Abstract
Context:
Bone mineral density (BMD) is lower in young amenorrheic athletes (AA) compared to eumenorrheic athletes (EA) and nonathletic controls and may contribute to fracture risk during a critical time of bone accrual. Abnormal bone microarchitecture is an independent determinant of fracture risk and has not been assessed in young athletes and nonathletes.
Objective:
We hypothesized that bone microarchitecture is impaired in AA compared to EA and nonathletes despite weight-bearing exercise.
Design and Setting:
We conducted this cross-sectional study at the Clinical Research Center of Massachusetts General Hospital.
Subjects and Outcome Measures:
We assessed BMD and bone microarchitecture in 50 subjects [16 AA, 18 EA, and 16 nonathletes (15–21 yr old)] using dual-energy x-ray absorptiometry and high-resolution peripheral quantitative computed tomography.
Results:
Groups did not differ for chronological age, bone age, body mass index, or vitamin D levels. Lumbar BMD Z-scores were lower in AA vs. EA and nonathletes; hip and femoral neck BMD Z-scores were highest in EA. At the weight-bearing tibia, athletes had greater total area, trabecular area, and cortical perimeter than nonathletes, whereas cortical area and thickness trended lower in AA. Trabecular number was lower and trabecular separation higher in AA vs. EA and nonathletes. At the non-weight-bearing radius, trabecular density was lower in AA vs. EA and nonathletes. Later menarchal age was an important determinant of impaired microarchitecture. After controlling for covariates, subject grouping accounted for 18–24% of the variability in tibial trabecular number and separation.
Conclusion:
In addition to low BMD, AA have impaired bone microarchitecture compared with EA and nonathletes. These are the first data to show abnormal bone microarchitecture in AA.
Adolescence and young adulthood are critical for bone acquisition toward attainment of peak bone mass, an important determinant of bone health and future fracture risk (1, 2). Conditions associated with low bone mineral density (BMD) and decreased bone accrual during adolescence and young adulthood are therefore concerning for both an immediate increase in fracture risk and impaired future bone health. Athleticism in young women has increased since implementation of Title IX (3), and although weight-bearing activities have beneficial effects on bone (4), female amenorrheic athletes (AA) have lower BMD than eumenorrheic athletes (EA) and nonathletic controls (5, 6), increasing their risk for fractures. Of concern, the prevalence of oligoamenorrhea is as high as 24% in adolescent athletes (7).
Reported data thus far are based on dual-energy x-ray absorptiometry (DXA) assessment of BMD. However, bone microarchitecture may provide additional information regarding bone strength not provided by DXA (8–12), and there are no data regarding bone microarchitecture in young AA. DXA is the most common modality used to determine bone health, given its precision, safety, low cost, and availability (13–15). However, it measures areal rather than volumetric BMD and is affected by stature and body composition, factors in a state of flux during adolescence (13, 14). Additionally, DXA does not provide direct measures of bone geometry and cannot differentiate between trabecular and cortical bone (14, 16).
Fracture risk is associated with abnormal bone microarchitecture even in the setting of normal BMD assessed by DXA, suggesting that microarchitecture may be a more sensitive measure of fracture risk (17). Alterations in microarchitecture in postmenopausal women contribute to fracture risk independent of low BMD, and women with a history of fractures have lower total and trabecular density, cortical thickness, trabecular number (Tb.N) and thickness (Tb.Th), and increased trabecular separation (Tb.Sp) compared with those without this history (18). Adolescents with anorexia nervosa (AN), a condition of low weight and amenorrhea, have lower trabecular bone volume, Tb.Th, and Tb.N, and increased Tb.Sp compared with normal-weight controls, even when BMD does not differ, and women with AN have lower bone strength compared with controls (19, 20). AA, however, may be normal weight and have the additional advantage of weight-bearing exercise (known to be beneficial to bone) (4). It is not known whether amenorrhea alone in these women contributes to impaired microarchitecture.
In this study, we sought to determine whether bone microarchitecture, assessed by high-resolution peripheral quantitative computed tomography (HRpQCT), differs in adolescent and young adult AA compared with EA and nonathletic controls and to find the determinants of microarchitecture in this population. We hypothesized that microarchitecture is impaired in AA compared with EA and nonathletes despite weight-bearing exercise and weight maintenance.
Subjects and Methods
Subject selection
We screened 92 girls and young women 14–21 yr old for this study (32 AA, 32 EA, and 28 nonathletes), of whom 16 AA, 18 EA, and 16 nonathletes met inclusion criteria. Subjects were recruited through medical clinics and advertising in local newspapers and colleges. Inclusion criteria included a bone age of at least 15 yr and body mass index (BMI) between the 10th and 90th percentiles. Amenorrhea (for AA) was defined as the absence of menses for at least 3 months within a period of oligomenorrhea (cycle length >6 wk) for at least 6 months, or the absence of menarche at age 16 yr or older. Eumenorrhea (for EA and nonathletes) was defined as at least nine menses (cycle length, 21–35 d) in the preceding year. Three EA had a past history of oligomenorrhea (but no amenorrhea) lasting 1–2 yr. Median duration of oligoamenorrhea in AA was 30 months. Only one AA had a history of primary amenorrhea, and other pathology was ruled out before study entry.
Athlete enrollment was limited to endurance athletes participating in at least 4 h of aerobic weight-bearing training of the legs or at least 20 miles of running weekly at least for the 6 preceding months to minimize variability from type of exercise. These criteria were modified for a young population based on published data in adult athletes after consultation with exercise physiologists (21). Cyclists and swimmers were excluded because their training does not include true weight bearing. Rowers and gymnasts were excluded given that these activities differ in the nature of weight bearing and impact (22–24). Nonathletic controls were eligible if weight-bearing exercise was no greater than 2 h/wk and if they did not participate in organized team sports. Exclusion criteria included the use of medications affecting bone metabolism and conditions other than endurance training that may cause amenorrhea. The study was approved by the Institutional Review Board of Partners HealthCare. Informed consent was obtained from subjects at least 18 yr old and parents of subjects younger than 18 yr. Informed assent was obtained from subjects younger than 18 yr.
Study procedures
Subjects underwent a complete history and physical examination. Labs were drawn to rule out exclusion criteria and measure 25-hydroxyvitamin D. Height was measured on a wall-mounted stadiometer as the average of three measurements, and weight on an electronic scale. BMI was calculated as weight (in kilograms) divided by height (in meters) squared. A detailed history of exercise activity was obtained to confirm that endurance criteria were met. Subjects had a hand x-ray to assess bone age. We used DXA (Hologic 4500; Hologic Inc., Waltham, MA) to assess lean mass, fat mass, and spine and hip BMD. Spine bone mineral apparent density (BMAD) was calculated using published methods (25). Subjects completed the Bouchard 3-d activity record over 2 weekdays and 1 weekend day. This is a validated method to assess 24-h energy expenditure (26), and it serves as an index of physical activity. For subjects meeting inclusion criteria, bone microarchitecture at the ultradistal radius and tibia (nondominant) was assessed using HRpQCT.
