Abstract
Background
Prolyl hydroxylase domain 2 (PHD2) has been implicated in several pathways of cell signaling, most notably in its regulation of hypoxia inducible factor (HIF)-1α stability. In normoxia, PHD2 hydroxylates proline residues on HIF-1α, rendering it inactive. However in hypoxia, PHD2 is inactive, HIF-1α is stabilized and downstream effectors such as VEGF and FGF-2 are produced to promote angiogenesis. In the present study we utilize RNAi to PHD2 to promote therapeutic angiogenesis in a diabetic wound model, presumably by the stabilization of HIF-1α.
Methods
Stented wounds were created on the dorsum of diabetic Lpr db/db mice. Mice were treated with PHD2 siRNA or nonsense siRNA. Wounds were measured photometrically on days 0–28. Wounds were harvested for histology, protein, and RNA analysis.
Results
Diabetic wounds treated with siRNA closed within 21 +/−1.2 days; sham treated closed in 28 +/−1.5 days. By day 7, Western blot revealed near complete suppression of PHD protein and corresponding increased HIF-1α. Angiogenic mediators VEGF and FGF-2 were elevated, corresponding to increased CD31 staining in the treated groups.
Conclusions
siRNA-mediated silencing of PHD2 increases HIF-1α and several mediators of angiogenesis. This corresponded to improved time to closure in diabetic wounds compared to sham treated wounds. These findings suggest that impaired wound healing in diabetes can be ameliorated with therapeutic angiogenesis.
Keywords: diabetic wound, PHD2, HIF-1
INTRODUCTION
Complications related to diabetes bear a tremendous financial impact on our healthcare system, as well as significant morbidity for the patients who live with them. Over 15% of diabetics suffer from chronic nonhealing wounds, which may lead to ischemic ulcers and ultimately1. While poor wound healing in diabetes is multifactorial, recent literature has shown that there is impairment in endothelial progenitor cell (EPC)-mediated neovascularization2. The reason for this is two-fold: not only is EPC mobilization from the bone marrow inhibited, but also mobilized EPCs demonstrate impaired homing to the site of injury.3 As a result, neovascularization is impaired ultimately contributing to dysfunctional wound healing.3–8
Neovascularization is the process by which new blood vessel formation occurs in response to tissue hypoxia in order to preserve adequate oxygen delivery and utilization in the event of tissue loss or destruction. In any wound, vasculogenesis and angiogenesis are important processes to healing. Vasculogenesis, thought to be limited to embryogenesis, is also essential for postnatal de novo formation of capillary networks. On contrast, angiogenesis is the sprouting of new blood vessels from existing ones.9, 10 Hypoxia-inducible factor 1α (HIF-1α) is the central regulator of the hypoxia-induced response which results in the upregulation of angiogenic factors such as VEGF, FGF-2, and angiopoeitin-2 thereby promoting neovascularization.11 While studies have evaluated the efficacy of individual angiogenic factors as possible treatment modalities, it has become clear that one factor alone is inadequate to create a milieu for growth of mature functional new vessels.12–15 Recently, increasing attention has been directed to HIF-1α, a major upstream regulator responsible for the multitude of effectors that mediate angiogenesis. Increasing or stabilizing the expression of HIF-1α has proven sufficient to induce angiogenesis, even in normoxia. 16
Prolyl hydroxylase domain proteins play a critical role in oxygen homeostasis. Specifically, prolyl hydroxylase domain (PHD) 2 has been implicated in the degradation of HIF-1α. 17–19 In the presence of oxygen, PHD2 hydroxylates HIF-1α on two specific proline residues, which results in its destruction by an E3 ubiquitin ligase containing the von Hippel Lindau tumor suppressor protein.1, 16 In hypoxia, PHD-2 is missing its co-substrate (oxygen), rendering it inactive. In turn, HIF-1α becomes stabilized, translocates to the nucleus and promotes the transcription of a variety of effectors such as VEGF and FGF-2, promoting angiogenesis and vasculogenesis12–15. The specificity of PHD2 to HIF-1α makes its inhibition a valuable target in the promotion of angiogenesis in a wound bed.
Posttranscriptional silencing of a gene via RNA interference (RNAi) has greatly contributed to the treatment possibilities for a variety of cutaneous disease states, such as diabetic ulcers. Studies have shown that small interfering RNAs (siRNAs) can be used to suppress the transcription of specific gene sequences, thereby inhibiting the production of a specific protein product20. Moreover, in a murine wound model, this silencing is transient and local, alleviating concerns of prolonged action and off-target effects. 22 Interestingly, Wu et. al. have been able to show, both in vitro and in vivo that RNAi mediated PHD-2 silencing upregulates angiogenic mediators and promotes angiogenesis, offering an intriguing tool for angiogenic therapy.13 Topical siRNA application for the modulation of local gene expression and treatment of diabetic wounds has not been studied extensively. We hypothesized that topical silencing of PHD-2 in a diabetic wound would improve wound healing and would be associated with improved neovascularization.
