Abstract
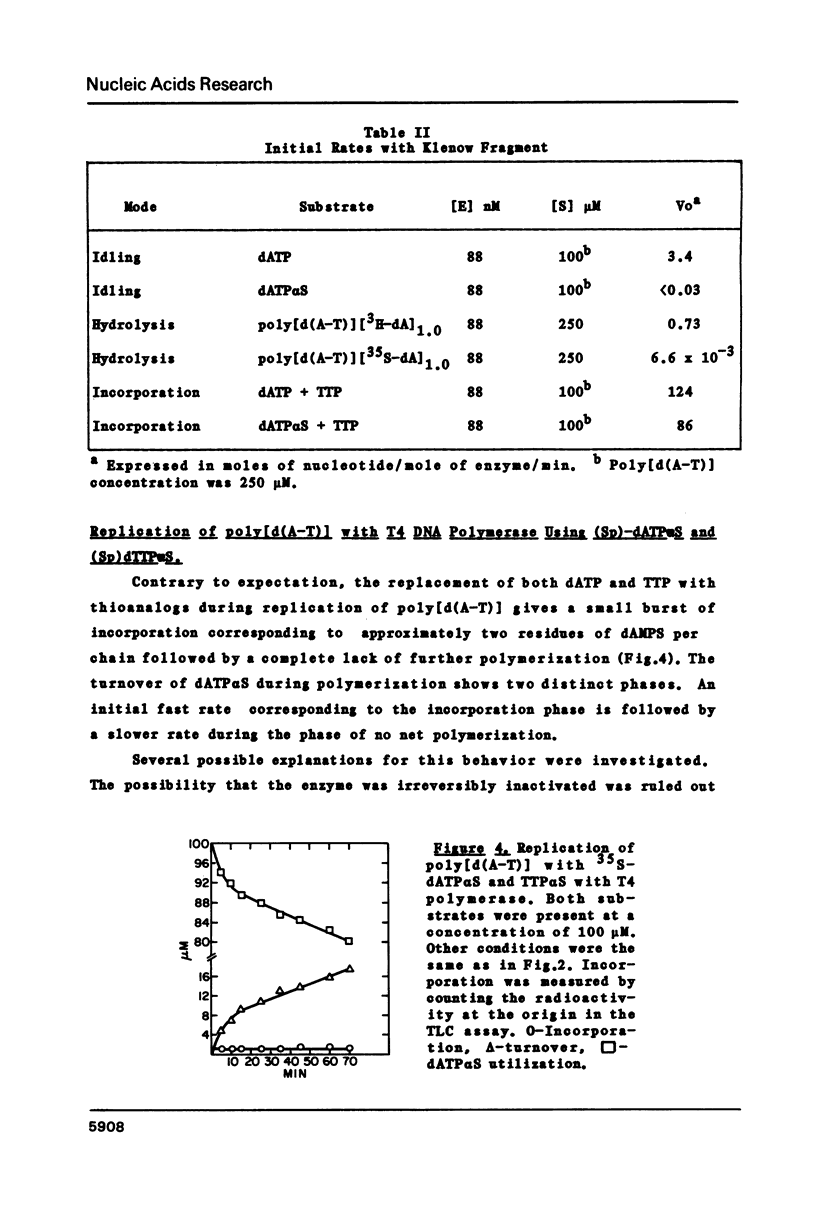
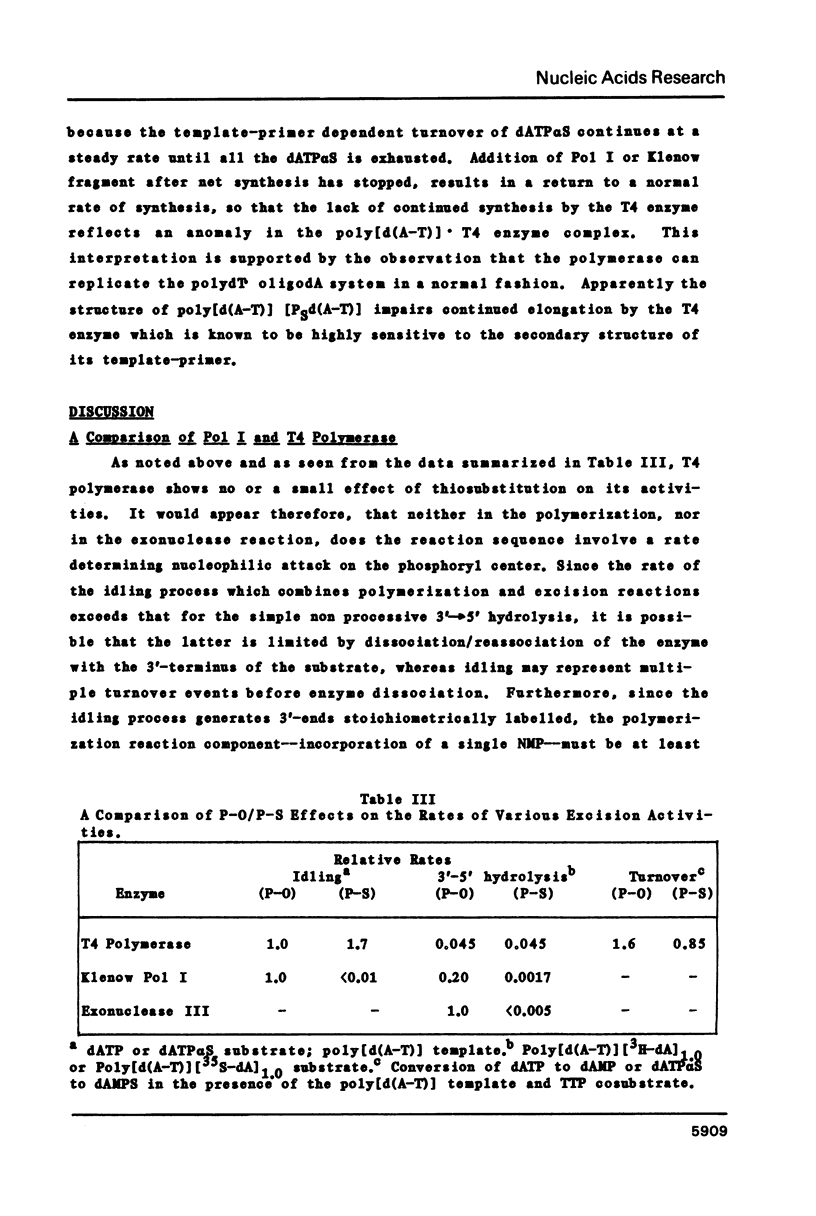
The 3'----5' exonuclease activities of T4 DNA polymerase and the Klenow fragment of Polymerase I towards the phosphoryl and thiophosphoryl 3',5' linkage were examined under comparable conditions of idling-turnover, duplex hydrolysis and turnover during polymerization. With the T4 enzyme there is a negligible effect of thiosubstitution on these activities; with the Klenow fragment there is a greater than one hundred-fold reduction in rate with the thiolinkage for the exonuclease but not polymerization activities. This inability to hydrolyze rapidly the thiophosphoryl linkage extends to the hydrolytic activity of Exonuclease III. The quantitation of the exonuclease activities of these three proteins under various conditions should aid in the successful employment of thiophosphoryl nucleoside triphosphates for their incorporation into DNA.