Measurement of bone microarchitecture
HR-pQCT was used to measure volumetric density, morphology, and microarchitecture at the ultradistal radius and tibia (XtremeCT; Scanco Medical AG, Bassersdorf, Switzerland) with an isotropic voxel size of 82 μm3 (27). Measurements were performed at the nondominant wrist and leg unless there was a history of fracture, in which case the nonfractured side was measured. Outcome variables computed by automated analysis included area and volumetric density for total, trabecular, and cortical regions; cortical thickness and perimeter (28); and Tb.N, Tb.Th, and Tb.Sp. The precision is 0.7–1.5% for densities and 2.5–4.4% for trabecular and cortical architecture. Effective radiation dose was 0.027 mSv.
Biochemical analysis
We used a chemiluminescent immunoassay to measure 25-hydroxyvitamin D (DiaSorin, Stillwater, MN; sensitivity ≤4 ng/ml; intraassay coefficient of variation, 2.9–5.5%) and estradiol (Beckman Coulter, Fullerton, CA; 20 pg/ml; precision, 12–21%). All other tests were performed using standard Labcorp assays.
Statistical methods
We used JMP (version 9; SAS Institute, Inc., Cary, NC) for all analyses and report data as means ± sd. For three-group comparisons, we performed an overall ANOVA, followed by a Tukey-Kramer analysis to assess between-group differences while controlling for multiple comparisons. Significance was defined as a two-tailed P < 0.05. We used multivariate analysis to further analyze differences among the groups after controlling for: 1) bone age (estimate of maturity; important because bone structure changes with increasing pubertal maturity); 2) bone age and BMI (marker of nutritional status); 3) bone age and lean mass (because lean mass is increased in athletes and has an impact on bone); 4) bone age and height (because bone area increases as height increases); and 5) bone age and age at menarche (indicates age at which sex steroid production is optimized). We used Pearson correlations to assess associations between microarchitecture measures and covariates (menarchal age, bone age, BMI, height, lean mass, and energy expenditure). We next performed stepwise regression modeling to determine the variability contributed by covariates to microarchitecture parameters. Variables entered into the model included study groups, bone age, menarchal age, and lean mass. Lean mass was tightly correlated with both height and energy expenditure; hence, only lean mass was included in the model. This was used in preference to height, given the known impact of muscle pull on bone. A P value of 0.1 was used to enter and leave the model.
Results
Subject characteristics
Groups did not differ for age, bone age, height, BMI, or vitamin D levels (Table 1). Menarchal age was higher in AA than nonathletes but did not differ between athletes. Compared with nonathletes, lean mass was higher in EA, whereas percentage body fat was lower in AA. Athlete groups did not differ for activity as indicated by daily energy expenditure. Although level of training did not differ between athletes, VO2 max was higher only in EA compared with controls. Reported caloric and vitamin D intake did not differ between groups (data not shown). Eleven AA, seven EA, and one nonathlete were taking vitamin supplements. Estradiol was lowest in AA.
Table 1.
Clinical characteristics in AA and EA and nonathletic controls
| AA | EA | NAC | P value | |
|---|---|---|---|---|
| n | 16 | 18 | 15 | |
| Age (yr) | 19.9 ± 1.7 | 18.7 ± 1.7 | 19.4 ± 1.2 | 0.08 |
| Bone age (yr) | 17.7 ± 0.7 | 17.5 ± 0.9 | 17.7 ± 0.9 | 0.70 |
| Age at menarche (yr) | 14.2 ± 2.5 | 12.8 ± 1.2 | 12.1 ± 1.7 | 0.006a |
| Height (cm) | 166.4 ± 5.8 | 165.8 ± 7.8 | 161.4 ± 7.6 | 0.10 |
| BMI (kg/m2) | 20.9 ± 2.4 | 22.2 ± 2.4 | 21.4 ± 2.4 | 0.19 |
| Lean mass (kg) | 44.9 ± 5.8 | 46.7 ± 8.1 | 39.8 ± 4.5 | 0.009c |
| Percentage body fat | 21.4 ± 4.5 | 23.1 ± 4.1 | 25.6 ± 4.9 | 0.03a |
| VO2 max (ml/kg/min) | 43.9 ± 9.2 | 46.7 ± 15.5 | 31.2 ± 10.9 | 0.03c |
| VO2 max % predicted (ml/kg/min) | 108.9 ± 21.3 | 113.2 ± 37.9 | 76.1 ± 26.3 | 0.03c |
| Energy expenditure (from Bouchard questionnaire) (calories/day) | 2919 ± 750 | 2835 ± 826 | 2179 ± 403 | 0.03a |
| Calcium (mg/dl) | 8.8 ± 0.3 | 8.8 ± 0.3 | 8.8 ± 0.4 | 0.93 |
| Vitamin D (ng/ml) | 34.8 ± 12.8 | 33.2 ± 15.5 | 23.7 ± 7.4 | 0.10 |
| Estradiol (pg/ml) | 34.5 ± 4.3 | 61.2 ± 10.5 | 125.6 ± 32.4 | 0.01a |
| Bone density measures | ||||
| Lumbar BMD (g/cm2) | 0.90 ± 0.13 | 1.03 ± 0.11 | 1.01 ± 0.13 | 0.009a,b |
| Lumbar BMD Z-score | −1.06 ± 1.31 | 0.26 ± 1.08 | 0.06 ± 1.23 | 0.007a,b |
| Lumbar BMAD (g/cm3) | 0.13 ± 0.02 | 0.15 ± 0.02 | 0.15 ± 0.02 | 0.004a,b |
| Lumbar BMAD Z-score | −1.66 ± 1.10 | −0.62 ± 1.05 | −0.36 ± 1.26 | 0.005a,b |
| Femoral neck BMD (g/cm2) | 0.82 ± 0.08 | 0.91 ± 0.12 | 0.83 ± 0.11 | 0.03 |
| Femoral neck BMD Z-score | −0.64 ± 0.80 | 0.21 ± 1.14 | −0.62 ± 1.01 | 0.02b,c |
| Hip BMD (g/cm2) | 0.95 ± 0.10 | 1.06 ± 0.12 | 0.94 ± 0.07 | 0.0007b,c |
| Hip BMD Z-score | −0.36 ± 0.93 | 0.80 ± 1.09 | −0.41 ± 0.66 | 0.0004b,c |
Vitamin D: ng/ml can be multiplied by 2.5 to get nmol/liter. NAC, Nonathletic controls.
P < 0.05 AA vs. NAC.
P < 0.05 AA vs. EA.
P < 0.05 EA vs. NAC.
Bone mineral density
Lumbar BMD and BMAD Z-scores were lower in AA compared with EA and nonathletes, whereas femoral neck and hip BMD Z-scores were highest in EA (Table 1). This beneficial effect of athletic activity on bone was lost in AA, who had significantly lower Z-scores than EA at the femoral neck and total hip. Differences persisted after controlling for bone age and height (data not shown). Additionally, after controlling for height, hip BMD measures were lower in AA than nonathletes (P = 0.02).
Bone microarchitecture
Microarchitecture parameters are described in Table 2, which also reports adjusted P values after controlling for bone age (measure of pubertal maturity), bone age and BMI (measure of nutritional status), bone age and lean mass, bone age and height, and bone age and menarchal age.
Table 2.
