SUMMARY
Protein tyrosine phosphatase 1B (PTP1B) plays important roles in down-regulation of insulin and leptin signaling and is an established therapeutic target for diabetes and obesity. PTP1B is regulated by reactive oxygen species (ROS) produced in response to various stimuli, including insulin. The reversibly oxidized form of the enzyme (PTP1B-OX) is inactive and undergoes profound conformational changes at the active site. We generated conformation-sensor antibodies, in the form of single chain variable fragments (scFvs), that stabilize PTP1B-OX and thereby inhibit its phosphatase function. Expression of conformation sensor scFvs as intrabodies enhanced insulin-induced tyrosyl phosphorylation of the β-subunit of the insulin receptor and its substrate IRS-1, and increased insulin induced phosphorylation of PKB/AKT. Our data suggest that stabilization of the oxidized, inactive form of PTP1B with appropriate therapeutic molecules may offer a novel paradigm for phosphatase drug development.
INTRODUCTION
In conjunction with the increased prevalence of obesity, diabetes has become a major cause of illness and premature death in most countries, mainly through the increased risk of cardiovascular disease. Type 2 diabetes, which is caused by insulin resistance resulting in loss of normal glucose homeostasis, accounts for >90% of all diabetes. It affects more than 220 million people worldwide and this number is likely to double by 2030 (WHO, 2009). At this time therapeutic options for treating diabetes and obesity are inadequate, and effective approaches to counter the disease are urgently needed.
Considerable interest grew in the potential of PTP1B as a therapeutic target for treating diabetes and obesity following the elucidation of its importance as a regulator of insulin and leptin signaling pathways. Gene targeting studies demonstrated that PTP1B-null mice are healthy, do not develop type 2 diabetes and are resistant to obesity when fed with a high fat diet (Elchebly et al., 1999; Klaman et al., 2000). PTP1B is also an inhibitor of leptin signaling (Cheng et al., 2002; Myers et al., 2001; Zabolotny et al., 2002). Furthermore, depletion of PTP1B expression with antisense oligonucleotides elicits anti-diabetic and anti-obesity effects in rodents (Rondinone et al., 2002; Zinker et al., 2002) as well as human subjects (Brandt, 2010).
PTP1B was the first member of the protein tyrosine phosphatase (PTP) superfamily to be identified and was purified to homogeneity from human placenta as a catalytic domain of 37 kDa (Tonks et al., 1988). Later, it was characterized as a ~50 kDa protein (435 amino acids), consisting of an N-terminal catalytic domain, followed by a C-terminal segment that serves a regulatory function and anchors the protein at the cytoplasmic face of the ER membrane (Tonks, 2003). It contains a signature catalytic motif, (I/V)HCXAGXXR(S/T)G, which is a highly conserved structural feature among PTPs, in which Cys215 and Arg221 are essential for catalytic activity (Tonks, 2003). PTP1B recognizes the activated insulin receptor as a substrate in vitro and in cells (Bandyopadhyay et al., 1997; Salmeen et al., 2000). Crystal structure and kinetic studies illustrate that PTP1B preferentially dephosphorylates the tandem tyrosine residues (pYpY1162/1163) in the activation loop of the β-subunit of insulin receptor (Salmeen et al., 2000). In addition, IRS-1 is also a potential substrate of PTP1B (Goldstein et al., 2000). Hence, PTP1B functions to downregulate insulin signaling.
The activity of PTP1B is regulated at multiple levels. The architecture of the PTP-active site is such that the cysteinyl residue has a pKa of 4.5–5.0 and is predominantly in the thiolate form at neutral pH, unlike the normal pKa of cysteine which is ~8 (Lohse et al., 1997). This property makes the active site cysteine a very good nucleophile, but also renders it prone to oxidation. Several labs have demonstrated that PTPs, including PTP1B, are transiently oxidized and reversibly inactivated by H2O2, and that this is important for induction of an optimal tyrosine phosphorylation response to a variety of physiological stimuli (Lee et al., 2002; Meng et al., 2002; Savitsky and Finkel, 2002). Insulin stimulation of mammalian cells leads to enhanced production of intracellular H2O2, which causes reversible oxidization and inhibition of PTP1B activity (Mahadev et al., 2001; Meng et al., 2004). Nox4, a member of the family of NADPH oxidases, was shown to mediate insulin-stimulated H2O2 generation and to regulate the insulin signaling cascade (Mahadev et al., 2004). Understanding the redox regulation of PTPs in a cellular context has been hampered by the absence of sensitive and robust methods for detecting the oxidized phosphatases and separating them from the background of reduced enzymes. Using antibody phage display we have generated conformation sensor antibodies in the form of single chain variable fragments (scFvs) to the reversibly oxidized form of PTP1B (PTP1B-OX) and applied them to understand the redox regulation of this phosphatase.
Mild oxidation of the active site cysteine of PTP1B produces a sulfenic acid (S-OH) intermediate that undergoes a rapid condensation reaction to produce a 5-atom cyclic sulfenyl-amide species, in which the sulfur atom of the catalytic cysteine is covalently linked to the main-chain nitrogen of the adjacent serine residue (Salmeen et al., 2003; Van Montfort et al., 2003). Formation of this sulfenyl-amide intermediate causes profound conformational changes in the active site that transiently inhibit substrate binding and catalysis. These structural changes, however, are reversible under reducing conditions. In order to maintain reversibility, the active site Cys residue should be oxidized no further than sulfenic acid (S-OH), as higher oxidation to sulfinic (S-O2H) or sulfonic (S-O3H) acid is generally irreversible (Salmeen et al., 2003; Van Montfort et al., 2003). The chemical change at the core of the catalytic site is accompanied by a conformational change in which the PTP loop containing the signature motif, and Tyr46 of the phosphotyrosine loop, which are both normally buried in the structure, now adopt solvent exposed positions. We hypothesized that a conformation-sensor antibody that recognizes the reversibly oxidized form of PTP1B (PTP1B-OX) may stabilize the inactive state and thereby inhibit phosphatase activity.
We have exploited the fact that a mutant form of PTP1B (CASA), in which the catalytic Cys and adjacent Ser residues are mutated to Ala, adopts a stable conformation that is identical to PTP1B-OX. We have used this as an antigen and, in this report, we describe the characterization of conformation-sensor single chain variable fragments (scFvs) that recognize the reversibly oxidized form of PTP1B and stabilize the inactive state to inhibit its reactivation by reducing agent. Using the conformation sensor scFvs as intracellular antibodies or “intrabodies” we have demonstrated that the activity of PTP1B can be attenuated selectively by stabilizing its reversibly oxidized conformation in cells in response to insulin-induced production of ROS. This results in enhanced and sustained signaling in response to insulin, suggesting that this strategy may provide a new approach to the design of PTP-directed inhibitors.
