were used, with centralized analysis of DXA scans to assure uniformity of results.
Many health conditions and medical therapies have been associated with poor bone acquisition. Primary bone disorders such as osteogenesis imperfecta as well as health conditions that involve chronic inflammation, malabsorption, immobility, hematological disorders, delayed sexual maturation, or gonadal insufficiency may adversely affect bone growth in childhood (14). These may include disorders such as Crohn's disease, cystic fibrosis, cerebral palsy, thalassemia, acute lymphocytic leukemia, and anorexia nervosa. Medical therapies, such as glucocorticoids, also threaten bone acquisition. Pediatric reference data are vital for identifying poor bone acquisition in children who are affected by chronic illness and its treatment.
A subset of the observations presented here was used to create previous reference curves (4). The revised and expanded curves described here closely correspond with the previous curves, which is an important consideration for clinicians who are already prospectively monitoring BMC or aBMD of children at risk for poor bone acquisition or who are receiving bone-active therapies. The median proximal femur aBMD values for the oldest age ranges in our study correspond approximately with published young adult reference ranges but differed in range of variation. This may be accounted for by use of different DXA technologies, sampling strategies, inclusion criteria, and statistical techniques for determining reference values as well as possible changes in aBMD during early adulthood. For all groups, differences in the −2 sd level between reference curves are relevant to longitudinal monitoring of at-risk individuals as they transition to adult care.
The recent NHANES TBBMC and TBaBMD data were acquired using Hologic DXA devices and analysis software similar to ours. NHANES and BMDCS reference curves for TBaBMD corresponded closely. For TBBMC, the NHANES and BMDCS median curves were similar, but the percentile ranges were greater in the NHANES curves compared with ours. The difference is peculiar because the NHANES BMC and aBMD data were obtained on the same subjects. It may be related to the construction of the reference curves because the shape of the curves will change as the number of degrees of freedom increases (15). Differences between NHANES and the BMDCS in sample size, study design, and selection criteria may also account for curve differences, although this would not explain why our results are similar to NHANES for aBMD but not for BMC. Of note, we previously reported that total body measurements varied by as much as 4–6% percent between centers (4). We did not apply corrections for center differences because we assumed this variability to be typical of that occurring at clinical centers at which our reference curves will be used for diagnostic purposes. The high degree of equipment-related variability in total body measurements suggests that the use of total body outcomes for diagnosing osteoporosis in childhood needs further scrutiny and validation.
Most DXA outcome distributions were skewed as denoted by L values that differed from one. Therefore, Z-score calculation based on a median and sd will give an inaccurate representation of a child's bone status relative to the reference population. We used the LMS technique to construct reference percentiles, which accounts for skewness and the nonlinear distribution. The Z-score calculation from the L, M, and S values (equation 2 above) is not simple but can be implemented in calculators and other programs to facilitate computations such as the one maintained on the BMDCS web site (www.bmdcspublic.com).
Children with health conditions that affect bone acquisition often have altered growth. Short or tall stature relative to age presents a challenge when interpreting DXA results because, on average, smaller bones have a lower BMC and areal aBMD than larger bones (5). We provided correction factors for adjusting BMC and aBMD for height status. Although the height adjustment method is a three-step process, it provides the least biased adjustment for short (or tall stature), especially among children who are within the age range when normal timing of puberty occurs. Comparison of the age-based bone Z-score and the height-adjusted bone Z-score provides the clinician with a frame of reference for the degree to which the bone Z-score is influenced by short (or tall) stature. Fracture studies in children have clearly demonstrated the importance of adjusting for body size in the association between DXA bone outcomes and fracture risk (17–21). Future studies are needed to evaluate the accuracy of the height Z-score adjustment method in identifying individual children at-risk for fracture.
The greater BMC and aBMD levels of Black vs. non-Black adults and children have been reported previously in studies using DXA (22–26), peripheral quantitative computed tomography (27, 28) and spine computed tomography (29, 30). We quantified the magnitude of the difference between Black and non-Black children using Z-scores based on non-Black reference curves. The Black cohort had mean Z-scores that were profoundly greater, ranging from 0.55 to 0.83. This result confirmed the need for separate curves so that bone health among children of African ancestry can be evaluated relative to their genetic potential for bone accrual. Greater BMC and aBMD in this group are consistent with lower fracture rates among people of African ancestry reported in studies in the United States (21, 31, 32), the United Kingdom (33), and South Africa (16). However, the relationship between fracture risk and BMC or aBMD Z-score among Black children remains to be determined.
The primary limitations of this study was the inability to acquire data on a randomly selected group of healthy children from all regions in the United States and the inability to apply these reference curves to the results from other DXA manufacturers. However, this robust sample, characterized using standardized methodology, offers the best pediatric reference data available.
