Abstract
In most animals, the Antero-Posterior (A-P) axis requires a gradient of Wnt signaling. Wnts are expressed posteriorly in many vertebrate and invertebrate embryos, forming a gradient of canonical Wnt/β-Catenin activity that is highest in the posterior and lowest in the anterior. One notable exception to this evolutionary conservation is in the Drosophila embryo, in which the A-P axis is established by early transcription factors of maternal origin. Despite this initial axial establishment, Drosophila still expresses Wingless (Wg), the main Drosophila Wnt homologue, in a strong posterior band early in embryogenesis. Since its discovery 30 years ago this posterior band of Wg has been largely ignored. In this study, we re-examined the onset of expression of the Wg posterior band in relation to the expression of Wg in other segments, and compared the timing of its expression to that of axial regulators such as gap and pair-rule genes. It was found that the posterior band of Wg is first detected in blastoderm at mid nuclear cycle 14, before the segment-polarity stripes of Wg are formed in other segments. The onset of the posterior band of Wg expression was preceded by that of the gap gene products Hunchback (hb) and Krüppel (Kr), and the pair-rule protein Even-skipped (Eve). Although the function of the posterior band of Wg was not analyzed in this study, we note that in temperature-sensitive Wg mutants, in which Wg is not properly secreted, the posterior band of Wg expression is diminished in strength, indicating a positive feedback loop required for Wg robust expression at the cellular blastoderm stage. We propose that this early posterior expression could play a role in the refinement of A-P patterning.
Keywords: Wnt, antero-posterior patterning, gap genes, pair-rule genes, evo-devo
1. Introduction
The antero-posterior (A-P) body axis of most animals is specified by evolutionarily conserved molecular mechanisms, as exemplified by the Hox genes (Doubule, 2007; Butts et al., 2008; De Robertis, 2008;). An important recent realization has been that the Wnt family of secreted growth factors function as global regulators of A-P pattern development in most animals. Wnts are expressed posteriorly in many vertebrate and invertebrate embryos, forming a gradient of canonical Wnt/β-Catenin activity that is highest in the posterior and lowest in the anterior, where extracellular Wnt antagonists such as Dickkopf (Dkk) and secreted Frizzled-Related Proteins (sFRP/Frzb) are expressed (Kiecker and Niehrs, 2001; Peterson and Reddien, 2009; Niehrs, 2010). The remarkable conservation of the A-P patterning system is best exemplified by planarians, in which knock-down of canonical Wnt signaling with β-catenin RNAi leads to regeneration of heads instead of tails (Gurley et al., 2008; Peterson and Reddien, 2008; Iglesias et al., 2008). Conversely, increasing canonical Wnt signaling using adenopolyposis coli RNAi causes the regeneration of tails instead of heads (Gurley et al., 2008).
One notable exception is the Drosophila embryo, in which the A-P and dorso-ventral (D-V) patterns are specified by maternal determinants deposited in the egg (Lawrence, 1992). Wingless, the main Drosophila Wnt homologue, functions in the specification of the polarity of individual segments during early embryogenesis, but is not thought to be involved in global A-P patterning. In view of the near universal role of Wnt in A-P patterning in animal development, it seemed puzzling that the Drosophila embryo would lack this regulation.
The wingless (wg) mutation was initially identified in adults that lacked one or both wings (Sharma and Chopra, 1976). The wg gene was then found to be required for the polarity of Drosophila segments in a classical screen of zygotic mutations that affect the embryonic cuticle (Nüsslein-Volhard and Wieschaus, 1980). In earlier investigations, wg transcripts and protein have been detected in each segment, just anterior to the parasegment boundary (Baker, 1987; van den Heuvel et al., 1989). Expression was also noted in the head region at ~85% egg length (EL) and in a posterior band at ~10% EL. Since these early studies, attention has been focused on the segmental function of wg. This was likely due to the fact that wg null mutants generate a striking cuticle phenotype consisting of a lawn of denticles in which all segmentation is lost. However, it was also briefly noted that in wg null flies the cuticle of the entire posterior region, and part of the anterior cuticle, were deleted (Baker, 1987).
The renewed interest in a global effect of Wnt signaling in A-P pattering prompted us to reinvestigate the posterior expression of wg mRNA and protein in the Drosophila early embryo. We found that the posterior band of Wg was expressed before the 14 Wg segmental stripes. When compared to the expression of the gap genes hunchback (hb) and Krüppel (Kr), or the pair-rule gene even-skipped (eve), the posterior Wg band appeared later, at mid nuclear cycle 14 stage, during cellularization of the blastoderm. Wg expression in the posterior band remained very prominent, stronger than in any segmental stripe, throughout early development, until hindgut invagination at the end of gastrulation. This early region of Wg expression in the posterior may represent an evolutionary residual atavism originating in insect ancestors that patterned the A-P axis via a posterior growth zone driven by Wnt signals.