RESULTS
PTP1B-CASA is Structurally Similar to PTP1B-OX
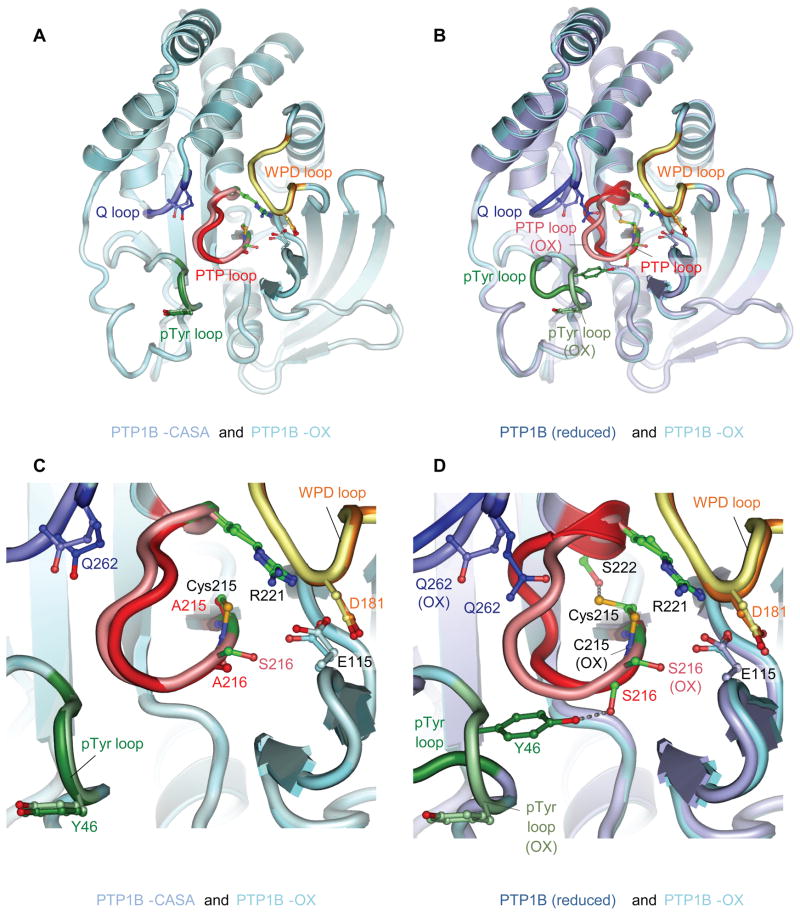
From comparative analysis of the crystal structure of PTP1B in the reduced and oxidized state (cyclic sulfenyl-amide), we hypothesized that mutation of both the catalytic Cys215 and the adjacent Ser216 to Ala would break two critical hydrogen-bonds and thereby may induce a conformational change similar to the effects of oxidation. The crystal structure of the mutated PTP1B (PTP1B-CASA) illustrated that the Tyr46 of the pTyr loop became solvent exposed and the PTP loop was also presented on the protein surface as seen in the oxidized enzyme (Figure 1 and Table S1). PTP1B-CASA was, therefore, used as a stable antigen to generate conformation-specific antibodies to test their potential to recognize specifically the oxidized form of PTP1B.
Figure 1. The Conformation of PTP1B-CASA Resembles that of PTP1B-OX.
(A) View of PTP1B-CASA superimposed onto PTP1B-OX (sulfenyl-amide species) (PDB code: 1OEM) (Salmeen et al., 2003). (B) View of PTP1B-CASA superimposed onto wild type reduced PTP1B (B) (PDB code: 2HNQ) (Barford et al., 1994a). In (A) and (B) the view is onto the catalytic site. (C) and (D) are the same superimpositions as (A) and (B) respectively, and show close-up views of the conformational changes at the catalytic site indicating that the PTP loop and Tyr46 of the pTyr loop become similarly solvent exposed in PTP1B-OX and PTP1B-CASA. In all figures the PTP loop, pTyr loop, WPD loop and Q loop of PTP1B-OX are shown as salmon, light green, yellow and light blue, respectively. The equivalent loops for PTP1B-CASA (A) and (C) and reduced PTP1B (B) and (D) are red, dark green, orange and blue. The Ca-Cb bond of Tyr46 of the reduced pTyr loop is obscured by the superimposition of the oxidized pTyr loop in Figure 1B and 1D. Crystallographic coordinates and structure factors of the PTP1B-CASA mutant have been deposited with the PDB with accession ID codes 3zv2 and r3zv2sf, respectively. Figures are produced using PyMOL.
See also Table S1.
Construction of Antibody Phage Display Library to Target PTP1B-OX
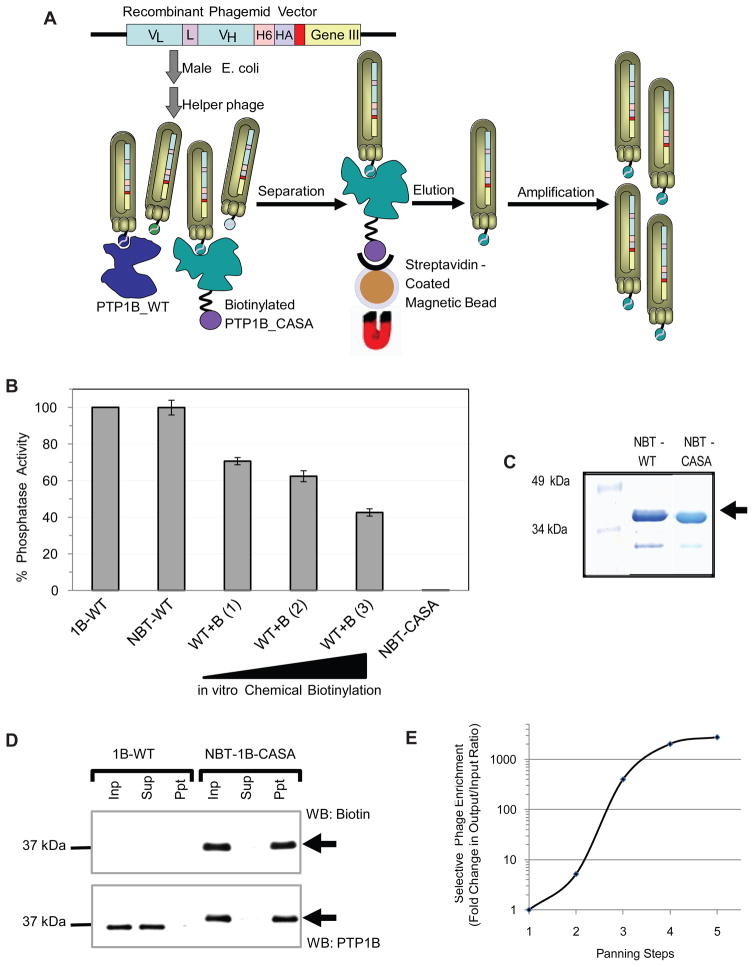
We have generated a phage display library displaying single chain variable fragments (scFvs) fused to phage surface protein pIII (Figure 2A and S1), from antibody genes collected from chickens immunized with PTP1B-CASA. We generated two different scFv constructs, one with a short linker sequence (GQSSRSS) and one with a long linker (GQSSRSSSGGGSSGGGGS) and mixed them together to generate an scFv library of ~2 ×107 total clones. To select for scFvs specific for the PTP1B-CASA mutant we employed a subtractive panning strategy (Figure 2A). Addition of molar excess of reduced wild type PTP1B to the library in solution would favor enrichment of CASA-specific scFvs over scFvs that recognize the common epitopes on both the oxidized and the reduced form of the enzyme. Initially, we generated biotinylated CASA mutant for use in subtractive panning by an in vitro chemical modification. This biotinylation method, however, adds biotin to free amine groups at random and we found that chemical biotinylation caused significant reduction of PTP activity (Figure 2B). To alleviate this problem we took the approach of site specific homogenous biotinylation of PTP1B in E. coli by using a biotinylation tag that we fused to the N-terminus of the phosphatase (Figure 2C). Biotinylation of PTP1B by this method generated a soluble protein and caused no reduction in activity when compared to the phosphatase activity of the non-modified enzyme (Figure 2B). This suggests that the in vivo biotinylation does not add biotin non-specifically to important residues in or around the catalytic site of PTP1B and N-terminally biotinylated PTP1B-CASA (NBT-PTP1B-CASA) was amenable to use for screening for conformation-sensor antibodies (Figure 2D).
Figure 2. Subtractive Panning to Enrich PTP1B-OX-Specific scFvs.
(A) Single chain variable fragments (scFvs) were cloned into the phagemid and transformed to E. coli. Infection with a helper phage (VCSM13) enabled bacteria to make phage particles expressing the scFvs fused to surface protein pIII. Molar excess (up to 50X) of wild type PTP1B was added under reducing condition, followed by biotinylated PTP1B-CASA. Phage-scFvs were then isolated by magnetic beads. The phage-scFvs were eluted, amplified and used for subsequent rounds of panning.
(B) Phosphatase activity of PTP1B (1-321), N-terminally biotinylated (NBT) in vivo or chemically biotinylated in vitro (WT+B) was measured using 32P-RCML as the substrate. Chemical biotinylation was performed at three protein: biotin ratios [(1) = 1:10, (2) = 1:20 and (3) = 1:30]. The activity of biotinylated PTP1B was compared to that of the untagged wild type enzyme (1–321), which was set as 100%. The error bars represent standard deviation from 4 phosphatase assays.
(C) PTP1B (1–321) (WT and CASA), N-terminally biotinylated in vivo in E. coli, was purified in a two-step purification scheme.
