Abstract
Excitatory synaptic transmission stimulates brain tissue glycolysis. This phenomenon is the signal detected in FDG-PET imaging and, through enhanced lactate production, is also thought to contribute to the fMRI signal. Using a method based on Förster resonance energy transfer in mouse astrocytes, we have recently observed that a small rise in extracellular K+ can stimulate glycolysis by >300% within seconds. The K+ response was blocked by ouabain, but intracellular engagement of the Na+/K+ ATPase pump with Na+ was ineffective, suggesting that the canonical feedback regulatory pathway involving the Na+ pump and ATP depletion is only permissive and that a second mechanism is involved. Because of their predominant K+ permeability and high expression of the electrogenic Na+/HCO3− cotransporter NBCe1, astrocytes respond to a rise in extracellular K+ with plasma membrane depolarization and intracellular alkalinization. In the present article, we show that a fast glycolytic response can be elicited independently of K+ by plasma membrane depolarization or by intracellular alkalinization. The glycolytic response to K+ was absent in astrocytes from NBCe1 null mice (Slc4a4) and was blocked by functional or pharmacological inhibition of the NBCe1. Hippocampal neurons acquired K+-sensitive glycolysis upon heterologous NBCe1 expression. The phenomenon could also be reconstituted in HEK293 cells by coexpression of the NBCe1 and a constitutively open K+ channel. We conclude that the NBCe1 is a key element in a feedforward mechanism linking excitatory synaptic transmission to fast modulation of glycolysis in astrocytes.
Introduction
Neural activity is accompanied by a transient imbalance between the usage of glucose and the usage of O2 (Fox et al., 1988). Reflecting this mismatch, a local surge in lactate concentration can be detected within seconds (Prichard et al., 1991; Hu and Wilson, 1997a; Caesar et al., 2008) that coincides with a decrease in interstitial glucose (Silver and Erecińska, 1994; Hu and Wilson, 1997b). Multiple roles have been ascribed to lactate in the brain, including preferential fueling of neurons (Pellerin and Magistretti, 1994; Suzuki et al., 2011), intercellular signaling (Lam et al., 2005; Shimizu et al., 2007; Gordon et al., 2008), and macromolecular synthesis (Rinholm et al., 2011), but there is no consensus on whether the lactate surge is produced by astrocytes or by neurons, nor there is agreement about the subcellular mechanisms that may be involved (Dienel and Cruz, 2004; Bonvento et al., 2005; Pellerin et al., 2007; Magistretti, 2009; Barros and Deitmer, 2010; Jolivet et al., 2010). Using an optical method that measures the glycolytic rate with high spatiotemporal resolution (Bittner et al., 2010), we have observed that small increases in extracellular K+, such as measured in brain tissue during afferent stimulation, can exert a strong stimulatory effect on astrocytic glycolysis, a process that evolves within seconds and is quickly reversible after K+ withdrawal (Bittner et al., 2011). K+-stimulated glycolysis was found to be sensitive to ouabain, suggesting a role for the canonical, negative feedback pathway that involves the Na+/K+ ATPase pump. However, glutamate, aspartate, and gramicidin, which increase intracellular Na+ and effectively stimulate the sodium pump (Chatton et al., 2000; Porras et al., 2008), failed to activate glycolysis in the short term (Bittner et al., 2011), implying that Na+ pump engagement is not sufficient for glycolytic stimulation and that a second signaling pathway may be involved.
Materials and Methods
Chemicals and tissue culture reagents were from Sigma. BCECF AM was obtained from Invitrogen. The construct coding for the sensor FLII12Pglu600μΔ6 (Takanaga et al., 2008) is available through www.addgene.org. Adenoviral vector Ad FLII12Pglu600μΔ6 was custom made by Vector Biolabs.
Animals, cell cultures, and tissue slices.
Animals used were mixed F1 male mice (C57BL/6J × CBA/J), kept in an animal room under SPF conditions at a room temperature of 20 ± 2°C, in a 12/12 h light/dark cycle with ad libitum access to food and water. NBCe1A null mice were mixed 129S6/SvEv and Black Swiss background, with wild-type age-matched littermates serving as controls. Animals were genotyped by PCR analysis (Gawenis et al., 2007). Experiments were approved by the Centro de Estudios Científicos Animal Care and Use Committee. Mixed cortical cultures of neuronal and glial cells (1- to 3-d-old neonates), cultures of hippocampal neurons (17.5 d embryos), and organotypic hippocampal slices (30–60 d adults) were prepared as detailed previously (Bittner et al., 2010). The hyperpolarized cell line HEKT2 line was created by stable transfection of the background potassium channel TASK2 (Niemeyer et al., 2001) (Mus musculus TASK-2, GenBank accession No. AF319542) in HEK293 cells using geneticin (900 μg/ml) for selection purposes. Cultures were transfected at days 5–7 (cortical) or 3–4 (hippocampal) with 5 μg of plasmid DNA, FLII12Pglu600μΔ6, and/or NBCe1A (Toye et al., 2006), using Lipofectamine 2000 (Invitrogen) or, alternatively, exposed to 5 × 106 PFU of FLII12Pglu600μΔ6 (cortical cultures and slices). For NBCe1A transfection, hippocampal neurons were cultured in the absence of bicarbonate/CO2 (HEPES-DMEM, Sigma). Measurements were performed after 24 h.
Protein extraction and immunoblot analysis.
HEK cells transfected with NBCe1A and primary culture cells were scraped into cold PBS (1×) followed by centrifugation at 5000 rpm for 5 min at 4°C. The cell pellet was then suspended in cold RIPA 1× (radioimmunoprecipitation assay) lysis buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Nonidet P40, 10 mm N-ethylmaleimide, 0.1 mm phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin A). After 30 min on ice, unlysed cells and nuclei were pelleted at 12,000 rpm for 15 min at 4°C. The protein concentration of the supernatant was determined by Bio-Rad Dc Protein Assay using BSA standards. For immunoblotting, protein samples (50 μg) were loaded onto 8% (w/v) SDS-polyacrylamide gels and electrotransferred onto nitrocellulose membranes. Blots were probed with a 1:1000 dilution of rabbit polyclonal anti-NBCe1A antibody (anti-SLC4A4 of Abcam) and visualized with a secondary antibody conjugated to peroxidase-labeled goat anti-rabbit antibody to a dilution of 1:25,000. The signal was revealed using a chemiluminescence kit (Thermo Scientific, Pierce) following the manufacturer's instructions.
Electrophysiological recordings.