Bone microarchitecture at the ultradistal radius and distal tibia in AA, EA, and nonathletic controls
| AA | EA | NAC | P | P* | P** | P† | P†† | P‡ | |
|---|---|---|---|---|---|---|---|---|---|
| n | 16 | 18 | 16 | ||||||
| Distal tibia | |||||||||
| Trabecular area (mm2) | 583.9 ± 106.0 | 577.9 ± 105.8 | 464.0 ± 120.7 | 0.005a,c | 0.005a,c | 0.006a,c | 0.14 | 0.03a,c | 0.06 |
| Trabecular area (% total area) | 83.0 ± 3.7 | 81.3 ± 3.2 | 78.4 ± 5.8 | 0.01a | 0.01a,c | 0.02a,c | 0.05a,c | 0.08 | 0.33 |
| Trabecular density (mg HA/cm3) | 192.3 ± 24.7 | 213.1 ± 29.2 | 202.6 ± 34.2 | 0.13 | 0.06 | 0.13 | 0.07 | 0.06 | 0.07 |
| Tb.N (1/mm) | 1.77 ± 0.26 | 2.04 ± 0.20 | 1.97 ± 0.25 | 0.007a,b | 0.007a,b | 0.02a,b | 0.004a,b | 0.006a,b | 0.04a,b |
| Tb.Th (mm) | 0.09 ± 0.02 | 0.09 ± 0.01 | 0.09 ± 0.02 | 0.54 | 0.46 | 0.50 | 0.25 | 0.30 | 0.22 |
| Tb.Sp (mm) | 0.48 ± 0.07 | 0.41 ± 0.04 | 0.43 ± 0.06 | 0.0001a,b | 0.002a,b | 0.006a,b | 0.001a,b | 0.002a,b | 0.01a,b |
| Cortical area (mm2) | 116.1 ± 20.5 | 130.5 ± 17.9 | 120.2 ± 20.1 | 0.09 | 0.07 | 0.30 | 0.095 | 0.07 | 0.11 |
| Cortical area (% total area) | 16.9 ± 4.0 | 18.7 ± 3.4 | 21.4 ± 5.9 | 0.02a | 0.02a,c | 0.02a,c | 0.07 | 0.11 | 0.45 |
| Cortical density (mg HA/cm3) | 870.7 ± 31.3 | 876.6 ± 36.4 | 902.4 ± 8.5 | 0.03a | 0.02a,c | 0.02a,c | 0.11 | 0.15 | 0.24 |
| Cortical thickness (mm) | 1.14 ± 0.22 | 1.27 ± 0.18 | 1.30 ± 0.26 | 0.10 | 0.08 | 0.15 | 0.08 | 0.14 | 0.68 |
| Cortical perimeter (mm) | 102.8 ± 7.6 | 103.3 ± 8.6 | 93.8 ± 9.3 | 0.003a,c | 0.004a,c | 0.004a,c | 0.19 | 0.01a,c | 0.03a,c |
| Total area (mm2) | 700.6 ± 104.6 | 708.4 ± 107.8 | 585.3 ± 117.0 | 0.003a,c | 0.004a,c | 0.005a,c | 0.19 | 0.02a,c | 0.03a,c |
| Total density (mg HA/cm3) | 308.1 ± 39.6 | 337.8 ± 45.3 | 353.9 ± 68.6 | 0.05 | 0.03a,b | 0.06 | 0.05a,c | 0.09 | 0.55 |
| Ultradistal radius | |||||||||
| Trabecular area (mm2) | 212.3 ± 53.3 | 231.3 ± 44.9 | 191.7 ± 41.7 | 0.06 | 0.08 | 0.11 | 0.73 | 0.22 | 0.12 |
| Trabecular area (% total area) | 79.4 ± 5.0 | 80.4 ± 3.7 | 75.6 ± 5.4 | 0.01c | 0.02a,c | 0.01a,c | 0.06 | 0.07 | 0.049 |
| Trabecular density (mg HA/cm3) | 158.1 ± 26.6 | 180.5 ± 30.5 | 188.8 ± 34.9 | 0.02a | 0.02a,b | 0.02a,b | 0.03a,b | 0.04a,b | 0.32 |
| Tb.N (1/mm) | 1.96 ± 0.26 | 2.04 ± 0.22 | 2.07 ± 0.21 | 0.41 | 0.40 | 0.33 | 0.54 | 0.54 | 0.73 |
| Tb.Th (mm) | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.11 | 0.08 | 0.12 | 0.08 | 0.14 | 0.59 |
| Tb.Sp (mm) | 0.45 ± 0.07 | 0.42 ± 0.05 | 0.41 ± 0.05 | 0.15 | 0.15 | 0.11 | 0.23 | 0.24 | 0.52 |
| Cortical area (mm2) | 47.9 ± 13.2 | 49.5 ± 9.3 | 56.6 ± 12.8 | 0.09 | 0.10 | 0.06 | 0.008a,c | 0.07 | 0.44 |
| Cortical area (% total area) | 18.7 ± 5.8 | 17.7 ± 4.3 | 23.0 ± 5.9 | 0.02c | 0.02a,c | 0.01a,c | 0.06 | 0.07 | 0.06 |
| Cortical density (mg HA/cm3) | 825.8 ± 64.6 | 815.5 ± 54.0 | 855.5 ± 53.1 | 0.12 | 0.15 | 0.12 | 0.34 | 0.40 | 0.21 |
| Cortical thickness (mm) | 0.71 ± 0.20 | 0.71 ± 0.15 | 0.86 ± 0.18 | 0.04 | 0.04a,c | 0.03a,c | 0.04a,c | 0.09 | 0.23 |
| Cortical perimeter (mm) | 67.6 ± 6.6 | 70.2 ± 6.6 | 66.4 ± 5.8 | 0.20 | 0.25 | 0.40 | 0.47 | 0.26 | 0.19 |
| Total area (mm2) | 265.4 ± 54.1 | 286.2 ± 45.5 | 251.8 ± 42.8 | 0.12 | 0.16 | 0.24 | 0.69 | 0.25 | 0.19 |
| Total density (mg HA/cm3) | 298.2 ± 52.6 | 306.1 ± 46.8 | 352.8 ± 67.9 | 0.01a | 0.02a,c | 0.02a,c | 0.049a,c | 0.07 | 0.20 |
NAC, Nonathletic control; HA, hydroxyapatite. Numbers in bold indicate significant differences among the groups.
Controlled for bone age.
Controlled for bone age and BMI.
Controlled for bone age and lean mass.
Controlled for bone age and height.
Controlled for bone age and age at menarche.
P < 0.05 AA vs. NAC.
P < 0.05 AA vs. EA.
P < 0.05 EA vs. NAC.
Distal tibia (weight-bearing bone)
Total density was lowest in AA compared with EA and nonathletes after controlling for bone age. Cortical density was lower in AA compared with nonathletes and lower in both groups of athletes compared with controls after controlling for bone age. Compared with nonathletes, both groups of athletes had greater total area, cortical perimeter, and trabecular area. Percentage of cortical area was lower in AA compared with nonathletes and in both groups of athletes compared with controls after controlling for bone age. Cortical thickness trended lower in AA. Trabecular density did not differ between groups but trended lower in AA. Tb.N was lower and Tb.Sp higher in AA compared with the other groups (Fig. 1).