(D) N-terminally biotinylated PTP1B-CASA mutant (NBT-CASA) or untagged wild type PTP1B (1B-WT) was incubated with Streptavidin coated magnetic beads and biotinylated proteins (and PTP1B) were detected in input (inp), supernatant (sup) and precipitated (ppt) samples by immunoblot.
(E) Enrichment of phage expressing PTP1B-CASA-specific scFvs was estimated in terms of phage output/input ratio after each round of panning.
See also Figure S1.
We performed five rounds of subtractive panning using NBT-PTP1B-CASA in the presence of molar excess of non-modified PTP1B-WT. Selective enrichment of phage expressing PTP1B-CASA-specific scFvs was observed in terms of increased phage output/input ratio after each round of panning. We observed ~1000-fold enrichment of specific scFv-expressing phage particles after the 4th round of panning (Figure 2E). To isolate individual phage particles expressing functional scFvs we sequenced ~400 phagemid constructs from the enriched library. More than 95% of the sequences were full-length functional scFvs, indicating that the cloning strategy and library display worked. Interestingly, diversity among the functional scFv sequences was found to be ~70% after the 2nd round of panning, whereas after the 4th round of panning it was 20%, suggesting that the library was enriched with specific scFvs after the panning steps. Sequences were sorted into groups on the basis of differences in their hypervariable regions. The selected scFv sequences (Figure S1B) also contain the 6-His and HA tags at the C-terminus.
Isolation of Candidate scFvs Specific for PTP1B-OX
We analyzed systematically individual bacterially purified scFvs from pools of phage enriched after the subtractive panning, by conducting a screen in solution, in order to preserve the conformational integrity of PTP1B-OX. In this screen we employed a phosphatase assay to assess the effect of scFv binding on the activity of PTP1B.
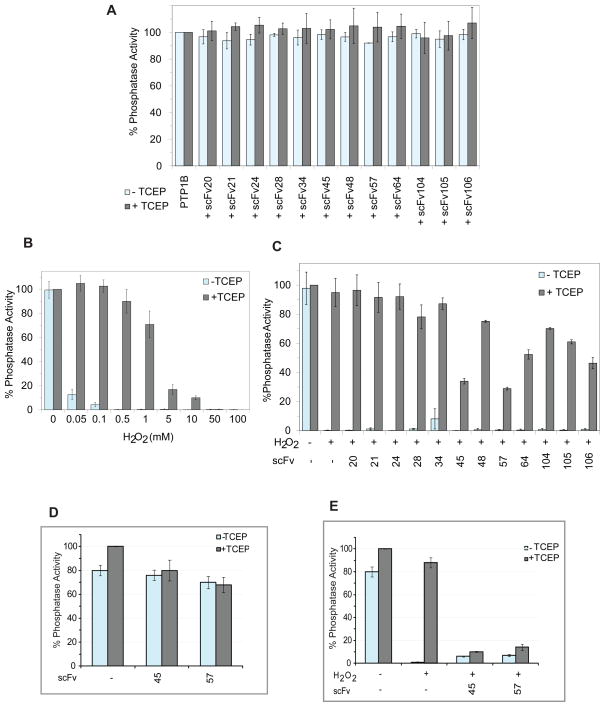
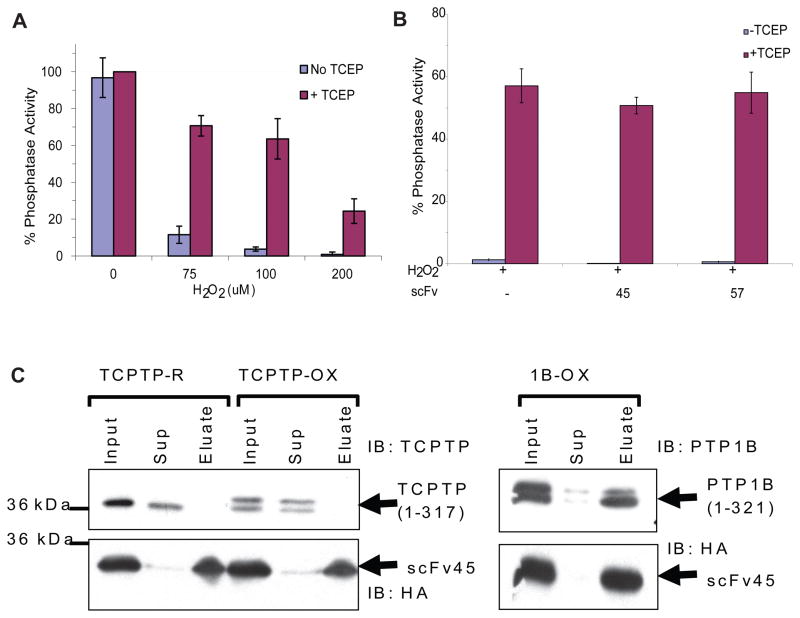
At first, we tested whether any of the scFvs used in this screen had direct inhibitory effect on phosphatase activity of PTP1B under reducing conditions. We observed, as expected, that none of the scFvs, from a randomly selected batch, had any such direct effect on activity (Figure 3A). To screen for PTP1B-OX-specific scFvs, we established conditions under which PTP1B was reversibly oxidized by H2O2 (Figure 3B). The ability of individual scFvs to stabilize the reversibly oxidized, inactive conformation of PTP1B was assessed by the ability of the scFv to inhibit the reactivation of the enzyme by reducing agent. Some of the scFvs demonstrated substantial inhibition of the restoration of PTP1B activity following addition of reducing agent (Figure 3C). In particular, scFvs 45 and 57, inhibited the reactivation by ~70%.
Figure 3. Screening for PTP1B-OX-Specific scFvs.
(A) Purified scFvs (750 nM) were incubated with PTP1B (1–321) (7.5 nM) with or without reducing agent TCEP (5 mM) and phosphatase activity was measured using 100 nM RCML, in which tyrosine was phosphorylated, as the substrate.
(B) PTP1B (1–321) (15 nM) was incubated with increasing concentration of H2O2. Aliquots of H2O2-treated PTP1B (5 nM final) were used in the phosphatase assay following buffer exchange, without or with TCEP, to observe the inactivation and reactivation of the enzyme, respectively.
(C) PTP1B (1–321) (15 nM) was reversibly oxidized by H2O2 (75 μM) and aliquots (7.5 nM) of this reversibly oxidized PTP1B were incubated with purified scFvs (750 nM). Reactivation with or without the presence of scFvs was measured by phosphatase assay in the presence of TCEP.
(D) Direct inhibitory effect of scFvs 45 or 57 (750 nM each) were assessed using PTP1B (1–394) (7.5 nM) in a similar phosphatase assay as in C.
(E) PTP1B (1–394) (15 nM) was reversibly oxidized with H2O2 (75 μM) and reactivation by TCEP with or without scFvs 45 and 57 was observed by phosphatase assay.
Error bars show standard deviation from three phosphatase assays.
See also Figure S2.
To generate the scFvs we utilized a truncated (1–321) form of PTP1B, which comprised the catalytic domain. To investigate further two of the candidate conformation sensor antibodies (scFv45 and 57), we tested their potential to inhibit reactivation of a longer form of recombinant PTP1B (1–394) that contained most of the non-catalytic C-terminal segment that is found in the protein in vivo. Both scFvs 45 and 57 demonstrated no significant direct inhibition of phosphatase activity of the reduced active PTP1B (1–394), whereas, when incubated with the reversibly oxidized form, both of these scFvs caused almost complete inhibition of reactivation (Figure 3D, 3E and S2). These results suggest that these scFvs can sequester oxidized PTP1B in its inactive conformation, thereby preventing reactivation by reducing agents with the effect of inhibiting phosphatase activity.