Full text
PDF
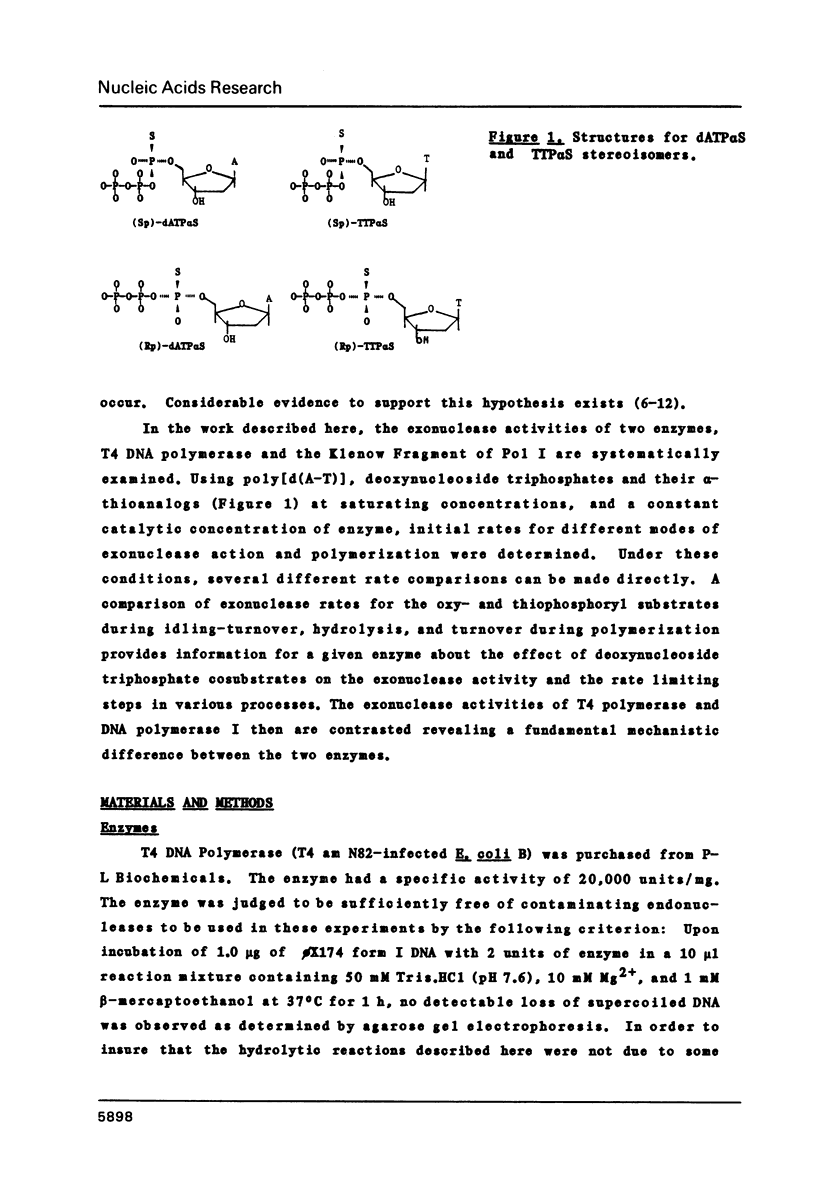




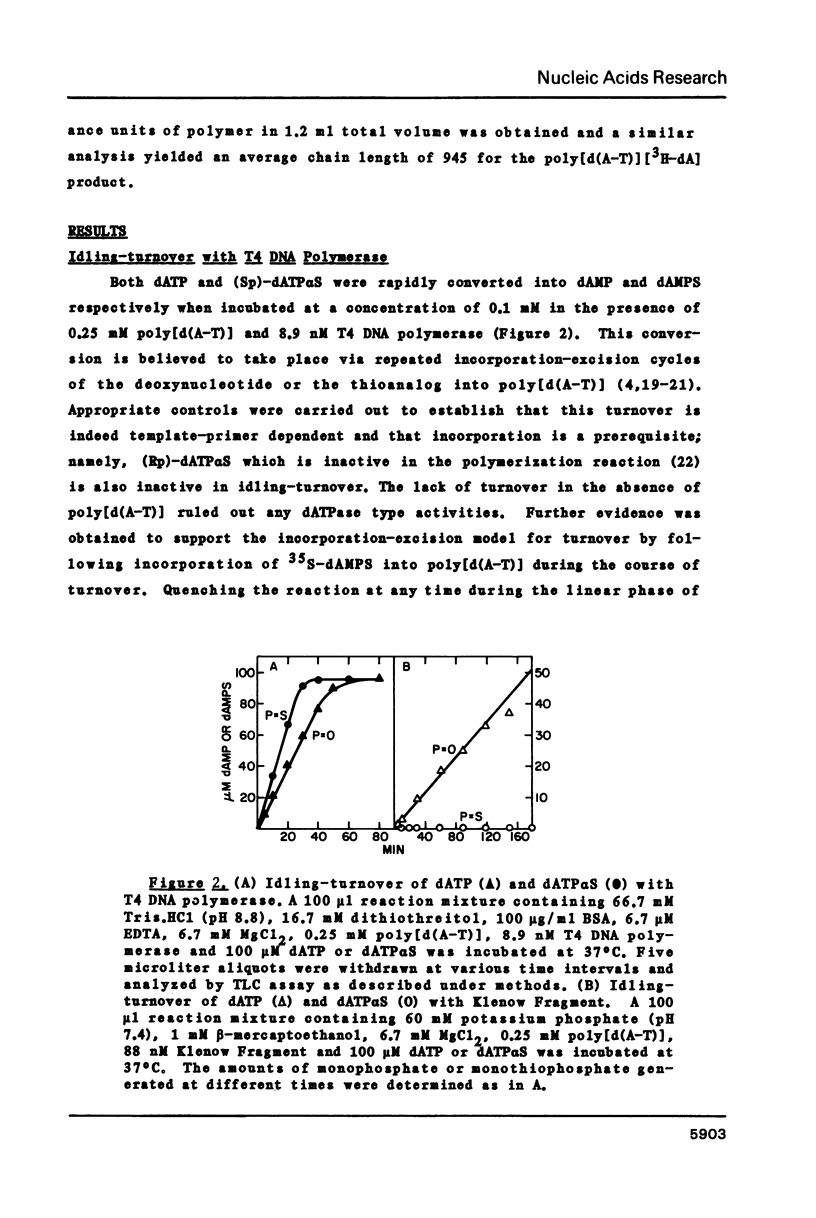

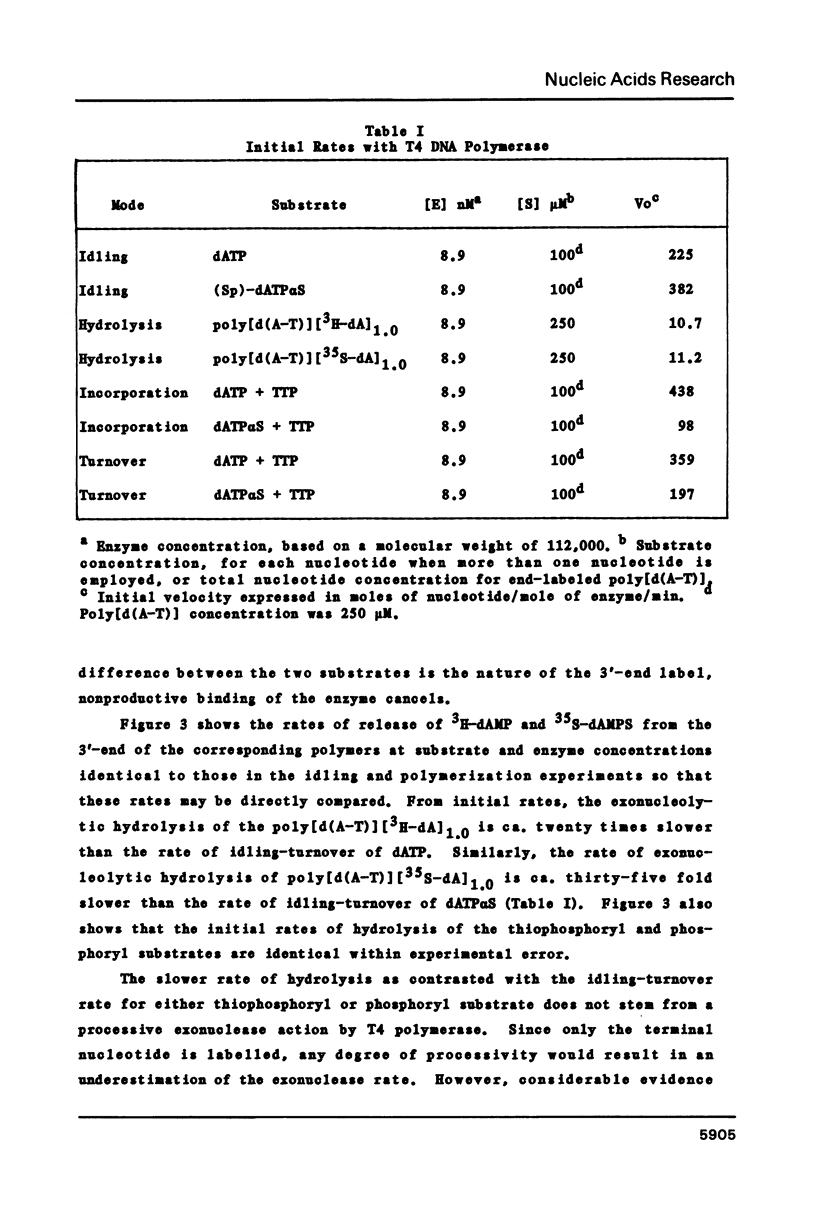
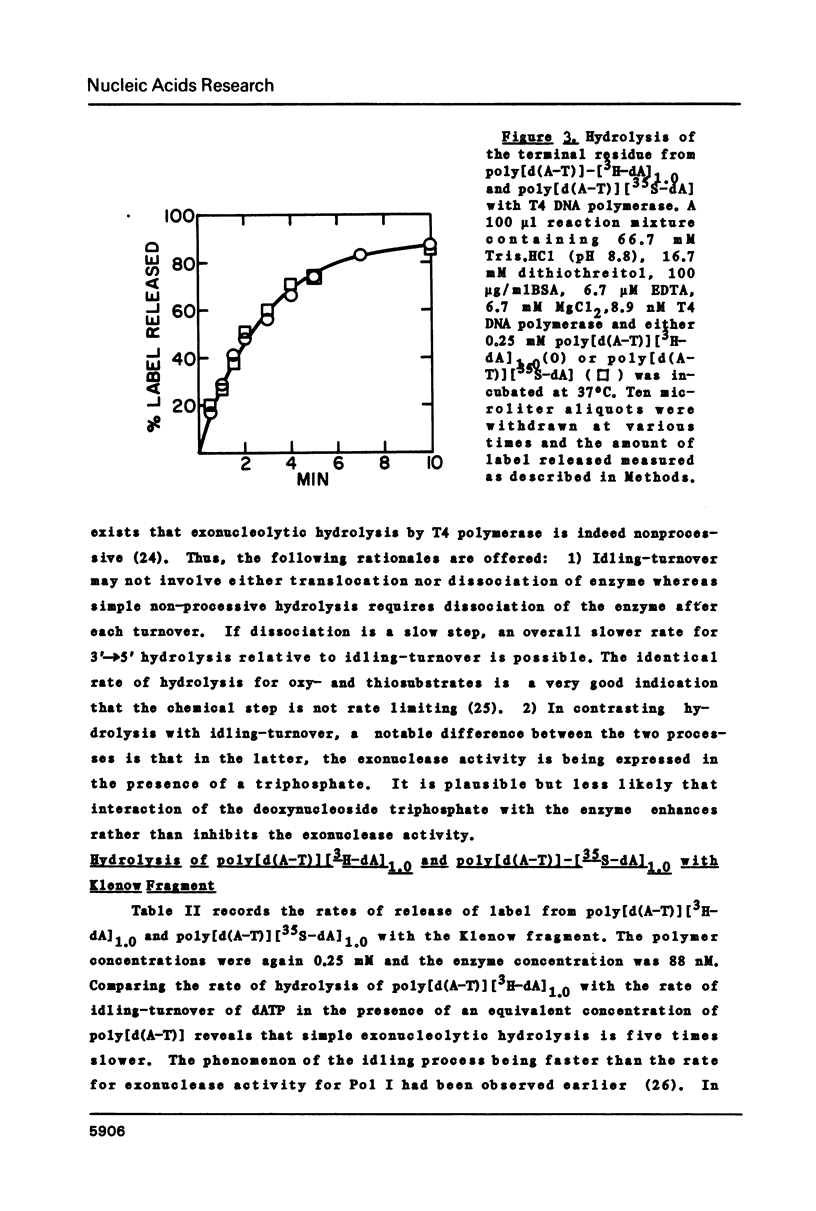



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessman M. J., Muzyczka N., Goodman M. F., Schnaar R. L. Studies on the biochemical basis of spontaneous mutation. II. The incorporation of a base and its analogue into DNA by wild-type, mutator and antimutator DNA polymerases. J Mol Biol. 1974 Sep 15;88(2):409–421. doi: 10.1016/0022-2836(74)90491-4. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3' leads to 5' exonuclease activity in deoxyribonucleic acid polymerases. J Biol Chem. 1972 Jan 10;247(1):241–248. [PubMed] [Google Scholar]
- Burgers P. M., Eckstein F. Diastereomers of 5'-O-adenosyl 3'-O-uridyl phosphorothioate: chemical synthesis and enzymatic properties. Biochemistry. 1979 Feb 20;18(4):592–596. doi: 10.1021/bi00571a007. [DOI] [PubMed] [Google Scholar]
- Chen J. T., Benkovic S. J. Synthesis and separation of diastereomers of deoxynucleoside 5'-O-(1-thio)triphosphates. Nucleic Acids Res. 1983 Jun 11;11(11):3737–3751. doi: 10.1093/nar/11.11.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton L. K., Goodman M. F., Branscomb E. W., Galas D. J. Error induction and correction by mutant and wild type T4 DNA polymerases. Kinetic error discrimination mechanisms. J Biol Chem. 1979 Mar 25;254(6):1902–1912. [PubMed] [Google Scholar]