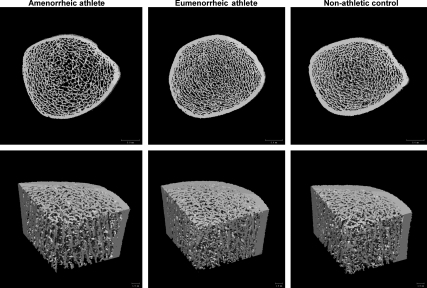
Fig. 1.
Representative images from an AA, an EA, and a nonathletic control. Upper panel, Two-dimensional slice through the tibia; lower panel, three-dimensional image of a bone “wedge,” allowing visualization of the trabecular and cortical compartments.
Ultradistal radius (non-weight-bearing bone)
Total density was lower in AA compared with nonathletes and in both groups of athletes compared with controls after controlling for bone age. Cortical density did not differ between groups. Trabecular density was lower in AA compared with nonathletes and in AA compared with both groups after controlling for bone age. Percentage of trabecular area was higher, and percentage of cortical area and cortical thickness were lower in both groups of athletes compared with controls, particularly after controlling for bone age.
Differences among groups after controlling for other covariates (BMI, lean mass, height, and menarchal age)
Differences among groups persisted after controlling for BMI, in addition to bone age (Table 2). Group differences for tibial total area, cortical perimeter, and trabecular area were no longer significant when we controlled for bone age and lean mass, but remained significant when we controlled for bone age and height or age at menarche. Differences for radial cortical area and thickness persisted when we controlled for bone age and lean mass, but were no longer significant after controlling for bone age and height or age at menarche. These data suggest that in weight-bearing bone (but not non-weight-bearing bone), greater total and trabecular areas in athletes are related to greater lean mass (and not height or age at menarche), whereas in non-weight-bearing bone (but not weight-bearing bone), differences in cortical area and thickness are related to differences in height and age at menarche (and not lean mass). Differences among groups for trabecular parameters persisted after controlling for BMI, height, lean mass, menarchal age, or energy expenditure, in addition to bone age (data not shown).
Correlation analysis of bone microarchitectural parameters with covariates of interest
We found significant associations between menarchal age and microarchitecture parameters (Table 3). At both sites, older menarchal age was associated with lower total, trabecular, and cortical density; lower cortical but greater trabecular and total area; lower cortical thickness; and greater Tb.Sp. At the radius, older menarchal age correlated with lower Tb.Th.
Table 3.
Simple correlations of bone microarchitectural measures with age at menarche, bone age, BMI, height, and lean mass for the group as a whole
| Bone age |
BMI |
Lean mass |
Height |
Age of menarche |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |
| Distal tibia | ||||||||||
| Trabecular area (mm2) | −0.27 | 0.06 | 0.65 | <0.0001 | 0.79 | <0.0001 | 0.50 | 0.0003 | ||
| Trabecular density (mg HA/cm3) | 0.32 | 0.02 | 0.24 | 0.09 | −0.39 | 0.006 | ||||
| Tb.N (1/mm) | −0.31 | 0.03 | 0.39 | 0.005 | −0.26 | 0.07 | ||||
| Tb.Th (mm) | 0.52 | 0.0001 | ||||||||
| Tb.Sp (mm) | 0.24 | 0.09 | −0.38 | 0.006 | 0.35 | 0.01 | ||||
| Cortical area (mm2) | 0.58 | <0.0001 | 0.45 | 0.001 | −0.28 | 0.05 | ||||
| Cortical density (mg HA/cm3) | 0.34 | 0.02 | −0.28 | 0.045 | −0.55 | <0.0001 | −0.42 | 0.003 | ||
| Cortical thickness (mm) | 0.41 | 0.003 | −0.33 | 0.02 | −0.48 | 0.0004 | ||||
| Cortical perimeter (mm) | 0.73 | <0.0001 | 0.83 | <0.0001 | 0.45 | 0.001 | ||||
| Total area (mm2) | −0.26 | 0.07 | 0.72 | <0.0001 | 0.82 | <0.0001 | 0.45 | 0.001 | ||
| Total density (mg HA/cm3) | 0.34 | 0.02 | 0.27 | 0.06 | −0.45 | 0.001 | −0.53 | 0.0001 | ||
| Ultradistal radius | ||||||||||
| Trabecular area (mm2) | −0.26 | 0.06 | 0.54 | <0.0001 | 0.60 | <0.0001 | 0.35 | 0.01 | ||
| Trabecular density (mg HA/cm3) | −0.32 | 0.03 | −0.52 | 0.0001 | ||||||
| Tb.N (1/mm) | −0.26 | 0.07 | ||||||||
| Tb.Th (mm) | 0.30 | 0.04 | −0.42 | 0.003 | ||||||
| Tb.Sp (mm) | 0.35 | 0.01 | ||||||||
| Cortical area (mm2) | 0.36 | 0.01 | −0.36 | 0.01 | ||||||
| Cortical density (mg HA/cm3) | 0.44 | 0.002 | −0.37 | 0.007 | −0.43 | 0.002 | ||||
| Cortical thickness (mm) | −0.47 | 0.0007 | ||||||||
| Cortical perimeter (mm) | 0.28 | 0.049 | 0.66 | <0.0001 | 0.64 | <0.0001 | 0.30 | 0.04 | ||
| Total area (mm2) | 0.61 | <0.0001 | 0.62 | <0.0001 | 0.29 | 0.047 | ||||
| Total density (mg HA/cm3) | 0.29 | 0.04 | −0.41 | 0.003 | −0.59 | <0.0001 | ||||
Only P values <0.1 are reported. HA, Hydroxyapatite.
At the tibia, bone age was positively associated with total, trabecular and cortical density and Tb.Th, and inversely with Tb.N. Similar associations were observed at the radius for total and cortical density and Tb.Th. BMI (marker of nutritional status) was positively associated with tibial cortical area and thickness and Tb.N, and inversely with Tb.Sp. Positive associations were also observed of BMI with radial cortical area and perimeter. Height was associated positively with total and trabecular area and cortical perimeter, and inversely with total and cortical density for both sites. Lean mass was associated with total, cortical and trabecular area and cortical perimeter at the tibia, and with total and trabecular area and cortical perimeter at the radius. Associations of energy expenditure with microarchitecture parameters were similar to those observed with lean mass and are not reported.
Regression modeling
We next performed regression modeling with age of menarche, bone age, lean mass, and subject group entered into the model to determine independent determinants of microarchitecture (Table 4). Total density was predicted by menarchal age and bone age, contributing to 44 and 40% of the variability at the radius and tibia, respectively. At the radius, cortical density was predicted by menarchal age and bone age (R2 = 0.36) and trabecular density by menarchal age (R2 = 0.27). At the tibia, cortical density was predicted by menarchal age, bone age, and lean mass (R2 = 0.33), and trabecular density by menarchal age and bone age (R2 = 0.28). For both sites, age of menarche and lean mass independently predicted cortical area (R2 = 0.23 and 0.37), whereas menarchal age, bone age, and lean mass predicted trabecular area (R2 = 0.44 and 0.64). Total area and cortical perimeter were predicted by bone age and lean mass at the radius (R2 = 0.45 and 0.47), and menarchal age, bone age, and lean mass at the tibia (R2 = 0.69 for both). Cortical thickness at the radius (R2 = 0.27) and tibia (R2 = 0.28) was predicted by menarchal age and bone age.