Candidate scFvs Bound to PTP1B-OX in vitro with High Affinity and Specificity
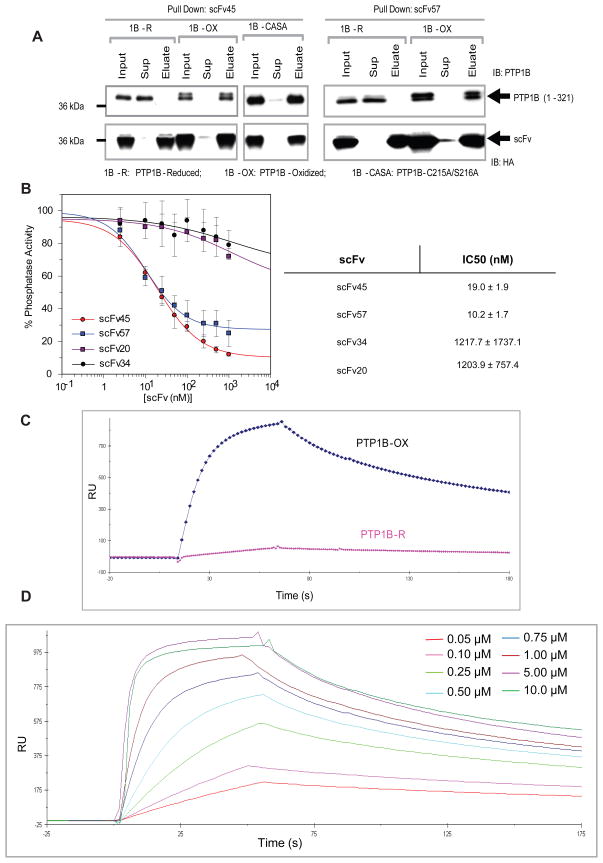
We tested whether the candidate scFvs from the in vitro screen bound to PTP1B-OX. In a Ni-NTA precipitation experiment in vitro, scFv45 and scFv57 interacted with PTP1B-OX or PTP1B-CASA, but not with reduced PTP1B (Figure 4A). A dose response analysis indicated that scFvs 45 and 57 displayed IC50s of 19 nM and 10 nM, respectively, for suppressing the reactivation of PTP1B-OX (Figure 4B). This result is consistent with the effects of scFvs 45 and 57 on the activity of PTP1B (Figure 3). We measured the binding constants for the interaction between scFv45 and PTP1B-OX by Surface Plasmon Resonance (SPR). Under reducing conditions, PTP1B did not show any significant binding to scFv45, further confirming that scFv45 binds specifically to PTP1B-OX but not to PTP1B in its reduced active conformation (Figure 4C). A dose response between increasing concentrations of PTP1B-OX (50 nM to 10 μM) and a fixed amount of scFv45 (500 nM) indicated that scFv45 bound to PTP1B-OX with high affinity (Kd = 46 nM) and the interaction had a slow off rate (Koff = 2.3 × 10−3 s−1) (Figure 4D). The interaction between the PTP1B-CASA mutant and scFv45 was comparable (Kd = 52 nM, Koff = 4.6 × 10−3s−1) (Figure S3A). The interaction between PTP1B-CASA and scFv45 was almost identical with or without the reducing agent, TCEP (Figure S3B), showing that the presence of the reducing agent does not affect PTP1B-scFv45 binding per se, rather it is the redox-dependent changes in conformation of the enzyme that are responsible for the specific interaction.
Figure 4. scFv45 binds Specifically to PTP1B-OX in vitro.
(A) PTP1B (1–321), reduced (1B-R), reversibly oxidized with H2O2 (1B-OX) or PTP1B-CASA mutant, were incubated with purified scFvs 45 and 57 and the protein complexes were precipitated with Ni-NTA agarose beads. Equivalent amounts (2.5 ng of PTP1B) of input, supernatant and eluate were subjected to immunoblot analysis.
(B) Increasing concentrations of scFvs 45, 57, 34, and 20 (2.5 nM to 1 mM) were incubated with PTP1B-OX and phosphatase activity was measured after adding TCEP (5 mM). IC50 values for the inhibition of PTP1B reduction and reactivation were determined using the Grafit software.
(C) Comparative SPR sensogram shows the interaction between scFv45 (500 nM) and either PTP1B-OX (1μM) or reduced PTP1B (1μM with 2 mM TCEP) (PTP1B-R).
(D) Different concentrations of PTP1B-OX were injected on immobilized scFv45 (500 nM) on Ni-NTA sensor chip using BIACORE 2000. The kinetic constants for binding were calculated with the BIAevaluation 3.1 software.
See also Figure S3.
TCPTP is the closest relative of PTP1B among the classical PTP family of enzymes and displays ~75% sequence identity with the catalytic domain of PTP1B (Iversen et al., 2002). Because of their close structural similarity (Figure S4), the potential for agents that target PTP1B to display overlapping specificity towards TCPTP has been a concern. It was shown previously that TCPTP is reversibly oxidized in mammalian cells, together with PTP1B, following insulin stimulation (Meng et al., 2004). Therefore, we tested the specificity of scFv45 for PTP1B-OX over TCPTP. Bacterially purified recombinant TCPTP was incubated with increasing concentrations of H2O2 and reactivation by reducing agent was determined by measuring phosphatase activity (Figure 5A). Incubation with scFv45, or scFv57, exerted no obvious effect on the reduction and reactivation of oxidized TCPTP (Figure 5B). In a pull down experiment with anti-HA antibody conjugated to agarose beads, we demonstrated that in-vitro oxidized TCPTP also did not bind to scFv45 (Figure 5C). These results demonstrate that conformation specific scFvs to PTP1B-OX did not bind the oxidized inactive form of TCPTP, highlighting their selectivity in recognition of specific epitope(s) on PTP1B-OX.
Figure 5. scFv45 Displays Specificity for PTP1B-OX over TCPTP-OX.
(A) Recombinant TCPTP (1–317) was reversibly oxidized by H2O2 and reactivated by TCEP (5 mM). Phosphatase assay was determined using 100 nM 32P-RCML and 5 nM of enzyme from each sample.
(B) Purified scFvs (750 nM) were incubated with oxidized TCPTP (7.5 nM) and phosphatase assay was determined in presence of TCEP (5 mM). [Error bars in A and B show standard deviation from six (A) and three (B) phosphatase assays].
(C) TCPTP was reversibly oxidized with H2O2 and binding with scFv45 was assessed by anti-HA-Agarose pull down. Equivalent amounts (4 ng of TCPTP) of input, supernatant and precipitate were analyzed by immunoblot. As a control, a parallel pull down was performed with reversibly oxidized PTP1B and scFv45.
See also Figure S4.
scFv45 Bound to PTP1B-OX in Mammalian Cells in Response to Insulin and H2O2
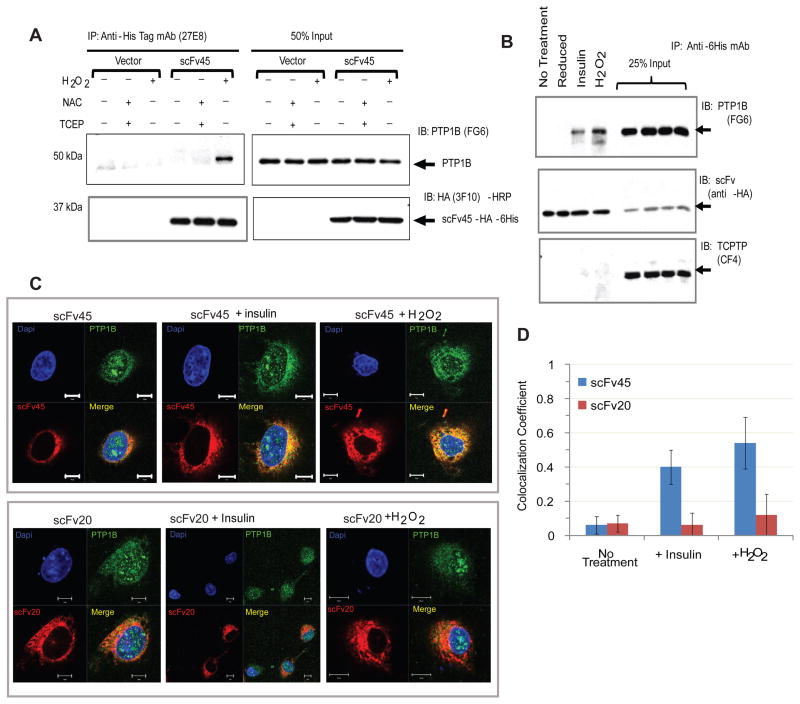
A major advantage of using phage display to generate PTP1B-OX specific antibodies is that the selected scFvs can be expressed inside mammalian cells as functional intracellular antibodies or “intrabodies”. We observed robust and stable expression of the PTP1B-OX conformation-sensor scFv45 in 293T cells (Figure 6). We generated a “mini” mammalian expression library by cloning additional individual scFvs into the pCDNA3.2/V5-GWD-TOPO vector from their corresponding phagemid constructs. Using a Ni-NTA pull down assay with equivalent amounts of scFv-transfected, H2O2-treated lysates, we identified more candidate scFvs that bound to endogenous PTP1B-OX and did not display any binding to PTP1B under reducing conditions (Figure S5). Interestingly, consistent with selective binding to PTP1B-OX in vitro, scFv45 expressed as an intrabody also bound to PTP1B-OX. The negative control scFv20, which did not stabilize and inhibit reactivation of PTP1B-OX in vitro (Figure 3), showed no binding to PTP1B-OX in cells. This indicates that PTP1B undergoes similar oxidation-induced conformational change in mammalian cells when treated with H2O2 and scFvs that bind PTP1B-OX in vitro were also functional when expressed in 293T cells.