METHODS
In Vitro murine fibroblast PHD2 silencing
Murine fibroblasts were harvested from diabetic Lepr db/db using an established fibroblast explant protocol and grown to confluence23. PHD2 siRNA (Applied Biosystems, Foster City, CA) was applied using RNAiFect (Qiagen, Valencia, CA) in a 6-well plate following the protocol listed in the manual. Transfection was allowed to continue for 24-hrs. Cells were harvested for mRNA quantification and protein.
In-Vivo topical silencing of PHD2 in a dorsal wound in diabetic (Lepr db/b) mice
After approval from The New York University Medical Center Animal Care Committee (IACUC #80812-01), diabetic Lepr db/db mice were obtained from Jackson Laboratories (Maine) and were housed in an animal care facility in accordance with the Division of Laboratory Animal Resources bylaws.
An established murine model of excisional wound healing was employed.24 Briefly, paired wounds (4-mm) were created on the dorsum of the mice and the perimeter of the wounds was marked with India ink. The wounds were then splinted open with 12-mm silicon stents to reduce wound contraction and allow the wounds to heal by secondary intention.
An agarose matrix was used as the delivery system for the siRNA.22 Briefly a 12 kDa FDA-approved 0.4% (weight/volume) agarose colloid matrix was created for the siRNA-liposomal transfection reagent complex. Experimental arms included 1) Topical siRNA PHD2 (n=10) and 2) Topical nonsense siRNA (n=12). Five animals from each group were sacrificed on days 7 and 14. The remaining animals were harvested on day 30, when the wounds were closed. Twenty pmol of PHD2 siRNA was delivered to the wound bed. Control wounds received the agarose gel with nonsense siRNA. The paired stents were then covered with a transparent sterile occlusive dressing (3M, St Paul, MN). Based on prior experiments that outlined optimal dosing routines, PHD-2 siRNA or nonsense siRNA, were applied on days 7, 14, and 21. At scheduled time intervals, the transparent dressing was removed and the wounds were photographed and assessed for contraction and healing.
RT-PCR
mRNA quantification was completed using real time RT-PCR. Wounds were harvested at days 7 and 14 post-wounding. Custom primers were created to PHD-2 (CGTCACGTTGATAACCCAAA), VEGF (TAACGATGAAGCCCTGGAGT), FGF-2 (CAAGGGAGTGTGTGCCAAC), and normalized to 18S (GTAACCCGTTGAACCCCATT). Quantification is expressed as fold-induction to normal untreated and unwounded skin.
Western Blot