Mouse cortical astrocytes from confluent day 7–9 cultures were subcultured into 35 mm Petri dishes and superfused with a bathing solution containing (in mm) 136 NaCl, 3 KCl, 1.25 CaCl2, 1.25 MgCl2, 2 glucose, 10 HEPES/Tris, pH 7.4, 300 mOsm (achieved by the addition of appropriate amounts of sucrose). The pipette solution contained (in mm) 35 KCl, 105 K-gluconate, 2 MgCl2, 0.5 CaCl2, 5 EGTA, 2 Na3ATP, and 10 HEPES, pH 7.3. HEK cells were seeded on 35 mm dishes and superfused with a bathing solution containing 110 mm NaCl, 5 mm KCl, 1.25 mm CaCl2, 1.25 mm MgSO4, 24 mm NaHCO3, and 10 mm HEPES/Tris, pH 7.4. Osmolarity was adjusted with sucrose to a final value of 324 mOsm. Pipette solution contained 132 mm K-gluconate, 8 mm KCl, 1 mm MgCl2, 15 mm NaHCO3, 10 mm EGTA, 1 mm Na3ATP, 0.1 mm GTP, and 10 mm HEPES, pH 7.2, 314 mOsm. Extracellular solutions were equilibrated with 5% CO2/95% O2. Higher K+ concentrations were obtained by equimolar replacement of Na+. Standard whole-cell patch-clamp recordings were performed using an EPC-7 (List Medical) amplifier. The bath was grounded via an agar bridge. Patch-clamp pipettes were made from thin borosilicate (hard) glass capillary tubing with an outside diameter of 1.5 mm (Harvard Apparatus) using a P-97 puller (Sutter Instruments). The pipettes had a resistance of 2.5–4 MΩ. Voltage and current signals from the amplifier were digitized using a computer equipped with a Digidata 1322A or 1200 AD/DA interface (Molecular Devices). The voltage pulse generator and analysis programs were from Molecular Devices. Corrections for changes in junction potential were made.
Glucose and pH imaging.
Experiments were performed at room temperature (22–25°C). The methodology to measure glucose and the glycolytic rate has been described in detail (Bittner et al., 2010). Previously, we showed that the acute modulation of astrocytic glycolysis by K+ is also present at 36°C (Bittner et al., 2011). Cells were imaged in a 95% air/5% CO2-gassed solution of the following composition (in mm): 112 NaCl, 1.25 CaCl2, 1.25 MgSO4, 1–2 glucose, 1 sodium lactate, 10 HEPES, and 24 NaHCO3, pH 7.4, with 3 mm KCl (astrocytes and neurons) or 5 mm KCl (HEK). Brain slices were superfused with a 95% O2/5% CO2-gassed buffer containing the following (in mm): 139.5 NaCl, 3 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 2 glucose, 1 sodium lactate, and 26 NaHCO3, pH 7.4. For Ba2+ experiments, MgSO4 was replaced by MgCl2 to avoid precipitation. Experiments with gramicidin and monensin were in the following solution (in mm): 136 NaCl, 3 KCl, 1.25 CaCl2, 1.25 MgCl2, 1–2 glucose, 1 sodium lactate, and 10 HEPES, pH 7.4. When using higher K+ concentrations, NaCl was adjusted to maintain isotonicity. Cultures were imaged with an Olympus IX70 or with an Olympus BX51 microscope respectively equipped with a 40× oil-immersion objective (NA 1.3) or with a 20× water-immersion objective (NA 0.95). The microscopes were equipped with Cairn monochromators and a Hamamatsu Orca camera controlled by Kinetics software or a Rollera camera controlled with Metafluor software. For nanosensor ratio measurements, cells were sequentially excited at 430 and 512 nm (0.2 and 0.025 s, respectively). Emissions were collected at 535 ± 15 nm. Citrine correction of pH effects were as detailed in the article describing the glycolytic rate method (Bittner et al., 2010). BCECF was ester loaded at 0.1 μm for 3–4 min and the signal was calibrated by exposing the cultures to solutions of different pH after permeabilizing the cells with 10 μg/ml nigericin and 20 μg/ml gramicidin in an intracellular buffer (Castro et al., 2004). BCECF was sequentially excited at 440 and 490 nm (0.1 and 0.05 s) and imaged at 535 ± 15 nm.
Statistical analysis.
All time courses shown represent single cells. Regression analyses were performed with the computer program SigmaPlot (Jandel). Data are presented as means ± SEM (n = number of experiments, with a minimum of 6 cells per experiment). Differences in mean values of paired samples were evaluated with the Student's t test. p values <0.05 were considered significant and are indicated with an asterisk (*).
Results
Acute modulation of glycolysis by intracellular pH
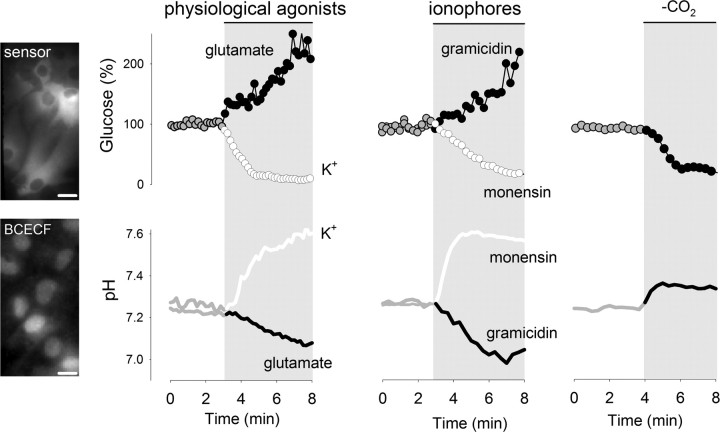
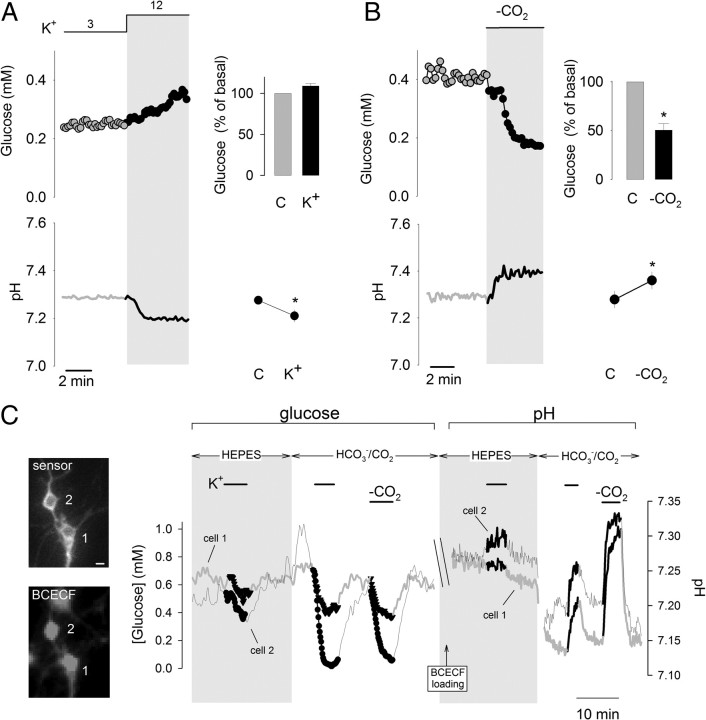
The short-term effects of glutamate and K+ on astrocytic glycolysis are opposite (Bittner et al., 2011), as are the effects of these molecules on intracellular pH (Fig. 1). This correlation, together with the notion that phosphofructokinase (PFK), the control point of glycolysis, is sensitive to pH (Trivedi and Danforth, 1966; Erecińska et al., 1995), suggested intracellular pH as a possible signal linking K+ and astrocytic glycolysis. To test this possibility, we first used the ionophores gramicidin and monensin, which cause opposite effects on intracellular pH; gramicidin transports protons into cells and acidifies, whereas monensin is a Na+/H+ exchanger that induces alkalinization. As shown previously, the concentration of glucose increased in response to gramicidin due to glucose transporter stimulation without changes in the rate of glycolysis (Bittner et al., 2011). In contrast, monensin caused a decrease in intracellular glucose (Fig. 1), with a glycolytic rate stimulation of 352 ± 151% (n = 3 experiments, p < 0.05) measured using the cytochalasin B protocol (see Materials and Methods).