Figure 6. scFv45 Bound to PTP1B-OX in H2O2 and Insulin-Treated 293T Cells.
(A) scFv45-expressing 293T cells were treated with H2O2 (1 mM) or NAC (20 mM) and cell lysates were prepared with or without TCEP (2 mM). From 1 mg of cell lysate, scFv45 was immunoprecipitated and binding to PTP1B, under oxidized or reduced conditions, was detected by immunoblot.
(B) In a similar experiment, 293T cells expressing scFv45 were treated with insulin (25 nM) or H2O2 (1 mM) or NAC (20 mM) and intrabody was immunoprecipitated from 1 mg of lysate. The membrane was stripped and re-probed with anti-TCPTP mouse monoclonal antibody (CF4).
(C) Cos1 cells transfected with scFv45 or scFv20 were treated with insulin (25 nM), or H2O2 (1mM) or left untreated. Fixed cells were processed for immunofluorescence and visualized by confocal microscopy with oil immersion (63X).
(D) Colocalization of PTP1B and scFv45 was analyzed by Zeiss (LSM 710) Colocalization Viewer Software. The numeric range for colocalization is set as 0 – 1, where “0” indicates no colocalization and “1” indicates colocalization of all pixels. Error bars indicate standard deviation from colocalization analysis of 25 individual cells.
See also Figure S5.
Furthermore, we demonstrated that scFv45 efficiently immunoprecipitated PTP1B-OX from cells treated with H2O2 but showed little or no interaction with PTP1B when cells were treated under reducing conditions (Figure 6A). These results are consistent with the earlier observation in vitro that scFv45 recognized distinct conformational epitopes formed by oxidation of PTP1B by H2O2. Stimulation of cells with insulin causes rapid and transient oxidation and inhibition of PTP1B, which facilitates increased phosphorylation of the insulin receptor-β subunit (Mahadev et al., 2001; Meng et al., 2004). We observed that scFv45 immunoprecipitated PTP1B from lysates of transfected 293T cells that were treated with insulin (Figure 6B). In cells that were untreated or were processed under reducing conditions, such interaction was absent. We demonstrated that scFv45 bound specifically to PTP1B-OX but not to TCPTP-OX in vitro (Figure 5). In mammalian cells, scFv45 displayed similar specificity towards endogenous PTP1B-OX, and did not interact with endogenous TCPTP following either insulin stimulation or H2O2 treatment (Figure 6B).
In order to visualize the interaction between PTP1B-OX and scFv45 in mammalian cells, we used immunofluorescence to examine whether there was co-localization of PTP1B and scFv45 in Cos1 cells following insulin stimulation or H2O2 treatment. Significant co-localization between PTP1B and scFv45 was observed, whereas such co-localization was absent between the negative control scFv20 and PTP1B (Figure 6C and 6D).
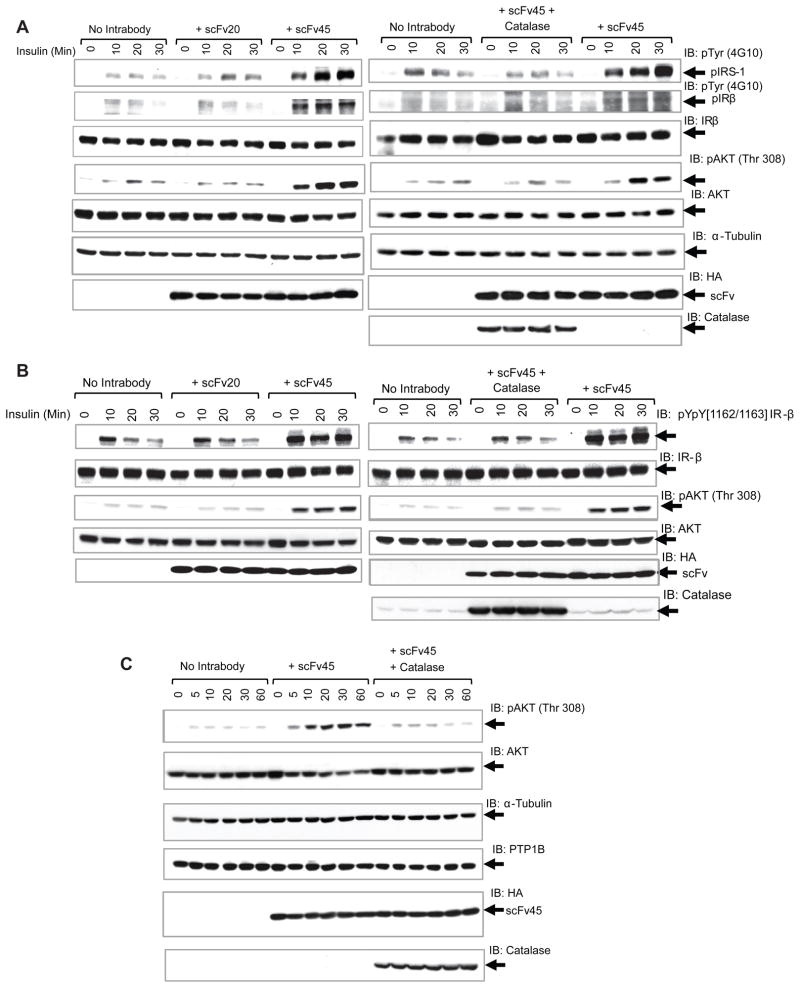
scFv45 Enhanced and Prolonged Tyrosine Phosphorylation in 293T Cells in Response to Insulin in a ROS-dependent Manner
We demonstrated that the conformation sensor antibody scFv45 sequestered reversibly oxidized PTP1B and prevented reactivation of the enzyme. Therefore, we tested whether such sequestration of a negative regulator of insulin receptor kinase had an impact on downstream signaling in mammalian cells. When 293T cells in which scFv45 was overexpressed were stimulated with insulin, the insulin receptor β subunit (IRβ) and insulin receptor substrate-1 (IRS-1) displayed enhanced and sustained tyrosine phosphorylation in comparison to cells without the ectopically expressed intrabody or with the negative control intrabody scFv20 (Figure 7A).
Figure 7. scFv45 Enhanced Insulin Signaling in 293T cells in a Redox-Dependent Manner.
In panel A, B and C, cells transiently expressing scFv45 or scFv20 or cotransfected with scFv45 and catalase were treated with insulin (25 nM) for the indicated times and total cell lysate (60 μg in A and C and 80 μg in B) was subjected to immunoblot analysis of the indicated proteins.
See also Figure S6 and S7.
Similar stimulatory effects of scFv45 were observed on insulin and EGF-induced signaling in HeLa cells (Figure S7). Interestingly, when catalase, the enzyme that catalyzes the decomposition of H2O2 to water and oxygen, was co-overexpressed in the cytosol together with scFv45 or the negative control scFv20, the enhanced tyrosine phosphorylation of both IRβ and IRS-1 was diminished (Figure 7A and S6B).