2. Results
2.1. The posterior band of Wg expression appears before the segmental stripes
The first expression of Wg protein appeared during cellularization of the blastoderm (Fig. 1), which occurs between 2 h 30 min to 3 h 15min after egg laying (AEL) (Wieschaus and Nüsslein-Volhard, 1998). At mid nuclear cycle 14 (Wieschaus and Nüsslein-Volhard, 1998; Lott et al., 2011), posterior Wg appeared in a band at ~10% egg length (EL) (Fig. 1A). Two patches of anterior expression at ~85% and 100% EL were present as well (Fig. 1A; van del Heuvel et al., 1989). Note that this expression occurred before the appearance of any segmental Wg stripes. As blastoderm stage progressed, the posterior band became stronger and remained the most prominent region of Wg expression during gastrulation and early germ-band extension (Fig. 1B-E). The segmental stripes appeared sequentially from the anterior to the posterior (with a weak pair-rule pattern) during the late cell cycle 14 blastoderm stage (Fig. 1C). At early gastrulation, all 14 of the segmental stripes were formed and posterior Wg maintained its expression in a strong band at ~10% EL (Fig. 1D). By late gastrulation, this posterior Wg band migrated dorsally to a position parallel to with the length of the embryo in a region called the midgut plate and (Fig. 1E-F). This region is known to invaginate at the extended germ-band and eventually forms the hindgut (van den Heuvel et al., 1989; Wieschaus and Nüsslein-Volhard, 1998; Wu and Lengyel 1998; Takashima and Murakami, 2001).
Fig. 1.
Expression of Wg protein in a posterior band of the early Drosophila embryo. (A) Early cellularization of the blastoderm (early-mid nuclear cycle 14) (Wieschaus and Nüsslein-Volhard, 1998) Wg expression starts in anterior and posterior regions (the asterisk marks the posterior band). (B) Mid nuclear cycle 14, expression in the posterior band has intensified. (C) Late nuclear cycle 14, the blastoderm becomes cellularized and the segmental Wg bands become weakly apparent; note that they arise with a pair-rule pattern. (D) By early gastrulation (Stage 6) the segment polarity stripes are established (displaying pair-rule variations in intensity) and the posterior band is the strongest signal; the ventral furrow has been formed. (E) As the midgut invaginates (Stage 7) posterior Wingless moves dorsally to form the midgut plate. (F) By late germ band extension (Stage 12) posterior Wg is found in the future hindgut.
In situ hybridization studies showed that wg mRNA followed a very similar pattern to that of its protein expression, with posterior wg transcripts being first detected at early nuclear cycle 14 (beginning of blastoderm cellularization) (Fig. 2A), and remaining strong through germ-band extension (Fig. 2B-E) (Baker, 1987, 1988).
Fig. 2.
Transcript distribution of wg in the early Drosophila embryo. (A) Early cellularization of the blastoderm (early nuclear cycle 14), wg mRNA is found in the posterior region, starting slightly before anterior wg expression and posterior protein expression (asterisk marks posterior band). (B) At mid nuclear cycle 14, before complete cellularization of the blastoderm, mRNA levels of the posterior band have increased and transcripts for segmental bands begin to appear in anterior segments. (C) Late nuclear stage 14, cellularization is not completed yet, as indicated by the absence of the cephalic furrow, which forms just after cellularization. (D) Early gastrulation, each segment has a thin stripe of wg mRNA and the posterior wg band is most intense, ventral view (stage 6). (E) Early germ band extension (stage 7) in which the posterior stripe remains strong and moves dorsally to form the midgut plate. (F) At full germ-band extension (stage 10) the posterior tissue expressing wg mRNA invaginates to later form the hindgut.
2.2. Expression of the Wg posterior band starts after gap and pair-rule genes
The focus of embryonic Wg expression in the literature has primarily been concerned with the 14 segmental stripes involved in polarization of the segments. This was likely due to the great interest in the mutant phenotypes of the cuticle. Inasmuch, little attention has been paid to the temporal expression of posterior Wg when compared to other early genes expressed in the blastoderm that are involved in embryo patterning, such as gap and pair-rule genes. In Fig. 3 A-E, we show that the posterior band of Wg appears subsequently to Hunchback (Hb) protein expression in the posterior region. Hb is already detectable during late cleavage stages of the syncytial blastoderm. Posterior Hb was detected at early cellularization in a broad stripe from 0-15% EL, while Wg expression was not detectable at this stage (compare Fig. 3A to 3C) (Bender et al., 1988). During cellularization of the blastoderm, the Hb stripe narrowed to ~10-15% EL (Fig. 3D, F) and Wg was found in a narrow band posterior to this Hb stripe (Fig. 3B, F). This shows that posterior Wg expression was activated after Hb expression, but prior to Wg segment polarity stripes.
Fig 3.