Figure 1.
Acute modulation of astrocytic glycolysis by intracellular pH. Images on the left show astrocytes expressing the glucose sensor FLII12Pglu600μΔ6 or loaded with BCECF, bars represent 10 μm. The effects of K+ (12 mm), glutamate (50 μm), monensin (100 μm), and gramicidin (20 μg/ml) on intracellular glucose concentration and pH were measured as described in Materials and Methods. Intracellular glucose and pH were measured during transition between HCO3− buffers of equal pH, with and without 5% CO2. All traces correspond to representative plots of at least three independent experiments.
The contrasting glycolytic effects of gramicidin and monensin seem at odds with a major controlling role for the Na+ pump, for both gramicidin and monensin augment intracellular Na+ and stimulate the pump. Control experiments with SBFI-loaded cells showed that gramicidin was even more effective than monensin in terms of the Na+ overload (data not shown), implying that the inhibitory effect of an acid pH can override the stimulatory effect of adenine nucleotides, a conclusion that was also reached in a study of astrocytic ischemia (Swanson et al., 1997). In a separate strategy to manipulate intracellular pH, CO2 was acutely withdrawn from the perfusate. This maneuver increased pH and decreased intracellular glucose concentration (Fig. 1), with a glycolytic rate stimulation of 150 ± 45% (n = 3 experiments, p < 0.05). These results show that intracellular pH can modulate astrocytic glycolysis on its own and that the effect can be fast and may surpass that of the Na+/sodium pump pathway.
Acute stimulation of glycolysis by membrane depolarization
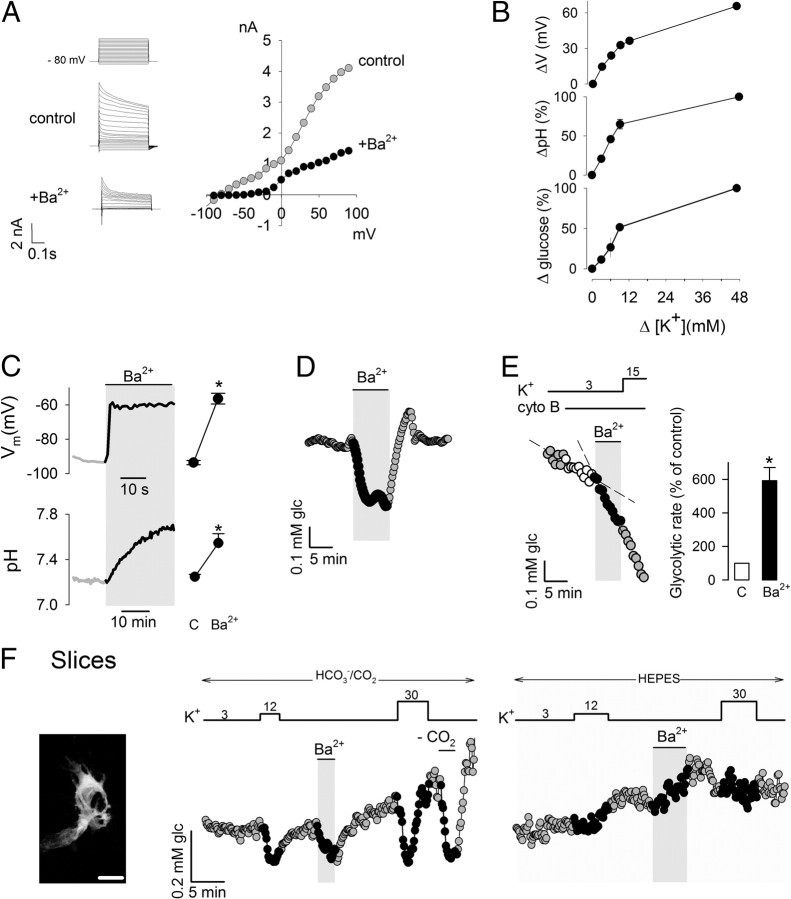
The pH change induced in astrocytes by a rise in extracellular K+ is mediated by plasma membrane depolarization, a response present in vivo that has been termed depolarization-induced alkalinization and requires expression of the NBCe1, an electrogenic membrane transporter that carries one Na+ and two HCO3− molecules per catalytic cycle (Chesler and Kraig, 1987; Chesler and Kraig, 1989; Deitmer and Szatkowski, 1990; Pappas and Ransom, 1994). Because of their dominant potassium permeability, the membrane potential in astrocytes is optimally sensitive to extracellular K+ (Fig. 2A,B). At rest, the membrane potential is close to the reversal potential of the NBCe1, and therefore, there is little or no HCO3− flux. Synaptic activity increases extracellular K+, astrocytes depolarize, HCO3− enters the cells through the NBCe1, and intracellular pH goes up. In agreement with functional NBCe1 expression in our cultures, intracellular pH was sensitive to small rises in extracellular K+ (Fig. 2B). Consistent with a metabolic role for NBCe1-mediated pH changes, the sensitivity to K+ of glucose usage was similar to that of membrane potential and pH (Fig. 2B). To depolarize the cells independently of K+, we applied Ba2+, which blocks K+ channels and is not an extracellular substrate for the Na+/K+ ATPase (Gatto et al., 2007). Figure 2C shows that Ba2+ exposure resulted in a membrane depolarization of >35 mV, which led to intracellular alkalinization (Fig. 2D). The extents of both depolarization and alkalinization were similar to those achieved by 12 mm K+. Supporting a metabolic role for the NBCe1, Ba2+ caused a strong acute stimulation of astrocytic glycolysis in primary culture cells and in tissue slices (Fig. 2E,F).
Figure 2.