Wounds were harvested at day 7 post-wounding for protein analysis by Western Blot. Primary rat anti-mouse antibodies for PHD-2 and rat anti-mouse HIF-1a antibodies (Cell Signaling, Danvers, MA) were used for quantitative protein analysis. Due to the difference in time course of healing between treated and control mice identified in preliminary experiments, day 7 was identified as a critical time point.
CD31 Immunofluorescence
The wound bed along with the surrounding 2 mm of unwounded skin was harvested on post-wounding days 7 and 21 and cut for frozen-sectioning. CD31 immunofluorescence staining was performed to evaluate endothelial staining.
Time to closure
The transparent dressing was removed and the wounds were photographed and assessed for contraction and healing. Photometric analysis was carried out on days 0, 3, 7, 10, 14, 21 and 28.
Statistical Analysis
All data are expressed as mean ± standard error of the mean. Students’ t-tests and one-way ANOVA were applied to assess for statistical significance. A p-value less than 0.05 determined statistical significance.
RESULTS
Diabetic murine fibroblast cells
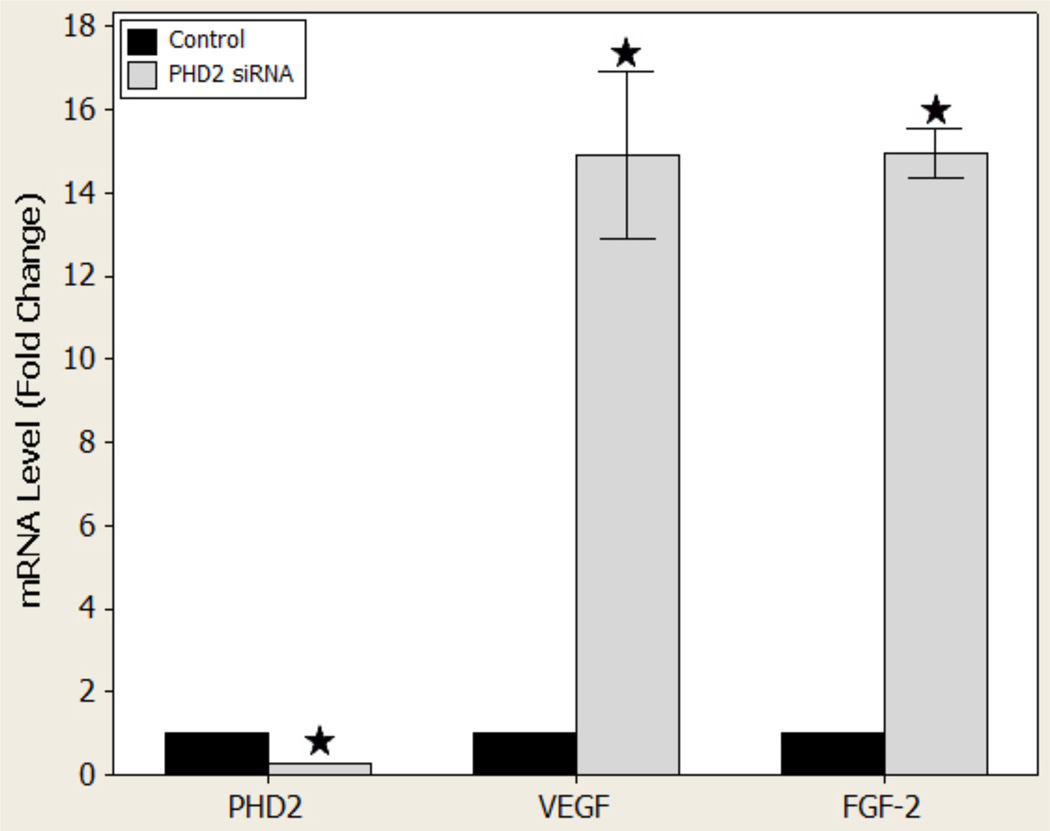
Following a single dose of PHD2 siRNA, PCR was employed to determine PHD2, VEGF, and FGF-2 mRNA levels in cultured diabetic murine fibroblast cells. Treatment yielded a 73 ± 0.075% reduction in the level of PHD2 mRNA (p < 0.001), demonstrating efficient knockdown of PHD2. Moreover, PHD2 silencing also resulted in a 14.90 ± 0.47 (p = 0.001) fold-increase in VEGF levels and a near equal 14.95 ± 0.138 (p < 0.001) fold-increase in FGF-2 mRNA levels, respectively (Figure 1).
Figure 1.
PHD-2, VEGF, and FGF-2 gene expression levels in vitro
mRNA analysis of PHD2 and angiogenic factor levels after treatment with nonsense or siPHD2
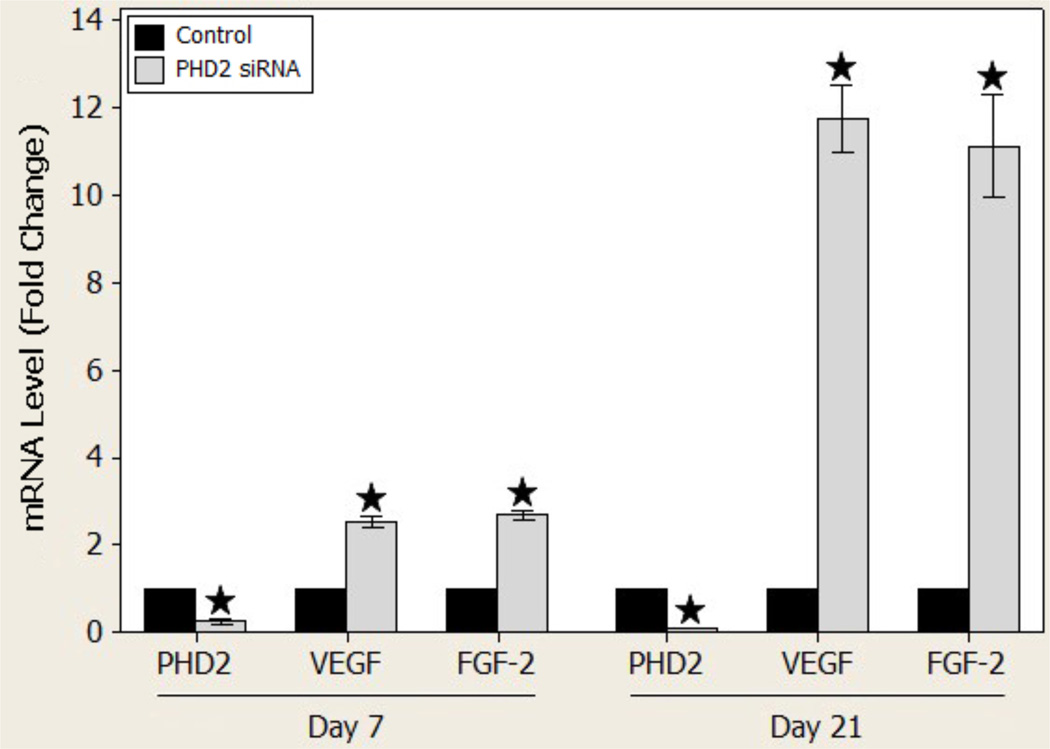
At day 7, after one treatment of topical PHD2 siRNA, diabetic wounds demonstrated a 75 ± 2% decrease in the level of PHD2 mRNA (p < 0.001) compared to controls. In contrast, VEGF mRNA levels were increased 2.51 ± 0.044 (p < 0.001) fold while FGF-2 levels were similarly increased 2.69 ± 0.039 (p < 0.001) fold.
At day 21, following 3 applications of PHD2 siRNA, PHD2 knockdown persisted with a 96.6 ± 0.069% reduction in the level of PHD2 mRNA (p < 0.001) compared to controls. Moreover, VEGF mRNA levels were increased strikingly to 11.78 ± 0.18 (p < 0.001) fold with a close increase of 11.15 ± 0.27 (p < 0.001) fold in FGF-2 mRNA levels (Figure 2).
Figure 2.