Wg protein expression appears after that of the gap gene products Hunchback (Hb) and Krüppel (Kr). (A, C, E) Early nuclear cycle 14 (Wieschaus and Nüsslein-Volhard, 1998), Hb expression is present in both anterior and posterior domains (green). Wg (in red) is not yet expressed at this time. (B, D, F) By mid nuclear cycle 14, Wg is expressed both anteriorly and posteriorly (arrow) and abuts the now narrower band of posterior Hb. (G, I, K) Krüppel expression is clearly defined by early nuclear cycle 14, at which time no Wg expression is detected. (H, J, L) By mid-nuclear cycle 14, posterior Wg expression (arrow) is detected at 10% EL of the embryo. DAPI staining was used to identify nuclei (blue).
The temporal expression of additional gap gene product, Krüppel (Kr), was also compared to that of posterior Wg (Fig. 3G-L). Kr protein is first detected during late syncytial blastoderm at 50-60% EL (Fig. 3G, K; Gual et al., 1987). At early nuclear cycle 14, Kr was well expressed but Wg protein was not detectable (Fig. 3G, I, K). By mid nuclear cycle 14, Kr expression was still strong and well defined, and the Wingless posterior band appeared just posterior to the Kr domain (Fig. 3H, J, L).
To more precisely define the temporal expression of Wg, its protein expression was also compared to the pair-rule protein Even-skipped (Eve) (Fig. 4). Eve antigen is first expressed at early blastoderm, where it gradually becomes more defined (Frasch et al., 1987). At early nuclear cycle 14, Eve is found in its seven characteristic stripes, while Wg was not yet detectable (Fig. 4A, C, E). At mid nuclear cycle 14 blastoderm, Wg protein became detectable posterior to the seventh Eve stripe (Fig. 4B, D, F, arrows). The Eve and Wg antibodies used in these double staining studies were both of mouse origin. We therefore performed the stainings sequentially, which allowed us to detect both proteins in the same embryo. The appearance of the seven weak green pair-rule stripes in green in the blastoderm (Fig. 4B), due to cross reaction of the secondary antibodies, was an unavoidable artifact of the staining procedure (see experimental procedures). We conclude that the posterior Wg band is expressed subsequently to Hb, Kr and Eve.
Fig. 4.
Wg expression starts after formation of the characteristic seven stripes of the pair-rule gene Even-skipped. (A, C, E) Early nuclear cycle 14, the seven stripes of Eve (red) are apparent; note that Wg is not yet expressed. (B, D, F) Mid nuclear cycle 14, Wg expression (in green) is detected in the posterior band. The posterior band of Wg is marked with an arrow. DAPI is used to mark nuclei (blue). Sequential staining with two mouse antibodies was used in this experiment (see Experimental Procedures).
2.3. Posterior Wg expression levels are decreased in a wg temperature-sensitive mutant
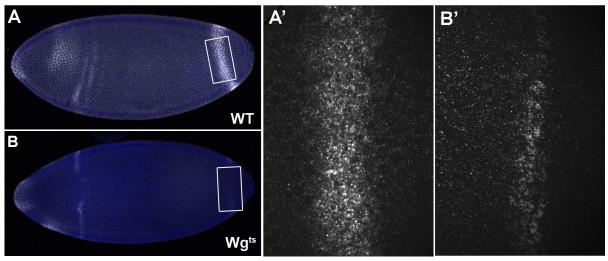
Is the posterior Wg band active in signaling? To answer this question we used the wg temperature-sensitive mutant wgts (Nüsslein-Volhard et al., 1984). Heterozygous flies were crossed at non-permissive temperature (25°C), and the resulting embryos stained for Wg protein expression at blastoderm stages. In about a quarter of the progeny, we observed that the expression of Wg was much less robust than in non-mutant embryos, particularly in the posterior (Fig. 5). In situ hybridization studies indicated this decrease was at the transcriptional level (data not shown). The decrease in Wg protein was apparent even before the start of Wg segmental expression, indicating that it results from signaling by the posterior Wg band (Fig. 5). The wgts mutant lacks the ability to be secreted; therefore, it cannot transmit the Wg signal but still makes full length protein that is recognizable by antibody staining (González et al., 1991). The wgts mutant has been used to show that Wg is required to establish segmental polarity independently of other segmental genes such as engrailed (en), but the function of the posterior Wg band was not investigated (Yoffe et al., 1995). Posterior Wg was thought to have no function prior to hindgut formation. The present results indicate that posterior Wg does signal during blastoderm, and is required to maintain high levels of its own expression in the posterior band.
Fig. 5.
Wg secretion mutants (wgts) have a less robust posterior stripe than wild-type embryos. (A, A’) Non-mutant embryo showing Wg protein expression (in white) at late nuclear cycle 14 in both the whole embryo and a higher magnification (boxed) of the posterior band region. (B, B’) wgts mutant embryo at the same stage showing Wg expression at late nuclear cycle 14 in both the whole embryo and an enlargement of the posterior band region alone. DAPI is used to identify the nuclei (blue). Note that the posterior Wg protein band is narrower and less intense in the mutant; this indicates that Wg does signal during blastoderm stages, increasing its own expression.
