Abstract
Objectives
Our aim was to examine the prevalence of arrhythmias and identify independent associations of time to arrhythmia development.
Background
Since introduction of the Fontan operation in 1971, long term results have steadily improved with newer modifications. However, atrial arrhythmias are frequent and contribute to ongoing morbidity and mortality. Data are lacking regarding the prevalence of arrhythmias and risk factors for their development in the current era.
Methods
The Pediatric Heart Network Fontan Cross-Sectional Study evaluated data from 7 centers, with 520 patients aged 6–18 years (mean 8.6±3.4 years after the Fontan operation), including echocardiograms, electrocardiograms, exercise testing, parent-reported Child Health Questionnaire (CHQ) results, and medical history.
Results
Supraventricular tachycardias were present in 9.4% of patients. Intra-atrial reentrant tachycardia (IART) was present in 7.3% (32/520). The hazard of IART decreased until 4–6 years post-Fontan, and then increased with age thereafter. Cardiac anatomy and resting heart rate (including marked bradycardia) were not associated with IART. We identified three independent associations of time to occurrence of IART: lower CHQ physical summary score (p<0.001); predominant rhythm (p=0.002; highest risk with paced rhythm), and type of Fontan operation (p=0.037; highest risk with atriopulmonary connection). Time to IART did not differ between patients with lateral tunnel and extracardiac conduit types of Fontan repair. Ventricular tachycardia was noted in 3.5% of patients.
Conclusions
Overall prevalence of IART was lower in this cohort (7.3%) than previously reported. Lower functional status, an atriopulmonary connection and paced rhythm were determined to be independently associated with development of IART after Fontan.
Keywords: Fontan, Intraatrial Reentrant Tachycardia, Arrhythmia, Congenital Heart Disease, Prevalence
Background
Since its introduction in 1971, the Fontan operation has consistently been the primary surgical technique used for palliation of patients with single ventricle physiology.(1) Morbidity and mortality in Fontan patients have decreased dramatically but rhythm abnormalities remain a significant problem (2–6). Previous studies of cohorts having undergone the Fontan operation have recognized that arrhythmias are an important contributor to morbidity. The most frequent of these is intra-atrial reentrant tachycardia (IART), seen in 16–22% of patients at 5 years’ follow-up (2,7,8). Detailed information regarding factors that influence the development of arrhythmias in this population is limited. Importantly, controversy exists in the pediatric cardiology and cardiovascular surgery community as to possible differences in arrhythmia burden between the lateral tunnel Fontan and the extracardiac conduit Fontan procedures.
Using data from the Pediatric Heart Network’s (PHN) Fontan Cross-Sectional Study, we examined the prevalence of arrhythmias in the current generation of Fontan survivors, as well as features of anatomy and surgical repair that may be associated with arrhythmia prevalence. We sought to identify any factors associated with development of IART, and any difference in atrial arrhythmia prevalence between the two most commonly performed Fontan procedures in the current era.
Methods
Funded by the National Heart, Lung, and Blood Institute, the PHN is a collective of pediatric cardiac centers in the United States and Canada, supported by a data coordinating center at the New England Research Institutes. The Fontan Cross-Sectional Study gathered data from seven centers in 2003–2004 and examined multiple clinical aspects of Fontan survivors, with a primary aim of exploring the correlations between functional outcomes and ventricular performance measures.(9–11) The study was approved by an Institutional Review Board or Research Ethics Board at each participating institution. Written informed consent was obtained from a parent or guardian and assent obtained where applicable.
Fontan Cross-Sectional Study
The Fontan cross-sectional study included surviving children aged 6–18 years who had undergone a Fontan procedure at least 6 months prior to entering the study (9). The subjects were identified through a search of all cases seen in the prior three years, and invited to participate. Study data and testing within 3 months of enrollment included echocardiograms, electrocardiograms (ECG), exercise testing results, health status questionnaires, and medical history from a review of the medical record. Patients were excluded if they had a co-existing non-cardiac health condition that would preclude participation in the study protocol or otherwise confound study endpoints.
Study Participants
A total of 1078 records were screened for potential participation in the Fontan Cross-Sectional Study, and 644 patients (60%) were found to be eligible. Of these, 546 consented (85%) to participate in the Fontan Cross-Sectional Study. From this study sample, a subgroup was selected for this analysis: 17 were excluded because they had no ECG or exercise test completed; 7 were excluded because they had undergone Fontan conversion which would alter the natural history of the Fontan operation, one patient was excluded due to second degree heart block, and one because the nature of the arrhythmia was indeterminate. Thus 520 patients were included in the analytic data set (Table 1).
Table 1.