PHD-2, VEGF, and FGF-2 gene expression levels on day 7 and day 21 in vivo
Assessment of PHD2 silencing and HIF-1α expression
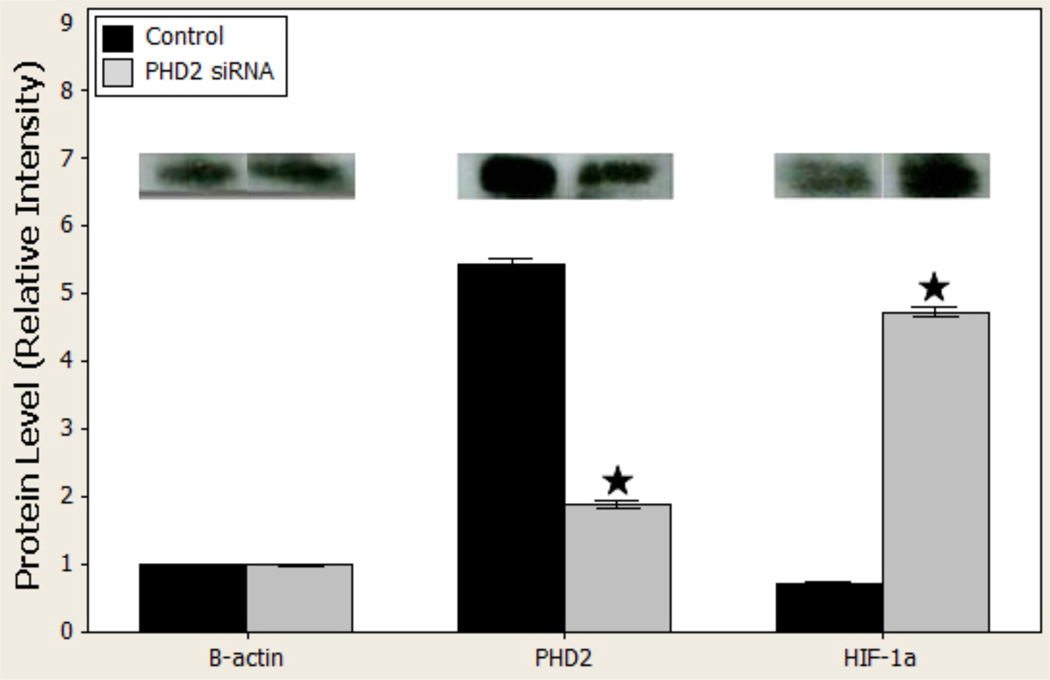
By day 7, treatment with PHD2 siRNA decreased PHD2 protein levels from 5.4675 ± 0.03 to 1.90 ± 0.22 (p < 0.001), and increased HIF-1α protein levels from 0.72 ± 0.0041 to 4.74 ± 0.025 (p < 0.001; Figure 3.
Figure 3.
b: Western blot gross images (above) and quantified relative to beta actin (below) in vivo
PHD2 silencing augments neovascularization
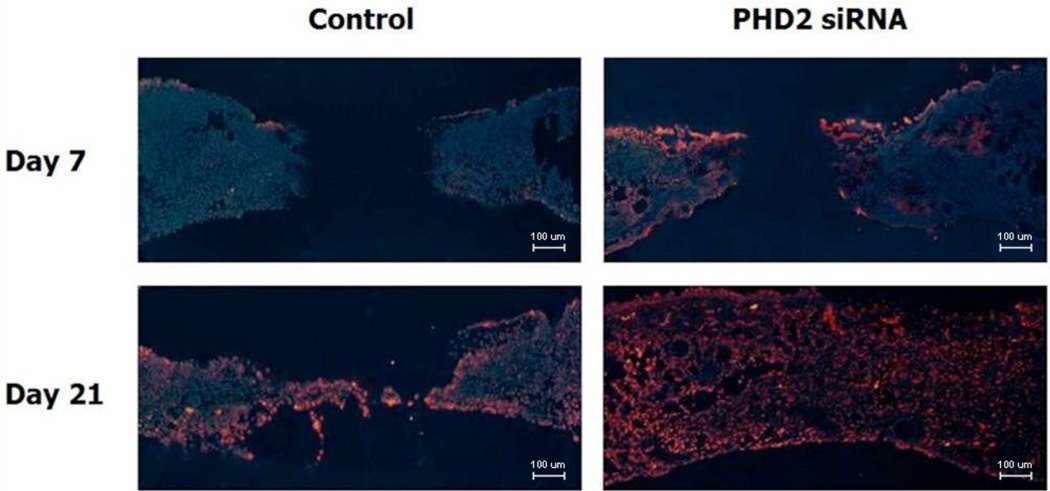
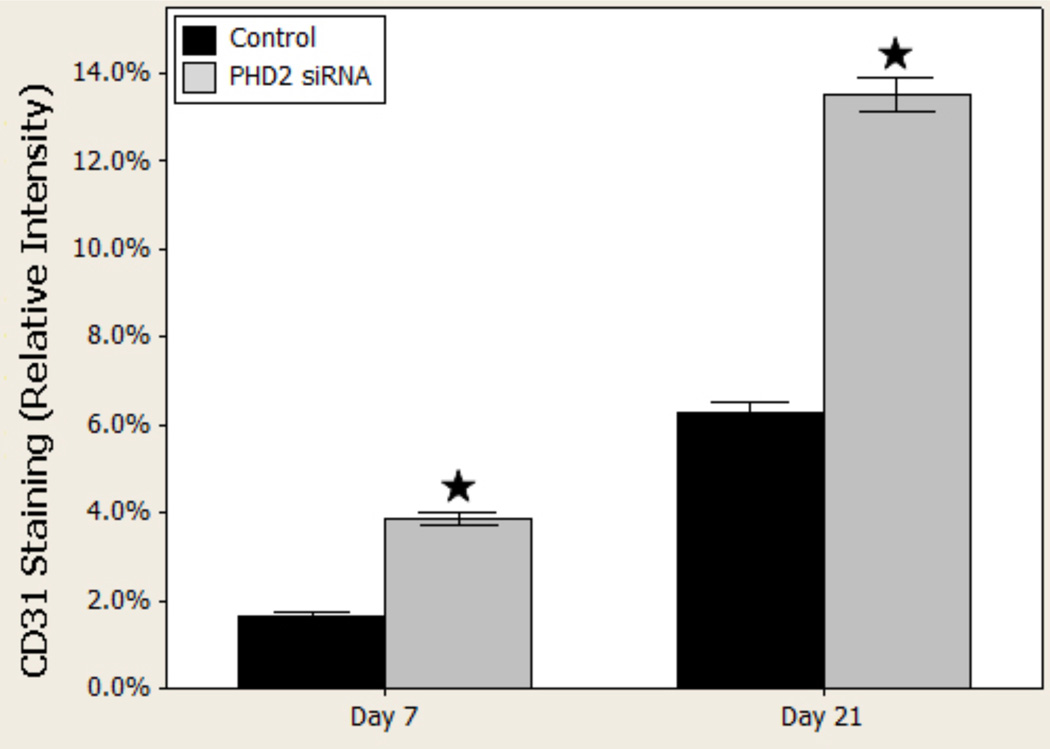
Immunohistochemistry targeting the cell surface marker CD31 revealed increased endothelial density in the PHD2 siRNA treated wounds (day 7, Figure 4). This qualitative finding was confirmed via quantitative analysis (Fisher’s Exact tests). Quantification of these results (percentage of wound bed containing CD31 positive cells) revealed more than twice as much CD31 staining on day 7 in the treated wounds compared to controls (p < 0.001). Although CD31 staining increased in both groups by day 21, the relationship between PHD-2 siRNA treated groups and nonsense treated controls persisted and CD31 staining remained more than twice the level in the PHD-2 siRNA groups (Figure 5).
Figure 4.
CD31 immunohistochemistry in vivo, 100×, demonstrates increased vascularity in treated vs. controls.
Figure 5.
CD31 quantified per high power field in vivo
Time to Closure
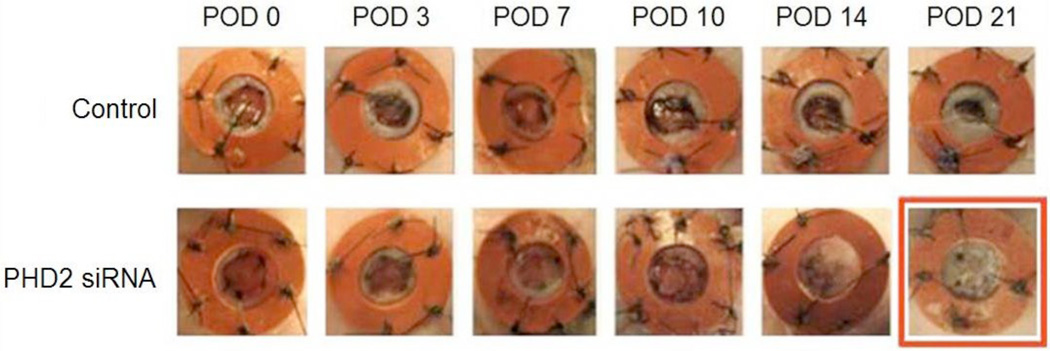
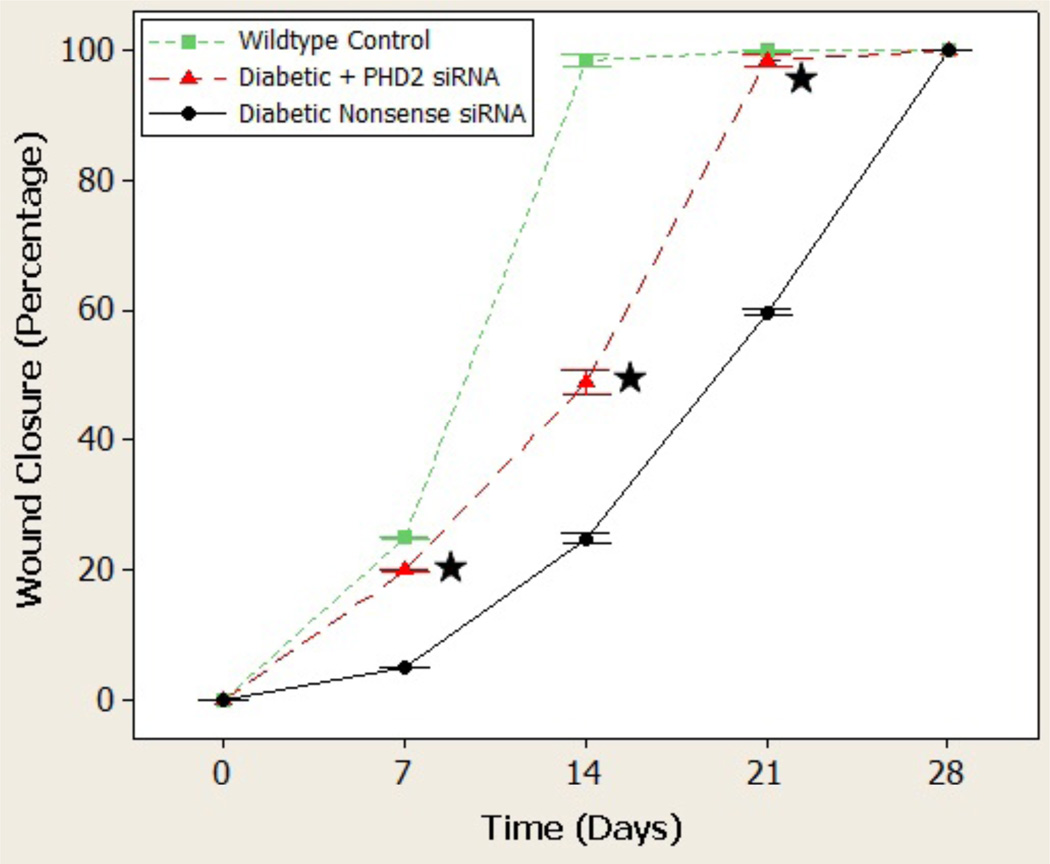