3. Discussion
The most prominent expression of Wg in the early Drosophila embryo appears in the posterior region before Wg expression starts in a segmental pattern. Nevertheless, this posterior band has received very little attention in the literature. This may be attributed to the analysis of wg cuticular phenotypes being centered on the dramatic segmental defects, with little emphasis given to posterior deletions initially reported in the larval cuticle (Baker, 1987). A-P patterning in Drosophila is established by maternal genes such as bicoid and nanos, which subsequently turn on gap and pair-rule genes (Rivera-Pomar and Jäckle, 1996). Our analyses show that expression of the posterior Wg band starts after gap and pair-rule genes are activated. Therefore, the posterior band of Wg cannot be responsible for the initiation of the overall A-P pattern. However, posterior Wg expression starts before that of the 14 Wg segmental stripes and is very is abundant. Thus, it can not be excluded that it may participate in a later refinement of A-P patterning.
In the case of D-V patterning in the Drosophila embryo, the Short-gastrulation BMP-binding protein (Sog) is secreted into the perivitelline space where it diffuses from the ventral to the dorsal side (O’Connor et al. 2006; Srinivasan et al., 2002; Wang and Ferguson, 2005). An attractive hypothesis would be that Wg protein might be secreted into the perivitelline space at cellular blastoderm and may diffuse over long distances, contributing to the regulation of A-P pattern. The methods used here do not have the sensitivity to test this hypothesis. Testing the function of Wg in the posterior blastoderm band would be difficult for us, since it would require loss-of-function manipulations targeting specifically the posterior Wg band without affecting segmental Wg expression. An RNAi against Wg has been proven effective in Wg knockdown (E. Eivers, H. Demagny, and E.M.D.R., submitted); however, an early driver in the posterior region would be necessary to utilize it in studies of the posterior. We tested several posterior drivers, but none drove GAL4 sufficiently early and specifically enough to knockdown the posterior band of Wg. Functional testing of the posterior Wg band at blastoderm stages will require development of new tools. It should be noted that Bejsovec and Martinez Arias (1991), using a wgts mutant, showed that inactivating Wg for the first four hours of development resulted in normal segment formation, when the posterior band is first expressed. However, in this case posterior Wg might still signal after four hours, since its expression persists until late gastrulation. This posterior region (10% EL) also expresses caudal, which overlaps with Wg expression, and gives rise to the cuticle of the analia (proctodeum) and to the hindgut (Calleja et al., 1996; Lengyel and Iwaki, 2002). At later stages, during organogenesis, posterior Wg functions mostly in hindgut development (Lengyel et al., 2002; Takashima and Murakami, 2001; van den Heuvel et al., 1989; Wu and Lengyel, 1998). As shown here, at blastoderm stages posterior Wg is active in signaling, for it is required to maintain high levels of its own expression in the posterior band as early as late cell cycle 14 (Fig. 5). Wg is known to form a positive autoregulatory loop upon its own expression during Drosophila segmentation (Manoukian et al., 1995, Yoffe et al., 1995).
The regulation of the early posterior band of wg is distinct from the regulation of wg responsible for segment polarity. Ingham and Hidalgo (1993) analyzed the regulation of wg by pair-rule genes, but did not comment as to why the posterior and anterior wg expression domains remained unaffected by any of the mutations studied. In their study, the posterior wg band remained robust throughout embryogenesis in the absence of many known regulators of segmental wg, such as ftz, paired (prd), eve, and hedgehog (hh), although only the segmental pattern was studied (Ingham and Hidalgo, 1993). Further studies will be required to identify the transcriptional regulators of the Wg posterior band.
There is extensive evidence that most animals use Wg to pattern the A-P body axis (Kiecker and Niehrs, 2001; De Robertis, 2008; Petersen and Reddien, 2009; Niehrs et al., 2010). In most animals, Wnts are expressed at the site of the blastopore formation which develops to posterior end of the A-P axis. Closely related to human development, the posterior expression of Wnt3a in mice is required for proper posterior patterning (Takada et al., 1994). In amphioxus embryos, genes of the A-P Wnt/Dkk/sFRP axis are deployed perpendicularly to those of the BMP/Chordin D-V pathway (Yu et al., 2007). Even in non-bilateral animals such as cnidarians (Hydra and sea anemones), a Wnt/Dkk axis determines the polarity of the body column (Guder et al., 2006). In the lophotrochozoan branch, planarians have a marked requirement for Wnt/β-Catenin signaling in the regeneration of the A-P axis (Gurley et al., 2008; Petersen and Reddien, 2008; Iglesias et al., 2008). When Wnt signaling is inhibited by β-catenin or dishevelled RNAi knockdown, planarians regenerate heads instead of tails; when Wnt signaling is increased by adenopolyposis coli knockdown, tails regenerate instead of heads. Wnt signaling is needed to sustain posterior development, and is involved in most aspects of planarian wound regeneration (De Robertis, 2010).