Table 4.
Regression modeling to determine independent predictors of bone microarchitecture parameters at the distal tibia
| Covariates | Parameter estimate | F ratio | P value | R2 | Cumulative R2 |
|---|---|---|---|---|---|
| Distal tibia | |||||
| Trabecular area (mm2) | |||||
| Intercept | 584.70 | ||||
| Lean mass (kg) | 10.29 | 40.5 | <0.0001 | 0.46 | |
| Age at menarche (yr) | 21.41 | 14.2 | 0.0005 | 0.10 | |
| Bone age (yr) | −43.91 | 10.2 | 0.003 | 0.08 | 0.64 |
| Trabecular density (mg HA/cm3) | |||||
| Intercept | 0.92 | ||||
| Age at menarche (yr) | −6.29 | 9.3 | 0.004 | 0.15 | |
| Bone age (yr) | 15.97 | 11.4 | 0.002 | 0.13 | |
| Subject grouping (AA vs. NAC and EA) | −4.04 | 2.9 | 0.07 | 0.08 | |
| Subject grouping (EA vs. NAC) | 9.45 | 4.5 | 0.04 | 0.37 | |
| Tb.N (1/mm) | |||||
| Intercept | 3.25 | ||||
| Subject grouping (AA vs. NAC and EA) | −0.12 | 11.5 | 0.001 | 0.18 | |
| Bone age (yr) | −0.10 | 5.7 | 0.02 | 0.08 | |
| Lean mass (kg) | 0.009 | 3.6 | 0.07 | 0.06 | 0.32 |
| Tb.Th (mm) | |||||
| Intercept | −0.09 | ||||
| Bone age (yr) | 0.01 | 20.4 | <0.0001 | 0.30 | 0.30 |
| Tb.Sp (mm) | |||||
| Intercept | 0.16 | ||||
| Subject grouping (AA vs. NAC and EA) | 0.03 | 8.9 | 0.005 | 0.24 | |
| Lean mass (kg) | −0.003 | 5.3 | 0.03 | 0.05 | |
| Age at menarche (yr) | 0.009 | 3.6 | 0.07 | 0.05 | |
| Bone age (yr) | 0.02 | 2.9 | 0.09 | 0.04 | 0.38 |
| Cortical area (mm2) | |||||
| Intercept | 109.75 | ||||
| Lean mass (kg) | 1.60 | 21.0 | <0.0001 | 0.19 | |
| Age at menarche (yr) | −4.39 | 12.8 | 0.0008 | 0.18 | 0.37 |
| Cortical density (mg HA/cm3) | |||||
| Intercept | 768.47 | ||||
| Age at menarche (yr) | −6.76 | 9.0 | 0.004 | 0.18 | |
| Bone age (yr) | 14.39 | 6.9 | 0.01 | 0.10 | |
| Lean mass (kg) | −1.15 | 3.2 | 0.08 | 0.05 | 0.33 |
| Cortical thickness (mm) | |||||
| Intercept | 0.86 | ||||
| Age at menarche (yr) | −0.057 | 15.9 | 0.0002 | 0.23 | |
| Bone age (yr) | 0.064 | 3.2 | 0.08 | 0.05 | 0.28 |
| Cortical perimeter (mm) | |||||
| Intercept | 93.25 | ||||
| Lean mass (kg) | 0.94 | 64.83 | <0.0001 | 0.57 | |
| Age at menarche (yr) | 1.30 | 10.1 | 0.003 | 0.06 | |
| Bone age (yr) | −2.92 | 8.6 | 0.005 | 0.06 | 0.69 |
| Total area (mm2) | |||||
| Intercept | 667.56 | ||||
| Lean mass (kg) | 11.78 | 61.4 | <0.0001 | 0.55 | |
| Bone age (yr) | −42.26 | 10.9 | 0.002 | 0.07 | |
| Age at menarche (yr) | 17.26 | 10.6 | 0.002 | 0.07 | 0.69 |
| Total density (mg HA/cm3) | |||||
| Intercept | 97.07 | ||||
| Age at menarche (yr) | −15.22 | 23.3 | <0.0001 | 0.28 | |
| Bone age (yr) | 24.65 | 9.6 | 0.003 | 0.12 | 0.40 |
| Distal radius | |||||
| Trabecular area (mm2) | |||||
| Intercept | 342.96 | ||||
| Lean mass (kg) | 3.44 | 17.5 | 0.0001 | 0.29 | |
| Bone age (yr) | −20.27 | 8.4 | 0.006 | 0.10 | |
| Age at menarche (yr) | 5.87 | 4.1 | 0.048 | 0.05 | 0.44 |
| Trabecular density (mg HA/cm3) | |||||
| Intercept | 288.85 | ||||
| Age at menarche (yr) | −8.65 | 17.2 | 0.0001 | 0.27 | 0.27 |
| Tb.N (1/mm) | |||||
| Intercept | 2.41 | ||||
| Age at menarche (yr) | −0.03 | 3.4 | 0.07 | 0.07 | 0.07 |
| Tb.Th (mm) | |||||
| Intercept | 0.027 | ||||
| Age at menarche (yr) | −0.0027 | 12.1 | 0.001 | 0.18 | |
| Bone age (yr) | 0.0046 | 5.4 | 0.02 | 0.08 | 0.26 |
| Tb.Sp (mm) | |||||
| Intercept | 0.29 | ||||
| Age at menarche (yr) | 0.011 | 6.7 | 0.01 | 0.12 | 0.12 |
| Cortical area (mm2) | |||||
| Intercept | 46.26 | ||||
| Age at menarche (yr) | −2.21 | 7.7 | 0.008 | 0.13 | |
| Lean mass (kg) | 0.81 | 11.9 | 0.001 | 0.10 | |
| Subject grouping (AA and EA vs. NAC) | −4.71 | 7.1 | 0.01 | 0.11 | 0.34 |
| Cortical density (mg HA/cm3) | |||||
| Intercept | 449.26 | ||||
| Age at menarche (yr) | −13.34 | 15.1 | 0.0003 | 0.18 | |
| Bone age (yr) | 31.58 | 13.3 | 0.0007 | 0.18 | 0.36 |
| Cortical thickness (mm) | |||||
| Intercept | 1.33 | ||||
| Age at menarche (yr) | −0.05 | 14.9 | 0.0004 | 0.22 | |
| Bone age (yr) | 0.05 | 3.3 | 0.07 | 0.05 | 0.27 |
| Cortical perimeter (mm) | |||||
| Intercept | 72.44 | ||||
| Lean mass (kg) | 0.61 | 38.0 | <0.0001 | 0.43 | |
| Bone age (yr) | −1.76 | 4.0 | 0.05 | 0.04 | 0.47 |
| Total area (mm2) | |||||
| Intercept | 399.3 | ||||
| Lean mass (kg) | 4.35 | 31.5 | <0.0001 | 0.36 | |
| Bone age (yr) | −18.21 | 7.1 | 0.01 | 0.09 | 0.45 |
| Total density (mg HA/cm3) | |||||
| Intercept | 144.91 | ||||
| Age at menarche (yr) | −16.15 | 21.9 | <0.0001 | 0.34 | |
| Bone age (yr) | 22.03 | 7.2 | 0.01 | 0.09 | |
| Subject grouping (AA and EA vs. NAC) | −12.24 | 2.9 | 0.09 | 0.04 | 0.47 |
HA, Hydroxyapatite.