Fontan Cross-Sectional Study Subject Characteristics
| Mean±SD or % |
|||||
|---|---|---|---|---|---|
| Characteristic | Total n |
All | History of IART |
No History of IART |
p- value |
| N | 520 | 38 | 482 | ||
| Age at study, years (median) | 520 | 11.9±3.4 (11.3) | 14.4±3.6 (15.6) | 11.7±3.3 (11.1) | <.001 |
| Age at most recent Fontan, years (median) | 520 | 3.4±1.9 (2.8) | 3.9±1.9 (3.4) | 3.3±1.9 (2.8) | 0.01 |
| Years since most recent Fontan (median) | 520 | 8.6±3.4 (8.2) | 10.7±3.9 (12.1) | 8.5±3.3 (8.1) | <.001 |
| Male | 315 | 61% | 55% | 61% | 0.50 |
| Race/Ethnicity | 0.27 | ||||
| Hispanic | 35 | 7% | 11% | 7% | |
| Non-Hispanic, white | 375 | 76% | 81% | 76% | |
| Non-Hispanic, black | 49 | 10% | 8% | 10% | |
| Non-Hispanic, other | 33 | 6% | 0% | 7% | |
| Unknown | 28 | ||||
| Type of Fontan Operation | 0.002 | ||||
| Atriopulmonary Connection | 72 | 14% | 37% | 12% | |
| Intracardiac Lateral Tunnel | 306 | 59% | 53% | 59% | |
| Extracardiac Lateral Tunnel | 62 | 12% | 8% | 12% | |
| Extracardiac Conduit | 69 | 13% | 3% | 14% | |
| Other | 11 | 2% | 0% | 2% | |
SD=standard deviation
IART = Intra-atrial reentrant tachycardia
Medical Record Review
Standardized data forms were used to extract pertinent data from the medical record regarding details of cardiac anatomy, type of surgical repair, as well as both early and late complications since Fontan surgery, including onset of supraventricular or ventricular arrhythmias. In all patients who were identified as having supraventricular arrhythmias the relevant rhythm documentation was reviewed at the local center and the arrhythmia classified more specifically as ectopic atrial tachycardia, atrioventricular reentrant tachycardia, or IART (a primary macroreentrant atrial arrhythmia).
Electrocardiogram and Exercise Testing
A standard 12-lead ECG was performed at rest in the supine position and recorded at 25 mm/sec sweep speed with a 10 mm/mV amplitude. Bradycardia was defined as a resting heart rate less than the 5th percentile for age (12). Predominant rhythm was classified as: atrial-based (sinus and atrial escape) vs. junctional escape vs. paced (14 with other or unknown type were excluded from analyses specific to predominant rhythm). Exercise testing was performed using a standard ramp protocol on an electronically braked cycle ergometer with continuously monitored 12-lead ECGs.
Echocardiography
Two-dimensional echocardiograms and Doppler evaluations of standard short- and long-axis views of the ventricle(s) were centrally interpreted by one of two readers, and included assessment of ventricular morphology, ventricular systolic and diastolic function, and atrioventricular and semilunar valve regurgitation. Total ejection fraction was expressed as a z-score relative to age in normal children (13).
Child Health Questionnaire (CHQ)
The CHQ Parent Report PF-50 has been validated in healthy children aged 5–18 years and in cardiac cohorts (10,14). This instrument provides summary scores for physical (CHQ-p) and psychosocial (CHQ-ps) well-being.
Statistical Methodology
Groups were defined by history versus no history of IART after the Fontan procedure. The Fisher exact test was used to compare the distributions of categorical variables by group, and two-sample t-test and Wilcoxon rank sum test were used for comparison of the distributions of continuous variables by group. Time to IART was defined as the number of years from the Fontan procedure to first IART diagnosis. Follow-up was censored at study enrollment for patients having no history of IART. The Kaplan-Meier method was used to estimate the distribution of time to first episode of IART after Fontan(15). Hazard estimation was performed using kernel-based smoothing, with bootstrapped estimates of the pointwise standard errors. Independent associations of time to development of IART were identified using multivariable stepwise Cox proportional hazards regression. All variables in Table 1–3 were considered as candidates in multivariate modeling if the p value was less than 0.20 on univariate analysis. A p-value of <0.05 was considered statistically significant. Analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC) and R (kernel-based hazard estimation).
Table 3.
Association of Anatomy and Function with History of IART
| Variable | n | History of IART |
n | No History of IART |
p-value* |
|---|---|---|---|---|---|
| Anatomic diagnosis | 38 | 482 | 0.24 | ||
| Single LV: DILV and TA | 47% | 36% | |||
| Single RV: DIRV; MA and HLHS | 13% | 28% | |||
| SV, Unbalanced AV canal defect | 3% | 4% | |||
| Other | 26% | 24% | |||
| SV, Heterotaxia syndrome | 11% | 7% | |||
| Ventricular morphology | 38 | 482 | 0.13 | ||
| Left ventricular | 61% | 49% | |||
| Right ventricular | 18% | 34% | |||
| Mixed | 21% | 17% | |||
| L loop anatomy | 38 | 32% | 482 | 18% | 0.05 |
| AV valve regurgitation | 37 | 78% | 74% | 0.70 | |
| AV valve regurgitation severity | 37 | 465 | 0.38 | ||
| None | 22% | 27% | |||
| Mild | 54% | 54% | |||
| Moderate/severe | 24% | 19% | |||
| Semilunar valve regurgitation | 22 | 55% | 279 | 49% | 0.66 |
| Semilunar valve regurgitation severity | 22 | 279 | 0.94 | ||
| None | 46% | 51% | |||
| Mild | 50% | 39% | |||
| Moderate | 5% | 9% | |||
| Echo ejection fraction, % | 27 | 58.1±10.7 | 366 | 58.3±10.4 | .95 |
| Echo ejection fraction z-score | 27 | −1.0±2.1 | 366 | −0.9±2.0 | .94 |
p-value is from Fisher exact test for categorical variables and Wilcoxon rank sum test for continuous variables. For valve regurgitation severity grade, the p-value is from the Mantel-Haenszel test for linear trend.