A recent study on the hemichordate Saccoglossus kowalevskii has shown that embryonic patterning of this basal deuterostome is also regulated by Wnt in a similar fashion to chordates. Darras et al. (2011) showed that Wnt has two signaling phases during embryonic development, early and late. At early cleavage, nuclear β-Catenin accumulates in vegetal cells, leading to the specification of the endomesoderm. The endomesoderm, at a later stage in development, secretes Wnt signals that are responsible for A-P axis formation in the ectoderm (Darras et al., 2011).
In a similar way, in Xenopus an early maternal Wnt signal causes accumulation of β-Catenin in the dorsal side at cleavage stages, establishing the Nieuwkoop center, which in turn leads to the induction of Spemann’s organizer (Heasman, 2006; Tao et al., 2005; Weaver and Kimelman, 2004). The organizer signaling center then induces axis development in Xenopus (De Robertis et al., 2000). The posteriorizing activity of Wnt only starts at later stages of development (Christian and Moon, 1993), when a gradient of Wnt/β-Catenin, maximal in the posterior, regulates Xenopus A-P patterning (Kiecker and Niehrs, 2001; Niehrs, 2010).
From the discussion above, it is clear that Wnt signaling is a determinant of A-P patterning in many animals. The expression pattern of Wingless in the posterior of the Drosophila blastoderm examined here might suggest that Wg may play a role in Drosophila A-P regulation, in addition to its well-known role in segment formation, but this will remain a hypothesis until functional experiments are carried out. As is the case in hemichordates and Xenopus, the posteriorizing effect of Wingless would not take place at the earliest stages of development, as posterior Wingless expression only starts after the gap and pair-rule genes have outlined the overall A-P pattern of the Drosophila embryo.
Drosophila is not the only insect to have posterior wg expression. In fact, many arthropods have been shown to exhibit posterior abdominal defects when canonical Wnt signaling is disrupted (Bolognesi et al., 2008; Murat et al., 2010). RNAi knockdown of just one Wg homologue in general is not sufficient to cause posterior defects, but when multiple Wnt family members are targeted, or a single Wnt gene in conjunction with a downstream signaling components - such as armadillo/β-Catenin, pangolin/TCF, or arrow/LRP6 - abdominal A-P patterning defects are observed (Angelini and Kaufman, 2005; Bolognesi et al., 2008; Miyawaki et al., 2004). In spiders, knockdown of a single gene, Wnt8, has been shown to cause defects in posterior development (McGregor et al., 2008). Drosophila, a highly derived long germ-band embryo, may have retained the posterior band of wg as an atavism inherited from a short germ-band ancestor in which the abdomen developed from a posterior growth zone that required Wnt signaling (Martin and Kimelman, 2009; Murat et al., 2010).
Although the function of the Wingless posterior band is yet to be deciphered, its presence in this region indicates an evolutionary conservation that spans the passage of large periods of time. There is general agreement that Urbilateria, the common ancestor for protostomes and deuterostomes, patterned its A-P body axis using a Wnt gradient (De Robertis, 2008; Niehrs, 2010). The striking posterior band of Drosophila Wg expression may represent a remnant of the evolutionary history of its ancestors.
4. Experimental Procedures
4.1. Fly stocks
The W1118 fly strain was used for all temporal immunostaining and in situ hybridization. Wgts mutant was used to visualize non-secreted Wg in the early embryo (Bloomington Stock Center, stock #7000). We attempted the use of marked balancers to identify the wgts mutants, however their expression was not detectable at the early cellular blastoderm stages used in this study. However, mutant embryos, at the non-permissive temperature, were identified by differences in Wg protein staining patterns which is intracellular and not secreted (van den Heuvel et al., 1991). All flies and embryos were grown at 25°C until collection and fixation.
4.2. Drosophila embryo immunostaining
Drosophila embryos were collected at the desired stage, dechorionated in 50% bleach and rinsed thoroughly in distilled H2O. Embryos were transferred to a glass scintillation vial containing 50% heptane, 50% PEMFA (100 mM PIPES, 2.0 mM EGTA, 1.0 mM MgSO4, pH to 6.9 using KOH and 4% formaldehyde) solution and gently rocked for 20 min. The lower PEMFA layer was removed and an equal volume of methanol was added to the remaining heptane solution. The vial was then vigorously shaken for 30 seconds and embryos were allowed to settle to the bottom. The methanol/heptane solution was removed and embryos were washed 3 times with 100% methanol. Embryos were stepwise rehydrated in 0.2% Triton X-100 in phosphate buffered saline (PBS) and incubated for 1 h with gentle rocking. After blocking for 1 h in blocking solution (1x PBS, 20% goat serum, 2.5% bovine serum albumin) antibody solutions were added to the embryos. For whole-mount embryo immunostaining, the primary antibodies used were the monoclonal mouse antibodies anti-Wg (1:200 from concentrated antibody, Developmental Studies Hybridoma Bank #4D4), and anti-Eve (1:100, from concentrated antibody, Developmental Studies Hybridoma Bank #3C10), or the polyclonal rabbit antibodies anti-Hb (1:1000), and anti-Kr (1:500) (both Hb and Kr antisera were generous gifts from H. Jäckle). Antibodies were incubated overnight in blocking solution at 4°C. Embryos were washed 4 times for 15 min each using PBS/0.2% Triton X-100 before applying secondary antibodies (anti-rabbit Alexa-488 conjugated, anti-mouse Alexa488 conjugated, anti-mouseCy3 conjugated, (1;1000, Jackson Labs)) for 1 h at room temperature. After washing 4 times with PBS/0.2% Triton X-100 for 15 min each, Drosophila embryos were mounted on glass slides using DAPI-containing Vectashield (Vector). Immunofluorescence was analyzed and photographed using a Zeiss Imager Z.1 microscope with Apotome.