Tb.N was determined by menarchal age at the radius (R2 = 0.07) and bone age and lean mass at the tibia (R2 = 0.14). Tb.Th was predicted by menarchal and bone age at the radius (R2 = 0.26) but only bone age at the tibia (R2 = 0.30). Tb.Sp was predicted by menarchal age at the radius (R2 = 0.12) and all measures at the tibia (R2 = 0.38). Even after controlling for menarchal age, bone age, and lean mass, subject grouping independently predicted cortical area (R2 = 0.11) and total density (R2 = 0.04) at the radius, and Tb.N (R2 = 0.18), Tb.Sp (R2 = 0.24), and trabecular density (R2 = 0.11) at the tibia.
Discussion
We demonstrate alterations in bone microarchitecture in young athletes compared with nonathletes, and in AA compared with EA and nonathletic controls. Our data indicate differences in the effect of athletic activity vs. amenorrhea on cortical and trabecular parameters in young women.
The greatest increases in bone mass occur during puberty (29–31), and decreased bone accrual during puberty and young adulthood may lead to lower peak bone mass and future increases in fracture risk. Adolescent AA have lower BMD than age-matched peers (5, 6), and factors contributing to low BMD in AA include estrogen deficiency (32) (because normal estrogen is key to skeletal homeostasis), low energy availability (33, 34), low fat mass and hormones involved in energy homeostasis (5, 35), hypercortisolemia (36–38), and possibly progesterone deficiency (39). Of importance, although EA have an advantage over nonathletes and AA at weight-bearing sites such as the hip, these girls also develop stress fractures, the reason for which remains unclear.
Our study demonstrates lower lumbar BMD and BMAD Z-scores in AA compared with EA and nonathletes, and higher total hip and femoral neck BMD Z-scores in EA compared with AA and nonathletes. These results are consistent with the hypothesis that amenorrhea attenuates bone anabolic effects of weight-bearing exercise in adolescent athletes (40, 41). Of importance, changes in BMD may not consistently and accurately reflect changes in bone microarchitecture, a sensitive measure of fracture risk independent of BMD in adults, particularly postmenopausal women (8, 11, 12, 17). Compared with women without a history of fractures and controls, those with a history of fractures have lower trabecular bone volume, Tb.N, and Tb.Th, and increased Tb.Sp (8–12). Also, adolescents with AN have impaired microarchitecture compared with controls even when DXA measures of BMD do not differ (19, 20). However, little is known about alterations in bone microarchitecture in adolescent AA.
Our study demonstrates for the first time the differing impact of athletic weight-bearing activity vs. amenorrhea (and associated estrogen deficiency) on bone microarchitecture in adolescent and young adult athletes. Weight-bearing exerts a significant influence on BMD in female athletes, with site-specific mechanical loading significantly affecting bone accrual (42, 43). Both groups of athletes in our study had greater total cross-sectional area, trabecular area, and cortical perimeter compared with nonathletes at the distal tibia, a weight-bearing site. In contrast, greater total area and cortical perimeter were not observed in athletes at the ultradistal radius (a non-weight-bearing site). This suggests that forces exerted on the tibia through weight-bearing exercise likely contribute to increased total cross-sectional area in athletes. Of note, greater bone mass and greater distance of this mass from the neutral axis are associated with greater moment of inertia (reflecting resistance of bone to bending) and lower strain at any given force (44). Thus, repetitive mechanical force through exercise may expand tibial bone area to decrease strain, thereby lowering accumulation of microdamage.
Notably, although total and trabecular area at the tibia was higher in both groups of athletes compared with nonathletes, tibial cortical area trended higher in EA vs. AA and nonathletes. We speculate that in EA, cortical perimeter expands outward in weight-bearing bone secondary to exercise (leading to greater total area), whereas expansion of the endocortical circumference is prevented by estradiol. In AA, cortical perimeter and total area similarly increase from effects of exercise. However, in the absence of estrogen, inhibition of endocortical bone resorption does not occur. Hence, endocortical circumference may continue to grow outward, consistent with our observation of a trend toward lower cortical thickness and cortical area as a percentage of total area in AA, similar to reports in postmenopausal women (45).
As expected, lean mass was overall higher in athletes than nonathletes, and highest in EA. After controlling for lean mass, groups no longer differed for tibial total and trabecular area and cortical perimeter. This is possibly because greater lean mass in athletes secondary to exercise (46, 47) is the primary driver of bone expansion, increasing resistance to mechanical forces. Consistent with this, on regression modeling, lean mass was an independent determinant of total, cortical, and trabecular area.
Despite greater total area and cortical perimeter in athletes, cortical volumetric BMD at the tibia was lower in both groups of athletes than nonathletes, particularly after adjusting for maturity and nutritional status. This may be consequent to a delay in mineral deposition in the expanding tibia of young athletes (48) or an increase in cortical porosity, which has been associated with a transient increase in fracture risk in adolescents (49).
Deleterious effects of amenorrhea on microarchitecture were also evident in AA at trabecular bone. AA had lower radial trabecular density, lower tibial Tb.N, and increased Tb.Sp compared with other groups, even after controlling for maturity (assessed by bone age), nutritional status (assessed by BMI), and lean mass (measure of athletic activity). This suggests a true negative relationship between amenorrhea and trabecular parameters, consistent with pQCT findings in retired gymnasts with a history of amenorrhea (50). Additionally, menarchal age was an important determinant of microarchitecture. Later menarchal age reflects delayed exposure of bone to estrogen and shorter duration of estrogen exposure during puberty, and later age at menarche has been reported among AA in other studies as well (51–53). Our data are consistent with those of Chevalley et al. (54) who reported that healthy women with delayed menarche have lower BMD (by DXA) and lower total and cortical volumetric BMD and cortical thickness (by HRpQCT). Interestingly, even after controlling for age at menarche (in addition to other covariates), differences in tibial trabecular parameters persisted across groups.
Limitations of our study include its cross-sectional nature. Also, associations do not prove causation, and a prospective study may better explain interactions of menstrual function and exercise on bone. Additionally, it would have been ideal to include a group of amenorrheic healthy nonathletes as another control group. However, such a group is difficult to identify, particularly one without associated risk factors for impaired bone health. Strengths include our careful selection of AA, EA, and nonathlete subjects. Athletes were selected based on strict criteria regarding weight-bearing endurance exercise of the legs to minimize variability of exercise forces on upper and lower extremities. A narrow age range was studied, and homogeneity was further achieved by presenting Z-scores and controlling for bone age. We factored height into our analysis by calculating BMAD and by controlling for height when comparing microarchitecture across groups. All HRpQCT data were collected by one operator, and DXA data were obtained on a single instrument.