Acute modulation of astrocytic glycolysis by membrane depolarization. A, The current/voltage relationship in cortical astrocytes was registered by patch clamping as described in Materials and Methods. Currents were recorded from a holding potential of −80 mV at variable 500 ms test pulses from −100 to +100 mV, in the absence and presence of 3 mm Ba2+. B, Effect of increasing K+ concentrations on membrane potential (n = 3 cells), pH (n = 3), and glucose concentration (n = 9 cells). Traces are representative of three independent experiments. Initial rates of pH increase and glucose decrease were estimated over 3 min. C, Membrane potential and intracellular pH was measured before and after exposure to 3 mm Ba2+. Bars summarize data from three experiments. D, Effect of 3 mm Ba2+ on intracellular glucose in a single astrocyte, representative of three similar experiments. E, Sequential effects of 3 mm Ba2+ and 15 mm K+ on the glycolytic rate of a single astrocyte. Bars summarize data from three experiments. F, Image on the left shows a 3D confocal reconstruction of two astrocytes expressing the glucose sensor FLII12Pglu600μΔ6 in an organotypical hippocampal slice. Bar represents 10 μm. Graphs illustrate experiments in slices testing the effects of 12 mm K+ and 3 mm Ba2+ on intracellular glucose in the presence and absence (HEPES) of HCO3−. Data are representative of three separate experiments.
NBCe1 involvement in K+-stimulated glycolysis
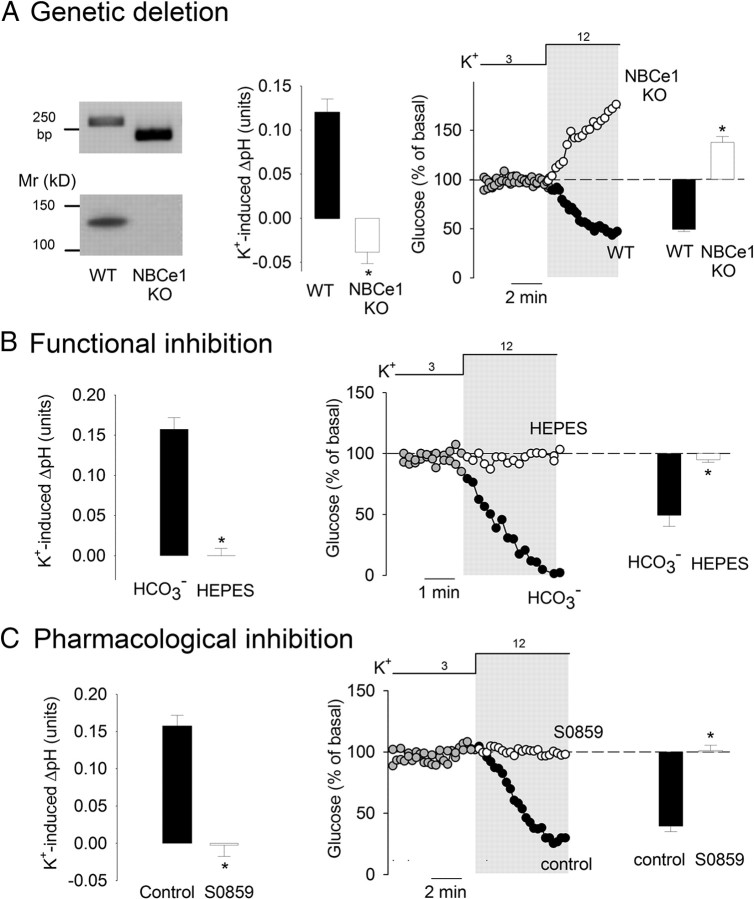
Three strategies were followed to study astrocytic glycolysis in the absence of NBCe1 activity. The NBCe1 is encoded by the Slc4a4 gene, for which a null mutant mouse is available (Gawenis et al., 2007). NBCe1 null mice die 2 weeks after birth due to a systemic acidosis of renal origin, but their brain tissue does not present gross morphological alterations as analyzed by light microscopy. Astrocytes cultured from neonatal NBCe1 null mice did not present detectable NBCe1 activity, measured as the rate of pH recovery after an acid load (data not shown). Figure 3A shows that, in contrast to astrocytes from wild-type littermates, astrocytes from NBCe1 null mice responded to high extracellular K+ with an increase in glucose concentration, consistent with an inhibition of glycolysis. In NBCe1 KO astrocytes, the alkalinization induced by CO2 withdrawal caused a decrease in glucose concentration similar to that observed in wild-type cells (data not shown), demonstrating that the glycolytic machinery was not directly compromised by NBCe1 deletion. Next, NBCe1 function was inhibited by replacing HCO3−/CO2 with a HEPES buffer, in which bicarbonate levels, though not zero, are greatly reduced as is NBCe1 activity (Deitmer and Rose, 1996). In HEPES buffer, extracellular K+ failed to alkalinize the cells and also failed to activate glucose metabolism, both in culture and in slices (Figs. 2F, 3B). Finally, NBCe1 activity was blocked pharmacologically with S0859, an N-cyanosulfonamide that reversibly abrogates NBCe1 activity in cardiomyocytes (Ch'en et al., 2008). In astrocytes, S0859 was found to be an effective NBCe1 blocker (data not shown). As shown in Figure 3C, S0859 inhibited the alkalinization induced by K+ and prevented the effect of K+ on glucose metabolism.
Figure 3.
NBCe1 is necessary for K+-stimulated glycolysis in astrocytes. A, Genetic deletion of NBCe1. The left panel illustrates the results of PCR genotyping of tail biopsies and Western blotting analysis of cultured astrocytes from wild-type (WT) and NBCe1 knock-out mice (KO). Bars in the middle represent the changes in intracellular pH elicited by a 3 min exposure to 12 mm K+ in WT (n = 3) and NBCe1 KO astrocytes (n = 7). The impact of a 3 min exposure to 12 mm K+ on glucose concentration in WT and NBCe1 KO astrocytes is illustrated by the time course and bar graph on the right (n = 3–4). B, Bicarbonate omission. Bars on the left illustrate pH changes elicited by a 3 min exposure to 12 mm K+ in the presence and absence (HEPES) of bicarbonate (n = 4). Middle, The effect of 12 mm K+ on glucose concentration was measured over time in the presence and absence (HEPES) of bicarbonate. Bars on the right show the change in intracellular glucose after 3 min (n = 3). C, S0859. Bars on the left illustrate pH changes elicited by a 3 min exposure to 12 mm K+ before and after a 4 min preincubation with 30 μm S0859 (n = 3). Middle, The effect of 12 mm K+ on glucose concentration was measured before and after a 4 min preincubation with 30 μm S0859 (n = 3). Bars on the right show the change in intracellular glucose after 3 min (n = 3).