In Figure 4B some artifactual bands present are due to the use of a sequential antibody staining procedure, which allowed us to perform double immunofluorescence using two antibodies of mouse origin. The anti-Eve antibody was incubated for 1 h with embryos and washed off as described above. After washing, a secondary anti-mouse-Cy3 was added for 1 h to detect Eve protein (in red). Anti-mouse-Cy3 was then washed off, and anti-Wg antibody added and was incubated for 1 h and then washed. A second secondary antibody, anti-mouse-Alexa488 (green signal) was then added for 1 h and washed as described above. Embryos were imaged as described above. The seven weak bands seen in the green fluorescent channel are due to the fact that trace amounts of the secondary antibody used to detect Wg also recognize the anti-Eve mouse antibodies, giving the appearance of seven weak stripes in the green channel (Fig. 4B). When the first Eve antibody was omitted, these artifactual Wg bands were not observed.
4.3 In situ hybridization
Embryo preparation, fixation, probe preparation and hybridization were performed as described in Kosman et al. (2004). A 400 bp region of wg cDNA obtained by PCR was cloned into pGEM vector. This vector was linearized with NdeI, purified by phenol/chloroform extraction, 2 × volumes of ethanol, 0.1 volume of sodium acetate pH 5.2 added, and precipitated at −20°C overnight . Plasmid DNA was pelleted in an Eppendorph centrifuge for 15min at maximal speed, washed with 70% ethanol, and centrifuged again for 2 min. The pellet was dried and resuspended in 20 μl nuclease-free water. Synthesis of the probe with T7 polymerase plus transcription buffer (Roche, #881-767) was performed using a digoxigenin (DIG) RNA labeling mix (Roche, #1-277-073). Because only a short fragment of wg cDNA was used to make the probe, no fragmentation of the probe was required. Following the synthesis reaction, DNAse was added for 15 min at 37°C and a spin column was used to purify the RNA (Quick Spin Column, Roche). The probe was quantified and stored at −20°C (1 μg of probe was used per hybridization reaction). Probes were detected using a primary sheep anti-DIG (Roche) and secondary anti-sheep Alexa488 conjugated antibody to amplify the signal (1:1000, Jackson Labs.)
Highlights.
>The expression of the posterior Wg band was compared to gap and pair-rule genes >Posterior Wg preceeded segment-polarity Wg but not gap and pair-rule genes >The posterior band of Wg may refine A-P patterning or represent an evo-devo atavism
Acknowledgements
The authors thank the Bloomington Stock Center for fly stocks; H. Jäckle and the Developmental Studies Hybridoma Bank for antibodies and, Diego Ploper, Radoslaw Dobrowolski, Philipp Vick, Edward Eivers and Matthew Denholtz for discussions and comments on the manuscript; This work was supported by the NIH (HD21502-24). E.M.D.R. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angelini DR, Kaufman TC. Functional analyses in the milkweed bug Oncopeltus fasciatus (Hemiptera) support a role for Wnt signaling in body segmentation but not appendage development. Dev. Biol. 2005;283:409–423. doi: 10.1016/j.ydbio.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Baker N. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: a spatial distribution of a transcript in embryos. EMBO J. 1987;6:1765–1773. doi: 10.1002/j.1460-2075.1987.tb02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. Localization of transcripts from the wingless gene in the whole Drosophila embryo. Development. 1988;103:289–298. doi: 10.1242/dev.103.2.289. [DOI] [PubMed] [Google Scholar]
- Bender M, Horikami S, Cribbs D, Kaufman T. Identification and expression of the gap segmentation gene hunchback in Drosophila melanogaster. Dev. Genet. 1988;9:715–732. doi: 10.1002/dvg.1020090604. [DOI] [PubMed] [Google Scholar]
- Bejsovec A, Martinez Arias A. Roles of wingless in pattering the larval epidermis of Drosophila. Development. 1991;113:471–485. doi: 10.1242/dev.113.2.471. [DOI] [PubMed] [Google Scholar]
- Bolognesi R, Farzana L, Fischer TD, Brown SJ. Multiple Wnt genes are required for segmentation in the short-germ embryo of Tribolium castaneum. Curr. Biol. 2008;18:1624–1629. doi: 10.1016/j.cub.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts T, Holland PWH, Ferrier DEK. The urbilaterian super-hox cluster. Trends Genet. 2008;24:259–262. doi: 10.1016/j.tig.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Calleja M, Moreno E, Pelaz S, Morata G. Visualization of gene expression in living adult Drosophila. Science. 1996;274:252–255. doi: 10.1126/science.274.5285.252. [DOI] [PubMed] [Google Scholar]