SV=single ventricle
MA=mitral atresia
TA=tricuspid atresia
AV=atrioventricular
DILV=double inlet left ventricle
DIRV=double inlet right ventricle
HLHS=hypoplastic left heart syndrome
IART=Intraatrial Reentrant Tachycardia
Results
Prevalence of Supraventricular Tachycardias
As recently described in our recent PHN publication (6), the overall prevalence of supraventricular tachyarrhythmia was 9.6%, with 50 of 521 patients having had a history of at least one episode following hospital discharge after the Fontan procedure. One subject had an indeterminate type of supraventricular tachyarrhythmia and was excluded. Among the remaining 49, 4 (0.8%) had ectopic atrial tachycardia, 7 (1.8%) had reentrant atrioventricular tachycardia, and 38 (7.3%, representing 78% of all those with supraventricular tachyarrhythmia) had IART.
Clinical Associations of Intra-Atrial Reentrant Tachycardia
Age and Time since Fontan Operation
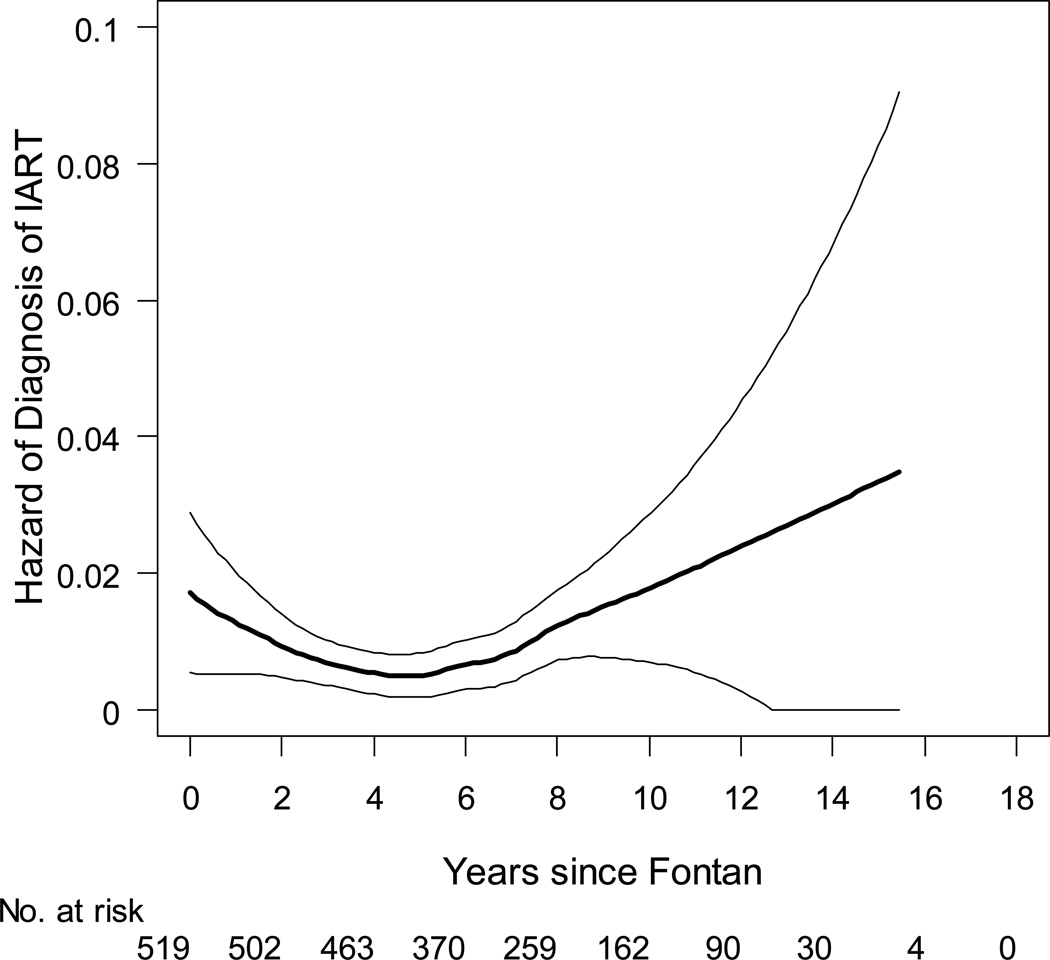
The prevalence of IART increased with age; the mean age of patients with IART was 14.4±3.6 years, while the mean age of those without IART was 11.7±3.3 years (p<0.001). The hazard of a first occurrence of IART was lowest 4–6 years after completion of the Fontan operation, with the risk of IART high in the first two years after Fontan and then increasing again in later childhood (Figure 1). Patients who underwent Fontan at an older age were also more likely to have a history of IART. However, these patients had more follow-up time post-Fontan during which IART might occur, because those who had Fontan at an older age (≥4 years) were in the upper age quartile (≥15 years) at study enrollment (Table 1).