Furthermore, we used a phospho-site-specific antibody to focus on the tandem residues (Y1162/Y1163) of the IRβ activation loop, which have been identified as substrates of PTP1B (Salmeen et al., 2000). Again, expression of scFv45 led to enhanced and sustained insulin-induced phosphorylation of these residues and this effect was attenuated by coexpression with catalase (Figure 7B). In contrast, any effects of scFv45 on the phosphorylation of Y1328, from the C-terminus of IRβ, were much less pronounced, suggesting preferential recognition of individual phosphorylation sites in IRβ by PTP1B (Figure S6C).
scFv45 Caused Increased AKT Phosphorylation in response to Insulin
In order to test for effects of the intrabody on downstream signaling, we used a phospho-specific antibody that recognizes phosphorylation of T308 in PKB/AKT as a read-out. Cells overexpressing scFv45 displayed enhanced and sustained PKB/AKT phosphorylation when treated with insulin (Figure 7A). This effect was maintained over a time course of 60 minutes (Figure 7C). When catalase was overexpressed together with scFv45, the stimulatory effects of the intrabody on PKB/AKT activation were ablated (Figure 7C). Furthermore, expression of the negative control scFv20 had no effect on PKB/AKT activation (Figure S6A). We found no change in PTP1B expression in any of these conditions, indicating that changes in the levels of PTP1B do not underlie the enhancement in signaling. Overall, our data are consistent with a model in which scFv45 binds and stabilizes endogenous PTP1B-OX, thereby effectively attenuating PTP1B activity and removing its inhibitory effect, with resulting enhancement of insulin signaling.
DISCUSSION
Dysfunctional insulin signaling results in insulin resistance, which is ultimately associated with metabolic syndromes, including type 2 diabetes and obesity (Saltiel and Kahn, 2001). Insulin induces activation of the insulin-receptor kinase through autophosphorylation (Saltiel and Pessin, 2002). Recruitment of IRS-1 to the receptor triggers activation of phosphatidylinositol 3-kinases (PI3K) and the stimulation of downstream effectors, such as phosphatidylinositol-dependent kinase 1 (PDK1) and PKB/AKT, leading to translocation of glucose transporter 4 (GLUT4) and glucose uptake, and inactivation of glycogen-synthase kinase 3 (GSK3) (Bryant et al., 2002). PTP1B is an important negative regulator of signaling; it dephosphorylates the tandem tyrosine residues (pY1162/pY1163) of activated IRβ (Salmeen et al., 2000), and IRS-1 (Goldstein et al., 2000), exerting a major influence on the duration and amplitude of the cellular response to insulin. Consequently, regulation of PTP1B activity would be expected to facilitate fine-tuning of insulin-induced signaling. Our data highlight the importance of reversible oxidation of PTP1B as one such regulatory mechanism and validate stabilization of the inactive phosphatase conformation as a potential therapeutic strategy.
A feature of the microbicidal function of phagocytes is the reduction of molecular oxygen by a nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) enzyme system, which generates superoxide (O2·−) that is converted to H2O2 (Bokoch and Zhao, 2006). The NOX enzymes are multi-protein complexes, the activity of which is tightly controlled. Non-phagocytic cells are now also known to contain similar NOX enzymes, which are capable of generating ROS, but at lower levels than associated with the killing of invading micro-organisms (Lambeth, 2004). Controlled production of reactive oxygen species (ROS), such as H2O2, has been observed in non-phagocytic cells in response to a number of ligands that act through receptor tyrosine kinases (Bae et al., 1997; Mahadev et al., 2001; Meng et al., 2004; Sundaresan et al., 1995). PTPs have been identified as direct targets of ROS, the presence of an essential low pKa catalytic cysteine residue rendering them exquisitely sensitive to oxidation and inactivation (Rhee, 2006). In particular, PTP1B is transiently inactivated by reversible oxidation of the active site cysteine following insulin-induced generation of H2O2 in mammalian cells (Mahadev et al., 2001; Meng et al., 2004).
PTP1B is targeted to the cytoplasmic face of membranes of the endoplasmic reticulum where it functions in a “dephosphorylation compartment” in which it acts to terminate signaling from receptor PTKs that have undergone endocytosis following ligand stimulation (Eden et al., 2010; Haj et al., 2002). This ER localization exposes PTP1B essentially to the entire cytoplasm and there have been reports of it exerting its effects from the perinuclear compartment (Romsicki et al., 2004) all the way to interactions with substrate at the plasma membrane (Nievergall et al., 2010). It has been suggested that PTP1B exists in spatially distinct subpopulations on the surface of the ER, some of which are associated with a reversible low-activity state that may define different signaling functions (Yudushkin et al., 2007). Reversible oxidation would be one mechanism to achieve this compartmentalization. Interestingly, NOX4, which is responsible for oxidation of PTP1B in response to insulin (Mahadev et al., 2004), was reported to co-localize with PTP1B on ER membranes (Martyn et al., 2006) and this co-localization was essential for signal-induced oxidation and inactivation of PTP1B (Chen et al., 2008). The reversibility of oxidation and inactivation is essential for this covalent modification to represent a means for regulation of tyrosine phosphorylation-dependent signaling. Our original structure of oxidized PTP1B suggests a molecular mechanism by which such reversibility may be achieved.
We observed that oxidation induced profound conformational changes in PTP1B, in which the active site cleft opens up both to expose critical catalytic residues and to present new binding surfaces on the protein (Salmeen et al., 2003). Upon soaking PTP1B crystals with stoichiometric quantities of H2O2, an unstable sulfenic acid (Cys–S-OH) is generated initially, which then undergoes a rapid condensation reaction to produce a cyclic sulfenyl-amide species (Salmeen et al., 2003). This modification, previously undetected in proteins, was generated when a covalent bond formed between the side chain S atom of Cys215 and the main chain N atom of Ser216, and resulted in disruption of critical H bonds that maintain the stability of the active site cleft. Independently, Van Montfort et al. reported the formation of the same structure when PTP1B crystals were soaked with 2-phenyl-isoxazolidine-3,5-dione, which is a peroxide generator and, thereby, an inhibitor of PTP1B (Tjernberg et al., 2004; Van Montfort et al., 2003). Incubation of crystals of the sulfenyl-amide form of PTP1B with reducing agent led to a quantitative reduction of the enzyme and a return to the catalytically active conformation. Thus, formation of this cyclic sulfenyl-amide intermediate may protect the enzyme from irreversible inactivation by higher-order oxidation and, by presenting the signature motif that contains the active site Cys residue on the surface of the protein, may facilitate reactivation by reducing agents in the cell (Tonks, 2005). We exploited the new binding surfaces that are unique to the oxidized form of the enzyme to generate scFv antibodies that were selective for the oxidized conformation of PTP1B in vitro. Our data illustrate that these scFvs also bound to PTP1B in mammalian cells in response to insulin-mediated ROS production or treatment with exogenous H2O2. These results indicate that the reversible redox modification involving formation of a cyclic sulfenyl-amide also occurs in cells, thereby removing concerns that the structure was an artifact of crystallization and focusing attention on its potential physiological importance.
Our study illustrates the importance of endogenously produced H2O2 and concomitant reversible inhibition of PTP1B activity in augmenting insulin signaling. We demonstrated that the conformation-sensor antibody scFv45 bound to the oxidized conformation of PTP1B that is produced following treatment with either insulin or H2O2, but no interaction was observed in untreated cells. Furthermore, scFv45 enhanced and prolonged tyrosine phosphorylation of both IRβ and IRS-1, and this effect was transduced downstream to yield a sustained increase in PKB/AKT phosphorylation and activation. Interestingly, scFv45 did not cause changes in basal tyrosine phosphorylation, or downstream signaling, indicating that it does not function as an insulin-mimetic to promote basal signaling in the absence of hormone, rather it functions as an insulin-sensitizer. This enhancement of insulin-induced signaling was attenuated when scFv45 was co-expressed with catalase, which has been shown previously to degrade H2O2 in cells (Irani et al., 1997), indicating that H2O2 production is required for the antibodies to exert their stimulatory effects on signaling. Therefore, our data suggest that insulin stimulates a rise in intracellular H2O2 that transiently oxidizes and inactivates PTP1B, tipping the delicate balance between PTP and PTK activity in favor of the kinase, to promote signaling. Expression of scFv45 stabilizes the oxidized conformation of PTP1B, delaying its reduction and reactivation, and further shifting the balance towards tyrosine phosphorylation, thereby promoting enhanced and sustained insulin signaling.