- Christian JL, Moon RT. Interactions between Xwnt-8 and Spemann organizer signaling pathways to generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 1993;7:13–28. doi: 10.1101/gad.7.1.13. [DOI] [PubMed] [Google Scholar]
- Darras S, Gerhart J, Terasaki M, Kirschner M, Lowe CJ. β-Catenin specifies the endomesoderm and defines the posterior organizer of the hemichordate Saccoglossus kowalevski. Development. 2011;138:959–970. doi: 10.1242/dev.059493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Larrain J, Oelgeschläger M, Wessely O. The establishment of Spemann’s organizer and pattering of the vertebrate embryo. Nat. Rev. Genet. 2000;1:171–181. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM. Evo-Devo: variations on ancestral themes. Cell. 2008;132:185–195. doi: 10.1016/j.cell.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM. Wnt signaling in axial patterning and regeneration: a lesson from Planaria. Sci. Signal. 2010;3:1–3. doi: 10.1126/scisignal.3127pe21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboule D. The rise and fall of Hox gene clusters. Development. 2007;134:2549–2560. doi: 10.1242/dev.001065. [DOI] [PubMed] [Google Scholar]
- Eivers E, Demagny H. Mad functions in the wingless pathway independently of c-terminal phosphorylation. Science Signal. submitted. [Google Scholar]
- Frasch M, Hoey T, Rushlow C, Doyle H, Levine M. Characterization and localization of the even-skipped protein of Drosophila. EMBO. 1987;6:749–759. doi: 10.1002/j.1460-2075.1987.tb04817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaul U, Seifert E, Reinhard S, Jäckle H. Analysis of Krüppel protein distribution during early Drosophila development reveals posttranscriptional regulation. Cell. 1987;50:639–647. doi: 10.1016/0092-8674(87)90037-7. [DOI] [PubMed] [Google Scholar]
- González F, Swales L, Bejsovec A, Skaer H, Arias AM. Secretion and movement of wingless protein in the epidermis of the Drosophila embryo. Mech. Dev. 1991;35:43–54. doi: 10.1016/0925-4773(91)90040-d. [DOI] [PubMed] [Google Scholar]
- Guder C, Philipp I, Lengfeld T, Watanabe H, Hobmayer B, Holtstien TW. The Wnt code: cnidarians signals all the way. Oncogene. 2006;25:7450–7460. doi: 10.1038/sj.onc.1210052. [DOI] [PubMed] [Google Scholar]
- Gurley KA, Rink JC, Alvarado A. Sánchez. β-Catenin defines head versus tail identity during planarian regeneration and homeostasis. Science. 2008;319:323–326. doi: 10.1126/science.1150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman J. Patterning the early Xenopus embryo. Development. 2006;13:1205–1217. doi: 10.1242/dev.02304. [DOI] [PubMed] [Google Scholar]
- Iglesias M, Gomez-Skarmeta JL, Saló E, Adell T. Silencing of Smed-βcatenin1 generates radial-like hypercephalized planarians. Development. 2008;135:1215–1221. doi: 10.1242/dev.020289. [DOI] [PubMed] [Google Scholar]
- Ingham PW, Hidalgo A. Regulation of wingless transcription in the Drosophila embryo. Development. 1993;117:283–291. doi: 10.1242/dev.117.1.283. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Niehrs C. A morphogen gradient of Wnt/β-Catenin signaling regulates A-P neural patterning in Xenopus. Development. 2001;128:4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- Kosman D, Mizutani CM, Lemons D, Cox WG, McGinnis W, Bier E. Multiplex detection of RNA expression in Drosophila embryos. Science. 2004;305:846. doi: 10.1126/science.1099247. [DOI] [PubMed] [Google Scholar]
- Lawrence PA. The Making of a Fly: the genetics of animal design. Blackwell Science Ltd; Oxford: 1992. [Google Scholar]
- Lengyel JA, Iwaki DD. It takes guts: the Drosophila hindgut as a model system for Organogenesis. Dev. Biol. 2002;243:1–19. doi: 10.1006/dbio.2002.0577. [DOI] [PubMed] [Google Scholar]
- Lott SE, Villalta JE, Schroth GP, Luo S, Tonkin LA, Eisen MB. Noncanonical compensation of Zygotic X transcription in Early Drosophila melanogaster development reavealed through single-embryo RNA-seq. Plos Biol. 2011;9:1–13. doi: 10.1371/journal.pbio.1000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoukian AS, Yoffe KB, Wilder EL, Perrimon N. The porcupine gene is required for wingless autoregulation in Drosophila. Development. 1995;121:4037–4044. doi: 10.1242/dev.121.12.4037. [DOI] [PubMed] [Google Scholar]