Figure 1.
Hazard of intraatrial reentrant tachycardia following the Fontan operation, with 95% confidence bands. Date of diagnosis was missing for one patient, thus the number at risk initially is 519.
Heart Rate
We found no association between the prevalence of IART and resting heart rate, bradycardia (see Methods), or maximum heart rate achieved on exercise test (Table 2).
Table 2.
Association of Predominant Rhythm on Resting ECG and Exercise Testing with History of IART
| Variable | n | History of IART |
n | No History of IART |
p-value‡ |
|---|---|---|---|---|---|
| Mean±SD (median) or % |
Mean±SD (median) or % |
||||
| Predominant Rhythm on ECG | 36 | 468 | <.001 | ||
| Atrial based rhythm | 67% | 87% | |||
| Junctional escape | 3% | 6% | |||
| Paced | 31% | 7% | |||
| Resting HR * | 11 | 75.3±15.4 (80.0) | 433 | 75.7±16.6 (75.0) | .87 |
| Resting HR < 5th percentile* | 11 | 27% | 433 | 28% | 1.00 |
| Max HR, bpm§ | 11 | 146±30 (152) | 359 | 157±21 (160) | .31 |
| %Predicted Max HR§ | 11 | 71.2±14.6 (73.0) | 359 | 75.7±10.1 (77.0) | .44 |
| %Predicted Max HR<75§ | 11 | 55% | 359 | 41% | .37 |
| P-axis, degrees† | 23 | 17.3±58.2 (40.0) | 421 | 29.3±49.8 (38.0) | .54 |
| P-axis 0 to 90† | 23 | 65% | 421 | 77% | .21 |
| Right Atrial Enlargement on ECG | 27 | 7% | 431 | 8% | 1.00 |
| Left Atrial Enlargement on ECG | 27 | 26% | 430 | 14% | .09 |
excluded patients with a paced rhythm and patients on heart rate medications
excluded patients with rate-responsive pacemakers and patients on heart rate medications
excluded patients with a paced or junctional rhythm
p-value is from Fisher exact test for categorical variables and Wilcoxon rank sum test for continuous variables
IART= Intraatrial Reentrant Tachycardia
Resting Rhythm
Predominant rhythm on resting ECG was associated with IART (p<0.001), with a higher prevalence of IART in patients with a paced rhythm (26% of those actively paced on ECG) compared to those with an atrial-based (6%) or junctional escape rhythm (3%). This effect was independent of age. Among those with atrial rhythm, there was no significant difference in the P-wave axis between those with and without IART (Table 2).
Anatomy, Cardiac Function and Protein-Losing Enteropathy (PLE)
There was no association of IART with anatomic diagnosis, or morphology of the systemic ventricle. The prevalence of IART was marginally higher in those with L-looping compared to those without (12% vs. 6%, p=0.052). Neither the degree of atrioventricular valve regurgitation nor the systemic ventricular ejection fraction was associated with a history of IART (Table 3). PLE occurred in 7.9% of those with IART and 2.9% of those without (p=0.12; p=0.19 after age adjustment) and was not significantly associated with rhythm (data not shown).
Functional Status Assessment
CHQ-p scores were lower (p=0.001) in those with IART than those without (Table 4). The difference remained significant after adjusting for age. There was no significant difference in psychosocial scores (CHQ-ps).
Table 4.
Multivariable Cox Regression Model for IART (N=464)*
| Cox Regression Model | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|
| CHQ Physical Summary score | 1.23 per 5-unit decrease | 1.09, 1.37 | <0.001 |
| Predominant Rhythm | 0.002 | ||
| Paced vs. atrial-based | 4.01 | 1.84, 8.75 | <0.001 |
| Paced vs. junctional escape | 4.85 | 0.61, 38.70 | 0.14 |
| Atrial-based vs. junctional escape | 1.21 | 0.16, 9.07 | 0.85 |
| Type of Fontan operation | 0.04 | ||
| Atriopulmonary Connection | - | ||
| Intracardiac lateral tunnel | 0.35 | 0.17, 0.75 | 0.007 |
| Extracardiac lateral tunnel | 0.75 | 0.20, 2.87 | 0.68 |
| Extracardiac conduit | 0.22 | 0.03, 1.77 | 0.16 |
The data of 56 of 520 subjects were excluded from the model: 11 patients with ‘other’ type of Fontan, 14 with other/unknown type of predominant rhythm 30 with missing CHQ score and 1 with unknown date of discharge after Fontan.