Gross imaging of the healing wounds taken at days 0, 3, 7, 10, 14, and 21 suggested an accelerated rate of time to wound closure in the PHD2 siRNA-treated group (Figure 6). This observation was confirmed by photometric analysis at days 0, 7, 14, 21, and 28 whereby treated diabetic wounds experienced a statistically significant increase in wound closure at every time point examined (Figure 6). Treated wounds began to heal as early day 14 (47%) whereas the control wounds were only 22% healed at the same time point. This relationship persisted such that by the time of closure of treated diabetic wounds, control wounds were only 61% closed. Overall, treated wounds were closed at 21 days compared to control wounds, which were not closed until 28 days. Serving as a positive control, wounds inflicted in the dorsa of wild type mice healed in 14 days.
Figure 6.
Gross images of wound healing demonstrate treated wounds closing by day 21, while sham treated wounds are not at the same time point.
DISCUSSION
Diabetics suffer from an impaired ability to mount a successful neovascular response following injury.3,4,27,28 The diabetic wound is hypoxic, yet HIF-1α levels have been shown to be reduced in diabetic wound healing29. This is counterintuitive for the following reason: in hypoxia, HIF-1α dimerizes in the nucleus with HIF-1β and binds to the hypoxia response element (HRE) on the promoter region of its target genes, transcriptionally activating them. Not coincidentally, two such gene targets are VEGF and FGF-2, which are key to the neovascular response. We sought to investigate whether the constitutive expression of HIF-1α through RNA interference of PHD2 could upregulate these gene targets to increase neovascularization in a wound bed and thus improve diabetic wound healing.
Impaired wound healing in diabetics is a well-documented phenomenon, and aberrant fibroblast function contributes to this process29–32. To test the efficacy of PHD2 knockdown as well as the ability to affect protein expression downstream of HIF-1α, we therefore chose to examine this relationship in vitro using murine diabetic fibroblasts. After just one dose of PHD2 siRNA, a 75% reduction in PHD2 expression was achieved. Additionally, a significant elevation in both VEGF and FGF2 was observed (p<.001).