- Das S. K., Fujimura R. K. Mechanism of 3' to 5' exonuclease associated with phage T5-induced DNA polymerase: processiveness and template specificity. Nucleic Acids Res. 1980 Feb 11;8(3):657–671. doi: 10.1093/nar/8.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S. K., Fujimura R. K. Mechanism of primer-template-dependent conversion of dNTP leads to dNMP by T5 DNA polymerase. J Biol Chem. 1980 Aug 10;255(15):7149–7154. [PubMed] [Google Scholar]
- Englund P. T. Analysis of nucleotide sequences at 3' termini of duplex deoxyribonucleic acid with the use of the T4 deoxyribonucleic acid polymerase. J Biol Chem. 1971 May 25;246(10):3269–3276. [PubMed] [Google Scholar]
- Galas D. J., Branscomb E. W. Enzymatic determinants of DNA polymerase accuracy. Theory of coliphage T4 polymerase mechanisms. J Mol Biol. 1978 Oct 5;124(4):653–687. doi: 10.1016/0022-2836(78)90176-6. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Nossal N. G. Control of mutation frequency by bacteriophage T4 DNA polymerase. II. Accuracy of nucleotide selection by the L88 mutator, CB120 antimutator, and wild type phage T4 DNA polymerases. J Biol Chem. 1976 Sep 10;251(17):5225–5232. [PubMed] [Google Scholar]