IART=Intraatrial Reentrant Tachycardia
CHQ= Child Health Questionnaire
Fontan Operation
The type of Fontan was associated with the presence of IART. Patients with an atriopulmonary connection were more likely to have IART (19%) than those with intracardiac lateral tunnel (7%), extracardiac lateral tunnel (5%), or extracardiac conduit (2%) type (p=0.002). The prevalence of IART did not differ between patients with an extracardiac conduit (2%) and those with a lateral tunnel procedure (6%, p=0.22).
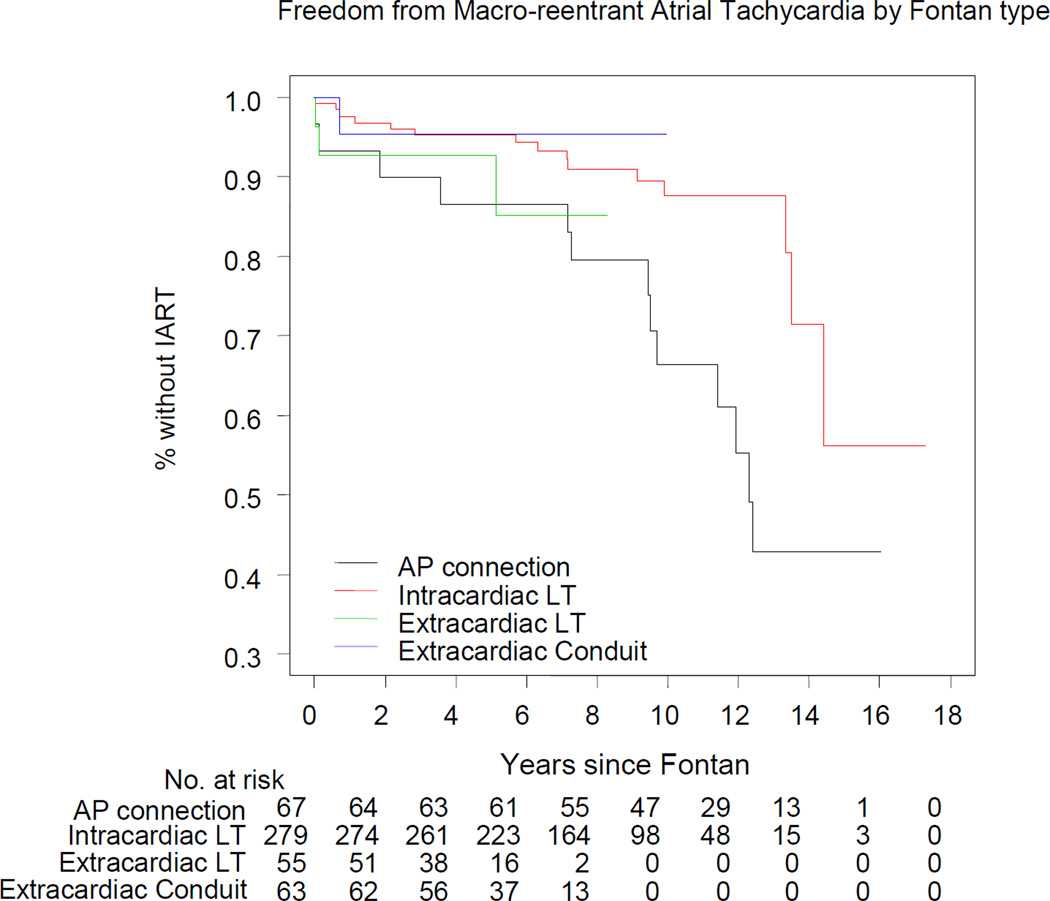
Because age has been noted to be a strong predictor of IART, and patients with atriopulmonary connections tended to be older, the association of IART with type of Fontan was reanalyzed by modeling time to first occurrence of IART as a function of number of years since the Fontan operation. We constructed a multivariable Cox regression model (N=464) to determine whether type of Fontan operation (four categories) was an independent association of time to occurrence of IART (Table 4). Among these types, only the atriopulmonary connection was independently associated with IART. There was no difference in the covariate-adjusted event-free distributions of time to IART for intracardiac lateral tunnel vs. extracardiac conduit (hazard ratio 1.58, 95% CI 0.20 to 12.24; p=0.66). Two additional factors were independently associated with IART: lower CHQ physical summary score and a paced rhythm on ECG. Figure 2 displays the covariate (CHQ and predominant rhythm)-adjusted event-free curves.
Figure 2.
Freedom from intraatrial reentrant tachycardia following the Fontan operation by type of Fontan procedure, adjusted for predominant rhythm and CHQ Physical Summary Score. Number of patients at risk in each surgical group is shown in the adjoining panel.