Antibody phage display is a powerful technique that allows target antigens to be preserved in their native state and, therefore, has the potential to yield antibodies that can recognize specific conformations of a target protein (Marasco, 1997). Furthermore, it permits the resulting antibodies to be expressed in cells as intrabodies, such as the scFvs used in this study, in which the variable heavy and light chains are linked directly, without the need for disulphide bonds. Therefore, these intrabodies are not only stable and functional in the overall reducing environment of the cell, but also the ability to express them overcomes the problems associated with delivering whole antibodies across the plasma membrane (Cardinale and Biocca, 2008). One example is the generation of intrabodies that detect specifically GTP-bound Rab6 in the Golgi and with which the dynamics of Rab6-GTP-positive transport intermediates could be followed by immunofluorescence (Nizak et al., 2003). The screening procedures in phage display permit great flexibility in selection conditions. In our study, we generated a library from a donor immunized with the mutant form of PTP1B that is locked in the oxidized conformation. We were able to enrich for conformation-sensor antibodies that recognized this structure specifically by screening with oxidized PTP1B in the presence of a molar excess of the reduced enzyme. Not only did the intrabodies recognize and stabilize the inactive conformation induced by a unique post-translational inhibitory modification on PTP1B, thereby potentiating insulin signaling, but also they did so with remarkable specificity. There are a number of gene duplications that occurred in the evolution of the PTP family. One such event generated two closely related phosphatases, PTP1B and TCPTP, and one spliced isoform of TCPTP is localized to the ER in a similar manner to PTP1B (Iversen et al., 2002). In fact, insulin induces the oxidation of TCPTP (Meng et al., 2004) and there are data suggesting distinct, yet complementary, roles for PTP1B and TCPTP in regulation of insulin signaling (Galic et al., 2005). Nevertheless, the scFvs generated in this study did not recognize TCPTP, highlighting their potential as specific probes of PTP1B function. Although TCPTP displays ~75% sequence identity to PTP1B in its core catalytic domain, rising to ~85% when conservative substitutions are considered, there are differences in surface residues between the two PTPs (Figure S4) that may contribute to this specificity.
Quite apart from their significance as reagents, these antibodies may influence strategies for development of PTP1B-directed therapeutics. The phenotype of the knockout mouse, together with structural and biochemical data from various groups, has established PTP1B as a key regulator of insulin and leptin signaling (Tonks, 2003). Consequently, it became a highly prized target in the pharmaceutical industry for therapeutic intervention in diabetes and obesity. Although there have been major programs in industry focused on developing small molecule inhibitors of PTP1B, these efforts have been frustrated by technical challenges arising from the chemical properties of the PTP active site (Zhang and Zhang, 2007). The susceptibility of PTPs to oxidation causes problems in high throughput screens. In addition, the tendency of potent active site-directed inhibitors to be highly charged, such as non-hydrolyzable pTyr mimetics, presents problems with respect to oral bioavailability that limit drug development potential. Nevertheless, considering the importance of PTP1B as a therapeutic target, there is a compelling need to explore innovative ideas and schemes to target the enzyme for generating novel, potent and selective inhibitors. The profound conformational change at the active site induced by oxidation provides new sites for developing chemical scaffolds or small molecule inhibitors that could recognize the reversibly oxidized conformation of PTP1B and lock it in the inactive state. Stabilization of the inactive PTP1B-OX conformation may potentiate insulin signaling in a similar manner to inhibiting the catalytically active form of the enzyme, analogous to stabilization of the inactive form of p210 BCR-ABL by Gleevec/Imatinib (Schindler et al., 2000). Also, if one assumes that in responding to insulin the cell targets for oxidation the pool of PTP1B that is important for regulation of the signaling response, then this strategy will also target that pool specifically, possibly also reducing complications of side effects that may accompany inhibition of the native enzyme as a whole. Furthermore, most potent active site-directed inhibitors of PTP1B show some degree of inhibition of TCPTP as well (Johnson et al., 2002). TCPTP is essential for normal hematopoiesis and TCPTP-null mice die as a result of hematopoietic defects within weeks of birth (You-Ten et al., 1997). Consequently, there was concern in drug discovery efforts to minimize the effects of any PTP1B inhibitors on TCPTP. The properties of our scFv intrabodies alleviate this concern. Problems of delivery to the appropriate tissues represent serious hurdles to the use of such intrabodies as therapeutic agents. Nevertheless, our data indicate that if it is possible to stabilize the oxidized, inactive form of PTP1B with an appropriate small molecule that mimics the effects of these antibodies, then this could provide a new strategy for PTP-directed drug development that would circumvent the difficulties that are faced when targeting the PTP active site with highly charged inhibitors.
MATERIALS AND METHODS
Construction of scFv Phage Display Library
Chickens were immunized with purified PTP1B-CASA. Total RNA was isolated from the spleen and bone marrow of the immunized animals. First-strand cDNA was synthesized from the total RNA and used for PCR amplification of the VL and VH genes, which were combined to form a full-length scFv construct.
Screening Individual scFvs as Conformation-Sensor Antibodies to PTP-OX
The scFv library was mixed with 10–50 fold molar excess of wild type PTP1B over the biotinylated PTP1B-CASA under reducing conditions. Phage-scFvs bound to biotinylated PTP1B-CASA were captured by streptavidin coated magnetic beads, eluted under acidic conditions, neutralized and amplified. A total of five rounds of panning were performed accordingly. Individual scFvs were expressed without the pIII fusion component in a nonsuppressor E. coli (TOP10F′) and purified with Ni-NTA. Recombinant PTP1B was reversibly oxidized and inactivated with H2O2, then incubated with purified scFvs. The effect of individual scFvs on stabilizing the reversibly oxidized conformation was assessed by phosphatase assay under reducing conditions.
Effect of PTP1B-OX-specific Intrabodies on Insulin Signaling
Intrabody constructs for scFv45 or scFv20 were transfected in 293T cells, which were stimulated with insulin for various times. Total proteins in the cell lysates were separated by SDS-PAGE and global tyrosyl phosphorylation was detected by anti-phosphotyrosine antibody 4G10. To detect specific tyrosyl phosphorylation of the IR-β subunit, we used rabbit polyclonal anti-insulin receptor [pYpY1162/1163] phospho-specific antibody. To test the effect of suppressing H2O2 levels on intrabody function, catalase was ectopically co-expressed with scFv45. Catalase expression was detected in the cell samples with anti-catalase rabbit polyclonal antibody. Phosphorylation of the AKT activation loop at residue Threonine 308 (T308) was observed with phospho-specific antibody.
Details of methods and materials are described in the Extended Experimental Procedures in the Supplemental Information section.
Supplementary Material
Acknowledgments
This work was supported by NIH grants CA53840 and GM55989 to NKT. The work in DB’s lab was supported by a grant from Cancer Research UK.