Conclusion
We have extended previously reported pediatric DXA reference curves to encompass the age range from 5 to 20 yr for Black and non-Black children. These robust reference curves are based on about 10,000 observations in well-characterized healthy children using standardized techniques. As noted by the International Society of Clinical Densitometry Pediatric Recommendations, interpretation of DXA results in children should be based on sex, age, and group-specific reference ranges using similar DXA technology and analysis software and should be adjusted for body size. The results presented here significantly improve the information needed by clinicians for assessing and monitoring bone health in children, especially as they transition through adolescence and into young adulthood.
Supplementary Material
Acknowledgments
We are indebted to our collaborators in the pediatric endocrine divisions of each Bone Mineral Density in Childhood Study Clinical Center. In particular, we acknowledge Drs. Andrea Kelly, David Langdon, Thomas Moshang, Steve Willi, Lorraine Katz, Charles Stanley, and Craig Alter and the faculty in the Division of Pediatric Endocrinology at Children's Hospital of Philadelphia; Lynda Fisher, Mitchell Geffner, Debra Jeandron, Steven Mittelman, Pisit Pitukcheewanont, and Francine Kaufman from Children's Hospital Los Angeles; Susan Rose, Frank Biro, Peggy Stenger, Debbie Elder, and James Heubi from Cincinnati Children's Hospital and Medical Center; Mary Horlick, Natasha Leibel, and Abeer Hassoun at Columbia University Medical Center-St. Luke's Hospital; and Jean-Claude Desmangles from Creighton University. We also acknowledge the guidance and advice from the Data Safety and Monitoring Board members: Drs. Clifford Rosen, Ralph D'Agostino, Ingrid Holm, James Reynolds, and Reginald Tsang.
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Contracts NO1-HD-1-3228, NO1-HD-1-3329, NO1-HD-1-3330, NO1-HD-1-3331, NO1-HD-1-3332 and NO1-HD-1-3333 and the Clinical and Translational Research Center Grant 5-MO1-RR-000240 and UL1 RR-026314.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aBMD
- Areal BMD
- BMC
- bone mineral content
- BMD
- bone mineral density
- BMDCS
- Bone Mineral Density in Childhood Study
- BMI
- body mass index
- DXA
- dual-energy x-ray absorptiometry
- Ht-Z
- height Z-score
- LMS
- power for the Box-Cox transformation, median, sd
- NHANES
- National Health and Nutrition Examination Surveys
- TBBMC
- total body BMC
- TBaBMD
- total body aBMD
- TBLH
- total body less head.
References
- 1. Bachrach L, Levine M, Cowell C, Shaw N. 2007. Clinical indications for the use of DXA in pediatrics. In: Sawyer A, Barchrach L, Fung EB. eds. Bone densitometry in growing patients. Totowa, NJ: Humana Press; 59–72 [Google Scholar]
- 2. Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, Lorenc RS, Tosi LL, Ward KA, Ward LM, Kalkwarf HJ. 2008. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom 11:43–58 [DOI] [PubMed] [Google Scholar]
- 3. Leonard MB, Zemel BS. 2002. Current concepts in pediatric bone disease. Pediatr Clin North Am 49:143–173 [DOI] [PubMed] [Google Scholar]
- 4. Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, Shepherd JA. 2007. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab 92:2087–2099 [DOI] [PubMed] [Google Scholar]
- 5. Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, Mahboubi S, Shepherd JA, Hangartner TN, Frederick MM, Winer KK, Kalkwarf HJ. 2010. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab 95:1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuczmarski R, Ogden CL, Grummer-Strawn LM. June 8, 2000. CDC Growth Charts—United States. Advance data from Vital and Health Statistics. Vol. 11 Hyattsville, MD: National Center for Health Statistics; 241–190 [Google Scholar]
- 7. Tanner JM. 1962. Growth at Adolescence. 2nd ed Oxford UK: Blackwell Scientific Publications [Google Scholar]
- 8. Zachmann M, Prader A, Kind HP, Häfliger H, Budliger H. 1974. Testicular volume during adolescence. Cross-sectional and longitudinal studies. Helv Paediatr Acta 29:61–72 [PubMed] [Google Scholar]
- 9. Cole TJ, Green PJ. 1992. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 11:1305–1319 [DOI] [PubMed] [Google Scholar]
- 10. Cole T, Pan H. 2002. LMS program, version 1.16. Cambridge, UK: Medical Research Council [Google Scholar]
- 11. Ogden CL, Flegal KM, Carroll MD, Johnson CL. 2002. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 288:1728–1732 [DOI] [PubMed] [Google Scholar]
- 12. Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr, Lindsay R. 1998. Updated data on proximal femur bone mineral levels of U.S. adults. Osteoporos Int 8:468–489 [DOI] [PubMed] [Google Scholar]
