Abstract
The PediaFlow pediatric ventricular assist device is a miniature magnetically levitated mixed flow pump under development for circulatory support of newborns and infants (3–15 kg) with a targeted flow range of 0.3–1.5 L/min. The first generation design of the PediaFlow (PF1) was manufactured with a weight of approximately 100 g, priming volume less than 2 mL, length of 51 mm, outer diameter of 28 mm, and with 5-mm blood ports. PF1 was evaluated in an in vitro flow loop for 6 h and implanted in ovines for three chronic experiments of 6, 17, and 10 days. In the in vitro test, normalized index of hemolysis was 0.0087 ± 0.0024 g/100L. Hemodynamic performance and blood biocompatibility of PF1 were characterized in vivo by measurements of plasma free hemoglobin, plasma fibrinogen, total plasma protein, and with novel flow cytometric assays to quantify circulating activated ovine platelets. The mean plasma free hemoglobin values for the three chronic studies were 4.6 ± 2.7, 13.3 ± 7.9, and 8.8 ± 3.3 mg/dL, respectively. Platelet activation was low for portions of several studies but consistently rose along with observed animal and pump complications. The PF1 prototype generated promising results in terms of low hemolysis and platelet activation in the absence of complications. Hemodynamic results validated the magnetic bearing design and provided the platform for design iterations to meet the objective of providing circulatory support for young children with exceptional biocompatibility.
Keywords: Pediatric ventricular assist devices, Biocompatibility assessment, Platelet activation, Hemolysis, Magnetic levitation
Ventricular assist devices (VADs) have proven to be an acceptable treatment modality as either a bridge to transplant or as destination therapy in adults (1,2). Despite the clinical success in adults, there is limited experience implanting VADs in small children, although these devices fill a clinical need (3,4). Approximately 36 000 children are born with congenital heart defects yearly in the United States, of which 9200 require invasive treatment to prevent death. The total-mention mortality for congenital heart disease is approximately 5500 (5).Cardiac transplant is an effective treatment for many of these children; however, its efficacy is marginalized due to the limited number and wide range of sizes of available donor hearts. Extracorporeal membrane oxygenation (ECMO) can provide temporary cardiopulmonary support but the duration of support is limited because of infection, bleeding, and thromboembolic complications (3,6,7). As a result, there is currently a 20–27% transplantation waiting list mortality for infants and children, the highest in solid organ transplantation (8,9). There is a need for devices that can provide longer durations of support that might allow recovery of the myocardium or to bridge the child to a transplant. Such devices must possess excellent blood biocompatibility as the lack of blood biocompatibility is the cause of many complications that diminish efficacy in adult VAD patients (10,11).
The pediatric heart transplant study demonstrated that VADs were an effective treatment modality in children as a bridge to transplant, with 77% of the VAD patients surviving to transplant, and further, that there was no difference in the 5-year survival of transplant patients supported by VAD when compared with patients who did not receive a VAD (3). The majority of the VADs utilized in this study, however, were adult devices that were implanted in older children. This pediatric study along with other reports have highlighted the need to miniaturize adult devices or develop new devices to provide cardiac support for infants and small children, observing that the vast majority of children listed for heart transplant and those who die after listing are under 2 years of age (3,4,8,12). Despite this clinical need, there are currently no Food and Drug Administration-approved VADs available for infants/small children in the United States (4,13).
The limitations of ECMO and dearth of donor hearts and VADs for small children were the motivations for the National Heart, Lung, and Blood Institute pediatric circulatory support program whose goal was to award contracts to institutions to develop novel circulatory support systems for infants and children (6). The University of Pittsburgh consortium, comprised of the University of Pittsburgh, Carnegie Mellon University, LaunchPoint Technologies, Inc., WorldHeart, Inc., and Children’s Hospital of Pittsburgh, was awarded a contract from this program to build the PediaFlow pediatric VAD (6,14).
The PediaFlow VAD is a mixed flow turbodynamic VAD that employs a magnetic suspension (4,14,15). The ultimate goal of this device is to deliver a flow rate between 0.3 and 1.5 L/min to serve a patient population from newborns to approximately 2 years old. The device aims for an implantation period of up to 6 months to provide cardiac support as a child awaits a transplant or myocardial recovery. The first generation design (PF1) weighs approximately 100 g and pumped a maximum of 660–810 mL/min against physiologic pressure in three ovine animal studies. It measures 51 mm in length, has a 28-mm outer diameter, and a pump priming volume of less than 2 mL. In concert with meeting the clinical design requirements stated above, the focus of PediaFlow VAD development is to achieve high levels of blood biocompatibility. To achieve this criterion, the PF1 flow path was developed using iterative computational fluid dynamics (CFD) to minimize areas of high shear and to possess smooth velocity vectors devoid of stagnation or recirculation zones (4,14,16). Heat generation caused by the pump was quantified and determined to be within acceptable limits (≤2°C temperature rise) during normal operation (17). Furthermore, magnetic bearings were chosen for the PediaFlow VAD to avoid the wear and heat generation associated with contact bearings (18,19). As contact bearings are also known to form a high shear region and may promote hemolysis as well as thrombus formation, the use of magnetic bearings in the PediaFlow VAD, we hypothesized, would significantly improve its potential for excellent biocompatibility in vivo (18–20).
Damage to red blood cells (hemolysis) and platelet activation are important parameters that are often measured to assess the biocompatibility of artificial organs (21–25). The use of platelet biocompatibility assays has demonstrated utility in evaluating mechanical pump design, potential surface coating modifications, and anticoagulation regimen providing guidance to potentially improve device hemocompatibility (22,23,26,27). To assess the biocompatibility of the PediaFlow PF1 design in this report, we employed assays for hemolysis (quantified by plasma free hemoglobin; plfHb), and plasma protein and fibrinogen concentrations, as well as recently developed flow cytometric assays to quantify ovine platelet activation during implantation in vivo (21,28,29).