Reconstitution of K+-stimulated glycolysis in HEK293 cells
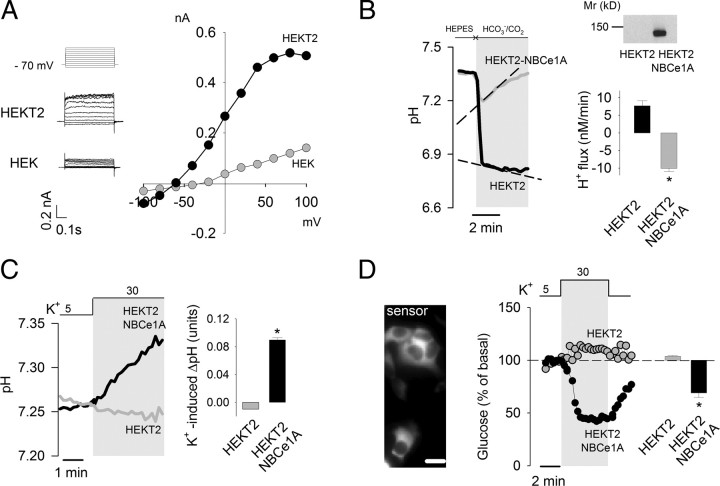
Having demonstrated a necessary role for the NBCe1 in the glycolytic effect of K+, we investigated the possible presence of additional astrocyte-specific factors using HEK293 cells as a template. To make membrane potential sensitive to extracellular K+, a stable cell line HEKT2 was first generated that overexpresses the background potassium channel TASK2. These cells show outward currents at depolarized membrane potentials (Fig. 4A) and a shift in resting membrane potential from −17 ± 3 mV (n = 5) to −61 ± 3 mV (n = 6). An increase in extracellular K+ from 5 to 30 mm depolarized HEKT2 cells by 30 ± 4 mV (n = 6), similar to the depolarization obtained in astrocytes while switching from 3 to 12 mm K+ (Fig. 2B). HEKT2 cells lack NBCe1 protein expression and detectable NBCe1 activity (Fig. 4B). Transient expression of human NBC1eA conferred HEKT2 with robust NBCe activity (Fig. 4B). A rise in extracellular K+ induced a small acidification in HEKT2 cells and a strong alkalinization in NBCe1A-expressing HEKT2 cells (Fig. 4C). Thus, coexpression of a potassium channel and NBCe1A replicates the phenomenon of depolarization-induced alkalinization. Whereas a rise in extracellular K+ did not affect glucose levels in HEKT2 cells, it did lead to strong glucose depletion in NBCe1A-expressing HEKT2 cells (Fig. 4D). These results show that the phenomenon of K+-stimulated glycolysis does not require astrocytic-specific factors other than a predominant K+ permeability and functional NBCe1.
Figure 4.
Assembly of K+-modulated glycolysis in HEK cells. A, The steady-state current/voltage relationship in HEK and HEKT2 cells was measured by patch clamp as described in Materials and Methods. The left panels show the actual currents recorded from a holding potential of −80 mV at different 500 ms test pulses from −100 to +100 mV. B, Protein expression in HEK and HEKT2 cells overexpressing NBCe1A was measured by Western blotting as described in Materials and Methods. NBCe1 activity was assessed by estimating the rate of acid extrusion in response to an acid load induced by switching from HEPES to HCO3−/CO2 in the presence of the NHE inhibitor amiloride (1 mm). The bar graph summarizes data from four experiments. C, The effect of K+ rise (from 5 to 30 mm) on intracellular pH was measured in HEKT2 and HEKT2-NBCe1A cells. The bar graph shows the change in pH after 3 min (n = 3). D, HEKT2 cells were cotransfected with cDNAs coding for the FRET glucose sensor and NBCe1A, bar represents 10 μm. The effect of K+ rise (from 5 to 30 mm) on intracellular glucose was measured in HEKT2 and HEKT2-NBCe1A cells. Bars show the change in glucose concentration after 3 min (n = 3).
Effects of K+ on neuronal glycolysis
Neurons, being the actual source of elevated K+ during activity, are also subject to the effects of K+. Figure 5A shows that in response to a K+ rise, cultured hippocampal neurons did not significantly modify their glucose concentration and acidified, reminiscent of the early effect of synaptic activity on neuronal pH (Chesler, 2003; Makani and Chesler, 2010; Svichar et al., 2011). CO2 removal resulted in acute glycolysis stimulation (Fig. 5B), consistent with previous long-term measurements of glycolysis with radioisotopes (Erecińska et al., 1995), so we tested whether the NBCe1 overexpression would endow them with K+-sensitive glycolysis as observed with HEK cells. Initially, NBCe1A expression was found to be toxic for neurons (as it was with wild-type HEK293 cells), perhaps due to unregulated influx of Na+ and bicarbonate. Thus NBCe1A-transfected neurons had to be cultured in HEPES DMEM medium without added CO2. Figure 5C illustrates a prolonged experiment with NBCe1A-transfected neurons in which glucose was first measured using the FRET sensor and then cells were loaded with BCECF to corroborate functional NBCe1A expression. First, it was observed that a rise in extracellular K+ caused a drop in glucose concentration that was >10 times faster in bicarbonate than in HEPES. Ensuing pH measurements in the same cells showed that K+ elicited an alkalinization that was also >10 times faster in bicarbonate than in HEPES. The acidification during the switch from HEPES to CO2/bicarbonate, which is due to fast CO2 diffusion into the cell, led to inhibition of glucose metabolism and higher intracellular glucose (Fig. 5C), an effect that had also been observed in astrocytes and HEK cells (data not shown). These results show that neuronal glycolysis is acutely sensitive to changes in pH and that the NBCe1 is sufficient to endow neurons with K+-stimulated glycolysis. Mature hippocampal neurons in culture express functional NBCe1, which leads to a delayed intracellular alkalinization in response to high extracellular K+ (Svichar et al., 2011). The lack of delayed pH rise in our cultures may be due to low or absent NBCe1 expression in younger neurons, and/or to dominant Ca2+-dependent acidification masking the NBCe1 response (Makani and Chesler, 2010).
Figure 5.
Lack of K+-stimulated glycolysis in hippocampal neurons is explained by absence of NBCe1. A, Time course of the effects of 12 mm K+ on neuronal glucose (top) and pH (bottom). Graphs on the right summarize the changes after 3 min of exposure (n = 3 or 4). B, Time course of the effect of CO2 removal on neuronal glucose (top) and pH (bottom). Changes after 3 min are summarized on the right (n = 3). C, Neurons were cotransfected with cDNAs coding for the FRET glucose sensor and NBCe1A, bars represent 10 μm. After monitoring glucose concentration, cells were AM loaded with BCECF, allowing for a series of pH measurements. The graph presents the effect of 12 mm K+ on glucose and pH in the presence and absence of HCO3−/CO2 in two neurons of different morphology. The acute alkalinization observed in response to high K+ demonstrates functional NBCe in these two neurons. Similar results were obtained in six independent experiments.