- Goodman M. F., Hopkins R., Gore W. C. 2-Aminopurine-induced mutagenesis in T4 bacteriophage: a model relating mutation frequency to 2-aminopurine incorporation in DNA. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4806–4810. doi: 10.1073/pnas.74.11.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., DeBrosse C., Benkovic S. J. Template-prime-dependent turnover of (Sp)-dATP alpha S by T4 DNA polymerase. The stereochemistry of the associated 3' goes to 5'-exonuclease. J Biol Chem. 1982 Jul 10;257(13):7689–7692. [PubMed] [Google Scholar]
- Hall Z. W., Lehman I. R. An in vitro transversion by a mutationally altered T4-induced DNA polymerase. J Mol Biol. 1968 Sep 28;36(3):321–333. doi: 10.1016/0022-2836(68)90158-7. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S., Nossal N. G. Hydrolysis of template and newly synthesized deoxyribonucleic acid by the 3' to 5' exonuclease activity of the T4 deoxyribonucleic acid polymerase. J Biol Chem. 1972 Jun 10;247(11):3393–3404. [PubMed] [Google Scholar]
- Hershfield M. S. On the role of deoxyribonucleic acid polymerase in determining mutation rates. Characterization of the defect in the T4 deoxyribonucleic acid polymerase caused by the ts L88 mutation. J Biol Chem. 1973 Feb 25;248(4):1417–1423. [PubMed] [Google Scholar]