MATERIALS AND METHODS
In vitro biocompatibility flow loop test
Blood collection
Ovine whole blood (540 mL) was collected by jugular venipuncture using an 18-G 1.5-in needle with syringe and stopcock into a blood bag containing 60 mL of anticoagulant citrate dextrose solution. Heparin (4 U/mL) was added to the blood reservoir postcollection (hematocrit = 28%).
Flow loop setup
A fluid dynamic test loop with a blood bag reservoir and the PF1 pediatric VAD was prepared with luer ports for pressure measurement at VAD inlet and outlet as well as blood temperature measurement. A magnetic stir bar was introduced into the reservoir, which was placed on a magnetic stirrer for constant mixing of the blood. The circuit was cleaned and the surfaces passivated by introducing, in sequence, detergent (Simple Green, Sunshine Makers, Inc., Huntington Beach, CA, USA), enzymatic detergent (Tergazyme, Alconox, Inc., White Plains, NY, USA), deionized water (twice), 1% bovine serum albumin solution in Tyrode’s buffer (Electron Microscopy Sciences, Hatfield, PA, USA), and saline. Each of these fluids was pumped by the PF1 pediatric VAD through the test loop at minimum speed (3000 rpm) for 15 min. Ovine blood (500 mL) was then added to the reservoir and circulated at 650–750 mL/min for 6 h at 10 500 rpm. Resistance of the circuit (a Hoffman clamp downstream of the pump) was set to achieve an afterload pressure of 80 mm Hg. A bag with the same ovine blood (~100 mL) was slowly rocked next to the circulation loop to serve as a control. A blood sample was taken at the start of the experiment immediately after the pump reached maximum speed (hour 0).The pump operated for 6 h and blood samples (3 mL) were collected hourly from the circuit and the control bag. Each time a blood sample was drawn from the flow loop, the volume was replaced with blood from the control blood bag to maintain the flow loop blood volume at 500 mL.
Assessment of plfHb
Collected blood samples from both the control bag and the flow loop were centrifuged for 15 min at 2200 × g to obtain plasma. Plasma was transferred to microcentrifuge tubes and centrifuged at 20 800 × g for 20 min in a microcentrifuge (Eppendorf 5417R, Eppendorf North America, Westbury, NY, USA). Plasma was then transferred to disposable semi-micro spectrophotometer cuvettes (Thermo Fisher Scientific, Inc., Waltham, MA, USA). PlfHb was measured for both samples at each time point using a spectrophotometer (Spectronic GENESYS 5, Thermo Fisher Scientific, Inc.) at 540-nm wavelength (30). The spectrophotometer was calibrated to zero using a blank solution according to established protocols. A hemoglobin calibration curve was obtained using standard dilutions of hemoglobin solution of known concentrations. “Blood damage” was then characterized by the normalized index of hemolysis using the difference between the plfHb from the flow loop and from the control bag recorded at each time point (31).
In vivo testing
Surgical procedure
Each sheep was prepared for surgery in compliance with the University of Pittsburgh Institutional Animal Care and Use Committee and National Institutes of Health (NIH) guidelines for the care and the use of laboratory animals. Anesthesia was induced with ketamine and maintained with inhalation isoflurane. The characteristics of each implant can be found in Table 1. An arterial line for pressure measurement was placed in the left carotid artery along with a venous line for drug and fluid administration in the jugular vein. A left thoracotomy was performed through the fourth intercostal space. The inflow cannula was measured and cut to length and assembled to the PF1 inflow connector. A felt-coated sewing ring was fixed on the apex of the heart with pledgeted sutures and the cannula was inserted through this ring in the left ventricle via a stab wound.After bolus administration of heparin (150 U/kg), the descending aorta was partially clamped using a vascular clamp (without cardiopulmonary bypass) and the outflow graft (Table 1) was anastomosed and fixed directly onto the pump outflow connector. The pump was started and set to run at maximum speed (Table 1). A 6PXL ultrasonic flow probe (Transonic Systems, Inc., Ithaca, NY, USA) was attached around the outflow graft. After closure of the chest, each animal was allowed to recover from anesthesia, and spontaneously ventilate. Marcaine (Hospira, Inc., Lake Forest, IL, USA) was used in the intercostal muscles and Banamine (Phoenix Pharmaceutical, Inc., St. Joseph, MO, USA; 25 mg intravenously) was used for analgesia. Heparin was not administered for the first 48 h following implant. Heparin was administered to maintain the activated clotting time at approximately 180 s and was discontinued whenever the hematocrit dropped below 20%.
TABLE 1.
PediaFlow (PF1) Implant Summary
| In vivo study # |
Animal weight (kg) |
Duration of support |
Inflow cannula |
Outflow cannula | Flow rate (L/min) |
Pump Speed (krpm) |
Mean Arterial Pressure (mm Hg) |
|---|---|---|---|---|---|---|---|
| 1 | 54 | 6 d | shortened 20Fr DLP | 6 mm Vascutek | 0.81 | 10.7 | 76.5 |
| 2 | 30 | 17 d | shortened 20Fr DLP | PVC + 6 mm Vascutek | 0.55 | 11.0 | 94.7 |
| 3 | 34 | 10 d | shortened 20Fr DLP | PVC + 6 mm Vascutek | 0.66 | 10.0 | 111.8 |
DLP cannula (Medtronic, Minneapolis,MN, USA).Vascutek outflow graft (Terumo Cardiovascular Systems Corp.,Ann Arbor,MI, USA). PVC (6 mm) bonded to Vascutek graft by silicon
Blood collection
Preoperative whole blood was collected from each ovine by jugular venipuncture using an 18-gauge 1.5-in needle with syringe and stopcock. The first 3 mL were added to sodium heparin tubes for plfHb and hemorheological parameter measurement. An additional 2.7 mL of blood for platelet activation assessment was drawn and added to monovettes containing 0.3 mL of 0.106 M trisodium citrate (Sarstedt, Newton, NC, USA). In the 10-day implant an indwelling catheter was inserted for preoperative blood collection. For this implant, samples were collected by withdrawing 20 mL of blood, then the sample volume, and then re-infusing the initial 20 mL of blood. Postoperative samples were collected daily through an indwelling arterial line that was placed during surgery for plfHb (reported in mg/dL) and hemorheology assessment. For platelet activation, samples were obtained on postoperative days 1, 2, and 3, and then twice weekly for the duration of each implant.