Discussion
NBCe1 participates in a feedforward pathway for glycolysis regulation
In most mammalian cell types, glycolysis is regulated via the Na+/K+ ATPase. This canonical mechanism is a negative feedback pathway, in which a Na+-stimulated pump decreases the ATP/(ADP+AMP) ratio, leading to allosteric activation of glycolytic enzymes and compensatory ATP synthesis. In astrocytes, the canonical pathway can be activated by extracellular glutamate via the Na+/glutamate cotransporter (Pellerin et al., 2007). In a recent article, we have reported that extracellular K+, which is released by active neurons, behaves as a fast agonist of glycolysis in astrocytes and that the effect is sensitive to inhibition of the sodium pump (Bittner et al., 2011). However, we show here that the glycolytic response to K+ is not mediated by the canonical pathway, which only plays a permissive role, but by an alternative cascade involving K+, membrane potential, the NBCe1, and intracellular pH, the KPNH pathway. Unlike the conventional pathway, the KPNH pathway is not a negative feedback but rather a feedforward, whereby an astrocyte, prompted by active neurons, anticipates its own needs for fuel in the form of ATP and the needs of neurons in the form of lactate. Considering the role proposed for lactate in neurovascular coupling (Gordon et al., 2008), the KPNH pathway may also help to anticipate the local need for oxygen. Astrocytes can also be depolarized by other mechanisms, for example, engagement of ionotropic glutamate receptors and α-1 adrenergic receptors (Bowman and Kimelberg, 1984; Kettenmann and Schachner, 1985; Bowman and Kimelberg, 1987), which may also result in glycolytic stimulation.
In support of a role for the KPNH pathway in the regulation of astrocyte glycolysis, the following experimental evidence was collected: (1) a close correlation between the effects of K+ on membrane potential, intracellular pH, and glycolysis; (2) the rise in pH and concurrent glycolytic activation induced by K+-independent membrane depolarization; (3) the abrogation of the metabolic effect of K+ by genetic, functional, and pharmacological inhibition of the NBCe1; and (4) the endowment of neurons and HEK cells with K+-dependent alkalinization and glycolysis stimulation by functional expression of the NBCe1. In favor of a physiological role for the KPNH pathway is the robustness of the K+ response, which is independent of resting metabolic rate, temperature (22–36°C), days in culture, and confluence. The phenomenon is also resistant to subculturing, independent of the presence of neurons and present in tissue slices (Bittner et al., 2011). Another argument is that all individual components of the cascade are in place in vivo, including K+-sensitive membrane potential, NBCe1 expression, and activity-dependent astrocytic alkalinization (Chesler and Kraig, 1987, 1989; Deitmer and Rose, 1996; Schmitt et al., 2000; Chesler, 2003). The observation that cortical activity in vivo results in astrocytic alkalinization shows that the effect of K+ surpasses that of acidifying factors such as glutamate and lactate. Judging from similar effects of pH on glycolysis in various contexts: astrocytes, HEK cells, neurons, synaptosomes, C6 glioma cells, muscle cells, and purified phosphofructokinase (Trivedi and Danforth, 1966; Fidelman et al., 1982; Erecińska et al., 1995; Swanson et al., 1997), the pH modulation of glycolysis appears to be a conserved microscopic property that is not likely to differ in vivo.
In addition, we observed that glycolysis in young neuronal cultures is insensitive to rises in extracellular K+ and that their lack of NBCe is accountable for the deficit. However, an elegant study reported recently that mature hippocampal neuron do express NBCe and respond to high extracellular K+ with a biphasic pH response: an early acidification, followed minutes later by a rebound alkalinization (Svichar et al., 2011). The biphasic response was dissected as the net result of two simultaneous and independent mechanisms: transient acid influx mediated by the PMCA (Makani and Chesler, 2010) and sustained but weaker acid extrusion mediated by the NBCe. The NBCe is expressed in some neuronal subpopulations in vivo (Majumdar and Bevensee, 2010). It remains to be established whether the biphasic pH response in mature neurons is accompanied by corresponding changes in glycolytic rate.
Interstitial K+ concentration
A relative unknown is the concentration of K+ reached in brain interstitium during activity. Although a maximum “ceiling” K+ concentration of 12 mm has been measured with microelectrodes in the brain interstice during electrical afferent stimulation (Kofuji and Newman, 2004), direct extrapolation to physiological conditions is not possible, for exogenous stimulation recruits many more fibers than the intrinsic activity, resulting in supraphysiological K+ release. An example of the levels reached by physiological K+ release is the 4.7 mm measured in the spinal cord (Heinemann et al., 1990), but this value is regarded as an underestimate because it was also measured with microelectrodes, whose tips are much larger than the interstice (100 μm vs 20 nm) and create a dead space that muffles the K+ rise (Fröhlich et al., 2008). The actual concentration of K+ at the synaptic cleft remains to be determined, but the fact that significant glycolytic effects were detected with a K+ rise of just 1 mm and a 3 mm rise achieved stimulations of 200–300% (Bittner et al., 2011) suggests that the phenomenon is of physiological relevance. Much higher extracellular K+ concentrations do occur in traumatic injury, ischemia, seizures, and spreading depression (Kofuji and Newman, 2004), conditions that are accompanied by high rates of glucose consumption and lactate production and in which the KPNH pathway is likely to play pathogenic roles. Interestingly, energy-deficient neurological conditions like stroke and hypoxia induce changes in NBCe1 expression levels (Majumdar and Bevensee, 2010). Arguably, K+-stimulated astrocytes may outcompete neighboring neurons for glucose, with ensuing oxidative stress and neuronal apoptosis (Bolaños et al., 2010). K+ may behave as a more general signal for fast neurometabolic coupling. Bergmann glial cells are rich in NBCe1, are highly permeable to K+, and are exposed to K+ released from glutamatergic synapses at Purkinje cell dendrites. Other regions of possible relevance are the neuromuscular junction, the Ranvier node, and the serotoninergic synapse, where neuronal K+ is released in the vicinity of glial cells. Astrocytes are heterogeneous in terms of K+ channel expression and resting membrane potential (McKhann et al., 1997), which may determine different basal NBCe1 activity, different pH responses to extracellular K+, and therefore different metabolic responses.