- Kunkel T. A., Eckstein F., Mildvan A. S., Koplitz R. M., Loeb L. A. Deoxynucleoside [1-thio]triphosphates prevent proofreading during in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6734–6738. doi: 10.1073/pnas.78.11.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A., Dube D. K., Beckman R. A., Koplitz M., Gopinathan K. P. On the fidelity of DNA replication. Nucleoside monophosphate generation during polymerization. J Biol Chem. 1981 Apr 25;256(8):3978–3987. [PubMed] [Google Scholar]
- Murray A. W., Atkinson M. R. Adenosine 5'-phosphorothioate. A nucleotide analog that is a substrate, competitive inhibitor, or regulator of some enzymes that interact with adenosine 5'-phosphate. Biochemistry. 1968 Nov;7(11):4023–4029. doi: 10.1021/bi00851a032. [DOI] [PubMed] [Google Scholar]
- Muzyczka N., Poland R. L., Bessman M. J. Studies on the biochemical basis of spontaneous mutation. I. A comparison of the deoxyribonucleic acid polymerases of mutator, antimutator, and wild type strains of bacteriophage T4. J Biol Chem. 1972 Nov 25;247(22):7116–7122. [PubMed] [Google Scholar]
- Price S. S., Schwing J. M., Englund P. T. The 3'-terminal heptanucleotide sequence of the l strand of T7 deoxyribonucleic acid. J Biol Chem. 1973 Oct 25;248(20):7001–7006. [PubMed] [Google Scholar]