We then applied these principles to our in vivo wound model as described above. Mirroring the in vitro work, knockdown was achieved to approximately 75% of pretreated levels after the initial dose of siRNA to PHD2. After 3 doses, the knockdown was near complete. Subsequently, VEGF and FGF2 expression was significantly elevated at both time points as well, and the effect was additive. Repeated treatment caused greater upregulation at 21 days. These findings are consistent with previous work by Wu et al13, but is unique in its application to the diabetic state (in vitro and in vivo) which is characterized by impaired neovascularization and wound healing.
Western blot was employed to demonstrate qualitative and quantitative knockdown of PHD2 and upregulation of HIF-1α in the wound bed. As figure 3 shows, relative to beta actin there is a significant reduction in PHD2 levels with concomitant increase in HIF-1α. We deduce that PHD2 silencing stabilized HIF-1α, which subsequently turned on the neovasculogenic cascade; however PHD2 may have a roll in this process independent of HIF-1α, which is not addressed in this paper but deserves future study. Other methods of HIF-1α stabilization, such as by hybridization with the VP16 adenovirus, could theoretically produce similar effects; however we aimed to utilize a simple topical application with foreseeable clinical relevance. Likewise, other methods of modulating PHD2 expression, such as with TGF-β33, would not be particularly effective in this circumstance; diabetic wounds characteristically have low levels of circulating TGF-β34.