pH as a second messenger for glycolysis modulation
By linking extracellular K+ to glycolysis, H+ behaves as a second messenger, though an unusual one that keeps its target under tonic inhibition. Other than the glucose transporter, flux control of glucose utilization is shared among three enzymes: hexokinase, PFK, and pyruvate kinase, and large increases in flux such as observed in response to K+ must involve the three of them. Phosphofructokinase is sensitive to pH both in vitro and in cells, with steep H+ concentration dependence (Trivedi and Danforth, 1966; Erecińska et al., 1995). Hexokinase may be recruited by the decrease in the concentration of its allosteric inhibitor, glucose-6-phosphate, depleted as a result of PFK stimulation. Pyruvate kinase is activated allosterically by its substrate phosphoenolpyruvate and by fructose-1,6-bisphosphate, both of which are expected to rise after PFK activation. Thus it seems feasible that the KPNH pathway converges onto a single direct target, PFK. An analogous, albeit slower, regulation of glycolysis by intracellular pH has been reported in skeletal muscle, where the stimulation of the Na+/H+ exchanger by insulin increases intracellular pH, decreases glucose-6-phosphate levels (i.e. PFK stimulation), and activates glycolysis over a period of minutes (Fidelman et al., 1982). A commanding role for pH over astrocytic glycolysis may help to understand energy coupling during inhibitory neurotransmission. For instance, compared with glutamatergic activity, GABAergic activity is energetically inexpensive (Attwell and Laughlin, 2001). But as for glutamate, GABA is taken up by astrocytes via a Na+-coupled transporter, and it is not obvious how GABA uptake could avoid activating the astrocytic sodium pump and thus stimulating glycolysis (Chatton et al., 2003). One important difference between GABAergic transmission and glutamatergic transmission is that the former does not release K+. Moreover, astrocytes possess HCO3−-permeable GABAA receptors, which when open release HCO3− and acidify the cells (Kaila et al., 1991), which, as observed for glutamate, aspartate, and gramicidin, may counteract the stimulatory effect of Na+. This putative GABAA-dependent glycolytic inhibition may perhaps match the inhibition of oxidative phosphorylation that follows intracellular acidification in astrocytes (Azarias et al., 2011). Excitatory and inhibitory neurotransmission crosstalk by opposite modulation of neuronal membrane potential; for the purposes of glycolysis modulation, excitatory and inhibitory neurotransmission may perhaps crosstalk by opposite modulation of astrocytic pH.
Footnotes
This work was partially funded by Fondecyt 1100936 (L.F.B.) and 3085021 (C.A). G.E.S. was supported by NIH Grants DK050594 and HL061974. I.R. is a Conicyt Fellow and received a “Beca de Apoyo a la Tesis Doctoral” from Conicyt. G.E.S. is supported by NIH Grant DK050594. The Centro de Estudios Científicos is funded by the Chilean Government through the Centers of Excellence Base Financing Program of Conicyt and Gobierno Regional de Los Ríos. We thank Karin Alegría for technical assistance, veterinary Dr. Viviana Bustos for assistance with NBCe1 KO animals, and Karen Everett for critical reading of this manuscript. S0859 was kindly provided by Juergen Puenter, Sanofi-Aventis, and human NBCe1A cDNA was kindly provided by Dr. Ashley Toye.
References
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Azarias G, Perreten H, Lengacher S, Poburko D, Demaurex N, Magistretti PJ, Chatton JY. Glutamate transport decreases mitochondrial pH and modulates oxidative metabolism in astrocytes. J Neurosci. 2011;31:3550–3559. doi: 10.1523/JNEUROSCI.4378-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros LF, Deitmer JW. Glucose and lactate supply to the synapse. Brain Res Rev. 2010;63:149–159. doi: 10.1016/j.brainresrev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Bittner CX, Loaiza A, Ruminot I, Larenas V, Sotelo-Hitschfeld T, Gutiérrez R, Córdova A, Valdebenito R, Frommer WB, Barros LF. High resolution measurement of the glycolytic rate. Front Neuroenergetics. 2010;2:26. doi: 10.3389/fnene.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner CX, Valdebenito R, Ruminot I, Loaiza A, Larenas V, Sotelo-Hitschfeld T, Moldenhauer H, San Martín A, Gutiérrez R, Zambrano M, Barros LF. Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J Neurosci. 2011;31:4709–4713. doi: 10.1523/JNEUROSCI.5311-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolaños JP, Almeida A, Moncada S. Glycolysis: a bioenergetic or a survival pathway? Trends Biochem Sci. 2010;35:145–149. doi: 10.1016/j.tibs.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Bonvento G, Herard AS, Voutsinos-Porche B. The astrocyte–neuron lactate shuttle: a debated but still valuable hypothesis for brain imaging. J Cereb Blood Flow Metab. 2005;25:1394–1399. doi: 10.1038/sj.jcbfm.9600127. [DOI] [PubMed] [Google Scholar]
- Bowman CL, Kimelberg HK. Excitatory amino acids directly depolarize rat brain astrocytes in primary culture. Nature. 1984;311:656–659. doi: 10.1038/311656a0. [DOI] [PubMed] [Google Scholar]
- Bowman CL, Kimelberg HK. Pharmacological properties of the norepinephrine-induced depolarization of astrocytes in primary culture: evidence for the involvement of an alpha 1-adrenergic receptor. Brain Res. 1987;423:403–407. doi: 10.1016/0006-8993(87)90872-9. [DOI] [PubMed] [Google Scholar]
- Caesar K, Hashemi P, Douhou A, Bonvento G, Boutelle MG, Walls AB, Lauritzen M. Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo. J Physiol. 2008;586:1337–1349. doi: 10.1113/jphysiol.2007.144154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J, Bittner CX, Humeres A, Montecinos VP, Vera JC, Barros LF. A cytosolic source of calcium unveiled by hydrogen peroxide with relevance for epithelial cell death. Cell Death Differ. 2004;11:468–478. doi: 10.1038/sj.cdd.4401372. [DOI] [PubMed] [Google Scholar]
- Chatton JY, Marquet P, Magistretti PJ. A quantitative analysis of L-glutamate-regulated Na+ dynamics in mouse cortical astrocytes: implications for cellular bioenergetics. Eur J Neurosci. 2000;12:3843–3853. doi: 10.1046/j.1460-9568.2000.00269.x. [DOI] [PubMed] [Google Scholar]
- Chatton JY, Pellerin L, Magistretti PJ. GABA uptake into astrocytes is not associated with significant metabolic cost: implications for brain imaging of inhibitory transmission. Proc Natl Acad Sci U S A. 2003;100:12456–12461. doi: 10.1073/pnas.2132096100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch'en FF, Villafuerte FC, Swietach P, Cobden PM, Vaughan-Jones RD. S0859, an N-cyanosulphonamide inhibitor of sodium-bicarbonate cotransport in the heart. Br J Pharmacol. 2008;153:972–982. doi: 10.1038/sj.bjp.0707667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- Chesler M, Kraig RP. Intracellular pH of astrocytes increases rapidly with cortical stimulation. Am J Physiol. 1987;253:R666–R670. doi: 10.1152/ajpregu.1987.253.4.R666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M, Kraig RP. Intracellular pH transients of mammalian astrocytes. J Neurosci. 1989;9:2011–2019. doi: 10.1523/JNEUROSCI.09-06-02011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer JW, Rose CR. pH regulation and proton signalling by glial cells. Prog Neurobiol. 1996;48:73–103. doi: 10.1016/0301-0082(95)00039-9. [DOI] [PubMed] [Google Scholar]