Assessment of blood and plasma parameters
Heparinized blood (3 mL) samples were used in hemorhelogical assays to measure blood parameters including plfHb, total blood hemoglobin, hematocrit, fibrinogen, and total plasma protein concentrations. Hematocrit was determined in a microhematocrit centrifuge (IEC MB Centrifuge, International Equipment Company, Needham Heights, MA, USA). Total blood hemoglobin concentration was measured in a hemoximeter (ABL 700 Series, Radiometer American, Inc., Westlake, OH, USA). The remaining sample was centrifuged at 9500 × g for 15 min at room temperature (ML Vanguard V6-500, Marketlab, Inc., Caledonia, MI, USA). The supernatant was then added to a 1.5 mL microcentrifuge tube and centrifuged again (Laboratory Centrifuge IEC MiniMax, International Equipment Company) at 15 000 × g for 12 min. The supernatant was transferred to a 1.5-mL microcentrifuge tube and then centrifuged at 20 800 × g for 20 min (Eppendorf 5417R, Eppendorf North America).The resulting plasma was then transferred to disposable semi-micro cuvettes (Thermo Fisher Scientific, Inc.) for measurement of plfHb as described above. Plasma total protein and fibrinogen concentrations were assessed using a benchtop refractometer (Kernco Instruments Co., Inc., El Paso, TX, USA). Fibrinogen provided an indirect assessment of the inflammatory state of the animal. Total plasma protein concentration was used as an indirect measure of blood dilution (along with hematocrit) and is also generally related to liver function.
Assessment of platelet activation
Blood (5 μL) was transferred from monovette tubes into 12 × 75 mm polystyrene tubes with 5 μL of 25 μg/mL of either coli S69 (IgG1 isotype control antibody, Washington State University Monoclonal Antibody Center, Pullman, WA, USA), MCA2419 (anti-human CD62P antibody-clone: Psel.KO.2.7, AbD Serotec, Raleigh, NC, USA), or MCA2420 (anti-human CD62P antibody-clone: Psel.KO.2.12, AbD Serotec), 5 μL of 50 μg/mL goat anti-mouse IgG-Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA), and 35 μL of Tyrode’s buffer (Electron Microscopy Sciences) with 1% bovine serum albumin (BSA) and 0.106 M sodium citrate and incubated for 20 min. The sample was washed with 1 mL of Tyrode’s buffer with 1% BSA and 0.106 M sodium citrate (washing buffer) and mixed. Samples were then centrifuged for 10 min at 132 × g. The supernatant was removed and the pellet resuspended. CAPP2A (Veterinary Medical Research & Development, Pullman, WA, USA), an antibody that binds to the surface of resting ovine platelets, was biotinylated previously (29). CAPP2A-biotin (5 μL of 7.5 μg/mL) and 5 μL of 73 μg/mL streptavidin-phycoerythrin (SA-PE; Invitrogen) were added to tubes and incubated for 20 min. Samples were then mixed with 1 mL of washing buffer and centrifuged at 132 × g. Following removal of the supernatant, samples were fixed with 1% paraformaldehyde. Recently, MCA2419 and MCA2420 as well as the IgG1 isotype control have become available, conjugated to a variety of different fluorochromes including Alexa Fluor 488. Employing the conjugated antibodies, goat anti-mouse IgG-PE (secondary antibody) could then be added to a un-biotinylated CAPP2A antibody, avoiding the need for SA-PE in the initial antibody incubation step and then the conjugated p-selectin or isotype control antibody could be added in the second incubation step.
Flow cytometric analysis was performed as previously described (29). In the 10-day implant, platelet activation after stimulation was also evaluated. These samples were prepared as above using 25 μL of Tyrode’s buffer with 1% BSA, 5 μL of 20-mM glycine-proline-arginine-proline (GPRP for inhibition of fibrin polymerization, Anaspec, San Jose, CA, USA) in phosphate-buffered saline (PBS), and 5 μL of either 200 μM of adenosine diphosphate (ADP; Calbiochem, San Diego, CA, USA) or 100 μM platelet activating factor (PAF; Sigma-Aldrich, St. Louis, MO, USA) instead of the 35 μl of citrated Tyrode’s buffer. In these stimulation studies, the use of Tyrode’s buffer without citrate along with GPRP is necessary as citrate will prevent the expression of additional p-selectin.
RESULTS
In vitro testing
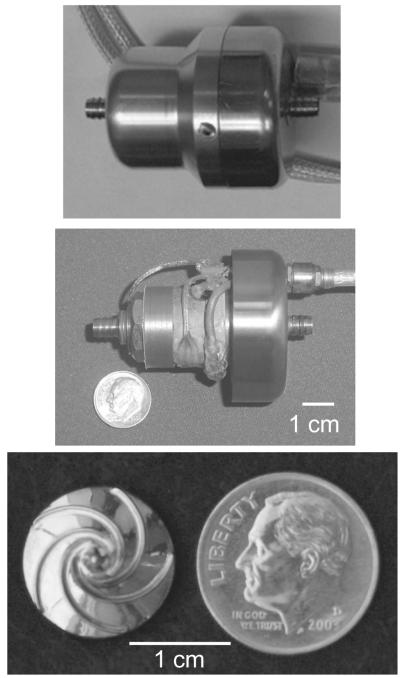
Figure 1 illustrates the PediaFlow PF1 pediatric VAD with its outer housing (top panel), without its outer housing (middle panel), and a close-up view of the impeller (bottom panel). In Fig. 2, the the steady-state in-vitro pressure-volumetric flow rate curve of PF1 is shown using a 35% glycerine/saline (vol) blood-analog for pump speeds of 4600–10 000 rpm. During the 6 h in vitro blood test, the pump successfully generated a mean flow of 700 mL/min against an 80 mm Hg afterload at 10 600 rpm. The mean hourly normalized index of hemolysis value was 0.0087 ± 0.0024 g/100 L, a clinically acceptable result (31).
FIG. 1.