- 13. Kelly TL, Wilson KE, Heymsfield SB. 2009. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One 4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bishop N, Braillon P, Burnham J, Cimaz R, Davies J, Fewtrell M, Hogler W, Kennedy K, Mäkitie O, Mughal Z, Shaw N, Vogiatzi M, Ward K, Bianchi ML. 2008. Dual-energy X-ray aborptiometry assessment in children and adolescents with diseases that may affect the skeleton: the 2007 ISCD Pediatric Official Positions. J Clin Densitom 11:29–42 [DOI] [PubMed] [Google Scholar]
- 15. van Buuren S, Fredriks M. 2001. Worm plot: a simple diagnostic device for modelling growth reference curves. Stat Med 20:1259–1277 [DOI] [PubMed] [Google Scholar]
- 16. Thandrayen K, Norris SA, Pettifor JM. 2009. Fracture rates in urban South African children of different ethnic origins: the Birth to Twenty cohort. Osteoporos Int 20:47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clark EM, Ness AR, Bishop NJ, Tobias JH. 2006. Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res 21:1489–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clark EM, Tobias JH, Ness AR. 2006. Association between bone density and fractures in children: a systematic review and meta-analysis. Pediatrics 117:e291–e297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goulding A, Cannan R, Williams SM, Gold EJ, Taylor RW, Lewis-Barned NJ. 1998. Bone mineral density in girls with forearm fractures. J Bone Miner Res 13:143–148 [DOI] [PubMed] [Google Scholar]
- 20. Flynn J, Foley S, Jones G. 2007. Can BMD assessed by DXA at age 8 predict fracture risk in boys and girls during puberty?: an eight-year prospective study. J Bone Miner Res 22:1463–1467 [DOI] [PubMed] [Google Scholar]
- 21. Kalkwarf HJ, Laor T, Bean JA. 2011. Fracture risk in children with a forearm injury is associated with volumetric bone density and cortical area (by peripheral QCT) and areal bone density (by DXA). Osteoporos Int 22:607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. 1999. Bone mineral acquisition in healthy Asian, Hispanic, Black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab 84:4702–4712 [DOI] [PubMed] [Google Scholar]
- 23. Harel Z, Gold M, Cromer B, Bruner A, Stager M, Bachrach L, Wolter K, Reid C, Hertweck P, Nelson A, Nelson D, Coupey S, Johnson C, Burkman R, Bone H. 2007. Bone mineral density in postmenarchal adolescent girls in the United States: associated biopsychosocial variables and bone turnover markers. J Adolesc Health 40:44–53 [DOI] [PubMed] [Google Scholar]
- 24. Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr, Lindsay RL. 1995. Proximal femur bone mineral levels of U.S. adults. Osteoporos Int 5:389–409 [DOI] [PubMed] [Google Scholar]
- 25. Ellis KJ. 1997. Body composition of a young, multiethnic, male population. Am J Clin Nutr 66:1323–1331 [DOI] [PubMed] [Google Scholar]
- 26. Ellis KJ, Abrams SA, Wong WW. 1997. Body composition of a young, multiethnic female population. Am J Clin Nutr 65:724–731 [DOI] [PubMed] [Google Scholar]
- 27. Leonard MB, Elmi A, Mostoufi-Moab S, Shults J, Burnham JM, Thayu M, Kibe L, Wetzsteon RJ, Zemel BS. 2010. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab 95:1681–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wetzsteon RJ, Hughes JM, Kaufman BC, Vazquez G, Stoffregen TA, Stovitz SD, Petit MA. 2009. Ethnic differences in bone geometry and strength are apparent in childhood. Bone 44:970–975 [DOI] [PubMed] [Google Scholar]
- 29. Gilsanz V, Roe TF, Mora S, Costin G, Goodman WG. 1991. Changes in vertebral bone density in black girls and white girls during childhood and puberty. N Engl J Med 325:1597–1600 [DOI] [PubMed] [Google Scholar]
- 30. Gilsanz V, Skaggs DL, Kovanlikaya A, Sayre J, Loro ML, Kaufman F, Korenman SG. 1998. Differential effect of race on the axial and appendicular skeletons of children. J Clin Endocrinol Metab 83:1420–1427 [DOI] [PubMed] [Google Scholar]
- 31. Cauley JA, Wampler NS, Barnhart JM, Wu L, Allison M, Chen Z, Hendrix S, Robbins J, Jackson RD. 2008. Incidence of fractures compared to cardiovascular disease and breast cancer: the Women's Health Initiative Observational Study. Osteoporos Int 19:1717–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lappe J, Davies K, Recker R, Heaney R. 2005. Quantitative ultrasound: use in screening for susceptibility to stress fractures in female army recruits. J Bone Miner Res 20:571–578 [DOI] [PubMed] [Google Scholar]
- 33. Donaldson LJ, Reckless IP, Scholes S, Mindell JS, Shelton NJ. 2008. The epidemiology of fractures in England. J Epidemiol Community Health 62:174–180 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.