Footnotes
Supplemental Information includes Supplemental Data (one Table and seven Figures with legends), Extended Experimental Procedures, and Supplemental References.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide - Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- Bandyopadhyay D, Kusari A, Kenner KA, Liu F, Chernoff J, Gustafson TA, Kusari J. Protein-tyrosine phosphatase 1B complexes with the insulin receptor in vivo and is tyrosine-phosphorylated in the presence of insulin. J Biol Chem. 1997;272:1639–1645. doi: 10.1074/jbc.272.3.1639. [DOI] [PubMed] [Google Scholar]
- Barford D, Flint AJ, Tonks NK. Crystal structure of human protein tyrosine phosphatase 1B. Science. 1994a;263:1397–1404. [PubMed] [Google Scholar]
- Barford D, Keller JC, Flint AJ, Tonks NK. Purification and crystallization of the catalytic domain of human protein tyrosine phosphatase 1B expressed in Escherichia coli. J Mol Biol. 1994b;239:726–730. doi: 10.1006/jmbi.1994.1409. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Zhao T. Regulation of the phagocyte NADPH oxidase by Rac GTPase. Antioxid Redox Signal. 2006;8:1533–1548. doi: 10.1089/ars.2006.8.1533. [DOI] [PubMed] [Google Scholar]
- Brandt TA, Crooke ST, Ackermann EJ, Xia X, Morgan ES, Liu Q, Geary RS, Bhanot S. ISIS 113715, a Novel PTP-1B Antisense Inhibitor, Improves Glycemic Control and Dyslipidemia and Increases Adiponectin Levels in T2DM Subjects Uncontrolled on Stable Sulfonylurea Therapy. Paper presented at: American Diabetes Association; Carlsbad, CA. 2010. [Google Scholar]
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- Cardinale A, Biocca S. The potential of intracellular antibodies for therapeutic targeting of protein-misfolding diseases. Trends Mol Med. 2008;14:373–380. doi: 10.1016/j.molmed.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell. 2002;2:497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- Eden ER, White IJ, Tsapara A, Futter CE. Membrane contacts between endosomes and ER provide sites for PTP1B-epidermal growth factor receptor interaction. Nat Cell Biol. 2010;12:267–272. doi: 10.1038/ncb2026. [DOI] [PubMed] [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Galic S, Hauser C, Kahn BB, Haj FG, Neel BG, Tonks NK, Tiganis T. Coordinated regulation of insulin signaling by the protein tyrosine phosphatases PTP1B and TCPTP. Mol Cell Biol. 2005;25:819–829. doi: 10.1128/MCB.25.2.819-829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BJ, Bittner-Kowalczyk A, White MF, Harbeck M. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. J Biol Chem. 2000;275:4283–4289. doi: 10.1074/jbc.275.6.4283. [DOI] [PubMed] [Google Scholar]
- Haj FG, Verveer PJ, Squire A, Neel BG, Bastiaens PI. Imaging sites of receptor dephosphorylation by PTP1B on the surface of the endoplasmic reticulum. Science. 2002;295:1708–1711. doi: 10.1126/science.1067566. [DOI] [PubMed] [Google Scholar]
- Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- Iversen LF, Moller KB, Pedersen AK, Peters GH, Petersen AS, Andersen HS, Branner S, Mortensen SB, Moller NP. Structure determination of T cell protein-tyrosine phosphatase. J Biol Chem. 2002;277:19982–19990. doi: 10.1074/jbc.M200567200. [DOI] [PubMed] [Google Scholar]
- Johnson TO, Ermolieff J, Jirousek MR. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat Rev Drug Discov. 2002;1:696–709. doi: 10.1038/nrd895. [DOI] [PubMed] [Google Scholar]
- Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD. Nox enzymes and the biology of reactive oxygen. Nature Reviews Immunology. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- Lohse DL, Denu JM, Santoro N, Dixon JE. Roles of aspartic acid 181 and serine-222 in intermediate formation and hydrolysis of the mammalian protein-tyrosine-phosphatase PTP1. Biochemistry. 1997;36:4568–4575. doi: 10.1021/bi963094r. [DOI] [PubMed] [Google Scholar]
- Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1B in vivo and enhances the early insulin action cascade. J Biol Chem. 2001;276:21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- Mahadev K, Motoshima H, Wu XD, Ruddy JM, Arnold RS, Cheng GJ, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco WA. Intrabodies: turning the humoral immune system outside in for intracellular immunization. Gene Ther. 1997;4:11–15. doi: 10.1038/sj.gt.3300346. [DOI] [PubMed] [Google Scholar]
- Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Meng TC, Buckley DA, Galic S, Tiganis T, Tonks NK. Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J Biol Chem. 2004;279:37716–37725. doi: 10.1074/jbc.M404606200. [DOI] [PubMed] [Google Scholar]
- Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- Minor W, Cymborowski M, Otwinowski Z, Chruszcz M. HKL-3000: the integration of data reduction and structure solution--from diffraction images to an initial model in minutes. Acta Crystallogr D Biol Crystallogr. 2006;62:859–866. doi: 10.1107/S0907444906019949. [DOI] [PubMed] [Google Scholar]
- Myers MP, Andersen JN, Cheng A, Tremblay ML, Horvath CM, Parisien JP, Salmeen A, Barford D, Tonks NK. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem. 2001;276:47771–47774. doi: 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- Nievergall E, Janes PW, Stegmayer C, Vail ME, Haj FG, Teng SW, Neel BG, Bastiaens PI, Lackmann M. PTP1B regulates Eph receptor function and trafficking. J Cell Biol. 2010;191:1189–1203. doi: 10.1083/jcb.201005035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizak C, Monier S, del Nery E, Moutel S, Goud B, Perez F. Recombinant antibodies to the small GTPase Rab6 as conformation sensors. Science. 2003;300:984–987. doi: 10.1126/science.1083911. [DOI] [PubMed] [Google Scholar]
- Rondinone CM, Trevillyan JM, Clampit J, Gum RJ, Berg C, Kroeger P, Frost L, Zinker BA, Reilly R, Ulrich R, et al. Protein tyrosine phosphatase 1B reduction regulates adiposity and expression of genes involved in lipogenesis. Diabetes. 2002;51:2405–2411. doi: 10.2337/diabetes.51.8.2405. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Romsicki Y, Reece M, Gauthier JY, Asante-Appiah E, Kennedy BP. Protein tyrosine phosphatase-1B dephosphorylation of the insulin receptor occurs in a perinuclear endosome compartment in human embryonic kidney 293 cells. J Biol Chem. 2004;279:12868–12875. doi: 10.1074/jbc.M309600200. [DOI] [PubMed] [Google Scholar]
- Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- Salmeen A, Andersen JN, Myers MP, Tonks NK, Barford D. Molecular basis for the dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Mol Cell. 2000;6:1401–1412. doi: 10.1016/s1097-2765(00)00137-4. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Pessin JE. Insulin signaling pathways in time and space. Trends Cell Biol. 2002;12:65–71. doi: 10.1016/s0962-8924(01)02207-3. [DOI] [PubMed] [Google Scholar]
- Savitsky PA, Finkel T. Redox regulation of Cdc25C. J Biol Chem. 2002;277:20535–20540. doi: 10.1074/jbc.M201589200. [DOI] [PubMed] [Google Scholar]
- Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of Abelson tyrosine kinase. Science. 2000;289:1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- Tjernberg A, Hallen D, Schultz J, James S, Benkestock K, Bystrom S, Weigelt J. Mechanism of action of pyridazine analogues on protein tyrosine phosphatase 1B (PTP1B) Bioorg Med Chem Lett. 2004;14:891–895. doi: 10.1016/j.bmcl.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Tonks NK. PTP1B: from the sidelines to the front lines! FEBS Lett. 2003;546:140–148. doi: 10.1016/s0014-5793(03)00603-3. [DOI] [PubMed] [Google Scholar]
- Tonks NK. Redox redux: Revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Tonks NK, Diltz CD, Fischer EH. Purification of the major protein-tyrosine-phosphatases of human placenta. J Biol Chem. 1988;263:6722–6730. [PubMed] [Google Scholar]
- Van Montfort RLM, Congreve M, Tisi D, Carr R, Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- WHO. Diabetes Fact Sheet. World Health Organization; 2009. [Google Scholar]
- You-Ten KE, Muise ES, Itie A, Michaliszyn E, Wagner J, Jothy S, Lapp WS, Tremblay ML. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J Exp Med. 1997;186:683–693. doi: 10.1084/jem.186.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudushkin IA, Schleifenbaum A, Kinkhabwala A, Neel BG, Schultz C, Bastiaens PI. Live-cell imaging of enzyme-substrate interaction reveals spatial regulation of PTP1B. Science. 2007;315:115–119. doi: 10.1126/science.1134966. [DOI] [PubMed] [Google Scholar]
- Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, et al. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2:489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhang ZY. PTP1B as a drug target: recent developments in PTP1B inhibitor discovery. Drug Discov Today. 2007;12:373–381. doi: 10.1016/j.drudis.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Zinker BA, Rondinone CM, Trevillyan JM, Gum RJ, Clampit JE, Waring JF, Xie N, Wilcox D, Jacobson P, Frost L, et al. PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. Proc Natl Acad Sci U S A. 2002;99:11357–11362. doi: 10.1073/pnas.142298199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.