Image of the PediaFlow PF1 device with and without its outer housing (top panel and middle panel) and the PF1 impeller (bottom panel).
FIG. 2.
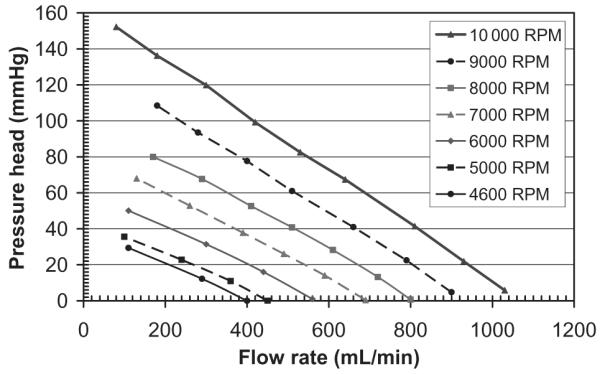
Pressure-volumetric flow rate curve of PediaFlow PF1 using a blood-analog for pump speeds of 4600–10 000 rpm.
In vivo testing
The implantation of the PF1 pediatric VAD is seen in Fig. 3A. Figure 3B shows a lateral chest radiograph following PF1 implant. Table 1 summarizes characteristics of each of the implants. At the end of the study, there was a positive blood culture for bacteria in the first implant, although the white blood cell count was within normal limits. White blood cell counts were normal at the end of study for the other two implants and their blood cultures were negative for any micro-organisms. The flow rate and pump speed are plotted for each implant in Fig. 4. In the first implant, pump flow started off at 0.77 LPM and remained relatively constant concluding at 0.85 LPM. Pump speed was constant at a mean of 10.68 krpm. In the second implant, pump flow was very low (0.3–0.4 LPM) for the first five days, beginning to increase on day 6 and reaching its maximum (0.67 LPM) on day 9 and remained at approximately this value through the conclusion of study. Pump speed in this implant starts at 10.6 krpm and begins to increase on day 9 and at the end of the study pump speed is approximately 11.5 krpm. Pump flow in the final study starts at a mean around 0.7 LPM. The flow signal is lost on days 4–7 due to a loss of acoustic coupling. The flow signal returns on day 8 and pump flow at the end of study is 0.6 LPM, while pump speed is constant throughout the study at a mean of approximately 10 krpm.
FIG. 3.
(A) Image of the PediaFlow PF1 during an implant procedure and (B) lateral chest radiograph following PF1 implant.
FIG. 4.
Flow rate and pump speed for the (A) first, (B) second, and (C) third PF1 implants (shaded area represents loss of acoustic signal).
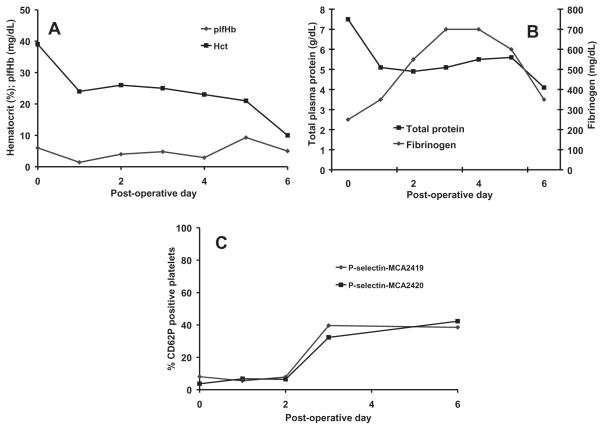
In the first chronic study, the outflow graft tore postoperatively because of a sharp cannula barb. This tear resulted in significant blood loss into the left chest with subsequent atelectasis which caused the animal to have labored breathing. The significant blood loss and respiratory compromise led to the implant being terminated on day 6. There were no renal infarcts or evidence of thromboembolism in other organs observed at necropsy. In Fig. 5, plfHb and hematocrit, total plasma protein and fibrinogen concentrations, and platelet activation results are presented for the first chronic implant. PlfHb was low for all 6 days of implantation with a mean value of <5 mg/dL. The hematocrit decreased following the implant and began to steadily decline on postoperative day 3 before a marked decrease on day 6 down to 10%. Total plasma protein concentration decreased postoperatively, but remained stable at approximately 5 g/dL for 5 days before decreasing to 4 g/dL on day 6. Fibrinogen concentration rose for the first 4 postoperative days (up to 700 mg/dL) and began to decrease on day 5. Platelet activation was very low at preoperative values through day 2, but on day 3 rose markedly and remained elevated at the conclusion of the implant.
FIG. 5.
(A) Hemolysis and hematocrit, (B) total plasma protein and fibrinogen, and (C) platelet activation (p-selectin expression on platelets) following the first PF1 chronic implant.
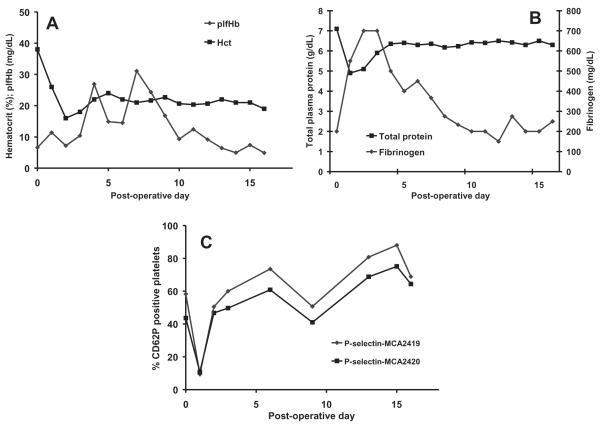
Technical challenges with the pump-cannula interface negatively impacted the second chronic implant resulting in the necessity of a very short inflow cannula with a sharp angle into the pump, and a subsequently long, tortuous outflow graft anastomosed to the aorta. Initially, very low pump outputs were observed for the target speed, which may have been due to kinking of the outflow graft which did eventually resolve. On day 15, there was a power outage in the animal facility, which, coupled with a failure in the PediaFlow uninterrupted power source, led to pump stoppage (15-min duration) and regurgitant flow through the pump. When power was restored, suspected thrombus embolization led to bowel and kidney infarction, which caused the gastrointestinal organs to fail and the animal to expire on day 17. Kidney, bowel, and gall bladder infarcts and an abnormal appearing liver were observed at necropsy. Left lung atelectasis and significant blood accumulation in the left chest were also observed. In Fig. 6, the plfHb and hematocrit, total plasma protein and fibrinogen concentrations, and platelet activation results for the second chronic implant are presented. The mean plfHb level for this implant was 13.3 ± 7.9 mg/dL. This parameter was substantially increased on days 4–9, but returned to baseline levels on day 10. The hematocrit steadily decreased following implantation and two blood transfusions were given to the animal (days 2 and 4). The hematocrit stabilized on day 4 at 20–22%. Total plasma protein concentration reduced postoperatively and began to rise on day 2 before stabilizing on day 4 at approximately 6 g/dL. The fibrinogen concentration significantly increased postoperatively (up to 700 mg/dL on days 2 and 3) and then slowly decreased returning to baseline on day 10. Platelet activation was elevated preoperatively and was highly elevated after day 1.