- Martin BL, Kimelman D. Wnt Signaling and the Evolution of Embryonic Posterior Development. Curr. Biol. 2009;19:R215–R219. doi: 10.1016/j.cub.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor AP, Pechmann M, Schwager EE, Feitosa NM, Kruck S, Aranda M, Damen WGM. Wnt8 is required for growth-zone establishment and development of opisthosomal segments in a spider. Curr. Biol. 2008;18:1619–1623. doi: 10.1016/j.cub.2008.08.045. [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Mito T, Sarashina I, Zhang H, Shinmyo Y, Ohuchi H, Noji S. Involvement of Wingless/Armadillo signaling in the posterior sequential segmentation in the cricket, Gryllus bimaculatus (Orthoptera), as revealed by RNAi analysis. Mech. Develop. 2004;121:119–130. doi: 10.1016/j.mod.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Murat S, Hopfen C, McGregor AP. The function and evolution of Wnt genes in arthropods. Arthropod Struct. Dev. 2010;39:446–452. doi: 10.1016/j.asd.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Niehrs C. On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development. 2010;137:845–857. doi: 10.1242/dev.039651. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Wieschaus E, Kluding H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. I. Zygotic loci on the second chromosome. Wilhelm Roux’s Arch, devl. Biol. 1984;193:267–282. doi: 10.1007/BF00848156. [DOI] [PubMed] [Google Scholar]
- O’Connor MB, Umulis D, Othmer HG, Blair SS. Shaping the BMP morphogen gradients in the Drosophila embryo and pupal wing. Development. 2006;133:183–193. doi: 10.1242/dev.02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW. Smed-βcatenin-1 is required for A-P blastema polarity in planarian regeneration. Science. 2008;319:327–330. doi: 10.1126/science.1149943. [DOI] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Rivera-Pomar R, Jäckle H. From gradients to stripes in Drosophila embryogenesis: filling in the gaps. Trends Genet. 1996;12:478–483. doi: 10.1016/0168-9525(96)10044-5. [DOI] [PubMed] [Google Scholar]
- Sharma RP, Chopra VL. Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev. Biol. 1976;48:461–465. doi: 10.1016/0012-1606(76)90108-1. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Rashka KE, Beir E. Creation of a Sog morphogen gradient in the Drosophila embryo. Dev. Cell. 2002;2:91–101. doi: 10.1016/s1534-5807(01)00097-1. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulated somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Takashima S, Murakami R. Regulation of pattern formation in the Drosophila hindgut by wg, hh, dpp, and en. Mech Dev. 2001;101:79–90. doi: 10.1016/s0925-4773(00)00555-4. [DOI] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal Wnt11 activates the canonical Wnt signaling pathways required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, Nusse R, Johnston P, Lawrence PA. Distribution of wingless gene product in Drosophila embryos: a protein involved in cell-cell communication. Cell. 1989;59:739–749. doi: 10.1016/0092-8674(89)90020-2. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, Harryman-Samos C, Klingensmith J, Perrimon N, Nusse R. Mutations in the segment polarity genes wingless and porcupine impair the secretion of wingless protein. EMBO J. 1993;12:5293–5302. doi: 10.1002/j.1460-2075.1993.tb06225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Ferguson EL. Spatial bistability of Dpp-receptor interactions during Drosophila dorsal-ventral patterning. Nature. 2005;434:229–234. doi: 10.1038/nature03318. [DOI] [PubMed] [Google Scholar]
- Weaver C, Kimelman D. Move it or lose it: axis specification in Xenopus. Development. 2004;131:3491–3499. doi: 10.1242/dev.01284. [DOI] [PubMed] [Google Scholar]
- Wieschaus E, Nüsslein-Volhard C. Looking at embryos. In: Roberts DB, editor. Drosophila: a practical approach. Oxford University Press; USA: 1998. [Google Scholar]
- Wu L, Lengyel JA. Role of caudal in the hindgut specification and gastrulation suggests homology between Drosophila amnioproctodeal invagination and vertebrate blastopore. Development. 1998;125:2433–2442. doi: 10.1242/dev.125.13.2433. [DOI] [PubMed] [Google Scholar]
- Yoffe KB, Manoukian AS, Wilder e.L., Brand AH, Perrimon N. Evidence for engrailed-independent wingless autoregulation in Drosophila. Dev. Biol. 1995;170:636–650. doi: 10.1006/dbio.1995.1243. [DOI] [PubMed] [Google Scholar]
- Yu J-K, Satou Y, Holland ND, Shin-l T, Kohara Y, Satoh N, Bronner-Fraser M, Holland LZ. Axial patterning in cephalochordates and the evolution of the organizer. Nature. 2007;445:613–617. doi: 10.1038/nature05472. [DOI] [PubMed] [Google Scholar]