Pacing
Overall, 68 of 520 patients (13%) had a pacemaker or defibrillator at study enrollment (although were not necessarily actively paced on resting ECG as described in previous section). Of these 68 patients, 19 (28%) had a history of IART. IART was determined to be the primary indication for pacemaker placement in 8 of 19. Other indications for device placement included bradycardia (26), sinus node dysfunction (7), ventricular tachycardia (VT) (5), junctional rhythm (2), low cardiac output (3) and complete heart block (1). No indication was recorded in nine patients, and seven had pacemaker implantation at the time of Fontan without further specific indication. Anatomic diagnosis was examined in relationship to pacemaker presence, and as would be anticipated, there was a strong association between pacemaker and the presence of ventricular L-looping (p=0.01). There was no association between pacemaker presence and other anatomic features, or any relationship to type of surgery.
Ventricular Tachycardia (VT)
Patients with VT were defined as having had at least one documented episode of VT or ventricular fibrillation causing symptoms or requiring therapy. VT was seen in 18 patients (3.5%). Because of the infrequency of these events, we did not have the power to detect clinical associations. We found no relationship between VT and valvar regurgitation (atrioventricular or semilunar) or ejection fraction. No other associations with VT were found to be significant, including indicators of chronotropic status, atrial enlargement, type of surgical repair, anatomic subgroup or ventricular morphology.
Discussion
Intra-Atrial Reentrant Tachycardia
Although long term results have improved with contemporary modifications of the Fontan operation, IART has remained a frequent (historically 16–41%) finding and contributes to ongoing morbidity and mortality.(2,5,7,16–19) In contrast to previous studies, we found only 7.3% of subjects had experienced IART at a mean of 8.6 years follow-up. These previous studies had mean follow-up periods of 3–11 years (median 5 years), with the highest rates of IART seen in those studies with longer follow-up periods and a greater proportion of atriopulmonary connection patients. This improvement is likely multifactorial, and changes in surgical strategy have probably played an important role. We found that patients with an atriopulmonary connection-type Fontan operation were at higher risk of developing IART when compared to other surgical strategies even after adjustment for functional status and predominant rhythm. However, we hypothesize that the majority of the risk in this subgroup is conferred by older age and patients with types of Fontan procedures other than an atriopulmonary connection were younger in this study. The high hazard that we observed at 10 years post-Fontan and beyond may have an anatomic, electrophysiologic, and hemodynamic basis. Nearly all Fontan patients have atrial incisions and suture lines which may provide the substrate for the development of IART over time. Second, elevated atrial pressures present in nearly all Fontan patients at various stages of palliation lead to atrial dilation and stretch. Atrial fibrosis occurs over time and can result in the development of both anatomic conduction barriers and regions of functional block. These conditions create an anatomic and physiologic substrate that facilitates the development and maintenance of intraatrial reentrant circuits. (7,18,20)
Interestingly, there was no significant difference in the event-free distributions of time to IART among the intracardiac lateral tunnel, extracardiac lateral tunnel, and the extracardiac conduit. It has been postulated that the extracardiac conduit, by excluding the majority of atrial tissue, would be less arrhythmogenic than the lateral tunnel.(21–23) However, this was not borne out in our cohort. It is important to note that the lateral tunnel and the extracardiac conduit Fontan patients represent a relatively young cohort. Longer follow-up is needed to substantiate these findings.
Sinus node dysfunction has been previously described as associated with IART. (7) In contrast, in the present study, even marked bradycardia (heart rate less than the 5th percentile for age) was not associated with IART. Thus, isolated bradycardia per se, as a measure of sinus node dysfunction, is not independently associated with the development of IART. However, this lack of association may be an artifact of excluding the patients with a paced rhythm from heart rate analysis for bradycardia, as this subgroup was more prevalent in the IART group. In contrast, chronotropic competence is not limited by pacemaker implantation. This measure of sinus node dysfunction was also not found to be associated with IART. Non-sinus intrinsic rhythm on ECG, for example atrial or junctional escape rhythm, did not associate with IART. We were not able to discern from our data whether patients had anti-tachycardia devices placed for IART or for bradycardia.
Lower CHQ physical summary score was the third measure independently associated with IART. Due to the cross-sectional nature of this study, it is not possible to identify causality. It may be that those patients with worse physical function have impaired hemodynamics which contribute to the development of IART. Alternatively the burden of IART itself may limit physical function. Further follow-up within this study cohort will be useful in identifying any causal relationship or link between these variables.
Ventricular Tachyarrhythmias
VT occurred in 3.5% of the population. Given the prevalence of VT, interpretation of possible associations must be undertaken cautiously. Although there was a trend towards lower ejection fraction in those with VT, this did not reach significance, and none of the patients with VT were found to have an ejection fraction of less than 30%. This should not be interpreted to mean that patients with Fontan palliation and low ejection fractions are at low risk for VT. Patients with single ventricle anatomy may be vulnerable to VT with ejection fractions higher than associated with increased risk in adult heart failure populations. A survival bias may have a particularly strong effect in this subset of patients: it may be that patients with a Fontan, low ejection fraction, and VT did not survive to be included in this cohort.