FIG. 6.
(A) Hemolysis and hematocrit, (B) total plasma protein and fibrinogen, and (C) platelet activation following the second PF1chronic implant.
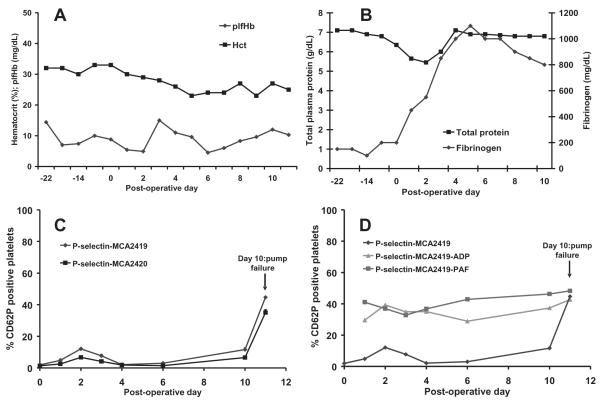
In our final PF1 implant, technical challenges connecting the cannula with the pump resulted in a long partial aortic clamping time and was potentially the cause for the kidney infarct and the putative ischemic injury that prevented the animal from being able to stand postoperatively. On day 10, the percutaneous cable experienced an electrical short; the animal appeared to receive an electrical shock, then the pump failed and the implant was terminated. A healed renal infarct was observed in the right kidney at necropsy. At necropsy, several small tears were observed in the cable jacket insulation along with fluid entrapment, the likely cause of the electrical short. Figure 7 illustrates the plfHb and hematocrit, total plasma protein and fibrinogen concentrations, and platelet activation results with and without exogenous stimulation with ADP and PAF for the third chronic implant. The later stimulation assays were newly developed and first assessed in vivo with our third animal. Mean plfHb for this implant was 8.8 ± 3.3 mg/dL. Hematocrit declined from its pre-operative value of 33 to 30% postoperatively and decreased slowly before stabilizing at approximately 25%. Total plasma protein concentration slightly decreased postoperatively but returned back to baseline on day 4. Fibrinogen concentration rose to extremely high levels of 1100 mg/dL on day 5 remaining at a very high level ~800–1000 mg/dL at the conclusion of the implant. Platelet activation rose slightly on days 1 and 2 and returned to baseline on day 4. Platelet activation began to rise on day 10 and sharply rose in the last data point on day 10. The mostly low platelet activation values in this implant were accompanied with an ability of the platelets to respond to application of agonists ADP and PAF for all but the final data point.
FIG. 7.
(A) Hemolysis and hematocrit, (B) total plasma protein and fibrinogen, (C) platelet activation, and (D) platelet activation on platelets following stimulation of blood with 20 μM adenosine diphosphate (ADP) and 10 μM platelet activating factor (PAF) in the third PF1 chronic implant.
DISCUSSION
In all three ovine studies, hematocrit decreased postoperatively and in the first two studies dropped precipitously. The precipitous drop in hematocrit in the first two studies may be explained by the observation of a large amount of blood in the left chest at necropsy. In the first study, the drop in hematocrit was attributed to an outflow graft tear. In the second study, however, no obvious tear sites in the graft or inflow cannula were observed, although there may have been bleeding at the pump/cannula connection site. Total plasma protein concentration also decreased postoperatively in each implant mostly due to blood dilution by the fluids given during surgery and postoperatively. The total protein rebounded during the postoperative course in the second and third studies. In the first implant, however, total plasma protein concentration continued to decrease as hematocrit decreased down to 10%. Fibrinogen concentration rose in all three studies reflecting the normal postsurgical inflammatory reaction process seen in this animal model (32,33). In the first two studies, fibrinogen began to decrease, returning to baseline levels. However, in the third implant it remained significantly above baseline at the time of implant termination. In this implant, the elevated fibrinogen might have been related to renal injury observed in this study as the creatinine in this animal was abnormally low, and the urea nitrogen value was near the lower limit of the normal range. Hepatic injury did not appear to be a concern in this implant because the aspartate aminotrans-ferase value was normal.
Typically, a plfHb level >10 mg/dL would be considered elevated; however, ovine cells are more fragile than human and bovine cells indicating that a slightly higher than 10 mg/dL plfHb level could still be considered satisfactory from the point of view of low potential mechanical damage to red blood cells produced by the pump (34). Hemolysis levels were low in the first and third PediaFlow PF1 pediatric VAD studies. In the second implant the mean plfHb level was slightly above 10 mg/dL due to several spikes in plfHb value. The major contributor to the hemolysis was the suspected outflow graft kink; as the flow rate increased (presumably due to the kink resolving), the plfHb decreased to very low levels. The mean plfHb beginning on day 10 (pump reachedits maximum flow on day 9) to the end of the implant was 7.8 ± 2.7 mg/dL. The need for several blood transfusions early in the postoperative period of this implant might have also impacted the elevated hemolysis levels observed early in the postoperative course. However, the baseline plfHb of the transfused blood was not measured before it was given to the sheep.