In conclusion, athletic activity is associated with greater total and trabecular area and greater cortical perimeter at the weight-bearing tibia, whereas amenorrhea is associated with lower trabecular bone density of the non-weight-bearing radius, lower total density and Tb.N, and greater trabecular separation at the tibia. Interestingly, subject grouping remains an independent determinant of Tb.N and separation at weight-bearing sites (accounting for 18–24% of the variability), and accounts for a smaller variability of cortical area and total density at non-weight-bearing sites, even after controlling for covariates such as menarchal age, bone age, and lean mass. Therefore, independent of BMD, microarchitecture provides information about bone parameters in the amenorrheic athlete.
Acknowledgments
This work was supported by National Institutes of Health Grants 1 UL1 RR025758-01 and 1 R01 HD060827-01A1.
Clinical Trial registration no.: NCT00946192.
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
- AA
- Amenorrheic athlete
- AN
- anorexia nervosa
- BMAD
- bone mineral apparent density
- BMD
- bone mineral density
- BMI
- body mass index
- DXA
- dual-energy x-ray absorptiometry
- EA
- eumenorrheic athlete
- HRpQCT
- high-resolution peripheral quantitative computed tomography
- Tb.N
- trabecular number
- Tb.Sp
- trabecular separation
- Tb.Th
- trabecular thickness.
References
- 1. Harel Z, Gold M, Cromer B, Bruner A, Stager M, Bachrach L, Wolter K, Reid C, Hertweck P, Nelson A, Nelson D, Coupey S, Johnson C, Burkman R, Bone H. 2007. Bone mineral density in postmenarchal adolescent girls in the United States: associated biopsychosocial variables and bone turnover markers. J Adolesc Health 40:44–53 [DOI] [PubMed] [Google Scholar]
- 2. Sabatier JP, Guaydier-Souquières G, Laroche D, Benmalek A, Fournier L, Guillon-Metz F, Delavenne J, Denis AY. 1996. Bone mineral acquisition during adolescence and early adulthood: a study in 574 healthy females 10–24 years of age. Osteoporos Int 6:141–148 [DOI] [PubMed] [Google Scholar]
- 3. Kaestner R, Xu X. 2007. Effects of Title IX and sports participation on girls' physical activity and weight. Adv Health Econ Health Serv Res 17:79–111 [PubMed] [Google Scholar]
- 4. Shafi SM, Malla MA, Salaam PA, Kirmani OS. 2009. Abdominal pregnancy as a cause of hemoperitoneum. J Emerg Trauma Shock 2:196–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Russell M, Stark J, Nayak S, Miller KK, Herzog DB, Klibanski A, Misra M. 2009. Peptide YY in adolescent athletes with amenorrhea, eumenorrheic athletes and non-athletic controls. Bone 45:104–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rencken ML, Chesnut CH, 3rd, Drinkwater BL. 1996. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA 276:238–240 [PubMed] [Google Scholar]
- 7. Nichols JF, Rauh MJ, Lawson MJ, Ji M, Barkai HS. 2006. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med 160:137–142 [DOI] [PubMed] [Google Scholar]
- 8. Link TM, Bauer J, Kollstedt A, Stumpf I, Hudelmaier M, Settles M, Majumdar S, Lochmüller EM, Eckstein F. 2004. Trabecular bone structure of the distal radius, the calcaneus, and the spine: which site predicts fracture status of the spine best? Invest Radiol 39:487–497 [DOI] [PubMed] [Google Scholar]
- 9. Link TM, Vieth V, Matheis J, Newitt D, Lu Y, Rummeny EJ, Majumdar S. 2002. Bone structure of the distal radius and the calcaneus vs BMD of the spine and proximal femur in the prediction of osteoporotic spine fractures. Eur Radiol 12:401–408 [DOI] [PubMed] [Google Scholar]
- 10. Kleerekoper M, Villanueva AR, Stanciu J, Rao DS, Parfitt AM. 1985. The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int 37:594–597 [DOI] [PubMed] [Google Scholar]
- 11. Patel PV, Prevrhal S, Bauer JS, Phan C, Eckstein F, Lochmüller EM, Majumdar S, Link TM. 2005. Trabecular bone structure obtained from multislice spiral computed tomography of the calcaneus predicts osteoporotic vertebral deformities. J Comput Assist Tomogr 29:246–253 [DOI] [PubMed] [Google Scholar]
- 12. Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. 2007. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res 22:425–433 [DOI] [PubMed] [Google Scholar]
- 13. Bachrach LK. 2007. Consensus and controversy regarding osteoporosis in the pediatric population. Endocr Pract 13:513–520 [DOI] [PubMed] [Google Scholar]
- 14. Bachrach LK. 2006. Measuring bone mass in children: can we really do it? Horm Res 65(Suppl 2):11–16 [DOI] [PubMed] [Google Scholar]
- 15. Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, Lorenc RS, Tosi LL, Ward KA, Ward LM, Kalkwarf HJ. 2008. Dual energy x-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom 11:43–58 [DOI] [PubMed] [Google Scholar]
- 16. Burrows M, Liu D, Perdios A, Moore S, Mulpuri K, McKay H. 2010. Assessing bone microstructure at the distal radius in children and adolescents using HR-pQCT: a methodological pilot study. J Clin Densitom 13:451–455 [DOI] [PubMed] [Google Scholar]
- 17. Lawson EA, Miller KK, Bredella MA, Phan C, Misra M, Meenaghan E, Rosenblum L, Donoho D, Gupta R, Klibanski A. 2010. Hormone predictors of abnormal bone microarchitecture in women with anorexia nervosa. Bone 46:458–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. 2008. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res 23:392–399 [DOI] [PubMed] [Google Scholar]
- 19. Bredella MA, Misra M, Miller KK, Madisch I, Sarwar A, Cheung A, Klibanski A, Gupta R. 2008. Distal radius in adolescent girls with anorexia nervosa: trabecular structure analysis with high-resolution flat-panel volume CT. Radiology 249:938–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walsh CJ, Phan CM, Misra M, Bredella MA, Miller KK, Fazeli PK, Bayraktar HH, Klibanski A, Gupta R. 2010. Women with anorexia nervosa: finite element and trabecular structure analysis by using flat-panel volume CT. Radiology 257:167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rickenlund A, Carlström K, Ekblom B, Brismar TB, Von Schoultz B, Hirschberg AL. 2004. Effects of oral contraceptives on body composition and physical performance in female athletes. J Clin Endocrinol Metab 89:4364–4370 [DOI] [PubMed] [Google Scholar]
- 22. Robinson TL, Snow-Harter C, Taaffe DR, Gillis D, Shaw J, Marcus R. 1995. Gymnasts exhibit higher bone mass than runners despite similar prevalence of amenorrhea and oligomenorrhea. J Bone Miner Res 10:26–35 [DOI] [PubMed] [Google Scholar]
- 23. Fehling PC, Alekel L, Clasey J, Rector A, Stillman RJ. 1995. A comparison of bone mineral densities among female athletes in impact loading and active loading sports. Bone 17:205–210 [DOI] [PubMed] [Google Scholar]