- Putney S. D., Benkovic S. J., Schimmel P. R. A DNA fragment with an alpha-phosphorothioate nucleotide at one end is asymmetrically blocked from digestion by exonuclease III and can be replicated in vivo. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7350–7354. doi: 10.1073/pnas.78.12.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON C. C., SCHILDKRAUT C. L., APOSHIAN H. V., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XIV. FURTHER PURIFICATION AND PROPERTIES OF DEOXYRIBONUCLEIC ACID POLYMERASE OF ESCHERICHIA COLI. J Biol Chem. 1964 Jan;239:222–232. [PubMed] [Google Scholar]
- Romaniuk P. J., Eckstein F. A study of the mechanism of T4 DNA polymerase with diastereomeric phosphorothioate analogues of deoxyadenosine triphosphate. J Biol Chem. 1982 Jul 10;257(13):7684–7688. [PubMed] [Google Scholar]
- Setlow P., Brutlag D., Kornberg A. Deoxyribonucleic acid polymerase: two distinct enzymes in one polypeptide. I. A proteolytic fragment containing the polymerase and 3' leads to 5' exonuclease functions. J Biol Chem. 1972 Jan 10;247(1):224–231. [PubMed] [Google Scholar]
- Thomas K. R., Olivera B. M. Processivity of DNA exonucleases. J Biol Chem. 1978 Jan 25;253(2):424–429. [PubMed] [Google Scholar]