- Deitmer JW, Szatkowski M. Membrane potential dependence of intracellular pH regulation by identified glial cells in the leech central nervous system. J Physiol. 1990;421:617–631. doi: 10.1113/jphysiol.1990.sp017965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Nutrition during brain activation: does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochem Int. 2004;45:321–351. doi: 10.1016/j.neuint.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Erecińska M, Deas J, Silver IA. The effect of pH on glycolysis and phosphofructokinase activity in cultured cells and synaptosomes. J Neurochem. 1995;65:2765–2772. doi: 10.1046/j.1471-4159.1995.65062765.x. [DOI] [PubMed] [Google Scholar]
- Fidelman ML, Seeholzer SH, Walsh KB, Moore RD. Intracellular pH mediates action of insulin on glycolysis in frog skeletal muscle. Am J Physiol. 1982;242:C87–C93. doi: 10.1152/ajpcell.1982.242.1.C87. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Fröhlich F, Bazhenov M, Iragui-Madoz V, Sejnowski TJ. Potassium dynamics in the epileptic cortex: new insights on an old topic. Neuroscientist. 2008;14:422–433. doi: 10.1177/1073858408317955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto C, Arnett KL, Milanick MA. Divalent cation interactions with Na,K-ATPase cytoplasmic cation sites: implications for the para-nitrophenyl phosphatase reaction mechanism. J Membr Biol. 2007;216:49–59. doi: 10.1007/s00232-007-9028-x. [DOI] [PubMed] [Google Scholar]
- Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/ J Biol Chem. 2007;282:9042–9052. doi: 10.1074/jbc.M607041200. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann U, Schaible HG, Schmidt RF. Changes in extracellular potassium concentration in cat spinal cord in response to innocuous and noxious stimulation of legs with healthy and inflamed knee joints. Exp Brain Res. 1990;79:283–292. doi: 10.1007/BF00608237. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wilson GS. A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J Neurochem. 1997a;69:1484–1490. doi: 10.1046/j.1471-4159.1997.69041484.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wilson GS. Rapid changes in local extracellular rat brain glucose observed with an in vivo glucose sensor. J Neurochem. 1997b;68:1745–1752. doi: 10.1046/j.1471-4159.1997.68041745.x. [DOI] [PubMed] [Google Scholar]
- Jolivet R, Allaman I, Pellerin L, Magistretti PJ, Weber B. Comment on recent modeling studies of astrocyte-neuron metabolic interactions. J Cereb Blood Flow Metab. 2010;30:1982–1986. doi: 10.1038/jcbfm.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K, Panula P, Karhunen T, Heinonen E. Fall in intracellular pH mediated by GABAA receptors in cultured rat astrocytes. Neurosci Lett. 1991;126:9–12. doi: 10.1016/0304-3940(91)90358-z. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Schachner M. Pharmacological properties of gamma-aminobutyric acid-, glutamate-, and aspartate-induced depolarizations in cultured astrocytes. J Neurosci. 1985;5:3295–3301. doi: 10.1523/JNEUROSCI.05-12-03295.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience. 2004;129:1045–1056. doi: 10.1016/j.neuroscience.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science. 2005;309:943–947. doi: 10.1126/science.1112085. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Role of glutamate in neuron-glia metabolic coupling. Am J Clin Nutr. 2009;90:875S–880S. doi: 10.3945/ajcn.2009.27462CC. [DOI] [PubMed] [Google Scholar]
- Majumdar D, Bevensee MO. Na-coupled bicarbonate transporters of the solute carrier 4 family in the nervous system: function, localization, and relevance to neurologic function. Neuroscience. 2010;171:951–972. doi: 10.1016/j.neuroscience.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makani S, Chesler M. Rapid rise of extracellular pH evoked by neural activity is generated by the plasma membrane calcium ATPase. J Neurophysiol. 2010;103:667–676. doi: 10.1152/jn.00948.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, 2nd, D'Ambrosio R, Janigro D. Heterogeneity of astrocyte resting membrane potentials and intercellular coupling revealed by whole-cell and gramicidin-perforated patch recordings from cultured neocortical and hippocampal slice astrocytes. J Neurosci. 1997;17:6850–6863. doi: 10.1523/JNEUROSCI.17-18-06850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer MI, Cid LP, Barros LF, Sepúlveda FV. Modulation of the two-pore domain acid-sensitive K+ channel TASK-2 (KCNK5) by changes in cell volume. J Biol Chem. 2001;276:43166–43174. doi: 10.1074/jbc.M107192200. [DOI] [PubMed] [Google Scholar]
- Pappas CA, Ransom BR. Depolarization-induced alkalinization (DIA) in rat hippocampal astrocytes. J Neurophysiol. 1994;72:2816–2826. doi: 10.1152/jn.1994.72.6.2816. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Porras OH, Ruminot I, Loaiza A, Barros LF. Na(+)-Ca(2+) cosignaling in the stimulation of the glucose transporter GLUT1 in cultured astrocytes. Glia. 2008;56:59–68. doi: 10.1002/glia.20589. [DOI] [PubMed] [Google Scholar]
- Prichard J, Rothman D, Novotny E, Petroff O, Kuwabara T, Avison M, Howseman A, Hanstock C, Shulman R. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci U S A. 1991;88:5829–5831. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, Attwell D. Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci. 2011;31:538–548. doi: 10.1523/JNEUROSCI.3516-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt BM, Berger UV, Douglas RM, Bevensee MO, Hediger MA, Haddad GG, Boron WF. Na/HCO3 cotransporters in rat brain: expression in glia, neurons, and choroid plexus. J Neurosci. 2000;20:6839–6848. doi: 10.1523/JNEUROSCI.20-18-06839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Watanabe E, Hiyama TY, Nagakura A, Fujikawa A, Okado H, Yanagawa Y, Obata K, Noda M. Glial Nax channels control lactate signaling to neurons for brain [Na+] sensing. Neuron. 2007;54:59–72. doi: 10.1016/j.neuron.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Silver IA, Erecińska M. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svichar N, Esquenazi S, Chen HY, Chesler M. Preemptive regulation of intracellular pH in hippocampal neurons by a dual mechanism of depolarization-induced alkalinization. J Neurosci. 2011;31:6997–7004. doi: 10.1523/JNEUROSCI.6088-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Farrell K, Stein BA. Astrocyte energetics, function, and death under conditions of incomplete ischemia: a mechanism of glial death in the penumbra. Glia. 1997;21:142–153. doi: 10.1002/(sici)1098-1136(199709)21:1<142::aid-glia16>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Takanaga H, Chaudhuri B, Frommer WB. GLUT1 and GLUT9 as major contributors to glucose influx in HepG2 cells identified by a high sensitivity intramolecular FRET glucose sensor. Biochim Biophys Acta. 2008;1778:1091–1099. doi: 10.1016/j.bbamem.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toye AM, Parker MD, Daly CM, Lu J, Virkki LV, Pelletier MF, Boron WF. The human NBCe1-A mutant R881C, associated with proximal renal tubular acidosis, retains function but is mistargeted in polarized renal epithelia. Am J Physiol Cell Physiol. 2006;291:C788–C801. doi: 10.1152/ajpcell.00094.2006. [DOI] [PubMed] [Google Scholar]
- Trivedi B, Danforth WH. Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem. 1966;241:4110–4112. [PubMed] [Google Scholar]