- 24. Mudd LM, Fornetti W, Pivarnik JM. 2007. Bone mineral density in collegiate female athletes: comparisons among sports. J Athl Train 42:403–408 [PMC free article] [PubMed] [Google Scholar]
- 25. Katzman DK, Bachrach LK, Carter DR, Marcus R. 1991. Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab 73:1332–1339 [DOI] [PubMed] [Google Scholar]
- 26. Hart TL, Ainsworth BE, Tudor-Locke C. 2011. Objective and subjective measures of sedentary behavior and physical activity. Med Sci Sports Exerc 43:449–456 [DOI] [PubMed] [Google Scholar]
- 27. Boutroy S, Bouxsein ML, Munoz F, Delmas PD. 2005. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90:6508–6515 [DOI] [PubMed] [Google Scholar]
- 28. Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. 2010. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res 25:882–890 [DOI] [PubMed] [Google Scholar]
- 29. Gilsanz V, Chalfant J, Kalkwarf H, Zemel B, Lappe J, Oberfield S, Shepherd J, Wren T, Winer K. 2011. Age at onset of puberty predicts bone mass in young adulthood. J Pediatr 158:100–105, 105.e1–e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Theintz G, Buchs B, Rizzoli R, Slosman D, Clavien H, Sizonenko PC, Bonjour JP. 1992. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab 75:1060–1065 [DOI] [PubMed] [Google Scholar]
- 31. Soyka LA, Fairfield WP, Klibanski A. 2000. Clinical review 117: hormonal determinants and disorders of peak bone mass in children. J Clin Endocrinol Metab 85:3951–3963 [DOI] [PubMed] [Google Scholar]
- 32. Gulekli B, Davies MC, Jacobs HS. 1994. Effect of treatment on established osteoporosis in young women with amenorrhoea. Clin Endocrinol (Oxf) 41:275–281 [DOI] [PubMed] [Google Scholar]
- 33. Ihle R, Loucks AB. 2004. Dose-response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res 19:1231–1240 [DOI] [PubMed] [Google Scholar]
- 34. De Souza MJ, West SL, Jamal SA, Hawker GA, Gundberg CM, Williams NI. 2008. The presence of both an energy deficiency and estrogen deficiency exacerbate alterations of bone metabolism in exercising women. Bone 43:140–148 [DOI] [PubMed] [Google Scholar]
- 35. Christo K, Cord J, Mendes N, Miller KK, Goldstein MA, Klibanski A, Misra M. 2008. Acylated ghrelin and leptin in adolescent athletes with amenorrhea, eumenorrheic athletes and controls: a cross-sectional study. Clin Endocrinol (Oxf) 69:628–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tabata I, Ogita F, Miyachi M, Shibayama H. 1991. Effect of low blood glucose on plasma CRF, ACTH, and cortisol during prolonged physical exercise. J Appl Physiol 71:1807–1812 [DOI] [PubMed] [Google Scholar]
- 37. Bullen BA, Skrinar GS, Beitins IZ, von Mering G, Turnbull BA, McArthur JW. 1985. Induction of menstrual disorders by strenuous exercise in untrained women. N Engl J Med 312:1349–1353 [DOI] [PubMed] [Google Scholar]
- 38. Laughlin GA, Yen SS. 1996. Nutritional and endocrine-metabolic aberrations in amenorrheic athletes. J Clin Endocrinol Metab 81:4301–4309 [DOI] [PubMed] [Google Scholar]
- 39. Prior JC, Vigna YM, Schechter MT, Burgess AE. 1990. Spinal bone loss and ovulatory disturbances. N Engl J Med 323:1221–1227 [DOI] [PubMed] [Google Scholar]
- 40. Lloyd T, Myers C, Buchanan JR, Demers LM. 1988. Collegiate women athletes with irregular menses during adolescence have decreased bone density. Obstet Gynecol 72:639–642 [PubMed] [Google Scholar]
- 41. Drinkwater BL, Bruemner B, Chesnut CH., 3rd 1990. Menstrual history as a determinant of current bone density in young athletes. JAMA 263:545–548 [PubMed] [Google Scholar]
- 42. Bennell KL, Malcolm SA, Khan KM, Thomas SA, Reid SJ, Brukner PD, Ebeling PR, Wark JD. 1997. Bone mass and bone turnover in power athletes, endurance athletes, and controls: a 12-month longitudinal study. Bone 20:477–484 [DOI] [PubMed] [Google Scholar]
- 43. Theodoropoulou A, Markou KB, Vagenakis GA, Benardot D, Leglise M, Kourounis G, Vagenakis AG, Georgopoulos NA. 2005. Delayed but normally progressed puberty is more pronounced in artistic compared with rhythmic elite gymnasts due to the intensity of training. J Clin Endocrinol Metab 90:6022–6027 [DOI] [PubMed] [Google Scholar]
- 44. Garrett WE, Kirkendall DT. 2000. Exercise and sport science. Philadelphia: Lippincott Williams, Wilkins [Google Scholar]
- 45. Riggs BL, Khosla S, Melton LJ., 3rd 2002. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302 [DOI] [PubMed] [Google Scholar]
- 46. Nichols DL, Sanborn CF, Bonnick SL, Gench B, DiMarco N. 1995. Relationship of regional body composition to bone mineral density in college females. Med Sci Sports Exerc 27:178–182 [PubMed] [Google Scholar]
- 47. Madsen KL, Adams WC, Van Loan MD. 1998. Effects of physical activity, body weight and composition, and muscular strength on bone density in young women. Med Sci Sports Exerc 30:114–120 [DOI] [PubMed] [Google Scholar]
- 48. Kirmani S, Christen D, van Lenthe GH, Fischer PR, Bouxsein ML, McCready LK, Melton LJ, 3rd, Riggs BL, Amin S, Müller R, Khosla S. 2009. Bone structure at the distal radius during adolescent growth. J Bone Miner Res 24:1033–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khosla S, Melton LJ, 3rd, Dekutoski MB, Achenbach SJ, Oberg AL, Riggs BL. 2003. Incidence of childhood distal forearm fractures over 30 years: a population-based study. JAMA 290:1479–1485 [DOI] [PubMed] [Google Scholar]
- 50. Ducher G, Eser P, Hill B, Bass S. 2009. History of amenorrhoea compromises some of the exercise-induced benefits in cortical and trabecular bone in the peripheral and axial skeleton: a study in retired elite gymnasts. Bone 45:760–767 [DOI] [PubMed] [Google Scholar]
- 51. Bale P, Doust J, Dawson D. 1996. Gymnasts, distance runners, anorexics body composition and menstrual status. J Sports Med Phys Fitness 36:49–53 [PubMed] [Google Scholar]
- 52. Georgopoulos NA, Roupas ND, Theodoropoulou A, Tsekouras A, Vagenakis AG, Markou KB. 2010. The influence of intensive physical training on growth and pubertal development in athletes. Ann NY Acad Sci 1205:39–44 [DOI] [PubMed] [Google Scholar]
- 53. Torstveit MK, Sundgot-Borgen J. 2005. Participation in leanness sports but not training volume is associated with menstrual dysfunction: a national survey of 1276 elite athletes and controls. Br J Sports Med 39:141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. 2008. Influence of age at menarche on forearm bone microstructure in healthy young women. J Clin Endocrinol Metab 93:2594–2601 [DOI] [PubMed] [Google Scholar]