In the bovine model of preclinical testing, the utility of flow cytometric platelet activation assays has demonstrated value in differentiating between animals that had uneventful postoperative VAD courses and those that had evidence of substantial thromboembolism or pump thrombotic deposition. These assays also were able to differentiate levels of circulating platelet activation between different VAD surface coatings (22,23,35). Given our limited experience in applying the recently developed ovine platelet activation assays in vivo, we sought to understand how these assays responded during device implant and evaluate their utility for in vivo biocompatibility assessment (29). In the first implant, platelet activation rose in concert with an outflow graft tear that appeared to occur on day 3 and resulted in significant blood loss. During the second implant, a kinked outflow graft significantly impeded flow and likely altered the flow path such that it became a nidus for platelet activation. The pronounced evidence of thromboembolic infarction observed at necropsy was reflected in the markedly increased sustained levels of platelet activation in this implant. Overall, the platelet activation data in the third chronic PediaFlow PF1 pediatric VAD implant were promising and were in contrast to the degree of platelet activation observed in the second chronic implant. The pump stopped on day 10 in the third implant as well, likely causing retrograde flow through the pump and presumably resulting in elevated platelet activation in the final blood collection data point. In all three studies, platelet activation rose or had sustained elevation in response to pump complications (graft tear, kinked outflow graft and pump stoppage, pump electrical short and pump stoppage). The sensitivity of the platelet activation assays to pump complications in this limited data set suggests the utility of these assays as a meaningful temporal test of biocompatibility in vivo, in line with the bovine experience.
Platelet activation can be caused due to changes in the animal physiology or can be precipitated by pump complications. One way to attribute platelet activation to the pump is to perform sham surgeries. A sham surgery involves undergoing the equivalent surgery required for a VAD implant on a healthy animal without actual placement of the device. Characterizing platelet activation in sham studies then enables the determination of platelet activation attributable to this surgery. With a group of such sham surgical animals studied, one could characterize temporal platelet activation expected from the implant surgery and attribute excess or extended platelet activation as being related to pump placement and subsequent device operation. While an earlier report performed such sham procedures with calf VAD implants, we did not perform sham surgeries in this study (23). It is also possible that for any animal, a bleeding or thrombotic complication unrelated to the pump could arise in the implant period and that the platelet activation assays could detect this phenomenon. For these implants, given the noted pump-related complications, it seems likely that the elevations in platelet activation seen were related to those complications, but other sources cannot be entirely dismissed.
In the development of artificial organs, hemolysis is nearly universally assessed during preclinical testing, whereas platelet activation is not. These two parameters, both relevant to potential clinical-use blood trauma generated by a device, are not necessarily changing the same way over the course of device implantation. In the first implant of this study, hemolysis remained low throughout the study while platelet activation was elevated after day 2. In the second implant, hemolysis and platelet activation were high; on day 10, however, hemolysis returned to baseline levels, while platelet activation remained highly elevated. In the final implant, hemolysis and platelet activation were both low until the final data point where platelet activation rose sharply following pump stoppage and the hemolytic markers remained relatively constant. These results illustrate the lack of agreement between the erythrocyte-related and platelet data. Considering the platelet activation data in concert with the hemolysis and blood protein data provides greater insight into the temporal course of VAD biocompatibility.
In the third implant, the use of in vitro stimulation with agonists was introduced to evaluate circulating platelet responsiveness. A low platelet activation result following stimulation would suggest dysfunctional platelets which cannot express its markers for platelet activation perhaps due to activation marker shedding. An inability for already activated platelets to further respond to agonists would suggest a setting where circulating platelets are already activated to a point beyond which there is a limited ability to respond further. For the third implant, the mostly low circulating platelet activation values were accompanied with an ability of these platelets to respond to agonists ADP and PAF. The response to these platelet agonists suggested that the low platelet activation levels observed in this implant reflected minimal impact of the PediaFlow PF1 pediatric VAD on platelets, suggesting the potential for good platelet biocompatibility. In contrast, the platelet activation value at the last data point in this implant was elevated and platelets from this data point were not able to respond further to stimulation.
In the second PF1 implant, preoperative platelet activation was very high and agreed with a pre-implant phenomenon that has been reported previously in ovines (32). To obtain preoperative samples in our first two implants required animal restraint along with jugular venipuncture which induces fear in the animal. Turner and Hodgetts observed stress and anesthesia result in changes in ovine jugular hematocrit as a result of sequestering of red blood cells in the spleen (36). The lack of tranquility in the sheep’s environment was also mentioned as a potential stress (36). These stresses then may have been responsible for the very high preoperative platelet activation in our second PF1 implant. In the first PF1 implant, the animal was housed in the animal facility for a much longer time than the animal of the second implant and had grown accustomed to its surroundings, perhaps contributing to the low preoperative platelet activation values. To alleviate artifact in the collection of preoperative platelet activation data, we have since adopted the practice of placing an indwelling catheter in the jugular vein preoperatively in the implant animal. The use of such a catheter reduces the potential for stress-induced artifact in preoperative sample collection by eliminating the need for animal restrainment and needle stick associated with a jugular venipuncture blood draw. It is worth noting that postoperative samples are also collected through a vascular access point. The placement of the indwelling catheter preoperatively was a potential cause for the low preoperative platelet activation values observed in the third PF1 implant.
Pump flow was measured by an ultrasonic flow probe. The ultrasonic flow probe relies on a continuous and complete coupling between the probe and the outflow graft. The probe is initially coupled to the graft in the perioperative period with ultrasound jelly and serous fluids from the inflammatory response to surgery. The coupling is broken as these materials resorb, and there is a period of signal loss until tissue growth and encapsulation reforms the coupling, which in our experience typically occurs within 7–14 days postoperatively. This phenomenon should have been observed in the first two implants; however, both of these implants unfortunately had significant blood loss into the chest and this fluid likely maintained continuous coupling.