CD31 stains endothelial cells in blood vessels and can be used to quantify angiogenesis.35 In this experiment, increased angiogenic factor expression translated to denser vessel growth, as confirmed by increased CD31 immunofluorescent staining in the treated wound samples. Moreover, and perhaps most importantly from a clinical perspective, siPHD2 treated wounds demonstrated decreased time to closure when compared with nonsense treated wounds and approached healing times of non-diabetic controls. We concluded silencing PHD2 improved diabetic wound healing, likely through a vasculogenic mechanism.
The choice to target the control of the upstream regulator HIF-1α rather than VEGF or FGF2 alone is based on its central role in oxygen sensing and the ability to affect both of these targets simultaneously. Several studies have shown that VEGF and FGF-2 are synergistic, leading to significantly more effective angiogenesis than when either cytokine is used alone.12–15 Despite an augmented vascular response with the isolated use of VEGF, the resultant vasculature is excessively permeable, 30 and clinical trials testing VEGF or FGF-2 alone have not been able to demonstrate meaningful clinical efficacy.14, 36,37 Interestingly, Elson et al showed that transgenic mice constitutively expressing HIF-1α in their skin demonstrated increased vascularity without hyperpermeability38. The choice to target PHD2 therefore could control not only the “master regulator” HIF-1α, but also several other critical mediators of angiogenesis as well.
Posttranscriptional gene silencing via RNA interference has great potential for the treatment of a variety of cutaneous disorders such as the diabetic ulcer, and this study demonstrated a novel in vivo approach to siRNA-mediated angiogenic therapy via a topical delivery system. The cell-specific action of PHD2 on HIF-1α, and the fundamental importance of HIF-1α to angiogenesis make PHD2 an attractive candidate for focused angiogenic therapy to a diabetic wound bed that is characterized by impaired vascularity.
We have previously demonstrated that the siRNA delivery system employed allows for repeated application and targeted gene knockdown, free of off-target effects. Moreover, this approach permits local therapy of a time-limited nature defined by a clinical endpoint, in this case wound healing. Our prior work confirmed that gene silencing was temporary, as expected given the nature of siRNA, and that no trace of gene knockdown could be detected three weeks after the last siRNA application.
In summary, the deficient hypoxic response to wound healing in diabetes can be attenuated by the upregulation of proangiogenic pathways via RNAi. One such pathway, stabilization of HIF-1α by silencing PHD2, is central to the improvement of diabetic wound healing through a neovascular mechanism. Interventions that promote angiogenesis may provide an important strategy to mitigate the aberrant injury response in diabetes.
Figure 7.
Wound closure quantified. Star denotes statistical significance (p<.05).
ACKNOWLEDGMENT
This project was supported in part by the Clinical and Translational Science Institute at NYU Langone Medical Center, NIH/NCRR 1UL1RR029893-01.
REFERENCES
- 1.Laing P. The development and complications of diabetic foot ulcers. Am J Surg. 1998;176:11S–19S. doi: 10.1016/s0002-9610(98)00182-2. [DOI] [PubMed] [Google Scholar]
- 2.Capla JM, Grogan RH, Callaghan MJ, no RD, Tepper OM, Ceradini DJ, Gurtner GC. Diabetes impairs endothelial progenitor cell-mediated blood vessel formation in response to hypoxia. Plast Reconstr Surg. 2006;119:59–70. doi: 10.1097/01.prs.0000244830.16906.3f. [DOI] [PubMed] [Google Scholar]
- 3.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106(22):2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 4.Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinç H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999 May 4;99(17):2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 5.Haffner SM, Lehto S, Ronnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 6.Shintani S, Murohara T, Ideka H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 8.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117–145. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- 9.Isner JM, Asahara T. Angiogenesis and Vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–1236. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 11.Bardos J, Ashcroft M. Negative and positive regulation of HIF-1: a complex network. Biochemica et Biophysica Acta. 2005;1775:107–120. doi: 10.1016/j.bbcan.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Pepper MS, Ferrara N, Orci L, Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun. 1992;189:824–831. doi: 10.1016/0006-291x(92)92277-5. [DOI] [PubMed] [Google Scholar]
- 13.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by the combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 14.Asahara T, Bauters C, Zheng LP, Takeshita S, Bunting S, Ferrara N, Symes JF, Isner JM. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation. 1995;92:11365–11371. doi: 10.1161/01.cir.92.9.365. [DOI] [PubMed] [Google Scholar]
- 15.Kano MR, Morishita Y, Iwata C, Iwasaka S, Watabe T, Ouchi Y, Miyazono K, Miyazawa K. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling. J Cell Sci. 2005;118:3759–3768. doi: 10.1242/jcs.02483. [DOI] [PubMed] [Google Scholar]