Limitations
The cross-sectional nature of this study limits the chronological data that are available and thus causality cannot be shown. All arrhythmia interpretation was conducted at the individual centers, with local electrophysiologists. All participating patients were cared for at tertiary care centers; however this is the case for the majority of Fontan patients and thus external validity can be expected to be reasonable. Due to the cross-sectional design of this study and a minimum enrollment age of 6 years, inferences from this study are subject to survivor bias; some associations may be attenuated because patients with the most severe course could not contribute to analysis. Finally, as noted above, we had limited power to detect differences by type of Fontan operation for events potentially occurring more than 6 years post-Fontan, because the subgroups undergoing lateral tunnel and extracardiac conduit procedures had shorter follow-up than the patients who underwent an atriopulmonary connection. Furthermore, the number of patients receiving an extracardiac conduit was relatively small, one-quarter the size of the cohort undergoing intracardiac lateral tunnel Fontan, rendering 80% power to detect hazard ratios for IART of 2.5; therefore our observed hazard ratio of 1.6 may have been not statistically significant due to power.
Conclusions
This contemporary cohort of Fontan survivors (mean 8.6 years post-procedure) represents one of the largest data sets available in this unique population. Overall prevalence of IART (7%) is lower in the current cohort than in previous reports. Independent associated factors of IART development include a paced rhythm, lower functional status, and an atriopulmonary connection Fontan, a previously suspected risk factor for atrial tachycardia. In our study, the atriopulmonary connection does confer a higher risk of IART; however some of that association is explained by the older age of the patients at the time of the study. We observed no significant difference in time to development of IART between the lateral tunnel and extracardiac types of surgery in this study.
ACKNOWLEDGEMENTS
Supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288, HL085057).
ABBREVIATIONS
- PHN
Pediatric Heart Network
- ECG
Electrocardiogram
- IART
Intra-atrial Reentrant Tachycardia
- CHQ
Child Health Questionnaire
- PLE
Protein Losing Enteropathy
- VT
Ventricular Tachycardia
Appendix
National Heart, Lung, and Blood Institute: Gail Pearson, Mario Stylianou, Judith Massicot-Fisher*, Marsha Mathis, Victoria Pemberton
Data Coordinating Center: New England Research Institutes, Lynn Sleeper, Steven Colan, Paul Mitchell*, Dianne Gallagher, Patti Nash, Gloria Klein, Minmin Lu
Network Chair: Lynn Mahony, University of Texas Southwestern Medical Center
Clinical Site Investigators: Children’s Hospital Boston, Jane Newburger (PI), Stephen Roth*, Roger Breitbart, Jonathan Rhodes, Jodi Elder, Ellen McGrath; Children’s Hospital of New York, Welton M. Gersony (PI), Seema Mital*, Beth Printz*, Ashwin Prakash*, Darlene Servedio*; Children’s Hospital of Philadelphia, Victoria Vetter (PI), Bernard J. Clark*, Mark Fogel, Steven Paridon, Jack Rychik, Margaret Harkins*, Jamie Koh; Duke University, Page A. W. Anderson (PI) - deceased, Rene Herlong*, Lynne Hurwitz, Jennifer S. Li, Ann Marie Nawrocki*; Medical University of South Carolina, J. Philip Saul (PI), Andrew M. Atz, Andrew D. Blaufox*, Girish Shirali, Jon Lucas*, Amy Blevins*; Primary Children’s Medical Center, Salt Lake City, Utah, LuAnn Minich (PI), Richard Williams, Linda Lambert, Michael Puchalski; Hospital for Sick Children, Toronto, Brian McCrindle (PI), Timothy Bradley, Kevin Roman*, Jennifer Russell, Shi-Joon Yoo, Elizabeth Radojewski, Nancy Slater
Core Laboratories:
Cardiac MRI, Children’s Hospital Boston: Tal Geva (Director); Andrew J. Powell Echocardiography, Children’s Hospital Boston: Steven Colan (Director), Marcy Schwartz*, Renee Margossian
Protocol Review Committee: Michael Artman, Chair; Dana Connolly, Timothy Feltes, Julie Johnson, Jeffrey Krischer, G. Paul Matherne.
Data and Safety Monitoring Board: John Kugler, Chair; Kathryn Davis, David J. Driscoll, Mark Galantowicz, Sally A. Hunsberger, Thomas J. Knight, Catherine L. Webb, Lawrence Wissow.
*no longer at the institution listed
Footnotes
The authors have no relationships to disclose.