The maximum flow rate for PF1 (0.81 LPM) was attained during our first implant. The design goal for the PediaFlow ultimately is to attain a maximum flow of 1.5 LPM. The PF1 was specifically designed to run at subcritical speeds, which limited our maximum speed and hence our maximum attainable flow. In our next PediaFlow design iteration, we will focus on designing the motor (while maintaining the current fluid path) for supercritical performance which may enable us to attain the target flows. Once we have re-optimized the motor, we can continue with additional implants to assess biocompatibility and hemodynamic performance. Although the fluid path will remain the same, the biocompatibility results may change from what we report in this study. Our hope would be for improvement in platelet and red blood cell biocompatibility in future studies; however, our hemolysis and platelet activation assays have demonstrated their ability to be sensitive markers of biocompatibility whether the results are positive or not.
The chronic evaluation of the PF1 provided valuable guidance toward improving the PediaFlow pediatric VAD in future design iterations. The in vivo experience validates earlier CFD work and feasibility of the design of a magnetically levitated turbodynamic pump to generate flow rates in the range necessary to provide support to the youngest cardiac patients. The first generation pump design was able to generate promising low hemolysis data overall and at times, promising platelet biocompatibility. Attaining this level of biocompatibility at low flow rates is encouraging. Future design iterations of the PediaFlow, however, must focus on achieving higher flow rates (1.5 L/min) in order to meet the design goal of providing cardiac support to children up to 2 years of age. Cannula/pump connection issues negatively impacted each implant, in particular the first two, and along with increasing the maximum flow rate these are the most significant issues to be addressed in the next design iteration. Pump miniaturization to ensure implantability in newborns and strengthening the cable jacket to prevent fluid penetration also needs to be addressed. Finally, there is ongoing research into covalently attaching a biomimetic coating onto the blood contacting surfaces of the PediaFlow pediatric VAD to further enhance biocompatibility (26,27).
CONCLUSIONS
The PediaFlow PF1 pediatric VAD pumped 660–810 mL/min during three chronic ovine implants. While this first generation pediatric VAD design had several limitations with respect to a limited flow range, cannula connections, and compromised driveline, the use of magnetic levitation and actuation to generate flow rates necessary to meet newborn patient cardiac demands was validated. Hemolysis level overall was low during the implants. Further, circulating platelet activation assays temporally reflected pump/cannula implant problems, pump stoppage, and animal postoperative complications, indicating the utility of these assays as sensitive markers of biocompatibility in the ovine model.
Acknowledgments
The authors would like to thank the PediaFlow consortium for their technical advice, support, and expertise in the development and evaluation of the first generation PediaFlow device (PF1). The authors would like to also thank the Center for Pre-clinical Studies for their animal care and management. This work was supported by NIH Contract No. HHSN268200448192C (N01-HV-48192), NIH SBIR Phase II Award R44 Hl071376-02, and the Commonwealth of Pennsylvania. Mr. Johnson was supported by a NIH Graduate Research Assistant Supplement Award and a United Negro College Fund MERCK Graduate Science Research Dissertation Fellowship. Mr. Woolley was supported by NIH training grant # T32-HL076124.
Footnotes
[Corrections made after online publication on 9 July 2010: The data in the “Inflow cannula” column, and “Mean Arterial Pressure” column for Table 1 have been amended.]
REFERENCES
- 1.Long JW, Kfoury AG, Slaughter MS, et al. Long-term destination therapy with the HeartMate XVE left ventricular assist device: improved outcomes since the REMATCH study. Congest Heart Fail. 2005;11:133–8. doi: 10.1111/j.1527-5299.2005.04540.x. [DOI] [PubMed] [Google Scholar]
- 2.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 3.Blume ED, Naftel DC, Bastardi HJ, Duncan BW, Kirklin JK, Webber SA. Outcomes of children bridged to heart transplantation with ventricular assist devices: a multi-institutional study. Circulation. 2006;113:2313–9. doi: 10.1161/CIRCULATIONAHA.105.577601. [DOI] [PubMed] [Google Scholar]
- 4.Borovetz HS, Badylak S, Boston JR, et al. Towards the development of a pediatric ventricular assist device. Cell Transplant. 2006;15(Suppl 1):S69–74. doi: 10.3727/000000006783982304. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin JT, Borovetz HS, Duncan BW, et al. The National Heart, Lung, and Blood Institute Pediatric Circulatory Support Program. Circulation. 2006;113:147–55. doi: 10.1161/CIRCULATIONAHA.105.571422. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin JT, Duncan BW. Ventricular assist devices for children. Prog Pediatr Cardiol. 2006;21:173–84. [Google Scholar]
- 8.Almond CS, Thiagarajan RR, Piercey GE, et al. Waiting list mortality among children listed for heart transplantation in the United States. Circulation. 2009;119:717–27. doi: 10.1161/CIRCULATIONAHA.108.815712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrow WR, Frazier E, Naftel DC. Survival after listing for cardiac transplantation in children. Prog Pediatr Cardiol. 2000;11:99–105. doi: 10.1016/s1058-9813(00)00041-2. [DOI] [PubMed] [Google Scholar]
- 10.Wagner W, Borovetz H, Griffith B. Implantable cardiac assist devices. In: Ratner B, Hoffman A, Schoen F, Lemons J, editors. Biomaterials Science: An Introduction to Materials in Medicine. Elsevier Academic Press; San Diego, CA: 2004. pp. 494–506. [Google Scholar]