- 16.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor-1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 17.Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- 18.Wu S, Nishiyama N, Kano M, Morishita Y, Miyazono K, Itaka K, Chung UI, Kataoka K. Enhancement of angiogenesis through stabilization of hypoxia-inducible factor-1 by silencing Prolyl hydroxylase domain-2 gene. Mol Ther. 2008;16:1227–1234. doi: 10.1038/mt.2008.90. [DOI] [PubMed] [Google Scholar]
- 19.Takeda K, Cowan A, Fong GH. Essential role of Prolyl hydroxylase domain protein-2 in oxygen homeostasis of the adult vascular system. Circulation. 2007;116:774. doi: 10.1161/CIRCULATIONAHA.107.701516. [DOI] [PubMed] [Google Scholar]
- 20.Scherr M, Morgan M, Eder M. Gene silencing mediated by small interfering RNAs in mammalian cells. Curr Med Chem. 2003;10:245. doi: 10.2174/0929867033368493. [DOI] [PubMed] [Google Scholar]
- 21.Galiano RD, Michaels J, 5th, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Rep Reg. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 22.Thanik VD, Greives MR, Lerman OZ, Seiser N, Dec W, Chang CC, Warren SM, Levine JP, Saadeh PB. Topical matrix-based siRNA silences local gene expression in a murine wound model. Gene Ther. 2007;14:1305–1308. doi: 10.1038/sj.gt.3302986. [DOI] [PubMed] [Google Scholar]
- 23.Takashima Akira. Current Protocols in Cell Biology. 1998:2.1.1–2.1.12. doi: 10.1002/0471143030.cb0201s00. www.currentprotocols.com/protocol/cb0201. [DOI] [PubMed] [Google Scholar]
- 24.Michaels J, Churgin SS, Blechman KM, Greives MR, Aarabi S, Galiano RD, Gurtner GC. db/db mice exhibit severe wound-healing impairments compared with other murine diabetic strains in a silicone-splinted excisional wound model. Wound Repair Regen. 2007;15(5):665–670. doi: 10.1111/j.1524-475X.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- 25.Feener EP, King GL. Vascular dysfunction in diabetes mellitus. Lancet. 1997;350:SI9. doi: 10.1016/s0140-6736(97)90022-2. [DOI] [PubMed] [Google Scholar]
- 26.Detmar M, Brown LF, Schon MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998;380:435–439. doi: 10.1046/j.1523-1747.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- 27.Mace KA, Yu DH, Paydar KZ, Boudreau N, Young DM. Wound Repair Regen. 2007;15(5):636–645. doi: 10.1111/j.1524-475X.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- 28.Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, Udelson JE, Gervino EV, Pike M, Whitehouse MJ, Moon T, Chronos NA. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double blind, randomized controlled clinical trial. Circulation. 2002;105:788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 29.Liu R, Bal HS, Desta T, Behl Y, Graves DT. Tumor necrosis factor-alpha mediates diabetes-enhanced apoptosis of matrix-producing cells and impairs diabetic healing. Am J Pathol. 2006;168(3):757–764. doi: 10.2353/ajpath.2006.050907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desta T, Li J, Chino T, Graves DT. Altered fibroblast proliferation and apoptosis in diabetic gingival wounds. J Dent Res. 2010;89(6):609–614. doi: 10.1177/0022034510362960. Epub 2010 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siqueira MF, Li J, Chehab L, Desta T, Chino T, Krothpali N, Behl Y, Alikhani M, Yang J, Braasch C, Graves DT. Impaired wound healing in mouse models of diabetes is mediated by TNF-alpha dysregulation and associated with enhanced activation of forkhead box O1 (FOXO1) Diabetologia. 2010;53(2):378–388. doi: 10.1007/s00125-009-1529-y. Epub 2009 Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol. 2003;162(1):303–312. doi: 10.1016/S0002-9440(10)63821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem. 2006;281(34):24171–24181. doi: 10.1074/jbc.M604507200. 25; Epub 2006 Jun 30. [DOI] [PubMed] [Google Scholar]
- 34.Velander P, Theopold C, Hirsch T, Bleiziffer O, Zuhaili B, Fossum M, Hoeller D, Gheerardyn R, Chen M, Visovatti S, Svensson H, Yao F, Eriksson E. Impaired wound healing in an acute diabetic pig model and the effects of local hyperglycemia. Wound Repair Regen. 2008;16:288–293. doi: 10.1111/j.1524-475X.2008.00367.x. [DOI] [PubMed] [Google Scholar]
- 35.Gleich LL, Biddinger PW, Duperier FD, Gluckman JL. Tumor angiogenesis as a prognostic indicator in T2–T4 oral cavity squamous cell carcinoma: a clinical–pathologic correlation. Head Neck. 1997;19:276–280. doi: 10.1002/(sici)1097-0347(199707)19:4<276::aid-hed5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 36.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. VIVA Investigators. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 37.Lee SH, Schloss DJ, Swain JL. Maintenance of vascular integrity in the embryo requires signaling through the fibroblast growth factor receptor. J Biol Chem. 2000;275:3367. doi: 10.1074/jbc.M004994200. [DOI] [PubMed] [Google Scholar]
- 38.Elson DA, Thurston G, Huang LE, Ginzinger DG, McDonald DM, Johnson RS, Arbeit JM. Induction of hypervascularity without leakage of inflammation in transgenic mice overexpressing hypoxia-inducible factor 1-α. Genes Dev. 2001;15:2520–2532. doi: 10.1101/gad.914801. [DOI] [PMC free article] [PubMed] [Google Scholar]