References
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–248. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelatt M, Hamilton RM, McCrindle BW, et al. Risk factors for atrial tachyarrhythmias after the Fontan operation. J Am Coll Cardiol. 1994;24:1735–1741. doi: 10.1016/0735-1097(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 3.Gardiner HM, Dhillon R, Bull C, de Leval MR, Deanfield JE. Prospective study of the incidence and determinants of arrhythmia after total cavopulmonary connection. Circulation. 1996;94:II17–II21. [PubMed] [Google Scholar]
- 4.Triedman JK, Saul JP, Weindling SN, Walsh EP. Radiofrequency ablation of intra-atrial reentrant tachycardia after surgical palliation of congenital heart disease. Circulation. 1995;91:707–714. doi: 10.1161/01.cir.91.3.707. [DOI] [PubMed] [Google Scholar]
- 5.Gentles TL, Mayer JE, Jr, Gauvreau K, et al. Fontan operation in five hundred consecutive patients: factors influencing early and late outcome. J Thorac Cardiovasc Surg. 1997;114:376–391. doi: 10.1016/s0022-5223(97)70183-1. [DOI] [PubMed] [Google Scholar]
- 6.Blaufox AD, Sleeper LA, Bradley DJ, et al. Functional status, heart rate, and rhythm abnormalities in 521 Fontan patients 6 to 18 years of age. J Thorac Cardiovasc Surg. 2008;136:100–107. doi: 10.1016/j.jtcvs.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fishberger SB, Wernovsky G, Gentles TL, et al. Factors that influence the development of atrial flutter after the Fontan operation. J Thorac Cardiovasc Surg. 1997;113:80–86. doi: 10.1016/s0022-5223(97)70402-1. [DOI] [PubMed] [Google Scholar]
- 8.Durongpisitkul K, Porter CJ, Cetta F, et al. Predictors of early- and late-onset supraventricular tachyarrhythmias after Fontan operation. Circulation. 1998;98:1099–1107. doi: 10.1161/01.cir.98.11.1099. [DOI] [PubMed] [Google Scholar]
- 9.Sleeper LA, Anderson P, Hsu DT, et al. Design of a large cross-sectional study to facilitate future clinical trials in children with the Fontan palliation. Am Heart J. 2006;152:427–433. doi: 10.1016/j.ahj.2006.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCrindle BW, Williams RV, Mitchell PD, et al. Relationship of patient and medical characteristics to health status in children and adolescents after the Fontan procedure. Circulation. 2006;113:1123–1129. doi: 10.1161/CIRCULATIONAHA.105.576660. [DOI] [PubMed] [Google Scholar]
- 11.Anderson PA, Sleeper LA, Mahony L, et al. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol. 2008;52:85–98. doi: 10.1016/j.jacc.2008.01.074. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davignon ARP, Coiselle E, et al. Normal ECG standards for infants and children. Pediatr Cardiol. 1979;1:123–152. [Google Scholar]
- 13.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–457. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 14.Landgraf JM, Abetz L, Ware JE. The Child Health Questionnaire (CHQ) User's Manual. Boston: Health Act; 1999. [Google Scholar]
- 15.Muller HG, Wang JL. Hazard rate estimation under random censoring with varying kernels and bandwidths. Biometrics. 1994;50:61–76. [PubMed] [Google Scholar]
- 16.Fishberger SB, Wernovsky G, Gentles TL, et al. Long-term outcome in patients with pacemakers following the Fontan operation. Am J Cardiol. 1996;77:887–889. doi: 10.1016/s0002-9149(97)89191-6. [DOI] [PubMed] [Google Scholar]
- 17.Freedom RM, Hamilton R, Yoo SJ, et al. The Fontan procedure: analysis of cohorts and late complications. Cardiology in the Young. 2000;10:307–331. doi: 10.1017/s1047951100009616. [DOI] [PubMed] [Google Scholar]
- 18.Gewillig M, Wyse RK, de Leval MR, Deanfield JE. Early and late arrhythmias after the Fontan operation: predisposing factors and clinical consequences. Br Heart J. 1992;67:72–79. doi: 10.1136/hrt.67.1.72. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghai A, Harris L, Harrison DA, Webb GD, Siu SC. Outcomes of late atrial tachyarrhythmias in adults after the Fontan operation. J Am Coll Cardiol. 2001;37:585–592. doi: 10.1016/s0735-1097(00)01141-4. [DOI] [PubMed] [Google Scholar]
- 20.Driscoll DJ, Offord KP, Feldt RH, et al. Five- to fifteen-year follow-up after Fontan operation. Circulation. 1992;85:469–496. doi: 10.1161/01.cir.85.2.469. [DOI] [PubMed] [Google Scholar]
- 21.Petrossian E, Reddy VM, McElhinney DB, et al. Early results of the extracardiac conduit Fontan operation. J Thorac Cardiovasc Surg. 1999;117:688–696. doi: 10.1016/S0022-5223(99)70288-6. [DOI] [PubMed] [Google Scholar]
- 22.Marcelletti CF, Hanley FL, Mavroudis C, et al. Revision of previous Fontan connections to total extracardiac cavopulmonary anastomosis: A multicenter experience. J Thorac Cardiovasc Surg. 2000;119:340–346. doi: 10.1016/S0022-5223(00)70190-5. [DOI] [PubMed] [Google Scholar]
- 23.Azakie A, McCrindle BW, Van Arsdell G, et al. Extracardiac conduit versus lateral tunnel cavopulmonary connections at a single institution: impact on outcomes. J Thorac Cardiovasc Surg. 2001;122:1219–1228. doi: 10.1067/mtc.2001.116947. [DOI] [PubMed] [Google Scholar]