- 11.Simon MA, Watson J, Baldwin JT, Wagner WR, Borovetz HS. Current and future considerations in the use of mechanical circulatory support devices. Annu Rev Biomed Eng. 2008;10:59–84. doi: 10.1146/annurev.bioeng.9.060906.151856. [DOI] [PubMed] [Google Scholar]
- 12.Uber BE, Webber SA, Morell VO, Antaki JF. Hemodynamic guidelines for design and control of a turbodynamic pediatric ventricular assist device. ASAIO J. 2006;52:471–8. doi: 10.1097/01.mat.0000227730.00085.36. [DOI] [PubMed] [Google Scholar]
- 13.Duncan BW. Mechanical circulatory support for infants and children with cardiac disease. Ann Thorac Surg. 2002;73:1670–7. doi: 10.1016/s0003-4975(01)03027-2. [DOI] [PubMed] [Google Scholar]
- 14.Wearden PD, Morell VO, Keller BB, et al. The PediaFlow pediatric ventricular assist device. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006;9:92–8. doi: 10.1053/j.pcsu.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Noh MD, Antaki JF, Ricci M, et al. Magnetic design for the PediaFlow ventricular assist device. Artif Organs. 2008;32:127–35. doi: 10.1111/j.1525-1594.2007.00501.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Borovetz H. Designing with heart. Ansys Advantage. 2007;1:s12–3. [Google Scholar]
- 17.Gardiner JM, Wu J, Noh MD, et al. Thermal analysis of the PediaFlow pediatric ventricular assist device. ASAIO J. 2007;53:65–73. doi: 10.1097/01.mat.0000247156.94587.6c. [DOI] [PubMed] [Google Scholar]
- 18.Hoshi H, Shinshi T, Takatani S. Third-generation blood pumps with mechanical noncontact magnetic bearings. Artif Organs. 2006;30:324–38. doi: 10.1111/j.1525-1594.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 19.Watterson PA, Woodard JC, Ramsden VS, Reizes JA. Ventr-Assist hydrodynamically suspended, open, centrifugal blood pump. Artif Organs. 2000;24:475–7. doi: 10.1046/j.1525-1594.2000.06606.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Gellman B, Koert A, et al. Computational and experimental evaluation of the fluid dynamics and hemocom-patibility of the CentriMag blood pump. Artif Organs. 2006;30:168–77. doi: 10.1111/j.1525-1594.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- 21.Kameneva MV, Watach MJ, Litwak P, et al. Chronic animal health assessment during axial ventricular assistance: importance of hemorheologic parameters. ASAIO J. 1999;45:183–8. doi: 10.1097/00002480-199905000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Snyder TA, Tsukui H, Kihara S, et al. Preclinical biocompatibility assessment of the EVAHEART ventricular assist device: coating comparison and platelet activation. J Biomed Mater Res A. 2007;81:85–92. doi: 10.1002/jbm.a.31006. [DOI] [PubMed] [Google Scholar]
- 23.Snyder TA, Watach MJ, Litwak KN, Wagner WR. Platelet activation, aggregation, and life span in calves implanted with axial flow ventricular assist devices. Ann Thorac Surg. 2002;73:1933–8. doi: 10.1016/s0003-4975(02)03549-x. [DOI] [PubMed] [Google Scholar]
- 24.Kihara S, Yamazaki K, Litwak KN, et al. In vivo evaluation of a MPC polymer coated continuous flow left ventricular assist system. Artif Organs. 2003;27:188–92. doi: 10.1046/j.1525-1594.2003.t01-2-06993.x. [DOI] [PubMed] [Google Scholar]
- 25.Matsubayashi H, Fastenau DR, McIntyre JA. Changes in platelet activation associated with left ventricular assist system placement. J Heart Lung Transplant. 2000;19:462–8. doi: 10.1016/s1053-2498(00)00088-7. [DOI] [PubMed] [Google Scholar]
- 26.Ye SH, Johnson CA, Jr, Woolley JR, Snyder TA, Gamble LJ, Wagner WR. Covalent surface modification of a titanium alloy with a phosphorylcholine-containing copolymer for reduced thrombogenicity in cardiovascular devices. J Biomed Mater Res A. 2009;91:18–28. doi: 10.1002/jbm.a.32184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye SH, Johnson CA, Jr, Woolley JR, et al. Surface modification of a titanium alloy with a phospholipid polymer prepared by a plasma-induced grafting technique to improve surface thromboresistance. Colloids Surf B Biointerfaces. 2009;74:96–102. doi: 10.1016/j.colsurfb.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marascalco PJ, Ritchie SP, Snyder TA, Kameneva MV. Development of standard tests to examine viscoelastic properties of blood of experimental animals for pediatric mechanical support device evaluation. ASAIO J. 2006;52:567–74. doi: 10.1097/01.mat.0000242248.66083.48. [DOI] [PubMed] [Google Scholar]
- 29.Johnson CA, Jr, Snyder TA, Woolley JR, Wagner WR. Flow cytometric assays for quantifying activated ovine platelets. Artif Organs. 2008;32:136–45. doi: 10.1111/j.1525-1594.2007.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wintrobe MM. Clinical Hematology. 6th Edition Lea & Febiger; Philadelphia: 1967. [Google Scholar]
- 31.Naito K, Mizuguchi K, Nose Y. The need for standardizing the index of hemolysis. Artif Organs. 1994;18:7–10. doi: 10.1111/j.1525-1594.1994.tb03292.x. [DOI] [PubMed] [Google Scholar]
- 32.Dasse KA, Gellman B, Kameneva MV, et al. Assessment of hydraulic performance and biocompatibility of a MagLev centrifugal pump system designed for pediatric cardiac or cardiopulmonary support. ASAIO J. 2007;53:771–7. doi: 10.1097/MAT.0b013e31815dbf66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kameneva MV, Antaki JF, Butler KC, et al. A sheep model for the study of hemorheology with assisted circulation. Effect of an axial flow blood pump. ASAIO J. 1994;40:959–63. [PubMed] [Google Scholar]
- 34.Jikuya T, Tsutsui T, Shigeta O, Sankai Y, Mitsui T. Species differences in erythrocyte mechanical fragility: comparison of human, bovine, and ovine cells. ASAIO J. 1998;44:M452–5. [PubMed] [Google Scholar]
- 35.Baker LC, Davis WC, Autieri J, et al. Flow cytometric assays to detect platelet activation and aggregation in device-implanted calves. J Biomed Mater Res. 1998;41:312–21. doi: 10.1002/(sici)1097-4636(199808)41:2<312::aid-jbm17>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 36.Turner AW, Hodgetts VE. The dynamic red cell storage function of the spleen in sheep. I. Relationship to fluctuations of jugular haematocrit. Aust J Exp Biol Med Sci. 1959;37:399–420. doi: 10.1038/icb.1959.42. [DOI] [PubMed] [Google Scholar]